Understanding the Salt-Dependent Outcome of Glycine Polymorphic Nucleation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurement of Solubility
2.3. Measurement of Nucleation Temperature and Metastable Zone Width
2.4. Solution Mediated Polymorphic Transformation
3. Results and Discussion
3.1. Solubility Data
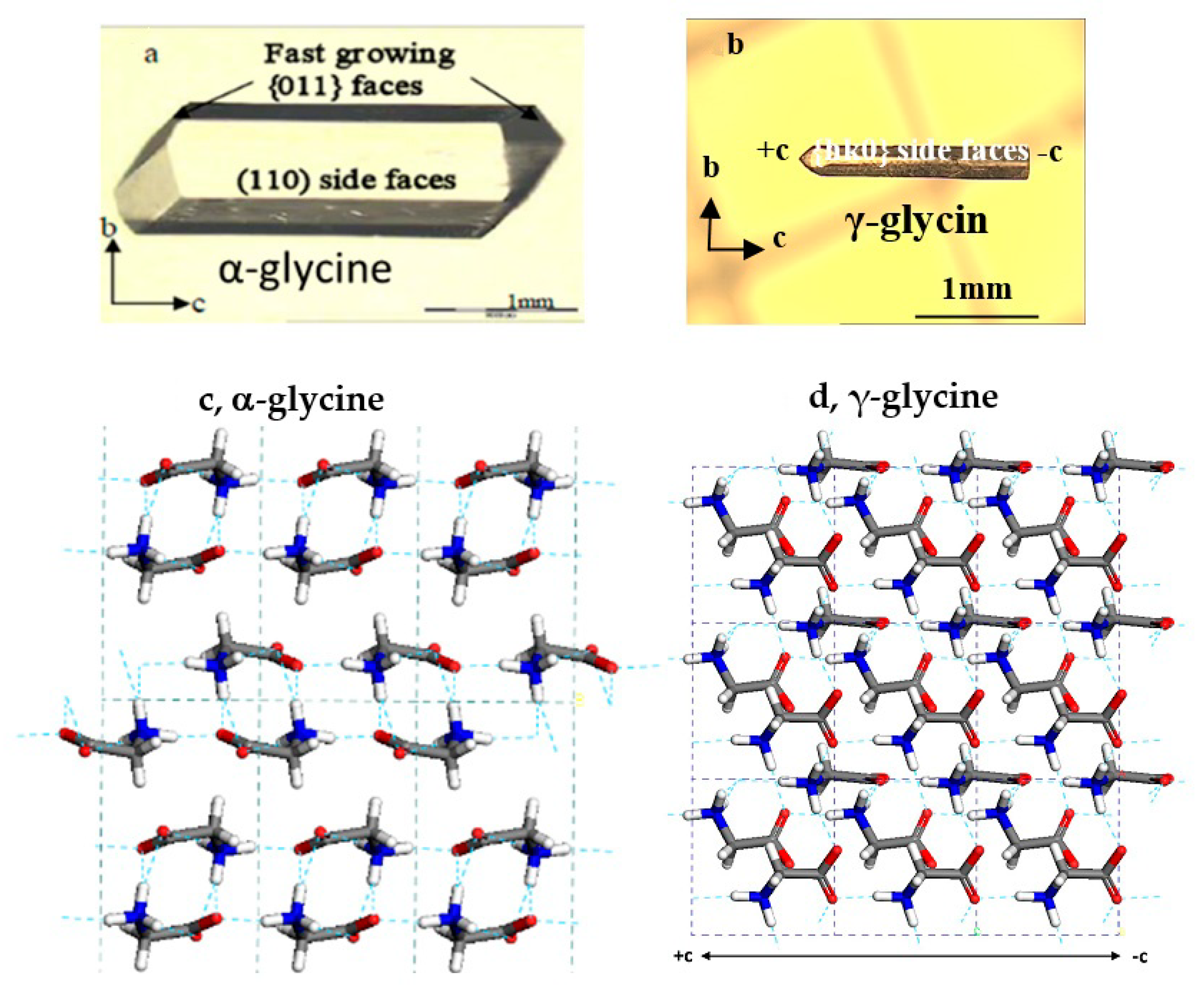
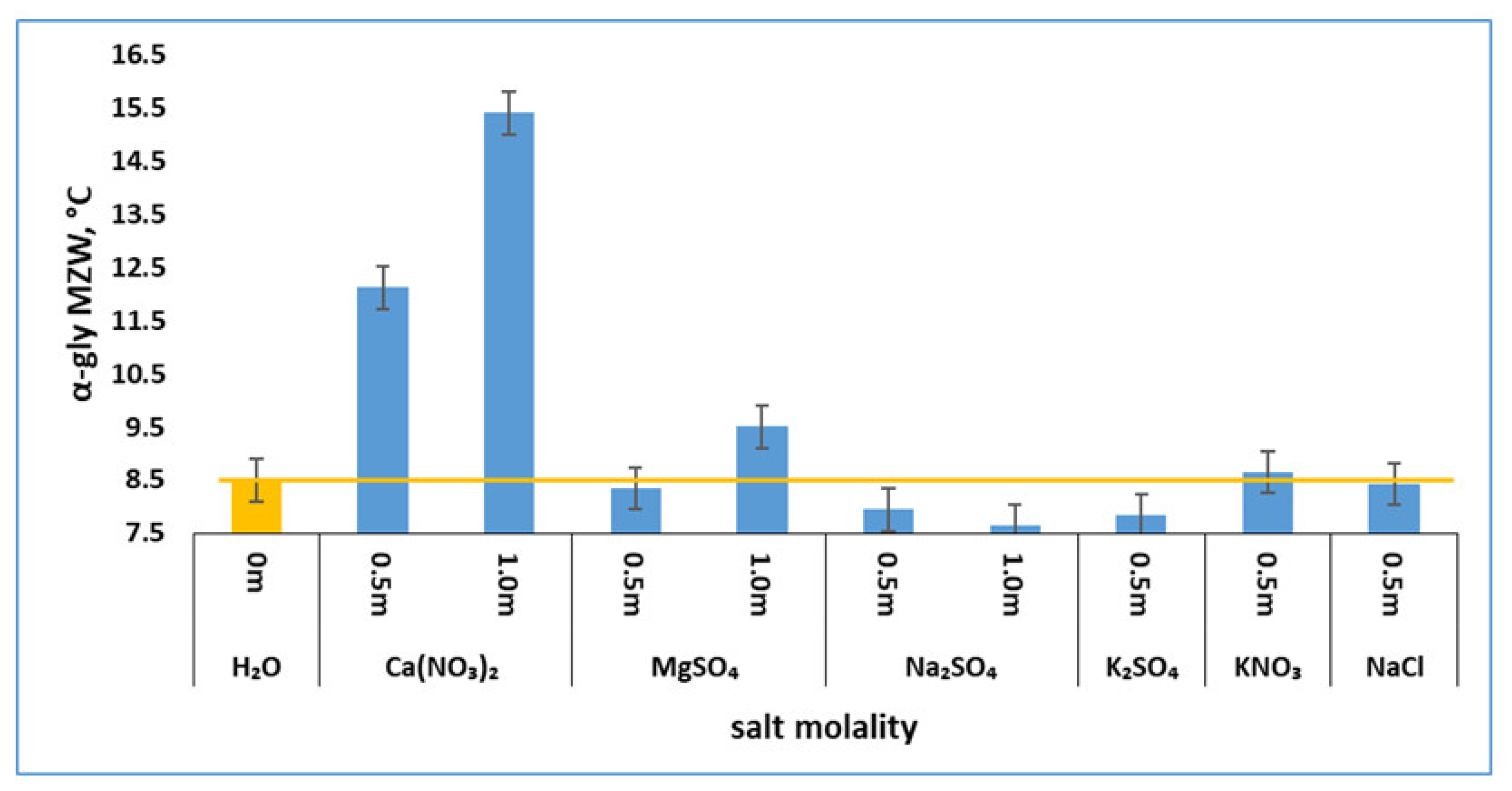
3.2. Effects of Salts on α-Glycine Primary Nucleation
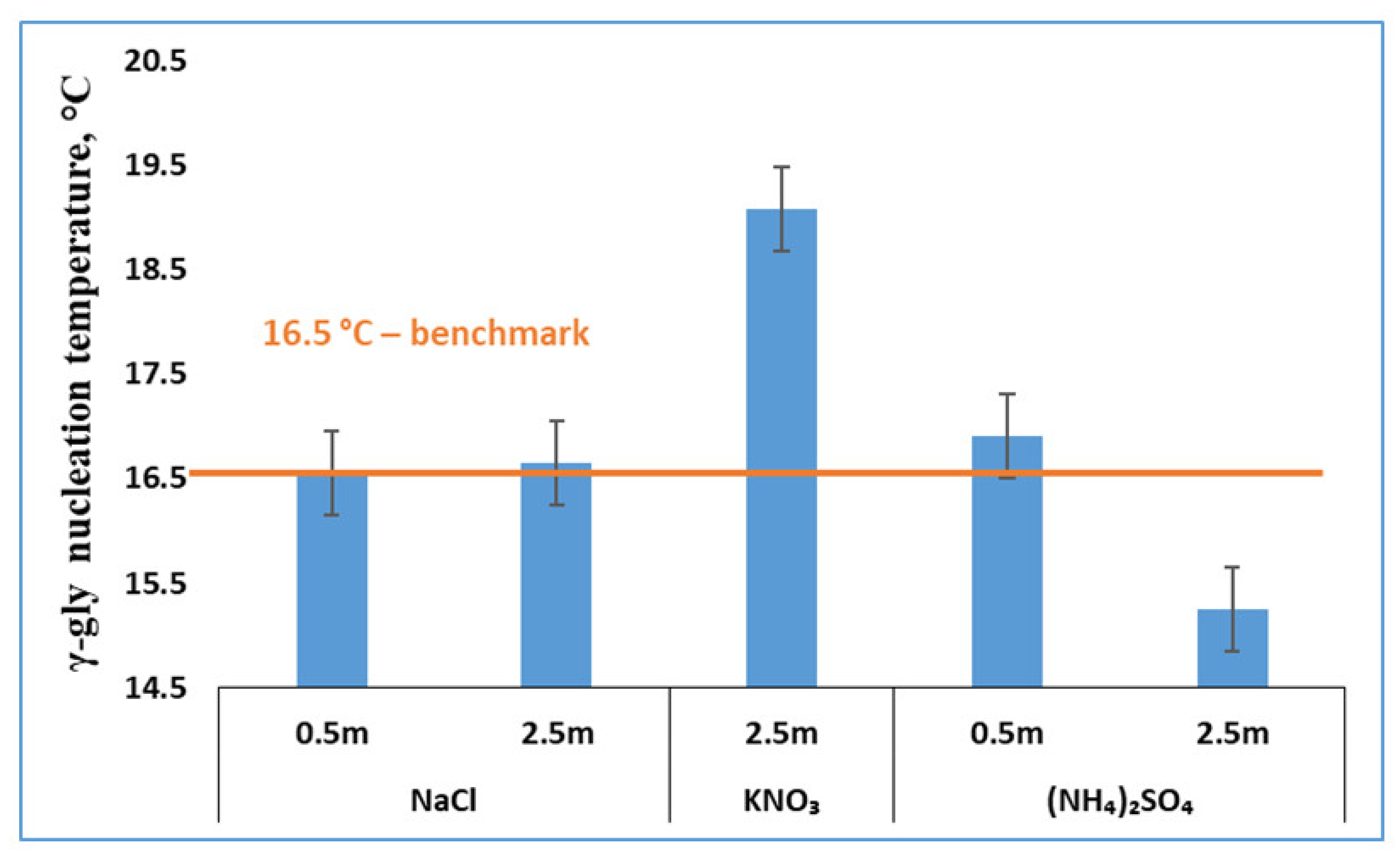
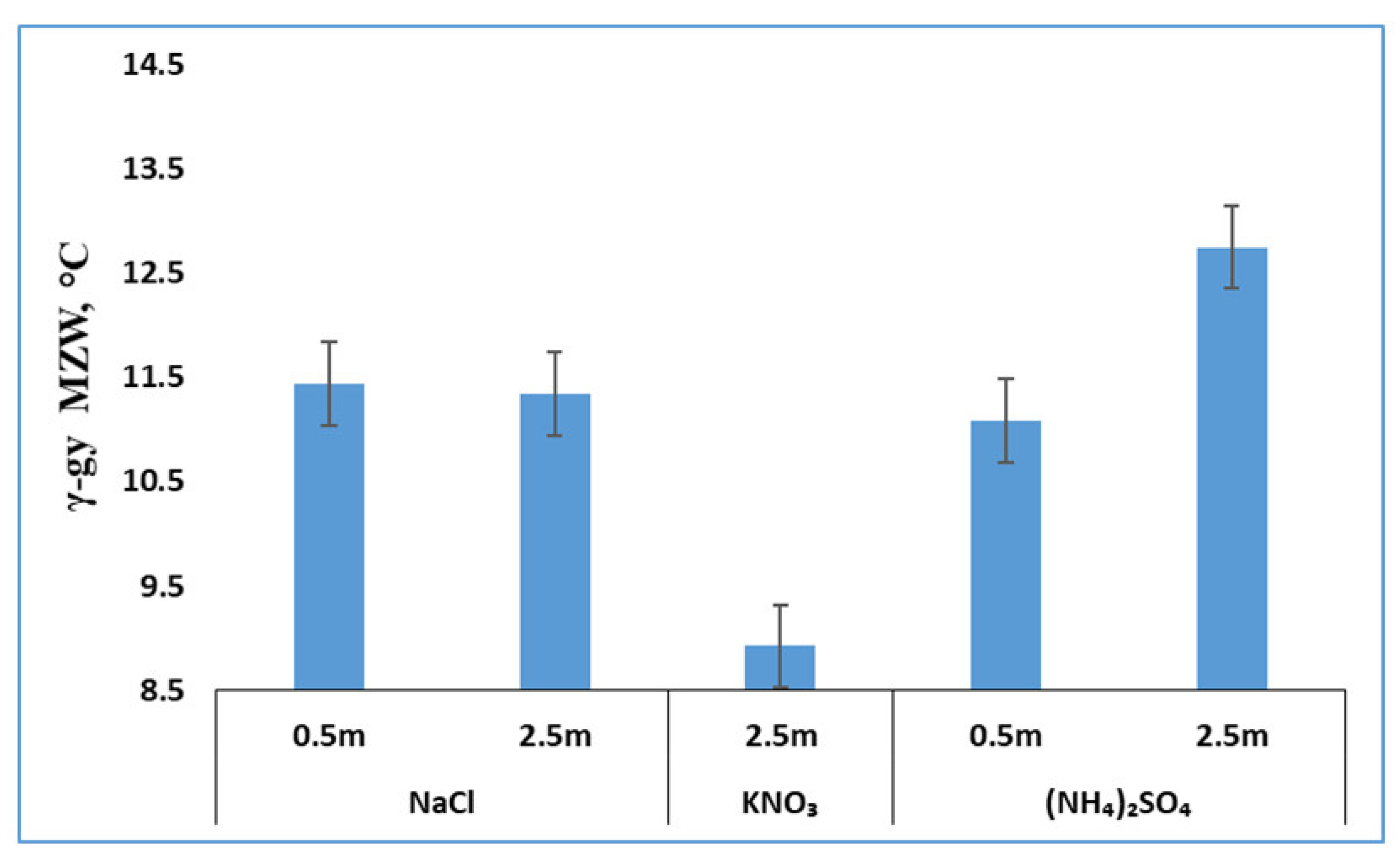
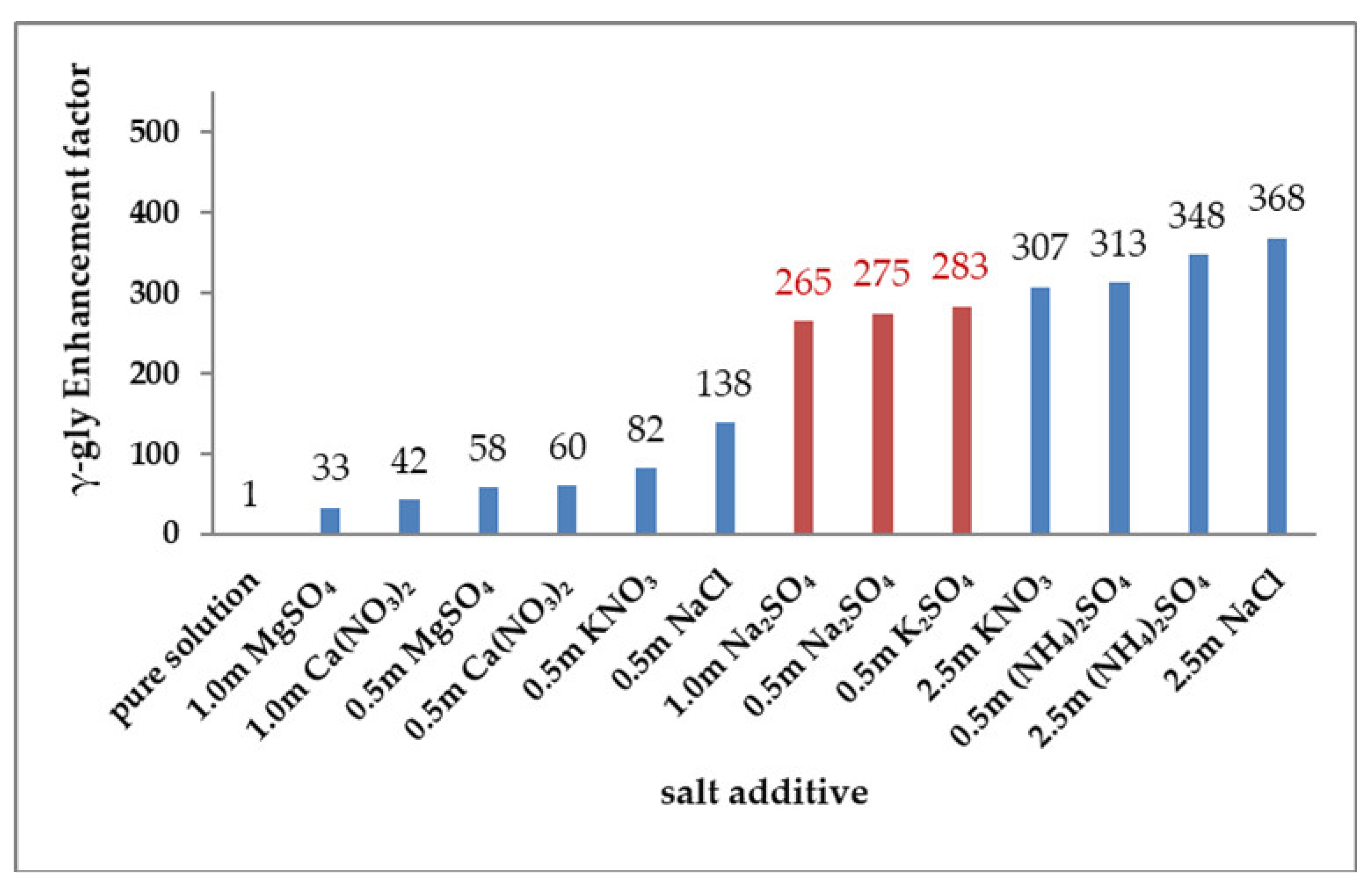
3.3. Effects of Salts on γ-Glycine Primary Nucleation
3.4. Effects of Salts Inferred from In-Depth Data Analyses
3.5. Explanation of the Salt-Dependent Polymorphic Shift
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoica, C.; Verwer, P.; Meekes, H.; Van Hoof, P.J.C.M.; Kaspersen, F.M.; Vlieg, E. Understanding the Effect of a Solvent on the Crystal Habit. Cryst. Growth Des. 2004, 4, 765–768. [Google Scholar] [CrossRef]
- Knapman, K. Polymorphic Predictions. Mod. Drug Discov. 2000, 3, 53–54. [Google Scholar]
- Huang, J.; Stringfellow, T.C.; Yu, L. Glycine Exists Mainly as Monomers, Not Dimers, in Supersaturated Aqueous Solutions: Implications for Understanding Its Crystallization and Polymorphism. J. Am. Chem. Soc. 2008, 130, 13973–13980. [Google Scholar] [CrossRef]
- Black, J.F.B.; Cardew, P.T.; Cruz-Cabeza, A.J.; Davey, R.J.; Gilks, S.E.; Sullivan, R.A. Crystal nucleation and growth in a polymorphic system: Ostwald’s rule, p-aminobenzoic acid and nucleation transition states. CrystEngComm 2018, 20, 768–776. [Google Scholar] [CrossRef]
- Yang, H.; Song, C.L.; Lim, Y.X.S.; Chen, W.; Heng, J.Y.Y. Selective crystallisation of carbamazepine polymorphs on surfaces with differing properties. CrystEngComm 2017, 19, 6573–6578. [Google Scholar] [CrossRef]
- Chew, J.W.; Black, S.N.; Chow, P.S.; Tan, R.B.H.; Carpenter, K.J. Stable polymorphs: Difficult to make and difficult to predict. CrystEngComm 2007, 9, 128–130. [Google Scholar] [CrossRef]
- Dowling, R.; Davey, R.J.; Curtis, R.; Han, G.; Poornachary, S.K.; Chow, P.S.; Tan, R.B.H. Acceleration of crystal growth rates: An unexpected effect of tailor-made additives. Chem. Commun. 2010, 46, 5924–5926. [Google Scholar] [CrossRef]
- Towler, C.S.; Davey, R.J.; Lancaster, R.W.; Price, C.J. Impact of Molecular Speciation on Crystal Nucleation in Polymorphic Systems: The Conundrum of γ Glycine and Molecular ‘Self Poisoning’. J. Am. Chem. Soc. 2004, 126, 13347–13353. [Google Scholar] [CrossRef]
- Han, G.; Thirunahari, S.; Chow, P.S.; Tan, R.B.H. Resolving the longstanding riddle of pH-dependent outcome of glycine polymorphic nucleation. CrystEngComm 2013, 15, 1218–1224. [Google Scholar] [CrossRef]
- Yang, X.; Lu, J.; Wang, X.-J.; Ching, C.-B. Effect of sodium chloride on the nucleation and polymorphic transformation of glycine. J. Cryst. Growth 2008, 310, 604–611. [Google Scholar] [CrossRef]
- Duff, N.; Dahal, Y.R.; Schmit, J.D.; Peters, B. Salting out the polar polymorph: Analysis by alchemical solvent transformation. J. Chem. Phys. 2014, 140, 014501. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Chow, P.S.; Tan, R.B.H. Effects of Common Inorganic Salts on Glycine Polymorphic Transformation: An Insight into Salt-Dependent Polymorphic Selectivity. Cryst. Growth Des. 2016, 16, 6499–6505. [Google Scholar] [CrossRef]
- Tang, W.; Mo, H.; Zhang, M.; Gong, J.; Wang, J.; Li, T. Glycine’s pH-Dependent Polymorphism: A Perspective from Self-Association in Solution. Cryst. Growth Des. 2017, 17, 5028–5033. [Google Scholar] [CrossRef]
- Ding, L.; Zong, S.; Dang, L.; Wang, Z.; Wei, H. Effects of inorganic additives on polymorphs of glycine in microdroplets. CrystEngComm 2018, 20, 164–172. [Google Scholar] [CrossRef]
- Han, G.; Chow, P.S.; Tan, R.B.H. Salt-dependent growth kinetics in glycine polymorphic crystallization. CrystEngComm 2016, 18, 462–470. [Google Scholar] [CrossRef]
- Iitaka, Y. The crystal structure of β-glycine. Acta Cryst. 1960, 13, 35–45. [Google Scholar] [CrossRef]
- Xavier, N.F.; Silva, A.M.; Bauerfeldt, G.F. What Rules the Relative Stability of α-, β-, and γ-Glycine Polymorphs? Cryst. Growth Des. 2020, 20, 4695–4706. [Google Scholar] [CrossRef]
- Perlovich, G.L.; Hansen, L.K.; Bauer-Brandl, A. The polymorphism of glycine: Thermochemical and structural aspects. J. Therm. Anal. Calorim. 2001, 66, 699–715. [Google Scholar] [CrossRef]
- Markel, A.L.; Achkasov, A.F.; Alekhina, T.A.; Prokudina, O.I.; Ryazanova, M.A.; Ukolova, T.N.; Efimov, V.M.; Boldyreva, E.V.; Boldyrev, V.V. Effects of the α- and γ-polymorphs of glycine on the behavior of catalepsy prone rats. Pharmacol. Biochem. Behav. 2011, 98, 234–240. [Google Scholar] [CrossRef]
- Weissbuch, I.; Leisorowitz, L.; Lahav, M. “Tailor-Made” and charge-transfer auxiliaries for the control of the crystal polymorphism of glycine. Adv. Mater. 1994, 6, 952–956. [Google Scholar] [CrossRef]
- Aber, J.E.; Arnold, S.; Garetz, B.A.; Myerson, A.S. Strong dc Electric Field Applied to Supersaturated Aqueous Glycine Solution Induces Nucleation of the γ Polymorph. Phys. Rev. Lett. 2005, 94, 145503. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Bhamidi, V.; Wilson, S.R.; Tan, R.B.H.; Kenis, P.J.A.; Zukoski, C.F. Direct Growth of γ-Glycine from Neutral Aqueous Solutions by Slow, Evaporation-Driven Crystallization. Cryst. Growth Des. 2006, 6, 1746–1749. [Google Scholar] [CrossRef]
- Di Profio, G.; Tucci, S.; Curcio, E.; Drioli, E. Selective Glycine Polymorph Crystallization by Using Microporous Membranes. Cryst. Growth Des. 2007, 7, 526–530. [Google Scholar] [CrossRef]
- Kim, J.-W.; Shim, H.-M.; Lee, J.-E.; Koo, K.-K. Interfacial Effect of Water/Oleic Acid Emulsion on Polymorphic Selection in the Cooling Crystallization of Glycine. Cryst. Growth Des. 2012, 12, 4739–4744. [Google Scholar] [CrossRef]
- Vesga, M.J.; McKechnie, D.; Mulheran, P.A.; Johnston, K.; Sefcik, J. Conundrum of γ glycine nucleation revisited: To stir or not to stir? CrystEngComm 2019, 21, 2234–2243. [Google Scholar] [CrossRef]
- Meirzadeh, E.; Dishon, S.; Weissbuch, I.; Ehre, D.; Lahav, M.; Lubomirsky, I. Solvent-Induced Crystal Polymorphism as Studied by Pyroelectric Measurements and Impedance Spectroscopy: Alcohols as Tailor-Made Inhibitors of α-Glycine. Angew. Chem. Int. Ed. 2018, 57, 4965–4969. [Google Scholar] [CrossRef]
- Dang, L.; Yang, H.; Black, S.; Wei, H. The Effect of Temperature and Solvent Composition on Transformation of β- to α-Glycine as Monitored in Situ by FBRM and PVM. Org. Process. Res. Dev. 2009, 13, 1301–1306. [Google Scholar] [CrossRef]
- Han, G.; Poornachary, S.K.; Chow, P.S.; Tan, R.B.H. Understanding Growth Morphology Changes of γ-Glycine and DL-Alanine Polar Crystals in Pure Aqueous Solutions. Cryst. Growth Des. 2010, 10, 4883–4889. [Google Scholar] [CrossRef]
- Han, G.; Chow, P.S.; Tan, R.B.H. Direct Comparison of α- and γ-Glycine Growth Rates in Acidic and Basic Solutions: New Insights into Glycine Polymorphism. Cryst. Growth Des. 2012, 12, 2213–2220. [Google Scholar] [CrossRef]
- Hamad, S.; Catlow, C.R.A. Are glycine cyclic dimers stable in aqueous solution? CrystEngComm 2011, 13, 4391–4399. [Google Scholar] [CrossRef]
- Hamad, S.; Hughes, C.E.; Catlow, C.R.A.; Harris, K.D.M. Clustering of Glycine Molecules in Aqueous Solution Studied by Molecular Dynamics Simulation. J. Phys. Chem. B 2008, 112, 7280–7288. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Trout, B.L. A Computational Study of the Mechanism of the Selective Crystallization of α- and β-Glycine from Water and Methanol−Water Mixture. J. Phys. Chem. B 2010, 114, 13764–13772. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S. X-ray study and IR spectra of glycine calcium nitrate dihydrate. Z. Krist. 1983, 163, 305–306. [Google Scholar]
- Bhat, M.N.; Dharmaprakash, S.M. New nonlinear optical material: Glycine sodium nitrate. J. Cryst. Growth 2002, 235, 511–516. [Google Scholar] [CrossRef]
| Salt Additive | Additive Concentration (Molality, m) | Ionic Strength of Solution | α-Glycine Solubility at 25 °C | γ-Glycine Solubility at 28 °C |
|---|---|---|---|---|
| NA | 0.0 | 0.0 | 25.03 | 25.00 |
| MgSO4 | 0.5 | 2.0 | 29.45 | 29.09 |
| MgSO4 | 1.0 | 4.0 | 32.26 | 32.10 |
| Ca(NO3)2 | 0.5 | 1.5 | 33.10 | 32.80 |
| Ca(NO3)2 | 1.0 | 3.0 | 40.81 | 40.53 |
| KNO3 | 0.5 | 0.5 | 26.84 | 26.80 |
| KNO3 | 2.5 | 2.5 | 30.80 | 30.53 |
| NaCl | 0.5 | 0.5 | 25.98 | 25.98 |
| NaCl | 2.5 | 2.5 | 28.31 | 28.02 |
| (NH4)2SO4 | 0.5 | 1.5 | 27.64 | 27.60 |
| (NH4)2SO4 | 2.5 | 7.5 | 29.49 | 29.51 |
| Na2SO4 | 0.5 | 1.5 | 27.47 | 27.45 |
| Na2SO4 | 1.0 | 3.0 | 28.50 | 28.52 |
| K2SO4 | 0.5 | 1.5 | 27.11 | 27.05 |
| Salt Additive | Probability (%) of α-Glycine Formation | Probability (%) of γ-Glycine Formation |
|---|---|---|
| NA (additive-free) | 100 | 0 |
| 0.5 m MgSO4 | 100 | 0 |
| 1 m MgSO4 | 100 | 0 |
| 0.5 m Ca(NO3)2 | 100 | 0 |
| 1 m Ca(NO3)2 | 100 | 0 |
| 0.5 m KNO3 | 100 | 0 |
| 2.5 m KNO3 | 0 | 100 |
| 0.5 m NaCl | 64 | 36 |
| 2.5 m NaCl | 0 | 100 |
| 0.5 m (NH4)2SO4 | 0 | 100 |
| 2.5 m (NH4)2SO4 | 0 | 100 |
| 0.5 m Na2SO4 | 100 | 0 |
| 1 m Na2SO4 | 100 | 0 |
| 0.5 m K2SO4 | 100 | 0 |
| Salt | By MZW (Primary Nucleation) | By SMPT (Secondary Nucleation) | |
|---|---|---|---|
| α-Glycine | γ-Glycine | γ-Glycine | |
| 1 m MgSO4 | inhibition | undetermined | promotion |
| 1 m Ca(NO3)2 | inhibition | undetermined | promotion |
| 0.5 m MgSO4 | insignificant | undetermined | promotion |
| 0.5 m Ca(NO3)2 | inhibition | undetermined | promotion |
| 0.5 m KNO3 | insignificant | undetermined | promotion |
| 0.5 m NaCl | insignificant | promotion (inferred) | promotion |
| 0.5–1 m Na2SO4 | promotion | undetermined | promotion |
| 0.5 m K2SO4 | promotion | undetermined | promotion |
| 2.5 m KNO3 | undetermined | promotion (inferred) | promotion |
| 0.5 m (NH4)2SO4 | inhibition (inferred) | promotion (inferred) | promotion |
| 2.5 m (NH4)2SO4 | inhibition (inferred) | undetermined effect | promotion |
| 2.5 m NaCl | inhibition (inferred) | promotion (inferred) | promotion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Chow, P.S.; Tan, R.B.H. Understanding the Salt-Dependent Outcome of Glycine Polymorphic Nucleation. Pharmaceutics 2021, 13, 262. https://doi.org/10.3390/pharmaceutics13020262
Han G, Chow PS, Tan RBH. Understanding the Salt-Dependent Outcome of Glycine Polymorphic Nucleation. Pharmaceutics. 2021; 13(2):262. https://doi.org/10.3390/pharmaceutics13020262
Chicago/Turabian StyleHan, Guangjun, Pui Shan Chow, and Reginald B. H. Tan. 2021. "Understanding the Salt-Dependent Outcome of Glycine Polymorphic Nucleation" Pharmaceutics 13, no. 2: 262. https://doi.org/10.3390/pharmaceutics13020262
APA StyleHan, G., Chow, P. S., & Tan, R. B. H. (2021). Understanding the Salt-Dependent Outcome of Glycine Polymorphic Nucleation. Pharmaceutics, 13(2), 262. https://doi.org/10.3390/pharmaceutics13020262






