Understanding In Vivo Fate of Nucleic Acid and Gene Medicines for the Rational Design of Drugs
Abstract
1. Introduction
2. History of Non-Viral Nucleic Acid and Gene Delivery
3. In Vivo Fate
3.1. Interaction with Blood Components
3.2. Distribution to Non-Target Tissues
3.3. Distribution to Target Tissue
3.4. Intracellular Trafficking
3.4.1. Uptake Pathway
3.4.2. Endosomal Escape
3.4.3. Subcellular Localization
3.4.4. Dissociation of Genes from Carriers
3.4.5. Autophagy
3.5. Metabolism and Excretion
3.6. Safety Concern
4. Rational Design
4.1. Controlling Size of Nanoparticles
4.2. Controlling the Interaction with Blood Components
4.3. Controlling the Circulation in Vasculatures
4.4. Selection of Administration Route
4.5. Considering Structures of Vessel Wall
4.6. Targeting Tissues/Cells
4.7. Enhancing Endosomal Escape Efficiency
4.8. Considering Intracellular Trafficking
4.9. Improving Effective Duration
4.10. Safety Issues
4.11. Helper Drugs
5. Evaluation Methods
5.1. Physicochemical Properties
5.2. Reporter Genes
5.3. Biodistribution
5.4. Subcellular Localization
5.5. Toxicity
6. Prospects and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonzaga-Jauregui, C.; Lupski, J.R.; Gibbs, R.A. Human Genome Sequencing in Health and Disease. Annu. Rev. Med. 2012, 63, 35–61. [Google Scholar] [CrossRef]
- Sarantis, P.; Koustas, E.; Papadimitropoulou, A.; Papavassiliou, A.G.; Karamouzis, M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020, 12, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu. Rev. Med. 2020, 71, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, S.; Karimi, M.H.; Lotfinia, M.; Gharibi, T.; Mahi-Birjand, M.; Kavi, E.; Hosseini, F.; Sineh Sepehr, K.; Khatami, M.; Bagheri, N.; et al. Potential drugs used in the antibody-drug conjugate (ADC) architecture for cancer therapy. J. Cell. Physiol. 2020, 235, 31–64. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Witztum, J.L.; Bennett, C.F.; Baker, B.F. RNA-Targeted Therapeutics. Cell Metab. 2018, 27, 714–739. [Google Scholar] [CrossRef]
- Lau, Y.-T.K.; Ramaiyer, M.; Johnson, D.E.; Grandis, J.R. Targeting STAT3 in Cancer with Nucleotide Therapeutics. Cancers 2019, 11, 1681. [Google Scholar] [CrossRef]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef]
- Kulabhusan, P.K.; Hussain, B.; Yüce, M. Current Perspectives on Aptamers as Diagnostic Tools and Therapeutic Agents. Pharmaceutics 2020, 12, 646. [Google Scholar] [CrossRef]
- Fang, L.; Du, W.W.; Lyu, J.; Dong, J.; Zhang, C.; Yang, W.; He, A.; Kwok, Y.S.S.; Ma, J.; Wu, N.; et al. Enhanced breast cancer progression by mutant p53 is inhibited by the circular RNA circ-Ccnb1. Cell Death Differ. 2018, 25, 2195–2208. [Google Scholar] [CrossRef]
- Xu, Y.; Qiu, A.; Peng, F.; Tan, X.; Wang, J.; Gong, X. Exosomal transfer of circular RNA FBXW7 ameliorates the chemoresistance to oxaliplatin in colorectal cancer by sponging miR-18b-5p. Neoplasma 2020, 200417N414, Epub ahead of print. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Hahm, S.H.; Lee, K.H. Retroviral gene therapy: Safety issues and possible solutions. Curr. Gene Ther. 2005, 5, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Shirley, J.L.; de Jong, Y.P.; Terhorst, C.; Herzog, R.W. Immune Responses to Viral Gene Therapy Vectors. Mol. Ther. 2020, 28, 709–722. [Google Scholar] [CrossRef]
- McCutchan, J.H.; Pagano, J.S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J. Natl. Cancer Inst. 1968, 41, 351–357. [Google Scholar]
- Johnson, S.M.; Bangham, A.D. Potassium permeability of single compartment liposomes with and without valinomycin. Biochim. Biophys. Acta 1969, 193, 82–91. [Google Scholar] [CrossRef]
- Johnson, S.M.; Bangham, A.D.; Hill, M.W.; Korn, E.D. Single bilayer liposomes. Biochim. Biophys. Acta 1971, 233, 820–826. [Google Scholar] [CrossRef]
- Bangham, A.D. Lipid bilayers and biomembranes. Annu. Rev. Biochem. 1972, 41, 753–776. [Google Scholar] [CrossRef]
- Mannino, R.J.; Allebach, E.S.; Strohl, W.A. Encapsulation of high molecular weight DNA in large unilamellar phospholipid vesicles. Dependence on the size of the DNA. FEBS Lett. 1979, 101, 229–232. [Google Scholar] [CrossRef]
- Dimitriadis, G.J. Entrapment of plasmid DNA in liposomes. Nucleic Acids Res. 1979, 6, 2697–2705. [Google Scholar] [CrossRef]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Liu, F.; Chu, S.; Liu, D. Characterization of cationic liposome-mediated gene transfer in vivo by intravenous administration. Hum. Gene Ther. 1997, 8, 1585–1594. [Google Scholar] [CrossRef]
- Semple, S.C.; Klimuk, S.K.; Harasym, T.O.; Dos Santos, N.; Ansell, S.M.; Wong, K.F.; Maurer, N.; Stark, H.; Cullis, P.R.; Hope, M.J.; et al. Efficient encapsulation of antisense oligonucleotides in lipid vesicles using ionizable aminolipids: Formation of novel small multilamellar vesicle structures. Biochim. Biophys. Acta 2001, 1510, 152–166. [Google Scholar] [CrossRef]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Myhre, J.L.; Chen, S.; Tam, Y.Y.C.; Danescu, A.; Richman, J.M.; Cullis, P.R. Design of lipid nanoparticles for in vitro and in vivo delivery of plasmid DNA. Nanomedicine 2017, 13, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Liu, F.; Song, Y.; Liu, D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999, 6, 1258–1266. [Google Scholar] [CrossRef]
- Zhang, G.; Budker, V.; Wolff, J.A. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum. Gene Ther. 1999, 10, 1735–1737. [Google Scholar] [CrossRef]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P.H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef]
- Reiss, M.; Jastreboff, M.M.; Bertino, J.R.; Narayanan, R. DNA-mediated gene transfer into epidermal cells using electroporation. Biochem. Biophys. Res. Commun. 1986, 137, 244–249. [Google Scholar] [CrossRef]
- Wells, J.M.; Li, L.H.; Sen, A.; Jahreis, G.P.; Hui, S.W. Electroporation-enhanced gene delivery in mammary tumors. Gene Ther. 2000, 7, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Nishikawa, M.; Thanaketpaisarn, O.; Yamashita, F.; Hashida, M. Hepatocyte-targeted gene transfer by combination of vascularly delivered plasmid DNA and in vivo electroporation. Gene Ther. 2005, 12, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Endoh, M.; Koibuchi, N.; Sato, M.; Morishita, R.; Kanzaki, T.; Murata, Y.; Kaneda, Y. Fetal gene transfer by intrauterine injection with microbubble-enhanced ultrasound. Mol. Ther. 2002, 5, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Takizawa, T.; Negishi, Y.; Hagisawa, K.; Tanaka, K.; Sawamura, K.; Utoguchi, N.; Nishioka, T.; Maruyama, K. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J. Control. Release 2007, 117, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Huang, L. Noninvasive gene delivery to the liver by mechanical massage. Hepatology 2002, 35, 1314–1319. [Google Scholar] [CrossRef]
- Mukai, H.; Kawakami, S.; Hashida, M. Renal press-mediated transfection method for plasmid DNA and siRNA to the kidney. Biochem. Biophys. Res. Commun. 2008, 372, 383–387. [Google Scholar] [CrossRef]
- Shimizu, K.; Kawakami, S.; Hayashi, K.; Kinoshita, H.; Kuwahara, K.; Nakao, K.; Hashida, M.; Konishi, S. In vivo site-specific transfection of naked plasmid DNA and siRNAs in mice by using a tissue suction device. PLoS ONE 2012, 7, e41319. [Google Scholar] [CrossRef]
- Hu, Y.; Haynes, M.T.; Wang, Y.; Liu, F.; Huang, L. A highly efficient synthetic vector: Nonhydrodynamic delivery of DNA to hepatocyte nuclei in vivo. ACS Nano 2013, 7, 5376–5384. [Google Scholar] [CrossRef]
- Goula, D.; Remy, J.S.; Erbacher, P.; Wasowicz, M.; Levi, G.; Abdallah, B.; Demeneix, B.A. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Ther. 1998, 5, 712–717. [Google Scholar] [CrossRef]
- Morimoto, K.; Nishikawa, M.; Kawakami, S.; Nakano, T.; Hattori, Y.; Fumoto, S.; Yamashita, F.; Hashida, M. Molecular weight-dependent gene transfection activity of unmodified and galactosylated polyethyleneimine on hepatoma cells and mouse liver. Mol. Ther. 2003, 7, 254–261. [Google Scholar] [CrossRef]
- Choi, J.S.; Nam, K.; Park, J.Y.; Kim, J.B.; Lee, J.K.; Park, J.S. Enhanced transfection efficiency of PAMAM dendrimer by surface modification with L-arginine. J. Control. Release 2004, 99, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Kataoka, K. Design concepts of polyplex micelles for in vivo therapeutic delivery of plasmid DNA and messenger RNA. J. Biomed. Mater. Res. A 2019, 107, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Chaya, H.; Toh, K.; Kim, B.S.; Hayashi, K.; Fukushima, S.; Nagata, T.; Yokota, T.; Kataoka, K.; Miyata, K. Structural tuning of oligonucleotides for enhanced blood circulation properties of unit polyion complexes prepared from two-branched poly(ethylene glycol)-block-poly(l-lysine). J. Control. Release 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Saw, P.E.; Song, E.W. siRNA therapeutics: A clinical reality. Sci. China Life Sci. 2020, 63, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liangcd, X.-J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 1–25. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020, 154–155, 37–63. [Google Scholar] [CrossRef]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine—United States, December 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 69, 1653–1656. [Google Scholar] [CrossRef]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine—United States, December 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1922–1924. [Google Scholar] [CrossRef]
- Liu, M.A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef]
- De Jong, W.; Leal, L.; Buyze, J.; Pannus, P.; Guardo, A.C.; Salgado, M.; Mothe, B.; Moltó, J.; Moron-Lopez, S.; Gálvez, C.; et al. Therapeutic Vaccine in Chronically HIV-1-Infected Patients: A Randomized, Double-Blind, Placebo-Controlled Phase IIa Trial with HTI-TriMix. Vaccines 2019, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Meleshko, A.N.; Petrovskaya, N.A.; Savelyeva, N.; Vashkevich, K.P.; Doronina, S.N.; Sachivko, N.V. Phase I clinical trial of idiotypic DNA vaccine administered as a complex with polyethylenimine to patients with B-cell lymphoma. Hum. Vaccines Immunother. 2017, 13, 1398–1403. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, E.H.; Kwak, G.; Chi, S.-G.; Kim, S.H.; Yang, Y. Exosomes: Cell-Derived Nanoplatforms for the Delivery of Cancer Therapeutics. Int. J. Mol. Sci. 2020, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.A. Are extracellular vesicles new hope in clinical drug delivery for neurological disorders? Neurochem. Int. 2021, 144, 104955. [Google Scholar] [CrossRef]
- Ortega, A.; Martinez-Arroyo, O.; Forner, M.J.; Cortes, R. Exosomes as Drug Delivery Systems: Endogenous Nanovehicles for Treatment of Systemic Lupus Erythematosus. Pharmaceutics 2020, 13, 3. [Google Scholar] [CrossRef]
- Lara, P.; Chan, A.; Cruz, L.J.; Quest, A.G.; Kogan, M.J. Exploiting the Natural Properties of Extracellular Vesicles in Targeted Delivery towards Specific Cells and Tissues. Pharmaceutics 2020, 12, 1022. [Google Scholar] [CrossRef]
- Yamayoshi, A.; Oyama, S.; Kishimoto, Y.; Konishi, R.; Yamamoto, T.; Kobori, A.; Harada, H.; Ashihara, E.; Sugiyama, H.; Murakami, A. Development of Antibody–Oligonucleotide Complexes for Targeting Exosomal MicroRNA. Pharmaceutics 2020, 12, 545. [Google Scholar] [CrossRef]
- Johannes, L.; Lucchino, M. Current Challenges in Delivery and Cytosolic Translocation of Therapeutic RNAs. Nucleic Acid Ther. 2018, 28, 178–193. [Google Scholar] [CrossRef]
- Ogris, M.; Wagner, E. Tumor-targeted gene transfer with DNA polyplexes. Somat. Cell Mol. Genet. 2002, 27, 85–95. [Google Scholar] [CrossRef]
- Alp, G.; Aydogan, N. Lipid-based mucus penetrating nanoparticles and their biophysical interactions with pulmonary mucus layer. Eur. J. Pharm. Biopharm. 2020, 149, 45–57. [Google Scholar] [CrossRef]
- Nishikawa, M.; Huang, L. Nonviral vectors in the new millennium: Delivery barriers in gene transfer. Hum. Gene Ther. 2001, 12, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Karmali, P.P.; Simberg, D. Interactions of nanoparticles with plasma proteins: Implication on clearance and toxicity of drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 343–357. [Google Scholar] [CrossRef]
- Fumoto, S.; Kawakami, S.; Ito, Y.; Shigeta, K.; Yamashita, F.; Hashida, M. Enhanced hepatocyte-selective in vivo gene expression by stabilized galactosylated liposome/plasmid DNA complex using sodium chloride for complex formation. Mol. Ther. 2004, 10, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, S.; Kawakami, S.; Shigeta, K.; Higuchi, Y.; Yamashita, F.; Hashida, M. Interaction with blood components plays a crucial role in asialoglycoprotein receptor-mediated in vivo gene transfer by galactosylated lipoplex. J. Pharmacol. Exp. Ther. 2005, 315, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Eliyahu, H.; Servel, N.; Domb, A.J.; Barenholz, Y. Lipoplex-induced hemagglutination: Potential involvement in intravenous gene delivery. Gene Ther. 2002, 9, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Huang, L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem. Biophys. Res. Commun. 1991, 179, 280–285. [Google Scholar] [CrossRef]
- Yang, J.P.; Huang, L. Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther. 1997, 4, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Nishioka, T.; Saito, H.; Baba, T.; Okuda, A.; Matsumoto, O.; Taga, T.; Yamashita, F.; Takakura, Y.; Hashida, M. Interaction between DNA-cationic liposome complexes and erythrocytes is an important factor in systemic gene transfer via the intravenous route in mice: The role of the neutral helper lipid. Gene Ther. 2001, 8, 677–686. [Google Scholar] [CrossRef]
- Tandia, B.M.; Vandenbranden, M.; Wattiez, R.; Lakhdar, Z.; Ruysschaert, J.M.; Elouahabi, A. Identification of human plasma proteins that bind to cationic lipid/DNA complex and analysis of their effects on transfection efficiency: Implications for intravenous gene transfer. Mol. Ther. 2003, 8, 264–273. [Google Scholar] [CrossRef]
- Tandia, B.M.; Lonez, C.; Vandenbranden, M.; Ruysschaert, J.M.; Elouahabi, A. Lipid mixing between lipoplexes and plasma lipoproteins is a major barrier for intravenous transfection mediated by cationic lipids. J. Biol. Chem. 2005, 280, 12255–12261. [Google Scholar] [CrossRef]
- Yoshikawa, N.; Sakamoto, K.; Mizuno, S.; Sakaguchi, J.; Miyamoto, H.; Mine, T.; Sasaki, H.; Fumoto, S.; Nishida, K. Multiple components in serum contribute to hepatic transgene expression by lipoplex in mice. J. Gene Med. 2011, 13, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Fumoto, S.; Nakashima, M.; Shimokawa, K.; Miyamoto, H.; Nishida, K. The role of fibronectin in pulmonary gene transfer following intravenous administration of lipoplex in mice. Biol. Pharm. Bull. 2013, 36, 1807–1813. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshikawa, N.; Fumoto, S.; Yoshikawa, K.; Hu, D.; Okami, K.; Kato, R.; Nakashima, M.; Miyamoto, H.; Nishida, K. Interaction of Lipoplex with Albumin Enhances Gene Expression in Hepatitis Mice. Pharmaceutics 2020, 12, 341. [Google Scholar] [CrossRef] [PubMed]
- Motta, S.; Rondelli, V.; Cantu’, L.; Del Favero, E.; Aureli, M.; Pozzi, D.; Caracciolo, G.; Brocca, P. What the cell surface does not see: The gene vector under the protein corona. Colloids Surf. B Biointerfaces 2016, 141, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Caracciolo, G.; Caruso, G.; Foglia, P.; Pozzi, D.; Samperi, R.; Laganà, A. Differential analysis of “protein corona” profile adsorbed onto different nonviral gene delivery systems. Anal. Biochem. 2011, 419, 180–189. [Google Scholar] [CrossRef]
- Caracciolo, G.; Pozzi, D.; Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Amenitsch, H.; Laganà, A. Evolution of the Protein Corona of Lipid Gene Vectors as a Function of Plasma Concentration. Langmuir 2011, 27, 15048–15053. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, A.L.; Caracciolo, G.; Caruso, G.; Foglia, P.; Pozzi, D.; Samperi, R.; Laganà, A. DNA affects the composition of lipoplex protein corona: A proteomics approach. Proteomics 2011, 11, 3349–3358. [Google Scholar] [CrossRef]
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R.; et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010, 18, 1357–1364. [Google Scholar] [CrossRef]
- Akita, H.; Noguchi, Y.; Hatakeyama, H.; Sato, Y.; Tange, K.; Nakai, Y.; Harashima, H. Molecular Tuning of a Vitamin E-Scaffold pH-Sensitive and Reductive Cleavable Lipid-like Material for Accelerated In Vivo Hepatic siRNA Delivery. ACS Biomater. Sci. Eng. 2015, 1, 834–844. [Google Scholar] [CrossRef]
- Dias, N.; Stein, C.A. Antisense oligonucleotides: Basic concepts and mechanisms. Mol. Cancer Ther. 2002, 1, 347–355. [Google Scholar]
- Yamamoto, T.; Nakatani, M.; Narukawa, K.; Obika, S. Antisense drug discovery and development. Future Med. Chem. 2011, 3, 339–365. [Google Scholar] [CrossRef] [PubMed]
- Khvorova, A.; Watts, J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017, 35, 238–248. [Google Scholar] [CrossRef]
- Wan, W.B.; Seth, P.P. The Medicinal Chemistry of Therapeutic Oligonucleotides. J. Med. Chem. 2016, 59, 9645–9667. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Vickers, T.; Lima, W.F.; Wu, H. Mechanisms of antisense drug action, an introduction. In Antisense Drug Technology: Principles, Strategies, and Applications, 2nd ed.; Crooke, S.T., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 3–46. [Google Scholar] [CrossRef]
- Crooke, S.T. Molecular Mechanisms of Antisense Oligonucleotides. Nucleic Acid Ther. 2017, 27, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Seth, P.P.; Vickers, T.A.; Liang, X.H. The Interaction of Phosphorothioate-Containing RNA Targeted Drugs with Proteins Is a Critical Determinant of the Therapeutic Effects of These Agents. J. Am. Chem. Soc. 2020, 142, 14754–14771. [Google Scholar] [CrossRef] [PubMed]
- Gaus, H.J.; Gupta, R.; Chappell, A.E.; Østergaard, M.E.; Swayze, E.E.; Seth, P.P. Characterization of the interactions of chemically-modified therapeutic nucleic acids with plasma proteins using a fluorescence polarization assay. Nucleic Acids Res. 2019, 47, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.Z.; Kim, T.W.; Hong, A.; Watanabe, T.A.; Gaus, H.J.; Geary, R.S. Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab. Dispos. 2007, 35, 460–468. [Google Scholar] [CrossRef]
- Shemesh, C.S.; Yu, R.Z.; Gaus, H.J.; Seth, P.P.; Swayze, E.E.; Bennett, F.C.; Geary, R.S.; Henry, S.P.; Wang, Y. Pharmacokinetic and Pharmacodynamic Investigations of ION-353382, a Model Antisense Oligonucleotide: Using Alpha-2-Macroglobulin and Murinoglobulin Double-Knockout Mice. Nucleic Acid Ther. 2016, 26, 223–235. [Google Scholar] [CrossRef]
- Matsumura, Y. The Drug Discovery by NanoMedicine and its Clinical Experience. Jpn. J. Clin. Oncol. 2014, 44, 515–525. [Google Scholar] [CrossRef]
- Charbe, N.B.; Amnerkar, N.D.; Ramesh, B.; Tambuwala, M.M.; Bakshi, H.A.; Aljabali, A.A.A.; Khadse, S.C.; Satheeshkumar, R.; Satija, S.; Metha, M.; et al. Small interfering RNA for cancer treatment: Overcoming hurdles in delivery. Acta Pharm. Sin. B 2020, 10, 2075–2109. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Harashima, H. The polyethyleneglycol dilemma: Advantage and disadvantage of PEGylation of liposomes for systemic genes and nucleic acids delivery to tumors. Biol. Pharm. Bull. 2013, 36, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Shum, P.; Thompson, D.H. Acid-triggered release via dePEGylation of DOPE liposomes containing acid-labile vinyl ether PEG-lipids. J. Control. Release 2003, 91, 187–200. [Google Scholar] [CrossRef]
- Terada, T.; Iwai, M.; Kawakami, S.; Yamashita, F.; Hashida, M. Novel PEG-matrix metalloproteinase-2 cleavable peptide-lipid containing galactosylated liposomes for hepatocellular carcinoma-selective targeting. J. Control. Release 2006, 111, 333–342. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Kogure, K.; Oishi, M.; Nagasaki, Y.; Kihira, Y.; Ueno, M.; Kobayashi, H.; Kikuchi, H.; Harashima, H. Development of a novel systemic gene delivery system for cancer therapy with a tumor-specific cleavable PEG-lipid. Gene Ther. 2007, 14, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Wasungu, L.; Nomden, A.; Stuart, M.C.; Polushkin, E.; Engberts, J.B.; Hoekstra, D. Interference of poly(ethylene glycol)-lipid analogues with cationic-lipid-mediated delivery of oligonucleotides; role of lipid exchangeability and non-lamellar transitions. Biochem. J. 2002, 366, 333–341. [Google Scholar] [CrossRef]
- Monck, M.A.; Mori, A.; Lee, D.; Tam, P.; Wheeler, J.J.; Cullis, P.R.; Scherrer, P. Stabilized plasmid-lipid particles: Pharmacokinetics and plasmid delivery to distal tumors following intravenous injection. J. Drug Target. 2000, 7, 439–452. [Google Scholar] [CrossRef]
- Ambegia, E.; Ansell, S.; Cullis, P.; Heyes, J.; Palmer, L.; MacLachlan, I. Stabilized plasmid-lipid particles containing PEG-diacylglycerols exhibit extended circulation lifetimes and tumor selective gene expression. Biochim. Biophys. Acta 2005, 1669, 155–163. [Google Scholar] [CrossRef]
- Akita, H.; Ishiba, R.; Togashi, R.; Tange, K.; Nakai, Y.; Hatakeyama, H.; Harashima, H. A neutral lipid envelope-type nanoparticle composed of a pH-activated and vitamin E-scaffold lipid-like material as a platform for a gene carrier targeting renal cell carcinoma. J. Control. Release 2015, 200, 97–105. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, W.; Liu, D.; Wu, J.; Guo, Z.; Ji, X.; Bharwani, Z.; Zhao, L.; Zhao, X.; Farokhzad, O.C.; et al. Surface De-PEGylation Controls Nanoparticle-Mediated siRNA Delivery In Vitro and In Vivo. Theranostics 2017, 7, 1990–2002. [Google Scholar] [CrossRef]
- Zhang, X.; Goel, V.; Attarwala, H.; Sweetser, M.T.; Clausen, V.A.; Robbie, G.J. Patisiran Pharmacokinetics, Pharmacodynamics, and Exposure-Response Analyses in the Phase 3 APOLLO Trial in Patients with Hereditary Transthyretin-Mediated (hATTR) Amyloidosis. J. Clin. Pharmacol. 2020, 60, 37–49. [Google Scholar] [CrossRef]
- Webb, M.S.; Saxon, D.; Wong, F.M.; Lim, H.J.; Wang, Z.; Bally, M.B.; Choi, L.S.; Cullis, P.R.; Mayer, L.D. Comparison of different hydrophobic anchors conjugated to poly(ethylene glycol): Effects on the pharmacokinetics of liposomal vincristine. Biochim. Biophys. Acta 1998, 1372, 272–282. [Google Scholar] [CrossRef]
- Lechanteur, A.; Furst, T.; Evrard, B.; Delvenne, P.; Hubert, P.; Piel, G. PEGylation of lipoplexes: The right balance between cytotoxicity and siRNA effectiveness. Eur. J. Pharm. Sci. 2016, 93, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Geary, R.S.; Norris, D.; Yu, R.; Bennett, C.F. Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, C.; Shi, S.; Jayaprakash, K.N.; Jayaraman, M.; Wang, G.; Pandey, R.K.; Rajeev, K.G.; Nakayama, T.; Charrise, K.; Ndungo, E.M.; et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007, 25, 1149–1157. [Google Scholar] [CrossRef]
- Nishina, T.; Numata, J.; Nishina, K.; Yoshida-Tanaka, K.; Nitta, K.; Piao, W.; Iwata, R.; Ito, S.; Kuwahara, H.; Wada, T.; et al. Chimeric Antisense Oligonucleotide Conjugated to α-Tocopherol. Mol. Ther. Nucleic Acids 2015, 4, e220. [Google Scholar] [CrossRef]
- Nishina, K.; Piao, W.; Yoshida-Tanaka, K.; Sujino, Y.; Nishina, T.; Yamamoto, T.; Nitta, K.; Yoshioka, K.; Kuwahara, H.; Yasuhara, H.; et al. DNA/RNA heteroduplex oligonucleotide for highly efficient gene silencing. Nat. Commun. 2015, 6, 7969. [Google Scholar] [CrossRef]
- Nishina, K.; Unno, T.; Uno, Y.; Kubodera, T.; Kanouchi, T.; Mizusawa, H.; Yokota, T. Efficient in vivo delivery of siRNA to the liver by conjugation of alpha-tocopherol. Mol. Ther. 2008, 16, 734–740. [Google Scholar] [CrossRef]
- Soutschek, J.; Akinc, A.; Bramlage, B.; Charisse, K.; Constien, R.; Donoghue, M.; Elbashir, S.; Geick, A.; Hadwiger, P.; Harborth, J.; et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 2004, 432, 173–178. [Google Scholar] [CrossRef]
- Hvam, M.L.; Cai, Y.; Dagnæs-Hansen, F.; Nielsen, J.S.; Wengel, J.; Kjems, J.; Howard, K.A. Fatty Acid-Modified Gapmer Antisense Oligonucleotide and Serum Albumin Constructs for Pharmacokinetic Modulation. Mol. Ther. 2017, 25, 1710–1717. [Google Scholar] [CrossRef]
- Nikan, M.; Osborn, M.F.; Coles, A.H.; Godinho, B.M.; Hall, L.M.; Haraszti, R.A.; Hassler, M.R.; Echeverria, D.; Aronin, N.; Khvorova, A. Docosahexaenoic Acid Conjugation Enhances Distribution and Safety of siRNA upon Local Administration in Mouse Brain. Mol. Ther. Nucleic Acids 2016, 5, e344. [Google Scholar] [CrossRef]
- Prakash, T.P.; Mullick, A.E.; Lee, R.G.; Yu, J.; Yeh, S.T.; Low, A.; Chappell, A.E.; Østergaard, M.E.; Murray, S.; Gaus, H.J.; et al. Fatty acid conjugation enhances potency of antisense oligonucleotides in muscle. Nucleic Acids Res. 2019, 47, 6029–6044. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Obika, S.; Shibata, M.A.; Yamamoto, T.; Nakatani, M.; Yamaoka, T.; Torigoe, H.; Harada-Shiba, M. Development of a 2′,4′-BNA/LNA-based siRNA for Dyslipidemia and Assessment of the Effects of Its Chemical Modifications In Vivo. Mol. Ther. Nucleic Acids 2012, 1, e45. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Yasuhara, H.; Wada, F.; Sawamura, M.; Waki, R.; Yamamoto, T.; Harada-Shiba, M.; Obika, S. Evaluation of the effects of chemically different linkers on hepatic accumulations, cell tropism and gene silencing ability of cholesterol-conjugated antisense oligonucleotides. J. Control. Release 2016, 226, 57–65. [Google Scholar] [CrossRef]
- Wada, F.; Yamamoto, T.; Ueda, T.; Sawamura, M.; Wada, S.; Harada-Shiba, M.; Obika, S. Cholesterol-GalNAc Dual Conjugation Strategy for Reducing Renal Distribution of Antisense Oligonucleotides. Nucleic Acid Ther. 2018, 28, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Sato, A.; Nishikawa, M.; Yamashita, F.; Hashida, M. Mannose receptor-mediated gene transfer into macrophages using novel mannosylated cationic liposomes. Gene Ther. 2000, 7, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Fumoto, S.; Nishikawa, M.; Yamashita, F.; Hashida, M. In vivo gene delivery to the liver using novel galactosylated cationic liposomes. Pharm. Res. 2000, 17, 306–313. [Google Scholar] [CrossRef]
- Nair, J.K.; Willoughby, J.L.; Chan, A.; Charisse, K.; Alam, M.R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kel’in, A.V.; Milstein, S.; et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. [Google Scholar] [CrossRef]
- Weingärtner, A.; Bethge, L.; Weiss, L.; Sternberger, M.; Lindholm, M.W. Less Is More: Novel Hepatocyte-Targeted siRNA Conjugates for Treatment of Liver-Related Disorders. Mol. Ther. Nucleic Acids 2020, 21, 242–250. [Google Scholar] [CrossRef]
- Matsuda, S.; Keiser, K.; Nair, J.K.; Charisse, K.; Manoharan, R.M.; Kretschmer, P.; Peng, C.G.; VKel’in, A.; Kandasamy, P.; Willoughby, J.L.; et al. siRNA conjugates carrying sequentially assembled trivalent N-acetylgalactosamine linked through nucleosides elicit robust gene silencing in vivo in hepatocytes. ACS Chem. Biol. 2015, 10, 1181–1187. [Google Scholar] [CrossRef]
- Rajeev, K.G.; Nair, J.K.; Jayaraman, M.; Charisse, K.; Taneja, N.; O’Shea, J.; Willoughby, J.L.; Yucius, K.; Nguyen, T.; Shulga-Morskaya, S.; et al. Hepatocyte-specific delivery of siRNAs conjugated to novel non-nucleosidic trivalent N-acetylgalactosamine elicits robust gene silencing in vivo. Chembiochem 2015, 16, 903–908. [Google Scholar] [CrossRef]
- Sharma, V.K.; Osborn, M.F.; Hassler, M.R.; Echeverria, D.; Ly, S.; Ulashchik, E.A.; Martynenko-Makaev, Y.V.; Shmanai, V.V.; Zatsepin, T.S.; Khvorova, A.; et al. Novel Cluster and Monomer-Based GalNAc Structures Induce Effective Uptake of siRNAs in Vitro and in Vivo. Bioconjug. Chem. 2018, 29, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.P.; Graham, M.J.; Yu, J.; Carty, R.; Low, A.; Chappell, A.; Schmidt, K.; Zhao, C.; Aghajan, M.; Murray, H.F.; et al. Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res. 2014, 42, 8796–8807. [Google Scholar] [CrossRef] [PubMed]
- Østergaard, M.E.; Yu, J.; Kinberger, G.A.; Wan, W.B.; Migawa, M.T.; Vasquez, G.; Schmidt, K.; Gaus, H.J.; Murray, H.M.; Low, A.; et al. Efficient Synthesis and Biological Evaluation of 5’-GalNAc Conjugated Antisense Oligonucleotides. Bioconjug. Chem. 2015, 26, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sawamura, M.; Wada, F.; Harada-Shiba, M.; Obika, S. Serial incorporation of a monovalent GalNAc phosphoramidite unit into hepatocyte-targeting antisense oligonucleotides. Bioorg. Med. Chem. 2016, 24, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, C.S.; Yu, R.Z.; Gaus, H.J.; Greenlee, S.; Post, N.; Schmidt, K.; Migawa, M.T.; Seth, P.P.; Zanardi, T.A.; Prakash, T.P.; et al. Elucidation of the Biotransformation Pathways of a Galnac3-conjugated Antisense Oligonucleotide in Rats and Monkeys. Mol. Ther. Nucleic Acids 2016, 5, e319. [Google Scholar] [CrossRef]
- Kinberger, G.A.; Prakash, T.P.; Yu, J.; Vasquez, G.; Low, A.; Chappell, A.; Schmidt, K.; Murray, H.M.; Gaus, H.; Swayze, E.E.; et al. Conjugation of mono and di-GalNAc sugars enhances the potency of antisense oligonucleotides via ASGR mediated delivery to hepatocytes. Bioorg. Med. Chem. Lett. 2016, 26, 3690–3693. [Google Scholar] [CrossRef]
- Watanabe, A.; Nakajima, M.; Kasuya, T.; Onishi, R.; Kitade, N.; Mayumi, K.; Ikehara, T.; Kugimiya, A. Comparative Characterization of Hepatic Distribution and mRNA Reduction of Antisense Oligonucleotides Conjugated with Triantennary N-Acetyl Galactosamine and Lipophilic Ligands Targeting Apolipoprotein B. J. Pharmacol. Exp. Ther. 2016, 357, 320–330. [Google Scholar] [CrossRef]
- Ämmälä, C.; Drury, W.J., 3rd; Knerr, L.; Ahlstedt, I.; Stillemark-Billton, P.; Wennberg-Huldt, C.; Andersson, E.M.; Valeur, E.; Jansson-Löfmark, R.; Janzén, D.; et al. Targeted delivery of antisense oligonucleotides to pancreatic β-cells. Sci. Adv. 2018, 4, eaat3386. [Google Scholar] [CrossRef]
- Wisse, E.; Jacobs, F.; Topal, B.; Frederik, P.; De Geest, B. The size of endothelial fenestrae in human liver sinusoids: Implications for hepatocyte-directed gene transfer. Gene Ther. 2008, 15, 1193–1199. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Yates, V.J.; el-Mishad, A.M.; McCormick, K.J.; Trentin, J.J. Isolation and characterization of an Avian adenovirus-associated virus. Infect. Immun. 1973, 7, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Pichon, C.; Billiet, L.; Midoux, P. Chemical vectors for gene delivery: Uptake and intracellular trafficking. Curr. Opin. Biotechnol. 2010, 21, 640–645. [Google Scholar] [CrossRef]
- Islam, M.A.; Firdous, J.; Choi, Y.-J.; Yun, C.-H.; Cho, C.-S. Regulation of endocytosis by non-viral vectors for efficient gene activity. J. Biomed. Nanotechnol. 2014, 10, 67–80. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Harashima, H. Endocytosis of gene delivery vectors: From clathrin-dependent to lipid raft-mediated endocytosis. Mol. Ther. 2013, 21, 1118–1130. [Google Scholar] [CrossRef] [PubMed]
- Spady, D.K. Hepatic clearance of plasma low density lipoproteins. Semin. Liver Dis. 1992, 12, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, J.; Yang, D.; Lu, M.; Yin, J. Physiological roles of asialoglycoprotein receptors (ASGPRs) variants and recent advances in hepatic-targeted delivery of therapeutic molecules via ASGPRs. Protein Pept. Lett. 2014, 21, 1025–1030. [Google Scholar] [CrossRef]
- Pelkmans, L.; Kartenbeck, J.; Helenius, A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell. Biol. 2001, 3, 473–483. [Google Scholar] [CrossRef]
- Pelkmans, L.; Helenius, A. Insider information: What viruses tell us about endocytosis. Curr. Opin. Cell Biol. 2003, 15, 414–422. [Google Scholar] [CrossRef]
- Henley, J.R.; Krueger, E.W.; Oswald, B.J.; McNiven, M.A. Dynamin-mediated internalization of caveolae. J. Cell Biol. 1998, 141, 85–99. [Google Scholar] [CrossRef]
- Damm, E.M.; Pelkmans, L.; Kartenbeck, J.; Mezzacasa, A.; Kurzchalia, T.; Helenius, A. Clathrin- and caveolin-1-independent endocytosis: Entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 2005, 168, 477–488. [Google Scholar] [CrossRef]
- Ugarte-Uribe, B.; Pérez-Rentero, S.; Lucas, R.; Aviñó, A.; Reina, J.J.; Alkorta, I.; Eritja, R.; Morales, J.C. Synthesis, cell-surface binding, and cellular uptake of fluorescently labeled glucose-DNA conjugates with different carbohydrate presentation. Bioconjug. Chem. 2010, 21, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Saltzman, W.M. Enhanced siRNA delivery into cells by exploiting the synergy between targeting ligands and cell-penetrating peptides. Biomaterials 2011, 32, 6194–6203. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Brussaard, A.B.; Yang, X.; Listerud, M.; Role, L.W. Uptake of antisense oligonucleotides and functional block of acetylcholine receptor subunit gene expression in primary embryonic neurons. Dev. Genet. 1993, 14, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Koller, E.; Vincent, T.M.; Chappell, A.; De, S.; Manoharan, M.; Bennett, C.F. Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hepatocytes. Nucleic Acids Res. 2011, 39, 4795–4807. [Google Scholar] [CrossRef]
- Crooke, S.T.; Wang, S.; Vickers, T.A.; Shen, W.; Liang, X.H. Cellular uptake and trafficking of antisense oligonucleotides. Nat. Biotechnol. 2017, 35, 230–237. [Google Scholar] [CrossRef]
- Liang, X.H.; Sun, H.; Shen, W.; Crooke, S.T. Identification and characterization of intracellular proteins that bind oligonucleotides with phosphorothioate linkages. Nucleic Acids Res. 2015, 43, 2927–2945. [Google Scholar] [CrossRef]
- Tanowitz, M.; Hettrick, L.; Revenko, A.; Kinberger, G.A.; Prakash, T.P.; Seth, P.P. Asialoglycoprotein receptor 1 mediates productive uptake of N-acetylgalactosamine-conjugated and unconjugated phosphorothioate antisense oligonucleotides into liver hepatocytes. Nucleic Acids Res. 2017, 45, 12388–12400. [Google Scholar] [CrossRef]
- Kaneda, Y.; Nakajima, T.; Nishikawa, T.; Yamamoto, S.; Ikegami, H.; Suzuki, N.; Nakamura, H.; Morishita, R.; Kotani, H. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol. Ther. 2002, 6, 219–226. [Google Scholar] [CrossRef]
- Farhood, H.; Serbina, N.; Huang, L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim. Biophys. Acta 1995, 1235, 289–295. [Google Scholar] [CrossRef]
- Guo, X.; Gagne, L.; Chen, H.; Szoka, F.C. Novel ortho ester-based, pH-sensitive cationic lipid for gene delivery in vitro and in vivo. J. Liposome Res. 2014, 24, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Kimura, S.; Sato, Y.; Harashima, H. Synergism between a cell penetrating peptide and a pH-sensitive cationic lipid in efficient gene delivery based on double-coated nanoparticles. J. Control. Release 2018, 275, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Takahashi, T.; Konishi, M.; Takata, N.; Gomi, M.; Shirane, D.; Miyama, R.; Hagiwara, S.; Yamasaki, Y.; Sakurai, Y.; et al. Self-Degradable Lipid-Like Materials Based on “Hydrolysis accelerated by the intra-Particle Enrichment of Reactant (HyPER)” for Messenger RNA Delivery. Adv. Funct. Mater. 2020, 30, 1910575. [Google Scholar] [CrossRef]
- Wagenaar, T.R.; Tolstykh, T.; Shi, C.; Jiang, L.; Zhang, J.; Li, Z.; Yu, Q.; Qu, H.; Sun, F.; Cao, H.; et al. Identification of the endosomal sorting complex required for transport-I (ESCRT-I) as an important modulator of anti-miR uptake by cancer cells. Nucleic Acids Res. 2015, 43, 1204–1215. [Google Scholar] [CrossRef] [PubMed]
- Döhner, K.; Sodeik, B. The Role of the Cytoskeleton during Viral Infection. Curr. Top. Microbiol. Immunol. 2005, 285, 67–108. [Google Scholar] [CrossRef] [PubMed]
- Ondrej, V.; Lukásová, E.; Falk, M.; Kozubek, S. The role of actin and microtubule networks in plasmid DNA intracellular trafficking. Acta Biochim. Pol. 2007, 54, 657–663. [Google Scholar] [CrossRef]
- Lechardeur, D.; Sohn, K.J.; Haardt, M.; Joshi, P.B.; Monck, M.; Graham, R.W.; Beatty, B.; Squire, J.; O’Brodovich, H.; Lukacs, G.L. Metabolic instability of plasmid DNA in the cytosol: A potential barrier to gene transfer. Gene Ther. 1999, 6, 482–497. [Google Scholar] [CrossRef]
- Wang, R.; Brattain, M.G. The maximal size of protein to diffuse through the nuclear pore is larger than 60kDa. FEBS Lett. 2007, 581, 3164–3170. [Google Scholar] [CrossRef]
- Dean, D.A. Import of plasmid DNA into the nucleus is sequence specific. Exp Cell Res. 1997, 230, 293–302. [Google Scholar] [CrossRef]
- Dean, D.A.; Dean, B.S.; Muller, S.; Smith, L.C. Sequence requirements for plasmid nuclear import. Exp. Cell Res. 1999, 253, 713–722. [Google Scholar] [CrossRef]
- Bai, H.; Lester, G.M.S.; Petishnok, L.C.; Dean, D.A. Cytoplasmic transport and nuclear import of plasmid DNA. Biosci. Rep. 2017, 37, BSR20160616. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T.; Vickers, T.A.; Liang, X.H. Phosphorothioate modified oligonucleotide-protein interactions. Nucleic Acids Res. 2020, 48, 5235–5253. [Google Scholar] [CrossRef] [PubMed]
- Weidner, D.A.; Valdez, B.C.; Henning, D.; Greenberg, S.; Busch, H. Phosphorothioate oligonucleotides bind in a non sequence-specific manner to the nucleolar protein C23/nucleolin. FEBS Lett. 1995, 366, 146–150. [Google Scholar] [CrossRef]
- Abdul-Manan, N.; Williams, K.R. hnRNP A1 binds promiscuously to oligoribonucleotides: Utilization of random and homo-oligonucleotides to discriminate sequence from base-specific binding. Nucleic Acids Res. 1996, 24, 4063–4070. [Google Scholar] [CrossRef] [PubMed]
- Buntz, A.; Killian, T.; Schmid, D.; Seul, H.; Brinkmann, U.; Ravn, J.; Lindholm, M.; Knoetgen, H.; Haucke, V.; Mundigl, O. Quantitative fluorescence imaging determines the absolute number of locked nucleic acid oligonucleotides needed for suppression of target gene expression. Nucleic Acids Res. 2019, 47, 953–969. [Google Scholar] [CrossRef]
- Leonetti, J.P.; Mechti, N.; Degols, G.; Gagnor, C.; Lebleu, B. Intracellular distribution of microinjected antisense oligonucleotides. Proc. Natl. Acad. Sci. USA 1991, 88, 2702–2706. [Google Scholar] [CrossRef]
- Fisher, T.L.; Terhorst, T.; Cao, X.; Wagner, R.W. Intracellular disposition and metabolism of fluorescently-labeled unmodified and modified oligonucleotides microinjected into mammalian cells. Nucleic Acids Res. 1993, 21, 3857–3865. [Google Scholar] [CrossRef]
- Zhang, W.; Kang, X.; Yuan, B.; Wang, H.; Zhang, T.; Shi, M.; Zheng, Z.; Zhang, Y.; Peng, C.; Fan, X.; et al. Nano-Structural Effects on Gene Transfection: Large, Botryoid-Shaped Nanoparticles Enhance DNA Delivery via Macropinocytosis and Effective Dissociation. Theranostics 2019, 9, 1580–1598. [Google Scholar] [CrossRef]
- Hama, S.; Akita, H.; Ito, R.; Mizuguchi, H.; Hayakawa, T.; Harashima, H. Quantitative comparison of intracellular trafficking and nuclear transcription between adenoviral and lipoplex systems. Mol. Ther. 2006, 13, 786–794. [Google Scholar] [CrossRef]
- Hama, S.; Akita, H.; Iida, S.; Mizuguchi, H.; Harashima, H. Quantitative and mechanism-based investigation of post-nuclear delivery events between adenovirus and lipoplex. Nucleic Acids Res. 2007, 35, 1533–1543. [Google Scholar] [CrossRef]
- Roberts, R.; Al-Jamal, W.T.; Whelband, M.; Thomas, P.; Jefferson, M.; van den Bossche, J.; Powell, P.P.; Kostarelos, K.; Wileman, T. Autophagy and formation of tubulovesicular autophagosomes provide a barrier against nonviral gene delivery. Autophagy 2013, 9, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Thomas, O.S.; Weber, W. Overcoming Physiological Barriers to Nanoparticle Delivery-Are We There Yet? Front. Bioeng. Biotechnol. 2019, 7, 415. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Ogawa, H.; Koujin, T.; Kobayashi, S.; Mori, C.; Hiraoka, Y.; Haraguchi, T. Depletion of autophagy receptor p62/SQSTM1 enhances the efficiency of gene delivery in mammalian cells. FEBS Lett. 2016, 590, 2671–2680. [Google Scholar] [CrossRef]
- Legendre, J.Y.; Szoka, F.C., Jr. Delivery of plasmid DNA into mammalian cell lines using pH-sensitive liposomes: Comparison with cationic liposomes. Pharm. Res. 1992, 9, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Maitani, Y.; Igarashi, S.; Sato, M.; Hattori, Y. Cationic liposome (DC-Chol/DOPE=1:2) and a modified ethanol injection method to prepare liposomes, increased gene expression. Int. J. Pharm. 2007, 342, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yue, J.; Huang, L.; Yang, M. Autophagy inhibitor Vacuolin-1 interferes with lipid-based small interference RNA delivery. Biochem. Biophys. Res. Commun. 2019, 510, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Geary, R.S.; Leeds, J.M.; Fitchett, J.; Burckin, T.; Truong, L.; Spainhour, C.; Creek, M.; Levin, A.A. Pharmacokinetics and metabolism in mice of a phosphorothioate oligonucleotide antisense inhibitor of C-raf-1 kinase expression. Drug Metab. Dispos. 1997, 25, 1272–1281. [Google Scholar] [PubMed]
- Wu, J.; Chen, J.; Feng, Y.; Tian, H.; Chen, X. Tumor microenvironment as the “regulator” and “target” for gene therapy. J. Gene Med. 2019, 21, e3088. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-G.; Han, Y.-H.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Subcellular Performance of Nanoparticles in Cancer Therapy. Int. J. Nanomed. 2020, 15, 675–704. [Google Scholar] [CrossRef]
- Post, N.; Yu, R.; Greenlee, S.; Gaus, H.; Hurh, E.; Matson, J.; Wang, Y. Metabolism and Disposition of Volanesorsen, a 2′-O-(2 methoxyethyl) Antisense Oligonucleotide, Across Species. Drug Metab. Dispos. 2019, 47, 1164–1173. [Google Scholar] [CrossRef]
- Geary, R.S.; Yu, R.Z.; Watanabe, T.; Henry, S.P.; Hardee, G.E.; Chappell, A.; Matson, J.; Sasmor, H.; Cummins, L.; Levin, A.A. Pharmacokinetics of a Tumor Necrosis Factor—A Phosphorothioate 2′-O-(2-Methoxyethyl) Modified Antisense Oligonucleotide: Comparison across Species. Drug Metab. Dispos. 2003, 31, 1419–1428. [Google Scholar] [CrossRef]
- Nidetz, N.F.; McGee, M.C.; Tse, L.V.; Li, C.; Cong, L.; Li, Y.; Huang, W. Adeno-associated viral vector-mediated immune responses: Understanding barriers to gene delivery. Pharmacol. Ther. 2020, 207, 107453. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Nakamura, S.-I.; Akitomo, K.; Shibata, H.; Terao, K.; Kawabata, K.; Hayakawa, T.; Mizuguchi, H. Transduction Properties of Adenovirus Serotype 35 Vectors After Intravenous Administration into Nonhuman Primates. Mol. Ther. 2008, 16, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Modlich, U.; Schambach, A.; Brugman, M.H.; Wicke, D.C.; Knoess, S.; Li, Z.; Maetzig, T.; Rudolph, C.; Schlegelberger, B.; Baum, C. Leukemia induction after a single retroviral vector insertion in Evi1 or Prdm16. Leukemia 2008, 22, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, F.; Inoue, R.; Nishino, Y.; Okuda, A.; Matsumoto, O.; Taga, T.; Yamashita, F.; Takakura, Y.; Hashida, M. Effect of DNA/liposome mixing ratio on the physicochemical characteristics, cellular uptake and intracellular trafficking of plasmid DNA/cationic liposome complexes and subsequent gene expression. J. Control. Release 2000, 66, 255–269. [Google Scholar] [CrossRef]
- Fischer, D.; Bieber, T.; Li, Y.; Elsässer, H.P.; Kissel, T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: Effect of molecular weight on transfection efficiency and cytotoxicity. Pharm. Res. 1999, 16, 1273–1279. [Google Scholar] [CrossRef]
- Aramaki, Y.; Takano, S.; Tsuchiya, S. Induction of apoptosis in macrophages by cationic liposomes. FEBS Lett. 1999, 460, 472–476. [Google Scholar] [CrossRef]
- Ito, Y.; Kawakami, S.; Charoensit, P.; Higuchi, Y.; Hashida, M. Evaluation of proinflammatory cytokine production and liver injury induced by plasmid DNA/cationic liposome complexes with various mixing ratios in mice. Eur. J. Pharm. Biopharm. 2009, 71, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Butash, K.A.; Natarajan, P.; Young, A.; Fox, D.K. Reexamination of the effect of endotoxin on cell proliferation and transfection efficiency. Biotechniques 2000, 29, 610–619. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Ogawa, Y.; Yamane, I.; Nishikawa, M.; Takakura, Y. Macrophage activation by a DNA/cationic liposome complex requires endosomal acidification and TLR9-dependent and -independent pathways. J. Leukoc. Biol. 2005, 77, 71–79. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Kanneganti, T.D. AIM2 inflammasome in infection, cancer, and autoimmunity: Role in DNA sensing, inflammation, and innate immunity. Eur. J. Immunol. 2016, 46, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Lila, A.S.A.; Ishida, T. Anti-PEG IgM Production via a PEGylated Nanocarrier System for Nucleic Acid Delivery. Methods Mol. Biol. 2019, 1943, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Sorlier, P.; Hartmann, D.J.; Denuzière, A.; Viton, C.; Domard, A. Preparation and development of anti-chitosan antibodies. J. Biomed. Mater. Res. A 2003, 67, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Le Guiner, C.; Stieger, K.; Snyder, R.O.; Rolling, F.; Moullier, P. Immune responses to gene product of inducible promoters. Curr. Gene Ther. 2007, 7, 334–346. [Google Scholar] [CrossRef]
- Li, L.; Petrovsky, N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev. Vaccines 2016, 15, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Litzinger, D.C.; Brown, J.M.; Wala, I.; Kaufman, S.A.; Van, G.Y.; Farrell, C.L.; Collins, D. Fate of cationic liposomes and their complex with oligonucleotide in vivo. Biochim. Biophys. Acta 1996, 1281, 139–149. [Google Scholar] [CrossRef]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; McCormack, M.P.; Wulffraat, N.; Leboulch, P.; Lim, A.; Osborne, C.S.; Pawliuk, R.; Morillon, E.; et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 2003, 302, 415–419. [Google Scholar] [CrossRef]
- Wang, Z.; Troilo, P.J.; Wang, X.; Griffiths, T.G.; Pacchione, S.J.; Barnum, A.B.; Harper, L.B.; Pauley, C.J.; Niu, Z.; Denisova, L.; et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004, 11, 711–721. [Google Scholar] [CrossRef]
- Smith, K.R. Gene therapy: Theoretical and bioethical concepts. Arch. Med. Res. 2003, 34, 247–268. [Google Scholar] [CrossRef]
- Frazier, K.S. Antisense oligonucleotide therapies: The promise and the challenges from a toxicologic pathologist’s perspective. Toxicol. Pathol. 2015, 43, 78–89. [Google Scholar] [CrossRef]
- Wittrup, A.; Lieberman, J. Knocking down disease: A progress report on siRNA therapeutics. Nat. Rev. Genet. 2015, 16, 543–552. [Google Scholar] [CrossRef]
- Swayze, E.E.; Siwkowski, A.M.; Wancewicz, E.V.; Migawa, M.T.; Wyrzykiewicz, T.K.; Hung, G.; Monia, B.P.; Bennett, C.F. Antisense oligonucleotides containing locked nucleic acid improve potency but cause significant hepatotoxicity in animals. Nucleic Acids Res. 2007, 35, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Burdick, A.D.; Sciabola, S.; Mantena, S.R.; Hollingshead, B.D.; Stanton, R.; Warneke, J.A.; Zeng, M.; Martsen, E.; Medvedev, A.; Makarov, S.S.; et al. Sequence motifs associated with hepatotoxicity of locked nucleic acid--modified antisense oligonucleotides. Nucleic Acids Res. 2014, 42, 4882–4891. [Google Scholar] [CrossRef]
- Dieckmann, A.; Hagedorn, P.H.; Burki, Y.; Brügmann, C.; Berrera, M.; Ebeling, M.; Singer, T.; Schuler, F. A Sensitive In Vitro Approach to Assess the Hybridization-Dependent Toxic Potential of High Affinity Gapmer Oligonucleotides. Mol. Ther. Nucleic Acids 2018, 10, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Burel, S.A.; Hart, C.E.; Cauntay, P.; Hsiao, J.; Machemer, T.; Katz, M.; Watt, A.; Bui, H.H.; Younis, H.; Sabripour, M.; et al. Hepatotoxicity of high affinity gapmer antisense oligonucleotides is mediated by RNase H1 dependent promiscuous reduction of very long pre-mRNA transcripts. Nucleic Acids Res. 2016, 44, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Kawakami, S.; Fumoto, S.; Yamashita, F.; Hashida, M. Effect of the particle size of galactosylated lipoplex on hepatocyte-selective gene transfection after intraportal administration. Biol. Pharm. Bull. 2006, 29, 1521–1523. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, S.; Ito, Y.; Fumoto, S.; Yamashita, F.; Hashida, M. Enhanced gene expression in lung by a stabilized lipoplex using sodium chloride for complex formation. J. Gene Med. 2005, 7, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Fuchigami, Y.; Hagimori, M.; Fumoto, S.; Miura, Y.; Kawakami, S. Efficient gene transfection to the brain with ultrasound irradiation in mice using stabilized bubble lipopolyplexes prepared by the surface charge regulation method. Int. J. Nanomed. 2018, 13, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Choi, S.H.; Kim, C.O.; Park, J.S.; Ahn, W.S.; Kim, C.K. Enhancement of polyethylene glycol (PEG)-modified cationic liposome-mediated gene deliveries: Effects on serum stability and transfection efficiency. J. Pharm. Pharmacol. 2003, 55, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Balbino, T.A.; Serafin, J.M.; Malfatti-Gasperini, A.A.; de Oliveira, C.L.; Cavalcanti, L.P.; de Jesus, M.B.; de La Torre, L.G. Microfluidic Assembly of pDNA/Cationic Liposome Lipoplexes with High pDNA Loading for Gene Delivery. Langmuir 2016, 32, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Tam, Y.Y.; Chen, S.; Hafez, I.M.; Cullis, P.R. Microfluidic Mixing: A General Method for Encapsulating Macromolecules in Lipid Nanoparticle Systems. J. Phys. Chem. B 2015, 119, 8698–8706. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Huang, L. Folate-targeted, anionic liposome-entrapped polylysine-condensed DNA for tumor cell-specific gene transfer. J. Biol. Chem. 1996, 271, 8481–8487. [Google Scholar] [CrossRef] [PubMed]
- Kurosaki, T.; Kitahara, T.; Fumoto, S.; Nishida, K.; Nakamura, J.; Niidome, T.; Kodama, Y.; Nakagawa, H.; To, H.; Sasaki, H. Ternary complexes of pDNA, polyethylenimine, and gamma-polyglutamic acid for gene delivery systems. Biomaterials 2009, 30, 2846–2853. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yahara, A.; Waki, R.; Yasuhara, H.; Wada, F.; Harada-Shiba, M.; Obika, S. Amido-bridged nucleic acids with small hydrophobic residues enhance hepatic tropism of antisense oligonucleotides in vivo. Org. Biomol. Chem. 2015, 13, 3757–3765. [Google Scholar] [CrossRef]
- Gaus, H.; Miller, C.M.; Seth, P.P.; Harris, E.N. Structural Determinants for the Interactions of Chemically Modified Nucleic Acids with the Stabilin-2 Clearance Receptor. Biochemistry 2018, 57, 2061–2064. [Google Scholar] [CrossRef]
- Manoharan, M.; Inamati, G.B.; Lesnik, E.A.; Sioufi, N.B.; Freier, S.M. Improving antisense oligonucleotide binding to human serum albumin: Dramatic effect of ibuprofen conjugation. Chembiochem 2002, 3, 1257–1260. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Akita, H.; Ito, E.; Hayashi, Y.; Oishi, M.; Nagasaki, Y.; Danev, R.; Nagayama, K.; Kaji, N.; Kikuchi, H.; et al. Systemic delivery of siRNA to tumors using a lipid nanoparticle containing a tumor-specific cleavable PEG-lipid. Biomaterials 2011, 32, 4306–4316. [Google Scholar] [CrossRef]
- Hayashi, J.Y.; Tamanoi, F. Exploiting Enzyme Alterations in Cancer for Drug Activation, Drug Delivery, and Nanotherapy. Enzymes 2017, 42, 153–172. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Hong, L.; Wang, Z.; Wei, X.; Shi, J.; Li, C. Antibodies against polyethylene glycol in human blood: A literature review. J. Pharmacol. Toxicol. Methods 2020, 102, 106678. [Google Scholar] [CrossRef]
- Fang, J.-L.; Beland, F.A.; Tang, Y.; Roffler, S.R. Flow cytometry analysis of anti-polyethylene glycol antibodies in human plasma. Toxicol. Rep. 2020, 8, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Maeda, T.; Sakamoto, H.; Takasaki, N.; Shigyo, M.; Ishida, T.; Kiwada, H.; Mizushima, Y.; Mizushima, T. Evasion of the Accelerated Blood Clearance Phenomenon by Coating of Nanoparticles with Various Hydrophilic Polymers. Biomacromolecules 2010, 11, 2700–2706. [Google Scholar] [CrossRef] [PubMed]
- Abu Lila, A.S.; Uehara, Y.; Ishida, T.; Kiwada, H. Application of Polyglycerol Coating to Plasmid DNA Lipoplex for the Evasion of the Accelerated Blood Clearance Phenomenon in Nucleic Acid Delivery. J. Pharm. Sci. 2014, 103, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ito, S.; Yoshino, F.; Suzuki, Y.; Zhao, L.; Komatsu, N. Polyglycerol Grafting Shields Nanoparticles from Protein Corona Formation to Avoid Macrophage Uptake. ACS Nano 2020, 14, 7216–7226. [Google Scholar] [CrossRef]
- Son, K.; Ueda, M.; Taguchi, K.; Maruyama, T.; Takeoka, S.; Ito, Y. Evasion of the accelerated blood clearance phenomenon by polysarcosine coating of liposomes. J. Control. Release 2020, 322, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, S.; Kawakami, S.; Hashida, M.; Nishida, K. Capter 1. Targeted Gene Delivery: Importance of Administration Routes. In Novel Gene Therapy Approaches; Wei, M., Good, D., Eds.; IntechOpen: London, UK, 2013; pp. 3–31. [Google Scholar] [CrossRef]
- Kawabata, K.; Takakura, Y.; Hashida, M. The fate of plasmid DNA after intravenous injection in mice: Involvement of scavenger receptors in its hepatic uptake. Pharm Res. 1995, 12, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Intratumoral injection of naked DNA. Methods Mol. Med. 2000, 35, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, S.; Nishi, J.; Nakamura, J.; Nishida, K. Gene therapy for gastric diseases. Curr. Gene Ther. 2008, 8, 187–200. [Google Scholar] [CrossRef][Green Version]
- Peter, G.; Katrin, W.; Niclas, C.; Dinko, R.; Catarina, N.; Jane, K.; Mikko, H.; Yanfeng, W.; Rosie, Y.; Stanley, R.; et al. Abstract 13307: An Oral Antisense Oligonucleotide for PCSK9 Inhibition in Humans. Circulation 2020, 142 (Suppl. 3), A13307. [Google Scholar] [CrossRef]
- Advances in RNAi Therapeutics Platform. Available online: https://www.alnylam.com/wp-content/uploads/2019/06/2019.06.22_Platform-Advances_FINAL.pdf (accessed on 19 January 2021).
- Raoof, A.A.; Ramtoola, Z.; McKenna, B.; Yu, R.Z.; Hardee, G.; Geary, R.S. Effect of sodium caprate on the intestinal absorption of two modified antisense oligonucleotides in pigs. Eur. J. Pharm. Sci. 2002, 17, 131–138. [Google Scholar] [CrossRef]
- Tillman, L.G.; Geary, R.S.; Hardee, G.E. Oral Delivery of Antisense Oligonucleotides in Man. J. Pharm. Sci. 2008, 97, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Kreiter, S.; Selmi, A.; Diken, M.; Koslowski, M.; Britten, C.M.; Huber, C.; Türeci, Ö.; Sahin, U. Intranodal Vaccination with Naked Antigen-Encoding RNA Elicits Potent Prophylactic and Therapeutic Antitumoral Immunity. Cancer Res. 2010, 70, 9031–9040. [Google Scholar] [CrossRef]
- Ita, K. Dermal/transdermal delivery of small interfering RNA and antisense oligonucleotides- advances and hurdles. Biomed. Pharmacother. 2017, 87, 311–320. [Google Scholar] [CrossRef]
- Koh, K.J.; Liu, Y.; Lim, S.H.; Loh, X.J.; Kang, L.; Lim, C.Y.; Phua, K.K.L. Formulation, characterization and evaluation of mRNA-loaded dissolvable polymeric microneedles (RNApatch). Sci. Rep. 2018, 8, 11842. [Google Scholar] [CrossRef]
- Yang, T.; Huang, D.; Li, C.; Zhao, D.; Li, J.; Zhang, M.; Chen, Y.; Wang, Q.; Liang, Z.; Liang, X.-J.; et al. Rolling microneedle electrode array (RoMEA) empowered nucleic acid delivery and cancer immunotherapy. Nano Today 2021, 36, 101017. [Google Scholar] [CrossRef]
- Boado, R.J. Blood-brain barrier transport of non-viral gene and RNAi therapeutics. Pharm. Res. 2007, 24, 1772–1787. [Google Scholar] [CrossRef]
- Danialou, G.; Comtois, A.S.; Matecki, S.; Nalbantoglu, J.; Karpati, G.; Gilbert, R.; Geoffroy, P.; Gilligan, S.; Tanguay, J.F.; Petrof, B.J. Optimization of regional intraarterial naked DNA-mediated transgene delivery to skeletal muscles in a large animal model. Mol. Ther. 2005, 11, 257–266. [Google Scholar] [CrossRef]
- Song, K.H.; Fan, A.C.; Hinkle, J.J.; Newman, J.; Borden, M.A.; Harvey, B.K. Microbubble gas volume: A unifying dose parameter in blood-brain barrier opening by focused ultrasound. Theranostics 2017, 7, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Negishi, Y.; Yamane, M.; Kurihara, N.; Endo-Takahashi, Y.; Sashida, S.; Takagi, N.; Suzuki, R.; Maruyama, K. Enhancement of Blood-Brain Barrier Permeability and Delivery of Antisense Oligonucleotides or Plasmid DNA to the Brain by the Combination of Bubble Liposomes and High-Intensity Focused Ultrasound. Pharmaceutics 2015, 7, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Oyama, N.; Fuchigami, Y.; Fumoto, S.; Sato, M.; Hagimori, M.; Shimizu, K.; Kawakami, S. Characterization of transgene expression and pDNA distribution of the suctioned kidney in mice. Drug Deliv. 2017, 24, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kawakami, S.; Suzuki, S.; Yamashita, F.; Hashida, M. Enhancement of immune responses by DNA vaccination through targeted gene delivery using mannosylated cationic liposome formulations following intravenous administration in mice. Biochem. Biophys. Res. Commun. 2004, 317, 992–999. [Google Scholar] [CrossRef]
- Hattori, Y.; Kawakami, S.; Nakamura, K.; Yamashita, F.; Hashida, M. Efficient gene transfer into macrophages and dendritic cells by in vivo gene delivery with mannosylated lipoplex via the intraperitoneal route. J. Pharmacol. Exp. Ther. 2006, 318, 828–834. [Google Scholar] [CrossRef]
- Yamamoto, T.; Sawamura, M.; Terada, C.; Kashiwada, K.; Wada, F.; Yamayoshi, A.; Obika, S.; Harada-Shiba, M. Effect of modular conjugation strategy for N-acetylgalactosamine-targeted antisense oligonucleotides. Nucleosides Nucleotides Nucleic Acids. 2020, 39, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Bragonzi, A.; Conese, M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol. Ther. 2005, 12, 468–4674. [Google Scholar] [CrossRef]
- Rejman, J.; Conese, M.; Hoekstra, D. Gene transfer by means of lipo- and polyplexes: Role of clathrin and caveolae-mediated endocytosis. J. Liposome Res. 2006, 16, 237–247. [Google Scholar] [CrossRef]
- Khalil, I.A.; Kogure, K.; Futaki, S.; Harashima, H. High density of octaarginine stimulates macropinocytosis leading to efficient intracellular trafficking for gene expression. J. Biol. Chem. 2006, 281, 3544–3551. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, S.; Nishi, J.; Ishii, H.; Wang, X.; Miyamoto, H.; Yoshikawa, N.; Nakashima, M.; Nakamura, J.; Nishida, K. Rac-mediated macropinocytosis is a critical route for naked plasmid DNA transfer in mice. Mol. Pharm. 2009, 6, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Futaki, S. Current Understanding of Direct Translocation of Arginine-Rich Cell-Penetrating Peptides and Its Internalization Mechanisms. Chem. Pharm. Bull. 2016, 64, 1431–1437. [Google Scholar] [CrossRef]
- Lehto, T.; Ezzat, K.; Wood, M.J.A.; El Andaloussi, S. Peptides for nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 106, 172–182. [Google Scholar] [CrossRef]
- Kuang, H.; Ku, S.H.; Kokkoli, E. The design of peptide-amphiphiles as functional ligands for liposomal anticancer drug and gene delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 80–101. [Google Scholar] [CrossRef] [PubMed]
- Sugar, I.P.; Neumann, E. Stochastic model for electric field-induced membrane pores. Electroporation. Biophys. Chem. 1984, 19, 211–225. [Google Scholar] [CrossRef]
- Chang, D.C.; Reese, T.S. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys. J. 1990, 58, 1–12. [Google Scholar] [CrossRef]
- Hu, Y.; Wan, J.M.; Yu, A.C. Membrane perforation and recovery dynamics in microbubble-mediated sonoporation. Ultrasound Med. Biol. 2013, 39, 2393–2405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gao, X.; Song, Y.K.; Vollmer, R.; Stolz, D.B.; Gasiorowski, J.Z.; Dean, D.A.; Liu, D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004, 11, 675–682. [Google Scholar] [CrossRef]
- Mine, T.; Ishii, H.; Nakajima, S.; Yoshikawa, N.; Miyamoto, H.; Nakashima, M.; Nakamura, J.; Fumoto, S.; Nishida, K. Rubbing gastric serosal surface enhances naked plasmid DNA transfer in rats and mice. Biol. Pharm. Bull. 2011, 34, 1514–1517. [Google Scholar] [CrossRef][Green Version]
- Fumoto, S.; Nakajima, S.; Mine, T.; Yoshikawa, N.; Kitahara, T.; Sasaki, H.; Miyamoto, H.; Nishida, K. Efficient in vivo gene transfer by intraperitoneal injection of plasmid DNA and calcium carbonate microflowers in mice. Mol. Pharm. 2012, 9, 1962–1970. [Google Scholar] [CrossRef]
- Durymanov, M.; Kamaletdinova, T.; Lehmann, S.E.; Reineke, J.J. Exploiting passive nanomedicine accumulation at sites of enhanced vascular permeability for non-cancerous applications. J. Control. Release 2017, 261, 10–22. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Kimura, N.; Maeki, M.; Sato, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Development of a Microfluidic-Based Post-Treatment Process for Size-Controlled Lipid Nanoparticles and Application to siRNA Delivery. ACS Appl. Mater. Interfaces 2020, 12, 34011–34020. [Google Scholar] [CrossRef] [PubMed]
- Hashiba, A.; Toyooka, M.; Sato, Y.; Maeki, M.; Tokeshi, M.; Harashima, H. The use of design of experiments with multiple responses to determine optimal formulations for in vivo hepatic mRNA delivery. J. Control. Release 2020, 327, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, S.; Nishida, K. Co-delivery Systems of Multiple Drugs Using Nanotechnology for Future Cancer Therapy. Chem. Pharm. Bull. 2020, 68, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Kaps, L.; Schuppan, D. Targeting Cancer Associated Fibroblasts in Liver Fibrosis and Liver Cancer Using Nanocarriers. Cells 2020, 9, 2027. [Google Scholar] [CrossRef] [PubMed]
- Dolor, A.; Szoka, F.C., Jr. Digesting a Path Forward: The Utility of Collagenase Tumor Treatment for Improved Drug Delivery. Mol. Pharm. 2018, 15, 2069–2083. [Google Scholar] [CrossRef] [PubMed]
- Cemazar, M.; Golzio, M.; Sersa, G.; Escoffre, J.-M.; Coer, A.; Vidic, S.; Teissie, J. Hyaluronidase and Collagenase Increase the Transfection Efficiency of Gene Electrotransfer in Various Murine Tumors. Hum. Gene Ther. 2012, 23, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Hattori, Y.; Kubo, M.; Maitani, Y. Collagenase-1 injection improved tumor distribution and gene expression of cationic lipoplex. Int. J. Pharm. 2012, 423, 428–434. [Google Scholar] [CrossRef]
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H.; et al. Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors. ACS Nano 2019, 13, 11008–11021. [Google Scholar] [CrossRef]
- Peng, J.Q.; Fumoto, S.; Suga, T.; Miyamoto, H.; Kuroda, N.; Kawakami, S.; Nishida, K. Targeted co-delivery of protein and drug to a tumor in vivo by sophisticated RGD-modified lipid-calcium carbonate nanoparticles. J. Control. Release 2019, 302, 42–53. [Google Scholar] [CrossRef]
- Suga, T.; Fuchigami, Y.; Hagimori, M.; Kawakami, S. Ligand peptide-grafted PEGylated liposomes using HER2 targeted peptide-lipid derivatives for targeted delivery in breast cancer cells: The effect of serine-glycine repeated peptides as a spacer. Int. J. Pharm. 2017, 521, 361–364. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Ghosh, A.; Maiti, S.; Ahir, M.; Debnath, G.H.; Gupta, P.; Bhattacharjee, M.; Ghosh, S.; Chattopadhyay, S.; Mukherjee, P.; et al. Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate angiogenesis and metastasis of triple-negative breast cancer. J. Control. Release 2020, 322, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.V.; Rho, J.G.; Um, W.; Ek, P.K.; Nguyen, V.Q.; Oh, B.H.; Kim, W.; Park, J.H. Hyaluronic Acid Nanoparticles as Nanomedicine for Treatment of Inflammatory Diseases. Pharmaceutics 2020, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Hattab, D.; Bakhtiar, A. Bioengineered siRNA-Based Nanoplatforms Targeting Molecular Signaling Pathways for the Treatment of Triple Negative Breast Cancer: Preclinical and Clinical Advancements. Pharmaceutics 2020, 12, 929. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, J.; Gu, P.; Fan, X. The application of nanoparticles in cancer immunotherapy: Targeting tumor microenvironment. Bioact. Mater. 2020, 6, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kawakami, S.; Higuchi, Y.; Maruyama, K.; Yamashita, F.; Hashida, M. Antitumor effect of nuclear factor-κB decoy transfer by mannose-modified bubble lipoplex into macrophages in mouse malignant ascites. Cancer Sci. 2014, 105, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Ultrasound-Mediated Drug/Gene Delivery in Solid Tumor Treatment. J. Healthc. Eng. 2013, 4, 223–254. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, S.; Kawakami, S. Combination of Nanoparticles with Physical Stimuli toward Cancer Therapy. Biol. Pharm. Bull. 2014, 37, 212–216. [Google Scholar] [CrossRef]
- Lee, S.; Han, H.; Koo, H.; Na, J.H.; Yoon, H.Y.; Lee, K.E.; Lee, H.; Kim, H.; Kwon, I.C.; Kim, K. Extracellular matrix remodeling in vivo for enhancing tumor-targeting efficiency of nanoparticle drug carriers using the pulsed high intensity focused ultrasound. J. Control. Release 2017, 263, 68–78. [Google Scholar] [CrossRef]
- Akinc, A.; Thomas, M.; Klibanov, A.M.; Langer, R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005, 7, 657–663. [Google Scholar] [CrossRef]
- Ukawa, M.; Akita, H.; Masuda, T.; Hayashi, Y.; Konno, T.; Ishihara, K.; Harashima, H. 2-Methacryloyloxyethyl phosphorylcholine polymer (MPC)-coating improves the transfection activity of GALA-modified lipid nanoparticles by assisting the cellular uptake and intracellular dissociation of plasmid DNA in primary hepatocytes. Biomaterials 2010, 31, 6355–6362. [Google Scholar] [CrossRef]
- Tanaka, H.; Sato, Y.; Harashima, H.; Akita, H. Cellular environment-responsive nanomaterials for use in gene and siRNA delivery: Molecular design for biomembrane destabilization and intracellular collapse. Expert Opin. Drug Deliv. 2016, 13, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Zanta, M.A.; Belguise-Valladier, P.; Behr, J.P. Gene delivery: A single nuclear localization signal peptide is sufficient to carry DNA to the cell nucleus. Proc. Natl. Acad. Sci. USA 1999, 96, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.M.; Dean, D.A. Cell-specific nuclear import of plasmid DNA in smooth muscle requires tissue-specific transcription factors and DNA sequences. Gene Ther. 2008, 15, 1107–1115. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, L.; Lin, X.; Barravecchia, M.; Dean, D.A. Identification of an alveolar type I epithelial cell-specific DNA nuclear import sequence for gene delivery. Gene Ther. 2016, 23, 734–742. [Google Scholar] [CrossRef]
- Tammam, S.N.; Azzazy, H.M.; Breitinger, H.G.; Lamprecht, A. Chitosan Nanoparticles for Nuclear Targeting: The Effect of Nanoparticle Size and Nuclear Localization Sequence Density. Mol. Pharm. 2015, 12, 4277–4289. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, W.; Qiu, Y.; Cespi, M.; Palmieri, G.F.; Mason, A.J.; Lam, J.K. Incorporation of a Nuclear Localization Signal in pH Responsive LAH4-L1 Peptide Enhances Transfection and Nuclear Uptake of Plasmid DNA. Mol. Pharm. 2016, 13, 3141–3152. [Google Scholar] [CrossRef]
- Pazmany, T.; Murphy, S.P.; Gollnick, S.O.; Brooks, S.P.; Tomasi, T.B. Activation of multiple transcription factors and fos and jun gene family expression in cells exposed to a single electric pulse. Exp. Cell Res. 1995, 221, 103–110. [Google Scholar] [CrossRef]
- Nishikawa, M.; Nakayama, A.; Takahashi, Y.; Fukuhara, Y.; Takakura, Y. Reactivation of silenced transgene expression in mouse liver by rapid, large-volume injection of isotonic solution. Hum. Gene Ther. 2008, 19, 1009–1020. [Google Scholar] [CrossRef]
- Un, K.; Kawakami, S.; Higuchi, Y.; Suzuki, R.; Maruyama, K.; Yamashita, F.; Hashida, M. Involvement of activated transcriptional process in efficient gene transfection using unmodified and mannose-modified bubble lipoplexes with ultrasound exposure. J. Control. Release 2011, 156, 355–363. [Google Scholar] [CrossRef]
- Mukai, H.; Kawakami, S.; Takahashi, H.; Satake, K.; Yamashita, F.; Hashida, M. Key physiological phenomena governing transgene expression based on tissue pressure-mediated transfection in mice. Biol. Pharm. Bull. 2010, 33, 1627–1632. [Google Scholar] [CrossRef][Green Version]
- Haraguchi, A.; Fuchigami, Y.; Kawaguchi, M.; Fumoto, S.; Ohyama, K.; Shimizu, K.; Hagimori, M.; Kawakami, S. Determining Transgene Expression Characteristics Using a Suction Device with Multiple Hole Adjusting a Left Lateral Lobe of the Mouse Liver. Biol. Pharm. Bull. 2018, 41, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Yew, N.S.; Zhao, H.; Przybylska, M.; Wu, I.H.; Tousignant, J.D.; Scheule, R.K.; Cheng, S.H. CpG-depleted plasmid DNA vectors with enhanced safety and long-term gene expression in vivo. Mol. Ther. 2002, 5, 731–738. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.P.; Argyros, O.; Coutelle, C.; Harbottle, R.P. Non-viral S/MAR vectors replicate episomally in vivo when provided with a selective advantage. Gene Ther. 2011, 18, 82–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nishimura, K.; Fumoto, S.; Fuchigami, Y.; Hagimori, M.; Maruyama, K.; Kawakami, S. Effective intraperitoneal gene transfection system using nanobubbles and ultrasound irradiation. Drug Deliv. 2017, 24, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, H.; Higuchi, Y.; Kawakami, S.; Yamashita, F.; Hashida, M. piggyBac transposon-mediated long-term gene expression in mice. Mol. Ther. 2010, 18, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Otani, Y.; Kawakami, S.; Mukai, H.; Fuchigami, Y.; Yamashita, F.; Hashida, M. Long-term in vivo gene expression in mouse kidney using φC31 integrase and electroporation. J. Drug Target. 2015, 23, 427–435. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miller, J.B.; Zhang, S.; Kos, P.; Xiong, H.; Zhou, K.; Perelman, S.S.; Zhu, H.; Siegwart, D.J. Non-Viral CRISPR/Cas Gene Editing In Vitro and In Vivo Enabled by Synthetic Nanoparticle Co-Delivery of Cas9 mRNA and sgRNA. Angew. Chem. Int. Ed. 2017, 56, 1059–1063. [Google Scholar] [CrossRef]
- Nair, J.K.; Attarwala, H.; Sehgal, A.; Wang, Q.; Aluri, K.; Zhang, X.; Gao, M.; Liu, J.; Indrakanti, R.; Schofield, S.; et al. Impact of enhanced metabolic stability on pharmacokinetics and pharmacodynamics of GalNAc–siRNA conjugates. Nucleic Acids Res. 2017, 45, 10969–10977. [Google Scholar] [CrossRef]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.; Turner, T.; Visseren, F.L.; et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef]
- Brown, C.R.; Gupta, S.; Qin, J.; Racie, T.; He, G.; Lentini, S.; Malone, R.; Yu, M.; Matsuda, S.; Shulga-Morskaya, S.; et al. Investigating the pharmacodynamic durability of GalNAc-siRNA conjugates. Nucleic Acids Res. 2020, 48, 11827–11844. [Google Scholar] [CrossRef]
- Shen, W.; De Hoyos, C.L.; Migawa, M.T.; Vickers, T.A.; Sun, H.; Low, A.; Bell, T.A., 3rd; Rahdar, M.; Mukhopadhyay, S.; Hart, C.E.; et al. Chemical modification of PS-ASO therapeutics reduces cellular protein-binding and improves the therapeutic index. Nat. Biotechnol. 2019, 37, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Griesenbach, U.; Meng, C.; Farley, R.; Gardner, A.; Brake, M.A.; Frankel, G.M.; Gruenert, D.C.; Cheng, S.H.; Scheule, R.K.; Alton, E.W. The role of doxorubicin in non-viral gene transfer in the lung. Biomaterials 2009, 30, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Un, K.; Kono, Y.; Yoshida, M.; Yamashita, F.; Kawakami, S.; Hashida, M. Enhancement of gene expression by transcriptional activation using doxorubicin-loaded liposome/pDNA complexes. Pharmazie 2012, 67, 400–405. [Google Scholar] [PubMed]
- Wang, S.; Fumoto, S.; Miyamoto, H.; Tanaka, M.; Nishida, K. Edaravone, a cytoprotective drug, enhances transgene expression mediated by lipoplexes in HepG2 cells and mice. Int. J. Pharm. 2018, 548, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Takiguchi, N.; Takahashi, Y.; Nishikawa, M.; Matsui, Y.; Fukuhara, Y.; Oushiki, D.; Kiyose, K.; Hanaoka, K.; Nagano, T.; Takakura, Y. Positive correlation between the generation of reactive oxygen species and activation/reactivation of transgene expression after hydrodynamic injections into mice. Pharm. Res. 2011, 28, 702–711. [Google Scholar] [CrossRef]
- Fumoto, S.; Nishida, K. Methods for Evaluating the Stimuli-Responsive Delivery of Nucleic Acid and Gene Medicines. Chem. Pharm. Bull. 2017, 65, 642–648. [Google Scholar] [CrossRef][Green Version]
- Kuchimaru, T.; Iwano, S.; Kiyama, M.; Mitsumata, S.; Kadonosono, T.; Niwa, H.; Maki, S.; Kizaka-Kondoh, S. A luciferin analogue generating near-infrared bioluminescence achieves highly sensitive deep-tissue imaging. Nat. Commun. 2016, 7, 11856. [Google Scholar] [CrossRef]
- Fumoto, S.; Nishimura, K.; Nishida, K.; Kawakami, S. Three-Dimensional Imaging of the Intracellular Fate of Plasmid DNA and Transgene Expression: ZsGreen1 and Tissue Clearing Method CUBIC Are an Optimal Combination for Multicolor Deep Imaging in Murine Tissues. PLoS ONE 2016, 11, e0148233. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, S.; Kinoshita, E.; Ohta, K.; Nakamura, K.I.; Hirayama, T.; Nagasawa, H.; Hu, D.; Okami, K.; Kato, R.; Shimokawa, S.; et al. A pH-Adjustable Tissue Clearing Solution That Preserves Lipid Ultrastructures: Suitable Tissue Clearing Method for DDS Evaluation. Pharmaceutics 2020, 12, E1070. [Google Scholar] [CrossRef]
- Hisazumi, J.; Kobayashi, N.; Nishikawa, M.; Takakura, Y. Significant role of liver sinusoidal endothelial cells in hepatic uptake and degradation of naked plasmid DNA after intravenous injection. Pharm. Res. 2004, 21, 1223–1228. [Google Scholar] [CrossRef]
- Shimizu, K.; Higuchi, Y.; Kozu, Y.; Hashida, M.; Konishi, S. Development of a suction device for stabilizing in vivo real-time imaging of murine tissues. J. Biosci. Bioeng. 2011, 112, 508–510. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.G.; König, K.; Halbhuber, K.J. Two-photon microscopy of deep intravital tissues and its merits in clinical research. J. Microsc. 2010, 238, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, S.; Kawakami, S.; Ishizuka, M.; Nishikawa, M.; Yamashita, F.; Hashida, M. Analysis of Hepatic Disposition of Native and Galactosylated Polyethylenimine Complexed with Plasmid DNA in Perfused Rat Liver. Drug Metab. Pharmacokinet. 2003, 18, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Fumoto, S.; Nakadori, F.; Kawakami, S.; Nishikawa, M.; Yamashita, F.; Hashida, M. Analysis of hepatic disposition of galactosylated cationic liposome/plasmid DNA complexes in perfused rat liver. Pharm. Res. 2003, 20, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.T. Nanoparticle-mediated delivery of oligonucleotides to the blood–brain barrier:in vitroandin situbrain perfusion studies on the uptake mechanisms. J. Drug Target. 2013, 21, 866–873. [Google Scholar] [CrossRef]
- Minchin, R.F.; Johnston, M.R.; Aiken, M.A.; Boyd, M.R. Pharmacokinetics of doxorubicin in isolated lung of dogs and humans perfused in vivo. J. Pharmacol. Exp. Ther. 1984, 229, 193–198. [Google Scholar]
- Sawai, K.; Miyao, T.; Takakura, Y.; Hashida, M. Renal Disposition Characteristics of Oligonucleotides Modified at Terminal Linkages in the Perfused Rat Kidney. Antisense Res. Dev. 1995, 5, 279–287. [Google Scholar] [CrossRef]
- Kakutani, T.; Yamaoka, K.; Hashida, M.; Sezaki, H. A new method for assessment of drug disposition in muscle: Application of statistical moment theory to local perfusion systems. J. Pharmacokinet. Biopharm. 1985, 13, 609–631. [Google Scholar] [CrossRef]
- Nomura, T.; Nakajima, S.; Kawabata, K.; Yamashita, F.; Takakura, Y.; Hashida, M. Intratumoral pharmacokinetics and in vivo gene expression of naked plasmid DNA and its cationic liposome complexes after direct gene transfer. Cancer Res. 1997, 57, 2681–2686. [Google Scholar]
- Akita, H.; Ito, R.; Khalil, I.A.; Futaki, S.; Harashima, H. Quantitative three-dimensional analysis of the intracellular trafficking of plasmid DNA transfected by a nonviral gene delivery system using confocal laser scanning microscopy. Mol. Ther. 2004, 9, 443–451. [Google Scholar] [CrossRef]
- Lambert, T.J.; Waters, J.C. Navigating challenges in the application of superresolution microscopy. J. Cell Biol. 2017, 216, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Lu, Q.; Wang, Y.; Xu, E.; Ho, A.; Singh, P.; Wang, Y.; Jiang, Z.; Yang, F.; Tietjen, G.T.; et al. Quantitating Endosomal Escape of a Library of Polymers for mRNA Delivery. Nano Lett. 2020, 20, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qu, Z.; Kim, S.; Shi, V.; Liao, B.; Kraft, P.; Bandaru, R.; Wu, Y.; Greenberger, L.M.; Horak, I.D. Down-modulation of cancer targets using locked nucleic acid (LNA)-based antisense oligonucleotides without transfection. Gene Ther. 2011, 18, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.A.; Hansen, J.B.; Lai, J.; Wu, S.; Voskresenskiy, A.; Høg, A.; Worm, J.; Hedtjärn, M.; Souleimanian, N.; Miller, P.; et al. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 2010, 38, e3. [Google Scholar] [CrossRef]
- Hori, S.-I.; Yamamoto, T.; Waki, R.; Wada, S.; Wada, F.; Noda, M.; Obika, S. Ca2+ enrichment in culture medium potentiates effect of oligonucleotides. Nucleic Acids Res. 2015, 43, e128. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, S.; Katsumi, H.; Suzuki, H.; Hirai, N.; Takashima, R.; Morishita, M.; Sakane, T.; Yamamoto, A. l-Cysteine and l-Serine Modified Dendrimer with Multiple Reduced Thiols as a Kidney-Targeting Reactive Oxygen Species Scavenger to Prevent Renal Ischemia/Reperfusion Injury. Pharmaceutics 2018, 10, 251. [Google Scholar] [CrossRef]
- Yamashita, S.; Katsumi, H.; Shimizu, E.; Nakao, Y.; Yoshioka, A.; Fukui, M.; Kimura, H.; Sakane, T.; Yamamoto, A. Dendrimer-based micelles with highly potent targeting to sites of active bone turnover for the treatment of bone metastasis. Eur. J. Pharm. Biopharm. 2020, 157, 85–96. [Google Scholar] [CrossRef]
- Nishimura, K.; Yonezawa, K.; Fumoto, S.; Miura, Y.; Hagimori, M.; Nishida, K.; Kawakami, S. Application of Direct Sonoporation from a Defined Surface Area of the Peritoneum: Evaluation of Transfection Characteristics in Mice. Pharmaceutics 2019, 11, 244. [Google Scholar] [CrossRef]
- Endo-Takahashi, Y.; Negishi, Y. Microbubbles and Nanobubbles with Ultrasound for Systemic Gene Delivery. Pharmaceutics 2020, 12, 964. [Google Scholar] [CrossRef]
- Negishi, Y.; Endo-Takahashi, Y.; Ishiura, S. Exon Skipping by Ultrasound-Enhanced Delivery of Morpholino with Bubble Liposomes for Myotonic Dystrophy Model Mice. Methods Mol. Biol. 2018, 1828, 481–487. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Oyama, N.; Fumoto, S.; Kinoshita, H.; Yamashita, F.; Shimizu, K.; Hashida, M.; Kawakami, S. Tissue suction-mediated gene transfer to the beating heart in mice. PLoS ONE 2020, 15, e0228203. [Google Scholar] [CrossRef] [PubMed]
- Lenk, G.M.; Jafar-Nejad, P.; Hill, S.F.; Huffman, L.D.; Smolen, C.E.; Wagnon, J.L.; Petit, H.; Yu, W.; Ziobro, J.; Bhatia, K.; et al. Scn8a Antisense Oligonucleotide Is Protective in Mouse Models of SCN8A Encephalopathy and Dravet Syndrome. Ann. Neurol. 2020, 87, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Krainer, A.R.; Cleveland, D.W. Antisense Oligonucleotide Therapies for Neurodegenerative Diseases. Annu. Rev. Neurosci. 2019, 42, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goel, V.; Robbie, G.J. Pharmacokinetics of Patisiran, the First Approved RNA Interference Therapy in Patients with Hereditary Transthyretin-Mediated Amyloidosis. J. Clin. Pharmacol. 2019, 60, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Tran, D.M.; Cavedon, A.; Cai, X.; Rajendran, R.; Lyle, M.J.; Martini, P.G.; Miao, C.H. Treatment of Hemophilia A Using Factor VIII Messenger RNA Lipid Nanoparticles. Mol. Ther. Nucleic Acids 2020, 20, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-assembled mRNA vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef] [PubMed]
- Seraj, S.; Lee, J.; Ahn, H.J. Systemic delivery of Eg5 shRNA-expressing plasmids using PEGylated DC-Chol/DOPE cationic liposome: Long-term silencing and anticancer effects in vivo. Biochem. Pharmacol. 2019, 166, 192–202. [Google Scholar] [CrossRef]
- Bazzani, R.P.; Pringle, I.A.; Connolly, M.M.; Davies, L.A.; Sumner-Jones, S.G.; Schleef, M.; Hyde, S.C.; Gill, D.R. Transgene sequences free of CG dinucleotides lead to high level, long-term expression in the lung independent of plasmid backbone design. Biomaterials 2016, 93, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ofri, R.; Averbukh, E.; Ezra-Elia, R.; Ross, M.; Honig, H.; Obolensky, A.; Rosov, A.; Hauswirth, W.W.; Gootwine, E.; Banin, E. Six Years and Counting: Restoration of Photopic Retinal Function and Visual Behavior Following Gene Augmentation Therapy in a Sheep Model of CNGA3 Achromatopsia. Hum. Gene Ther. 2018, 29, 1376–1386. [Google Scholar] [CrossRef]
- Al-Zaidy, S.A.; Mendell, J.R. From Clinical Trials to Clinical Practice: Practical Considerations for Gene Replacement Therapy in SMA Type 1. Pediatr. Neurol. 2019, 100, 3–11. [Google Scholar] [CrossRef]
- Verdera, H.C.; Kuranda, K.; Mingozzi, F. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol. Ther. 2020, 28, 723–746. [Google Scholar] [CrossRef] [PubMed]
- Breunig, M.; Lungwitz, U.; Liebl, R.; Goepferich, A. Breaking up the correlation between efficacy and toxicity for nonviral gene delivery. Proc. Natl. Acad. Sci. USA 2007, 104, 14454–14459. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, S.; Ding, Y.-F.; Yin, H.; Wang, L.H.; Wang, R. Macrocycle-wrapped polyethylenimine for gene delivery with reduced cytotoxicity. Biomater. Sci. 2018, 6, 1031–1039. [Google Scholar] [CrossRef]
- Iredale, J.P. Models of liver fibrosis: Exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Investig. 2007, 117, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Böttger, R.; Pauli, G.; Chao, P.-H.; Al Fayez, N.; Hohenwarter, L.; Li, S.D. Lipid-based nanoparticle technologies for liver targeting. Adv. Drug Deliv. Rev. 2020, 154-155, 79–101. [Google Scholar] [CrossRef]
- Niemietz, C.; Nadzemova, O.; Zibert, A.; Schmidt, H.H.-J. APOE polymorphism in ATTR amyloidosis patients treated with lipid nanoparticle siRNA. Amyloid 2020, 27, 45–51. [Google Scholar] [CrossRef]
- Ghiselli, G.; Schaefer, E.J.; Gascon, P.; Breser, H.B., Jr. Type III hyperlipoproteinemia associated with apolipoprotein E deficiency. Science 1981, 214, 1239–1241. [Google Scholar] [CrossRef]
- Tokunaga, A.; Miyamoto, H.; Fumoto, S.; Nishida, K. Effect of renal ischaemia/reperfusion-induced acute kidney injury on pharmacokinetics of midazolam in rats. J. Pharm. Pharmacol. 2019, 71, 1792–1799. [Google Scholar] [CrossRef]
- Tokunaga, A.; Miyamoto, H.; Fumoto, S.; Nishida, K. Effect of Chronic Kidney Disease on Hepatic Clearance of Drugs in Rats. Biol. Pharm. Bull. 2020, 43, 1324–1330. [Google Scholar] [CrossRef]
- Nakano, T.; Katsuki, S.; Chen, M.; Decano, J.L.; Halu, A.; Lee, L.H.; Pestana, D.V.S.; Kum, A.S.T.; Kuromoto, R.K.; Golden, W.S.; et al. Uremic Toxin Indoxyl Sulfate Promotes Proinflammatory Macrophage Activation via the Interplay of OATP2B1 and Dll4-Notch Signaling. Circulation 2019, 139, 78–96. [Google Scholar] [CrossRef]
- Li, Y.; Yan, J.; Wang, M.; Lv, J.; Yan, F.; Chen, J. Uremic toxin indoxyl sulfate promotes proinflammatory macrophage activation by regulation of β-catenin and YAP pathways. J. Mol. Histol. 2021, 1–9, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Ogawa, K.; Kawaguchi, M.; Fumoto, S.; Mukai, H.; Kawakami, S. Suppression of Peritoneal Fibrosis by Sonoporation of Hepatocyte Growth Factor Gene-Encoding Plasmid DNA in Mice. Pharmaceutics 2021, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Fumoto, S.; Miyamoto, H.; Chen, Y.; Kuroda, N.; Nishida, K. One-step formation of lipid-polyacrylic acid-calcium carbonate nanoparticles for co-delivery of doxorubicin and curcumin. J. Drug Target. 2017, 25, 704–714. [Google Scholar] [CrossRef] [PubMed]
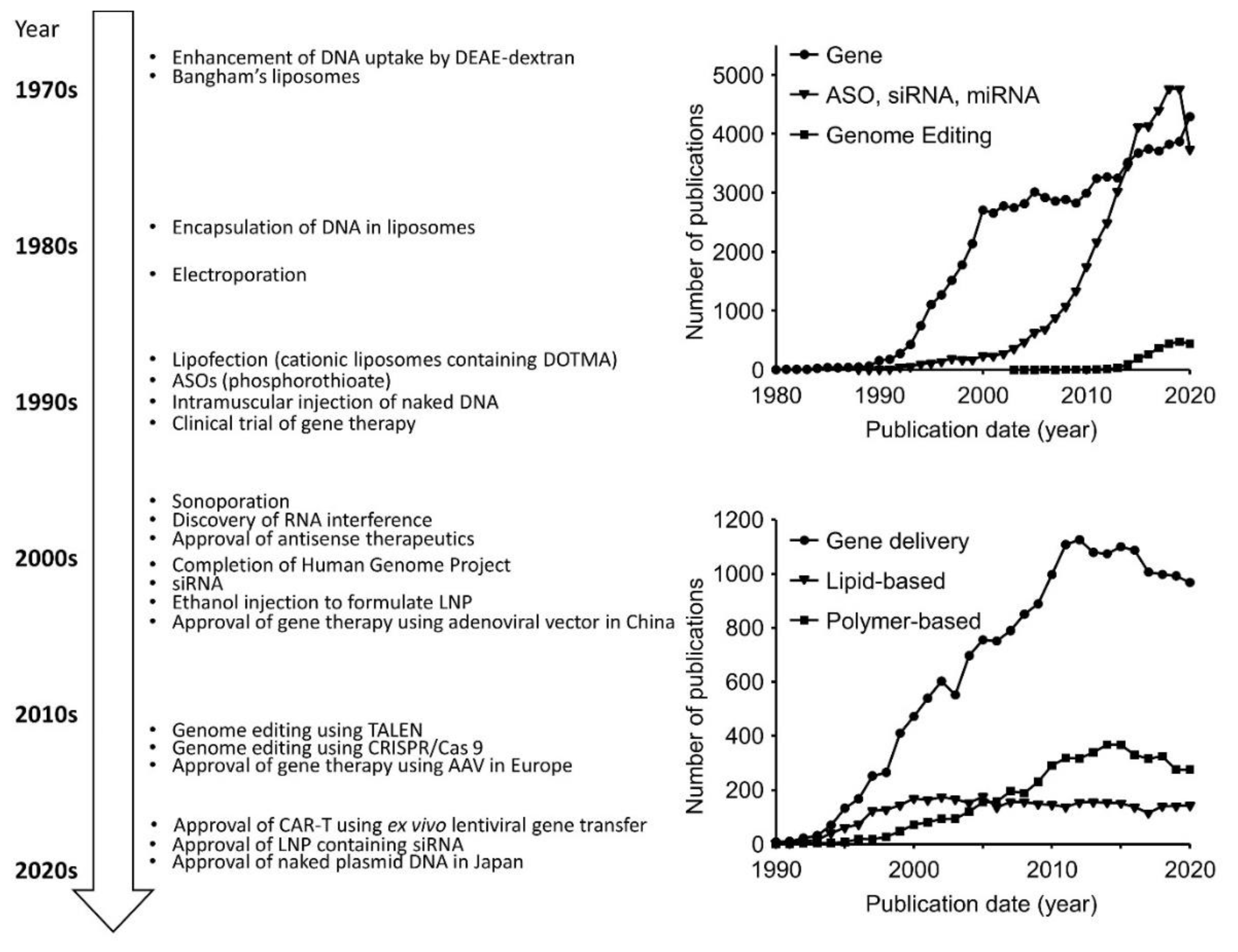

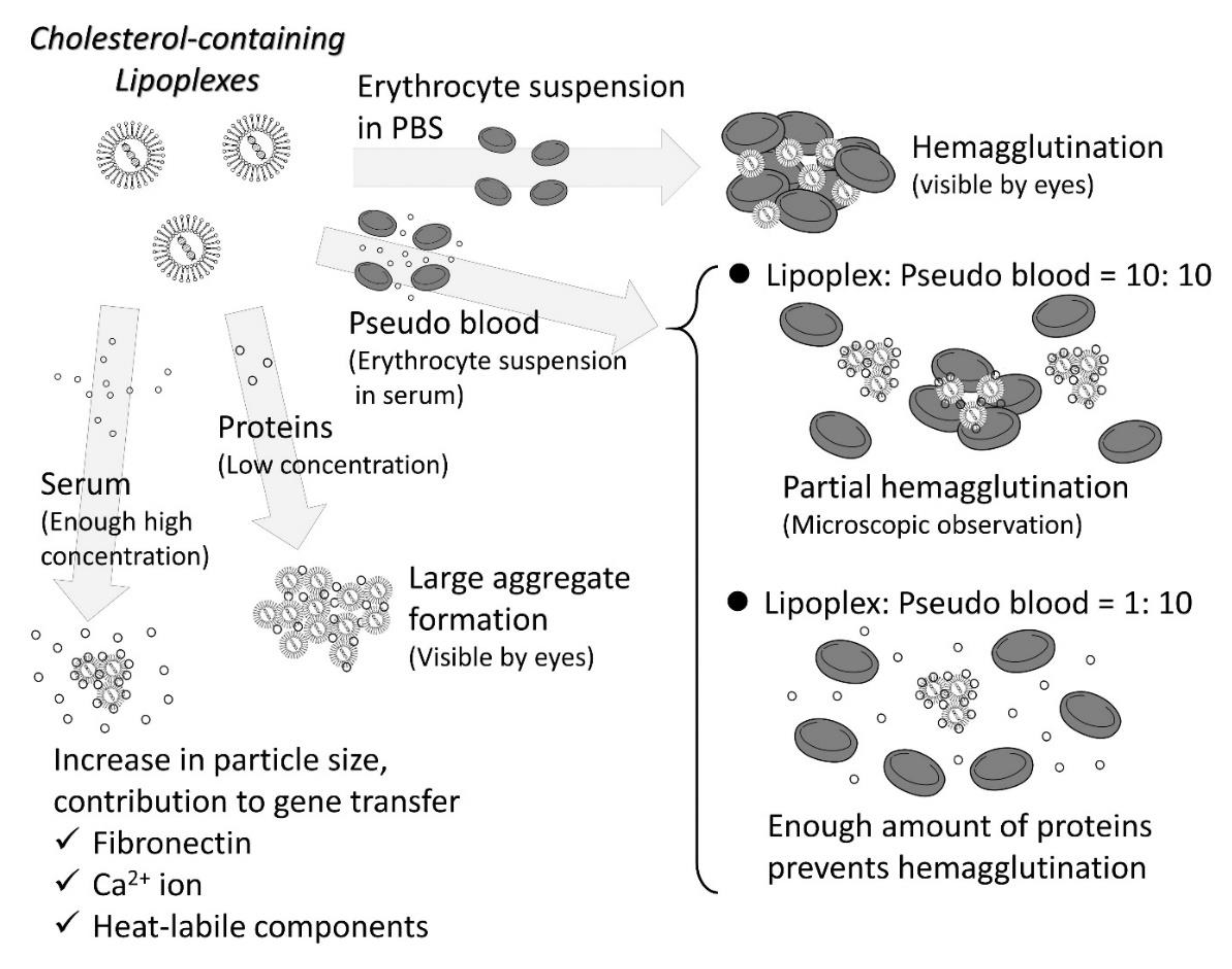
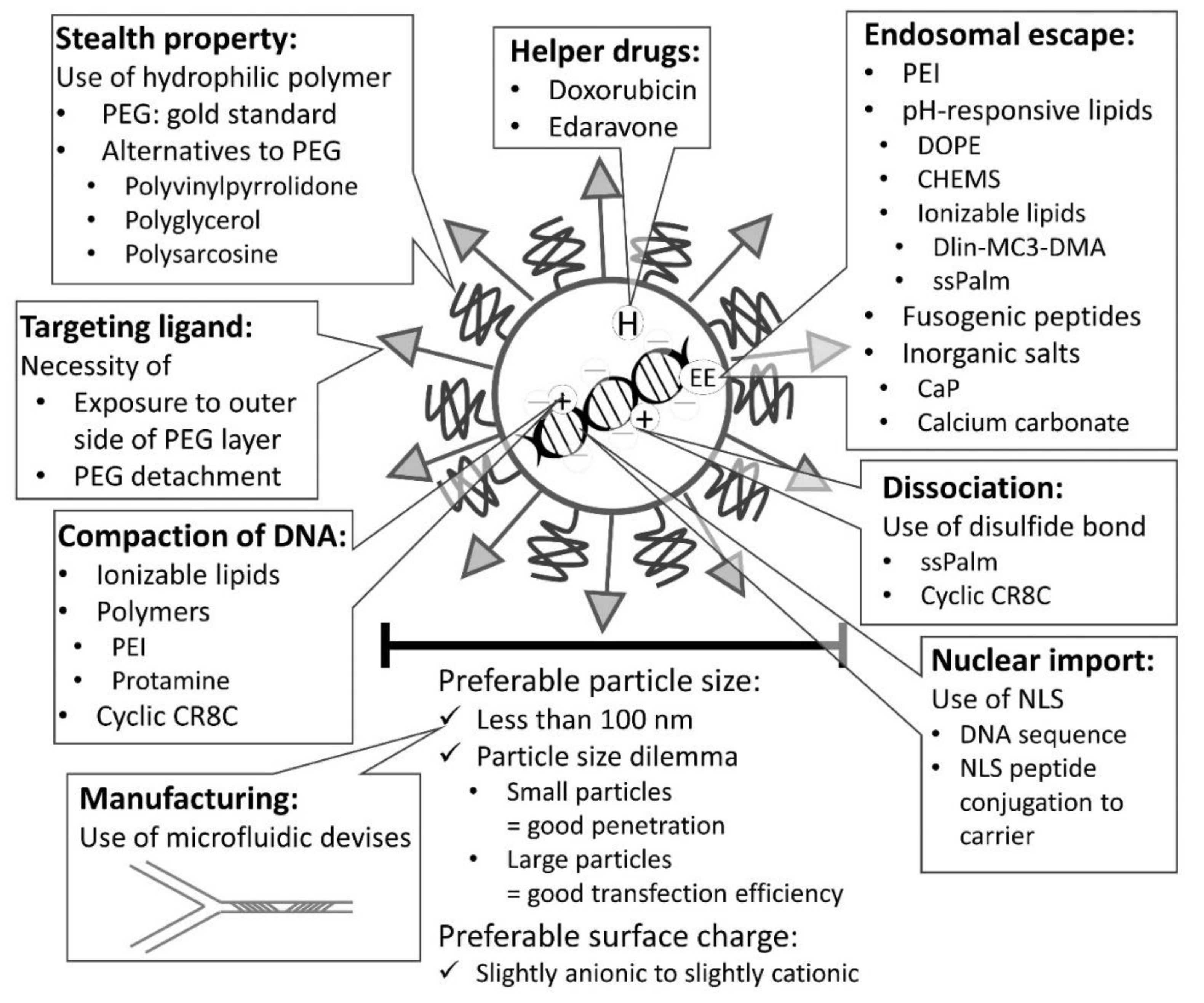
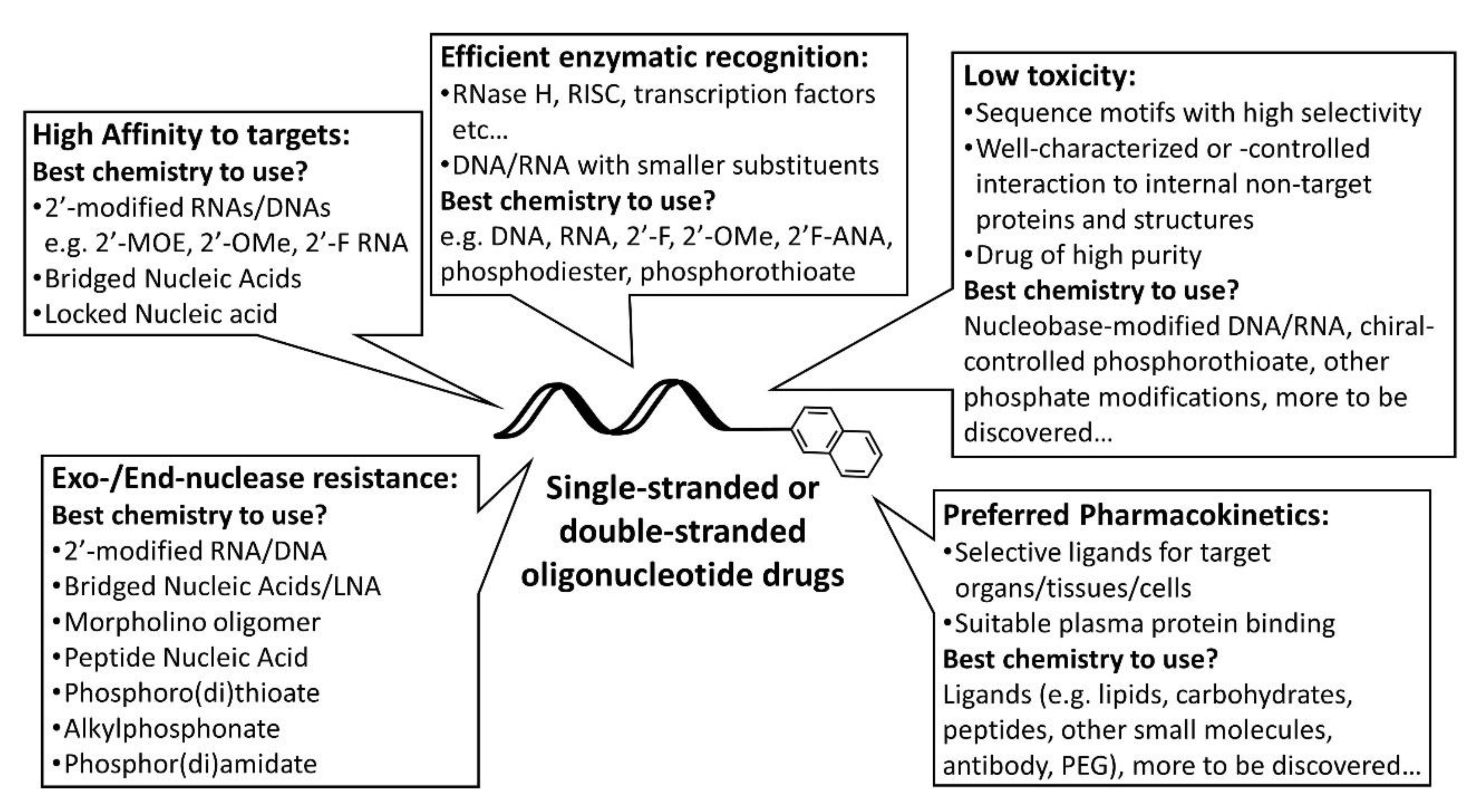
| Processes/Properties | Methods | Note |
|---|---|---|
| Physicochemical properties | ||
| Particle size | Dynamic light scattering | Most popular Necessity of solvent viscosity and refractive index |
| Nano tracking analysis | Determination of the particle concentration: possible | |
| ζ potential | Laser doppler electrophoresis | Necessity of solvent viscosity, refractive index and dielectric constant |
| Morphology | Electron microscopy | Cryo EM: preferable |
| Complexation/Encapsulation | Agarose gel retardation assay | Solution composition: to be considered |
| EtBr intercalation | Relatively low sensitivity | |
| Picogreen/Ribogreen assay | Determination of encapsulation efficiency: possible | |
| In vivo fate | ||
| Interaction with blood components | Microscopy (phase contrast/ differential interference contrast) | Aggregation/hemagglutination |
| Proteomics (LC-MS/MS) | Technique: required | |
| Blood/tissue concentration | Radioisotopes | High sensitivity Controlled area: necessary |
| Fluorescent labeling | Non-RI Multiplex: possible | |
| Local disposition | Tissue perfusion system | Technique: required |
| Spatial distribution in tissues | Fluorescent labeling/fluorescent protein, tissue clearing, confocal/ multi-photon/super-resolution microscopy | Multiplex: possible Relationship with biological events and states: analyzable |
| Efficacy of oligonucleotide drugs | Ca2+ enrichment in medium | Prediction of in vivo efficacy by in vitro experiments |
| Gymnosis | Prediction of in vivo efficacy by in vitro experiments | |
| Gene expression | ||
| Reporter genes | Luciferase (Firefly, Renilla, Gaussia, Synthetic), luminometer | Cost-effective, high sensitivity |
| Fluorescent protein Fluorescent/confocal microscopy | Determination of positive cells and spatial distribution | |
| Therapeutic genes | Realtime PCR | More direct than reporter gene assay Procedure: cumbersome |
| Cellular trafficking | ||
| Association/uptake | Fluorescent labeling | Separating association and uptake: possible, but difficult |
| Endosome/lysosome/cytosolic localization | Fluorescent probes (Lysotracker etc.) Confocal/super-resolution microscopy | Resolution: important Quantitation: possible, but difficult |
| Dissociation | Different color labeling of nucleic acids/genes and carriers Confocal/super-resolution microscopy | Quantitation: possible, but difficult |
| Autophagy | Fluorescent probes (DAL green etc.) or anti-LC3 antibody Confocal/super-resolution microscopy | Quantitation: possible, but difficult |
| Nuclear localization | Fluorescent labeling, nuclear stain (DAPI etc.) Confocal/super-resolution microscopy | Quantitation: possible, but difficult |
| Toxicity | ||
| Inflammation | ELISA (serum cytokines) Plate reader | Kits: available |
| Hepatitis | Serum AST and ALT, photometer | Kits: available |
| Renal function | Serum creatinine test, photometer | Kits: available |
| Others | Thin tissue section Hematoxylin and eosin staining | Procedure: cumbersome |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fumoto, S.; Yamamoto, T.; Okami, K.; Maemura, Y.; Terada, C.; Yamayoshi, A.; Nishida, K. Understanding In Vivo Fate of Nucleic Acid and Gene Medicines for the Rational Design of Drugs. Pharmaceutics 2021, 13, 159. https://doi.org/10.3390/pharmaceutics13020159
Fumoto S, Yamamoto T, Okami K, Maemura Y, Terada C, Yamayoshi A, Nishida K. Understanding In Vivo Fate of Nucleic Acid and Gene Medicines for the Rational Design of Drugs. Pharmaceutics. 2021; 13(2):159. https://doi.org/10.3390/pharmaceutics13020159
Chicago/Turabian StyleFumoto, Shintaro, Tsuyoshi Yamamoto, Kazuya Okami, Yuina Maemura, Chisato Terada, Asako Yamayoshi, and Koyo Nishida. 2021. "Understanding In Vivo Fate of Nucleic Acid and Gene Medicines for the Rational Design of Drugs" Pharmaceutics 13, no. 2: 159. https://doi.org/10.3390/pharmaceutics13020159
APA StyleFumoto, S., Yamamoto, T., Okami, K., Maemura, Y., Terada, C., Yamayoshi, A., & Nishida, K. (2021). Understanding In Vivo Fate of Nucleic Acid and Gene Medicines for the Rational Design of Drugs. Pharmaceutics, 13(2), 159. https://doi.org/10.3390/pharmaceutics13020159








