Evaluation of Current Amikacin Dosing Recommendations and Development of an Interactive Nomogram: The Role of Albumin
Abstract
1. Introduction
2. Materials and Methods
2.1. Pharmacokinetic Analysis
2.2. Amikacin Dosage Guidelines Evaluation
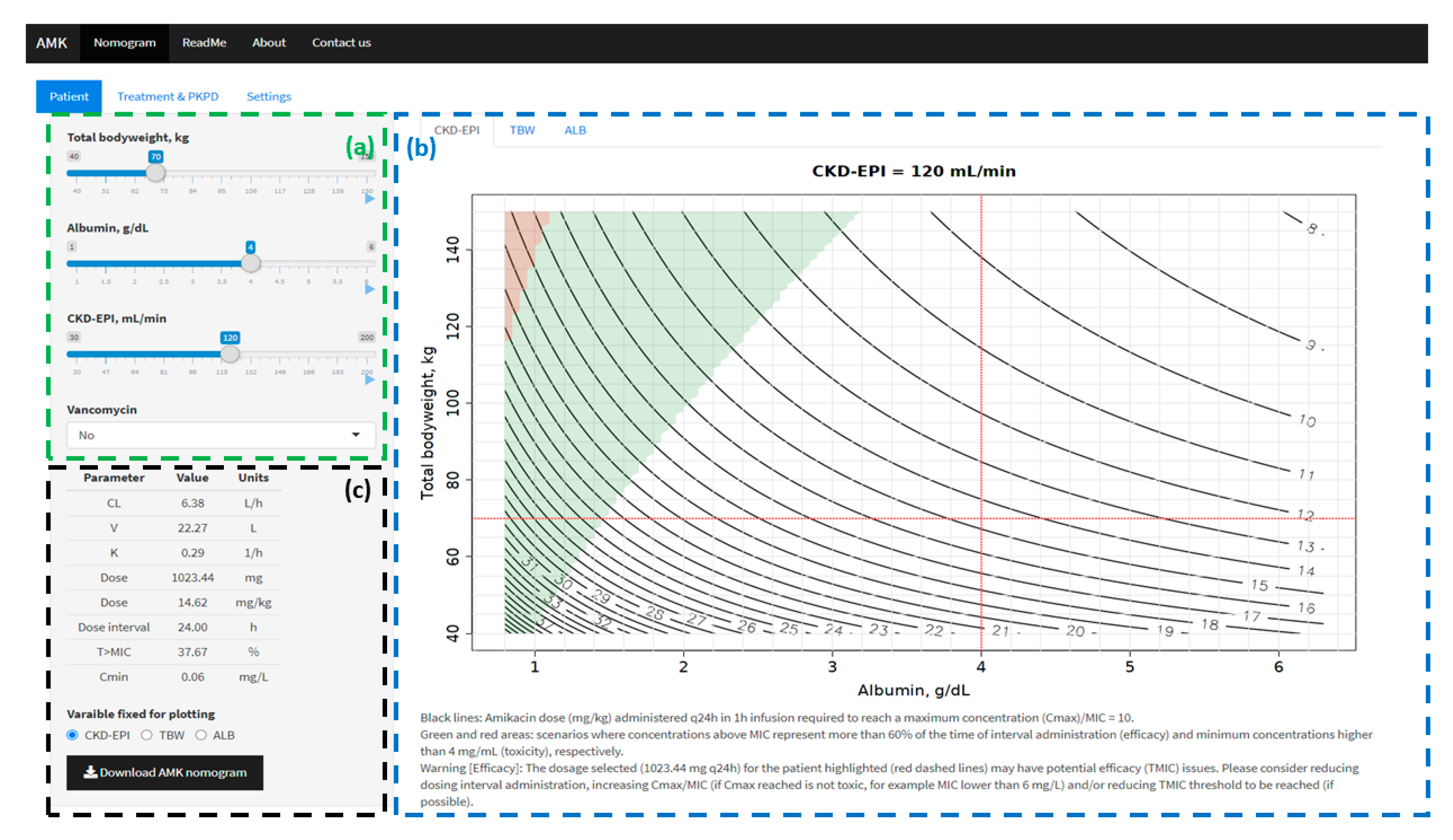
2.3. AMKnom: Interactive Amikacin Nomograms
- A.
- Input menu (left panel): information was displayed in three tabs: (i) Patient: TBW (kg), ALB (g/dL), CKD-EPI (mL/min) and co-medication with vancomycin (yes/no); (ii) Treatment (time of infusion (h), dosing interval (h) and MIC (mg/L)) and PKPD thresholds (Cmax/MIC, T>MIC and Cmin); (iii) Graphical settings. PK parameters and PKPD target values for the specific scenario defined (patient, treatment and PKPD thresholds) are summarized at the bottom of patient tab.
- B.
- Graphical output (main panel): amikacin dose expressed in mg/kg (black solid lines) required to reach the selected Cmax/MIC threshold at steady state (Treatment & PKPD tab of input menu) was calculated for all possible values of two of the following variables: TBW, ALB and CKD-EPI. For each combination of two variables, the remaining variable was fixed to the value introduced in the patient tab of the input menu. Three different tabs are defined based on the fixed variable (CKD-EPI, TBW and ALB). For each dosing scenario using the Cmax/MIC criterion to define the dose, the T>MIC and Cmin were calculated. A green area was drawn when T>MIC complied with the threshold defined in Treatment & PKPD tab (input menu). A red area was drawn when Cmin was equal or higher than the toxicity threshold (Cmin) defined in Treatment & PKPD tab (input menu). A specific scenario defined in input menu is represented across the three graphical representations.
2.4. Evaluation of the Impact of Intrinsic Factors on Amikacin Dosing Regimens
3. Results
3.1. Amikacin Dosage Guidelines Evaluation
3.2. AMKnom: Interactive Amikacin Nomograms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruiz, J.; Ramirez, P.; Company, M.J.; Gordon, M.; Villarreal, E.; Concha, P.; Aroca, M.; Frasquet, J.; Remedios-Marqués, M.; Castellanos-Ortega, Á. Impact of amikacin pharmacokinetic/pharmacodynamic index on treatment response in critically ill patients. J. Glob. Antimicrob. Resist. 2018, 12, 90–95. [Google Scholar] [CrossRef]
- White, B.P.; Lomaestro, B.; Pai, M.P. Optimizing the initial amikacin dosage in adults. Antimicrob. Agents Chemother. 2015, 59, 7094–7096. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Lawson, C.D.; Zelenitsky, S.; Findlay, B.; Schweizer, F.; Adam, H.; Walkty, A.; Rubinstein, E.; Gin, A.S.; Hoban, D.J.; et al. Comparison of the next-generation aminoglycoside plazomicin to gentamicin, tobramycin and amikacin. Expert Rev. Anti. Infect. Ther. 2012, 10, 459–473. [Google Scholar] [CrossRef]
- Bland, C.M.; Pai, M.P.; Lodise, T.P. Reappraisal of contemporary pharmacokinetic and pharmacodynamic principles for informing aminoglycoside dosing. Pharmacotherapy 2018, 38, 1229–1238. [Google Scholar] [CrossRef]
- Touchard, C.; Aubry, A.; Eloy, P.; Bréchot, N.; Lebreton, G.; Franchineau, G.; Besset, S.; Hékimian, G.; Nieszkowska, A.; Leprince, P.; et al. Predictors of insufficient peak amikacin concentration in critically ill patients on extracorporeal membrane oxygenation. Crit. Care 2018, 22, 1–9. [Google Scholar] [CrossRef]
- De Winter, S.; Wauters, J.; Meersseman, W.; Verhaegen, J.; Van Wijngaerden, E.; Peetermans, W.; Annaert, P.; Verelst, S.; Spriet, I. Higher versus standard amikacin single dose in emergency department patients with severe sepsis and septic shock: A randomised controlled trial. Int. J. Antimicrob. Agents 2018, 51, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Mensa, J.; Soriano, A.; García-Sánchez, J.E.; Letang, E.; López-Suñé, E.; Marco, F.; Llinares, E.; Barberán, J. Therapeutic Guide Mensa 2020; Antares: Barcelona, Spain, 2020; ISBN 978-84-88825-29-2. [Google Scholar]
- Medication Safety Queensland. Aminoglycoside dosing in adults. Department of Health. Available online: https://www.health.qld.gov.au/__data/assets/pdf_file/0019/713323/aminoglycoside-guidelines.pdf (accessed on 16 December 2020).
- Gilbert, D.N.; Chambers, D.N.; Eliopoulos, G.M.; Saag, M.S.; Pavia, A.T.; Black, D.; Freedman, D.O.; Kim, K.; Schwartz, B.S. The Sanford Guide to Antimicrobial Therapy 2019: 50 Years: 1969–2019; Editorial Médica A.W.E.E.S.A: Madrid, Spain, 2019; ISBN 978-987-639-059-0. [Google Scholar]
- UpToDate. Available online: https://www.uptodate.com/contents/search (accessed on 16 December 2020).
- Zazo, H.; Martín-Suárez, A.; Lanao, J.M. Evaluating amikacin dosage regimens in intensive care unit patients: A pharmacokinetic/pharmacodynamic analysis using Monte Carlo simulation. Int. J. Antimicrob. Agents 2013, 42, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Hoo, G.S.R.; Liew, Y.X.; Kwa, A.L.-H. Optimisation of antimicrobial dosing based on pharmacokinetic and pharmacodynamic principles. Indian J. Med. Microbiol. 2017, 35, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Ben Romdhane, H.; Ben Fredj, N.; Chaabane, A.; Ben Aicha, S.; Chadly, Z.; Ben Fadhel, N.; Boughattas, N.; Aouam, K. Interest of therapeutic drug monitoring of aminoglycosides administered by a monodose regimen. Néphrol. Ther. 2019, 15, 110–114. [Google Scholar] [CrossRef]
- Pérez-Blanco, J.S.; Sáez Fernández, E.M.; Calvo, M.V.; Lanao, J.M.; Martín-Suárez, A. Amikacin initial dosage in patients with hypoalbuminaemia: An interactive tool based on a population pharmacokinetic approach. J. Antimicrob. Chemother. 2020, 75, 2222–2231. [Google Scholar] [CrossRef]
- Burdet, C.; Pajot, O.; Couffignal, C.; Armand-Lefèvre, L.; Foucrier, A.; Laouénan, C.; Wolff, M.; Massias, L.; Mentré, F. Population pharmacokinetics of single-dose amikacin in critically ill patients with suspected ventilator-associated pneumonia. Eur. J. Clin. Pharmacol. 2015, 71, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Matar, K.M.; Al-lanqawi, Y.; Abdul-Malek, K.; Jelliffe, R. Amikacin population pharmacokinetics in critically ill Kuwaiti patients. BioMed Res. Int. 2013, 2013, 202818:1–202818:8. [Google Scholar] [CrossRef]
- Jang, S.B.; Lee, Y.J.; Park, M.S.; Song, Y.G.; Kim, J.-H.; Kim, H.K.; Ahn, B.S.; Park, K. Population pharmacokinetics of amikacin in a Korean clinical population. Int. J. Clin. Pharmacol. Ther. 2011, 49, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Delattre, I.K.; Musuamba, F.T.; Nyberg, J.; Taccone, F.S.; Laterre, P.-F.; Verbeeck, R.K.; Jacobs, F.; Wallemacq, P.E. Population pharmacokinetic modeling and optimal sampling strategy for bayesian estimation of amikacin exposure in critically ill septic patients. Ther. Drug Monit. 2010, 32, 749–756. [Google Scholar] [CrossRef]
- Lugo-Goytia, G.; Castañeda-Hernández, G. Bayesian approach to control of amikacin serum concentrations in critically ill patients with sepsis. Ann. Pharmacother. 2000, 34, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Joubert, P.; Bressolle, F.; Gouby, A.; Douçot, P.Y.; Saissi, G.; Gomeni, R. A population approach to the forecasting of amikacin plasma and urinary levels using a prescribed dosage regimen. Eur. J. Drug Metab. Pharmacokinet. 1999, 24, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Fdez de Gatta, M.M.; Calvo, M.V.; Caballero, D.; Dominguez-Gil, A.; Lanao, J.M. Population pharmacokinetics of amikacin in patients with haematological malignancies. J. Antimicrob. Chemother. 1999, 44, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Romano, S.; Fdez de Gatta, M.D.M.; Calvo, V.; Mendez, E.; Domínguez-Gil, A.; Lanao, J.M. Influence of clinical diagnosis in the population pharmacokinetics of amikacin in intensive care unit patients. Clin. Drug Investig. 1998, 15, 435–444. [Google Scholar] [CrossRef]
- Tod, M.; Lortholary, O.; Seytre, D.; Uzzan, B.; Guillevin, L.; Casassus, P.; Petitjean, O. Population pharmacokinetic study of amikacin administered once or twice daily to febrile, severely neutropenic adults. Antimicrob. Agents Chemother. 1998, 42, 849–856. [Google Scholar] [CrossRef]
- Lugo, G.; Castañeda-Hernández, G. Amikacin bayesian forecasting in critically ill patients with sepsis and cirrhosis. Ther. Drug Monit. 1997, 19, 271–276. [Google Scholar] [CrossRef]
- Lugo, G.; Castañeda-Hernández, G. Relationship between hemodynamic and vital support measures and pharmacokinetic variability of amikacin in critically ill patients with sepsis. Crit. Care Med. 1997, 25, 806–811. [Google Scholar] [CrossRef]
- Debord, J.; Pessis, C.; Voultoury, J.C.; Marquet, P.; Lotfi, H.; Merle, L.; Lachâtre, G. Population pharmacokinetics of amikacin in intensive care unit patients studied by NPEM algorithm. Fundam. Clin. Pharmacol. 1995, 9, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Maire, P.; Barbaut, X.; Girard, P.; Mallet, A.; Jelliffe, R.W.; Berod, T. Preliminary results of three methods for population pharmacokinetic analysis (NONMEM, NPML, NPEM) of amikacin in geriatric and general medicine patients. Int. J. Biomed. Comput. 1994, 36, 139–141. [Google Scholar] [CrossRef]
- Debord, J.; Voultoury, J.C.; Lachatre, G.; Gay, C.; Favereau, J.P.; Gay, R. Population pharmacokinetic parameters for bayesian monitoring of amikacin therapy in intensive care unit patients. Eur. J. Clin. Pharmacol. 1992, 43, 435–436. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.M.; Ramírez, M.; Cueva, L.; Alvarez, S.; de Loza, R.; Gamba, G. Low serum albumin and the increased risk of amikacin nephrotoxicity. Rev. Investig. Clin. 1994, 46, 37–43. [Google Scholar]
- Medicine Online Information Center of Spanish Agency for Medicines and Health Products. Available online: https://cima.aemps.es/cima/publico/lista.html (accessed on 26 January 2020).
- Stankowicz, M.S.; Ibrahim, J.; Brown, D.L. Once-daily aminoglycoside dosing: An update on current literature. Am. J. Health Syst. Pharm. 2015, 72, 1357–1364. [Google Scholar] [CrossRef]
- Freeman, C.D.; Nicolau, D.P.; Belliveau, P.P.; Nightingale, C.H. Once-daily dosing of aminoglycosides: Review and recommendations for clinical practice. J. Antimicrob. Chemother. 1997, 39, 677–686. [Google Scholar] [CrossRef]
- Mavros, M.N.; Polyzos, K.A.; Rafailidis, P.I.; Falagas, M.E. Once versus multiple daily dosing of aminoglycosides for patients with febrile neutropenia: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2011, 66, 251–259. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. Clinical Breakpoints and Dosing of Antibiotics. 2020. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 16 December 2020).
- De Montmollin, E.; Bouadma, L.; Gault, N.; Mourvillier, B.; Mariotte, E.; Chemam, S.; Massias, L.; Papy, E.; Tubach, F.; Wolff, M.; et al. Predictors of insufficient amikacin peak concentration in critically ill patients receiving a 25 mg/kg total body weight regimen. Intensive Care Med. 2014, 40, 998–1005. [Google Scholar] [CrossRef]
- Gálvez, R.; Luengo, C.; Cornejo, R.; Kosche, J.; Romero, C.; Tobar, E.; Illanes, V.; Llanos, O.; Castro, J. Higher than recommended amikacin loading doses achieve pharmacokinetic targets without associated toxicity. Int. J. Antimicrob. Agents 2011, 38, 146–151. [Google Scholar] [CrossRef]
- Duszynska, W.; Taccone, F.; Hurkacz, M.; Kowalska-Krochmal, B.; Wiela-Hojeńska, A.; Kübler, A. Therapeutic drug monitoring of amikacin in septic patients. Crit. Care 2013, 17, R165:1–R165:10. [Google Scholar] [CrossRef]
- Bartal, C.; Danon, A.; Schlaeffer, F.; Reisenberg, K.; Alkan, M.; Smoliakov, R.; Sidi, A.; Almog, Y. Pharmacokinetic dosing of aminoglycosides: A controlled trial. Am. J. Med. 2003, 114, 194–198. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. 2012. Available online: https://www.r-project.org/doc/R-SDLC.pdf (accessed on 27 January 2021).
- Wojciechowski, J.; Hopkins, A.; Upton, R. Interactive pharmacometric applications using R and the Shiny package. CPT: Pharmacomet. Syst. Pharmacol. 2015, 4, 146–159. [Google Scholar] [CrossRef]
- Darwich, A.S.; Ogungbenro, K.; Vinks, A.A.; Powell, J.R.; Reny, J.-L.; Marsousi, N.; Daali, Y.; Fairman, D.; Cook, J.; Lesko, L.J.; et al. Why has model-informed precision dosing not yet become common clinical reality? lessons from the past and a roadmap for the future. Clin. Pharmacol. Ther. 2017, 101, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Guerber, F.; Scheen, A.; Ellam, T.; Bouquegneau, A.; Guergour, D.; Mariat, C.; Pottel, H. Discrepancies between the Cockcroft–Gault and Chronic Kidney Disease Epidemiology (CKD-EPI) equations: Implications for refining drug dosage adjustment strategies. Clin. Pharmacokinet. 2017, 56, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Sáez Fernández, E.M.; Pérez-Blanco, J.S.; Lanao, J.M.; Calvo, M.V.; Martín-Suárez, A. Evaluation of renal function equations to predict amikacin clearance. Expert Rev. Clin. Pharmacol. 2019, 12, 805–813. [Google Scholar] [CrossRef]
- Levey, A.; Inker, L. Assessment of glomerular filtration rate in health and disease: A state of the art review. Clin. Pharmacol. Ther. 2017, 102, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Paraboni, L. Pharmacokinetics/pharmacodynamics of antibacterials in the intensive care unit: Setting appropriate dosing regimens. Int. J. Antimicrob. Agents 2008, 32, 294–301. [Google Scholar] [CrossRef]
- Margarson, M.P.; Soni, N. Serum Albumin: Touchstone or totem? Anaesthesia 1998, 53, 789–803. [Google Scholar] [CrossRef]
- Blackburn, L.M.; Tverdek, F.P.; Hernandez, M.; Bruno, J.J. First-dose pharmacokinetics of aminoglycosides in critically ill haematological malignancy patients. Int. J. Antimicrob. Agents 2015, 45, 46–53. [Google Scholar] [CrossRef]
- Alhadab, A.A.; Ahmed, M.A.; Brundage, R.C. Amikacin pharmacokinetic-pharmacodynamic analysis in pediatric cancer patients. Antimicrob. Agents Chemother. 2018, 62, e01781-17:1–e01781-17:12. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, C.M.T.; Wead, S.; Stockmann, C.; Healy, D.; Spigarelli, M.G.; Neely, A.; Kagan, R. Amikacin population pharmacokinetics among paediatric burn patients. Burns 2014, 40, 311–318. [Google Scholar] [CrossRef]
- Yu, T.; Stockmann, C.; Healy, D.P.; Olson, J.; Wead, S.; Neely, A.N.; Kagan, R.J.; Spigarelli, M.G.; Sherwin, C.M.T. Determination of optimal amikacin dosing regimens for pediatric patients with burn wound sepsis. J. Burn Care Res. 2015, 36, e244–e252. [Google Scholar] [CrossRef] [PubMed]
- Boidin, C.; Jenck, S.; Bourguignon, L.; Torkmani, S.; Roussey-Jean, A.; Ledochowski, S.; Marry, L.; Ammenouche, N.; Dupont, H.; Marçon, F.; et al. Determinants of amikacin first peak concentration in critically ill patients. Fundam. Clin. Pharmacol. 2018, 32, 669–677. [Google Scholar] [CrossRef]
- Taccone, F.; Laterre, P.-F.; Spapen, H.; Dugernier, T.; Delattre, I.; Layeux, B.; De Backer, D.; Wittebole, X.; Wallemacq, P.; Vincent, J.-L.; et al. Revisiting the loading dose of amikacin for patients with severe sepsis and septic shock. Crit. Care 2010, 14, R53:1–R53:10. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, K.; Hamishehkar, H.; Najmeddin, F.; Ahmadi, A.; Hazrati, E.; Honarmand, H.; Mojtahedzadeh, M. High-dose amikacin for achieving serum target levels in critically ill elderly patients. Infect. Drug Resist. 2018, 11, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Hagihara, M.; Hirai, J.; Sakanashi, D.; Suematsu, H.; Nishiyama, N.; Koizumi, Y.; Yamagishi, Y.; Matsuura, K.; Mikamo, H. Evaluation of amikacin pharmacokinetics and pharmacodynamics for pptimal initial dosing regimen. Drugs R&D 2017, 17, 177–187. [Google Scholar] [CrossRef]
- Kale-Pradhan, P.B.; Buckler, V.; Bush, P.W. Effect of body weight on aminoglycoside pharmacokinetics in patients with hypoalbuminemia. Am. J. Health Syst. Pharm. 1997, 54, 2201–2203. [Google Scholar] [CrossRef]
- Crass, R.L.; Ross, B.E.; Derstine, B.A.; Lichty, M.; Sullivan, J.A.; Su, G.L.; Wang, S.C.; Pai, M.P. Measurement of skeletal muscle area improves estimation of aminoglycoside clearance across body size. Antimicrob. Agents Chemother. 2018, 62, e00441-18:1–e00441-18:10. [Google Scholar] [CrossRef]
- Bonate, P.L. Pharmacokinetic-Pharmacodynamic Modeling and Simulation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006; ISBN 978-0-387-27199-6. [Google Scholar]
- Leykin, Y.; Miotto, L.; Pellis, T. Pharmacokinetic considerations in the obese. Best Pract. Res. Clin. Anaesthesiol. 2011, 25, 27–36. [Google Scholar] [CrossRef]
- Prado, C.; Maia, Y.; Ormsbee, M.; Sawyer, M.; Baracos, V. Assessment of nutritional status in cancer—The relationship between body composition and pharmacokinetics. Anticancer Agents Med. 2013, 13, 1197–1203. [Google Scholar] [CrossRef]
- Hollenberg, S.M. Vasoactive drugs in circulatory shock. Am. J. Respir. Crit. Care Med. 2011, 183, 847–855. [Google Scholar] [CrossRef]
- Tormo, C.; Abad, F.J.; Ronchera-Oms, C.L.; Parra, V.; Jiménez, N.V. Critically-ill patients receiving total parenteral nutrition show altered amikacin pharmacokinetics. Clin. Nutr. 1995, 14, 254–259. [Google Scholar] [CrossRef]
- Germovsek, E.; Barker, C.I.; Sharland, M. What do I need to know about aminoglycoside antibiotics? Arch. Dis. Child Educ. Pract. Ed. 2017, 102, 89–93. [Google Scholar] [CrossRef]
- Bertino, J.S.; Booker, L.A.; Franck, P.A.; Jenkins, P.L.; Franck, K.R.; Nafziger, A.N. Incidence of and significant risk factors for aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J. Infect. Dis. 1993, 167, 173–179. [Google Scholar] [CrossRef]
- Wicha, S.G.; Kees, M.G.; Solms, A.; Minichmayr, I.K.; Kratzer, A.; Kloft, C. TDMx: A novel web-based open-access support tool for optimising antimicrobial dosing regimens in clinical routine. Int. J. Antimicrob. Agents 2015, 45, 442–444. [Google Scholar] [CrossRef] [PubMed]
- InsightRX—Precision Dosing Done Right. Available online: https://insight-rx.com/ (accessed on 26 December 2020).
- Thirion, D.J.G.; Pasche, V.; Matouk, E.; Marsot, A. Amikacin nomogram for treatment of adult cystic fibrosis exacerbations based on an external evaluation of a population pharmacokinetic model. Pediatr. Pulmonol. 2020, 55, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Jelliffe, R.W. Creatinine clearance: Bedside estimate. Ann. Intern. Med. 1973, 79, 604–605. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Zarowitz, B.J.; Peterson, E.L.; Dumler, F. Predictability of creatinine clearance estimates in critically ill patients. Crit. Care Med. 1993, 21, 1487–1495. [Google Scholar] [CrossRef]
- Cockcroft, D.W.; Gault, H. Prediction of creatinine clearance from serum creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]


| Creatinine Clearance (mL/min) | |||||||
|---|---|---|---|---|---|---|---|
| Guide | ≤10 | 10–20 | 20–30 | 30–40 | 40–60 | 60–80 | >80 |
| EUCAST | 25–30 (24) | 25–30 (24) | 25–30 (24) | 25–30 (24) | 25–30 (24) | 25–30 (24) | 25–30 (24) |
| Mensa | 7.5–10 (48) | - | 12 (48) | - | 12 (24) ƍ | 15–20 (24) | 15–20 (24) |
| Queensland | 16 Δ | 16 Δ | 16 Δ | 16 Δ | 16–20 (36) | 20 (24) ♦ | 20 (24) ♦ |
| Sanford | 3 (72) † | 4 (48) | 7.5 (48) | 4 (24) | 7.5 (24) | 12 (24) | 15 (24) |
| UpToDate | 7.5 (48–72) ¶ | 7.5 (24–72) ¶ | 7.5 (24–72) ¶ | 15 (48) | 15 (36) | 15 (24) | 15 (24) & |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Blanco, J.S.; Sáez Fernández, E.M.; Calvo, M.V.; Lanao, J.M.; Martín-Suárez, A. Evaluation of Current Amikacin Dosing Recommendations and Development of an Interactive Nomogram: The Role of Albumin. Pharmaceutics 2021, 13, 264. https://doi.org/10.3390/pharmaceutics13020264
Pérez-Blanco JS, Sáez Fernández EM, Calvo MV, Lanao JM, Martín-Suárez A. Evaluation of Current Amikacin Dosing Recommendations and Development of an Interactive Nomogram: The Role of Albumin. Pharmaceutics. 2021; 13(2):264. https://doi.org/10.3390/pharmaceutics13020264
Chicago/Turabian StylePérez-Blanco, Jonás Samuel, Eva María Sáez Fernández, María Victoria Calvo, José M. Lanao, and Ana Martín-Suárez. 2021. "Evaluation of Current Amikacin Dosing Recommendations and Development of an Interactive Nomogram: The Role of Albumin" Pharmaceutics 13, no. 2: 264. https://doi.org/10.3390/pharmaceutics13020264
APA StylePérez-Blanco, J. S., Sáez Fernández, E. M., Calvo, M. V., Lanao, J. M., & Martín-Suárez, A. (2021). Evaluation of Current Amikacin Dosing Recommendations and Development of an Interactive Nomogram: The Role of Albumin. Pharmaceutics, 13(2), 264. https://doi.org/10.3390/pharmaceutics13020264