Cyclic Di-Adenosine Monophosphate: A Promising Adjuvant Candidate for the Development of Neonatal Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Human Adult and Cord Blood
2.3. In Vitro Assays Using Human Blood
2.4. Immunizations
2.5. Lymphocyte Isolation, In Vitro Antigen Re-Stimulation, and Intracellular Cytokine Staining
2.6. Viral Challenge
2.7. Data Processing
2.8. Reagents
2.9. Statistical Analysis
3. Results
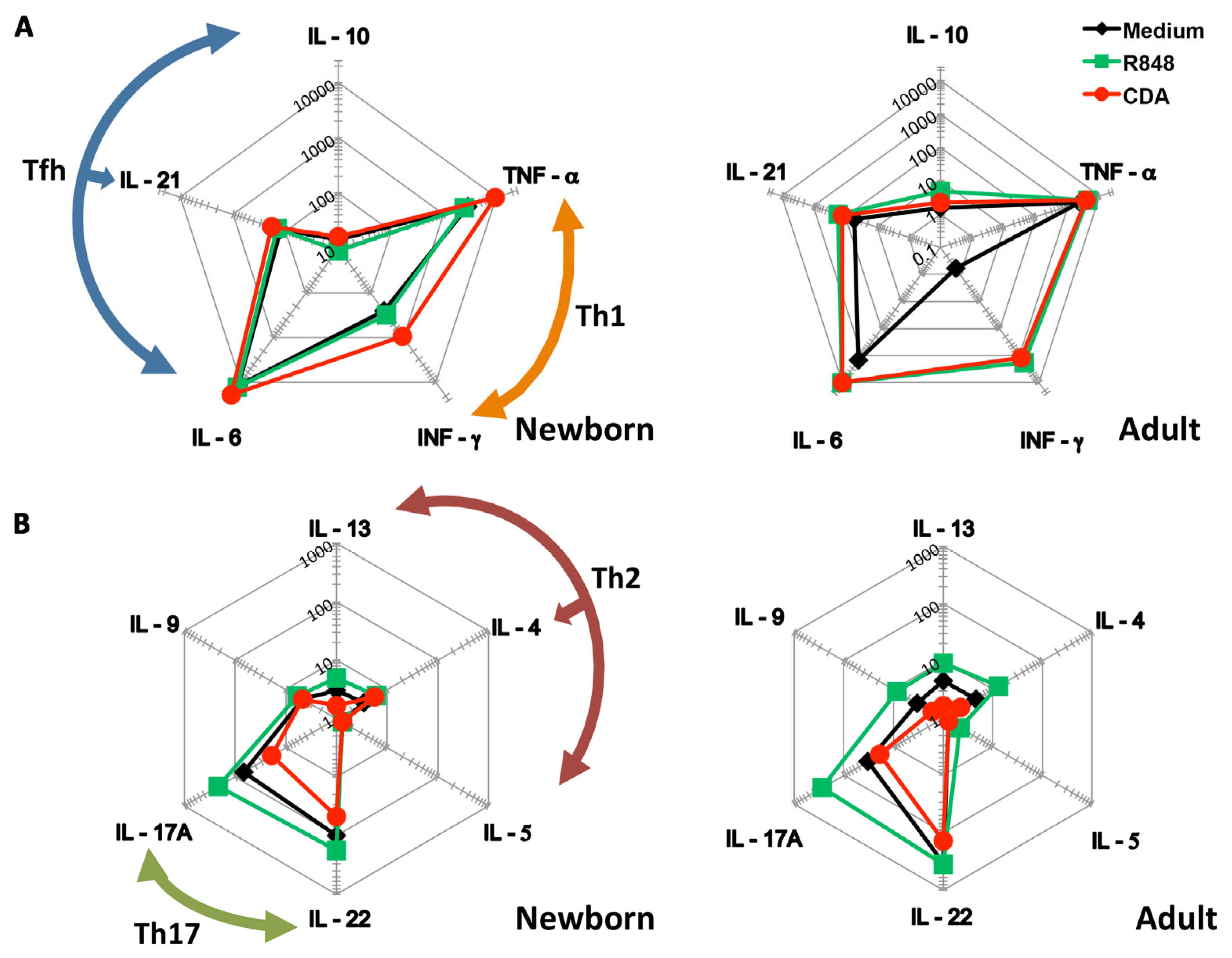
3.1. CDA Stimulates a Th1 Cytokine Profile on Cells Derived from Human Cord Blood
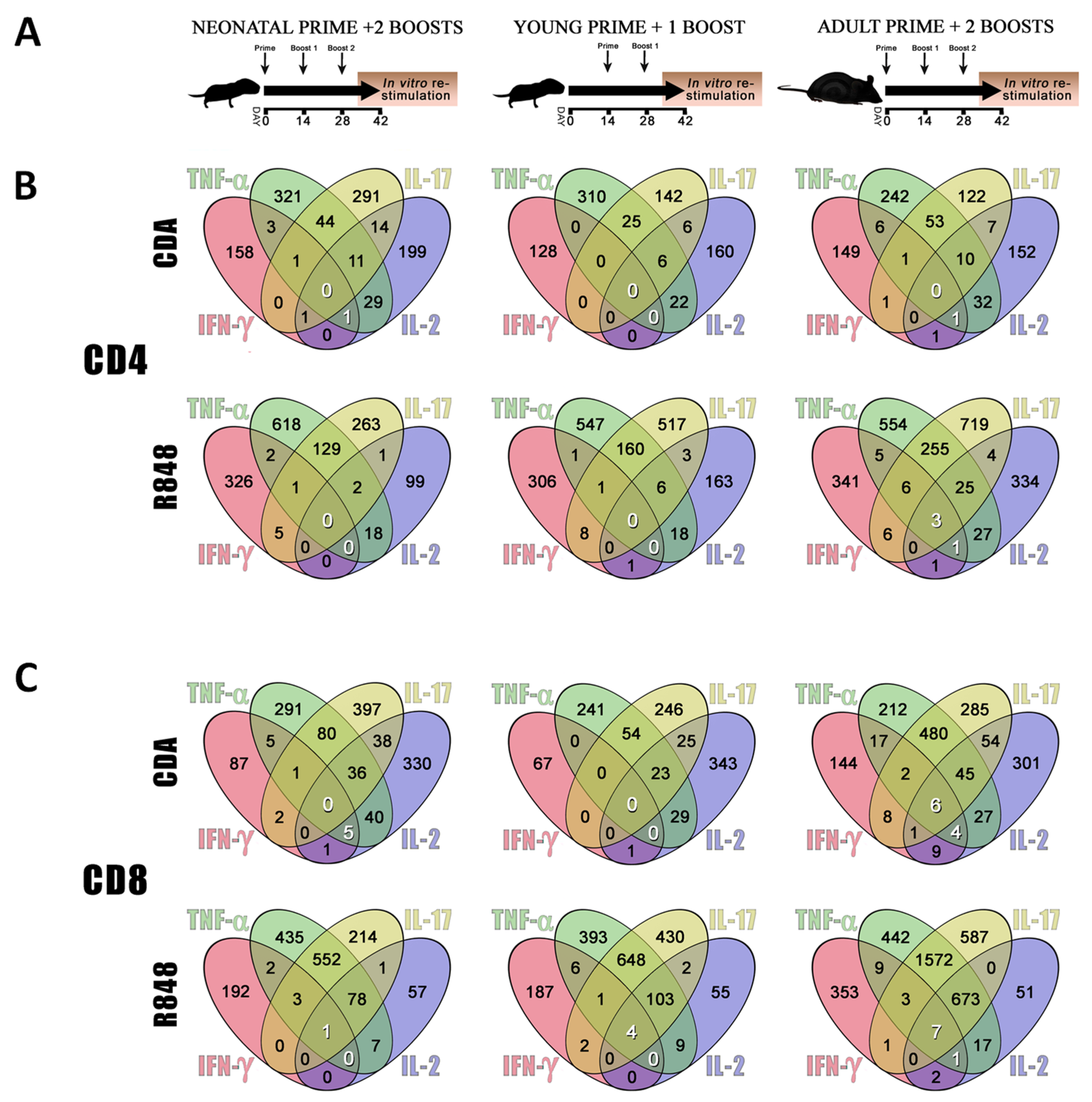
3.2. CDA-Adjuvanted Neonatal Dose Elicits Multiple-Cytokine Producers among Antigen-Specific T Cells
3.3. A CDA-Adjuvanted Neonatal Vaccine Induces B Cell Activation and Maturation
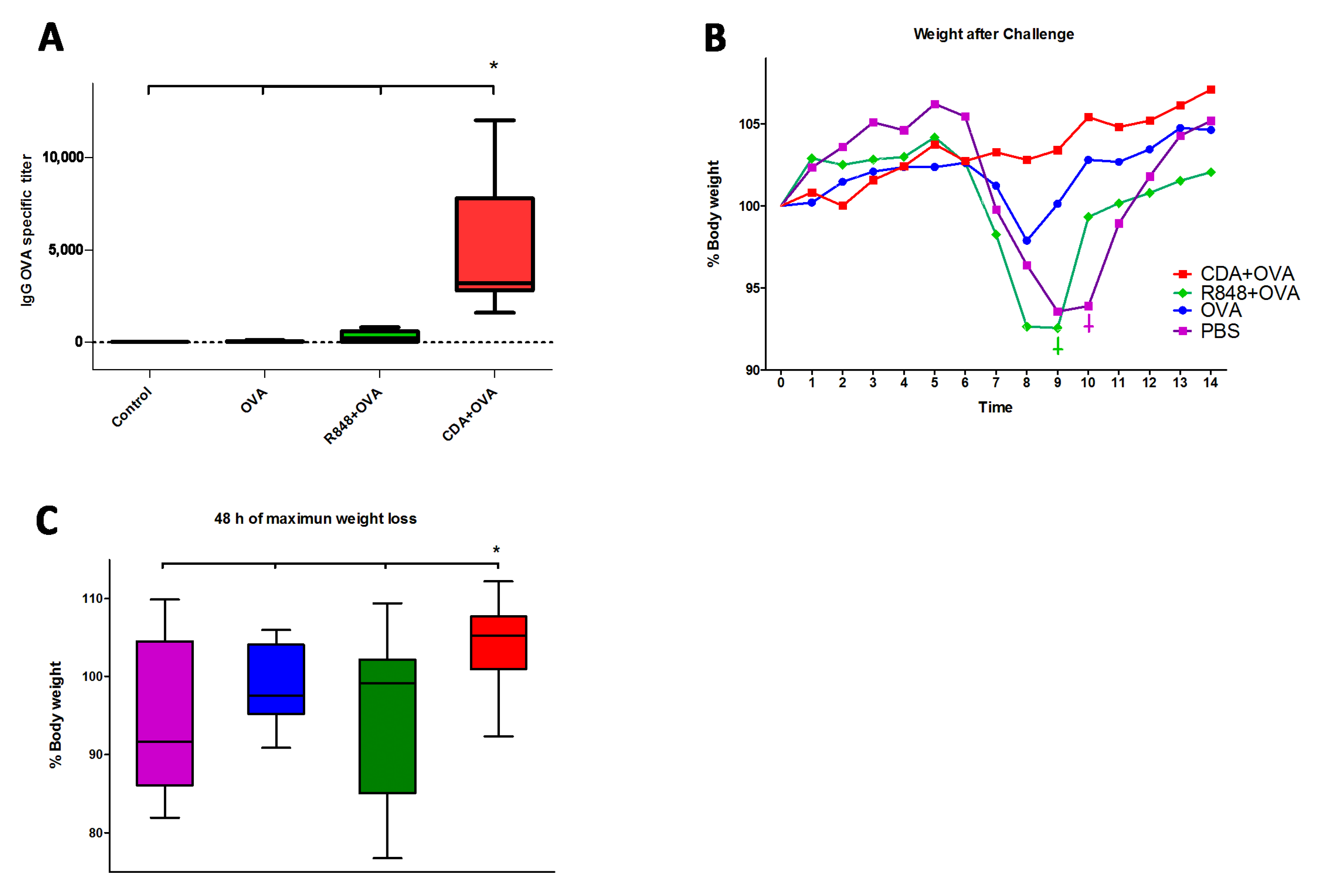
3.4. Neonatal CDA Vaccination Promotes a Protective Immune Response in Adult Mice
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Guy, B. The perfect mix: Recent progress in adjuvant research. Nat. Rev. Microbiol. 2007, 5, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Bonam, S.R.; Bhunia, D.; Muller, S.; Nerella, S.G.; Alvala, M.; Halmuthur Mahabalarao, S.K. Novel trisaccharide based phospholipids as immunomodulators. Int. Immunopharmacol. 2019, 74, 105684. [Google Scholar] [CrossRef] [PubMed]
- Dey, B.; Dey, R.J.; Cheung, L.S.; Pokkali, S.; Guo, H.; Lee, J.-H.; Bishai, W.R. A bacterial cyclic dinucleotide activates the cytosolic surveillance pathway and mediates innate resistance to tuberculosis. Nat. Med. 2015, 21, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.-D.; Sotelo-Troha, K.; von Moltke, J.; Monroe, K.M.; Rae, C.S.; Brubaker, S.W.; Hyodo, M.; Hayakawa, Y.; Woodward, J.J.; Portnoy, D.A.; et al. The N-ethyl-N-nitrosourea-induced Goldenticket mouse mutant reveals an essential function of Sting in the in vivo interferon response to Listeria monocytogenes and cyclic dinucleotides. Infect. Immun. 2011, 79, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Parvatiyar, K.; Zhang, Z.; Teles, R.M.; Ouyang, S.; Jiang, Y.; Iyer, S.S.; Zaver, S.A.; Schenk, M.; Zeng, S.; Zhong, W.; et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat. Immunol. 2012, 13, 1155–1161. [Google Scholar] [CrossRef]
- Levy, D.E.; Marié, I.J.; Durbin, J.E. Induction and function of type I and III interferon in response to viral infection. Curr. Opin. Virol. 2011, 1, 476–486. [Google Scholar] [CrossRef]
- Fulginiti, V.A.; Eller, J.J.; Sieber, O.F.; Joyner, J.W.; Minamitani, M.; Meiklejohn, G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 1969, 89, 435–448. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Black, R.E. Global maternal, newborn, and child health—So near and yet so far. N. Engl. J. Med. 2013, 369, 2226–2235. [Google Scholar] [CrossRef]
- Blencowe, H.; Vos, T.; Lee, A.C.C.; Philips, R.; Lozano, R.; Alvarado, M.R.; Cousens, S.; Lawn, J.E. Estimates of neonatal morbidities and disabilities at regional and global levels for 2010: Introduction, methods overview, and relevant findings from the Global Burden of Disease study. Pediatr. Res. 2013, 74 (Suppl. 1), 4–16. [Google Scholar] [CrossRef]
- Morris, M.C.; Surendran, N. Neonatal Vaccination: Challenges and Intervention Strategies. Neonatology 2016, 109, 161–169. [Google Scholar] [CrossRef]
- Saso, A.; Kampmann, B. Vaccine responses in newborns. Semin. Immunopathol. 2017, 39, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.J.; Rashid, M.M.; Kabir, Y.; Raqib, R.; Ahmad, S.M. On birth single dose live attenuated OPV and BCG vaccination induces gut cathelicidin LL37 responses at 6 week of age: A natural experiment. Vaccine 2015, 33, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Darmochwal-Kolarz, D.; Serafin, A.; Tabarkiewicz, J.; Kolarz, B.; Rolinski, J.; Oleszczuk, J. The expressions of co-stimulatory molecules are altered on putative antigen-presenting cells in cord blood. Am. J. Reprod. Immunol. 2013, 69, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Darmochwal-Kolarz, D.; Rolinski, J.; Buczkowski, J.; Tabarkiewicz, J.; Leszczynska-Gorzelak, B.; Zych, I.; Oleszczuk, J. CD1c(+) immature myeloid dendritic cells are predominant in cord blood of healthy neonates. Immunol. Lett. 2004, 91, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, T.R.; Levy, O.; Montgomery, R.R.; Goriely, S. Innate immune function by Toll-like receptors: Distinct responses in newborns and the elderly. Immunity 2012, 37, 771–783. [Google Scholar] [CrossRef] [PubMed]
- De Serres, G.; Boulianne, N.; Defay, F.; Brousseau, N.; Benoît, M.; Lacoursière, S.; Guillemette, F.; Soto, J.; Ouakki, M.; Ward, B.J.; et al. Higher risk of measles when the first dose of a 2-dose schedule of measles vaccine is given at 12–14 months versus 15 months of age. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 55, 394–402. [Google Scholar] [CrossRef]
- Carazo Perez, S.; De Serres, G.; Bureau, A.; Skowronski, D.M. Reduced Antibody Response to Infant Measles Vaccination: Effects Based on Type and Timing of the First Vaccine Dose Persist After the Second Dose. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2017, 65, 1094–1102. [Google Scholar] [CrossRef]
- Tomljenovic, L.; Shaw, C.A. Mechanisms of aluminum adjuvant toxicity and autoimmunity in pediatric populations. Lupus 2012, 21, 223–230. [Google Scholar] [CrossRef]
- Shaw, C.A.; Tomljenovic, L. Aluminum in the central nervous system (CNS): Toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol. Res. 2013, 56, 304–316. [Google Scholar] [CrossRef]
- Olson, M.R.; Varga, S.M. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol. 2007, 179, 5415–5424. [Google Scholar] [CrossRef] [PubMed]
- Hermann, E.; Truyens, C.; Alonso-Vega, C.; Even, J.; Rodriguez, P.; Berthe, A.; Gonzalez-Merino, E.; Torrico, F.; Carlier, Y. Human fetuses are able to mount an adultlike CD8 T-cell response. Blood 2002, 100, 2153–2158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Silvestri, N.; Whitton, J.L.; Hassett, D.E. Neonates mount robust and protective adult-like CD8(+)-T-cell responses to DNA vaccines. J. Virol. 2002, 76, 11911–11919. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marchant, A.; Appay, V.; Van Der Sande, M.; Dulphy, N.; Liesnard, C.; Kidd, M.; Kaye, S.; Ojuola, O.; Gillespie, G.M.A.; Vargas Cuero, A.L.; et al. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J. Clin. Investig. 2003, 111, 1747–1755. [Google Scholar] [CrossRef]
- Grohskopf, L.A.; Sokolow, L.Z.; Broder, K.R.; Walter, E.B.; Bresee, J.S.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2017–2018 Influenza Season. MMWR Recomm. Rep. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2017, 66, 1–20. [Google Scholar] [CrossRef]
- Block, S.L.; Yi, T.; Sheldon, E.; Dubovsky, F.; Falloon, J. A randomized, double-blind noninferiority study of quadrivalent live attenuated influenza vaccine in adults. Vaccine 2011, 29, 9391–9397. [Google Scholar] [CrossRef]
- Greenberg, D.P.; Robertson, C.A.; Landolfi, V.A.; Bhaumik, A.; Senders, S.D.; Decker, M.D. Safety and immunogenicity of an inactivated quadrivalent influenza vaccine in children 6 months through 8 years of age. Pediatr. Infect. Dis. J. 2014, 33, 630–636. [Google Scholar] [CrossRef]
- Fries, L.; Shinde, V.; Stoddard, J.J.; Thomas, D.N.; Kpamegan, E.; Lu, H.; Smith, G.; Hickman, S.P.; Piedra, P.; Glenn, G.M. Immunogenicity and safety of a respiratory syncytial virus fusion protein (RSV F) nanoparticle vaccine in older adults. Immun. Ageing 2017, 14, 8. [Google Scholar] [CrossRef]
- Müller, U.; Steinhoff, U.; Reis, L.F.; Hemmi, S.; Pavlovic, J.; Zinkernagel, R.M.; Aguet, M. Functional role of type I and type II interferons in antiviral defense. Science 1994, 264, 1918–1921. [Google Scholar] [CrossRef]
- Dowling, D.J.; Tan, Z.; Prokopowicz, Z.M.; Palmer, C.D.; Matthews, M.-A.H.; Dietsch, G.N.; Hershberg, R.M.; Levy, O. The ultra-potent and selective TLR8 agonist VTX-294 activates human newborn and adult leukocytes. PLoS ONE 2013, 8, e58164. [Google Scholar] [CrossRef]
- Topham, D.J.; Castrucci, M.R.; Wingo, F.S.; Belz, G.T.; Doherty, P.C. The role of antigen in the localization of naive, acutely activated, and memory CD8(+) T cells to the lung during influenza pneumonia. J. Immunol. 2001, 167, 6983–6990. [Google Scholar] [CrossRef] [PubMed]
- Debock, I.; Jaworski, K.; Chadlaoui, H.; Delbauve, S.; Passon, N.; Twyffels, L.; Leo, O.; Flamand, V. Neonatal follicular Th cell responses are impaired and modulated by IL-4. J. Immunol. 2013, 191, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Darrah, P.A.; Patel, D.T.; De Luca, P.M.; Lindsay, R.W.B.; Davey, D.F.; Flynn, B.J.; Hoff, S.T.; Andersen, P.; Reed, S.G.; Morris, S.L.; et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007, 13, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Casartelli, N.; Lemoine, S.; Mozeleski, B.; Azria, E.; Le Ray, C.; Schwartz, O.; Launay, O.; Leclerc, C.; Lo-Man, R. Plasmacytoid dendritic cells engagement by influenza vaccine as a surrogate strategy for driving T-helper type 1 responses in human neonatal settings. J. Infect. Dis. 2014, 210, 424–434. [Google Scholar] [CrossRef]
- Holbrook, B.C.; Kim, J.R.; Blevins, L.K.; Jorgensen, M.J.; Kock, N.D.; D’Agostino, R.B.J.; Aycock, S.T.; Hadimani, M.B.; King, S.B.; Parks, G.D.; et al. A Novel R848-Conjugated Inactivated Influenza Virus Vaccine Is Efficacious and Safe in a Neonate Nonhuman Primate Model. J. Immunol. 2016, 197, 555–564. [Google Scholar] [CrossRef]
- van Haren, S.D.; Dowling, D.J.; Foppen, W.; Christensen, D.; Andersen, P.; Reed, S.G.; Hershberg, R.M.; Baden, L.R.; Levy, O. Age-Specific Adjuvant Synergy: Dual TLR7/8 and Mincle Activation of Human Newborn Dendritic Cells Enables Th1 Polarization. J. Immunol. 2016, 197, 4413–4424. [Google Scholar] [CrossRef]
- van Haren, S.D.; Ganapathi, L.; Bergelson, I.; Dowling, D.J.; Banks, M.; Samuels, R.C.; Reed, S.G.; Marshall, J.D.; Levy, O. In vitro cytokine induction by TLR-activating vaccine adjuvants in human blood varies by age and adjuvant. Cytokine 2016, 83, 99–109. [Google Scholar] [CrossRef]
- Ganapathi, L.; Van Haren, S.; Dowling, D.J.; Bergelson, I.; Shukla, N.M.; Malladi, S.S.; Balakrishna, R.; Tanji, H.; Ohto, U.; Shimizu, T.; et al. The Imidazoquinoline Toll-Like Receptor-7/8 Agonist Hybrid-2 Potently Induces Cytokine Production by Human Newborn and Adult Leukocytes. PLoS ONE 2015, 10, e0134640. [Google Scholar] [CrossRef]
- Holbrook, B.C.; D’Agostino, R.B.J.; Tyler Aycock, S.; Jorgensen, M.J.; Hadimani, M.B.; Bruce King, S.; Alexander-Miller, M.A. Adjuvanting an inactivated influenza vaccine with conjugated R848 improves the level of antibody present at 6 months in a nonhuman primate neonate model. Vaccine 2017, 35, 6137–6142. [Google Scholar] [CrossRef]
- Holbrook, B.C.; Aycock, S.T.; Machiele, E.; Clemens, E.; Gries, D.; Jorgensen, M.J.; Hadimani, M.B.; King, S.B.; Alexander-Miller, M.A. An R848 adjuvanted influenza vaccine promotes early activation of B cells in the draining lymph nodes of non-human primate neonates. Immunology 2018, 153, 357–367. [Google Scholar] [CrossRef]
- Zhang, X.; Deriaud, E.; Jiao, X.; Braun, D.; Leclerc, C.; Lo-Man, R. Type I interferons protect neonates from acute inflammation through interleukin 10-producing B cells. J. Exp. Med. 2007, 204, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Jans, J.; Pettengill, M.; Kim, D.; van der Made, C.; de Groot, R.; Henriet, S.; de Jonge, M.I.; Ferwerda, G.; Levy, O. Human newborn B cells mount an interferon-α/β receptor-dependent humoral response to respiratory syncytial virus. J. Allergy Clin. Immunol. 2017, 139, 1997–2000.e4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Levy, O.; Zarember, K.A.; Roy, R.M.; Cywes, C.; Godowski, P.J.; Wessels, M.R. Selective impairment of TLR-mediated innate immunity in human newborns: Neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J. Immunol. 2004, 173, 4627–4634. [Google Scholar] [CrossRef] [PubMed]
- Lirussi, D.; Ebensen, T.; Schulze, K.; Trittel, S.; Duran, V.; Liebich, I.; Kalinke, U.; Guzmán, C.A. Type I IFN and not TNF, is Essential for Cyclic Di-nucleotide-elicited CTL by a Cytosolic Cross-presentation Pathway. EBioMedicine 2017, 22, 100–111. [Google Scholar] [CrossRef]
- Lirussi, D.; Ebensen, T.; Schulze, K.; Reinhard, E.; Trittel, S.; Riese, P.; Prochnow, B.; Guzmán, C.A. Rapid in vivo assessment of adjuvant’s cytotoxic t lymphocytes generation capabilities for vaccine development. J. Vis. Exp. 2018, 2018. [Google Scholar] [CrossRef]
- Ebensen, T.; Libanova, R.; Schulze, K.; Yevsa, T.; Morr, M.; Guzmán, C.A. Bis-(3’,5’)-cyclic dimeric adenosine monophosphate: Strong Th1/Th2/Th17 promoting mucosal adjuvant. Vaccine 2011, 29, 5210–5220. [Google Scholar] [CrossRef]
- Gold, M.C.; Robinson, T.L.; Cook, M.S.; Byrd, L.K.; Ehlinger, H.D.; Lewinsohn, D.M.; Lewinsohn, D.A. Human neonatal dendritic cells are competent in MHC class I antigen processing and presentation. PLoS ONE 2007, 2, e957. [Google Scholar] [CrossRef]
- Ariffin, J.K.; Sweet, M.J. Differences in the repertoire, regulation and function of Toll-like Receptors and inflammasome-forming Nod-like Receptors between human and mouse. Curr. Opin. Microbiol. 2013, 16, 303–310. [Google Scholar] [CrossRef]
- Mastelic, B.; Kamath, A.T.; Fontannaz, P.; Tougne, C.; Rochat, A.-F.; Belnoue, E.; Combescure, C.; Auderset, F.; Lambert, P.-H.; Tacchini-Cottier, F.; et al. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J. Immunol. 2012, 189, 5764–5772. [Google Scholar] [CrossRef]
- Elahi, S.; Ertelt, J.M.; Kinder, J.M.; Jiang, T.T.; Zhang, X.; Xin, L.; Chaturvedi, V.; Strong, B.S.; Qualls, J.E.; Steinbrecher, K.A.; et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013, 504, 158–162. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lirussi, D.; Weissmann, S.F.; Ebensen, T.; Nitsche-Gloy, U.; Franz, H.B.G.; Guzmán, C.A. Cyclic Di-Adenosine Monophosphate: A Promising Adjuvant Candidate for the Development of Neonatal Vaccines. Pharmaceutics 2021, 13, 188. https://doi.org/10.3390/pharmaceutics13020188
Lirussi D, Weissmann SF, Ebensen T, Nitsche-Gloy U, Franz HBG, Guzmán CA. Cyclic Di-Adenosine Monophosphate: A Promising Adjuvant Candidate for the Development of Neonatal Vaccines. Pharmaceutics. 2021; 13(2):188. https://doi.org/10.3390/pharmaceutics13020188
Chicago/Turabian StyleLirussi, Darío, Sebastian Felix Weissmann, Thomas Ebensen, Ursula Nitsche-Gloy, Heiko B. G. Franz, and Carlos A. Guzmán. 2021. "Cyclic Di-Adenosine Monophosphate: A Promising Adjuvant Candidate for the Development of Neonatal Vaccines" Pharmaceutics 13, no. 2: 188. https://doi.org/10.3390/pharmaceutics13020188
APA StyleLirussi, D., Weissmann, S. F., Ebensen, T., Nitsche-Gloy, U., Franz, H. B. G., & Guzmán, C. A. (2021). Cyclic Di-Adenosine Monophosphate: A Promising Adjuvant Candidate for the Development of Neonatal Vaccines. Pharmaceutics, 13(2), 188. https://doi.org/10.3390/pharmaceutics13020188







