Abstract
Natural prodrugs derived from different natural origins (e.g., medicinal plants, microbes, animals) have a long history in traditional medicine. They exhibit a broad range of pharmacological activities, including anticancer effects in vitro and in vivo. They have potential as safe, cost-effective treatments with few side effects, but are lacking in solubility, bioavailability, specific targeting and have short half-lives. These are barriers to clinical application. Nanomedicine has the potential to offer solutions to circumvent these limitations and allow the use of natural pro-drugs in cancer therapy. Mesoporous silica nanoparticles (MSNs) of various morphology have attracted considerable attention in the search for targeted drug delivery systems. MSNs are characterized by chemical stability, easy synthesis and functionalization, large surface area, tunable pore sizes and volumes, good biocompatibility, controlled drug release under different conditions, and high drug-loading capacity, enabling multifunctional purposes. In vivo pre-clinical evaluations, a significant majority of results indicate the safety profile of MSNs if they are synthesized in an optimized way. Here, we present an overview of synthesis methods, possible surface functionalization, cellular uptake, biodistribution, toxicity, loading strategies, delivery designs with controlled release, and cancer targeting and discuss the future of anticancer nanotechnology-based natural prodrug delivery systems.
1. Introduction
In 2001, Vallet-Regi et al. [1] introduced a mesoporous silica material called MCM-41 that can be used as a drug carrier. The nanostructure (e.g., pore size) of MCM-41 can be optimized using different surfactants. Since then, many efforts and attempts have been made to synthesize versatile mesoporous silica nanoparticles (MSNs) with different nanostructures and morphologies to meet the demand for pharmaceutical and medical applications. The history of the synthesis of mesoporous silica materials dates back to 1992, when they were discovered by the Mobile Oil Corporation [2]. Silica is one of the most abundant minerals in the Earth’s crust and is also found in the food chain and the human body [3]. As a biomaterial, silica is extensively used in many applications such as dentistry, orthopedics, and dermatology. MSNs have a characteristic mesoporous nanostructure that offers many advantages for medical applications in disease diagnosis and therapy [4]. The unique features include easy synthesis, the possibility of various surface modifications, the ability to obtain a tunable particle size, uniform pore size, high surface area to pore volume, good biocompatibility, and chemical stability [5,6,7,8,9]. In addition, easy functionalization to achieve magnetic, fluorescent, and photothermal properties increases the chance of using MSNs in bioimaging. MSN nanostructures can provide excellent nanoplatforms to fabricate smart drug delivery systems (DDSs) with a high drug loading capacity and stimuli-responsive drug release effect compared to other nanocarriers [6,10]. Several nanocarriers have been used to deliver and control drug release, including niosomes, liposomes, dendrimers, lipid nanoparticles, and polymeric nanoparticles, but most of them have low stability and need external stabilization during synthesis. In contrast, MSNs have a strong Si-O bond that makes them stable (chemically and mechanically) to external responses in the surrounding environment [11,12,13]. It is generally accepted that encapsulation of drugs or therapeutic agents into MSNs can enhance their therapeutic activity, solubility, and bioavailability, as indicated by many studies [14,15,16,17,18,19,20].
A consequence of these advantages is that MSNs have gained much attention and popularity in DDSs during the last few decades for the delivery of cargo to specific sites in the organism. A large number of in vivo studies indicate the high biocompatibility/safety profile and low toxicity of MSNs if they are synthesized using an optimized way [21,22,23]. A careful optimization process is needed because many details of the nanostructure of engineered MSNs, i.e., size, shape, surface, presence of surfactant, and other factors like dose, administration route affect the safety profile. According to many animal studies, the toxicity of MSNs can be diminished by optimizing the synthesis parameters and surface modification, resulting in safe nanoparticles [24,25].
The administration route is an important characteristic for constructing any DDS. MSNs can be applied via different routes, including oral and intravenous injection [26,27,28,29,30]. Many choices in the development of pharmaceutical formulations depend on the target tissues and organs in the human body. An important advantage of DDS-based MSNs is that the amorphous forms of silica and silicates are generally recognized as safe materials for use as oral delivery ingredients (up to 1500 mg per day) according to the US Food and Drug Administration and the European Food Safety Authority [27]. MSNs are promising materials because they exhibit low toxicity levels in animals when applied, i.e., orally, injection [31].
The global market for nanomedicine accounts for 5% when novel nanomedicines translated from the lab to the clinics are concerned [32]. Recently, the first clinical trial in humans was conducted with oral delivery of fenofibrate formulation based on the ordered mesoporous silica [33].
Despite these promising results for nanotechnology application in building DDSs, most research for targeted cancer therapy has been focused on drugs and therapeutic molecules of a synthetic nature. Combating cancers with synthetic drugs is an established therapy, however, progress in this area of medicine is slow and the treatments are frequently associated with undesirable effects: side effects and also insufficient patient compliance. For this reason, extensive research is carried out to apply natural prodrugs (known also as natural products and natural agents) in anticancer therapies.
Nature is a huge source of therapeutic substances, which can be derived from plants, microbes, and animals. Natural medicines account for 60% of anticancer agents used in clinical applications [34]. For example, vincristine, taxanes, and camptothecin are used in the treatment and prevention of cancer. There are still hundreds of promising new active natural anticancer agents to be discovered and renewed for cancer therapy [35,36,37]. The main advantages to using and developing natural prodrugs are that they offer safe, cost-effective, and have versatile pharmacological properties [38]. The main limitations for their use in cancer therapy are their poor water solubility, low bioavailability, short half-life, and non-specific targeting.
Nanotechnology offers many ways to overcome these obstacles [39,40,41,42,43,44]. Natural pro-drugs can be embedded into MSNs, which can serve as effective nanocarriers for the delivery of anticancer natural prodrugs to target cancers. In this review, we present an overview of synthesis methods, surface functionalization, as well as biodistribution, biocompatibility, toxicity, biological performance. Additionally, drug loading and release strategies, and active targeting approaches for MSNs will be addressed. We also discuss delivery and controlled release systems for selected prodrugs using MSNs.
Available data provide considerable evidence that MSNs allow the limitations associated with prodrugs, such as poor water solubility, poor bioavailability, and low specific targeting ability, to be overcome. Compared to organic delivery systems (e.g., lipid nanoparticles, polymeric nanoparticles) [45,46], the delivery of natural prodrugs by means of MSNs allows high drug loading and permits multifunctional delivery or co-delivery systems. Generally, MSNs allow long-term release compared to organic nanoparticles. This is because the prodrugs are trapped inside nano-pores. In the case of encapsulation of prodrugs into organic nanoparticles, fast degradation of the organic substance leads to quick pro-drug release. The MSN-based nanomedicine technology is mature enough to be extended to thousands of prodrugs not yet investigated in clinical applications.
To the best of our knowledge, this is the first review considering MSNs as delivery systems for anticancer natural prodrugs. The need for such a review is a consequence of rapid development in the field. This review may help researchers accelerate research and development of this important field of nanomedicine and, ultimately, clinical applications.
2. Synthesis of Mesoporous Silica Nanostructures
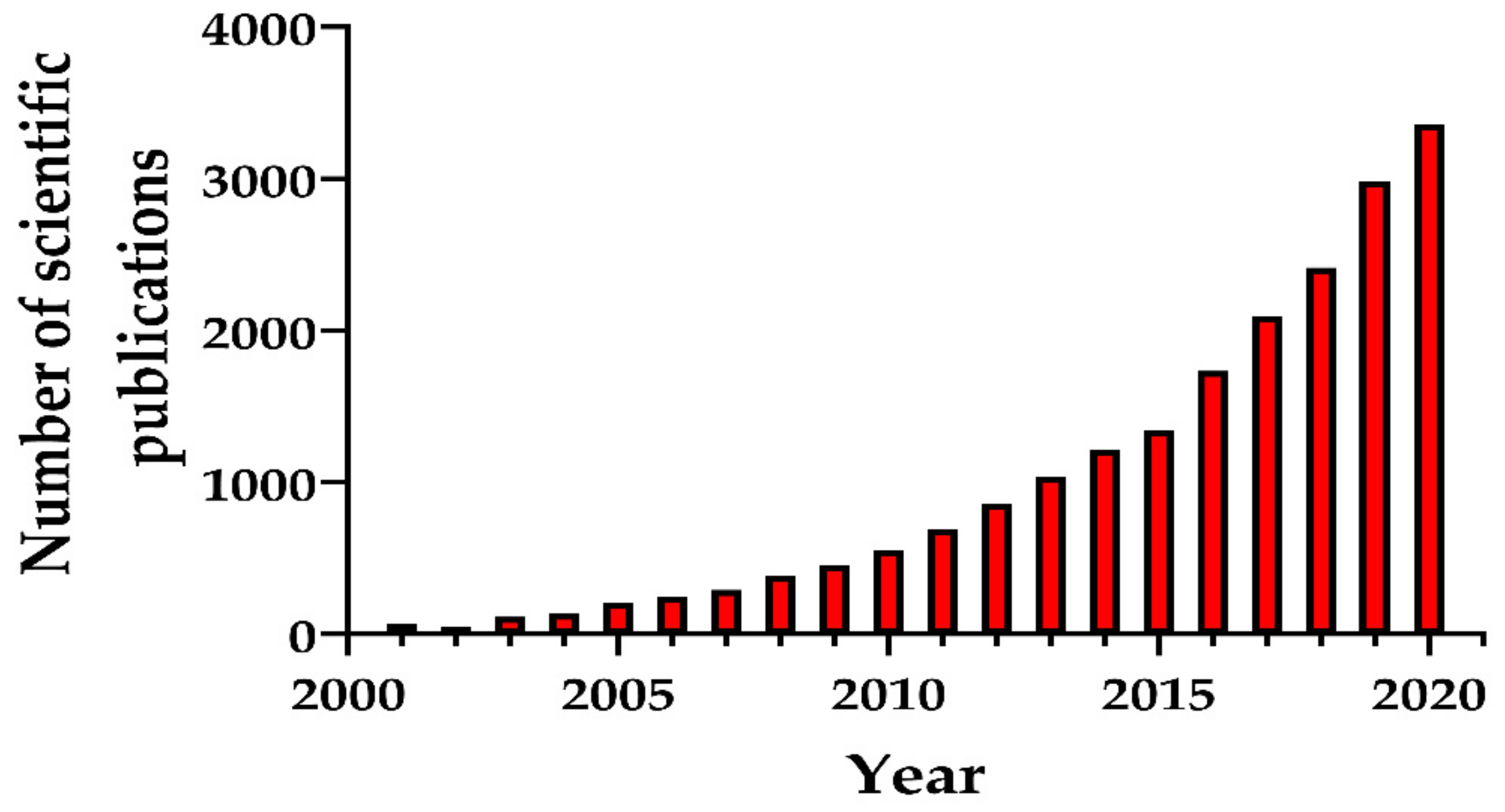
Numerous synthesis methods have been developed to obtain MSNs with different morphological, structural, and pore geometry. Particular attention was paid to the production of biocompatible MSNs for medicine. Figure 1 presents the number of scientific publications (research articles, review articles, and book chapters) as an indicator of the growth in MSN synthesis methods due to their emergence as nanostructures for various promising applications.
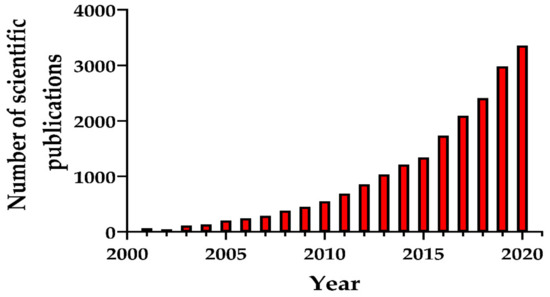
Figure 1.
Number of scientific publications (research papers, reviews, book chapters) during the period 2001–2020 found by entering key words “mesoporous silica nanoparticles and synthesis”. The search was performed in ScienceDirect 10 September 2020.
2.1. Discovery, Synthesis, and Properties of MSNs
Porous materials (natural or artificial) are characterized by the presence of pores, including cavities, channels, or interstices. The properties of these materials vary depending on the characteristics of their pores: size, arrangement/structure, shape, porosity, and chemical composition. They have been extensively studied in different areas, including water purification, gas separation, catalysts, energy storage, adsorbents, electronics, engineering, tissue engineering, and drug delivery systems, among others [47]. Depending on the predominant pore size, the International Union of Pure and Applied Chemistry (IUPAC) classifies porous materials into three categories as shown in Table 1 [48,49].

Table 1.
Classification of porous materials by pore size.
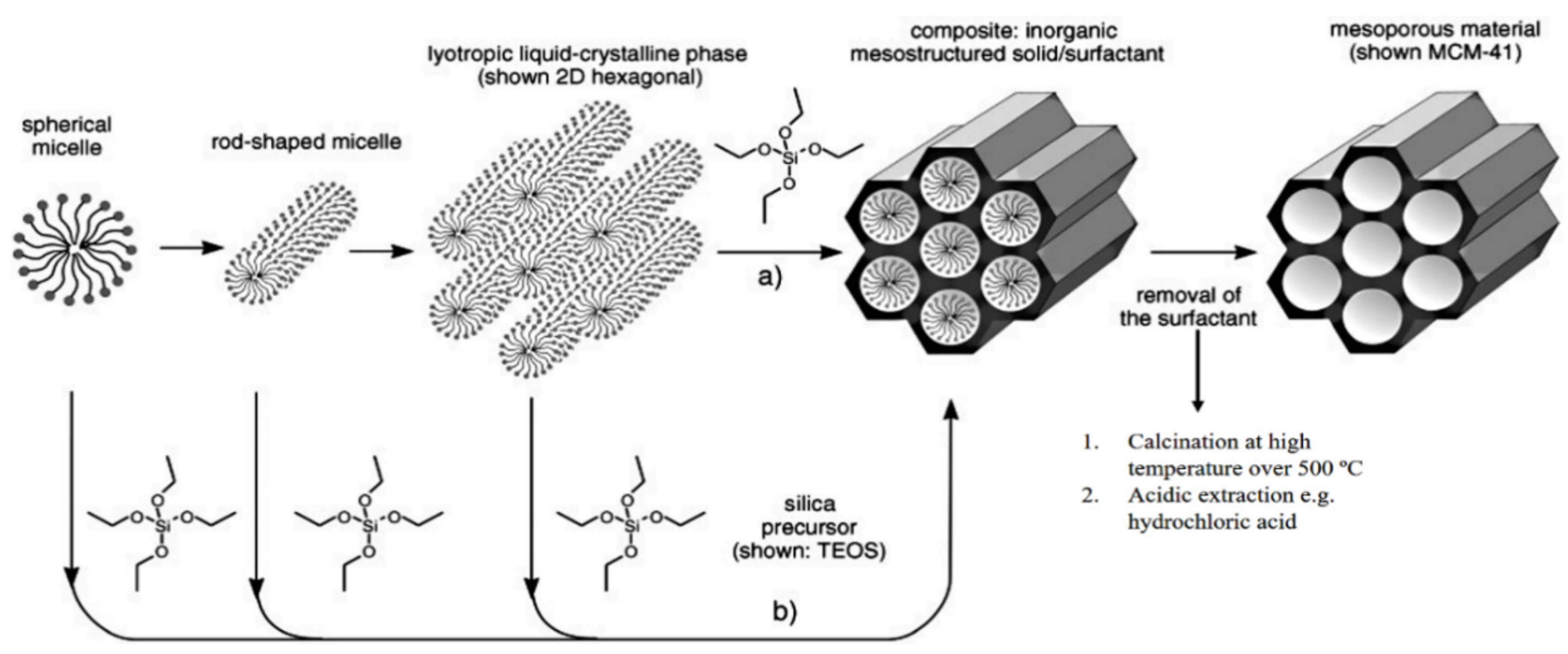
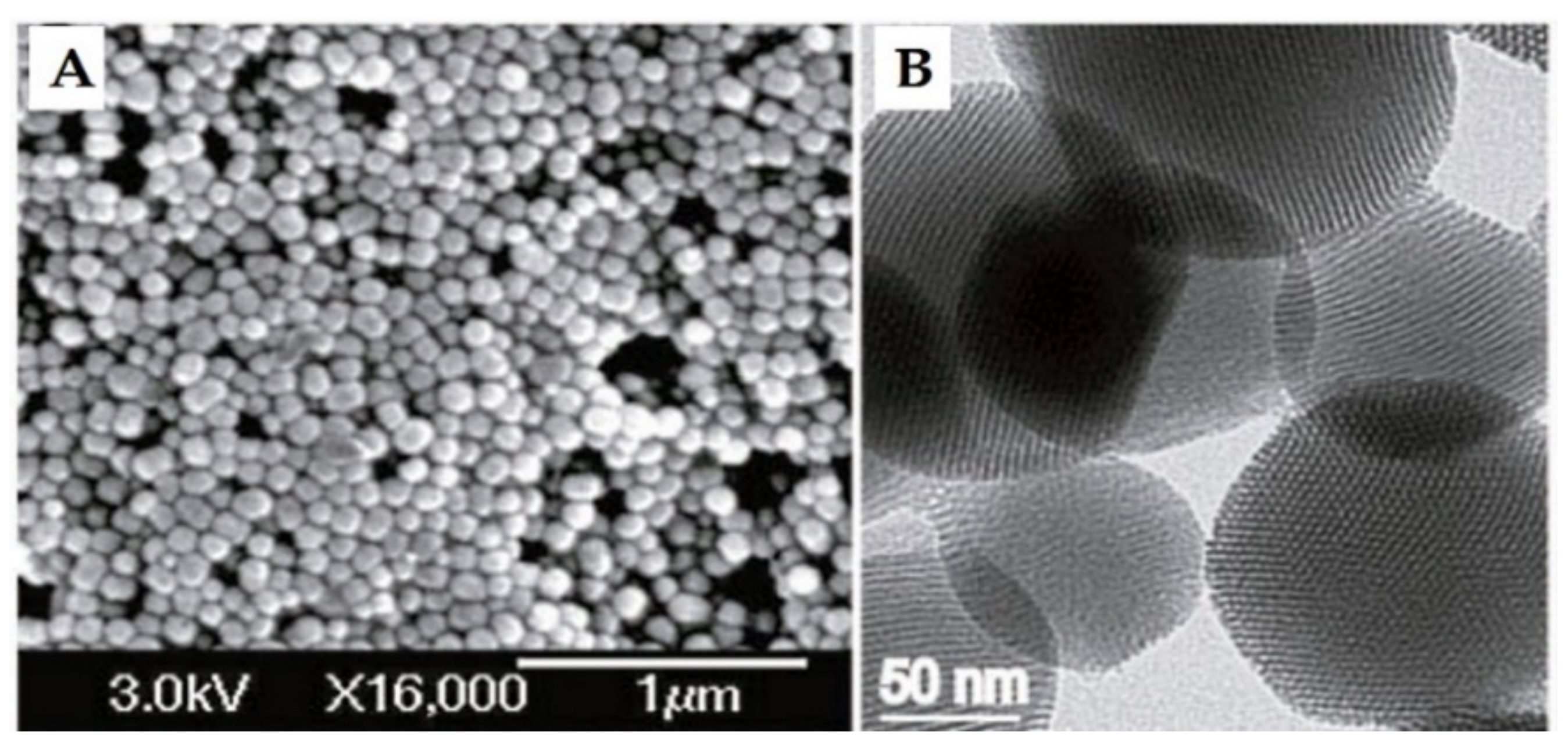
The history of MSN materials dates back to the early 1990s, when the Kuroda group at Waseda University and researchers from the Mobil Company discovered Mobil crystalline materials (MCMs), nanoparticles with a hexagonal porous structure [2]. In 1992 with the discovery of MCM-41, a material prepared using the cooperative assembly of surfactant with silicates, a breakthrough in the area of ordered mesoporous structures and their successful preparation occurred [50,51]. In addition, an ionic template, such as cetyltrimethylammonium bromide (CTAB), could be employed as a structure-directing agent to produce MCM-41 and MCM-48 with pore sizes of 2 to 10 nm [50,51]. MCM-41 has a hexagonal pore shape and MCM-84 has a cubic pore shape. For DDSs purpose, MCM-41 is considered to be one of the most widely explored materials. The synthesis mechanism for MCM-41 is shown in Figure 2 and electron microscope images in Figure 3.
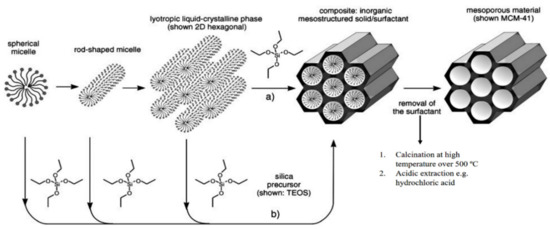
Figure 2.
The formation mechanism for mesoporous materials by structure-directing agents. (a) True liquid–crystal template mechanism. (b) Cooperative liquid–crystal template mechanism. Reproduced with permission from [52], WILEY-VCH Verlag GmbH and Co. KGaA, 2006.
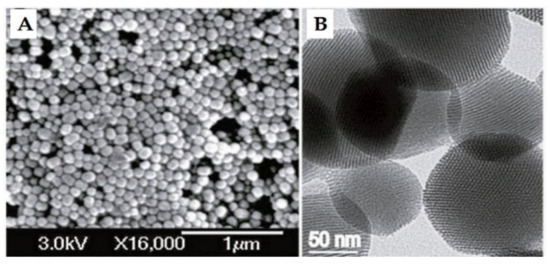
Figure 3.
(A) Scanning electron microscopy (SEM) and (B) transmission electron microscopy (TEM) of MCM-41 material. Reproduced with permission from [23], Wiley-VCH Verlag GmbH and Co. KGaA, 2010.
In 1996, another kind of MSN was discovered that has a non-ordered pore structure, named KIT-1 (Korea Advanced Institute of Science and Technology Number 1) [53]. The KIT family currently has many members, such as KIT-6, which has a hexagonal arrangement of pores [54], and KIT-5, which has a cubic ordered structure [55]. In 1998, the SBA-15 type (pore size 4–6 nm) MSNs introduced by Santa Barbara Amorphous (SBA), which have a hexagonal or cubic pore structure, were developed by means of nonionic surfactants in acidic conditions [56]. The cubic SBA-11, 3D hexagonal SBA-12, hexagonal SBA-15, and SBA-16 are mainly prepared based on non-ionic triblock copolymers, such as alkyl poly(ethylene oxide) (PEO) oligomeric surfactants and poly(alkylene oxide) block copolymers [10]. The typical synthesis of SBA-15 is dependent on tetramethyl-orthosilicate (TMOS) or tetraethyl-orthosilicate (TEOS) as the silica precursor reacting with a series of block-copolymer surfactants as structure-directing agents. The MCM and SBA materials are recognized as the first generation of hexagonally ordered pore structures and are the common MSNs used in research. A variety of strategies have been designed to attain tunable pore sizes (from less than 2 nm up to 30 nm). In this scenario, the adjustments are made depending on the surfactant template’s properties [57], pore swelling agents, such as mesitylene [50], or hydrothermal treatments [58].
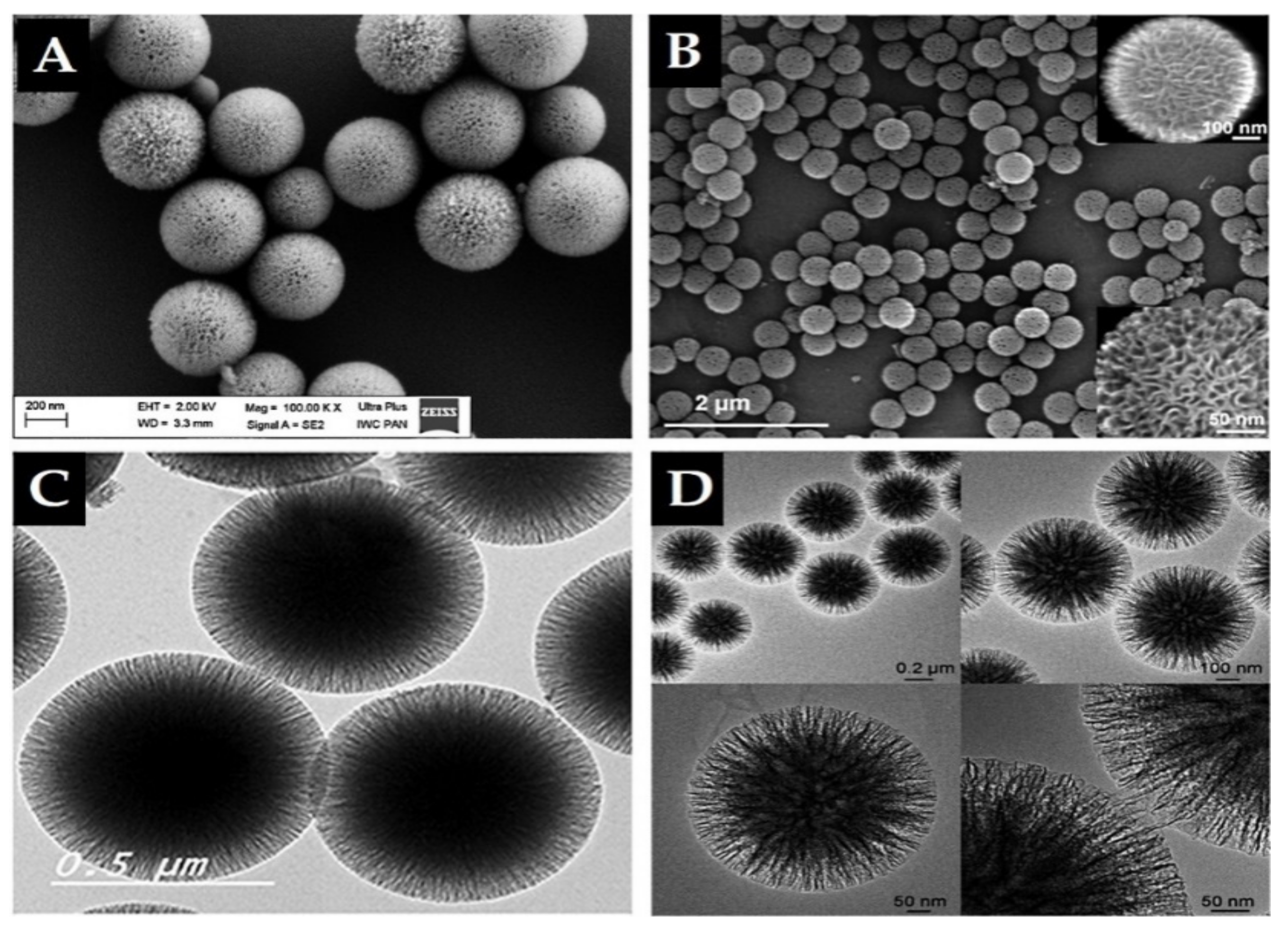
Importantly, in 2010, high surface-area silica nanospheres with a fibrous morphology and non-ordered pore structure were discovered by a research group of the Catalysis Center at King Abdullah University of Science and Technology (KAUST Catalysis Center, KCC) [59]. This material, KCC-1, features a high surface area due to the presence of dendrimeric silica fibers and their respective channels, making KCC-1 a first-of-its-kind material. It is a spherical particle with 3D tomography, a uniform size ranging from 250 nm to 500 nm, high surface area, and large pore size in a non-ordered structure (Figure 4). Synthesis of KCC-1 [59] was accomplished by a microwave-assisted, templated, solvothermal strategy using cetylpyridinium bromide (CPB) or cetyltrimethylammonium bromide (CTAB) as a surfactant (template), 1-pentanol as a co-surfactant, TEOS as the silica source, urea (catalyst-hydrolyzing agent), and a mixture of the cyclohexane solvent and water (as the reaction solvent). The chemicals were introduced to the reaction system stepwise with mixing and microwave-assisted heating applied (in a closed vessel >1200 °C) for a predetermined time for the reaction. Finally, the solution was filtered or centrifuged, washed, and the obtained material calcinated at high temperature (>550 °C). Many research groups changed the surface of substances used in the synthesis in addition to the parameters. For example, Bayal et al. [60] showed that changing the concentrations of urea, surfactant (CTAB instead of CPB), or solvent (1-pentanol), the reaction time, or temperature can result in various particle sizes, fiber densities, surface areas, and pore volumes for KCC-1. Such easy manipulation and controlled synthesis of this material make KCC-1 a good solution for versatile applications in the environment, energy, biology, medicine, and other fields [42,43,61,62,63,64,65,66,67,68]. KCC-1 could be recommended for different small or large drug/therapeutic agents, possibly for any design and pathological disorder due to KCC-1 s unique physicochemical features. Our research team is among the first to study KCC-1 for DDSs [42,43,68,69], and we think that research on KCC-1 will increase soon. In the literature, there are references to “spherical wrinkled mesoporous silica” (WMS) [70,71,72] and KCC-1 is known also “dendritic fibrous nano-silica” (DFNS) [73]. They were all obtained based on changing the synthesis conditions and parameters of the original synthesis method for KCC-1 particles.
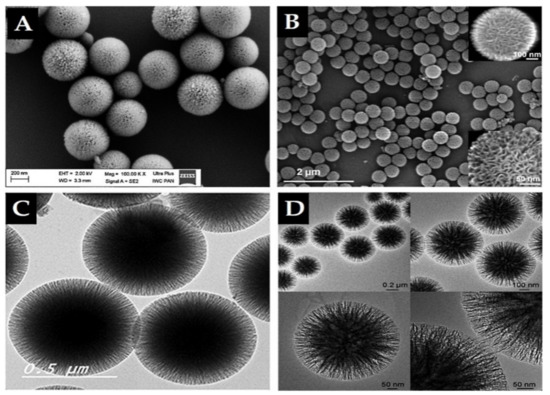
Figure 4.
Electron microscope images of prepared KCC-1 material. (A,B) Scanning electron microscopy (SEM). (C,D) Transmission electron microscopy (TEM). Note, the dendritic fibrous 3D mesopore structure is clearly seen by SEM in B. A and C reproduced from [42,43], Impact Journals, 2018 and MDPI, 2020. B and D reproduced with permission from [59], WILEY-VCH Verlag GmbH and Co. KGaA, 2010.
Unlimited opportunities exist for the synthesis of MSNs in pure, doped, composite, and modified forms by employing different templates (soft and hard), conditions, and methods [74].
Due to the unique properties of the KCC-1 family, they offer a wide range of possible applications. It seems that KCC-1 has comparable potential as the commonly used members of the MCM and SBA families, as well as Stober silica, solid silica discovered before all the families [73]. Table 2 presents the major physicochemical properties for fibrous KCC-1, MCM-41, SBA-15, and others. Below, we highlight the common and promising families that could be favored for drug delivery and medical applications. Numerous interesting review articles have been published on MSN synthesis strategies and applications that we recommend for further reading [10,22,32,73,75,76,77,78,79,80,81,82,83].

Table 2.
The physicochemical properties of the most common mesoporous silica nanoparticles (MSNs) synthesized by various approaches.
2.2. Surface Modification of MSNs for Drug Delivery
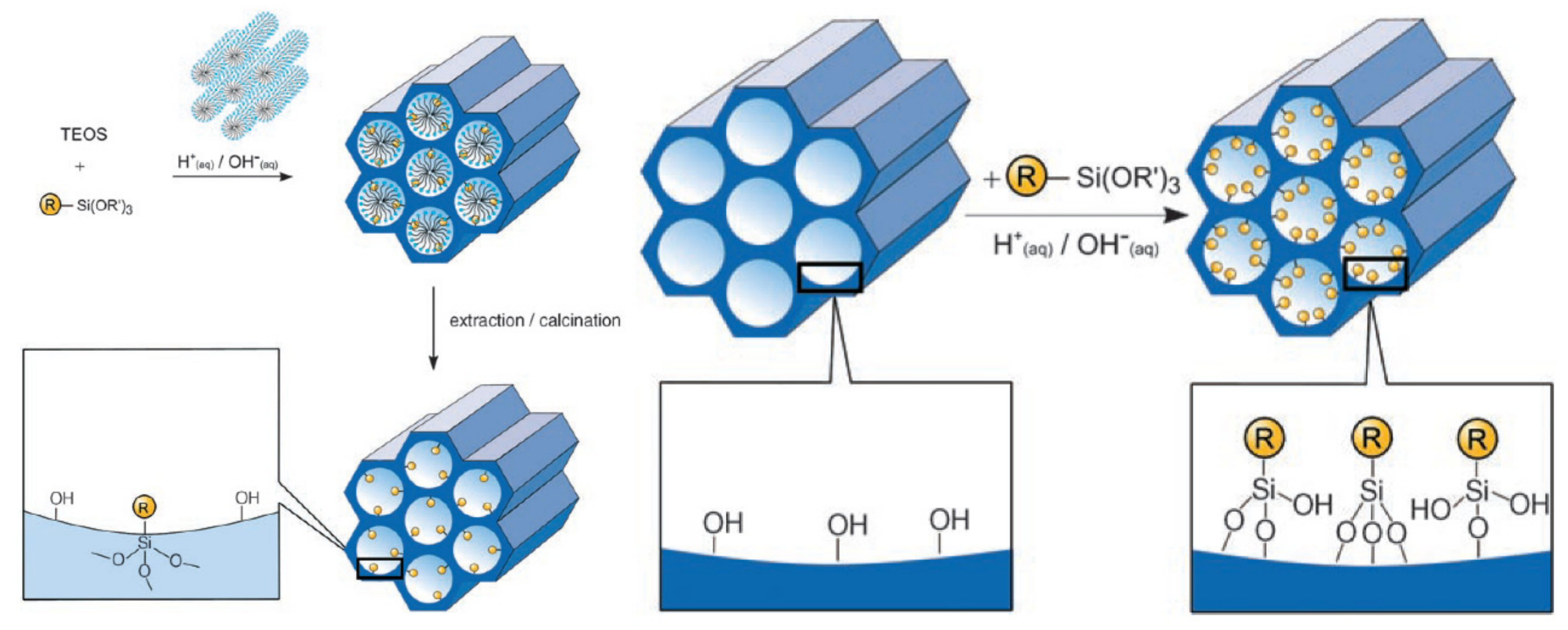
The keystone in the development of DDSs is to functionalize their surface [84,85] to increase their drug loading and release, leading to high therapeutic effects. The surface chemistry modulates the interaction of MSNs with the surrounding media. The MSNs have a high density of silanol groups (Si-OH) on their surface, allowing surface modification by various organic functionalities (e.g., silanes, polymers, proteins, and targeting moieties). Thus, MSNs can load various drugs with high capacity and release them in a sustained or controlled manner. A variety of functional groups can be used, such as amine, carboxylate, phosphonate, polyethylene glycol, octadecyl, thiol, carboxylic acid, and octadecyl groups. To introduce functional groups on the surface of MSNs, covalent bonding and electrostatic interactions are generally used [86]. The common approach to modify MSNs is to use organic silane groups via direct covalent attachment by means of co-condensation or post-synthetic grafting.
The co-condensation method is referred to as a one-pot synthesis method [87,88] as presented in Figure 5A. The desired functional group of silanes, such as 3-aminopropyl-triethoxysilane (APTES “NH2”) is added during the sol-gel synthesis process together with the silica source (e.g., TEOS). Next, the template is removed (Figure 5A) [52,87,89]. To remove the surfactant template, an extractive method using alcoholic/acidic solution under reflux can be used [90]. Removing the template anchors the organic residue covalently to the porous walls of the MSNs. This approach has the advantages of easy preparation, more homogeneous distribution of organic units, and high drug loading [52,83]. Despite these advantages, disadvantages are a potential change in the mesoscopic order, disordering the porosity and reducing the pore diameter, pore volume, and specific surface areas [52].
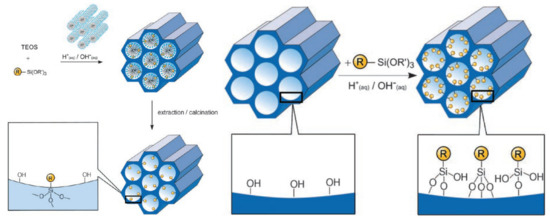
Figure 5.
A schematic presentation of the organic functionalization methods for mesoporous silica materials. (A) Co-condensation method and (B) grafting method. Reproduced with permission from [52], WILEY-VCH Verlag GmbH and Co. KGaA, 2006.
A post-synthetic approach refers to the subsequent modification of the inner/outer surface of MSNs by covalent and electrostatic interactions. The modification is usually achieved after surfactant removal from MSNs (Figure 5B). The most remarkable advantages of this approach are selective functionalization (either external or internal surfaces) and retention of the mesostructure of MSNs during synthesis. The major disadvantages include reduced pore size and non-homogeneous distribution of functional groups into/onto pores [52,91,92].
2.3. The Biological Performance of MSNs
2.3.1. Cellular Uptake
Any nanocarriers have to cross the cell membrane boundary to enter cells, allowing the therapeutic effects of the delivered drugs. The internalization of nanoparticles carrying therapeutic agents into cells represents the initial step in successful drug delivery [93,94]. The acting mechanisms and surface chemistry of nanocarriers are the major parameters in designing a preferred DDS for any pathological disease [78]. Nanoparticles mainly access the cell interior via simple diffusion or translocation as an energy-dependent process [95]. The most common mechanism of their internalization is the energy-dependent endocytosis, which allows the uptake of nanoparticles and submicron particles from an extracellular environment to the cell plasma membrane [96]. The mechanisms can generally be classified into phagocytosis, pinocytosis, micropinocytosis, receptor-mediated endocytosis, clathrin-mediated endocytosis, caveolin-mediated endocytosis, and others (e.g., Arf-6, Rho-A or IL2Rb-dependent pathway, flotillin, or CDC42 (CLIC/GEEC)-dependent endocytosis) [93]. The intracellular uptake and trafficking mechanisms by which nanoparticles are internalized in cells vary broadly depending on many factors, including size, shape, charge, and surface modification. Therefore, these factors should be taken into consideration in constructing DDSs.
Size of MSNs
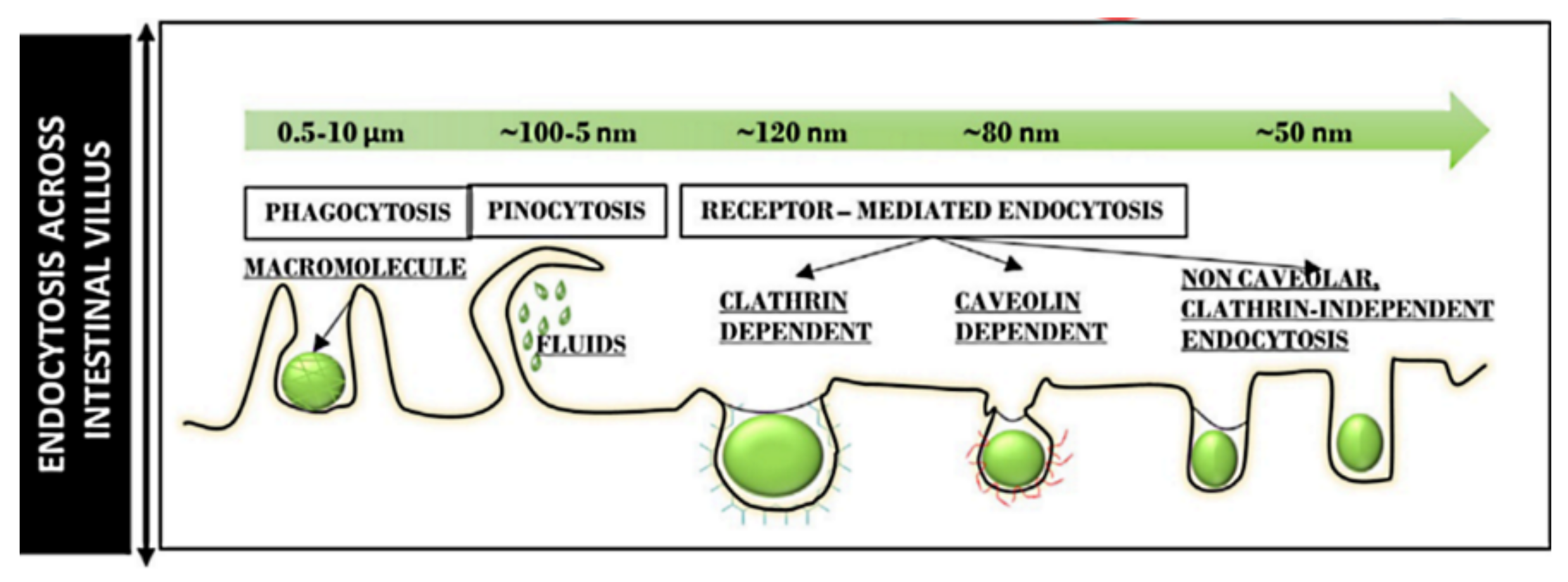
Particle size determines the intracellular uptake of MSNs (Figure 6) [97]. It is generally accepted that particles with the smaller size of 50 nm can internalize into cells via non-phagocytosis [98]. Nanoparticles up to 150 and 200 nm in size are internalized by pinocytosis, such as clathrin-mediated endocytosis and caveolin-mediated endocytosis, respectively [99,100]. In contrast, particles from 250 nm to 3 μm in size can internalize the cells by macropinocytosis and phagocytosis [101]. It is also accepted that the microparticles are efficiently taken up through phagocytosis but the process depends also on other parameters, such as geometry, surface charges, and functional groups of microparticles [102]. Particles with sizes ranging from 30 to 50 nm internalize also efficiently via receptor-mediated endocytosis [103]. Despite extensive investigations exploring the relationship between particle size and uptake pathways, the results are inconsistent [101,104,105,106]. The main reason for such contradictions can be attributed to the complexity of control of structural parameters, such as shape and surface charges. For successful internalization, particles should avoid degradation (within endosomal/lysosomal vesicles) and release their cargo in the cytoplasm [107]. Therefore, particle size is important in tailoring DDSs. It is also important for their intersections with the reticulo-endothelial system (RES), which is responsible for elimination of nanoparticles from the body, and prolong the circulation time in the blood. In this context, several studies have shown that increasing the particle size increases clearance from the body, reducing the therapeutic impact [108,109,110,111,112].
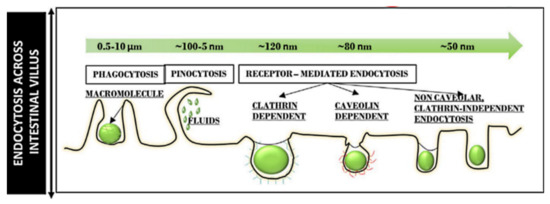
Figure 6.
Different endocytosis pathways across the intestinal villus for particles of different sizes. Reproduced with permission from [97], Elsevier Inc., 2020.
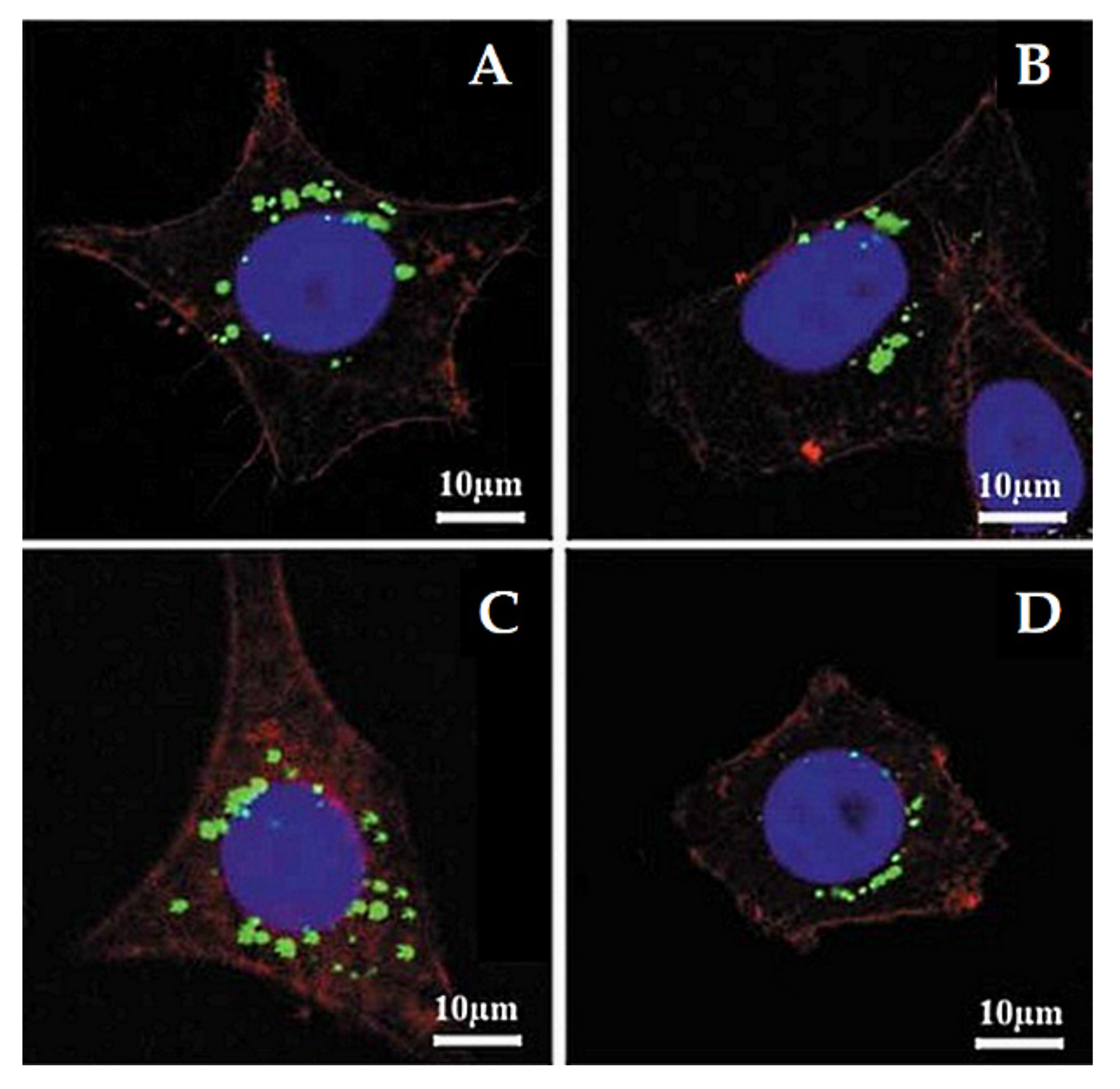
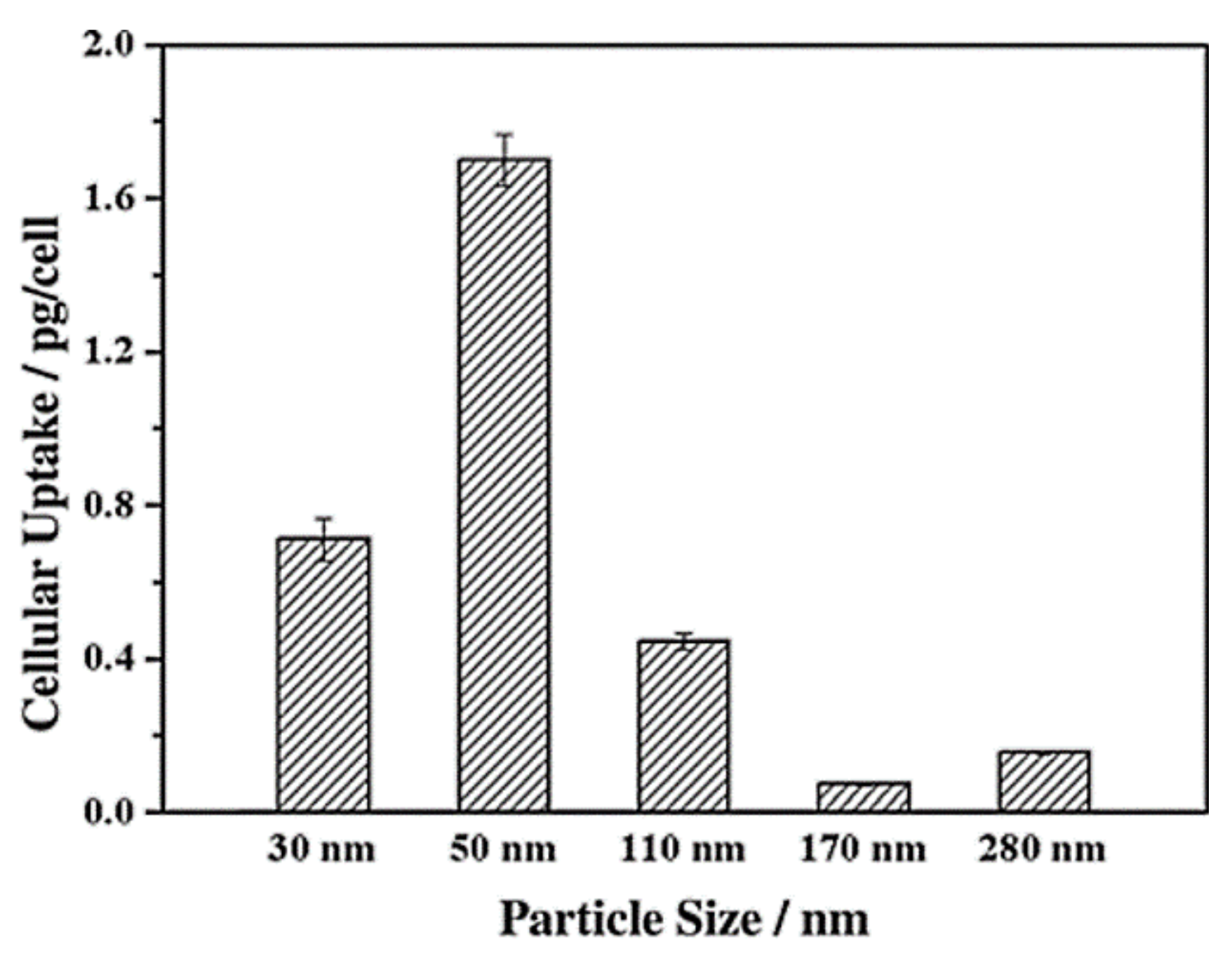
Lu et al. [103] investigated the impact of various sizes (30, 50, 110, 170 nm) of MSNs on cellular uptake by HeLa cancer cells using MSNs labeled with fluorescein isothiocyanate (FITC) green fluorescence (MSN-FITC) and confocal laser scanning microscopy. They found that the MSNs were internalized as non-uniform green-fluorescent aggregates in the perinuclear region, and no MSNs penetrated the nucleus (Figure 7). Quantifying the internalization of MSNs, they concluded that the cellular uptake is highly particle size-dependent, observing the order 50 > 30 > 110 > 280 > 170 nm (Figure 8). Haddick et al. [113] demonstrated that MSNs with a size of 160 nm had the fastest cellular internalization in T24 bladder cancer cells through receptor-mediated cellular internalization compared to 60, 80, 100, and 130 nm, leading to the highest level of gene knock-down for antitumoral effects. Yang et al. [114] tested different sizes of rod-shaped SBA-15 (from 80 to 200 nm) and spherical MCM-41 particles, as well as their intracellular uptake in human osteosarcoma cancer cells (KHOS). They found that the cellular uptake efficiency depends on the particle size and shape.
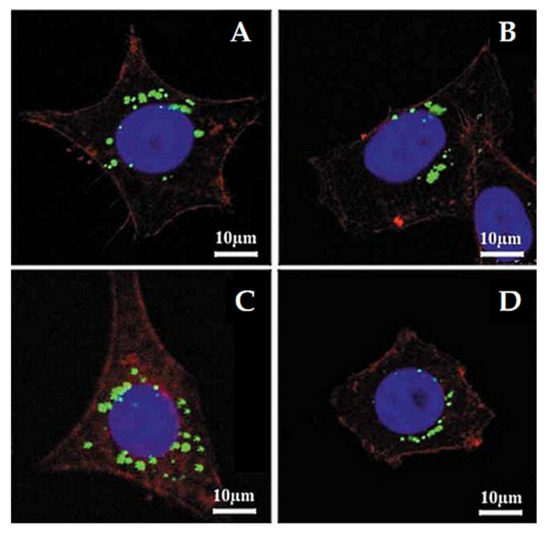
Figure 7.
Confocal laser microscopy images of HeLa cells after incubation with different sizes of MSNs labeled with fluorescein isothiocyanate (FITC) green fluorescence (MSN-FITC) (100 µg mL−1, green) for 5 h at 37 °C. (A) 170 nm, (B) 110 nm, (C) 50 nm, and (D) 30 nm. The cell skeleton was stained with rhodamine-phalloidin (red), and the cell nucleus with 4′,6-diamidino-2-phenylindole (DAPI; blue). Reproduced with permission from [103], WILEY-VCH Verlag GmbH and Co. KGaA, 2009.
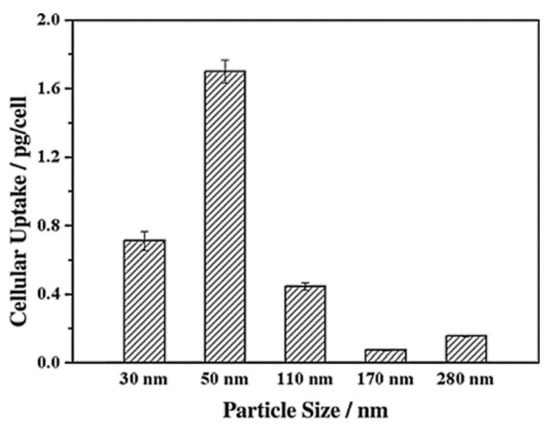
Figure 8.
Cellular uptake of FITC-MSN-x based on nanoparticle size. Reproduced with permission from [103], WILEY-VCH Verlag GmbH and Co. KGaA, 2009.
Surface Charges of MSNs
Another critical factor influencing the cellular uptake of nanoparticles is the surface charge. MSNs are characterized by silanol groups permitting to add different functional groups, modifying their surface to be either cationic or anionic [115]. Most cells have a negatively charged cell membrane, enhancing the uptake of positively charged nanoparticles. Several studies have shown that positively charged nanoparticles internalize with higher uptake than neutral and negatively charged nanoparticles [116,117,118,119]. Furthermore, neutral nanoparticles usually have lower cellular uptake compared to negatively charged nanoparticles [98,120]. As a result of the internalization of nanoparticles by cells, their interaction with the cell membrane can occur by means of gelation of membranes (with negatively charged nanoparticles) or fluidity of membranes (with positively charged nanoparticles) [121,122]. On the one hand, the positively charged nanoparticles mainly enter cells via micropinocytosis; on the other hand, the negatively charged nanoparticles always enter cells by clathrin- or caveolae-independent endocytosis [123].
Positively charged MSNs generally exhibit higher endocytosis efficiency compared to negatively charged MSNs due to the higher affinity for the negatively charged cell membranes. Jambhrunkar et al. [124] prepared MCM-41 with negative and positive charges for delivering curcumin. They found that the positively charged MCM-41-NH2 had more efficient uptake in the human squamous cell carcinoma cell line (SCC25) than negatively charged particles. Baghirov et al. [125] studied spherical and rod-shaped MSNs that were either non-modified or modified with a poly(ethylene glycol)-poly(ethylene imine) (PEG-PEI) block copolymer in in vitro models of the blood–brain barrier. The results showed that the modified MSN-PEG-PEI particles exhibited robust uptake in RBE4 rat brain endothelial cells and Madin–Darby canine kidney epithelial cells. Our group performed a comprehensive study of cellular uptake using two types of MSNs: KCC-1 and MCM-41 (non-modified, positive charges with -NH2, and folic acid ligands) [42]. The FA-conjugated MSNs exhibited higher cellular uptake than MSNs-NH2 and non-modified MSNs.
Morphological Structures of MSNs
The morphological structures (i.e., different shapes) play an important role in the cellular uptake and trafficking of nanoparticles into cells or organs. Trewyn et al. [126] studied the impact of different MSN shapes on cellular uptake in vitro, finding that a tubular structure achieves more efficient uptake by both cancer and normal cells than those of spherical morphology. Huang et al. [127] investigated the effect of three differently shaped particles on non-specific cellular uptake by human melanoma (A375) cells. Their results proved that particles with a larger aspect ratio are efficiently internalized by cells in large amounts at faster rates. Another study tested the core–shell MSNs with spherical or rod-like shapes for cellular uptake, showing that a rod shape results in more internalization by cells than a spherical shape [128] It is generally accepted that this effect could be due to the larger contact area of the rod than a sphere, permitting high favored internalization of nanoparticles in cell membranes [116,128] Furthermore, rod-shaped MSNs exhibit superior intracellular uptake compared to spherical MSNs [129]. The shape of the nanoparticles can allow a specific mechanism of intracellular uptake. In this context, Hao et al. [130] reported that the spherical particles are taken up by cells via clathrin-mediated endocytosis, whereas the rod-shaped particles enter cells through caveolae-mediated endocytosis.
Other Features of MSNs
One significant characteristic of any nanocarrier delivery system is hydrophobicity. Nanoparticles that have a hydrophobic nature exhibit a higher affinity for interacting with the cell membrane than those with a hydrophilic nature, contributing to improved cellular uptake [94].
2.3.2. Biocompatibility and Biodistribution of MSNs
Any DDSs introduced into clinical investigations should exhibit biocompatibility with body tissues and organs. The biocompatibility is dependent on many MSN characteristics, such as size, shape, surface functionality, porosity, route of administration, and structure (Figure 9) [131].
Figure 9.
Schematic illustration of the biocompatibility and biotranslocation of MSNs and the main physical–chemical characteristics. These highly influence the cellular uptake, intracellular translocation, and cytotoxicity on the in vitro level, and the biodistribution, biodegradation, excretion, and toxicity on the in vivo level. Reproduced with permission from [131], WILEY-VCH Verlag GmbH and Co. KGaA, 2012.
Most animal studies indicate the high biocompatibility and safety of MSNs [31,132,133]. The degree of biocompatibility of MSNs can vary according to many factors such as synthesis conditions, suitable structural features, and appropriate route at the right dosage [8,133,134,135,136,137]. As with other nanomaterials, for future translation to clinical applications, the safety aspects of MSNs should be considered carefully for each type [133]. Below, we present some studies highlighting the biocompatibility of MSNs in vitro and in vivo. Park et al. [138] investigated the biodistribution and biocompatibility of MSNs intravenously injected into mice at 20 mg/kg. The histopathological examination showed no significant toxicity compared to the control group. Their studies also indicated that MSNs are mostly cleared from the liver, spleen, heart, kidneys, brain, and lungs after 4 weeks. Hudson et al. [139] examined the biocompatibility of non-modified MSNs with particle sizes of ~150 (pores about 3 nm), 800 nm (pores about 7 nm), and ~4 µm (pores about 16 nm) at different does/concentrations. In vitro results in mesothelial cells showed that the cytotoxicity depends on the concentration; increasing concentration increases cytotoxicity towards cells. For in vivo studies, mcice were injected (intra-peritoneal, intra-peritoneal, and subcutaneous) at single dose of 30 mg/mL per mouse. The biocompatibility of MSNs in vivo depends on the dose and the route of administration. The subcutaneous injection of MSNs in rats indicates good biocompatibility, whereas intraperitoneal and intravenous injections at very high dose ~1.2 g/kg is lethal for mice due to toxicity or distress necessitating euthanasia, but at dose of ~40 mg/kg is safe. This severe systemic toxicity can be mitigated by further surface modification of the MSNs. Lu et al. [23] evaluated various doses of MSNs intravenously injected in mice (twice per week) for 14 days, they concluded that dose at 50 mg/kg is well tolerated in mice, no toxicity, no apparent abnormalities on the histopathological level or lesions were observed. They also revealed that this dose is adequate for the pharmacological application in cancer therapy.
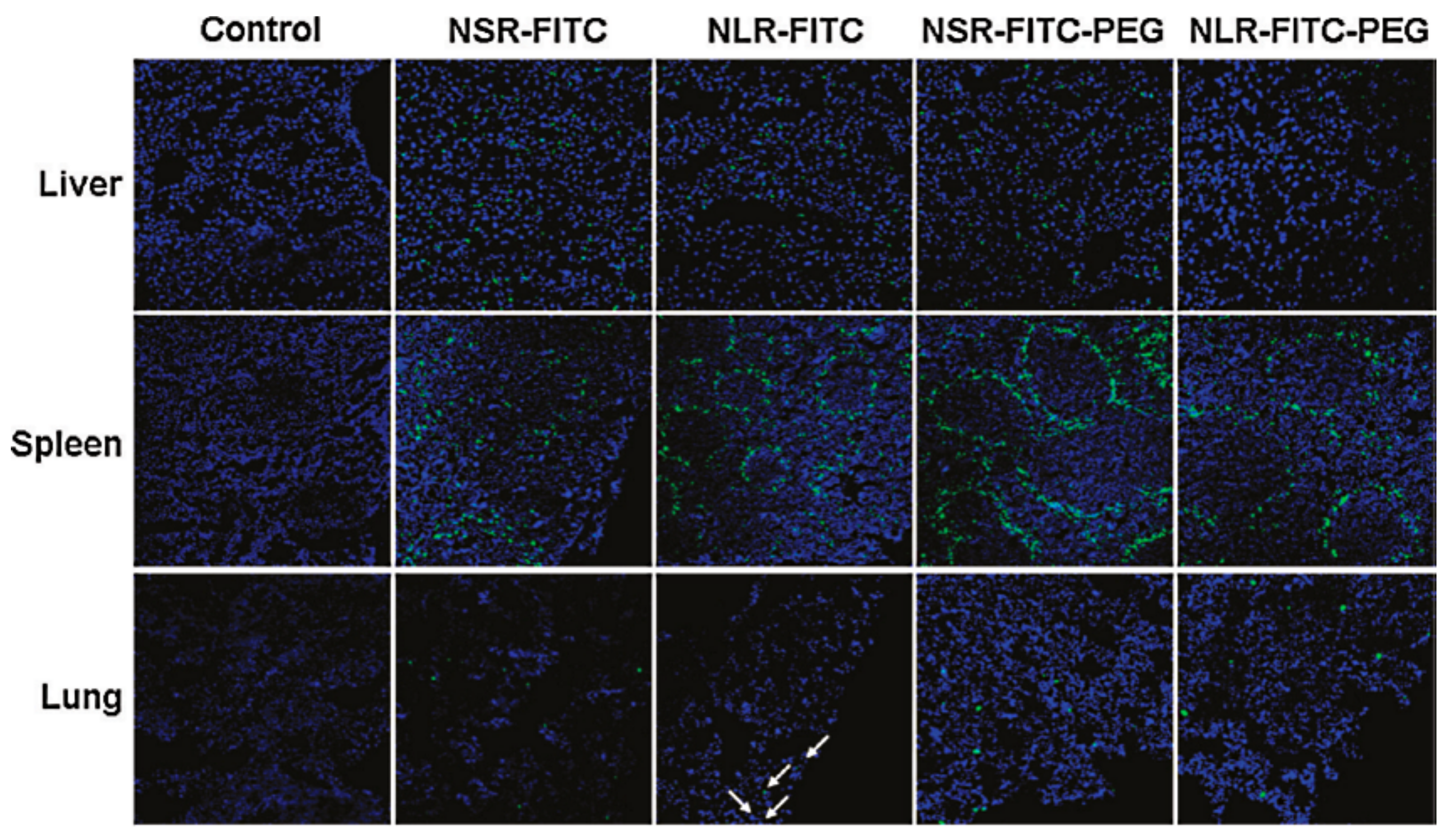
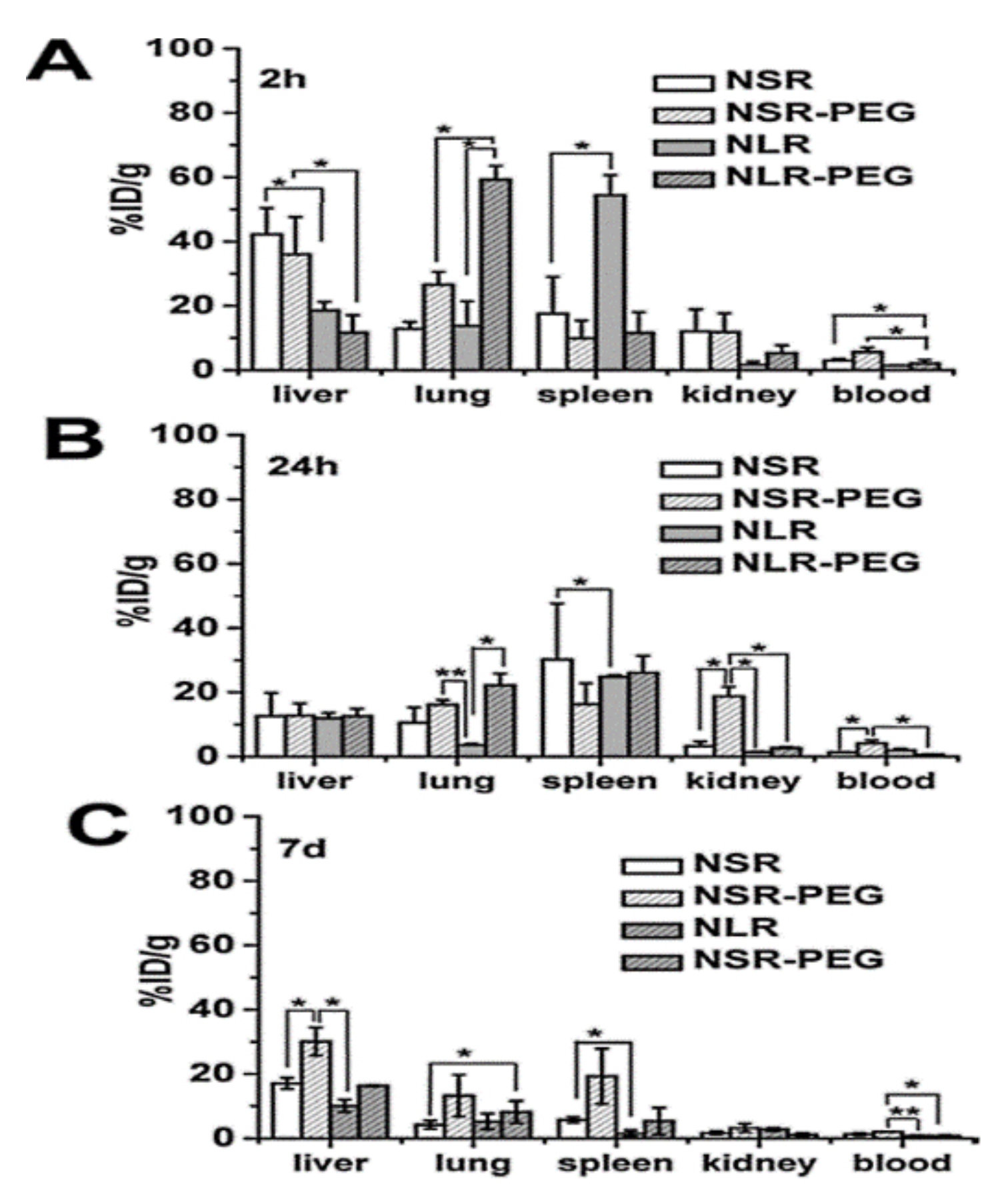
Huang et al. [30] evaluated the biocompatibility of differently shaped and PEGylated MSNs (Figure 10, Figure 11 and Figure 12), measuring various blood and serum biochemical indicators 24 h and 18 days after injection of MSNs at a dose of 20 mg/kg. All hematology markers were within normal ranges without any considerable toxicity, showing excellent biocompatibility. The results indicated that these particles do not influence liver function, and other parameters were also in the normal range. Concerning the quantitative determination of biodistribution and clearance, approximately 80% of MSNs are trapped in RES of the liver, spleen, and lung after 2 h of administration. Comparing the Si contents of different organs (at 2 h, 24 h, and 7 days), the Si content obviously decreased over time, indicating the possible degradation and clearance of MSNs from the liver, spleen, lung, and kidney. Moreover, the circulation time of MSNs in blood shows that long rod MSN (NLR) has a longer blood circulation time than short rod MSN (NSR), and the effect of surface modification by PEGylation is partially dependent on the shape.
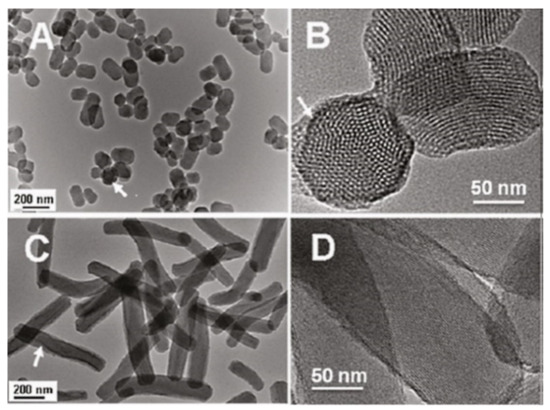
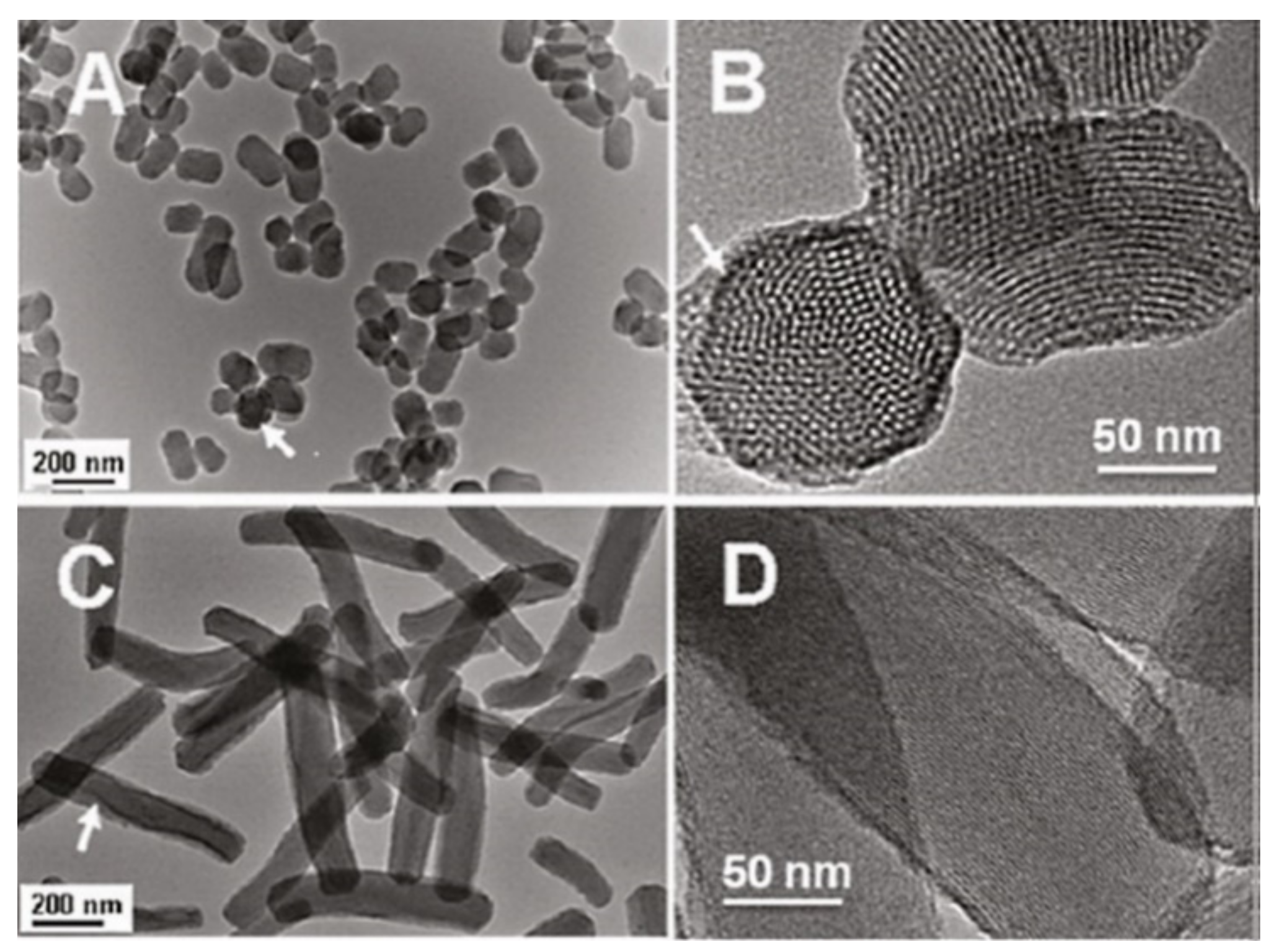
Figure 10.
Characterization of short rod MSN labeled with FITC (NSRFITC) and long rod MSN labeled FITC (NLRFITC). (A) TEM image of NSRFITC. (B) TEM image showing the mesostructure of NSRFITC. (C) TEM image of NLRFITC. (D) TEM image showing the mesostructure of NLRFITC. Arrows denote FITC embedded in a particle. Reproduced with permission from [30], American Chemical Society, 2011.
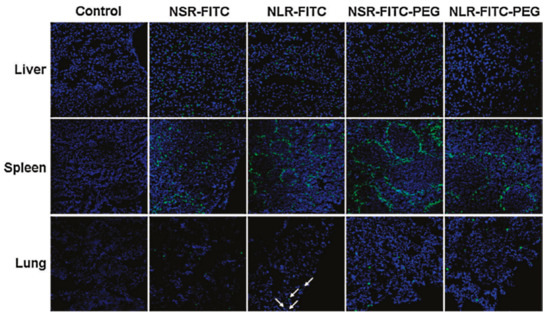
Figure 11.
Biodistribution of differently shaped and poly(ethylene glycol) (PEG)ylated MSNFITC in liver, spleen, and lung observed by confocal microscopy 2 h after intravenous injection. Arrows denote NLRFITC distribution in the lung. Reproduced with permission from [30], American Chemical Society, 2011.
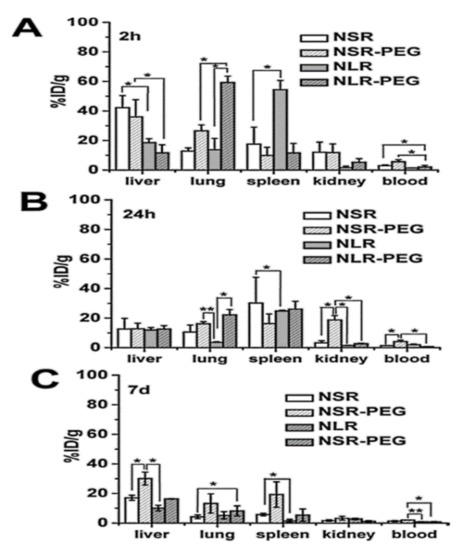
Figure 12.
Quantitative analysis of differently shaped and PEGylated MSNs in organs and blood by ICPOES. Relative Si contents in liver, spleen, and kidney at (A) 2 h, (B) 24 h, and (C) 7 d post-injection. Data are the mean ± SD from three separate experiments. * p < 0.05; ** p < 0.01 for the comparison of Si contents of differently shaped and PEGylated MSNs in organs and blood. Reproduced with permission from [30], American Chemical Society, 2011.
Yildirim et al. [140] evaluated the interactions of MSNs with different surface functional groups (ionic, polar, neutral, and hydrophobic) on blood parameters (hemolytic activity, thrombogenicity, and adsorption of blood proteins) to understand their biocompatibility. They concluded that the blood compatibility of MSNs positively improves with surface functional groups. Table 3 shows some data reported on the biocompatibility, biodistribution, and clearance of MSNs in vitro and in vivo.

Table 3.
The biocompatibility, biodistribution, and clearance of MSNs with different shapes, sizes, and surface modifications in vitro or in vivo (injection or oral administration).
2.3.3. Toxicity of MSNs
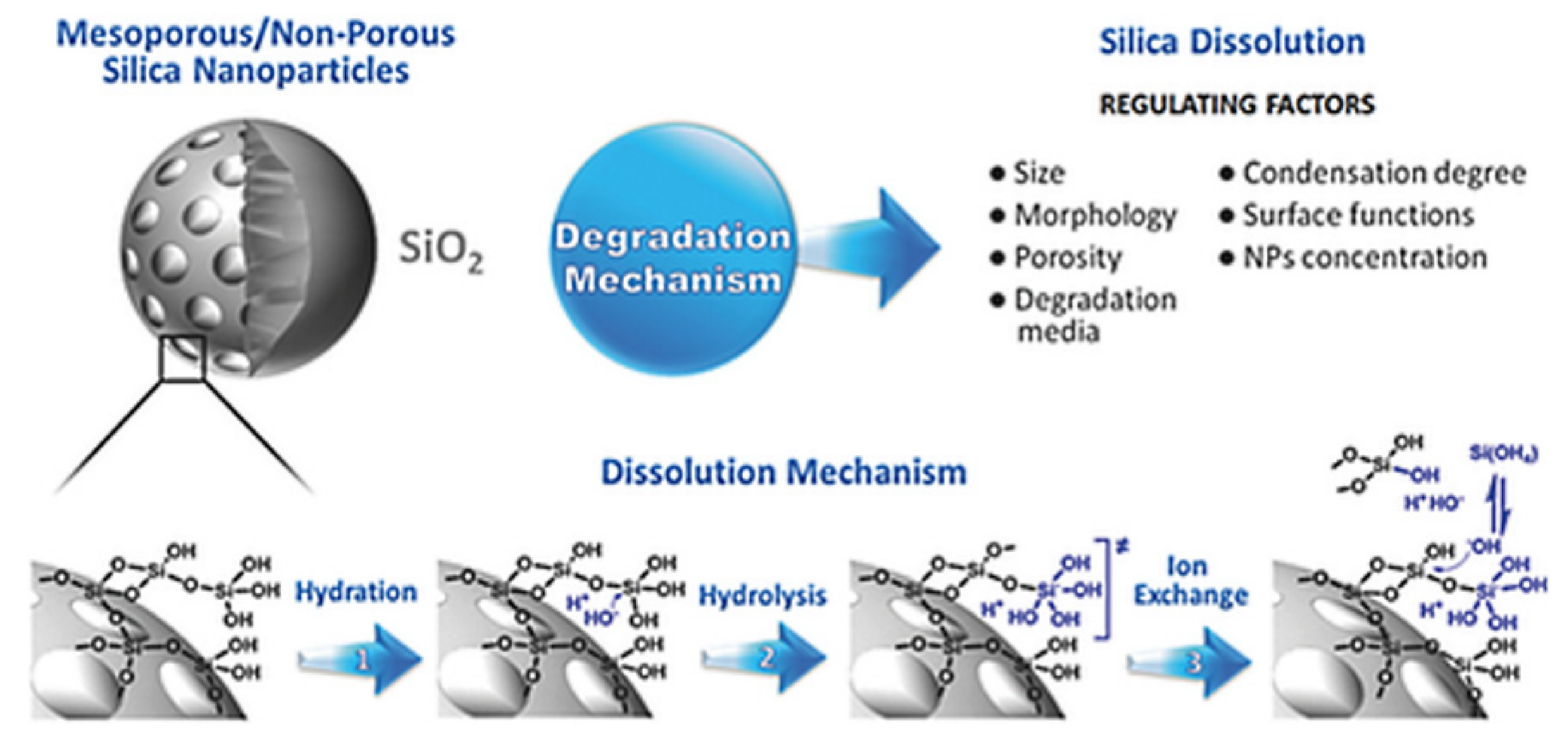
For preclinical and further clinical investigations, nanocarriers should be optimized to avoid undesirable characteristics (e.g., toxicity, side effects, non-specific interactions) and to allow good biological performance [131]. As one of the most abundant materials on Earth, silica (or silicon dioxide) in crystalline form can be found in nature as sand or quartz [149]. In contrast, the amorphous form is present in biological materials, including plants, cells, microbes (e.g., bacteria), vertebrates, and invertebrates [150]. Silica is also endogenous to human tissues, such as cartilage and bone [151]. Several efforts are underway to identify the toxicity of both the crystalline and amorphous forms of silica in different methods of application [10]. Crystalline silica mainly results in toxicity as a result of breathing fine crystalline powders created by the extraction of stone materials in soil [86]. Because it is found in vegetables, whole grains, and seafood, silica is a considerable part of the human diet (approximately 20–50 mg silicon/day for Western populations and reaching 200 mg/day for people whose diet is mainly plant-based as in China and India) [152]. Furthermore, after ingestion of silica, it circulates in the blood plasma and is absorbed in the form of silicic acid; up to 41% of silicic acid is excreted in the urine [153]. Silica nanomaterials are hydrolytically unstable and dissolve into the soluble form of silicic acid (Si(OH)4, pKa 9.6) [152]. This can occur through three different processes: hydration, hydrolysis, and ion-exchange [154]. A schematic representation of silica degradation is shown in Figure 13 [155]. Silicic acid has good bioavailability, contributing many health benefits, such as maintaining bone health [154,156,157]. The FDA has approved silica as “generally recognized as safe” for use in food additives and pharmaceutical products [86,155]. Silica nanoparticles have also been approved by the FDA for cancer imaging in clinical trials [158] and MSNs being developed with high potential for DDSs in clinical investigations [159].
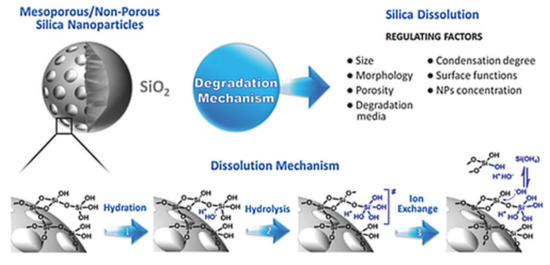
Figure 13.
Schematic representation of the intact and degraded structures of silica material nanoparticles with the mechanisms and regulating factors underlying degradation. Reproduced from [155], WILEY-VCH Verlag GmbH and Co. KGaA, 2017.
The biosafety of engineered MSNs has been confirmed by several studies. As shown in the literature, MSNs have insignificant toxicity, and the degree of toxicity is identified as low from in vivo studies. Additionally, even such insignificant toxicity can be reduced with the optimization of the synthesis process. However, a few reported data [160,161,162,163] provide contrary reports. The plausible reason for this is that there are many factors affecting the biocompatibility and safety of MSNs (e.g., shape, size, surface functional groups, physicochemical properties). For example, the method of removing the surfactant/template after MSNs synthesis (by calcination or by refluxing) influences the final cytotoxicity [139]. According to a number of in vivo experiments, a coherent message regarding the toxicity of MSNs is that that the toxicity depends on the dose/concentration used. For example, Hudson et al. [139] investigated the toxicity for MSNs (single dose) in vivo, they evaluated various doses and administration routes. They concluded that a very high dose (1.2 g/kg) is lethal for mice compared to the half dose which is well-tolerated and safe when applied by intraperitoneal or intravenous injection. Liu et al. [164] studied the single and repeated dose of MSNs via intravenous administration in mice. In the single-dose toxicity investigations, they found that the LD50 is higher than 1000 mg/kg. They also demonstrated that the groups that received low doses of MSNs did not show any behavioral, hematology, and pathological changes, whereas the groups that received high doses (1280 mg/kg) did not survive. In the repeated dose toxicity experiments, the mice groups were given continuously for 14 days followed by observation for a month. The results display that no mortality and no remarkable changes (in pathology or blood parameters) were detected. They also reported that the treatment of MSNs at daily doses (80 mg/kg) for 14 days is safe without any adverse effects in animals. Fu et al. [29] evaluated toxicity of MSNs (110 nm) in ICR mice treated by different routes: hypodermic, intramuscular, intravenous injections, and oral administration. They found that the oral route is well tolerated in mice even when increased to 5000 mg/kg compared to the intravenous route which shows the least threshold. As such results and others available from literature generated evidence to show that MSNs are well tolerated and safe in animals by various routes of administrations, i.e., oral, and intravenous injections [29,133,164,165]. However, there is no doubt that optimized production of MSNs and the final nanoformulation can achieve good biocompatibility and safe nanoparticles for treating diseases. Table 4 lists some studies that have explored the toxicity of MSNs and their delivery systems. For more reading concerning the toxicity and biosafety of MSNs, there are several extensive reviews [10,137,151,166,167,168]. The toxicity of any material/object, including MSNs, in a given environment is dependent on the dose [168]. As reviewed by Croissant et al. [168], there are mainly two mechanisms governing the toxicity of MSNs on the cellular level [88]. The first mechanism is surface silanolates that lead to membranolysis after the electrostatic interactions between MSNs and phospholipids of the cell membrane occur [169]. The second mechanism is reactive oxygen species (ROS) generation, which leads to cell death (by necrosis or apoptosis) by means of membranolysis [170]. Reducing the possible toxicity and improving the biosafety of MSNs can be achieved by optimizing the synthesis properties of MSNs for drug delivery and biomedical applications.

Table 4.
The toxicity and biosafety of MSNs of various size, shape, surface modification, and route of administration in in vivo studies.
3. Drug Loading and Release Strategies
3.1. Drug Loading Strategies
A unique feature of MSNs (e.g., large pore volume, high surface, pores, stability) makes them one of the most common nanocarriers exploited for drug delivery with a high drug loading capacity for a variety of drugs. Generally, drugs or therapeutic molecules can be loaded into MSNs with or without pore-capping. In the first technique without pore-capping, hydrophobic or hydrophilic therapeutic agents directly load MSNs with covalent or noncovalent bonding or electrostatic interactions. Loading of drugs or therapeutic agents into the mesopore network of MSNs delivers them to target tissues while simultaneously saving them from undesirable factors found in the surrounding environment (e.g., enzymatic degradation in the body) [9]. To load a suitable amount of drug, MSNs are immersed in the desired stock solution of the drug or therapeutic agent under stirring/shaking, during which the drug loading is highly driven by the concentration gradient, the competition between drug (adsorbate) and MSNs (adsorbent), adsorbate and solvent, and adsorbent and solvent [177,178]. As such, a loading process has been reported with a variety of drugs, such as camptothecin (hydrophobic anticancer molecule) [90], doxorubicin (Dox) hydrochloride [179], curcumin [69], quercetin [68], 5-fluorouracil (5-FU) [180], erythromycin [181], alendronate [182], silymarin [183], and paclitaxel (PTX) [184]. Importantly, the degree of drug loading can be maximized by choosing the desired solvent for the drug, modifying the MSN surface, and adjusting the loading parameters (e.g., time, temperature) [10,86,185].
In the second strategy with capping as the “gatekeeper” for the pore openings of MSNs [168], the first stage is to engineer the outer surface of MSNs via many techniques: molecular or supramolecular functionalization, capping with nanoparticles, and coating with polymer, protein, or lipid. This approach can control the release and delivery of therapeutic agents. In the molecular or supramolecular approach, caps are mainly rotaxanes, pseudorotaxanes, and others consisting of a long chain-like molecule that is threaded via a cyclic molecule [186]. Under certain conditions (e.g., pH, redox), the cyclic molecule can attract rotaxane (to one end of it), with the presence of a stimulus allowing it to slide to the other end. By attaching the thread near the pore opening on MSNs, the sliding cyclic molecule blocks the pore when it is near the particle or opens if it slides away. The idea of nanoparticles as gatekeepers was pioneered by Lin and co-workers [187,188,189,190] with many nanoparticles, such as iron oxide nanoparticles and gold nanoparticles. These small nanoparticles can graft on top of MSNs loaded with cargos through chemical bonding upon cleavage of the chemical bonds linking the nanoparticles with MSNs. Consequently, under certain conditions (pH, redox), external stimuli can trigger the release of cargos in a controlled manner. Next, in the coating strategy, different types of biomaterials, such as polymer, proteins, and lipids, can be introduced onto the surface of MSNs loaded with drugs. Drug release can occur upon degradation of these biomaterials or changing the surrounding environment stimuli, either external or internal [191,192,193]. Table 5 lists some examples of reported studies on prodrug loading in MSNs and their loading capacity. Table 6 provides the different loading strategies and their relationship to stimulate release under various conditions for MSNs, showing the connection between loading and release effects.

Table 5.
Loading capacity for natural prodrugs into MSNs established as recent drug delivery systems for natural medicinal substances.

Table 6.
Different loading strategies and their relationships to stimuli release under various conditions for MSNs.
3.2. Drug Delivery Strategies
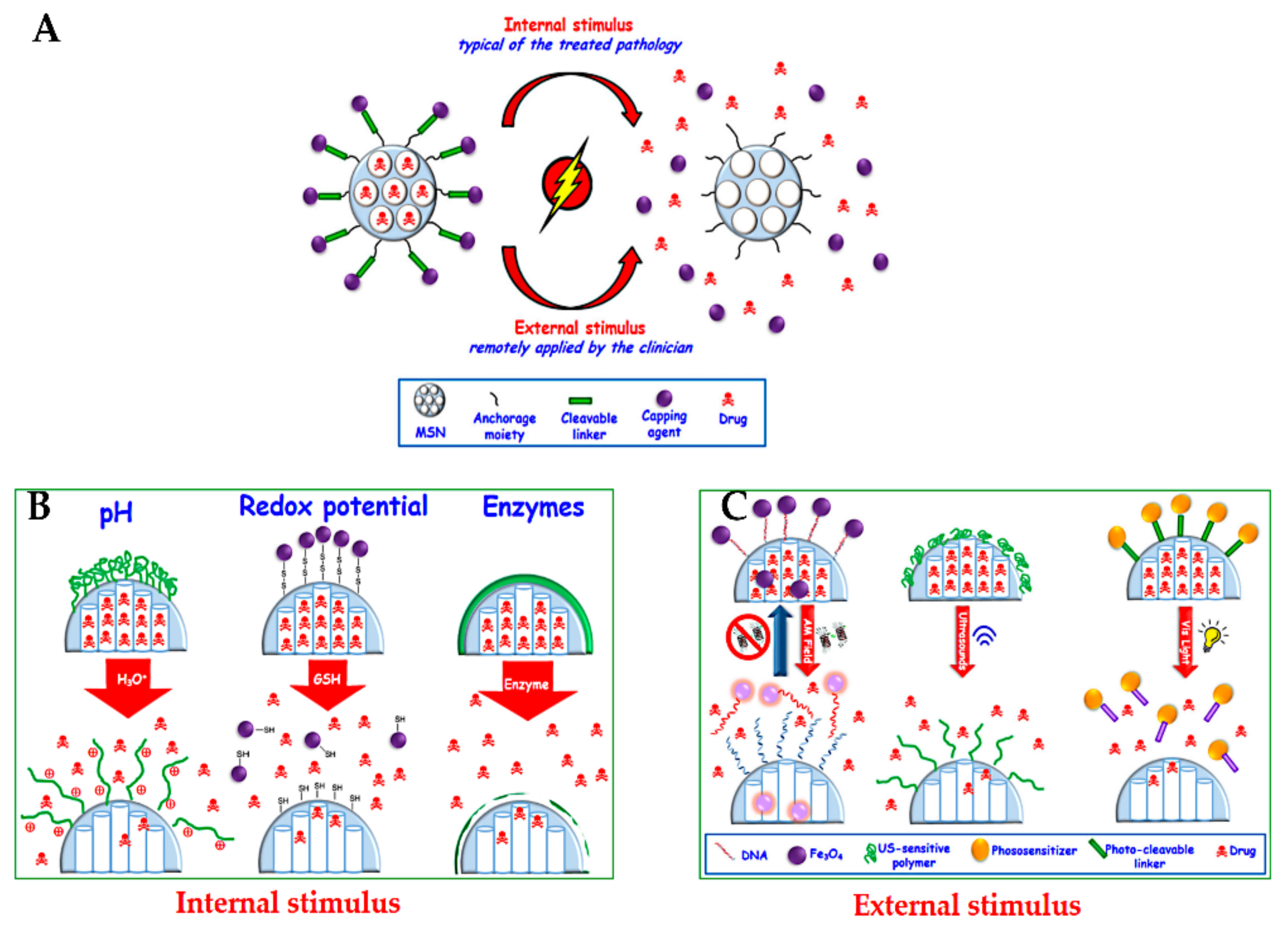
In this section, we provide a summary of delivery strategies that have been developed to treat cancer. This topic is well discussed in several reports for MSN delivery systems, and the readers are referred to these selected reviews [15,32,79,168,218,219,220,221]. Open pores on MSNs, the so-called cavities due to their porous structure, are not only used to load therapeutic agents, but also allow them to diffuse out in the surrounding solution. Closing these pores loaded with therapeutic agents is an essential step to avoid their premature release into the blood vessels, protecting from several side effects because of non-specific release [221]. Much effort has been made in controlled delivery systems with the stimulated or responsive release of therapeutic agents under certain conditions. Two major common strategies for delivering drugs have been reported by internal stimuli release (typical of the treated pathology), such as pH, redox potential, and enzymes, or by external stimuli (remotely applied by the clinician), such as magnetic fields, ultrasound, and light (Figure 14) [32].
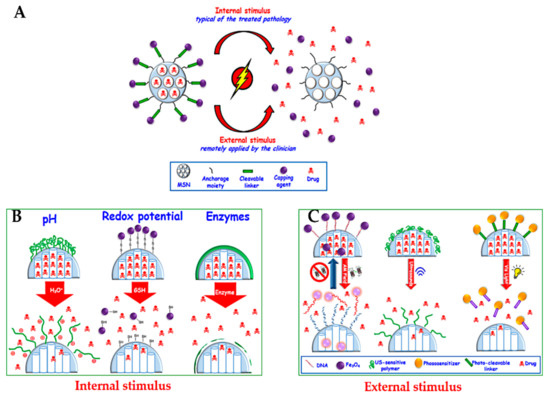
Figure 14.
(A) Schematic representation of stimuli-responsive release of drugs from MSNs. (B) Internal stimuli-responsive release. (C) External stimuli-responsive release. Reproduced from [81], MDPI, 2017.
3.2.1. Internal Stimuli-Responsive Drug Release from MSNs
pH-Responsive Release
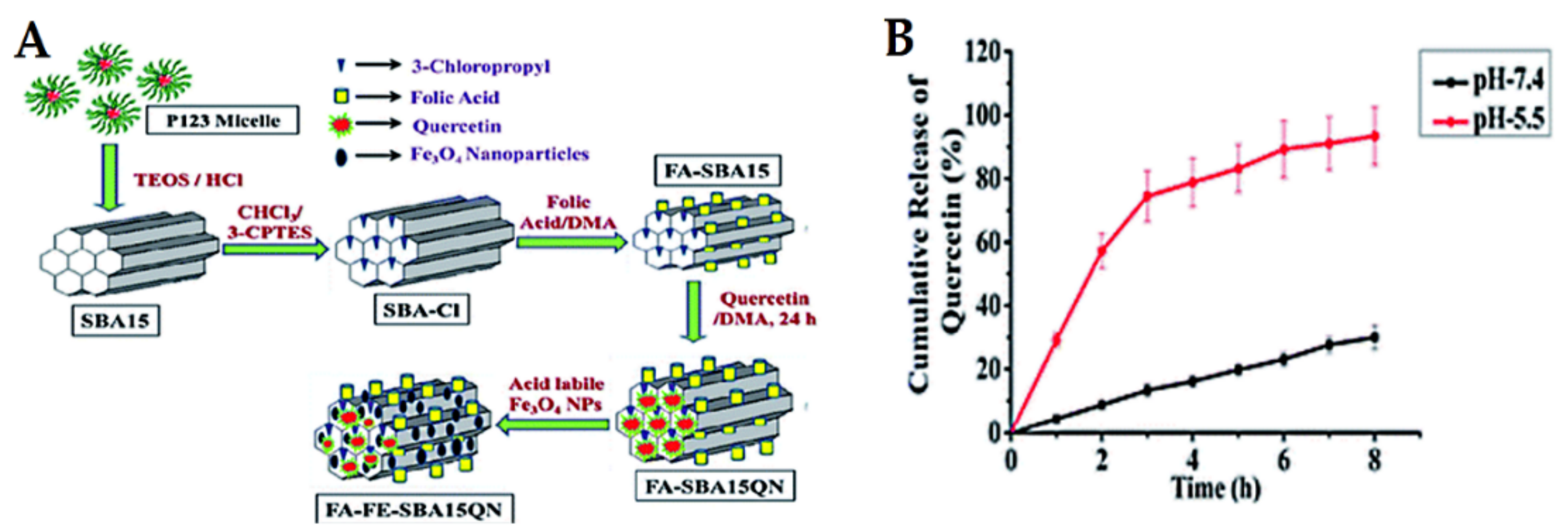
Cancer is well-known for its acidic tumor microenvironment with a lower pH than healthy cells/tissues. Consequently, pH-sensitive release is one of the approaches used in cancer nanomedicines. The most investigated pH-responsive delivery systems for anticancer therapeutic drugs have been inspired by applying diverse techniques and vary according to the loading strategies. In this section, we focus on some examples of recent studies published for natural anticancer prodrugs with pH-sensitive release. Nasab et al. [222] fabricated MSNs (MCM-41) capped with chitosan polymer and subsequently loaded with curcumin. This pH-responsive design depends on the degradation of chitosan, allowing high curcumin release at a low pH of 5.5 and resulting in low release at normal physiological pH (7.4). This is favorable for killing U87MG glioblastoma cancer cells. Mishra et al. [223] synthesized MSNs (SBA-15), followed by folic acid functionalization and further loading with quercetin and acid-labile magnetic nanoparticles (Figure 15). The system was investigated in vitro and in vivo in HCT-116 human colorectal carcinoma cells. The results showed that quercetin release was a pH-dependent effect, increasing with decreasing pH. Eventually, the system exhibits promising chemo-theranostic effects for managing colon carcinoma. In this context, Rashidi et al. [224] reported that the release of gallic acid (GA) from MSNs strongly depends on the pH levels of the release media. Furthermore, a pH-sensitive delivery system for ursolic acid prodrug was synthesized by incorporating an acid-sensitive linkage between the drug and MSNs [200]. This sustained release of ursolic acid enhances the anticancer effects against hepatocellular carcinoma cancer. A pH-responsive release of evodiamine and berberine was also achieved by loading them into lipid-coated MSNs [225]. In another strategy using Fe3O4 nanoparticles as gatekeepers, artemisinin is initially loaded into the inner space of hollow MSNs and Fe3O4 capped onto the pore outlets through acid-labile acetal linkers. The results proved that the system is stable under neutral conditions at pH 7.4 (no release), but it releases the prodrug upon exposure to the acidic lysosomal compartment (pH 3.8–5.0). The acetal linkers can be hydrolyzed under acidic conditions. This delivery system has an efficient and desirable anticancer action [226].
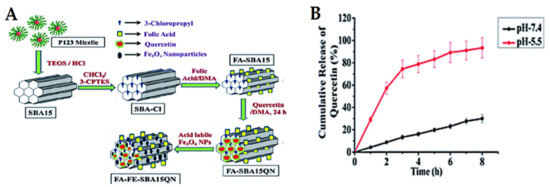
Figure 15.
(A) Schematic representation of the delivery design for quercetin “FA-FE-SBA15QN”. (B) The release kinetics of quercetin from FA-FE-SBA15QN at different pH (7.4 and 5.5). The values are represented as the mean ± SEM. Reproduced with permission from [223], The Royal Society of Chemistry, 2020. This article is licensed under a Creative Commons Attribution-Non-commercial 3.0 Unported License.
Redox-Responsive Release
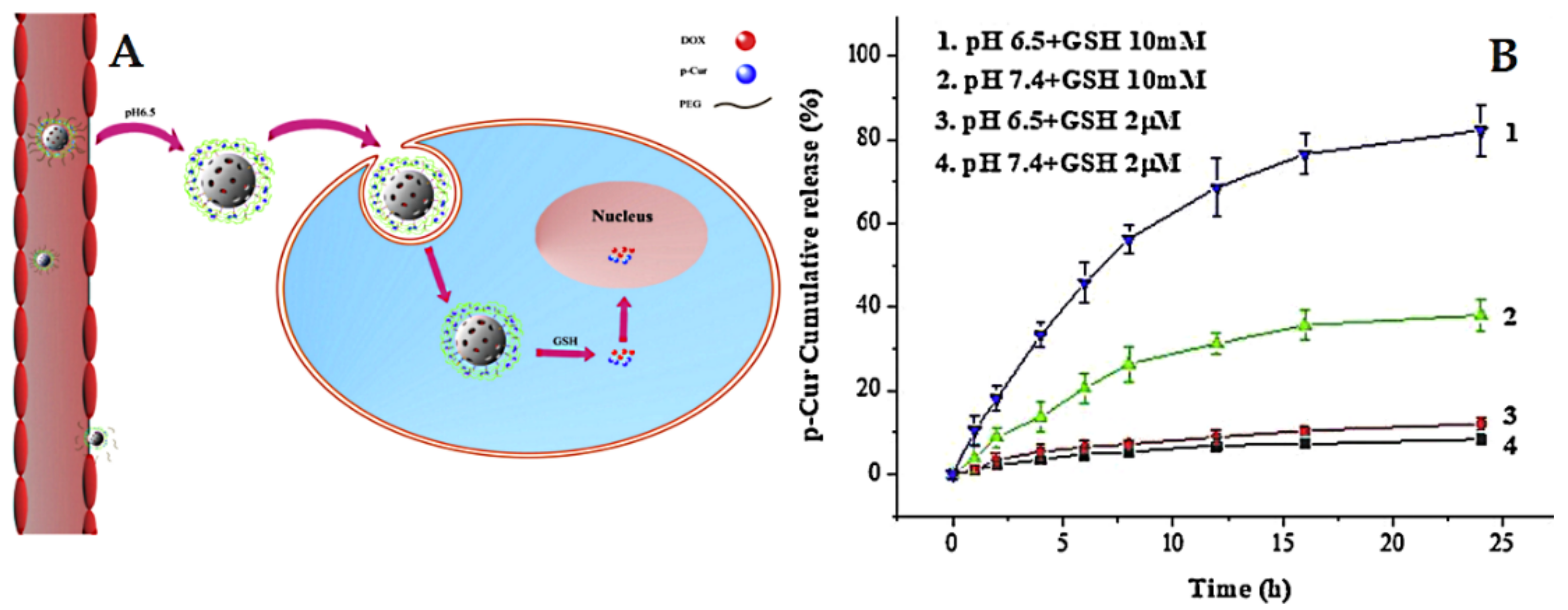
The delivery systems that consider redox-sensitive release are popular in cancer-targeted therapy. They take advantage of intracellular conditions and rely specifically on the presence of glutathione (GSH) with a high level of expression in cancer cells compared to normal cells [227]. For example, Lin et al. [228] prepared pH and redox dual-stage responsive release of curcumin with Dox through specific cleavable PEGylation and hydrogel coating (crosslinked by disulfide bonds). The used MSNs were loaded with Dox, whereas the curcumin was encapsulated in a hydrogel coating. The results indicated that dual-responsive release by means of GSH and pH allows efficient and specific cancer targeting (Figure 16). In another example, Xu et al. [229] developed a stimuli-responsive delivery for curcumin gatekeepers based on MSNs characterized by large pores (named LP). In this design, curcumin is anchored to the surface of LP using thiol-ene as the click chemistry approach, followed by a coating of the pluronic polymer (F127) on the surface by means of self-assembly. The release studies proved that curcumin exhibits a redox-responsive release depending on the absence or presence of GSH at different pH levels.
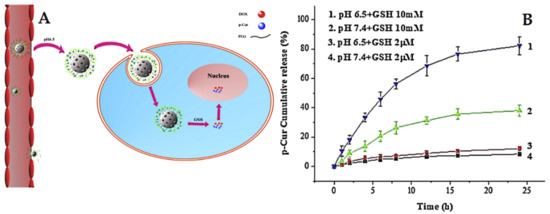
Figure 16.
(A) Illustration of the dual-response release of p-Cur and Dox co-delivery. (B) In vitro release profiles of Cur from MSN/SP/bPEG at 37 °C. Error bars indicate standard deviation. Reproduced with permission from [228], Elsevier B.V, 2019.
Enzyme-Responsive Release
In the human body, many chemicals and enzymes are inherently expressed during pathological conditions, including cancers, which are explored to trigger drug release from numerous MSN types [10]. A delivery system tailored for anticancer treatment with enzyme-responsive release, in which matrix metalloproteinase (MMP) substrate peptide containing PLGLAR, which is sensitive to MMPs, is immobilized onto amine-modified MSNs and further capped with bovine serum albumin by covalent bonding. The results revealed that the nanoplatform delivery exhibits enzyme-triggered release of drug and efficiently inhibits tumor growth in vivo. MMP enzyme-trigger release of cisplatin-based MSNs was reported by Vaghasiya [230]. The system constructed by coating collagen on the surface of drug-loaded MSNs eventually results in sensitive enzyme release.
3.2.2. External Stimuli-Responsive Drug Release from MSNs
Responsive Release Using Magnetic Fields
This approach is largely employed for responsive release due to the magnetic guidance by a permanent magnetic field and locally increases the internal temperature by changing the magnetic field potential [32]. The delivery systems concerning this method widely use magnetic nanoparticles (superparamagnetic iron oxide-SPIONs) 5–10 nm in size as a core and mesoporous silica shell permitting drug loading and release [231]. Regarding natural prodrugs, the nano platform developed by Janus MSNs consists of magnetic nanoparticles to achieve magnetic targeting and delivery of berberine. This system produces a sustained release and exerts extraordinarily site-specific internalization into hepatocellular carcinoma cells, facilitating a high antitumor effect against liver cancer due to an external magnetic field [232]. Another very recent example is Asgari et al. [233] developing a novel in situ encapsulation delivery for curcumin consisting of magnetite-silica core-shell nanocomposites. The system could be effective for clinical application by means of magnetic hyperthermia therapy. In addition, nanoparticles of DNA-capped magnetic mesoporous silica composite exhibit temperature-dependent release of Dox and magnetic hyperthermia effects against cancer [234].
Responsive Release of Drugs Using Light
As a non-invasive and spatiotemporal strategy, different wavelengths of ultraviolet, visible, or near-infrared light can be employed to trigger and control drugs from MSNs. The main advantages are easy application by the clinician and focalization to the target tissue [235,236,237].
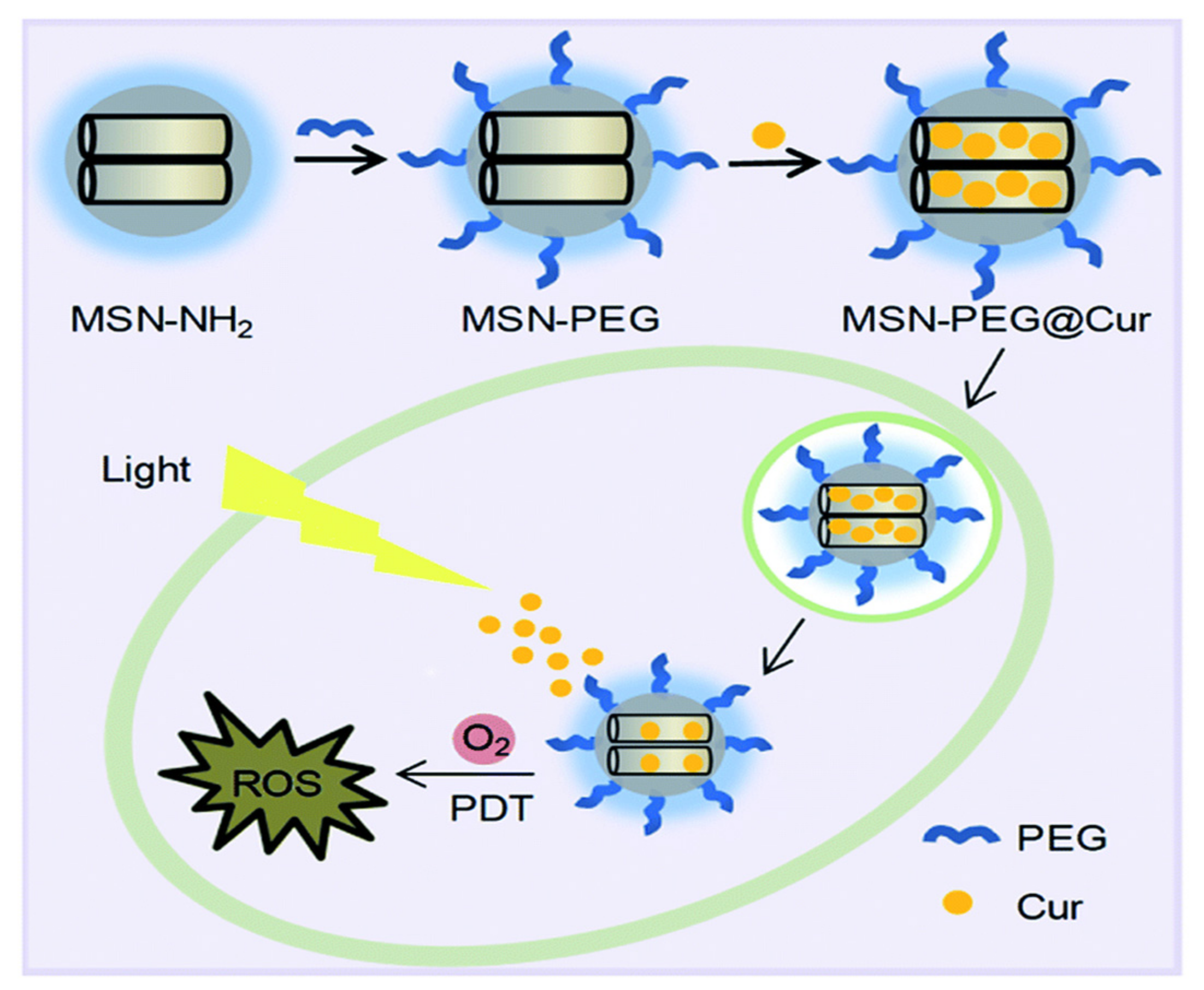
Kuang et al. [238] developed a curcumin delivery system by means of photodynamic therapy, achieving PEGylated MSNs loaded with curcumin (Figure 17). The results demonstrated that the developed system, “MSN-PEG@Cur”, exhibits efficient endocytosis into cells and the release of curcumin. As a photodynamic therapy, it promptly generates ROS upon irradiation, allowing effective treatment for cancer. In another example, Li et al. [239] preloaded berberine into folic acid-modified Janus gold MSNs. The in vitro and in vivo results demonstrated that the delivery system verifies sustained release dependent on light and an efficient anti-tumor effect with good biosafety for normal tissue. Feng et al. [225] fabricated a dual delivery platform for evodiamine and berberine loaded into lipid-coated MSNs with thermo-sensitive release. Their results suggest that the temperature-responsive release is promising for both hydrophobic and hydrophilic drugs. Using an important natural prodrug of capsaicin, the main ingredient in red or hot chili pepper, Yu et al. [240] reported a novel design of NIR-triggered plasmonic nanodot-capped MSNs for inhibiting metastasis of human papillary thyroid carcinoma. The nanoplatform consisting of gold nanodot-capped MSNs loaded the prodrug. The results depicted that the delivery of capsaicin by the developed nanoformulation exhibited strong cytotoxicity against the FTC-133 and B-CPAP cell lines compared to free capsaicin.
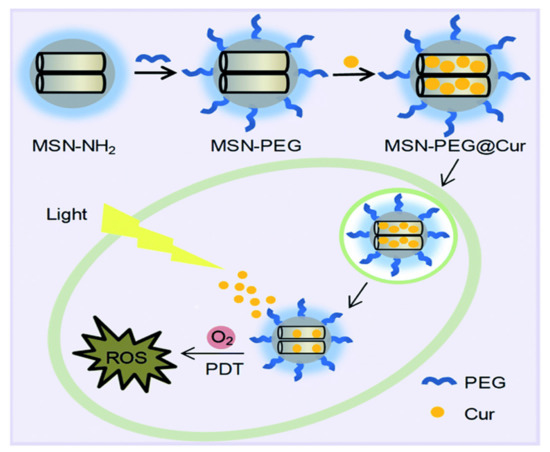
Figure 17.
The preparation process for MSN-PEG@Cur and schematic representation of the intracellular photodynamic therapy (PDT) process after endocytosis of MSN-PEG@Cur. Reproduced with permission from [238], The Royal Society of Chemistry, 2020. This article is licensed under a Creative Commons Attribution-Non-commercial 3.0 Unported License.
Responsive Release of Drugs by Ultrasound
Ultrasound is considered an interesting and efficient approach to trigger the release of drugs from MSNs. The main advantages include deep penetration of living tissues without causing damage, and it is non-invasive and can be concentrated to the desired tissue [32,241]. In this approach, drugs can be released from pores of MSNs due to the thermal effect of ultrasound radiation on chemical bonds and thermosensitive polymers while closing in the absence of a radiation effect [242,243,244]. An example is MSNs modified with amine groups covered by sodium alginate polymer and subsequently loaded with a model cargo (rhodamine B) [245]. The results indicated that rhodamine B releases based on changing the ultrasound potential (ultrasound on–off responsiveness).
4. Selective Targeting Strategies for Cancer
One of the hottest areas in delivery systems is the delivery of drugs or therapeutic agents directly to specific tissues where the desired therapy is required. The main goal of nanomedicine application for cancers is avoiding the expected side effects from drugs and damaging the healthy cells surrounding the tumor site [21,246]. Two routes have been used depending on nano-particulate delivery for cancers, passive and active selective targeting.
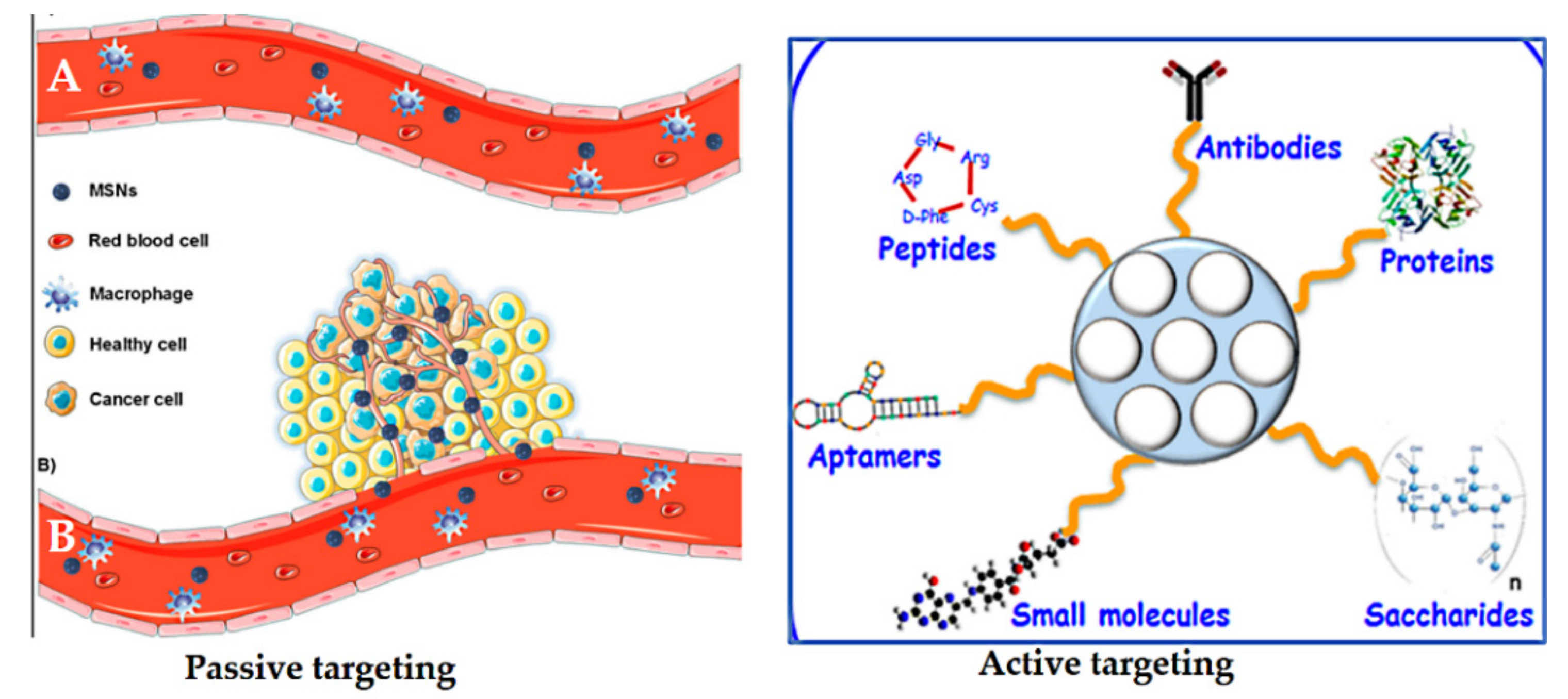
Passive targeting was first postulated by Matsumura and Maeda in 1986 [247]. Nanoparticles can accumulate in tumor tissue by the enhanced permeability and retention (EPR) effect. They hypothesized that the localization of macromolecules and particles of certain sizes differ, which is attributed to the tumor microenvironment, the relatively slow elimination rate, and poor lymphatic drainage. Particle size, surface charge, or hydrophobicity can be mediated by the so-called EPR effect, or passive targeting [248,249] (Figure 18). Passive targeting is due to abnormalities in tumor blood vessels, which have wide interendothelial junctions with pores (700 nm). Injected nanoparticles travel through the bloodstream and accumulate in the tumor interstitium because of this characteristic of tumor vessels [247,249]. The nanoparticles already located in the tumor would remain there because of the ineffective lymphatic drainage with the fast growth of the tumor tissue [221]. However, the EPR effect is often not efficient enough to selectively deliver and reduce the side effects of anticancer drugs [250].
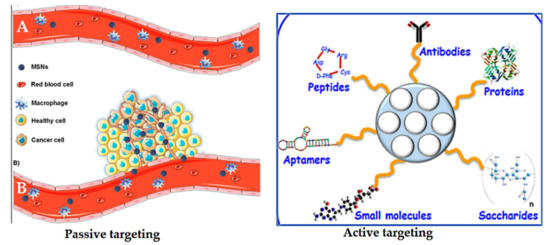
Figure 18.
Schematic representation of the enhanced permeability and retention (EPR) effect (lift side). (A) Normal blood vessels (no fenestrations), showing that MSNs remain in the bloodstream. (B) Tumor tissues (defective blood vessels present) showing that MSNs leak out through the endothelial gap–gap and eventually accumulate in the tumor. On the right is a schematic depiction of active targeting with a variety of possibilities depending on the MSNs. Reproduced from [32,259], MDPI, 2020.
Active targeting is used to enhance the ability of a nanoparticle delivery platform carrying drugs to be taken up and bind to cancer cells via specific receptors on their surfaces compared to normal cells [251]. It is well known that some tumor cells overexpress certain receptors on their surface. Thus, nano-delivery systems functionalized with various ligands permit a high affinity for receptors facilitating specific retention and uptake by cancer cells. Thus, the role of targeting ligands is to allow the nanocarriers to selectively enter the cancerous cells, but not normal cells. This not only reduces the administration dosage, but also diminishes toxic side effects of drugs during circulation [252]. Many ligands have been investigated to functionalize/decorate nano-delivery systems based on MSNs for selectively targeting cancers (Figure 18). These include antibodies, proteins, peptides, aptamers, small molecules, and saccharides [221]. For example, transferrin [237], folic acid [42], epidermal growth factor (EGF) [253], methotrexate [254], RGD-type peptide [255], anti-HER2/neu [256], hyaluronic acid [257], and mannose [258].
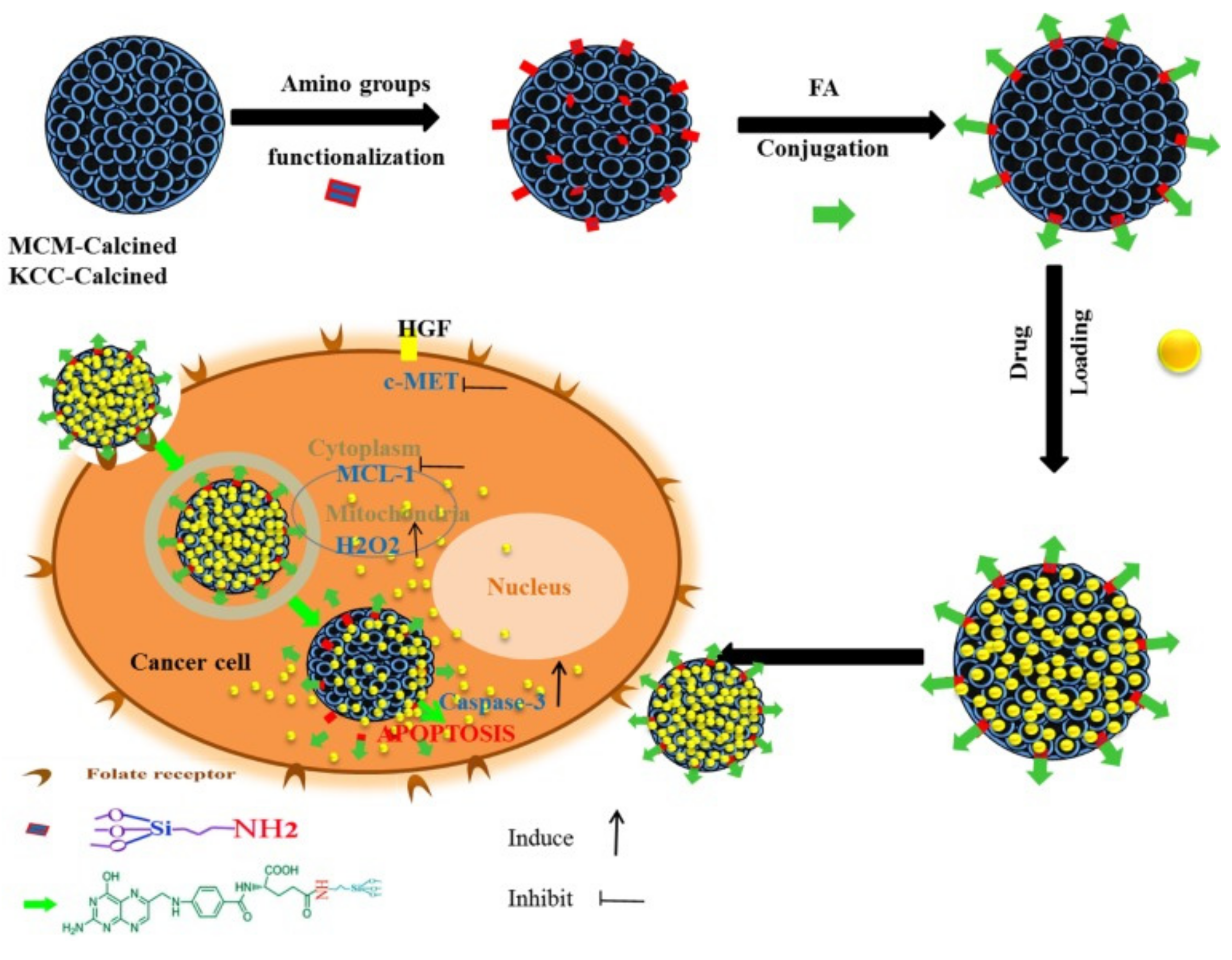
As an example, Kundu et al. [203] designed targeted delivery for umbelliferone prodrug, with the system consisting of umbelliferone loaded in MSNs and capped with a pH-sensitive poly acrylic acid and further grafted with folic acid on the surface. The delivery with folic acid conjugation increases the anticancer potential of umbelliferone against breast cancer cells. In another example, Yinxue et al. [199] investigated myricetin prodrug (Myr)-loaded MSNs combined with multidrug resistance protein (MRP-1) siRNA and the surface modified with folic acid to treat non-small cell lung cancer (NSCLC). In vivo fluorescence demonstrated that folic acid-conjugated MSNs with Myr and MRP-1 nanoparticles could specifically accumulate at tumor sites. Compared to free Myr and MSNs combined with MRP-1/Myr nanoparticles, folic acid-conjugated MSNs loaded with Myr and MRP-1 nanoparticles could more effectively suppress tumor growth with few side effects. Overall, a folic acid-conjugated nanoparticle system could provide a novel and effective platform for the treatment of NSCLC. We also reported a targeted delivery system consisting of folic acid conjugated to amine-modified MSNs (KCC-1 and MCM-41) and subsequently loaded with various prodrugs (curcumin, colchicine, and quercetin) [42]. The nanoformulation containing curcumin exhibited the highest anticancer activity against liver cancer cells through apoptosis via caspase-3, H2O2, c-MET, and MCL-1 (Figure 19). Table 7 lists some other examples of targeted delivery systems for anticancer natural prodrugs.
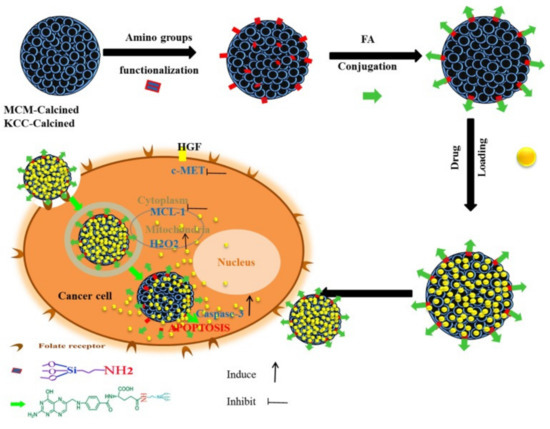
Figure 19.
Schematic representation of the preparation, internalization, and anticancer mechanism of action of the prepared nanosystem in human liver carcinoma (HepG2) cells. This schematic shows the prodrug release into cancer cells and the main anticancer action for inducing apoptosis via activation of caspase-3 for killing HepG2 cancer cells proposed by assistance from important signaling pathways (c-MET, MCL-1, and H2O2). Reproduced from [42], Impact Journals, 2018.

Table 7.
Some examples of targeted delivery systems for anticancer natural prodrugs using MSNs.
5. Motivation towards Natural Anticancer Agents
Nature is a great source of thousands of chemical substances/compounds generally considered natural products, as well as natural prodrugs if they are used for treating diseases [269,270]. Natural products (of natural origin) and herbal medicines have been used in traditional and modern medicine to treat cancer, and account for nearly 60% of pharmaceutical drugs [271,272,273,274,275,276,277,278,279]. Natural prodrugs provide medical effects against cancers as either chemotherapeutics or chemopreventive drugs. Regarding chemotherapeutics, anticancer natural prodrugs have been utilized for various cancer treatments and are becoming rising stars in the field of drug discovery for their contributions [280]. Some available drugs used in clinical applications for cancer patients diagnosed with different cancers are derived from plants, including vincristine, vinblastine, topotecan, and taxol [281]. There are also some examples of anticancer drugs originating from microbes, including Dox, daunorubicin, and bleomycin. Regarding cancer prevention, there are numerous natural substances (e.g., in fruits and vegetables) that have also been applied in cancer prevention along with human health enhancement with no detectable side effects [282]. To achieve cancer prevention goals, by completely preventing or delaying cancer, the main strategies that can be used, such as maintenance (healthy lifestyle), avoidance (exposure to toxicants/carcinogens), and dietary consumption (chemopreventive substances to drugs) [283]. There is no doubt that prevention leads to better management and treatment of tumor growth and the risk for developing metastases, secondary tumors, and recurrence [283]. Eliminating cancer, decreasing metastasis, reducing reappearance, and improving patient survival are key to curing cancers [284].
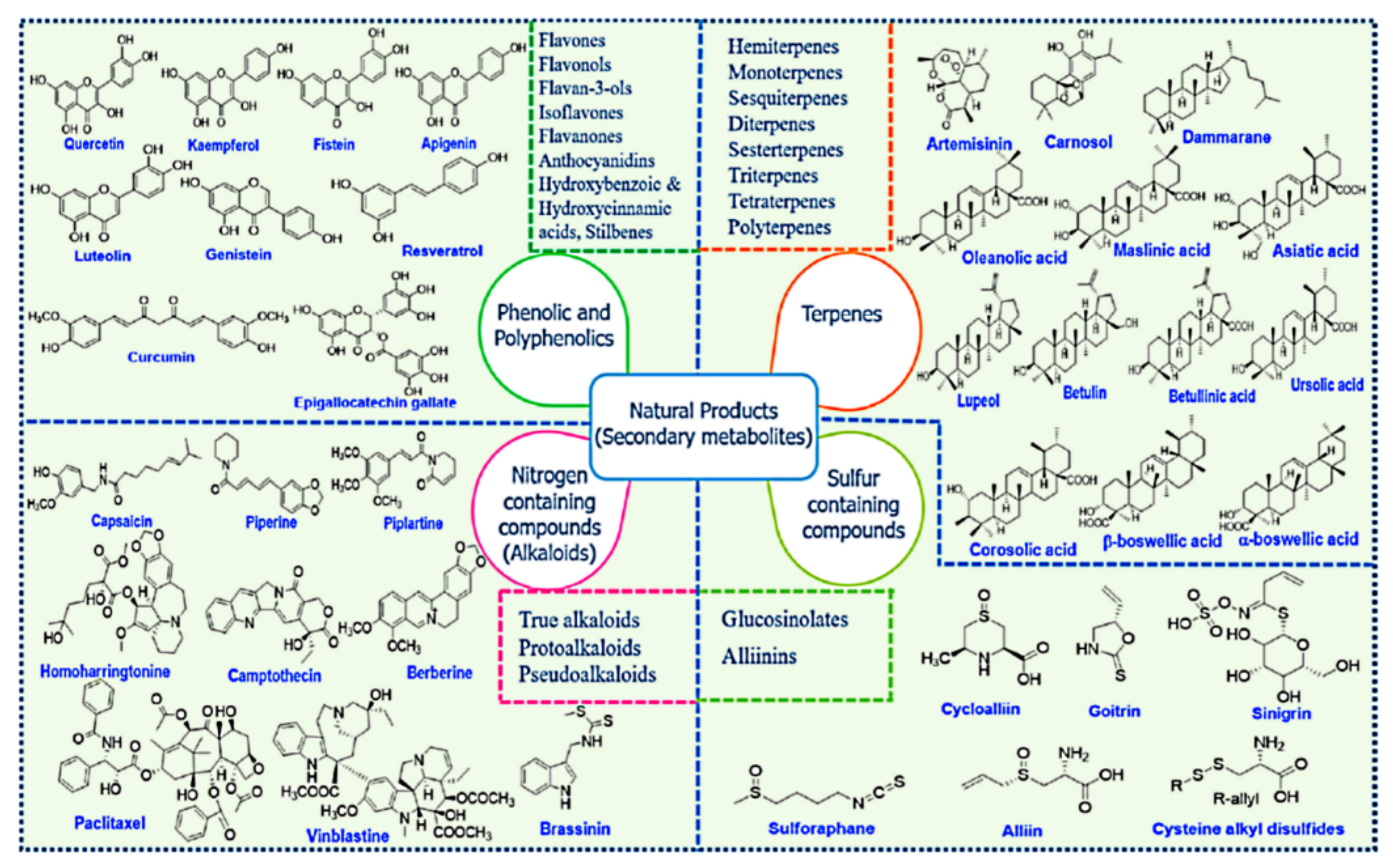
Among the main natural sources, plants are a considerable domain for supplying a variety of natural products with diverse chemical structures with a wide range of health benefits. The natural products are the main secondary metabolites produced by plants and can be classified into four major classes: phenolics and polyphenolics, terpenes, nitrogen-containing alkaloids, and sulfur-containing compounds (Figure 20) [285,286,287].
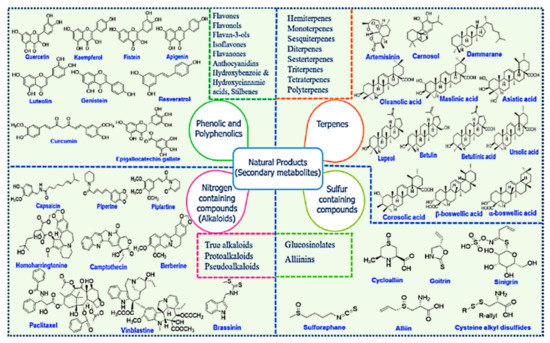
Figure 20.
Chemical structures of various classes of natural compounds (prodrugs). Reproduced with permission from [285], Elsevier Ltd., 2019.
In recent years, attention has been focused on solving the problems associated with natural prodrug substances to increase their use in cancers and other pathological disorders. As an advanced strategy, nanotechnology application in medicine, called nanomedicine, is a promising approach being developed to overcome the limitations of natural prodrugs and improve their efficiency in cancer therapy. The advent of nanomedicine for cancer therapy occurred recently, and the rate of its progress and transformation in cancer treatments has also been rapid [285]. This technology can solve the major drawbacks of natural anticancer prodrugs, including low aqueous solubility, low bioavailability, multidrug resistance, and non-specific targeting. The developed nanoformulations for delivery of natural anticancer prodrugs are intentionally being explored with several classes of prodrugs based on various organic and inorganic nanocarriers [285,288,289,290,291,292,293,294,295,296,297,298]. By reviewing in vitro and in vivo cancer models in the literature, it seems that nanoplatforms for delivering anticancer natural prodrugs have potentially improved the therapeutic activity, specific targeting, solubility, and bioavailability, and reduced side effects. The better patient response and survival are accompanied by possible enhancement of the pharmacological impacts and clinical outcome. Below, we discuss the delivery systems that have been established for select anticancer natural prodrugs employing MSNs.
5.1. Curcumin
Curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a natural hydrophobic polyphenol compound, and is the major constituent derived from turmeric rhizome (Curcuma longa L.). Turmeric is a well-known spice in the kitchen and has a long history in traditional medicine for a wide range of diseases. Curcumin has numerous pharmacological activities, including anticancer, antiviral, antioxidant, anti-inflammatory, wound healing, and antimicrobial, among others [299,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315].
Despite these potential pharmacological activities, the pharmacokinetics of curcumin show inherently poor solubility and bioavailability because of the limited absorption, rapid metabolism, and quick systematic elimination [316,317,318,319]. To take advantage of the medical actions of curcumin and improve the inherent limitations, versatile nanoplatform delivery systems have been constructed and studied, including MSNs. Concerning MSNs for curcumin delivery contribution, MSN-based nanosystems show great promise for combating cancers and will be seen soon in clinical stages.
Ma’mani and co-workers [196] fabricated guanidine-functionalized PEGylated KIT-6 MSNs 60–70 nm in size for delivery of curcumin to breast cancer cells. The system exhibited pH-sustained release of curcumin with long-term anticancer efficacy in human breast cancer cells (MCF-7 human breast adenocarcinoma cells, 4T1 mouse breast cancer cells, and MCF10A human mammary epithelial cells). A similar trend was observed for MSNs, namely MSU-2 and MCM-41 loaded with curcumin showing significant anticancer effects against different cancer cells (A549 human lung carcinoma cells, MCF-7 human breast cancer cells, and B16F10 mouse melanoma cells) compared to pure curcumin [320]. In further investigations, they found that the plausible mechanism contributing to anticancer effects is the generation of intracellular ROS and the induction of apoptosis. Lin et al. [228] tailored a co-delivery system of Dox loaded into MSNs as the core and curcumin loaded into the polymeric coating shell. The results indicate the long duration of blood circulation due to the PEG shell, GSH-sensitive release effect for drugs, and high cellular uptake resulting in synergistic anticancer effects through enhanced apoptosis of Hela cells. As an interesting nanoplatform, the fabricated lipid bilayer-coated curcumin-based MSNs unveiled a controllable and highly biocompatible theranostic nanosystem for cancer delivery [321]. Another recent strategy for building a delivery system for curcumin is by loading the prodrug into amino-MSNs using APTES silanes (KIL-2 and KIT-6), then coated by polyelectrolyte polymer complex by means of the layer-by-layer technique [197]. Based on the comparative data from this study, the nanoformulation exerts an anticancer effect on human cell lines, namely HL-60, EJ, and HEK-293, compared to free curcumin, demonstrating the promising delivery of prodrug with a sustained release effect. Considering active cancer-targeting designs, our group constructed selective targeted anticancer delivery of curcumin using MSNs (KCC-1-NH2-FA-CUR and MCM-41-NH2-FA-CUR) showing selective targeting of liver cancer cells (HepG2). The killing mechanism was found to be apoptosis [42]. The aspartic acid-functionalized PEGylated MSN-graphene oxide loaded with curcumin exhibited pH-sensitive release and excellent killing of breast cancer cells (MCF-7) [322]. With the occurrence of drug resistance in come cancers, silver-decorated SBA-15 (as metal-doped nanocomposites) coated with melanin-like polydopamine was used to deliver curcumin [323]. They found that the utilization of a nanoplatform containing curcumin enhances anticancer efficiency against select cancer cells (HeLa and taxol-resistant NSCLC (A549/TAX) compared to free curcumin.
To verify the antitumor action against breast cancer in vivo, Gao et al. investigated PEGylated lipid bilayer-coated MSNs for a dual-delivery of PTX and curcumin with prolonged release to determine their pharmacokinetic properties, uptake, subcellular localization, biodistribution and tumor site targeting, and effectiveness [324]. The delivery system could significantly increase the anti-tumor effect either by intravenous or intratumoral administration compared to free drug. The nanoplatform effectively led to the accumulation of the nanoformulation carrying drugs in the tumor site, resulting in highly efficient therapeutic effects in breast cancer. As such evidence of utilization of curcumin for co-delivery systems is important for further improvements and reducing side effects and drug resistance in cancers, which is the main issue for conventional cancer therapy. Sun et al. [325] conducted a study of cancer targeting by means of folic acid and PEI-modified-MSNs for curcumin; they concluded that the system exhibits sustained release (pH-sensitive delivery), which is suitable for antineoplastic drugs. Several studies have reported the delivery of curcumin in different cancers in vitro or in vivo (Table 8).

Table 8.
Delivery designs for curcumin in cancer (in vitro/in vivo studies) based on mesoporous silica nanoparticles (MSNs).
5.2. Quercetin
Quercetin is a dietary flavonoid compound derived from plants (e.g., medicinal plants, vegetable, fruits). It is a 3,3′,4′,5,7-pentahydroxyflvanone named by the International Union of Pure and Applied Chemistry (IUPAC) [337]. Quercetin has unique biological properties that play an important role in mental/physical performance, as well as reducing infection risk [338]. It has shown numerous pharmacological actions, including anti-oxidant, anti-microbial, anti-diabetic, anti-inflammatory, anti-cancer, anti-Alzheimer, psychostimulant, mitochondrial biogenesis stimulant, lipid peroxidation inhibitor, platelet aggregation inhibitor, and capillary permeability inhibitor, among others [339,340,341,342,343,344,345,346,347,348]. The dietary intake of quercetin varies in many countries. The estimated intake dosage of flavonoid (quercetin accounts for nearly 75%) ranges from 50–800 mg/day according to the consumption of fruits, vegetables, tea, and herbals [349]. In addition, quercetin is safe with a single dose of up to 4000 mg orally and up to 100 mg via intravenous administration [350]. Quercetin is an excellent free radical scavenging antioxidant [344] and is considered one of the most effective antioxidants [351]. Consequently, quercetin exhibits promising effects against cancer [339,352] in vitro and in vivo [353,354,355,356,357,358,359,360]. Nevertheless, its potential impacts in clinical applications are drastically limited due to its poor solubility, low bioavailability, and instability [361]. According to the pharmacokinetics of quercetin in humans, only ~2% is bioavailable (from single dose) with an absorption rate of 3 to 17% (from 100 mg applied in individual healthy persons) [337]. The factors affecting oral bioavailability are low absorption, extensive metabolism, and/or rapid elimination, in addition to low solubility and non-selective targeting of cancers. Several nanoplatform delivery systems focus on overcoming these challenges to introduce quercetin into clinical applications soon for cancer [362,363,364,365,366,367,368].
The use of MSNs to develop new delivery systems for quercetin against cancers has attracted many research groups. Liu et al. [369] fabricated a system for dual delivery of PTX and quercetin into MSNs to overcome multidrug resistance in breast cancer. The nanosystem exhibited CD44 receptor-mediated active targeting for MCF-7/ADR cells. At the same time, the addition of quercetin with PTX significantly improves the sensitivity of MCF-7/ADR cells to PTX, providing a solution to multidrug resistance in breast cancer. Huang et al. [370] designed a novel nanoformulation consisting of quercetin-loaded MSNs coating cancer cell membranes for enhanced tumor targeting and radiotherapy. In vitro and in vivo investigations revealed that the system has many advantages, including excellent tumor targeting ability and efficient inhibition of tumor growth. The platform fulfills innovative ideas for targeting cancer and improving therapy. In another attempt, polydopamine-coated hollow MSNs combining Dox hydrochloride with quercetin efficiently overcame multidrug resistance in taxol and Dox double-resistant human colorectal cancer cells (HCT-8/TAX cells) [371]. Fang et al. [262] developed a hyaluronic acid-modified MSNs that co-deliver quercetin and Dox to enhance the efficacy of chemotherapy for gastric carcinoma. They found that the system enables stability, sustained release, and selective killing effects. An in vivo study disclosed that the co-delivery significantly enhances the anticancer efficacy compared to a single drug, showing the importance of quercetin in clinical application. In this context, Murugan et al. [264] loaded topotecan into the pores of MSNs, followed by poly(acrylic acid)-chitosan as an outer layer to further conjugate quercetin, and then grafting with arginine-glycine-aspartic acid (cRGD) peptide on the surface as targeting ligands for cancers. The system released the drugs as a function of pH and uptake occurred through integrin receptor-mediated endocytosis, enabling efficient anti-tumor effects in multidrug resistant breast cancer cells and animal studies. As far as active targeting and bioavailability are concerned, MSNs conjugated with folic acid and loaded with quercetin exhibit higher cellular uptake and more quercetin bioavailability in breast cancer cells, as well as an enhanced antitumor effect through apoptosis [265]. These studies demonstrate the prospective application of quercetin in cancers by means of single or co-delivery, facilitating efficient targeting and antitumor effects, creating new possibilities for clinical applications.
5.3. Resveratrol
Resveratrol (RSV, 3,5,4′-trihydroxy-trans-stilbene) is a natural polystilbene and non-flavonoid polyphenol. As a phytoestrogen compound, RSV is present in a wide range of plants and is abundant in extracts from the grape skin and other fruits and vegetables. RSV has been reported to exert multiple pharmacological effects, including anti-inflammatory, anti-viral, anti-microbial, anti-Alzheimer, anticancer, cardioprotective, neuroprotective, and immunomodulatory actions [372,373,374,375,376,377,378,379,380,381,382,383,384,385,386]. Concerning the anticancer effects on the preclinical level, RSV has also been reported to possess important antitumor actions in several preclinical animal models [387,388,389,390,391,392,393,394,395,396,397,398]. The clinical prospective of RSV has also been evaluated in a few clinical trials. The first clinical trial by Nguyen et al. [399] indicated that the freeze-dried grape powder (containing RSV) effectively inhibits colon cancer in patients. In addition, Patel et al. [374] showed that a daily dose of RSV at 0.5 or 1.0 g produces sufficient anticarcinogenic effects in colorectal cancer. Furthermore, Howells et al. [400] demonstrated that RSV given at micronized formulation with 5.0 g daily for 14 days in patients with colorectal cancer and hepatic metastases prevented malignancies by increasing apoptosis.
Despite promising preclinical (in vitro and in vivo) and prospective clinical results as an anticancer agent, RSV still has many challenges due to the pharmacokinetics, metabolism, bioavailability, and toxicity in cancer patients [374,401]. These associated properties prevent translation into more clinical trials and human benefits. In addition, RSV has shown poor bioavailability due to its quick extensive metabolism, and large doses (up to 5 g/day) should be applied to provide anticancer therapeutic activity [402]. Such high doses result in adverse effects (e.g., diarrhea, nausea, and abdominal pain with >1 g/day) [402]. As the poor bioavailability limits the RSV activity, there are various approaches for overwhelming the bioavailability, including co-delivery with piperine prodrug [403], micronized powders [403], and nanoplatform delivery [404,405,406,407]. Application of nanomedicine can improve the stability and bioavailability, and minimize side effects of RSV, which is making RSV a prospective candidate for treating many diseases, including cancers.
Few investigations in recent years have used MSNs for the delivery of RSV. Chaudhary et al. [408] loaded RSV into MSN-modified phosphonate or MSN-modified amine to improve the anti-proliferative activity and sensitization of drug-resistant prostate cancer. The RSV is released as a function of pH, and the phosphonate-modified nanoparticles effectively kill cancer cells better than amine-modified nanoparticles. Hu et al. [267] constructed a dual delivery system for anti-miR21 and RSV using MSNs conjugated with hyaluronic acid to target gastric carcinoma through overexpression of the CD44 receptor on cell membranes. They found that this nanoformulation has a superior anticancer effect due to synergistic effects specifically delivered by combining anti-miR21 and RSV in gastric cancer cells. Furthermore, Summerlin et al. [409] encapsulated RSV in colloidal MCM-48 and found that the nanoformulation enhances saturated solubility (∼95%) and release effect compared to pure RSV. The nanoformulation also possesses a higher killing ability for HT-29 and LS147T colon cancer cells compared to pure RSV by mediating the PARP and cIAP1 pathways.
5.4. Berberine
Berberine is an isoquinoline alkaloid found in a handful of plants widely used in botanical medical practice, such as Hydrastis canadensis (Goldenseal), Berberis aquifolium (Oregon grape), Berberis vulgaris (Barberry), and Coptis chinensis (Chinese Goldthread) [410,411]. Versatile pharmacological activities have been reported for berberine, including anti-viral, anti-microbial, anticancer, anti-diabetic, anti-diarrhea, and anti-inflammatory, and treatment for congestive heart failure, cardiac arrhythmia, and hypertension. Recently, berberine extract or pure compound has gained much attention in the newly published research and is among the top pharmaceutical supplements on shelves [412]. The preclinical evidence from huge studies reveals the capability of berberine to treat many diseases [411,413,414,415,416,417,418,419]. Thus, berberine is clinically studied for many diseases, such as diabetes [410,420,421,422,423]. Particular attention has been given to berberine in cancers, so it is expected to be one of the most common natural compounds under the scope of extensive clinical investigations of cancers [424]. The main challenges in translating berberine to the clinical application are low solubility, poor aqueous solubility, slight absorption, and low bioavailability. There are some strategies to deal with these limitations, such as producing berberine hydrochloride to increase its solubility. Another approach is encapsulating berberine into nanocarriers for nanoplatform delivery [425,426,427].
Berberine loaded into folic acid-conjugated gold-MSNs shows superb anticancer effects, good biosafety, and protection of normal tissue in vitro and in vivo for chemo-radiotherapy of liver cancer [239]. Another conformation obtained by Feng et al. [225] showed that MSNs based on dual delivery of hydrophobic prodrugs with berberine and evodiamine through thermo/pH-responsiveness improves antitumor effects in vitro and in vivo. Other results propose that the berberine-loaded Janus nanocarriers (MSNs containing iron oxide) driven by a magnetic field provide an effective and safe approach against hepatocellular carcinoma [232]. As with other drugs, berberine can be released depending on different conditions; by disulfide bond linking, berberine releases from MSNs under glutathione conditions upon breakage of the disulfide bond, promoting the anticancer action against liver cancer [428].
5.5. Thymoquinone
Thymoquinone (TQ, 2-methyl-5-isopropyl-1,4-benzoquinone), a monoterpene diketone compound, is the main active component in essential oil (volatile oil) of Nigella sativa L. (known as black seed or black cumin). TQ was isolated for the first time in 1963 [429] and exhibits various pharmacological activities in vitro and in preclinical investigations. The most reported activities are anticancer, antioxidant, anti-microbial, neuroprotective, anti-inflammatory, anti-microbial, and anti-diabetic [430,431,432,433,434,435,436,437,438,439,440]. A considerable amount of available data from preclinical studies encourage the translation of TQ into clinical settings. There is no doubt of the promising anticancer effects of TQ, but the lack of bioavailability and pharmacokinetic parameters delay the use of TQ in clinical applications. The main issues are low bioavailability, solubility, biodistribution in the body, rapid metabolism, and excretion [441,442,443]. In recent years, several strategies have been investigated to improve these limitations, such as the development of novel analogs [444], use of different routes (e.g., oral, intraperitoneal, intravenous), and nano-delivery systems [296,445,446].
Few delivery systems have been designed for TQ based on MSNs. The TQ-loaded MSNs produce more anticancer effects against MCF-7 and HeLa cells than pure TQ [447]. In addition, both TQ-loaded MSNs and pure TQ exert their anticancer activity by means of ROS-mediated apoptosis. To enhance the targeting ability towards glioma cells, we fabricated core–shell nanoformulations [44], with the core consisting of MSNs loaded with TQ and the shell consisting of whey protein–Arabic gum or chitosan–stearic acid complex. Interestingly, TQ releases as a function of pH and induces selective killing of cancer cells compared to normal cells. Furthermore, the core–shell nanoformulations significantly kill glioma cancer cells via apoptosis-mediated pathways due to caspase-3 activation, cytochrome c triggers, and cell cycle arrest at G2/M signaling. In this sense, the efficient anticancer effects against brain cancers can be attributed to the distribution of TQ-loaded MSNs [448]. The study showed that encapsulating TQ in MSNs improves delivery to some brain areas, including the cortex, thalamus, hypothalamus, and midbrain, but reduces its delivery to the cerebellum compared to pure TQ. The results also indicated that neither free TQ nor MSN-TQ reaches the hippocampus. Thus, MSNs potentially target TQ to certain brain areas.
5.6. Gallic Acid
GA (3,4,5-trihydroxybenzoic acid) is one of the most abundant phenolic acids present in plants (e.g., fruits and medicinal plants. GA can be isolated from different plants of Quercus spp. and has extensive applications in the food and pharmaceutical industries [449]. The therapeutic uses include antimicrobial [450], anticancer, gastrointestinal disease, cardiovascular disease, metabolic disease, neuropsychological disease, and other miscellaneous diseases [449,451,452,453,454,455]. GA has a potential antioxidant action modulated by various signaling pathways (e.g., inflammatory cytokines, and enzymatic and nonenzymatic antioxidants) that lead to its therapeutic effects [453]. However, as with many prodrugs, limitations still exist for clinical use of GA and to confirm its therapeutic outcomes. Several nanostructures have been used to fabricate delivery systems to solve these limitations and achieve effectiveness to translate GA into clinical investigations [456,457,458,459,460].
Only a few studies have been reported on MSN nanosystems for GA. MSNs functionalized with amino acid and coated with chitosan exhibit a high loading capacity of ~20–38%, leading to better killing potency of MCF-7cells than pure GA [195]. GA is an unstable molecule under specific pH; by encapsulating it in MSNs, the release of GA can be controlled by media with different pH and released in the presence of higher antioxidant activity [224]. With respect to the anticancer effect, incorporation of GA into MSNs by means of covalent bonding increases its activity against HeLa and KB cells, with a killing efficiency of up to 67% [461]. Thus, GA-loaded MSNs easily internalize into Caco-2 cells, releasing GA to enhance cytotoxic effects against colon cancer [462].
5.7. Essential Oils
Among the plant natural prodrugs, the essential oils (also known as volatile oils) have particular importance in many sectors (e.g., pharmaceutical, cosmetic, agricultural, and food) [463,464]. With a long history in many cultures, essential oils can be used for different purposes [465]. For example, Ancient Egyptians used essential oils as early as 4500 BC for cosmetics and ointments [466]. They made a mixture of many herbals containing essential oils (e.g., aniseed, cedar, onion, myrrh, grapes, etc.) as preparations in perfume or medicine. In recent years, the most important use of essential oils has been aromatherapy due to their curative effects [467]. Essential oils are complex mixtures of volatile compounds found especially in aromatic plants, such as clary sage (Salvia sclarea L.), eucalyptus (Eucalyptus globulus Labill.), geranium (Pelargonium graveolens L.), lavender (Lavandula officinalis Chaix), lemon (Citrus limon L.), peppermint (Mentha piperita L.), roman chamomile (Anthemis nobilis L.), rosemary (Rosmarinus officinalis L.), basil (Ocimum basilicum), rosemary (Rosmarinus officinalis), and ginger (Zingiber officinale). The essential oils are obtained from plant sources by several methods, such as hydrodistillation, steam distillation, cold pressing, solvent extraction, microwave-assisted processing, and carbon dioxide extraction. Concerning their chemical composition, essential oils were originally characterized as monoterpene and sesquiterpene hydrocarbons together with their oxygenated derivatives, besides the aliphatic aldehydes, alcohols, and ester structures [466]. Due to the chemical compositions of essential oils with versatile compounds that possess many roles and modes of action in various pharmacological entities and therapeutics, including anticancer, cardiovascular disease treatment, anti-bacterial, anti-viral, anti-oxidants, analgesics, and antidiabetics [468,469]. The main applications are enhanced transdermal drug delivery due to their skin penetration, and aroma and massage therapy [470]. Essential oil compounds have been reported to have potential anticancer effects in vitro and in animal models [471,472,473,474,475,476,477]. However, essential oils generally have low stability, high volatility, and a high risk of deterioration by exposure to direct heat, humidity, light, or oxygen [478]. Nanoformulations are a recent strategy being developed for essential oils and their constituents to solve these problems [463,479,480,481,482].
To the best of our knowledge, no anticancer nanoformulations have been designed for essential oils and their constituents. MSNs are efficient particles for the high loading of essential oil substances. Melendez-Rodriguez et al. demonstrated that eugenol, an important component in various essential oils of herbs, is efficiently encapsulated in pores of MSNs up to 50 wt.%. by means of vapor adsorption [483]. Ebadollahi et al. [484] reported that the loading of essential oils of thymus species into MCM-41 increases their stability and persistence up to 20 days. Furthermore, Janatova et al. [485] demonstrated that different encapsulated essential oil components in MCM-41 provide long-term effects through controlled release compared to the same pure substances. In addition, Jobdeedamrong et al. [486] showed that the release of essential oils (peppermint, thyme, cinnamon, and clove oil) is controlled by loading them into MSN-functionalized particles and grafting them with hyaluronic acid. Confirmation of delayed volatilization was reported for lavender oil loaded into MSNs [487]. Jin et al. [488] showed that MCM-41 modified nanoparticles enable high loading of pepper fragrant along with bactericidal activities against different microbes. Thus, the incorporation of essential oils from different herbs could be used effectively for infectious diseases [489,490] and treating biofilm [491].
5.8. Other Natural Products
Artemisinin is a sesquiterpene lactone derived from Artemisia annua. It is used as an antimalarial for treating multi-drug-resistant strains of falciparum malaria. It has also shown promising anticancer effects [492]. Artemisinin loaded into pores of hollow MSNs and capped with Fe3O4 nanoparticles act as gatekeepers [226]. The system shows a pH-dependent release effect, with stable release achieved at pH 7.4 and higher artemisinin release at low pH (3.8–5.0). This system exhibits excellent anticancer efficacy. Another multifunctional nanocarrier, Fe3O4@C/Ag@mSiO2 loaded with a high amount of artemisinin, allows pH-stimuli release and more killing of HeLa cancer cells compared to free artemisinin [493].
Some natural prodrugs are toxic compounds, and this toxicity prevents them from being used to treat cancers. An important example is colchicine, a natural alkaloid derived mainly from Colchicum automnale. It has long been used clinically to treat gout and familial Mediterranean fever. Colchicine is an important antimitotic prodrug and efficiently kills cancer cells [494], but the major challenge to its use is its toxicity. Earlier, Cauda et al. reported a one-step fabrication of colchicine-loaded in lipid bilayer-coated MSNs, making the system more stable and leading to effective microtubule depolymerization upon cell uptake [495]. We also loaded colchicine into folic acid-conjugated MCM-41 and KCC-1 for anticancer and antioxidant effects, obtaining higher anticancer effects than with free colchicine [42]. Very recently, we developed a novel DDS for colchicine. The system consisted of KCC-1-functionalized with phosphonate groups and loaded with colchicine, and subsequently coated with folic acid chitosan–glycine complex (MSNsPCOL/CG-FA) [43]. This nanoformulation revealed enhanced selective killing towards cancer cells compared to free colchicine in this order: colon cancer (HCT116) > liver cancer (HepG2) > prostate cancer (PC3). As its cytotoxicity is a major concern, the system is also promising because it exhibits low cytotoxicity (4%) compared to free colchicine (~60%) in normal BJ1 cells. The main mechanism of action was studied in detail for HCT116 cells, indicating primarily intrinsic apoptosis as a result of enhanced antimitotic effects with a contribution of genetic regulation by MALAT 1 and mir-205 and immunotherapy effects by Ang-2 protein and PD-1.
Loading glabridin, a prodrug compound obtained from the root extract of Glycyrrhiza glabra, on MSNs leads to remarkable improvement in its saturation solubility and dissolution velocity [496]. In this context, loading of breviscapine in MSNs significantly improves the solubility and bioavailability [17]. In addition, Ibrahim et al. [183] concluded that incorporating silymarin in MSNs within a lyophilized tablet remarkably increases the dissolution rate and saturation solubility. Similarly, loading of glabridin in MSNs improves the saturation solubility and dissolution velocity [496]. The biological activity, including anticancer activity, of polyphenols and flavonoids obtained from black chokeberry fruits is efficient compared to the free forms when loaded in MCM-41 and ZnO-MCM-41 [497] Co-delivery of topotecan and quercetin by MSNs results in pH-responsive release, subsequently increasing the intracellular release in cancer cells. Ultimately, it induces notable molecular activation (structural changes in tumor cell: endoplasmic reticulum, nucleus, and mitochondria) leading to cancer cell death [264]. Similar evidence has been obtained with targeted delivery of epigallocatechin-3-gallate-loaded MSNs for breast cancer treatment in vivo [266].
6. Conclusions and Future Perspective
Engineered MSNs with a variety of nanostructures are important inorganic nanocarriers for drug delivery in nanomedicine applications. MSNs have various unique physiochemical properties, including high pore volume, high specific surface area and porosity. In addition, various organic functional groups can be used for their surface modification by facile processes. MSNs are generally accepted to have good biocompatibility, being safe and showing no-significant side effects. The toxicity of MSNs as in the case of any drug or nanomaterial depends on dose/concentration, material properties, application routes. The degree of toxicity is low as indicated by several studies if the synthesis is performed in optimized conditions or overdose is avoided. Additionally, according to many animal studies, the toxicity of MSNs can diminish by optimizing the synthesis parameters and surface modification. Most in vivo studies generate data stipulating that the suggested average dose of 50 mg/kg is well tolerated in animals and safe without any toxicity or apparent abnormalities. This is considered as an adequate dose to be used, e.g., in cancer therapy. This dose can be increased for oral route administration compared to intraperitoneal or intravenous injection. Importantly, the use of MSN-drug-loaded nanoformulations can allow the use of an even higher dose (three or more times) [174]. As with other nanomaterials, for future translation to clinical applications, the safety aspects of MSNs should be considered carefully for each type because many nanostructures are reported. Recently, the first clinical trial in humans was conducted with oral delivery of fenofibrate formulation based on the ordered mesoporous silica [33].
MSNs can be used as multifunctional targeted anticancer delivery systems, delivering a variety of drugs, therapeutic proteins, and antibodies. Furthermore, due to their nanoporous structure, MSNs have a high loading capacity for therapeutic agents and are excellent nanocarriers for internal- and external-responsive release of drugs (e.g., pH, GSH, redox, light, magnetic direction, and ultrasound). The available data indicate that the use of MSNs as prodrug nanocarriers can overcome the present barriers in their application: poor water solubility, low bioavailability, and insufficient targeting. Therefore, the available literature suggests a high potential of MSNs as natural prodrug delivery vehicles. The present pre-clinical and clinical tests show that MSNs are promising drug delivery carriers from a biocompatibility/safety perspective, opening the door towards the clinical nanomedicine application for cancer therapy.
For future research directions, we suggest the importance of co-delivery systems in which two or more anticancer natural prodrugs are combined, as well as exploring thousands of natural prodrugs that have not been thoroughly investigated yet. Furthermore, scientists can investigate loading MSNs with crude extract from plant materials. This can also be explored in synergistic therapy with crude extract containing many prodrug components together. Particularly promising prodrug substances are essential oils applied using MSN-based delivery systems. Their traditional use is only limited to cosmetics and some pharmaceutical applications. The essential oil nanoformulations will add value to cancer therapy.
The core–shell nanoformulations containing a core of MSNs loaded with prodrugs and a shell of organic substances, such as chitosan, Arabic gum, or others, are highly recommended to establish prodrug delivery systems. As an important parameter, the stability and dispersibility of nanoformulations should be taken into consideration because they affect the biological performance and therapeutic actions. Additionally, we think that the large-scale production for each type of MSNs will lead to obtaining safe material by optimizing and stabilizing the material parameters. In our opinion, animal and reported clinical studies open the doors to develop MSNs-based nanoformulations to be translated into clinical evaluations for cancers soon.
Author Contributions
Conceptualization, K.A. and W.L.; literature review, K.A.; writing—original draft preparation, K.A.; writing-review and editing, K.A. and W.L.; visualization, K.A. and W.L.; supervision, W.L. Both authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Institute of High Pressure Physics (IHPP), Polish Academy of Sciences (PAS), Poland and The APC was also funded by IHPP.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank the Institute of High Pressure Physics (IHPP), Polish Academy of Sciences (PAS), Poland, for the financial support.
Conflicts of Interest
The authors declare no conflict of interest regarding the publication of this paper.
References
- Vallet-Regi, M.; Rámila, A.; del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Sol-Gel Silica Nanoparticles in Medicine: A Natural Choice. Design, Synthesis and Products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef]
- Pednekar, P.P.; Godiyal, S.C.; Jadhav, K.R.; Kadam, V.J. Chapter 23—Mesoporous silica nanoparticles: A promising multifunctional drug delivery system. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 593–621. [Google Scholar]
- Le Hoang Doan, T.; Mai, N.X.D.; Matsumoto, K.; Tamanoi, F. Chapter 4—Tumor Targeting and Tumor Growth Inhibition Capability of Mesoporous Silica Nanoparticles in Mouse Models. In The Enzymes; Tamanoi, F., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 44, pp. 61–82. [Google Scholar]
- Mitran, R.-A.; Deaconu, M.; Matei, C.; Berger, D. Chapter 11—Mesoporous Silica as Carrier for Drug-Delivery Systems. In Nanocarriers for Drug Delivery; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 351–374. [Google Scholar]
- Mekaru, H.; Lu, J.; Tamanoi, F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv. Drug Deliv. Rev. 2015, 95, 40–49. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous silica nanoparticles in medicine—Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef]
- Kwon, S.; Singh, R.K.; Perez, R.A.; Abou Neel, E.A.; Kim, H.W.; Chrzanowski, W. Silica-based mesoporous nanoparticles for controlled drug delivery. J. Tissue Eng. 2013, 4, 2041731413503357. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef]
- Pérez-Esteve, É.; Ruiz-Rico, M.; de la Torre, C.; Llorca, E.; Sancenón, F.; Marcos, M.D.; Amorós, P.; Guillem, C.; Martínez-Máñez, R.; Barat, J.M. Stability of different mesoporous silica particles during an in vitro digestion. Microporous Mesoporous Mater. 2016, 230, 196–207. [Google Scholar] [CrossRef]
- Riikonen, J.; Xu, W.; Lehto, V.-P. Mesoporous systems for poorly soluble drugs—Recent trends. Int. J. Pharm. 2018, 536, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous silica materials: From physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef]
- Ambrogi, V.; Perioli, L.; Pagano, C.; Latterini, L.; Marmottini, F.; Ricci, M.; Rossi, C. MCM-41 for furosemide dissolution improvement. Microporous Mesoporous Mater. 2012, 147, 343–349. [Google Scholar] [CrossRef]
- Yang, G.; Li, Z.; Wu, F.; Chen, M.; Wang, R.; Zhu, H.; Li, Q.; Yuan, Y. Improving Solubility and Bioavailability of Breviscapine with Mesoporous Silica Nanoparticles Prepared Using Ultrasound-Assisted Solution-Enhanced Dispersion by Supercritical Fluids Method. Int. J. Nanomed. 2020, 15, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.A.; Ahern, R.J.; Dontireddy, R.; Ryan, K.B.; Crean, A.M. Mesoporous silica formulation strategies for drug dissolution enhancement: A review. Expert Opin. Drug Deliv. 2016, 13, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sailaja Chirravuri, S.V.; Shastri, N.R. Impact of surface area of silica particles on dissolution rate and oral bioavailability of poorly water soluble drugs: A case study with aceclofenac. Int. J. Pharm. 2014, 461, 459–468. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, X.; Zu, Y.; Wang, L.; Deng, Y.; Wu, M.; Wang, H. Enhanced Solubility and Bioavailability of Apigenin via Preparation of Solid Dispersions of Mesoporous Silica Nanoparticles. Iran. J. Pharm. Res. 2019, 18, 168–182. [Google Scholar]
- Zhang, Q.; Wang, X.; Li, P.-Z.; Nguyen, K.T.; Wang, X.-J.; Luo, Z.; Zhang, H.; Tan, N.S.; Zhao, Y. Biocompatible, Uniform, and Redispersible Mesoporous Silica Nanoparticles for Cancer-Targeted Drug Delivery In Vivo. Adv. Funct. Mater. 2014, 24, 2450–2461. [Google Scholar] [CrossRef]
- Argyo, C.; Weiss, V.; Bräuchle, C.; Bein, T. Multifunctional Mesoporous Silica Nanoparticles as a Universal Platform for Drug Delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, Biodistribution, and Drug-Delivery Efficiency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Cha, B.G.; Cho, Y.; Min, J.; Park, E.B.; Kang, S.J.; Kim, J. Extra-Large Pore Mesoporous Silica Nanoparticles for Directing in Vivo M2 Macrophage Polarization by Delivering IL-4. Nano Lett. 2017, 17, 2747–2756. [Google Scholar] [CrossRef] [PubMed]
- Malvindi, M.A.; Brunetti, V.; Vecchio, G.; Galeone, A.; Cingolani, R.; Pompa, P.P. SiO2 nanoparticles biocompatibility and their potential for gene delivery and silencing. Nanoscale 2012, 4, 486–495. [Google Scholar] [CrossRef]
- Abeer, M.M.; Rewatkar, P.; Qu, Z.; Talekar, M.; Kleitz, F.; Schmid, R.; Lindén, M.; Kumeria, T.; Popat, A. Silica nanoparticles: A promising platform for enhanced oral delivery of macromolecules. J. Control. Release 2020, 326, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Diab, R.; Canilho, N.; Pavel, I.A.; Haffner, F.B.; Girardon, M.; Pasc, A. Silica-based systems for oral delivery of drugs, macromolecules and cells. Adv. Colloid Interface Sci. 2017, 249, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, R.; Cheney, D.L.; Grunberger, J.W.; Yazdimamaghani, M.; Jedrzkiewicz, J.; Isaacson, K.J.; Dobrovolskaia, M.A.; Ghandehari, H. One-year chronic toxicity evaluation of single dose intravenously administered silica nanoparticles in mice and their Ex vivo human hemocompatibility. J. Control. Release 2020, 324, 471–481. [Google Scholar] [CrossRef]
- Fu, C.; Liu, T.; Li, L.; Liu, H.; Chen, D.; Tang, F. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 2013, 34, 2565–2575. [Google Scholar] [CrossRef]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar] [CrossRef]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Manzano, M. Mesoporous Silica Nanoparticles for Drug Delivery: Current Insights. Molecules 2018, 23, 47. [Google Scholar] [CrossRef]
- Bukara, K.; Schueller, L.; Rosier, J.; Martens, M.A.; Daems, T.; Verheyden, L.; Eelen, S.; Van Speybroeck, M.; Libanati, C.; Martens, J.A.; et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur. J. Pharm. Biopharm. 2016, 108, 220–225. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Marston, A. The Search for New Drugs from Higher Plants. Chim. Int. J. Chem. 2007, 61, 322–326. [Google Scholar] [CrossRef][Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Ji, H.-F.; Li, X.-J.; Zhang, H.-Y. Natural products and drug discovery. EMBO Rep. 2009, 10, 194–200. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar] [CrossRef]
- Aljuffali, I.A.; Fang, C.L.; Chen, C.H.; Fang, J.Y. Nanomedicine as a Strategy for Natural Compound Delivery to Prevent and Treat Cancers. Curr. Pharm. Des. 2016, 22, 4219–4231. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. The uses of resveratrol for neurological diseases treatment and insights for nanotechnology based-drug delivery systems. Int. J. Pharm. 2020, 119832. [Google Scholar] [CrossRef]
- AbouAitah, K.; Swiderska-Sroda, A.; Farghali, A.A.; Wojnarowicz, J.; Stefanek, A.; Gierlotka, S.; Opalinska, A.; Allayeh, A.K.; Ciach, T.; Lojkowski, W. Folic acid-conjugated mesoporous silica particles as nanocarriers of natural prodrugs for cancer targeting and antioxidant action. Oncotarget 2018, 9, 26466–26490. [Google Scholar] [CrossRef]
- AbouAitah, K.; Hassan, H.A.; Swiderska-Sroda, A.; Gohar, L.; Shaker, O.G.; Wojnarowicz, J.; Opalinska, A.; Smalc-Koziorowska, J.; Gierlotka, S.; Lojkowski, W. Targeted Nano-Drug Delivery of Colchicine against Colon Cancer Cells by Means of Mesoporous Silica Nanoparticles. Cancers 2020, 12, 144. [Google Scholar] [CrossRef]
- Shahein, S.A.; Aboul-Enein, A.M.; Higazy, I.M.; Abou-Elella, F.; Lojkowski, W.; Ahmed, E.R.; Mousa, S.A.; AbouAitah, K. Targeted anticancer potential against glioma cells of thymoquinone delivered by mesoporous silica core-shell nanoformulations with pH-dependent release. Int. J. Nanomed. 2019, 14, 5503–5526. [Google Scholar] [CrossRef]
- Bisht, S.; Feldmann, G.; Soni, S.; Ravi, R.; Karikar, C.; Maitra, A.; Maitra, A. Polymeric nanoparticle-encapsulated curcumin (“nanocurcumin”): A novel strategy for human cancer therapy. J. Nanobiotechnol. 2007, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Pool, H.; Quintanar, D.; Figueroa, J.D.D.; Marinho Mano, C.; Bechara, J.E.H.; Godínez, L.A.; Mendoza, S. Antioxidant Effects of Quercetin and Catechin Encapsulated into PLGA Nanoparticles. J. Nanomater. 2012, 2012, 145380. [Google Scholar] [CrossRef]
- Fang, Q.; Sculley, J.; Zhou, H.C.J.; Zhu, G. 5.01—Porous Metal–Organic Frameworks. In Comprehensive Nanoscience and Technology; Andrews, D.L., Scholes, G.D., Wiederrecht, G.P., Eds.; Academic Press: Amsterdam, The Netherlands, 2011; pp. 1–20. [Google Scholar]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Inagaki, S.; Fukushima, Y.; Kuroda, K. Synthesis of highly ordered mesoporous materials from a layered polysilicate. J. Chem. Soc. Chem. Commun. 1993, 680–682. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-Based Mesoporous Organic–Inorganic Hybrid Materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Ryoo, R.; Kim, J.M.; Ko, C.H.; Shin, C.H. Disordered Molecular Sieve with Branched Mesoporous Channel Network. J. Phys. Chem. 1996, 100, 17718–17721. [Google Scholar] [CrossRef]
- Zhou, B.; Li, C.Y.; Qi, N.; Jiang, M.; Wang, B.; Chen, Z.Q. Pore structure of mesoporous silica (KIT-6) synthesized at different temperatures using positron as a nondestructive probe. Appl. Surf. Sci. 2018, 450, 31–37. [Google Scholar] [CrossRef]
- Deka, J.R.; Lin, Y.-H.; Kao, H.-M. Ordered cubic mesoporous silica KIT-5 functionalized with carboxylic acid groups for dye removal. RSC Adv. 2014, 4, 49061–49069. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548. [Google Scholar] [CrossRef] [PubMed]
- Widenmeyer, M.; Anwander, R. Pore Size Control of Highly Ordered Mesoporous Silica MCM-48. Chem. Mater. 2002, 14, 1827–1831. [Google Scholar] [CrossRef]
- Lin, L.-C.; Thirumavalavan, M.; Wang, Y.-T.; Lee, J.-F. Surface area and pore size tailoring of mesoporous silica materials by different hydrothermal treatments and adsorption of heavy metal ions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 369, 223–231. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Cha, D.; Zhang, X.; Basset, J.M. High-Surface-Area Silica Nanospheres (KCC-1) with a Fibrous Morphology. Angew. Chem. Int. Ed. 2010, 49, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Bayal, N.; Singh, B.; Singh, R.; Polshettiwar, V. Size and Fiber Density Controlled Synthesis of Fibrous Nanosilica Spheres (KCC-1). Sci. Rep. 2016, 6, 24888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qian, Y.; Ahn, W.-S. Catalytic dehydrogenation of formic acid over palladium nanoparticles immobilized on fibrous mesoporous silica KCC-1. Chin. J. Catal. 2019, 40, 1704–1712. [Google Scholar] [CrossRef]
- Zarei, F.; Marjani, A.; Soltani, R. Novel and green nanocomposite-based adsorbents from functionalised mesoporous KCC-1 and chitosan-oleic acid for adsorption of Pb(II). Eur. Polym. J. 2019, 119, 400–409. [Google Scholar] [CrossRef]
- Soleymani, J.; Hasanzadeh, M.; Somi, M.H.; Shadjou, N.; Jouyban, A. Highly sensitive and specific cytosensing of HT 29 colorectal cancer cells using folic acid functionalized-KCC-1 nanoparticles. Biosens. Bioelectron. 2019, 132, 122–131. [Google Scholar] [CrossRef]
- Abbasy, L.; Mohammadzadeh, A.; Hasanzadeh, M.; Ehsani, M.; Mokhtarzadeh, A. Biosensing of prostate specific antigen (PSA) in human plasma samples using biomacromolecule encapsulation into KCC-1-npr-NH2: A new platform for prostate cancer detection. Int. J. Biol. Macromol. 2020, 154, 584–595. [Google Scholar] [CrossRef]
- Ali, Z.; Tian, L.; Zhao, P.; Zhang, B.; Ali, N.; Khan, M.; Zhang, Q. Immobilization of lipase on mesoporous silica nanoparticles with hierarchical fibrous pore. J. Mol. Catal. B Enzym. 2016, 134, 129–135. [Google Scholar] [CrossRef]
- Follmann, H.D.M.; Oliveira, O.N.; Martins, A.C.; Lazarin-Bidóia, D.; Nakamura, C.V.; Rubira, A.F.; Silva, R.; Asefa, T. Nanofibrous silica microparticles/polymer hybrid aerogels for sustained delivery of poorly water-soluble camptothecin. J. Colloid Interface Sci. 2020, 567, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Wang, D.; Xu, L.; Wu, P. One-pot synthesis of primary amides on bifunctional Rh(OH)x/TS-1@KCC-1 catalysts. Chin. J. Catal. 2013, 34, 2057–2065. [Google Scholar] [CrossRef]
- AbouAitah, K.E.A.; Farghali, A.A.; Swiderska-Sroda, A.; Lojkowski, W.; Razin, A.M. Mesoporous silica materials in drug delivery system: pH/glutathione-responsive release of poorly water-soluble pro-drug quercetin from two and three-dimensional pore-structure nanoparticles. J. Nanomed. Nanotechnol. 2016, 7. [Google Scholar] [CrossRef]
- AbouAitah, K.E.A.; Farghali, A.A.; Swiderska-Sroda, A.; Lojkowski, W.; Razin, A.M. pH-controlled release system for curcumin based on functionalized dendritic mesoporous silica nanoparticles. J. Nanomed. Nanotechnol. 2016, 7, 1–11. [Google Scholar]
- Lin, J.; Peng, C.; Ravi, S.; Siddiki, A.K.M.N.A.; Zheng, J.; Balkus, K.J. Biphenyl Wrinkled Mesoporous Silica Nanoparticles for pH-Responsive Doxorubicin Drug Delivery. Materials 2020, 13, 1998. [Google Scholar] [CrossRef]
- Moon, D.-S.; Lee, J.-K. Tunable Synthesis of Hierarchical Mesoporous Silica Nanoparticles with Radial Wrinkle Structure. Langmuir 2012, 28, 12341–12347. [Google Scholar] [CrossRef]
- Wang, R.; Habib, E.; Zhu, X.X. Synthesis of wrinkled mesoporous silica and its reinforcing effect for dental resin composites. Dent. Mater. 2017, 33, 1139–1148. [Google Scholar] [CrossRef]
- Maity, A.; Polshettiwar, V. Dendritic Fibrous Nanosilica for Catalysis, Energy Harvesting, Carbon Dioxide Mitigation, Drug Delivery, and Sensing. ChemSusChem 2017, 10, 3866–3913. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Choma, J.; Jaroniec, M. Major advances in the development of ordered mesoporous materials. Chem. Commun. 2020, 56, 7836–7848. [Google Scholar] [CrossRef]
- Xu, C.; Lei, C.; Yu, C. Mesoporous Silica Nanoparticles for Protein Protection and Delivery. Front. Chem. 2019, 7, 290. [Google Scholar] [CrossRef]
- Douroumis, D.; Onyesom, I.; Maniruzzaman, M.; Mitchell, J. Mesoporous silica nanoparticles in nanotechnology. Crit. Rev. Biotechnol. 2013, 33, 229–245. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
- Farjadian, F.; Roointan, A.; Mohammadi-Samani, S.; Hosseini, M. Mesoporous silica nanoparticles: Synthesis, pharmaceutical applications, biodistribution, and biosafety assessment. Chem. Eng. J. 2019, 359, 684–705. [Google Scholar] [CrossRef]
- Castillo, R.R.; Lozano, D.; González, B.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery: An update. Expert Opin. Drug Deliv. 2019, 16, 415–439. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Co-Delivery of Drugs and Nucleic Acids in Oncology: A Review. Pharmaceutics 2020, 12, 526. [Google Scholar] [CrossRef]
- Aquib, M.; Farooq, M.A.; Banerjee, P.; Akhtar, F.; Filli, M.S.; Boakye-Yiadom, K.O.; Kesse, S.; Raza, F.; Maviah, M.B.J.; Mavlyanova, R.; et al. Targeted and stimuli–responsive mesoporous silica nanoparticles for drug delivery and theranostic use. J. Biomed. Mater. Res. Part A 2019, 107, 2643–2666. [Google Scholar] [CrossRef]
- Hadipour Moghaddam, S.P.; Mohammadpour, R.; Ghandehari, H. In vitro and in vivo evaluation of degradation, toxicity, biodistribution, and clearance of silica nanoparticles as a function of size, porosity, density, and composition. J. Control. Release 2019, 311–312, 1–15. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef]
- Nieto, A.; Colilla, M.; Balas, F.; Vallet-Regí, M. Surface Electrochemistry of Mesoporous Silicas as a Key Factor in the Design of Tailored Delivery Devices. Langmuir 2010, 26, 5038–5049. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Rahikkala, A.; Rosenholm, J.M.; Santos, H.A. Chapter 16—Biofunctionalized Mesoporous Silica Nanomaterials for Targeted Drug Delivery. In Biomedical Applications of Functionalized Nanomaterials; Sarmento, B., das Neves, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 489–520. [Google Scholar]
- Stein, A.; Melde, B.J.; Schroden, R.C. Hybrid Inorganic–Organic Mesoporous Silicates—Nanoscopic Reactors Coming of Age. Adv. Mater. 2000, 12, 1403–1419. [Google Scholar] [CrossRef]
- Tarn, D.; Ashley, C.E.; Xue, M.; Carnes, E.C.; Zink, J.I.; Brinker, C.J. Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility. Acc. Chem. Res. 2013, 46, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Wiench, J.W.; Yoo, J.-C.; Pruski, M.; Lin, V.S.Y. Organic Functionalization and Morphology Control of Mesoporous Silicas via a Co-Condensation Synthesis Method. Chem. Mater. 2003, 15, 4247–4256. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Zink, J.I.; Tamanoi, F. Mesoporous Silica Nanoparticles as a Delivery System for Hydrophobic Anticancer Drugs. Small 2007, 3, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Sayari, A.; Hamoudi, S. Periodic Mesoporous Silica-Based Organic−Inorganic Nanocomposite Materials. Chem. Mater. 2001, 13, 3151–3168. [Google Scholar] [CrossRef]
- Wu, S.-H.; Hung, Y.; Mou, C.-Y. Mesoporous silica nanoparticles as nanocarriers. Chem. Commun. 2011, 47, 9972–9985. [Google Scholar] [CrossRef] [PubMed]
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharm. Sci. 2013, 8, 1–10. [Google Scholar] [CrossRef]
- Borbás, E.; Sinkó, B.; Tsinman, O.; Tsinman, K.; Kiserdei, É.; Démuth, B.; Balogh, A.; Bodák, B.; Domokos, A.; Dargó, G.; et al. Investigation and Mathematical Description of the Real Driving Force of Passive Transport of Drug Molecules from Supersaturated Solutions. Mol. Pharm. 2016, 13, 3816–3826. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of Endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed]
- Rewatkar, P.; Kumeria, T.; Popat, A. Chapter 5—Size, shape and surface charge considerations of orally delivered nanomedicines. In Nanotechnology for Oral Drug Delivery; Martins, J.P., Santos, H.A., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 143–176. [Google Scholar]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle Uptake: The Phagocyte Problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef]
- Lu, F.; Wu, S.-H.; Hung, Y.; Mou, C.-Y. Size Effect on Cell Uptake in Well-Suspended, Uniform Mesoporous Silica Nanoparticles. Small 2009, 5, 1408–1413. [Google Scholar] [CrossRef]
- Gratton, S.E.A.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef]
- Min, Y.; Akbulut, M.; Kristiansen, K.; Golan, Y.; Israelachvili, J. The role of interparticle and external forces in nanoparticle assembly. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 38–49. [Google Scholar]
- Wei, P.; Zhang, L.; Lu, Y.; Man, N.; Wen, L. C60 (Nd) nanoparticles enhance chemotherapeutic susceptibility of cancer cells by modulation of autophagy. Nanotechnology 2010, 21, 495101. [Google Scholar] [CrossRef]
- Song, L.; Wengang, L.; Niveen, M.K. Stimuli responsive nanomaterials for controlled release applications. Nanotechnol. Rev. 2012, 1, 493–513. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.K.; Islam, M.R.; Choudhury, Z.S.; Mostafa, A.; Kadir, M.F. Nanotechnology based approaches in cancer therapeutics. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 043001. [Google Scholar] [CrossRef]
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Maruyama, K. Intracellular targeting delivery of liposomal drugs to solid tumors based on EPR effects. Adv. Drug Deliv. Rev. 2011, 63, 161–169. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Haddick, L.; Zhang, W.; Reinhard, S.; Möller, K.; Engelke, H.; Wagner, E.; Bein, T. Particle-Size-Dependent Delivery of Antitumoral miRNA Using Targeted Mesoporous Silica Nanoparticles. Pharmaceutics 2020, 12, 505. [Google Scholar] [CrossRef]
- Yang, Y.; Karmakar, S.; Zhang, J.; Yu, M.; Mitter, N.; Yu, C. Synthesis of SBA-15 rods with small sizes for enhanced cellular uptake. J. Mater. Chem. B 2014, 2, 4929–4934. [Google Scholar] [CrossRef]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical Properties Determine Nanomaterial Cellular Uptake, Transport, and Fate. Acc. Chem. Res. 2013, 46, 622–631. [Google Scholar] [CrossRef]
- Panariti, A.; Miserocchi, G.; Rivolta, I. The effect of nanoparticle uptake on cellular behavior: Disrupting or enabling functions? Nanotechnol. Sci. Appl. 2012, 5, 87–100. [Google Scholar] [CrossRef]
- Adjei, I.M.; Sharma, B.; Labhasetwar, V. Nanoparticles: Cellular uptake and cytotoxicity. Adv. Exp. Med. Biol 2014, 811, 73–91. [Google Scholar] [CrossRef]
- Marano, F.; Hussain, S.; Rodrigues-Lima, F.; Baeza-Squiban, A.; Boland, S. Nanoparticles: Molecular targets and cell signalling. Arch. Toxicol. 2011, 85, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Austin, G.A.; Chonn, A.; Lin, L.; Lee, K.C. Uptake of liposomes by cultured mouse bone marrow macrophages: Influence of liposome composition and size. Biochim. Biophys. Acta (BBA) Biomembr. 1991, 1061, 56–64. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Miranda, O.R.; Thompson, M.A.; Pabelick, C.M.; Bhattacharya, R.; Robertson, J.D.; Rotello, V.M.; Prakash, Y.S.; Mukherjee, P. Effect of Nanoparticle Surface Charge at the Plasma Membrane and Beyond. Nano Lett. 2010, 10, 2543–2548. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Bae, S.C.; Granick, S. Nanoparticle-induced surface reconstruction of phospholipid membranes. Proc. Natl. Acad. Sci. USA 2008, 105, 18171–18175. [Google Scholar] [CrossRef]
- Dausend, J.; Musyanovych, A.; Dass, M.; Walther, P.; Schrezenmeier, H.; Landfester, K.; Mailänder, V. Uptake Mechanism of Oppositely Charged Fluorescent Nanoparticles in HeLa Cells. Macromol. Biosci. 2008, 8, 1135–1143. [Google Scholar] [CrossRef]
- Jambhrunkar, S.; Qu, Z.; Popat, A.; Yang, J.; Noonan, O.; Acauan, L.; Ahmad Nor, Y.; Yu, C.; Karmakar, S. Effect of Surface Functionality of Silica Nanoparticles on Cellular Uptake and Cytotoxicity. Mol. Pharm. 2014, 11, 3642–3655. [Google Scholar] [CrossRef]
- Baghirov, H.; Karaman, D.; Viitala, T.; Duchanoy, A.; Lou, Y.-R.; Mamaeva, V.; Pryazhnikov, E.; Khiroug, L.; de Lange Davies, C.; Sahlgren, C.; et al. Feasibility Study of the Permeability and Uptake of Mesoporous Silica Nanoparticles across the Blood-Brain Barrier. PLoS ONE 2016, 11, e0160705. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Nieweg, J.A.; Zhao, Y.; Lin, V.S.Y. Biocompatible mesoporous silica nanoparticles with different morphologies for animal cell membrane penetration. Chem. Eng. J. 2008, 137, 23–29. [Google Scholar] [CrossRef]
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 2010, 31, 438–448. [Google Scholar] [CrossRef]
- Dias, D.R.; Moreira, A.F.; Correia, I.J. The effect of the shape of gold core–mesoporous silica shell nanoparticles on the cellular behavior and tumor spheroid penetration. J. Mater. Chem. B 2016, 4, 7630–7640. [Google Scholar] [CrossRef] [PubMed]
- Pada, A.-K.; Desai, D.; Sun, K.; Prakirth Govardhanam, N.; Törnquist, K.; Zhang, J.; Rosenholm, J.M. Comparison of Polydopamine-Coated Mesoporous Silica Nanorods and Spheres for the Delivery of Hydrophilic and Hydrophobic Anticancer Drugs. Int. J. Mol. Sci. 2019, 20, 3408. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Li, L.; Tang, F. Shape matters when engineering mesoporous silica-based nanomedicines. Biomater. Sci. 2016, 4, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Niculescu, V.-C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Walsh, L.J.; Xu, C. Biomedical application of mesoporous silica nanoparticles as delivery systems: A biological safety perspective. J. Mater. Chem. B 2020, 8, 9863–9876. [Google Scholar] [CrossRef]
- Trewyn, B.G.; Giri, S.; Slowing, I.I.; Lin, V.S.Y. Mesoporous silica nanoparticle based controlled release, drug delivery, and biosensor systems. Chem. Commun. 2007, 3236–3245. [Google Scholar] [CrossRef]
- Manzano, M.; Colilla, M.; Vallet-Regí, M. Drug delivery from ordered mesoporous matrices. Expert Opin Drug Deliv 2009, 6, 1383–1400. [Google Scholar] [CrossRef]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous Silica Nanoparticles for Intracellular Controlled Drug Delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef]
- Asefa, T.; Tao, Z. Biocompatibility of Mesoporous Silica Nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Ozgur, E.; Bayindir, M. Impact of mesoporous silica nanoparticle surface functionality on hemolytic activity, thrombogenicity and non-specific protein adsorption. J. Mater. Chem. B 2013, 1, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cano, I.; Candela-Noguera, V.; Herrera, G.; Cejalvo, J.M.; Lluch, A.; Marcos, M.D.; Sancenon, F.; Eroles, P.; Martínez-Máñez, R. Biocompatibility and internalization assessment of bare and functionalised mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2021, 310. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Gao, F.; Li, Y.; Shi, J. In vivo Biodistribution and Urinary Excretion of Mesoporous Silica Nanoparticles: Effects of Particle Size and PEGylation. Small 2011, 7, 271–280. [Google Scholar] [CrossRef]
- Yu, T.; Hubbard, D.; Ray, A.; Ghandehari, H. In vivo biodistribution and pharmacokinetics of silica nanoparticles as a function of geometry, porosity and surface characteristics. J. Control. Release 2012, 163, 46–54. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Fu, C.; Tan, L.; Meng, X.; Liu, H. Biodistribution, excretion, and toxicity of mesoporous silica nanoparticles after oral administration depend on their shape. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1915–1924. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef]
- Janßen, H.C.; Warwas, D.P.; Dahlhaus, D.; Meißner, J.; Taptimthong, P.; Kietzmann, M.; Behrens, P.; Reifenrath, J.; Angrisani, N. In vitro and in vivo accumulation of magnetic nanoporous silica nanoparticles on implant materials with different magnetic properties. J. Nanobiotechnol. 2018, 16, 96. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Yang, B.; Li, J.; Xu, L.; Liu, H.; Li, S.; Xu, J.; Yang, M.; Wei, M. Evaluation of biomimetically synthesized mesoporous silica nanoparticles as drug carriers: Structure, wettability, degradation, biocompatibility and brain distribution. Mater. Sci. Eng. C 2019, 94, 453–464. [Google Scholar] [CrossRef]
- Cho, M.; Cho, W.-S.; Choi, M.; Kim, S.J.; Han, B.S.; Kim, S.H.; Kim, H.O.; Sheen, Y.Y.; Jeong, J. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol. Lett. 2009, 189, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Bauernfeind, F.; Halle, A.; Samstad, E.O.; Kono, H.; Rock, K.L.; Fitzgerald, K.A.; Latz, E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 2008, 9, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.H.P.; Handreck, K.A. Silica In Soils, Plants, and Animals. In Advances in Agronomy; Norman, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1967; Volume 19, pp. 107–149. [Google Scholar]
- Rosenholm, J.M.; Mamaeva, V.; Sahlgren, C.; Lindén, M. Nanoparticles in targeted cancer therapy: Mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine 2012, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R. Silicon: The Health Benefits of a Metalloid. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 451–473. [Google Scholar]
- Ehrlich, H.; Demadis, K.D.; Pokrovsky, O.S.; Koutsoukos, P.G. Modern Views on Desilicification: Biosilica and Abiotic Silica Dissolution in Natural and Artificial Environments. Chem. Rev. 2010, 110, 4656–4689. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.R. The chemistry of silica and its potential health benefits. J. Nutr. Health Aging 2007, 11, 94–97. [Google Scholar] [PubMed]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef]
- Carlisle, E.M. Silicon: A Possible Factor in Bone Calcification. Science 1970, 167, 279. [Google Scholar] [CrossRef]
- Jugdaohsingh, R. Silicon and bone health. J. Nutr Health Aging 2007, 11, 99–110. [Google Scholar]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef]
- Quan, G.; Pan, X.; Wang, Z.; Wu, Q.; Li, G.; Dian, L.; Chen, B.; Wu, C. Lactosaminated mesoporous silica nanoparticles for asialoglycoprotein receptor targeted anticancer drug delivery. J. Nanobiotechnol. 2015, 13, 7. [Google Scholar] [CrossRef]
- Napierska, D.; Thomassen, L.C.J.; Lison, D.; Martens, J.A.; Hoet, P.H. The nanosilica hazard: Another variable entity. Part. Fibre Toxicol. 2010, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Di Pasqua, A.J.; Sharma, K.K.; Shi, Y.-L.; Toms, B.B.; Ouellette, W.; Dabrowiak, J.C.; Asefa, T. Cytotoxicity of mesoporous silica nanomaterials. J. Inorg. Biochem. 2008, 102, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yun, H.-S.; Kim, S.-H. The comparative effects of mesoporous silica nanoparticles and colloidal silica on inflammation and apoptosis. Biomaterials 2011, 32, 9434–9443. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, L.; Jin, M.; Du, Z.; Liu, X.; Guo, C.; Li, Y.; Huang, P.; Sun, Z. Size-dependent cytotoxicity of amorphous silica nanoparticles in human hepatoma HepG2 cells. Toxicol. In Vitro 2011, 25, 1343–1352. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Teng, X.; Huang, X.; Liu, H.; Chen, D.; Ren, J.; He, J.; Tang, F. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials 2011, 32, 1657–1668. [Google Scholar] [CrossRef]
- Carvalho, G.C.; Sábio, R.M.; de Cássia Ribeiro, T.; Monteiro, A.S.; Pereira, D.V.; Ribeiro, S.J.L.; Chorilli, M. Highlights in Mesoporous Silica Nanoparticles as a Multifunctional Controlled Drug Delivery Nanoplatform for Infectious Diseases Treatment. Pharm. Res. 2020, 37, 191. [Google Scholar] [CrossRef]
- Petushkov, A.; Ndiege, N.; Salem, A.K.; Larsen, S.C. Chapter 7—Toxicity of Silica Nanomaterials: Zeolites, Mesoporous Silica, and Amorphous Silica Nanoparticles. In Advances in Molecular Toxicology; Fishbein, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 4, pp. 223–266. [Google Scholar]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef]
- Nash, T.; Allison, A.C.; Harington, J.S. Physico-Chemical Properties of Silica in Relation to its Toxicity. Nature 1966, 210, 259–261. [Google Scholar] [CrossRef]
- Zhang, H.; Dunphy, D.R.; Jiang, X.; Meng, H.; Sun, B.; Tarn, D.; Xue, M.; Wang, X.; Lin, S.; Ji, Z.; et al. Processing Pathway Dependence of Amorphous Silica Nanoparticle Toxicity: Colloidal vs Pyrolytic. J. Am. Chem. Soc. 2012, 134, 15790–15804. [Google Scholar] [CrossRef]
- Bhavsar, D.; Patel, V.; Sawant, K. Systematic investigation of in vitro and in vivo safety, toxicity and degradation of mesoporous silica nanoparticles synthesized using commercial sodium silicate. Microporous Mesoporous Mater. 2019, 284, 343–352. [Google Scholar] [CrossRef]
- Rascol, E.; Daurat, M.; Da Silva, A.; Maynadier, M.; Dorandeu, C.; Charnay, C.; Garcia, M.; Lai-Kee-Him, J.; Bron, P.; Auffan, M.; et al. Biological Fate of Fe3O4 Core-Shell Mesoporous Silica Nanoparticles Depending on Particle Surface Chemistry. Nanomaterials 2017, 7, 162. [Google Scholar] [CrossRef]
- Rascol, E.; Pisani, C.; Dorandeu, C.; Nyalosaso, J.L.; Charnay, C.; Daurat, M.; Da Silva, A.; Devoisselle, J.-M.; Gaillard, J.-C.; Armengaud, J.; et al. Biosafety of Mesoporous Silica Nanoparticles. Biomimetics 2018, 3, 22. [Google Scholar] [CrossRef]
- Nasr, S.S.; Nasra, M.M.A.; Hazzah, H.A.; Abdallah, O.Y. Mesoporous silica nanoparticles, a safe option for silymarin delivery: Preparation, characterization, and in vivo evaluation. Drug Deliv. Transl. Res. 2019, 9, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Rawat, N.; Sandhya; Subaharan, K.; Eswaramoorthy, M.; Kaul, G. Comparative in vivo toxicity assessment places multiwalled carbon nanotubes at a higher level than mesoporous silica nanoparticles. Toxicol. Ind. Health 2016, 33, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, M.S.; Lee, D.; Kwon, T.K.; Khang, D.; Yun, H.S.; Kim, S.H. The comparative immunotoxicity of mesoporous silica nanoparticles and colloidal silica nanoparticles in mice. Int. J. Nanomed. 2013, 8, 147–158. [Google Scholar] [CrossRef]
- Limnell, T.; Santos, H.A.; Mäkilä, E.; Heikkilä, T.; Salonen, J.; Murzin, D.Y.; Kumar, N.; Laaksonen, T.; Peltonen, L.; Hirvonen, J. Drug delivery formulations of ordered and nonordered mesoporous silica: Comparison of three drug loading methods. J. Pharm. Sci. 2011, 100, 3294–3306. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Lindén, M. Towards establishing structure-activity relationships for mesoporous silica in drug delivery applications. J. Control. Release 2008, 128, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, L.; Wang, J.; Jiang, X.; Li, X.; Hu, Z.; Ji, Y.; Wu, X.; Chen, C. Mesoporous Silica-Coated Gold Nanorods as a Light-Mediated Multifunctional Theranostic Platform for Cancer Treatment. Adv. Mater. 2012, 24, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Chen, L.; Li, C.; He, C.; He, L.; Kong, L. Functionalization of Hollow Mesoporous Silica Nanoparticles for Improved 5-FU Loading. J. Nanomater. 2015, 2015, 872035. [Google Scholar] [CrossRef]
- Doadrio, J.C.; Sousa, E.M.B.; Izquierdo-Barba, I.; Doadrio, A.L.; Perez-Pariente, J.; Vallet-Regí, M. Functionalization of mesoporous materials with long alkyl chains as a strategy for controlling drug delivery pattern. J. Mater. Chem. 2006, 16, 462–466. [Google Scholar] [CrossRef]
- Balas, F.; Manzano, M.; Horcajada, P.; Vallet-Regí, M. Confinement and Controlled Release of Bisphosphonates on Ordered Mesoporous Silica-Based Materials. J. Am. Chem. Soc. 2006, 128, 8116–8117. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.H.; Smått, J.-H.; Govardhanam, N.P.; Ibrahim, H.M.; Ismael, H.R.; Afouna, M.I.; Samy, A.M.; Rosenholm, J.M. Formulation and optimization of drug-loaded mesoporous silica nanoparticle-based tablets to improve the dissolution rate of the poorly water-soluble drug silymarin. Eur. J. Pharm. Sci. 2020, 142, 105103. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liang, S.; Long, M.; Xu, H. Mesoporous silica nanoparticles as potential carriers for enhanced drug solubility of paclitaxel. Mater. Sci. Eng. C 2017, 78, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Cheng, C.-A.; Deng, T.; Lin, F.-C.; Cai, Y.; Zink, J.I. Supramolecular Nanomachines as Stimuli-Responsive Gatekeepers on Mesoporous Silica Nanoparticles for Antibiotic and Cancer Drug Delivery. Theranostics 2019, 9, 3341–3364. [Google Scholar] [CrossRef]
- Torney, F.; Trewyn, B.G.; Lin, V.S.Y.; Wang, K. Mesoporous silica nanoparticles deliver DNA and chemicals into plants. Nat. Nanotechnol. 2007, 2, 295–300. [Google Scholar] [CrossRef]
- Giri, S.; Trewyn, B.G.; Stellmaker, M.P.; Lin, V.S.Y. Stimuli-Responsive Controlled-Release Delivery System Based on Mesoporous Silica Nanorods Capped with Magnetic Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 5038–5044. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Trewyn, B.G.; Lin, V.S.Y. Mesoporous silica nanoparticles: Structural design and applications. J. Mater. Chem. 2010, 20, 7924–7937. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.Y. A Mesoporous Silica Nanosphere-Based Carrier System with Chemically Removable CdS Nanoparticle Caps for Stimuli-Responsive Controlled Release of Neurotransmitters and Drug Molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef]
- Chang, B.; Sha, X.; Guo, J.; Jiao, Y.; Wang, C.; Yang, W. Thermo and pH dual responsive, polymer shell coated, magnetic mesoporous silica nanoparticles for controlled drug release. J. Mater. Chem. 2011, 21, 9239–9247. [Google Scholar] [CrossRef]
- Chang, B.; Chen, D.; Wang, Y.; Chen, Y.; Jiao, Y.; Sha, X.; Yang, W. Bioresponsive Controlled Drug Release Based on Mesoporous Silica Nanoparticles Coated with Reductively Sheddable Polymer Shell. Chem. Mater. 2013, 25, 574–585. [Google Scholar] [CrossRef]
- Croissant, J.G.; Zhang, D.; Alsaiari, S.; Lu, J.; Deng, L.; Tamanoi, F.; AlMalik, A.M.; Zink, J.I.; Khashab, N.M. Protein-gold clusters-capped mesoporous silica nanoparticles for high drug loading, autonomous gemcitabine/doxorubicin co-delivery, and in-vivo tumor imaging. J. Control. Release 2016, 229, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Aptamer functionalized curcumin-loaded human serum albumin (HSA) nanoparticles for targeted delivery to HER-2 positive breast cancer cells. Int. J. Biol. Macromol. 2019, 130, 109–116. [Google Scholar] [CrossRef]
- Iraji, S.; Ganji, F.; Rashidi, L. Surface modified mesoporous silica nanoparticles as sustained-release gallic acid nano-carriers. J. Drug Deliv. Sci. Technol. 2018, 47, 468–476. [Google Scholar] [CrossRef]
- Ma’mani, L.; Nikzad, S.; Kheiri-Manjili, H.; Al-Musawi, S.; Saeedi, M.; Askarlou, S.; Foroumadi, A.; Shafiee, A. Curcumin-loaded guanidine functionalized PEGylated I3ad mesoporous silica nanoparticles KIT-6: Practical strategy for the breast cancer therapy. Eur. J. Med. Chem. 2014, 83, 646–654. [Google Scholar] [CrossRef]
- Szegedi, Á.; Shestakova, P.; Trendafilova, I.; Mihayi, J.; Tsacheva, I.; Mitova, V.; Kyulavska, M.; Koseva, N.; Momekova, D.; Konstantinov, S.; et al. Modified mesoporous silica nanoparticles coated by polymer complex as novel curcumin delivery carriers. J. Drug Deliv. Sci. Technol. 2019, 49, 700–712. [Google Scholar] [CrossRef]
- Sattary, M.; Amini, J.; Hallaj, R. Antifungal activity of the lemongrass and clove oil encapsulated in mesoporous silica nanoparticles against wheat’s take-all disease. Pestic. Biochem. Physiol. 2020, 170, 104696. [Google Scholar] [CrossRef]
- Song, Y.; Zhou, B.; Du, X.; Wang, Y.; Zhang, J.; Ai, Y.; Xia, Z.; Zhao, G. Folic acid (FA)-conjugated mesoporous silica nanoparticles combined with MRP-1 siRNA improves the suppressive effects of myricetin on non-small cell lung cancer (NSCLC). Biomed. Pharmacother. 2020, 125, 109561. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Liu, Y.; Fan, L.; Lin, L.; Xu, Y.; Chen, S.; Shao, J. pH-Sensitive mesoporous silica nanoparticles anticancer prodrugs for sustained release of ursolic acid and the enhanced anti-cancer efficacy for hepatocellular carcinoma cancer. Eur. J. Pharm. Sci. 2017, 96, 456–463. [Google Scholar] [CrossRef]
- Lungare, S.; Hallam, K.; Badhan, R.K.S. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 513, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous silica as topical nanocarriers for quercetin: Characterization and in vitro studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef]
- Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Tumor targeted delivery of umbelliferone via a smart mesoporous silica nanoparticles controlled-release drug delivery system for increased anticancer efficiency. Mater. Sci. Eng. C 2020, 116, 111239. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, B.; Shang, Y.; Huang, X.; Dong, H.; Liu, H.; Chen, W.; Gui, R.; Li, J. Development of a nano-drug delivery system based on mesoporous silica and its anti-lymphoma activity. Appl. Nanosci. 2020, 10, 3431–3442. [Google Scholar] [CrossRef]
- Angelos, S.; Khashab, N.M.; Yang, Y.-W.; Trabolsi, A.; Khatib, H.A.; Stoddart, J.F.; Zink, J.I. pH Clock-Operated Mechanized Nanoparticles. J. Am. Chem. Soc. 2009, 131, 12912–12914. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Angelos, S.; Dichtel, W.R.; Coskun, A.; Yang, Y.-W.; Zink, J.I.; Stoddart, J.F. Enzyme-Responsive Snap-Top Covered Silica Nanocontainers. J. Am. Chem. Soc. 2008, 130, 2382–2383. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Tseng, H.-R.; Wong, J.W.; Stoddart, J.F.; Zink, J.I. An Operational Supramolecular Nanovalve. J. Am. Chem. Soc. 2004, 126, 3370–3371. [Google Scholar] [CrossRef]
- Angelos, S.; Johansson, E.; Stoddart, J.F.; Zink, J.I. Mesostructured Silica Supports for Functional Materials and Molecular Machines. Adv. Funct. Mater. 2007, 17, 2261–2271. [Google Scholar] [CrossRef]
- Khashab, N.M.; Belowich, M.E.; Trabolsi, A.; Friedman, D.C.; Valente, C.; Lau, Y.; Khatib, H.A.; Zink, J.I.; Stoddart, J.F. pH-Responsive mechanised nanoparticles gated by semirotaxanes. Chem. Commun. 2009, 5371–5373. [Google Scholar] [CrossRef]
- Croissant, J.; Zink, J.I. Nanovalve-Controlled Cargo Release Activated by Plasmonic Heating. J. Am. Chem. Soc. 2012, 134, 7628–7631. [Google Scholar] [CrossRef]
- Croissant, J.; Chaix, A.; Mongin, O.; Wang, M.; Clément, S.; Raehm, L.; Durand, J.-O.; Hugues, V.; Blanchard-Desce, M.; Maynadier, M.; et al. Two-Photon-Triggered Drug Delivery via Fluorescent Nanovalves. Small 2014, 10, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Ambrogio, M.W.; Thomas, C.R.; Zhao, Y.-L.; Zink, J.I.; Stoddart, J.F. Mechanized Silica Nanoparticles: A New Frontier in Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Lu, X.; Yuan, Y.; Qian, J.; Zhou, H.; Shi, J.; Liu, C. A magnetic, reversible pH-responsive nanogated ensemble based on Fe3O4 nanoparticles-capped mesoporous silica. Biomaterials 2011, 32, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Zhao, X.; Agarwal, A.; Mueller, L.J.; Feng, P. pH-responsive nanogated ensemble based on gold-capped mesoporous silica through an acid-labile acetal linker. J. Am. Chem. Soc. 2010, 132, 1500–1501. [Google Scholar] [CrossRef]
- Liu, M.; Sun, X.; Liao, Z.; Li, Y.; Qi, X.; Qian, Y.; Fenniri, H.; Zhao, P.; Shen, J. Zinc oxide end-capped Fe(3)O(4)@mSiO(2) core-shell nanocarriers as targeted and responsive drug delivery system for chemo-/ions synergistic therapeutics. Drug Deliv. 2019, 26, 732–743. [Google Scholar] [CrossRef]
- Liu, M.C.; Liu, B.; Chen, X.L.; Lin, H.C.; Sun, X.Y.; Lu, J.Z.; Li, Y.Y.; Yan, S.Q.; Zhang, L.Y.; Zhao, P. Calcium carbonate end-capped, folate-mediated Fe(3)O(4)@mSiO(2) core-shell nanocarriers as targeted controlled-release drug delivery system. J. Biomater. Appl. 2018, 32, 1090–1104. [Google Scholar] [CrossRef]
- Butler, K.S.; Durfee, P.N.; Theron, C.; Ashley, C.E.; Carnes, E.C.; Brinker, C.J. Protocells: Modular Mesoporous Silica Nanoparticle-Supported Lipid Bilayers for Drug Delivery. Small 2016, 12, 2173–2185. [Google Scholar] [CrossRef]
- Kumar, P.; Tambe, P.; Paknikar, K.M.; Gajbhiye, V. Mesoporous silica nanoparticles as cutting-edge theranostics: Advancement from merely a carrier to tailor-made smart delivery platform. J. Control. Release 2018, 287, 35–57. [Google Scholar] [CrossRef]
- Bakhshian Nik, A.; Zare, H.; Razavi, S.; Mohammadi, H.; Torab Ahmadi, P.; Yazdani, N.; Bayandori, M.; Rabiee, N.; Izadi Mobarakeh, J. Smart drug delivery: Capping strategies for mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2020, 299, 110115. [Google Scholar] [CrossRef]
- Baeza, A.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin. Drug Deliv. 2015, 12, 319–337. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous silica nanoparticles in nanomedicine applications. J. Mater. Sci. Mater. Med. 2018, 29, 65. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Nasab, N.; Hassani Kumleh, H.; Beygzadeh, M.; Teimourian, S.; Kazemzad, M. Delivery of curcumin by a pH-responsive chitosan mesoporous silica nanoparticles for cancer treatment. Artif. Cellsnanomed. Biotechnol. 2018, 46, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Manna, K.; Kayal, U.; Saha, M.; Chatterjee, S.; Chandra, D.; Hara, M.; Datta, S.; Bhaumik, A.; Das Saha, K. Folic acid-conjugated magnetic mesoporous silica nanoparticles loaded with quercetin: A theranostic approach for cancer management. RSC Adv. 2020, 10, 23148–23164. [Google Scholar] [CrossRef]
- Rashidi, L.; Vasheghani-Farahani, E.; Rostami, K.; Ganji, F.; Fallahpour, M. Mesoporous silica nanoparticles with different pore sizes for delivery of pH-sensitive gallic acid. Asia-Pac. J. Chem. Eng. 2014, 9, 845–853. [Google Scholar] [CrossRef]
- Feng, Y.; Li, N.-X.; Yin, H.-L.; Chen, T.-Y.; Yang, Q.; Wu, M. Thermo- and pH-responsive, Lipid-coated, Mesoporous Silica Nanoparticle-based Dual Drug Delivery System To Improve the Antitumor Effect of Hydrophobic Drugs. Mol. Pharm. 2019, 16, 422–436. [Google Scholar] [CrossRef]
- Fu, J.; Zhu, Y. Lysosomes activating chain reactions against cancer cells with a pH-switched prodrug/procatalyst co-delivery nanosystem. J. Mater. Chem. B 2017, 5, 996–1004. [Google Scholar] [CrossRef]
- Russo, A.; DeGraff, W.; Friedman, N.; Mitchell, J.B. Selective modulation of glutathione levels in human normal versus tumor cells and subsequent differential response to chemotherapy drugs. Cancer Res. 1986, 46, 2845–2848. [Google Scholar]
- Lin, J.-T.; Ye, Q.-B.; Yang, Q.-J.; Wang, G.-H. Hierarchical bioresponsive nanocarriers for codelivery of curcumin and doxorubicin. Colloids Surf. B Biointerfaces 2019, 180, 93–101. [Google Scholar] [CrossRef]
- Xu, X.; Lü, S.; Gao, C.; Feng, C.; Wu, C.; Bai, X.; Gao, N.; Wang, Z.; Liu, M. Self-fluorescent and stimuli-responsive mesoporous silica nanoparticles using a double-role curcumin gatekeeper for drug delivery. Chem. Eng. J. 2016, 300, 185–192. [Google Scholar] [CrossRef]
- Vaghasiya, K.; Ray, E.; Sharma, A.; Katare, O.P.; Verma, R.K. Matrix Metalloproteinase-Responsive Mesoporous Silica Nanoparticles Cloaked with Cleavable Protein for “Self-Actuating” On-Demand Controlled Drug Delivery for Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 4987–4999. [Google Scholar] [CrossRef]
- Baeza, A.; Guisasola, E.; Ruiz-Hernández, E.; Vallet-Regí, M. Magnetically Triggered Multidrug Release by Hybrid Mesoporous Silica Nanoparticles. Chem. Mater. 2012, 24, 517–524. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.S.; Chang, Z.M.; Li, L.; Zhang, Y.; Lu, M.M.; Zheng, X.; Li, M.; Shao, D.; Li, J.; et al. Berberine-loaded Janus nanocarriers for magnetic field-enhanced therapy against hepatocellular carcinoma. Chem. Biol. Drug Des. 2017, 89, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.; Miri, T.; Soleymani, M.; Barati, A. A novel method for in situ encapsulation of curcumin in magnetite-silica core-shell nanocomposites: A multifunctional platform for drug delivery and magnetic hyperthermia therapy. J. Mol. Liq. 2020, 114731. [Google Scholar] [CrossRef]
- Zhu, Y.; Tao, C. DNA-capped Fe3O4/SiO2 magnetic mesoporous silica nanoparticles for potential controlled drug release and hyperthermia. RSC Adv. 2015, 5, 22365–22372. [Google Scholar] [CrossRef]
- Mal, N.K.; Fujiwara, M.; Tanaka, Y. Photocontrolled reversible release of guest molecules from coumarin-modified mesoporous silica. Nature 2003, 421, 350–353. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.P.; Zhao, Y.-L.; Khashab, N.M.; Khatib, H.A.; Stoddart, J.F.; Zink, J.I. Light-Operated Mechanized Nanoparticles. J. Am. Chem. Soc. 2009, 131, 1686–1688. [Google Scholar] [CrossRef]
- Martínez-Carmona, M.; Baeza, A.; Rodriguez-Milla, M.A.; García-Castro, J.; Vallet-Regí, M. Mesoporous silica nanoparticles grafted with a light-responsive protein shell for highly cytotoxic antitumoral therapy. J. Mater. Chem. B 2015, 3, 5746–5752. [Google Scholar] [CrossRef]
- Kuang, G.; Zhang, Q.; He, S.; Liu, Y. Curcumin-loaded PEGylated mesoporous silica nanoparticles for effective photodynamic therapy. RSC Adv. 2020, 10, 24624–24630. [Google Scholar] [CrossRef]
- Li, X.D.; Wang, Z.; Wang, X.R.; Shao, D.; Zhang, X.; Li, L.; Ge, M.F.; Chang, Z.M.; Dong, W.F. Berberine-loaded Janus gold mesoporous silica nanocarriers for chemo/radio/photothermal therapy of liver cancer and radiation-induced injury inhibition. Int. J. Nanomed. 2019, 14, 3967–3982. [Google Scholar] [CrossRef]
- Yu, T.; Tong, L.; Ao, Y.; Zhang, G.; Liu, Y.; Zhang, H. Novel design of NIR-triggered plasmonic nanodots capped mesoporous silica nanoparticles loaded with natural capsaicin to inhibition of metastasis of human papillary thyroid carcinoma B-CPAP cells in thyroid cancer chemo-photothermal therapy. J. Photochem. Photobiol. B 2019, 197, 111534. [Google Scholar] [CrossRef]
- Sirsi, S.R.; Borden, M.A. State-of-the-art materials for ultrasound-triggered drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pelletier, M.; Zhang, H.; Xia, H.; Zhao, Y. High-Frequency Ultrasound-Responsive Block Copolymer Micelle. Langmuir 2009, 25, 13201–13205. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; Cabañas, M.V.; Manzano, M.; Vallet-Regí, M. Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers. ACS Nano 2015, 9, 11023–11033. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.L.; de la Torre, P.; Victoria Cabañas, M.; Manzano, M.; Grau, M.; Flores, A.I.; Vallet-Regí, M. Vectorization of ultrasound-responsive nanoparticles in placental mesenchymal stem cells for cancer therapy. Nanoscale 2017, 9, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Xia, H. Ultrasound Reversible Response Nanocarrier Based on Sodium Alginate Modified Mesoporous Silica Nanoparticles. Front. Chem. 2019, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Ding, X.; Hu, Y.; Wu, S.; Xiang, Y.; Zeng, Y.; Zhang, B.; Yan, H.; Zhang, H.; Zhu, L.; et al. Engineering a Hollow Nanocontainer Platform with Multifunctional Molecular Machines for Tumor-Targeted Therapy in Vitro and in Vivo. ACS Nano 2013, 7, 10271–10284. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Nakamura, H.; Fang, J.; Maeda, H. Development of next-generation macromolecular drugs based on the EPR effect: Challenges and pitfalls. Expert Opin. Drug Deliv. 2015, 12, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Philipp, A.; Meyer, M.; Wagner, E. Extracellular targeting of synthetic therapeutic nucleic acid formulations. Curr. Gene 2008, 8, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.Y.; Tan, S.Y.; Zhao, Y. Recent advances in biocompatible nanocarriers for delivery of chemotherapeutic cargoes towards cancer therapy. Org. Biomol. Chem. 2014, 12, 4776–4806. [Google Scholar] [CrossRef] [PubMed]
- Mickler, F.M.; Möckl, L.; Ruthardt, N.; Ogris, M.; Wagner, E.; Bräuchle, C. Tuning nanoparticle uptake: Live-cell imaging reveals two distinct endocytosis mechanisms mediated by natural and artificial EGFR targeting ligand. Nano Lett. 2012, 12, 3417–3423. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Peuhu, E.; Bate-Eya, L.T.; Eriksson, J.E.; Sahlgren, C.; Lindén, M. Cancer-Cell-Specific Induction of Apoptosis Using Mesoporous Silica Nanoparticles as Drug-Delivery Vectors. Small 2010, 6, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, Z.F.; Wang, Y.; Chen, W.H.; Luo, G.F.; Cheng, S.X.; Zhuo, R.X.; Zhang, X.Z. Multifunctional envelope-type mesoporous silica nanoparticles for tumor-triggered targeting drug delivery. J. Am. Chem. Soc. 2013, 135, 5068–5073. [Google Scholar] [CrossRef]
- Shen, Y.; Li, M.; Liu, T.; Liu, J.; Xie, Y.; Zhang, J.; Xu, S.; Liu, H. A dual-functional HER2 aptamer-conjugated, pH-activated mesoporous silica nanocarrier-based drug delivery system provides in vitro synergistic cytotoxicity in HER2-positive breast cancer cells. Int. J. Nanomed. 2019, 14, 4029–4044. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Zhu, W.; Sun, C.; Di, D.; Zhang, Y.; Wang, P.; Wang, Z.; Wang, S. Dual-stimuli responsive hyaluronic acid-conjugated mesoporous silica for targeted delivery to CD44-overexpressing cancer cells. Acta Biomater. 2015, 23, 147–156. [Google Scholar] [CrossRef]
- Brevet, D.; Gary-Bobo, M.; Raehm, L.; Richeter, S.; Hocine, O.; Amro, K.; Loock, B.; Couleaud, P.; Frochot, C.; Morère, A.; et al. Mannose-targeted mesoporous silica nanoparticles for photodynamic therapy. Chem. Commun. 2009, 1475–1477. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Vallet-Regí, M. Influence of the Surface Functionalization on the Fate and Performance of Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 916. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Zhang, Y.; Zhang, K.; Xie, J.; Liu, Y.; Li, W.; Feng, N. Curcumin-loaded redox-responsive mesoporous silica nanoparticles for targeted breast cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 921–935. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Hadadzadeh, H.; Farrokhpour, H.; Amirghofran, Z. Immobilization of gold nanoparticles on folate-conjugated dendritic mesoporous silica-coated reduced graphene oxide nanosheets: A new nanoplatform for curcumin pH-controlled and targeted delivery. Soft Matter 2018, 14, 2400–2410. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, S.; Xue, X.; Zhu, X.; Song, S.; Wang, B.; Jiang, L.; Qin, M.; Liang, H.; Gao, L. Quercetin and doxorubicin co-delivery using mesoporous silica nanoparticles enhance the efficacy of gastric carcinoma chemotherapy. Int. J. Nanomed. 2018, 13, 5113–5126. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.N.P.; Puvvada, N.; Rajput, S.; Sarkar, S.; Mahto, M.K.; Yallapu, M.M.; Pathak, A.; Emdad, L.; Das, S.K.; Reis, R.L.; et al. Targeting of EGFR, VEGFR2, and Akt by Engineered Dual Drug Encapsulated Mesoporous Silica-Gold Nanoclusters Sensitizes Tamoxifen-Resistant Breast Cancer. Mol. Pharm. 2018, 15, 2698–2713. [Google Scholar] [CrossRef] [PubMed]
- Murugan, C.; Rayappan, K.; Thangam, R.; Bhanumathi, R.; Shanthi, K.; Vivek, R.; Thirumurugan, R.; Bhattacharyya, A.; Sivasubramanian, S.; Gunasekaran, P.; et al. Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in breast cancer cells: An improved nanomedicine strategy. Sci. Rep. 2016, 6, 34053. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Ghosh, S.; Chowdhury, S.; Pandey, B.; Sil, P.C. Targeted delivery of quercetin loaded mesoporous silica nanoparticles to the breast cancer cells. Biochim. Biophys. Acta 2016, 1860, 2065–2075. [Google Scholar] [CrossRef]
- Ding, J.; Yao, J.; Xue, J.; Li, R.; Bao, B.; Jiang, L.; Zhu, J.J.; He, Z. Tumor-Homing Cell-Penetrating Peptide Linked to Colloidal Mesoporous Silica Encapsulated (-)-Epigallocatechin-3-gallate as Drug Delivery System for Breast Cancer Therapy in Vivo. ACS Appl. Mater. Interfaces 2015, 7, 18145–18155. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Qiu, Y.; Liu, Y.; Ding, M.; Zhang, Y. Anti-miRNA21 and resveratrol-loaded polysaccharide-based mesoporous silica nanoparticle for synergistic activity in gastric carcinoma. J. Drug Target. 2019, 27, 1135–1143. [Google Scholar] [CrossRef]
- Del Favero, G.; Bialas, F.; Grabher, S.; Wittig, A.; Bräuer, B.; Gerthsen, D.; Echalier, C.; Kamalov, M.; Marko, D.; Becker, C.F.W. Silica particles with a quercetin–R5 peptide conjugate are taken up into HT-29 cells and translocate into the nucleus. Chem. Commun. 2019, 55, 9649–9652. [Google Scholar] [CrossRef]
- Havel, H.A. Where Are the Nanodrugs? An Industry Perspective on Development of Drug Products Containing Nanomaterials. AAPS J. 2016, 18, 1351–1353. [Google Scholar] [CrossRef]
- Havel, H.; Finch, G.; Strode, P.; Wolfgang, M.; Zale, S.; Bobe, I.; Youssoufian, H.; Peterson, M.; Liu, M. Nanomedicines: From Bench to Bedside and Beyond. AAPS J. 2016, 18, 1373–1378. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. Int. J. Kuwait Univ. Health Sci. Cent. 2016, 25 (Suppl. S2), 41–59. [Google Scholar] [CrossRef] [PubMed]
- Nwodo, J.N.; Ibezim, A.; Simoben, C.V.; Ntie-Kang, F. Exploring Cancer Therapeutics with Natural Products from African Medicinal Plants, Part II: Alkaloids, Terpenoids and Flavonoids. Anticancer Agents Med. Chem. 2016, 16, 108–127. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Tew, K.D.; Ba, G.N.; Mathé, G. Polyphenols: Do they play a role in the prevention of human pathologies? Biomed. Pharm. 2002, 56, 200–207. [Google Scholar] [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lu, J.J.; Huang, M.Q.; Bao, J.L.; Chen, X.P.; Wang, Y.T. Terpenoids: Natural products for cancer therapy. Expert Opin. Investig. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R., Jr.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef]
- Kumar, V.; Bhatt, P.C.; Rahman, M.; Kaithwas, G.; Choudhry, H.; Al-Abbasi, F.A.; Anwar, F.; Verma, A. Fabrication, optimization, and characterization of umbelliferone β-D-galactopyranoside-loaded PLGA nanoparticles in treatment of hepatocellular carcinoma: In vitro and in vivo studies. Int. J. Nanomed. 2017, 12, 6747–6758. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef]
- Rajesh, E.; Sankari, L.S.; Malathi, L.; Krupaa, J.R. Naturally occurring products in cancer therapy. J. Pharm. Bioallied. Sci. 2015, 7, S181–S183. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Natural products for cancer chemotherapy. Microb. Biotechnol. 2011, 4, 687–699. [Google Scholar] [CrossRef]
- Reddy, L.; Odhav, B.; Bhoola, K.D. Natural products for cancer prevention: A global perspective. Pharmacol. Ther. 2003, 99, 1–13. [Google Scholar] [CrossRef]
- Zubair, H.; Azim, S.; Ahmad, A.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Cancer Chemoprevention by Phytochemicals: Nature’s Healing Touch. Molecules 2017, 22, 395. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.; Newman, B.; Sun, D. Targeting cancer stem cells with natural products. Curr. Drug Targets 2012, 13, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef]
- Harborne, J.B. Classes and functions of secondary products from plants. In Chemicals from Plants; World Scientific/Imperial College Press: Singapore, 1999; pp. 1–25. [Google Scholar]
- Rahman, M.; Ahmad, M.Z.; Kazmi, I.; Akhter, S.; Afzal, M.; Gupta, G.; Sinha, V.R. Emergence of nanomedicine as cancer targeted magic bullets: Recent development and need to address the toxicity apprehension. Curr. Drug Discov. Technol. 2012, 9, 319–329. [Google Scholar] [CrossRef]
- Rahman, M.; Ahmad, M.Z.; Kazmi, I.; Akhter, S.; Afzal, M.; Gupta, G.; Jalees Ahmed, F.; Anwar, F. Advancement in multifunctional nanoparticles for the effective treatment of cancer. Expert Opin. Drug Deliv. 2012, 9, 367–381. [Google Scholar] [CrossRef]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Leonarduzzi, G.; Testa, G.; Sottero, B.; Gamba, P.; Poli, G. Design and development of nanovehicle-based delivery systems for preventive or therapeutic supplementation with flavonoids. Curr. Med. Chem. 2010, 17, 74–95. [Google Scholar] [CrossRef]
- Jeetah, R.; Bhaw-Luximon, A.; Jhurry, D. Nanopharmaceutics: Phytochemical-based controlled or sustained drug-delivery systems for cancer treatment. J. Biomed. Nanotechnol. 2014, 10, 1810–1840. [Google Scholar] [CrossRef]
- Gohulkumar, M.; Gurushankar, K.; Rajendra Prasad, N.; Krishnakumar, N. Enhanced cytotoxicity and apoptosis-induced anticancer effect of silibinin-loaded nanoparticles in oral carcinoma (KB) cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 41, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, D.; Jung, K.-H.; Zhang, H.; Nannapaneni, S.; Wang, X.; Amin, A.R.M.R.; Chen, Z.; Chen, Z.G.; Shin, D.M. Luteolin nanoparticle in chemoprevention: In vitro and in vivo anticancer activity. Cancer Prev. Res. 2014, 7, 65–73. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Al Jaouni, S.K.; Li, W.; Mousa, S.A. Protective Roles of Thymoquinone Nanoformulations: Potential Nanonutraceuticals in Human Diseases. Nutrients 2018, 10, 1369. [Google Scholar] [CrossRef] [PubMed]
- Ballout, F.; Habli, Z.; Rahal, O.N.; Fatfat, M.; Gali-Muhtasib, H. Thymoquinone-based nanotechnology for cancer therapy: Promises and challenges. Drug Discov. Today 2018, 23, 1089–1098. [Google Scholar] [CrossRef]
- Hazra, B.; Kumar, B.; Biswas, S.; Pandey, B.N.; Mishra, K.P. Enhancement of the tumour inhibitory activity, in vivo, of diospyrin, a plant-derived quinonoid, through liposomal encapsulation. Toxicol. Lett. 2005, 157, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An “old-age” disease with an “age-old” solution. Cancer Lett. 2008, 267, 133–164. [Google Scholar] [CrossRef]
- Nirmala, C.; Puvanakrishnan, R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol. Cell. Biochem. 1996, 159, 85–93. [Google Scholar] [CrossRef]
- Jordan, W.C.; Drew, C.R. Curcumin—A natural herb with anti-HIV activity. J. Natl. Med. Assoc. 1996, 88, 333. [Google Scholar]
- Phan, T.T.; See, P.; Lee, S.T.; Chan, S.Y. Protective effects of curcumin against oxidative damage on skin cells in vitro: Its implication for wound healing. J. Trauma 2001, 51, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The Curry Spice Curcumin Reduces Oxidative Damage and Amyloid Pathology in an Alzheimer Transgenic Mouse. J. Neurosci. 2001, 21, 8370. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Arablou, T.; Kolahdouz-Mohammadi, R. Curcumin and endometriosis: Review on potential roles and molecular mechanisms. Biomed. Pharm. 2018, 97, 91–97. [Google Scholar] [CrossRef]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Sharma, O.P. Antioxidant activity of curcumin and related compounds. Biochem. Pharm. 1976, 25, 1811–1812. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Mandal, D.; Sen, G.S.; Pal, S.; Banerjee, S.; Lahiry, L.; Finke, J.H.; Tannenbaum, C.S.; Das, T.; Sa, G. Tumor-induced oxidative stress perturbs nuclear factor-kappaB activity-augmenting tumor necrosis factor-alpha-mediated T-cell death: Protection by curcumin. Cancer Res. 2007, 67, 362–370. [Google Scholar] [CrossRef]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef]
- De, R.; Kundu, P.; Swarnakar, S.; Ramamurthy, T.; Chowdhury, A.; Nair, G.B.; Mukhopadhyay, A.K. Antimicrobial activity of curcumin against Helicobacter pylori isolates from India and during infections in mice. Antimicrob. Agents Chemother. 2009, 53, 1592–1597. [Google Scholar] [CrossRef]
- Mun, S.H.; Joung, D.K.; Kim, Y.S.; Kang, O.H.; Kim, S.B.; Seo, Y.S.; Kim, Y.C.; Lee, D.S.; Shin, D.W.; Kweon, K.T.; et al. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef]
- Neelofar, K.; Shreaz, S.; Rimple, B.; Muralidhar, S.; Nikhat, M.; Khan, L.A. Curcumin as a promising anticandidal of clinical interest. Can. J. Microbiol. 2011, 57, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Khalil, O.A.K.; de Faria Oliveira, O.M.M.; Vellosa, J.C.R.; de Quadros, A.U.; Dalposso, L.M.; Karam, T.K.; Mainardes, R.M.; Khalil, N.M. Curcumin antifungal and antioxidant activities are increased in the presence of ascorbic acid. Food Chem. 2012, 133, 1001–1005. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, Z.M.; Wang, F.L.; Zhang, Z.S.; Liu, X.Z.; Zhai, D.D.; Chen, W.D. Curcumin and its promise as an anticancer drug: An analysis of its anticancer and antifungal effects in cancer and associated complications from invasive fungal infections. Eur. J. Pharm. 2016, 772, 33–42. [Google Scholar] [CrossRef]
- Ravindranath, V.; Chandrasekhara, N. Absorption and tissue distribution of curcumin in rats. Toxicology 1980, 16, 259–265. [Google Scholar] [CrossRef]
- Ravindranath, V.; Chandrasekhara, N. In vitro studies on the intestinal absorption of curcumin in rats. Toxicology 1981, 20, 251–257. [Google Scholar] [CrossRef]
- Ravindranath, V.; Chandrasekhara, N. Metabolism of curcumn-studies with [3H]curcumin. Toxicology 1981, 22, 337–344. [Google Scholar] [CrossRef]
- Gupta, N.K.; Dixit, V.K. Bioavailability Enhancement of Curcumin by Complexation with Phosphatidyl Choline. J. Pharm. Sci. 2011, 100, 1987–1995. [Google Scholar] [CrossRef] [PubMed]
- Bollu, V.S.; Barui, A.K.; Mondal, S.K.; Prashar, S.; Fajardo, M.; Briones, D.; Rodríguez-Diéguez, A.; Patra, C.R.; Gómez-Ruiz, S. Curcumin-loaded silica-based mesoporous materials: Synthesis, characterization and cytotoxic properties against cancer cells. Mater. Sci. Eng. C 2016, 63, 393–410. [Google Scholar] [CrossRef]
- Datz, S.; Engelke, H.; Schirnding, C.V.; Nguyen, L.; Bein, T. Lipid bilayer-coated curcumin-based mesoporous organosilica nanoparticles for cellular delivery. Microporous Mesoporous Mater. 2016, 225, 371–377. [Google Scholar] [CrossRef]
- Rahmatolahzadeh, R.; Hamadanian, M.; Ma’mani, L.; Shafiee, A. Aspartic acid functionalized PEGylated MSN@GO hybrid as an effective and sustainable nano-system for in-vitro drug delivery. Adv. Med. Sci. 2018, 63, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cai, L.; Tian, Z.; Wu, Y.; Chen, J. Phytochemical Curcumin-Coformulated, Silver-Decorated Melanin-like Polydopamine/Mesoporous Silica Composites with Improved Antibacterial and Chemotherapeutic Effects against Drug-Resistant Cancer Cells. ACS Omega 2020, 5, 15083–15094. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Fan, K.; Jin, Y.; Zhao, L.; Wang, Q.; Tang, Y.; Xu, H.; Liu, Z.; Wang, S.; Lin, J.; et al. PEGylated lipid bilayer coated mesoporous silica nanoparticles co-delivery of paclitaxel and curcumin leads to increased tumor site drug accumulation and reduced tumor burden. Eur. J. Pharm. Sci. 2019, 140, 105070. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, N.; Yang, L.-Y.; Ouyang, X.-K.; Huang, F. Folic Acid and PEI Modified Mesoporous Silica for Targeted Delivery of Curcumin. Pharmaceutics 2019, 11, 430. [Google Scholar] [CrossRef]
- Mashayekhi, S.; Rasoulpoor, S.; Shabani, S.; Esmaeilizadeh, N.; Serati-Nouri, H.; Sheervalilou, R.; Pilehvar-Soltanahmadi, Y. Curcumin-loaded mesoporous silica nanoparticles/nanofiber composites for supporting long-term proliferation and stemness preservation of adipose-derived stem cells. Int. J. Pharm. 2020, 587, 119656. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shao, L.; Usman, I.; Hu, Y.; Pan, A.; Liang, S.; Xu, H. A pH-responsive dissociable mesoporous silica-based nanoplatform enabling efficient dual-drug co-delivery and rapid clearance for cancer therapy. Biomater. Sci. 2020, 8, 3418–3429. [Google Scholar] [CrossRef]
- Wibowo, F.R.; Saputra, O.A.; Lestari, W.W.; Koketsu, M.; Mukti, R.R.; Martien, R. pH-Triggered Drug Release Controlled by Poly(Styrene Sulfonate) Growth Hollow Mesoporous Silica Nanoparticles. ACS Omega 2020, 5, 4261–4269. [Google Scholar] [CrossRef]
- Kong, Z.L.; Kuo, H.P.; Johnson, A.; Wu, L.C.; Chang, K.L.B. Curcumin-Loaded Mesoporous Silica Nanoparticles Markedly Enhanced Cytotoxicity in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 2918. [Google Scholar] [CrossRef]
- Harini, L.; Srivastava, S.; Gnanakumar, G.P.; Karthikeyan, B.; Ross, C.; Krishnakumar, V.; Kannan, V.R.; Sundar, K.; Kathiresan, T. An ingenious non-spherical mesoporous silica nanoparticle cargo with curcumin induces mitochondria-mediated apoptosis in breast cancer (MCF-7) cells. Oncotarget 2019, 10, 1193–1208. [Google Scholar] [CrossRef]
- Chen, C.; Sun, W.; Wang, X.; Wang, Y.; Wang, P. Rational design of curcumin loaded multifunctional mesoporous silica nanoparticles to enhance the cytotoxicity for targeted and controlled drug release. Mater. Sci. Eng. C 2018, 85, 88–96. [Google Scholar] [CrossRef]
- Lv, Y.; Li, J.; Chen, H.; Bai, Y.; Zhang, L. Glycyrrhetinic acid-functionalized mesoporous silica nanoparticles as hepatocellular carcinoma-targeted drug carrier. Int. J. Nanomed. 2017, 12, 4361–4370. [Google Scholar] [CrossRef] [PubMed]
- Harini, L.; Karthikeyan, B.; Srivastava, S.; Suresh, S.B.; Ross, C.; Gnanakumar, G.; Rajagopal, S.; Sundar, K.; Kathiresan, T. Polyethylenimine-modified curcumin-loaded mesoporus silica nanoparticle (MCM-41) induces cell death in MCF-7 cell line. IET Nanobiotechnol. 2017, 11, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Nawale, L.; Sarkar, D.; Badiger, M.V. Carboxymethyl Cellulose-Grafted Mesoporous Silica Hybrid Nanogels for Enhanced Cellular Uptake and Release of Curcumin. Gels 2017, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Liu, Q.; Yang, L.; Zhu, R.; Yu, C.; Wang, S. Rational Design of Multifunctional Dendritic Mesoporous Silica Nanoparticles to Load Curcumin and Enhance Efficacy for Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 26511–26523. [Google Scholar] [CrossRef]
- Kotcherlakota, R.; Barui, A.K.; Prashar, S.; Fajardo, M.; Briones, D.; Rodríguez-Diéguez, A.; Patra, C.R.; Gómez-Ruiz, S. Curcumin loaded mesoporous silica: An effective drug delivery system for cancer treatment. Biomater. Sci. 2016, 4, 448–459. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the dietary flavonoid quercetin upon performance and health. Curr. Sports Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef]
- Lamson, D.W.; Brignall, M.S. Antioxidants and cancer, part 3: Quercetin. Altern. Med. Rev. 2000, 5, 196–208. [Google Scholar]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharm. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Choi, H.J.; Song, J.H.; Park, K.S.; Kwon, D.H. Inhibitory effects of quercetin 3-rhamnoside on influenza A virus replication. Eur. J. Pharm. Sci. 2009, 37, 329–333. [Google Scholar] [CrossRef]
- Rotelli, A.E.; Guardia, T.; Juárez, A.O.; de la Rocha, N.E.; Pelzer, L.E. Comparative study of flavonoids in experimental models of inflammation. Pharm. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A.; Srinivasan, B.P. Retinal neuroprotective effects of quercetin in streptozotocin-induced diabetic rats. Exp. Eye Res. 2014, 125, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.T.; Ding, C.; Zhou, N.; Xu, C. Quercetin protects gastric epithelial cell from oxidative damage in vitro and in vivo. Eur. J. Pharm. 2015, 754, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, L.; Arias, N.; Teresa Macarulla, M.; Gracia, A.; Portillo, M.P. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J. 2011, 4, 189–198. [Google Scholar]
- Yang, L.; Hu, Z.; Zhu, J.; Liang, Q.; Zhou, H.; Li, J.; Fan, X.; Zhao, Z.; Pan, H.; Fei, B. Systematic Elucidation of the Mechanism of Quercetin against Gastric Cancer via Network Pharmacology Approach. BioMed Res. Int. 2020, 2020, 3860213. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Chun, O.K.; Chung, S.J.; Song, W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007, 137, 1244–1252. [Google Scholar] [CrossRef]
- Gugler, R.; Leschik, M.; Dengler, H.J. Disposition of quercetin in man after single oral and intravenous doses. Eur. J. Clin. Pharm. 1975, 9, 229–234. [Google Scholar] [CrossRef]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [CrossRef]
- Thangasamy, T.; Sittadjody, S.; Burd, R. Quercetin: A potential complementary and alternative cancer therapy. In Complementary and Alternative Therapies and the Aging Population; Elsevier: Amsterdam, The Netherlands, 2009; pp. 563–584. [Google Scholar]
- Novo, M.C.; Osugui, L.; dos Reis, V.O.; Longo-Maugéri, I.M.; Mariano, M.; Popi, A.F. Blockage of Wnt/β-catenin signaling by quercetin reduces survival and proliferation of B-1 cells in vitro. Immunobiology 2015, 220, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell. Biosci. 2020, 10, 32. [Google Scholar] [CrossRef]
- Fernández-Palanca, P.; Fondevila, F.; Méndez-Blanco, C.; Tuñón, M.J.; González-Gallego, J.; Mauriz, J.L. Antitumor Effects of Quercetin in Hepatocarcinoma In Vitro and In Vivo Models: A Systematic Review. Nutrients 2019, 11, 2875. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.; Břetislav, G.; Pavel, S.; Pavla, U. Potential of the Flavonoid Quercetin to Prevent and Treat Cancer—Current Status of Research. Klin. Onkol. 2018, 31, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharm. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Niazvand, F.; Orazizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of Quercetin-Loaded Nanoparticles on MCF-7 Human Breast Cancer Cells. Medicina 2019, 55, 114. [Google Scholar] [CrossRef]
- Nam, J.-S.; Sharma, A.R.; Nguyen, L.T.; Chakraborty, C.; Sharma, G.; Lee, S.-S. Application of Bioactive Quercetin in Oncotherapy: From Nutrition to Nanomedicine. Molecules 2016, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Lou, M.; Zhang, L.-N.; Ji, P.-G.; Feng, F.-Q.; Liu, J.-H.; Yang, C.; Li, B.-F.; Wang, L. Quercetin nanoparticles induced autophagy and apoptosis through AKT/ERK/Caspase-3 signaling pathway in human neuroglioma cells: In vitro and in vivo. Biomed. Pharmacother. 2016, 84, 1–9. [Google Scholar] [CrossRef]
- Rajesh Kumar, S.; Priyatharshni, S.; Babu, V.N.; Mangalaraj, D.; Viswanathan, C.; Kannan, S.; Ponpandian, N. Quercetin conjugated superparamagnetic magnetite nanoparticles for in-vitro analysis of breast cancer cell lines for chemotherapy applications. J. Colloid Interface Sci. 2014, 436, 234–242. [Google Scholar] [CrossRef]
- Ren, K.-W.; Li, Y.-H.; Wu, G.; Ren, J.-Z.; Lu, H.-B.; Li, Z.-M.; Han, X.-W. Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells. Int. J. Oncol. 2017, 50, 1299–1311. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, B.; Wang, T.; Wan, J.; Zhang, Y.; Huang, J.; Shen, Y. Enhancing the anti-ovarian cancer activity of quercetin using a self-assembling micelle and thermosensitive hydrogel drug delivery system. RSC Adv. 2018, 8, 21229–21242. [Google Scholar] [CrossRef]
- Liu, M.; Fu, M.; Yang, X.; Jia, G.; Shi, X.; Ji, J.; Liu, X.; Zhai, G. Paclitaxel and quercetin co-loaded functional mesoporous silica nanoparticles overcoming multidrug resistance in breast cancer. Colloids Surf. B Biointerfaces 2020, 196, 111284. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, T.; Zhu, D.; Huang, Q. Enhanced Tumor Targeting and Radiotherapy by Quercetin Loaded Biomimetic Nanoparticles. Front. Chem. 2020, 8, 225. [Google Scholar] [CrossRef]
- Shao, M.; Chang, C.; Liu, Z.; Chen, K.; Zhou, Y.; Zheng, G.; Huang, Z.; Xu, H.; Xu, P.; Lu, B. Polydopamine coated hollow mesoporous silica nanoparticles as pH-sensitive nanocarriers for overcoming multidrug resistance. Colloids Surf. B Biointerfaces 2019, 183, 110427. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef]
- Shukla, Y.; Singh, R. Resveratrol and cellular mechanisms of cancer prevention. Ann. N. Y. Acad. Sci. 2011, 1215, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, T.N.; Ornstrup, M.J.; Poulsen, M.M.; Jørgensen, J.O.; Hougaard, D.M.; Cohen, A.S.; Neghabat, S.; Richelsen, B.; Pedersen, S.B. Resveratrol reduces the levels of circulating androgen precursors but has no effect on, testosterone, dihydrotestosterone, PSA levels or prostate volume. A 4-month randomised trial in middle-aged men. Prostate 2015, 75, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.S.L.; Tan, L.T.-H.; Chan, K.-G.; Yap, W.H.; Pusparajah, P.; Chuah, L.-H.; Ming, L.C.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Resveratrol-Potential Antibacterial Agent against Foodborne Pathogens. Front. Pharmacol. 2018, 9, 102. [Google Scholar] [CrossRef]
- Perrone, D.; Fuggetta, M.P.; Ardito, F.; Cottarelli, A.; De Filippis, A.; Ravagnan, G.; De Maria, S.; Lo Muzio, L. Resveratrol (3,5,4’-trihydroxystilbene) and its properties in oral diseases. Exp. Med. 2017, 14, 3–9. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef]
- Jung, C.M.; Heinze, T.M.; Schnackenberg, L.K.; Mullis, L.B.; Elkins, S.A.; Elkins, C.A.; Steele, R.S.; Sutherland, J.B. Interaction of dietary resveratrol with animal-associated bacteria. FEMS Microbiol. Lett. 2009, 297, 266–273. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharm. 2007, 224, 274–283. [Google Scholar] [CrossRef]
- Sawda, C.; Moussa, C.; Turner, R.S. Resveratrol for Alzheimer’s disease. Ann. N. Y. Acad. Sci. 2017, 1403, 142–149. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- De Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, Y.X.; Janakiraman, N.; Chapman, R.A.; Gautam, S.C. Immunomodulatory activity of resveratrol: Suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production. Biochem. Pharm. 2001, 62, 1299–1308. [Google Scholar] [CrossRef]
- Trung, L.Q.; An, D.T.T. Is Resveratrol a Cancer Immunomodulatory Molecule? Front. Pharmacol. 2018, 9, 1255. [Google Scholar] [CrossRef] [PubMed]
- Berge, G.; Øvrebø, S.; Eilertsen, E.; Haugen, A.; Mollerup, S. Analysis of resveratrol as a lung cancer chemopreventive agent in A/J mice exposed to benzo[a]pyrene. Br. J. Cancer 2004, 91, 1380–1383. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Chen, H.A.; Chen, P.S.; Cheng, Y.J.; Hsu, W.H.; Chang, Y.W.; Chen, Y.H.; Jan, Y.; Hsiao, M.; Chang, T.Y.; et al. MiR-520h-mediated FOXC2 regulation is critical for inhibition of lung cancer progression by resveratrol. Oncogene 2013, 32, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.T.; Tian, Q.Z.; Guan, L.; Zhou, Y.; Huang, X.E.; Zhang, H. In vitro and in vivo evaluation of the antitumor efficiency of resveratrol against lung cancer. Asian Pac. J. Cancer Prev. 2013, 14, 1703–1706. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Chen, Q.; Fu, J.; Shankar, S.; Srivastava, R.K. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS ONE 2011, 6, e25166. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Chen, Y.F.; Hsu, W.H.; Yang, C.W.; Kao, C.H.; Tsai, T.F. Resveratrol helps recovery from fatty liver and protects against hepatocellular carcinoma induced by hepatitis B virus X protein in a mouse model. Cancer Prev. Res. 2012, 5, 952–962. [Google Scholar] [CrossRef]
- Carbó, N.; Costelli, P.; Baccino, F.M.; López-Soriano, F.J.; Argilés, J.M. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem. Biophys. Res. Commun. 1999, 254, 739–743. [Google Scholar] [CrossRef]
- Chatterjee, K.; Mukherjee, S.; Vanmanen, J.; Banerjee, P.; Fata, J.E. Dietary Polyphenols, Resveratrol and Pterostilbene Exhibit Antitumor Activity on an HPV E6-Positive Cervical Cancer Model: An in vitro and in vivo Analysis. Front. Oncol. 2019, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Huderson, A.C.; Myers, J.N.; Niaz, M.S.; Washington, M.K.; Ramesh, A. Chemoprevention of benzo(a)pyrene-induced colon polyps in ApcMin mice by resveratrol. J. Nutr. Biochem. 2013, 24, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Hudson, T.S.; Wang, T.C.; Remsberg, C.M.; Davies, N.M.; Takahashi, Y.; Kim, Y.S.; Seifried, H.; Vinyard, B.T.; Perkins, S.N.; et al. Differential effects of resveratrol on androgen-responsive LNCaP human prostate cancer cells in vitro and in vivo. Carcinogenesis 2008, 29, 2001–2010. [Google Scholar] [CrossRef] [PubMed]
- Bove, K.; Lincoln, D.W.; Tsan, M.F. Effect of resveratrol on growth of 4T1 breast cancer cells in vitro and in vivo. Biochem. Biophys. Res. Commun. 2002, 291, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Garvin, S.; Ollinger, K.; Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006, 231, 113–122. [Google Scholar] [CrossRef]
- Bhat, K.P.; Lantvit, D.; Christov, K.; Mehta, R.G.; Moon, R.C.; Pezzuto, J.M. Estrogenic and antiestrogenic properties of resveratrol in mammary tumor models. Cancer Res. 2001, 61, 7456–7463. [Google Scholar]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25–37. [Google Scholar]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Patel, K.R.; Scott, E.; Brown, V.A.; Gescher, A.J.; Steward, W.P.; Brown, K. Clinical trials of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 161–169. [Google Scholar] [CrossRef]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Pangeni, R.; Sahni, J.K.; Ali, J.; Sharma, S.; Baboota, S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014, 11, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Ansari, K.A.; Vavia, P.R.; Trotta, F.; Cavalli, R. Cyclodextrin-based nanosponges for delivery of resveratrol: In vitro characterisation, stability, cytotoxicity and permeation study. AAPS Pharmscitech. 2011, 12, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Nastiti, C.; Ponto, T.; Mohammed, Y.; Roberts, M.S.; Benson, H.A.E. Novel Nanocarriers for Targeted Topical Skin Delivery of the Antioxidant Resveratrol. Pharmaceutics 2020, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Yen, F.L.; Huang, H.W.; Wu, T.H.; Ko, H.H.; Tzeng, W.S.; Lin, C.C. Resveratrol nanoparticle system improves dissolution properties and enhances the hepatoprotective effect of resveratrol through antioxidant and anti-inflammatory pathways. J. Agric. Food Chem 2012, 60, 4662–4671. [Google Scholar] [CrossRef]
- Chaudhary, Z.; Subramaniam, S.; Khan, G.M.; Abeer, M.M.; Qu, Z.; Janjua, T.; Kumeria, T.; Batra, J.; Popat, A. Encapsulation and Controlled Release of Resveratrol Within Functionalized Mesoporous Silica Nanoparticles for Prostate Cancer Therapy. Front. Bioeng. Biotechnol. 2019, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Summerlin, N.; Qu, Z.; Pujara, N.; Sheng, Y.; Jambhrunkar, S.; McGuckin, M.; Popat, A. Colloidal mesoporous silica nanoparticles enhance the biological activity of resveratrol. Colloids Surf. B Biointerfaces 2016, 144, 1–7. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020. [Google Scholar] [CrossRef]
- Cicero, A.F.; Baggioni, A. Berberine and Its Role in Chronic Disease. Adv. Exp. Med. Biol 2016, 928, 27–45. [Google Scholar] [CrossRef]
- Schor, J.; Fabno, N. Clinical Applications for Berberine: Potential therapeutic applications in metabolic syndrome, type 2 diabetes, and dyslipidemia. Nat. Med. J. 2012, 4. Available online: https://www.naturalmedicinejournal.com/print/587 (accessed on 1 January 2021).
- Zych, M.; Wojnar, W.; Kielanowska, M.; Folwarczna, J.; Kaczmarczyk-Sedlak, I. Effect of Berberine on Glycation, Aldose Reductase Activity, and Oxidative Stress in the Lenses of Streptozotocin-Induced Diabetic Rats In Vivo-A Preliminary Study. Int. J. Mol. Sci. 2020, 21, 4278. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, P.; Tao, S.; Deng, Y.; Li, X.; Lan, T.; Zhang, X.; Guo, F.; Huang, W.; Chen, F.; et al. Berberine inhibits aldose reductase and oxidative stress in rat mesangial cells cultured under high glucose. Arch. Biochem. Biophys. 2008, 475, 128–134. [Google Scholar] [CrossRef]
- Zhu, N.; Li, J.; Li, Y.; Zhang, Y.; Du, Q.; Hao, P.; Cao, X.; Li, L. Berberine Protects Against Simulated Ischemia/Reperfusion Injury-Induced H9C2 Cardiomyocytes Apoptosis In Vitro and Myocardial Ischemia/Reperfusion-Induced Apoptosis In Vivo by Regulating the Mitophagy-Mediated HIF-1α/BNIP3 Pathway. Front. Pharm. 2020, 11, 367. [Google Scholar] [CrossRef]
- Zhao, G.L.; Yu, L.M.; Gao, W.L.; Duan, W.X.; Jiang, B.; Liu, X.D.; Zhang, B.; Liu, Z.H.; Zhai, M.E.; Jin, Z.X.; et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharm. Sin. 2016, 37, 354–367. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Chi, C.-W.; Liu, T.-Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004, 203, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yi, X.; Ghanam, K.; Zhang, S.; Zhao, T.; Zhu, X. Berberine decreases cholesterol levels in rats through multiple mechanisms, including inhibition of cholesterol absorption. Metabolism 2014, 63, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Long, Y.; Ni, L.; Yuan, X.; Yu, N.; Wu, R.; Tao, J.; Zhang, Y. Anticancer effect of berberine based on experimental animal models of various cancers: A systematic review and meta-analysis. BMC Cancer 2019, 19, 589. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metab. Clin. Exp. 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Cicero, A.; Ertek, S. Metabolic and cardiovascular effects of berberine: From preclinical evidences to clinical trial results. Clin. Lipidol. 2009, 4, 553–563. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A. Role of phytochemicals in the management of metabolic syndrome. Phytomedicine 2016, 23, 1134–1144. [Google Scholar] [CrossRef]
- Cristiana, C.; Placido, F.; Silvia, S.; Aldo Roda and Arrigo, F.G.C. Berberine: New Insights from Pharmacological Aspects to Clinical Evidences in the Management of Metabolic Disorders. Curr. Med. Chem. 2016, 23, 1460–1476. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The Anti-Cancer Mechanisms of Berberine: A Review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Sahibzada, M.U.K.; Sadiq, A.; Faidah, H.S.; Khurram, M.; Amin, M.U.; Haseeb, A.; Kakar, M. Berberine nanoparticles with enhanced in vitro bioavailability: Characterization and antimicrobial activity. Drug Des. Dev. Ther. 2018, 12, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mirhadi, E.; Rezaee, M.; Malaekeh-Nikouei, B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed. Pharm. 2018, 104, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wu, Z.; Zhao, Z.; Wu, C.; Yuan, M.; Su, Z.; Wang, Y.; Wang, Z. Berberine-Loaded Nanostructured Lipid Carriers Enhance the Treatment of Ulcerative Colitis. Int. J. Nanomed. 2020, 15, 3937–3951. [Google Scholar] [CrossRef]
- Yue, J.; Wang, Z.; Shao, D.; Chang, Z.; Hu, R.; Li, L.; Luo, S.-Z.; Dong, W.-F. Cancer cell membrane-modified biodegradable mesoporous silica nanocarriers for berberine therapy of liver cancer. RSC Adv. 2018, 8, 40288–40297. [Google Scholar] [CrossRef]
- Schneider-Stock, R.; Fakhoury, I.H.; Zaki, A.M.; El-Baba, C.O.; Gali-Muhtasib, H.U. Thymoquinone: Fifty years of success in the battle against cancer models. Drug Discov. Today 2014, 19, 18–30. [Google Scholar] [CrossRef]
- Gurung, R.L.; Lim, S.N.; Khaw, A.K.; Soon, J.F.; Shenoy, K.; Mohamed Ali, S.; Jayapal, M.; Sethu, S.; Baskar, R.; Hande, M.P. Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PLoS ONE 2010, 5, e12124. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.; Ocker, M.; Kuester, D.; Krueger, S.; El-Hajj, Z.; Diestel, A.; Evert, M.; El-Najjar, N.; Peters, B.; Jurjus, A.; et al. Thymoquinone reduces mouse colon tumor cell invasion and inhibits tumor growth in murine colon cancer models. J. Cell Mol. Med. 2008, 12, 330–342. [Google Scholar] [CrossRef]
- Jakaria, M.; Cho, D.Y.; Ezazul Haque, M.; Karthivashan, G.; Kim, I.S.; Ganesan, P.; Choi, D.K. Neuropharmacological Potential and Delivery Prospects of Thymoquinone for Neurological Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 1209801. [Google Scholar] [CrossRef]
- Ahmad, A.; Mishra, R.K.; Vyawahare, A.; Kumar, A.; Rehman, M.U.; Qamar, W.; Khan, A.Q.; Khan, R. Thymoquinone (2-Isoprpyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharm. J. 2019, 27, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Al-Omar, F.A.; Nagi, M.N. Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur. J. Pharmacol. 2006, 543, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Umar, S.; Zargan, J.; Umar, K.; Ahmad, S.; Katiyar, C.K.; Khan, H.A. Modulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar rats. Chem. Biol. Interact. 2012, 197, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.C.; Kumar, A.P.; Sethi, G.; Tan, K.H.B. Thymoquinone: Potential cure for inflammatory disorders and cancer. Biochem. Pharmacol. 2012, 83, 443–451. [Google Scholar] [CrossRef]
- Asaduzzaman Khan, M.; Tania, M.; Fu, S.; Fu, J. Thymoquinone, as an anticancer molecule: From basic research to clinical investigation. Oncotarget 2017, 8, 51907–51919. [Google Scholar] [CrossRef]
- Chaieb, K.; Kouidhi, B.; Jrah, H.; Mahdouani, K.; Bakhrouf, A. Antibacterial activity of Thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement. Altern. Med. 2011, 11, 29. [Google Scholar] [CrossRef]
- Kassab, R.B.; El-Hennamy, R.E. The role of thymoquinone as a potent antioxidant in ameliorating the neurotoxic effect of sodium arsenate in female rat. Egypt. J. Basic Appl. Sci. 2017, 4, 160–167. [Google Scholar] [CrossRef]
- Bule, M.; Nikfar, S.; Amini, M.; Abdollahi, M. The antidiabetic effect of thymoquinone: A systematic review and meta-analysis of animal studies. Food Res. Int. 2020, 127, 108736. [Google Scholar] [CrossRef]
- El-Najjar, N.; Ketola, R.A.; Nissilä, T.; Mauriala, T.; Antopolsky, M.; Jänis, J.; Gali-Muhtasib, H.; Urtti, A.; Vuorela, H. Impact of protein binding on the analytical detectability and anticancer activity of thymoquinone. J. Chem. Biol. 2011, 4, 97–107. [Google Scholar] [CrossRef]
- Pathan, S.A.; Jain, G.K.; Zaidi, S.M.; Akhter, S.; Vohora, D.; Chander, P.; Kole, P.L.; Ahmad, F.J.; Khar, R.K. Stability-indicating ultra-performance liquid chromatography method for the estimation of thymoquinone and its application in biopharmaceutical studies. Biomed. Chromatogr. 2011, 25, 613–620. [Google Scholar] [CrossRef]
- Salmani, J.M.; Asghar, S.; Lv, H.; Zhou, J. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents, pH and light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef] [PubMed]
- Darakhshan, S.; Bidmeshki Pour, A.; Hosseinzadeh Colagar, A.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharm. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Rathore, C.; Upadhyay, N.; Kaundal, R.; Dwivedi, R.P.; Rahatekar, S.; John, A.; Dua, K.; Tambuwala, M.M.; Jain, S.; Chaudari, D.; et al. Enhanced oral bioavailability and hepatoprotective activity of thymoquinone in the form of phospholipidic nano-constructs. Expert Opin. Drug Deliv. 2020, 17, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Abukhader, M.M.; Khan, S.A. Thymoquinone and Nanoparticles: A Promising Approach for the Clinical Trials. J. Bionanosci. 2017, 11, 258–265. [Google Scholar] [CrossRef]
- Goel, S.; Mishra, P. Thymoquinone loaded mesoporous silica nanoparticles retard cell invasion and enhance in vitro cytotoxicity due to ROS mediated apoptosis in HeLa and MCF-7 cell lines. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109881. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Fathy, M.M.; Abd-elbadia, R.A.; Elshemey, W.M. Targeting of Thymoquinone-loaded mesoporous silica nanoparticles to different brain areas: In vivo study. Life Sci. 2019, 222, 94–102. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Li, Z.-J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.-G.; Aibai, S. Antifungal Activity of Gallic Acid In Vitro and In Vivo. Phytother. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef]
- Subramanian, A.P.; John, A.A.; Vellayappan, M.V.; Balaji, A.; Jaganathan, S.K.; Supriyanto, E.; Yusof, M. Gallic acid: Prospects and molecular mechanisms of its anticancer activity. RSC Adv. 2015, 5, 35608–35621. [Google Scholar] [CrossRef]
- Akbari, G. Molecular mechanisms underlying gallic acid effects against cardiovascular diseases: An update review. Avicenna J. Phytomed. 2020, 10, 11–23. [Google Scholar]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X19874174. [Google Scholar] [CrossRef]
- Shabani, S.; Rabiei, Z.; Amini-Khoei, H. Exploring the multifaceted neuroprotective actions of gallic acid: A review. Int. J. Food Prop. 2020, 23, 736–752. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- de Cristo Soares Alves, A.; Mainardes, R.M.; Khalil, N.M. Nanoencapsulation of gallic acid and evaluation of its cytotoxicity and antioxidant activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.A.; Mahmood, T.; Menaa, F.; Shahzad, Y.; Yousaf, A.M.; Hussain, T.; Ray, S.D. New Perspectives on the Efficacy of Gallic Acid in Cosmetics & Nanocosmeceuticals. Curr. Pharm. Des. 2018, 24, 5181–5187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, D.; Zhu, Z.; Sun, Y. Improved Neuroprotective Effects of Gallic Acid-Loaded Chitosan Nanoparticles Against Ischemic Stroke. Rejuvenation Res. 2019, 23, 284–292. [Google Scholar] [CrossRef]
- Shah, S.T.; Yehya, W.A.; Saad, O.; Simarani, K.; Chowdhury, Z.; Alhadi, A.A.; Al-Ani, L.A. Surface Functionalization of Iron Oxide Nanoparticles with Gallic Acid as Potential Antioxidant and Antimicrobial Agents. Nanomaterials 2017, 7, 306. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Nanoparticle mediated brain targeted delivery of gallic acid: In vivo behavioral and biochemical studies for improved antioxidant and antidepressant-like activity. Drug Deliv. 2012, 19, 378–391. [Google Scholar] [CrossRef]
- Lewandowski, D.; Ruszkowski, P.; Pińska, A.; Schroeder, G.; Kurczewska, J. SBA-15 Mesoporous Silica Modified with Gallic Acid and Evaluation of Its Cytotoxic Activity. PLoS ONE 2015, 10, e0132541. [Google Scholar] [CrossRef]
- Rashidi, L.; Vasheghani-Farahani, E.; Soleimani, M.; Atashi, A.; Rostami, K.; Gangi, F.; Fallahpour, M.; Tahouri, M.T. A cellular uptake and cytotoxicity properties study of gallic acid-loaded mesoporous silica nanoparticles on Caco-2 cells. J. Nanopart. Res. 2014, 16, 2285. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Loizzo, M.R.; Ademiluyi, A.O.; Sharifi-Rad, R.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- Andrade, M.A.; Braga, M.A.; Cesar, P.H.S.; Trento, M.V.C.; Espósito, M.A.; Silva, L.F.; Marcussi, S. Anticancer Properties of Essential Oils: An Overview. Curr. Cancer Drug Targets 2018, 18, 957–966. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic Potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer Properties of Essential Oils and Other Natural Products. Evid.-Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential Oils and Their Constituents as Anticancer Agents: A Mechanistic View. BioMed Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef]
- Fitsiou, E.; Pappa, A. Anticancer Activity of Essential Oils and Other Extracts from Aromatic Plants Grown in Greece. Antioxidants 2019, 8, 290. [Google Scholar] [CrossRef]
- Bhalla, Y.; Gupta, V.K.; Jaitak, V. Anticancer activity of essential oils: A review. J. Sci. Food Agric. 2013, 93, 3643–3653. [Google Scholar] [CrossRef] [PubMed]
- Bayala, B.; Bassole, I.H.; Scifo, R.; Gnoula, C.; Morel, L.; Lobaccaro, J.-M.A.; Simpore, J. Anticancer activity of essential oils and their chemical components—A review. Am. J. Cancer Res. 2014, 4, 591–607. [Google Scholar] [PubMed]
- Manjamalai, A.; Kumar, M.J.; Grace, V.M. Essential oil of Tridax procumbens L induces apoptosis and suppresses angiogenesis and lung metastasis of the B16F-10 cell line in C57BL/6 mice. Asian Pac. J. Cancer Prev. 2012, 13, 5887–5895. [Google Scholar] [CrossRef]
- Manjamalai, A.; Grace, V.M. The chemotherapeutic effect of essential oil of Plectranthus amboinicus (Lour) on lung metastasis developed by B16F-10 cell line in C57BL/6 mice. Cancer Investig. 2013, 31, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Froiio, F.; Ginot, L.; Paolino, D.; Lebaz, N.; Bentaher, A.; Fessi, H.; Elaissari, A. Essential Oils-Loaded Polymer Particles: Preparation, Characterization and Antimicrobial Property. Polymers 2019, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Al-Khalifa, A.R.; Aouf, A.; Boukhebti, H.; Farouk, A. Effect of nanoencapsulation on volatile constituents, and antioxidant and anticancer activities of Algerian Origanum glandulosum Desf. essential oil. Sci. Rep. 2020, 10, 2812. [Google Scholar] [CrossRef]
- Attallah, O.A.; Shetta, A.; Elshishiny, F.; Mamdouh, W. Essential oil loaded pectin/chitosan nanoparticles preparation and optimization via Box–Behnken design against MCF-7 breast cancer cell lines. RSC Adv. 2020, 10, 8703–8708. [Google Scholar] [CrossRef]
- De Matos, S.P.; Teixeira, H.F.; de Lima, Á.A.N.; Veiga-Junior, V.F.; Koester, L.S. Essential Oils and Isolated Terpenes in Nanosystems Designed for Topical Administration: A Review. Biomolecules 2019, 9, 138. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Bernardos, A.; Martínez-Máñez, R.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Electrospun Antimicrobial Films of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Containing Eugenol Essential Oil Encapsulated in Mesoporous Silica Nanoparticles. Nanomaterials 2019, 9, 227. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Sendi, J.J.; Aliakbar, A. Efficacy of Nanoencapsulated Thymus eriocalyx and Thymus kotschyanus Essential Oils by a Mesoporous Material MCM-41 Against Tetranychus urticae (Acari: Tetranychidae). J. Econ. Entomol. 2017, 110, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Janatova, A.; Bernardos, A.; Smid, J.; Frankova, A.; Lhotka, M.; Kourimská, L.; Pulkrabek, J.; Kloucek, P. Long-term antifungal activity of volatile essential oil components released from mesoporous silica materials. Ind. Crop. Prod. 2015, 67, 216–220. [Google Scholar] [CrossRef]
- Jobdeedamrong, A.; Jenjob, R.; Crespy, D. Encapsulation and Release of Essential Oils in Functional Silica Nanocontainers. Langmuir 2018, 34, 13235–13243. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yeom, Y.E.; Kim, D.; Kim, J.; Lee, C. Delayed Volatilization of Lavender Essential Oil Using Mesoporous Silica Nanoparticles. Polymer 2019, 43, 327–330. [Google Scholar] [CrossRef]
- Jin, L.; Teng, J.; Hu, L.; Lan, X.; Xu, Y.; Sheng, J.; Song, Y.; Wang, M. Pepper fragrant essential oil (PFEO) and functionalized MCM-41 nanoparticles: Formation, characterization, and bactericidal activity. J. Sci. Food Agric. 2019, 99, 5168–5175. [Google Scholar] [CrossRef]
- Bravo Cadena, M.; Preston, G.M.; Van der Hoorn, R.A.L.; Townley, H.E.; Thompson, I.P. Species-specific antimicrobial activity of essential oils and enhancement by encapsulation in mesoporous silica nanoparticles. Ind. Crop. Prod. 2018, 122, 582–590. [Google Scholar] [CrossRef]
- Bernardos, A.; Marina, T.; Žáček, P.; Pérez-Esteve, É.; Martínez-Mañez, R.; Lhotka, M.; Kouřimská, L.; Pulkrábek, J.; Klouček, P. Antifungal effect of essential oil components against Aspergillus niger when loaded into silica mesoporous supports. J. Sci. Food Agric. 2015, 95, 2824–2831. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.; Bravo Cadena, M.; Townley, H.E.; Fricker, M.D.; Thompson, I.P. Effective delivery of volatile biocides employing mesoporous silicates for treating biofilms. J. R. Soc. Interface 2017, 14, 20160650. [Google Scholar] [CrossRef]
- Crespo-Ortiz, M.P.; Wei, M.Q. Antitumor activity of artemisinin and its derivatives: From a well-known antimalarial agent to a potential anticancer drug. J. Biomed. Biotechnol. 2012, 2012, 247597. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Wang, H.B.; Zhou, J.J.; Zhang, W.J.; Chen, Q.W. Multifunctional mesoporous nanoparticles as pH-responsive Fe(2+) reservoirs and artemisinin vehicles for synergistic inhibition of tumor growth. Biomaterials 2014, 35, 6498–6507. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, P.R.; Mondhe, D.M. Potential anticancer role of colchicine-based derivatives: An overview. Anticancer Drugs 2017, 28, 250–262. [Google Scholar] [CrossRef]
- Cauda, V.; Engelke, H.; Sauer, A.; Arcizet, D.; Bräuchle, C.; Rädler, J.; Bein, T. Colchicine-Loaded Lipid Bilayer-Coated 50 nm Mesoporous Nanoparticles Efficiently Induce Microtubule Depolymerization upon Cell Uptake. Nano Lett. 2010, 10, 2484–2492. [Google Scholar] [CrossRef]
- Hespeler, D.; Kaltenbach, J.; Pyo, S.M. Glabridin smartPearls—Silica selection, production, amorphous stability and enhanced solubility. Int. J. Pharm. 2019, 561, 228–235. [Google Scholar] [CrossRef]
- Buda, V.; Brezoiu, A.M.; Berger, D.; Pavel, I.Z.; Muntean, D.; Minda, D.; Dehelean, C.A.; Soica, C.; Diaconeasa, Z.; Folescu, R.; et al. Biological Evaluation of Black Chokeberry Extract Free and Embedded in Two Mesoporous Silica-Type Matrices. Pharmaceutics 2020, 12, 838. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).