Cyclodextrin-Based Supramolecular Complexes of Osteoinductive Agents for Dental Tissue Regeneration
Abstract
1. Introduction
2. Regenerative Treatment and Drugs
2.1. Regenerative Treatment Using Cells and Signaling Proteins
2.2. Small-Molecule Osteoinductive Agents
3. Inclusion Complexes of β-Cyclodextrin with Osteoinductive Drugs
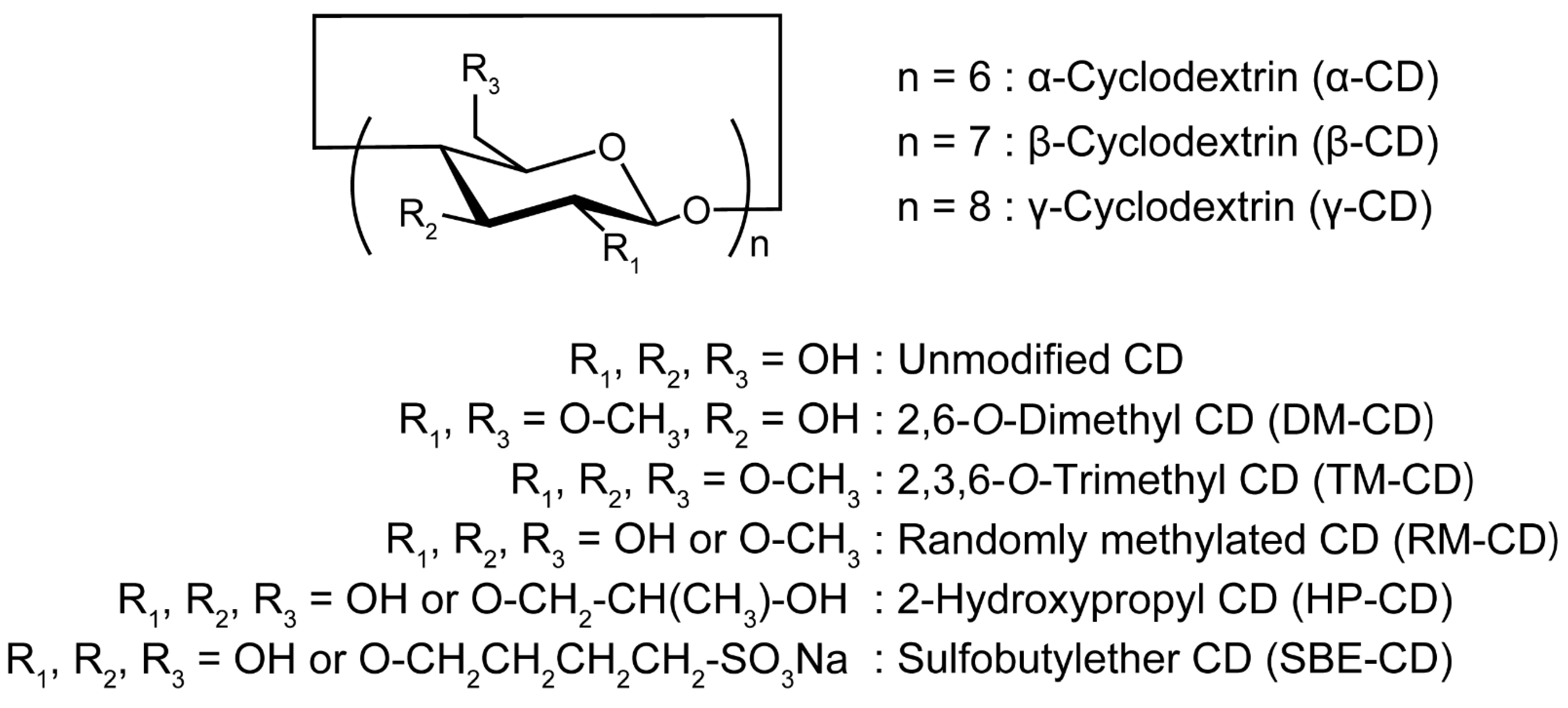
3.1. Cyclodextrins and Inclusion Complexes
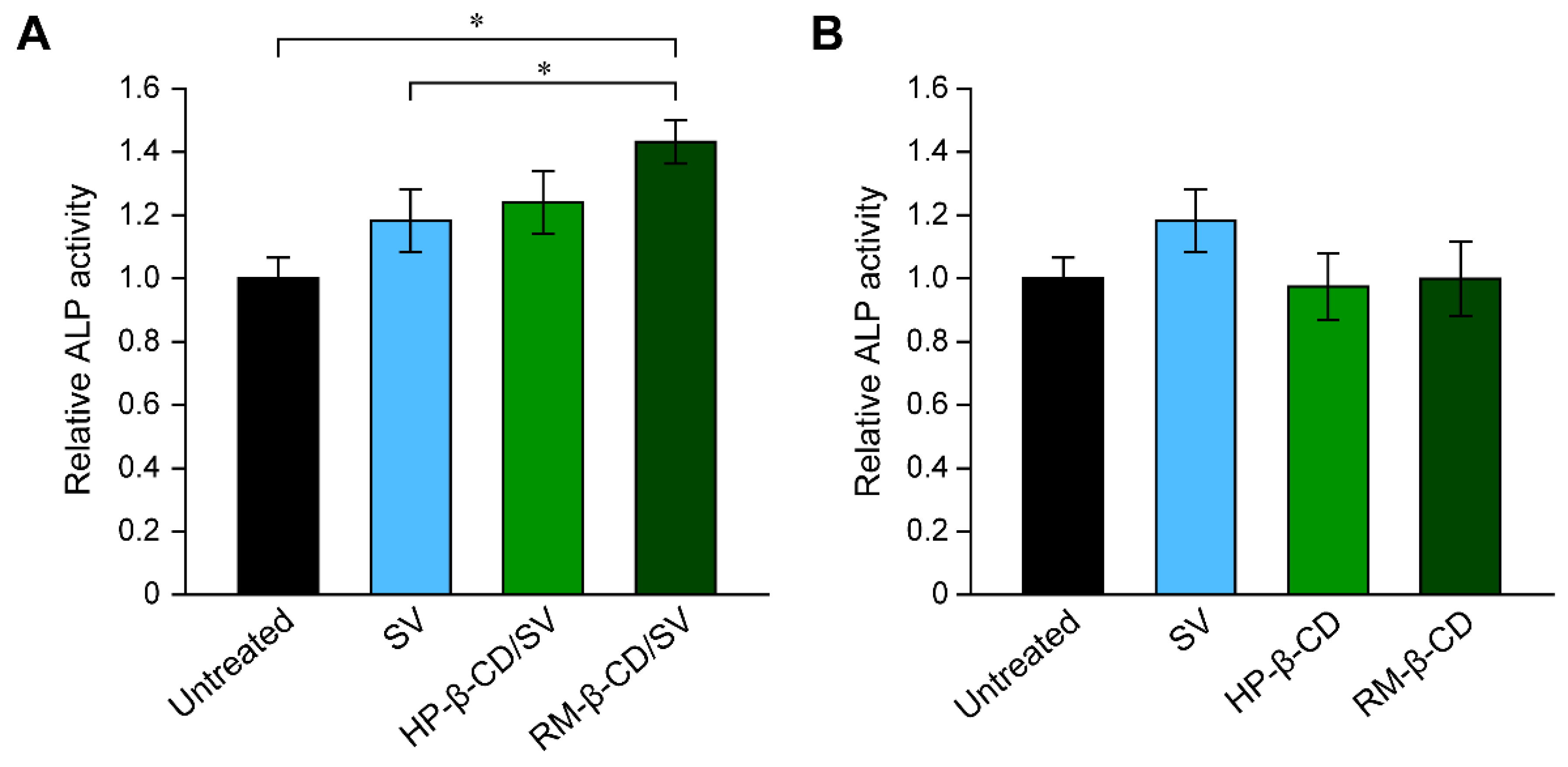
3.2. Inclusion Complex of Simvastatin
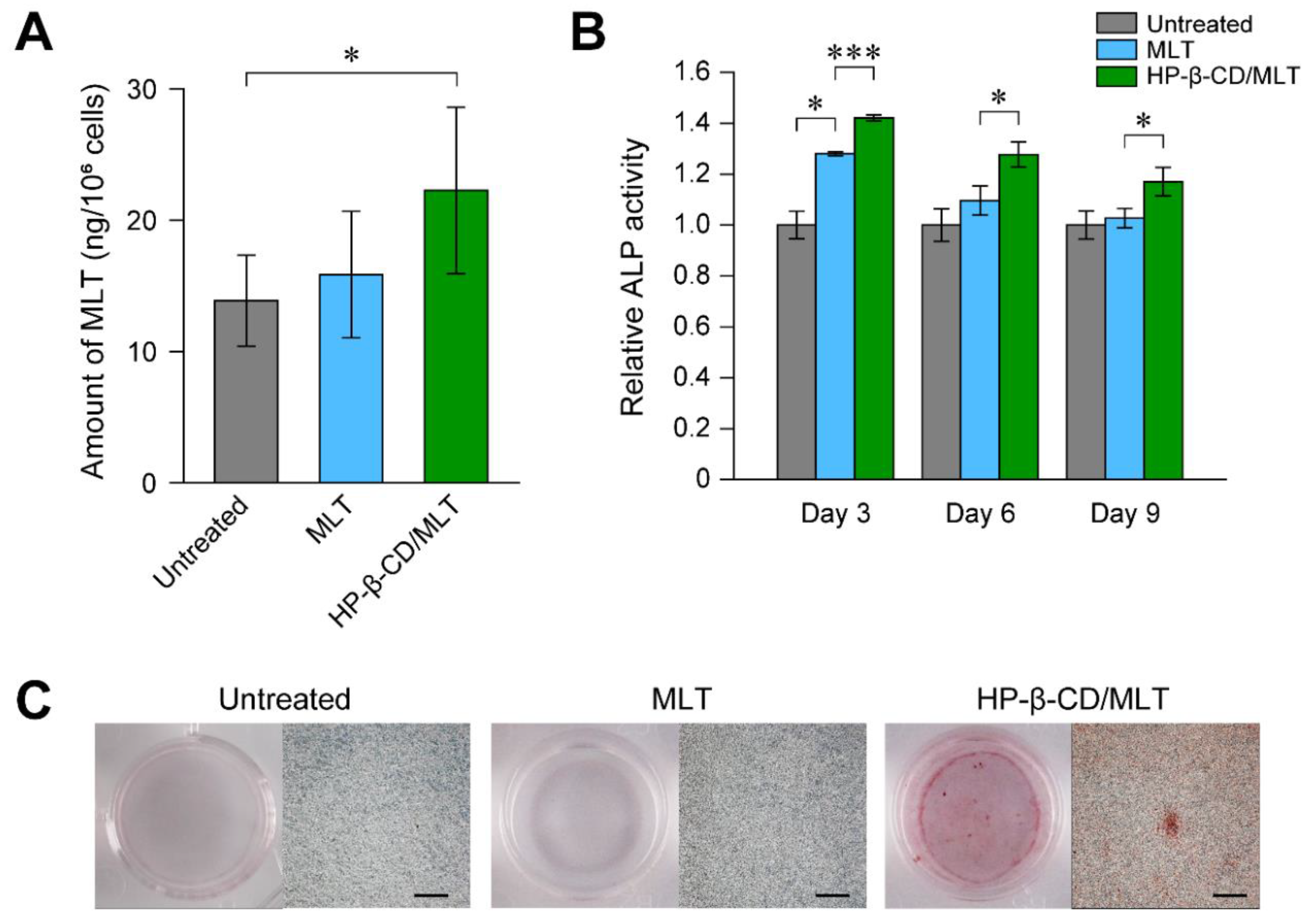
3.3. Inclusion Complexes of Other Osteoinductive Drugs
4. Enhanced Therapeutic Effects of Growth Factors Using Anionic Polymers
4.1. Heparin and Other Anionic Polymers
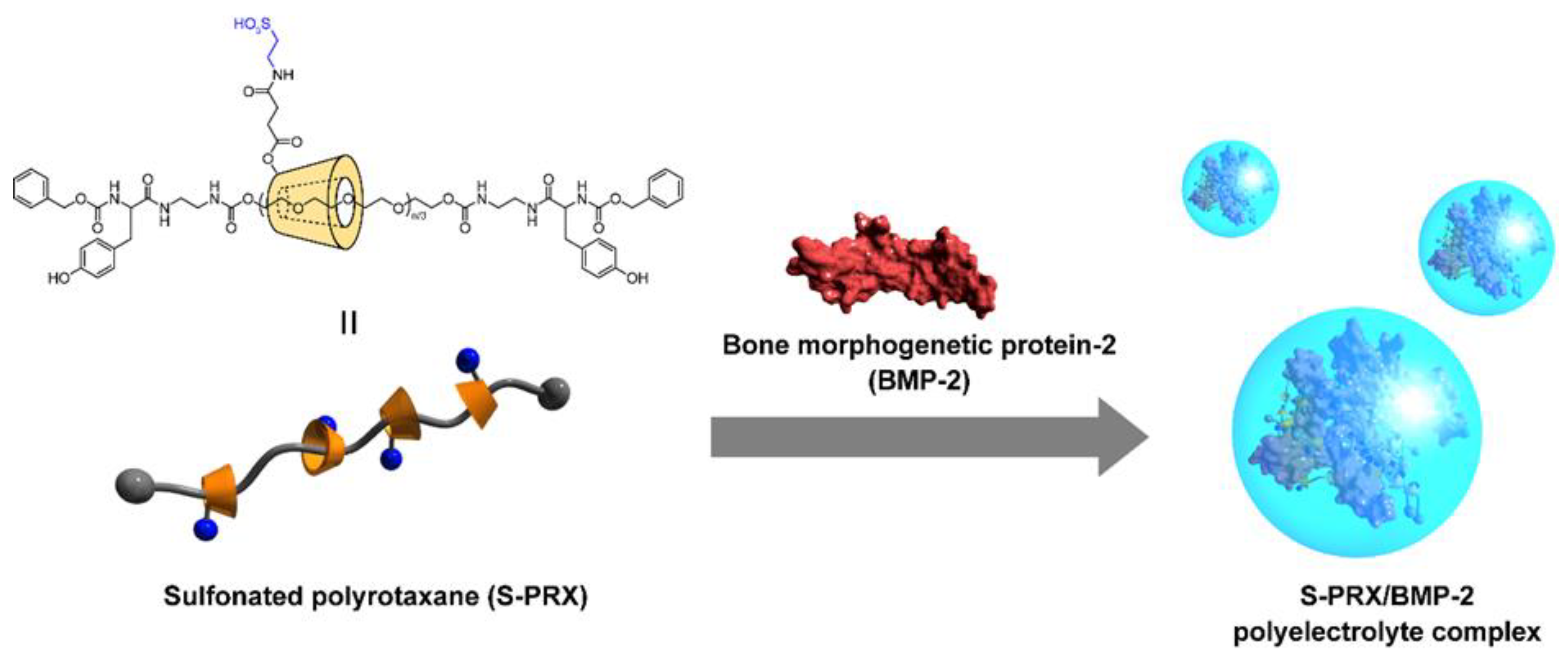
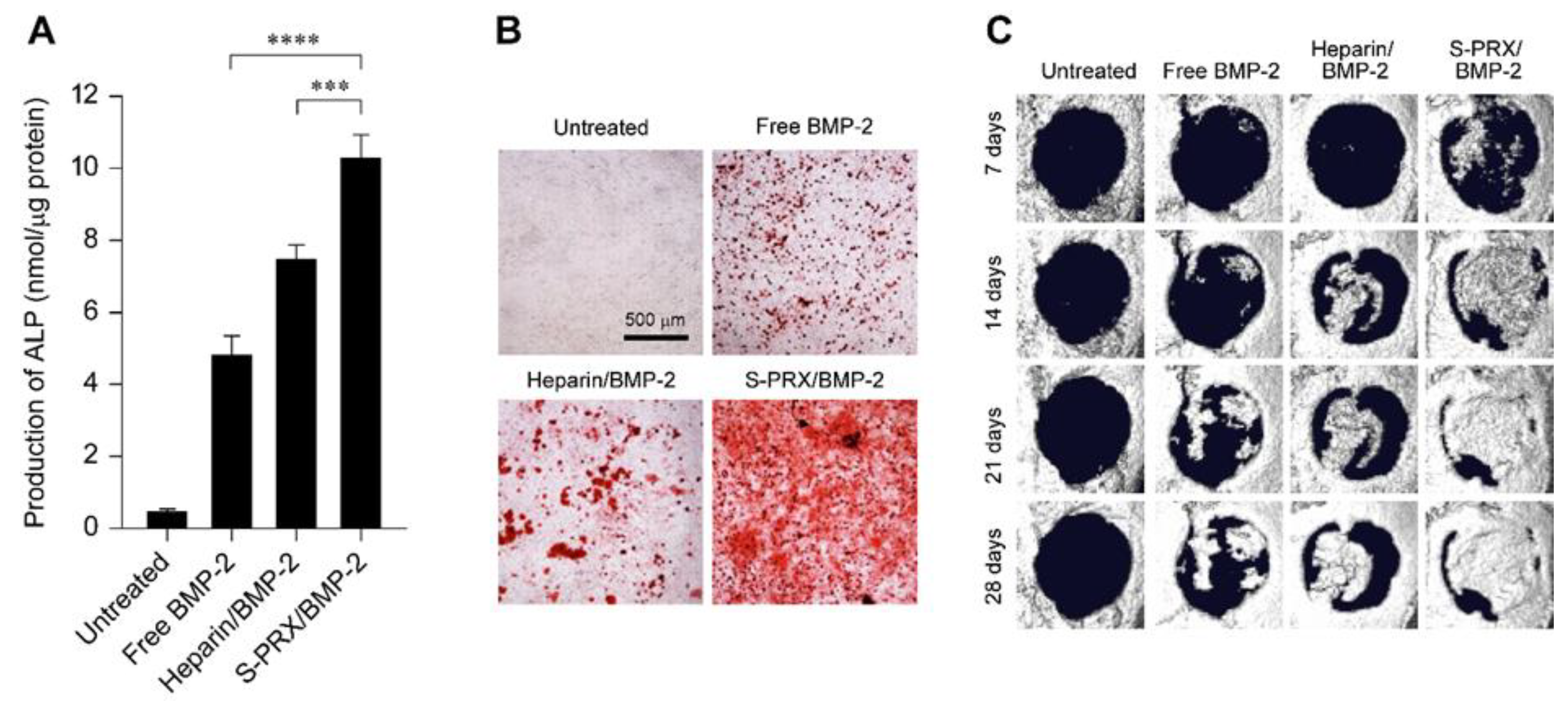
4.2. Supramolecular Polyrotaxane for Polyelectrolyte Complexation with BMP-2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petersen, P.E. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century—The approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2003, 31, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Yagiela, J.A.; Dowd, F.J.; Johnson, B.; Mariotti, A.; Neidle, E.A. (Eds.) Pharmacology and Therapeutics for Dentistry-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Madl, C.M.; Heilshorn, S.C.; Blau, H.M. Bioengineering strategies to accelerate stem cell therapeutics. Nat. Cell Biol. 2018, 557, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Silva, E.A.; Mooney, D.J. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J. R. Soc. Interface 2010, 8, 153–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Zhang, J.; Zhang, M.; An, Y.; Chen, F.; Wu, Z.-F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010, 31, 7892–7927. [Google Scholar] [CrossRef]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissuesvs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef]
- Ceccarelli, G.; Presta, R.; Benedetti, L.; De Angelis, M.G.C.; Lupi, S.M.; Baena, R.R.Y. Emerging Perspectives in Scaffold for Tissue Engineering in Oral Surgery. Stem Cells Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Moioli, E.K.; Clark, P.A.; Xin, X.; Lal, S.; Mao, J.J. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 308–324. [Google Scholar] [CrossRef]
- De Witte, T.-M.; E Fratila-Apachitei, L.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Sundelacruz, S.; Kaplan, D.L. Stem cell- and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin. Cell Dev. Biol. 2009, 20, 646–655. [Google Scholar] [CrossRef]
- Dawson, J.I.; Oreffo, R.O.C. Bridging the regeneration gap: Stem cells, biomaterials and clinical translation in bone tissue engineering. Arch. Biochem. Biophys. 2008, 473, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Li, R.H.; Friess, W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Stocum, D.L.; Zupanc, G.K. Stretching the limits: Stem cells in regeneration science. Dev. Dyn. 2008, 237, 3648–3671. [Google Scholar] [CrossRef]
- Togel, F.; Westenfelder, C. Adult bone marrow–derived stem cells for organ regeneration and repair. Dev. Dyn. 2007, 236, 3321–3331. [Google Scholar] [CrossRef]
- Tuan, R.S. Biology of Developmental and Regenerative Skeletogenesis. Clin. Orthop. Relat. Res. 2004, 427, S105–S117. [Google Scholar] [CrossRef] [PubMed]
- Satija, N.K.; Singh, V.K.; Verma, Y.K.; Gupta, P.; Sharma, S.; Afrin, F.; Sharma, M.; Sharma, P.; Tripathi, R.P.; Gurudutta, G.U. Mesenchymal stem cell-based therapy: A new paradigm in regenerative medicine. J. Cell. Mol. Med. 2009, 13, 4385–4402. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Washio, K.; Yoshida, T.; Ishikawa, I.; Ando, T.; Yamato, M.; Okano, T. Cell sheet engineering and its application for periodontal regeneration. J. Tissue Eng. Regen. Med. 2015, 9, 343–356. [Google Scholar] [CrossRef]
- Babensee, J.E.; McIntire, L.V.; Mikos, A.G. Growth Factor Delivery for Tissue Engineering. Pharm. Res. 2000, 17, 497–504. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by Autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef]
- Bleuming, S.A.; He, X.C.; Kodach, L.L.; Hardwick, J.C.; Koopman, F.A.; Kate, F.J.T.; Van Deventer, S.J.; Hommes, D.W.; Peppelenbosch, M.P.; Offerhaus, G.J.; et al. Bone Morphogenetic Protein Signaling Suppresses Tumorigenesis at Gastric Epithelial Transition Zones in Mice. Cancer Res. 2007, 67, 8149–8155. [Google Scholar] [CrossRef] [PubMed]
- Even, J.; Eskander, M.; Kang, J. Bone Morphogenetic Protein in Spine Surgery: Current and Future Uses. J. Am. Acad. Orthop. Surg. 2012, 20, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Rickard, D.; Sullivan, T.; Shenker, B.; Leboy, P.; Kazhdan, I. Induction of Rapid Osteoblast Differentiation in Rat Bone Marrow Stromal Cell Cultures by Dexamethasone and BMP-2. Dev. Biol. 1994, 161, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Rieß, J.; Gelse, K.; Kloss, F.; Von Der Mark, K.; Wiltfang, J.; Neukam, F.W.; Schneider, H. Bone regeneration in critical size defects by cell-mediated BMP-2 gene transfer: A comparison of adenoviral vectors and liposomes. Gene Ther. 2003, 10, 1089–1098. [Google Scholar] [CrossRef]
- Rosen, V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev. 2009, 20, 475–480. [Google Scholar] [CrossRef]
- Nakashima, M.; Reddi, A.H. The application of bone morphogenetic proteins to dental tissue engineering. Nat. Biotechnol. 2003, 21, 1025–1032. [Google Scholar] [CrossRef]
- Zara, J.N.; Siu, R.K.; Zhang, X.; Shen, J.; Ngo, R.; Lee, M.; Li, W.; Chiang, M.; Chung, J.; Kwak, J.; et al. High Doses of Bone Morphogenetic Protein 2 Induce Structurally Abnormal Bone and Inflammation In Vivo. Tissue Eng. Part A 2011, 17, 1389–1399. [Google Scholar] [CrossRef]
- Kanatani, M.; Sugimoto, T.; Kaji, H.; Kobayashi, T.; Nishiyama, K.; Fukase, M.; Kumegawa, M.; Chihara, K. Stimulatory effect of bone morphogenetic protein-2 on osteoclast-like cell formation and bone-resorbing activity. J. Bone Miner. Res. 2009, 10, 1681–1690. [Google Scholar] [CrossRef]
- Feldman, G.J.; Billings, P.C.; Patel, R.V.; Caron, R.J.; Guenther, C.; Kingsley, D.M.; Kaplan, F.S.; Shore, E.M. Over-expression ofBMP4 andBMP5 in a child with axial skeletal malformations and heterotopic ossification: A new syndrome. Am. J. Med. Genet. Part A 2007, 143, 699–706. [Google Scholar] [CrossRef]
- Glassman, S.D.; Carreon, L.; Djurasovic, M.; Campbell, M.J.; Puno, R.M.; Johnson, J.R.; Dimar, J.R. Posterolateral lumbar spine fusion with INFUSE bone graft. Spine J. 2007, 7, 44–49. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials 2003, 24, 4375–4383. [Google Scholar] [CrossRef]
- Zhu, G.; Mallery, S.R.; Schwendeman, S.P. Stabilization of proteins encapsulated in injectable poly (lactide- co-glycolide). Nat. Biotechnol. 2000, 18, 52–57. [Google Scholar] [CrossRef] [PubMed]
- McKay, W.F.; Peckham, S.M.; Badura, J.M. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE® Bone Graft). Int. Orthop. 2007, 31, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J.; Marx, R.E.; Nevins, M.; Triplett, G.; Lazaro, E.; Lilly, L.C.; Alder, M.; Nummikoski, P. A feasibility study evaluat-ing rhBMP-2/absorbable collagen sponge for maxillary sinus floor augmentation. Int. J. Periodontics Restor. Dent. 1997, 17, 11–25. [Google Scholar]
- Boyne, P.J.; Lilly, L.C.; Marx, R.E.; Moy, P.K.; Nevins, M.; Spagnoli, D.B.; Triplett, R.G. De Novo Bone Induction by Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) in Maxillary Sinus Floor Augmentation. J. Oral Maxillofac. Surg. 2005, 63, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Fiorellini, J.P.; Howell, T.H.; Cochran, D.; Malmquist, J.; Lilly, L.C.; Spagnoli, D.; Toljanic, J.; Jones, A.; Nevins, M. Randomized Study Evaluating Recombinant Human Bone Morphogenetic Protein-2 for Extraction Socket Augmentation. J. Periodontol. 2005, 76, 605–613. [Google Scholar] [CrossRef]
- Armelin, H.A. Pituitary Extracts and Steroid Hormones in the Control of 3T3 Cell Growth. Proc. Natl. Acad. Sci. USA 1973, 70, 2702–2706. [Google Scholar] [CrossRef]
- Finklestein, S.P.; Plomaritoglou, A. Growth factors. In Head Trauma: Basic, Preclinical, and Clinical Directions; Miller, L.P., Hayes, R.L., Newcomb, J.K., Eds.; Wiley: New York, NY, USA, 2001; pp. 165–187. [Google Scholar]
- Blaber, M.; Disalvo, J.; Thomas, K.A. X-ray Crystal Structure of Human Acidic Fibroblast Growth Factor. Biochemistry 1996, 35, 2086–2094. [Google Scholar] [CrossRef]
- Ornitz, D.M.; Itoh, N. Fibroblast growth factors. Genome Biol. 2001, 2, 1–12. [Google Scholar] [CrossRef]
- Vlodavsky, I.; Korner, G.; Ishai-Michaeli, R.; Bashkin, P.; Bar-Shavit, R.; Fuks, Z. Extracellular matrix-resident growth factors and enzymes: Possible involvement in tumor metastasis and angiogenesis. Cancer Metastasis Rev. 1990, 9, 203–226. [Google Scholar] [CrossRef]
- Green, P.J.; Walsh, F.S.; Doherty, P. Promiscuity of fibroblast growth factor receptors. BioEssays 1996, 18, 639–646. [Google Scholar] [CrossRef]
- Kitamura, M.; Nakashima, K.; Kowashi, Y.; Fujii, T.; Shimauchi, H.; Sasano, T.; Furuuchi, T.; Fukuda, M.; Noguchi, T.; Shibutani, T.; et al. Periodontal Tissue Regeneration Using Fibroblast Growth Factor -2: Randomized Controlled Phase II Clinical Trial. PLoS ONE 2008, 3, e2611. [Google Scholar] [CrossRef] [PubMed]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Heijl, L.; Heden, G.; Svardstrom, G.; Ostgren, A. Enamel matrix derivative (EMDOGAINR) in the treatment of intrabony periodontal defects. J. Clin. Periodontol. 1997, 24, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review. Eur. J. Oral Implant. 2009, 2, 247–266. [Google Scholar]
- Hammarstrom, L. Enamel matrix, cementum development and regeneration. J. Clin. Periodontol. 1997, 24, 658–668. [Google Scholar] [CrossRef]
- Bowers, G.M.; Chadroff, B.; Carnevale, R.; Mellonig, J.; Corio, R.; Emerson, J.; Stevens, M.; Romberg, E. Histologic Evaluation of New Attachment Apparatus Formation in Humans. J. Periodontol. 1989, 60, 683–693. [Google Scholar] [CrossRef]
- Burg, K.J.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Kimelman, N.; Pelled, G.; Helm, G.A.; Huard, J.; Schwarz, E.M.; Gazit, Z. Review: Gene- and Stem Cell–Based Therapeutics for Bone Regeneration and Repair. Tissue Eng. 2007, 13, 1135–1150. [Google Scholar] [CrossRef]
- Mundy, G.R.; Garrett, R.; Harris, S.; Chan, J.; Chen, D.; Rossini, G.; Boyce, B.; Zhao, M.; Gutierrez, G. Stimulation of Bone Formation in Vitro and in Rodents by Statins. Science 1999, 286, 1946–1949. [Google Scholar] [CrossRef]
- Endo, A. The discovery and development of HMG-CoA reductase inhibitors. J. Lipid Res. 1992, 33, 1569–1582. [Google Scholar] [CrossRef]
- Tobert, J.A. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K.; Laufs, U. Pleiotropic Effects of Statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Tanigo, T.; Takaoka, R.; Tabata, Y. Sustained release of water-insoluble simvastatin from biodegradable hydrogel augments bone regeneration. J. Control. Release 2010, 143, 201–206. [Google Scholar] [CrossRef]
- Nath, S.D.; Son, S.; Sadiasa, A.; Min, Y.K.; Lee, B.T. Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int. J. Pharm. 2013, 443, 87–94. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, C.; Zang, G.; Sun, H. The effect of simvastatin on remodelling of the alveolar bone following tooth extraction. Int. J. Oral Maxillofac. Surg. 2008, 37, 170–176. [Google Scholar] [CrossRef]
- Roth, J.A.; Kim, B.-G.; Lin, W.-L.; Cho, M.-I. Melatonin Promotes Osteoblast Differentiation and Bone Formation. J. Biol. Chem. 1999, 274, 22041–22047. [Google Scholar] [CrossRef]
- Koyama, H.; Nakade, O.; Takada, Y.; Kaku, T.; Lau, K.-H.W. Melatonin at Pharmacologic Doses Increases Bone Mass by Suppressing Resorption Through Down-Regulation of the RANKL-Mediated Osteoclast Formation and Activation. J. Bone Miner. Res. 2002, 17, 1219–1229. [Google Scholar] [CrossRef]
- Han, Y.; Kim, Y.-M.; Kim, H.S.; Lee, K.Y. Melatonin promotes osteoblast differentiation by regulating Osterix protein stability and expression. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Kim, C.H.; Yoo, Y.-M. Fluid shear stress and melatonin in combination activate anabolic proteins in MC3T3-E1 osteoblast cells. J. Pineal Res. 2013, 54, 453–461. [Google Scholar] [CrossRef]
- Balmayor, E. Targeted delivery as key for the success of small osteoinductive molecules. Adv. Drug Deliv. Rev. 2015, 94, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.T.; Ashe, K.M.; Henry, N.; Kan, H.M.; Lo, K.W.-H. Delivery of small molecules for bone regenerative engineering: Preclinical studies and potential clinical applications. Drug Discov. Today 2014, 19, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Uekama, K.; Hirayama, F.; Irie, T. Cyclodextrin Drug Carrier Systems. Chem. Rev. 1998, 98, 2045–2076. [Google Scholar] [CrossRef]
- Brewster, M.E.; Loftsson, T. Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 2007, 59, 645–666. [Google Scholar] [CrossRef]
- Liu, X.-M.; Wiswall, A.T.; Rutledge, J.E.; Akhter, M.P.; Cullen, D.M.; Reinhardt, R.A.; Wang, D. Osteotropic β-cyclodextrin for local bone regeneration. Biomaterials 2008, 29, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.; Fakes, M.; Serajuddin, A. Differentiation of 3-Hydroxy-3-methylglutaryl-coenzyme A Reductase Inhibitors by Their Relative Lipophilicity. Pharm. Pharmacol. Commun. 1999, 5, 269–271. [Google Scholar] [CrossRef]
- Ungaro, F.; Giovino, C.; Catanzano, O.; Miro, A.; Mele, A.; Quaglia, F.; La Rotonda, M.I. Use of cyclodextrins as solubilizing agents for simvastatin: Effect of hydroxypropyl-β-cyclodextrin on lactone/hydroxyacid aqueous equilibrium. Int. J. Pharm. 2011, 404, 49–56. [Google Scholar] [CrossRef]
- Jun, S.W.; Kim, M.-S.; Kim, J.-S.; Park, H.J.; Lee, S.; Woo, J.-S.; Hwang, S.-J. Preparation and characterization of simvastatin/hydroxypropyl-β-cyclodextrin inclusion complex using supercritical antisolvent (SAS) process. Eur. J. Pharm. Biopharm. 2007, 66, 413–421. [Google Scholar] [CrossRef]
- Süle, A.; Szente, L.; Csempesz, F. Enhancement of Drug Solubility in Supramolecular and Colloidal Systems. J. Pharm. Sci. 2009, 98, 484–494. [Google Scholar] [CrossRef]
- Csempesz, F.; Süle, A.; Puskás, I. Induced surface activity of supramolecular cyclodextrin–statin complexes: Relevance in drug delivery. Colloids. Surf. A Physicochem. Eng. Asp. 2010, 354, 308–313. [Google Scholar] [CrossRef]
- Yoshinari, M.; Matsuzaka, K.; Hashimoto, S.; Ishihara, K.; Inoue, T.; Oda, Y.; Ide, T.; Tanaka, T. Controlled release of simvastatin acid using cyclodextrin inclusion system. Dent. Mater. J. 2007, 26, 451–456. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Terauchi, M.; Inada, T.; Tonegawa, A.; Tamura, A.; Yamaguchi, S.; Harada, K.; Yui, N. Supramolecular inclusion complexation of simvastatin with methylated β-cyclodextrins for promoting osteogenic differentiation. Int. J. Biol. Macromol. 2016, 93, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Kodama, T.; Konishi, K.; Abe, K.; Asami, S.; Oikawa, S. Compactin and Simvastatin, but Not Pravastatin, Induce Bone Morphogenetic Protein-2 in Human Osteosarcoma Cells. Biochem. Biophys. Res. Commun. 2000, 271, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Matsunuma, A.; Kawane, T.; Horiuchi, N. Simvastatin Promotes Osteoblast Differentiation and Mineralization in MC3T3-E1 Cells. Biochem. Biophys. Res. Commun. 2001, 280, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Matsunuma, A.; Kurahashi, I.; Yanagawa, T.; Yoshida, H.; Horiuchi, N. Induction of osteoblast differentiation indices by statins in MC3T3-E1 cells. J. Cell. Biochem. 2004, 92, 458–471. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Masson, M. Cyclodextrins in topical drug formulation: Theory and practice. Int. J. Pharm. 2001, 225, 15–30. [Google Scholar] [CrossRef]
- Terauchi, M.; Tamura, A.; Yamaguchi, S.; Yui, N. Enhanced cellular uptake and osteogenic differentiation efficiency of melatonin by inclusion complexation with 2-hydroxypropyl β-cyclodextrin. Int. J. Pharm. 2018, 547, 53–60. [Google Scholar] [CrossRef]
- Jahed, V.; Vasheghani-Farahani, E.; Bagheri, F.; Zarrabi, A.; Fink, T.; Larsen, K.L. Enhanced Cellular Uptake Of Phenamil Through Inclusion Complex With Histidine Functionalized β-Cyclodextrin As Penetrative Osteoinductive Agent. Int. J. Nanomed. 2019, 14, 8221–8234. [Google Scholar] [CrossRef]
- Bongiorno, D.; Ceraulo, L.; Mele, A.; Panzeri, W.; Selva, A.; Liveri, V.T. Structural and physicochemical characterization of the inclusion complexes of cyclomaltooligosaccharides (cyclodextrins) with melatonin. Carbohydr. Res. 2002, 337, 743–754. [Google Scholar] [CrossRef]
- Babu, R.J.; Dayal, P.; Singh, M. Effect of cyclodextrins on the complexation and nasal permeation of melatonin. Drug Deliv. 2008, 15, 381–388. [Google Scholar] [CrossRef]
- Maeda, H.; Ogawa, Y.; Nakayama, H. Inclusion complexes of melatonin with modified cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2013, 78, 217–224. [Google Scholar] [CrossRef]
- Vlachou, M.; Papamichael, M.; Siamidi, A.; Fragouli, I.; Afroudakis, P.A.; Kompogennitaki, R.; Dotsikas, Y. Comparative In Vitro Controlled Release Studies on the Chronobiotic Hormone Melatonin from Cyclodextrins-Containing Matrices and Cyclodextrin: Melatonin Complexes. Int. J. Mol. Sci. 2017, 18, 1641. [Google Scholar] [CrossRef]
- Arisaka, Y.; Yui, N. Tethered bone morphogenetic protein-2 onto sulfonated-polyrotaxane based surfaces promotes osteogenic differentiation of MC3T3-E1 cells. J. Biomater. Sci. Polym. Ed. 2017, 28, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.W.-H.; Ulery, B.D.; Ashe, K.M.; Laurencin, C.T. Studies of bone morphogenetic protein-based surgical repair. Adv. Drug Deliv. Rev. 2012, 64, 1277–1291. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.C.; Pomerantz, J.H.; Brunet, L.J.; Kim, J.-B.; Chou, Y.-F.; Wu, B.M.; Harland, R.; Blau, H.M.; Longaker, M.T. Noggin Suppression Enhancesin VitroOsteogenesis and Acceleratesin VivoBone Formation. J. Biol. Chem. 2007, 282, 26450–26459. [Google Scholar] [CrossRef]
- Cowan, C.M.; Shi, Y.-Y.; Aalami, O.O.; Chou, Y.-F.; Mari, C.; Thomas, R.; Quarto, N.; Contag, C.H.; Wu, B.; Longaker, M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat. Biotechnol. 2004, 22, 560–567. [Google Scholar] [CrossRef]
- Martino, M.M.; Briquez, P.S.; Ranga, A.; Lutolf, M.P.; Hubbell, J.A. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc. Natl. Acad. Sci. USA 2013, 110, 4563–4568. [Google Scholar] [CrossRef]
- Takada, T.; Katagiri, T.; Ifuku, M.; Morimura, N.; Kobayashi, M.; Hasegawa, K.; Ogamo, A.; Kamijo, R. Sulfated Polysaccharides Enhance the Biological Activities of Bone Morphogenetic Proteins. J. Biol. Chem. 2003, 278, 43229–43235. [Google Scholar] [CrossRef]
- Zhao, B.; Katagiri, T.; Toyoda, H.; Takada, T.; Yanai, T.; Fukuda, T.; Chung, U.-I.; Koike, T.; Takaoka, K.; Kamijo, R. Heparin Potentiates the in Vivo Ectopic Bone Formation Induced by Bone Morphogenetic Protein-2. J. Biol. Chem. 2006, 281, 23246–23253. [Google Scholar] [CrossRef]
- Macdonald, M.L.; Samuel, R.E.; Shah, N.J.; Padera, R.F.; Beben, Y.M.; Hammond, P.T. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials 2011, 32, 1446–1453. [Google Scholar] [CrossRef]
- Bhakta, G.; Rai, B.; Lim, Z.X.; Hui, J.H.; Stein, G.S.; Van Wijnen, A.J.; Nurcombe, V.; Prestwich, G.D.; Cool, S.M. Hyaluronic acid-based hydrogels functionalized with heparin that support controlled release of bioactive BMP-2. Biomaterials 2012, 33, 6113–6122. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yun, Y.-P.; Han, Y.-K.; Lee, D.-W.; Ohe, J.-Y.; Lee, B.-S.; Song, H.-R.; Park, K.; Choi, B.-J. Osteogenesis induction of periodontal ligament cells onto bone morphogenic protein-2 immobilized PCL fibers. Carbohydr. Polym. 2014, 99, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Hettiaratchi, M.H.; Krishnan, L.; Rouse, T.; Chou, C.; McDevitt, T.C.; Guldberg, R.E. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci. Adv. 2020, 6, eaay1240. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Song, S.-H.; Yun, Y.P.; Choi, B.-J.; Kwon, I.K.; Bae, M.S.; Moon, H.-J.; Kwon, Y.-D. The effect of immobilization of heparin and bone morphogenic protein-2 (BMP-2) to titanium surfaces on inflammation and osteoblast function. Biomaterials 2011, 32, 366–373. [Google Scholar] [CrossRef]
- Damus, P.S.; Hicks, M.; Rosenberg, R.D. Anticoagulant Action of Heparin. Nat. Cell Biol. 1973, 246, 355–357. [Google Scholar] [CrossRef]
- Jin, L.; Abrahams, J.P.; Skinner, R.; Petitou, M.; Pike, R.N.; Carrell, R.W. The anticoagulant activation of antithrombin by heparin. Proc. Natl. Acad. Sci. USA 1997, 94, 14683–14688. [Google Scholar] [CrossRef]
- Kanzaki, S.; Ariyoshi, W.; Takahashi, T.; Okinaga, T.; Kaneuji, T.; Mitsugi, S.; Nakashima, K.; Tsujisawa, T.; Nishihara, T. Dual effects of heparin on BMP-2-induced osteogenic activity in MC3T3-E1 cells. Pharmacol. Rep. 2011, 63, 1222–1230. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyauchi, S.; Tawada, A.; Anada, T.; Matsuzaka, S.; Suzuki, O. Oversulfated chondroitin sulfate-E binds to BMP-4 and enhances osteoblast differentiation. J. Cell. Physiol. 2008, 217, 769–777. [Google Scholar] [CrossRef]
- Bramono, D.S.; Murali, S.; Rai, B.; Ling, L.; Poh, W.T.; Lim, Z.X.; Stein, G.S.; Nurcombe, V.; Van Wijnen, A.J.; Cool, S.M. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2). Bone 2012, 50, 954–964. [Google Scholar] [CrossRef]
- Kisiel, M.; Klar, A.S.; Ventura, M.; Buijs, J.; Mafina, M.-K.; Cool, S.M.; Hilborn, J. Complexation and Sequestration of BMP-2 from an ECM Mimetic Hyaluronan Gel for Improved Bone Formation. PLoS ONE 2013, 8, e78551. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, J.; Wang, J.; Yao, W.; Liu, C.; Chen, J.; Cao, X. Enhanced bioactivity of bone morphogenetic protein-2 with low dose of 2-N, 6-O-sulfated chitosan in vitro and in vivo. Biomaterials 2009, 30, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Peschel, D.; Zhang, K.; Fischer, S.; Groth, T. Modulation of osteogenic activity of BMP-2 by cellulose and chitosan derivatives. Acta Biomater. 2012, 8, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Paluck, S.J.; McGahran, A.J.; Maynard, H.D. Poly(vinyl sulfonate) Facilitates bFGF-Induced Cell Proliferation. Biomacromolecules 2015, 16, 2684–2692. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, M.; Tamura, A.; Tonegawa, A.; Yamaguchi, S.; Yoda, T.; Yui, N. Polyelectrolyte Complexes between Polycarboxylates and BMP-2 for Enhancing Osteogenic Differentiation: Effect of Chemical Structure of Polycarboxylates. Polymers 2019, 11, 1327. [Google Scholar] [CrossRef] [PubMed]
- Paluck, S.J.; Nguyen, T.H.; Maynard, H.D. Heparin-Mimicking Polymers: Synthesis and Biological Applications. Biomacromolecules 2016, 17, 3417–3440. [Google Scholar] [CrossRef]
- Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Polymeric Rotaxanes. Chem. Rev. 2009, 109, 5974–6023. [Google Scholar] [CrossRef]
- Wenz, G.; Han, A.B.-H.; Müller, A. Cyclodextrin Rotaxanes and Polyrotaxanes. Chem. Rev. 2006, 106, 782–817. [Google Scholar] [CrossRef]
- Huang, F.; Gibson, H.W. Polypseudorotaxanes and polyrotaxanes. Prog. Polym. Sci. 2005, 30, 982–1018. [Google Scholar] [CrossRef]
- Tamura, A.; Yui, N. Threaded macromolecules as a versatile framework for biomaterials. Chem. Commun. 2014, 50, 13433–13446. [Google Scholar] [CrossRef]
- Kidowaki, M.; Zhao, C.; Kataoka, T.; Ito, K. Thermoreversible sol-gel transition of an aqueous solution of polyrotaxane composed of highly methylated ?-cyclodextrin and polyethylene glycol. Chem. Commun. 2006, 21, 4102–4103. [Google Scholar] [CrossRef]
- Tonegawa, A.; Tamura, A.; Yui, N. Emerging Nanoassembly of Polyrotaxanes Comprising Acetylated α-Cyclodextrins and High-Molecular-Weight Axle Polymer. ACS Macro Lett. 2019, 8, 826–834. [Google Scholar] [CrossRef]
- Tamura, A.; Ohashi, M.; Yui, N. Oligo(ethylene glycol)-modified β-cyclodextrin-based polyrotaxanes for simultaneously modulating solubility and cellular internalization efficiency. J. Biomater. Sci. Polym. Ed. 2017, 28, 1124–1139. [Google Scholar] [CrossRef] [PubMed]
- Ooya, T.; Choi, H.S.; Yamashita, A.; Yui, N.; Sugaya, Y.; Kano, A.; Maruyama, A.; Akita, H.; Ito, R.; Kogure, A.K.; et al. Biocleavable Polyrotaxane−Plasmid DNA Polyplex for Enhanced Gene Delivery. J. Am. Chem. Soc. 2006, 128, 3852–3853. [Google Scholar] [CrossRef] [PubMed]
- Shibaguchi, K.; Tamura, A.; Terauchi, M.; Matsumura, M.; Miura, H.; Yui, N. Mannosylated Polyrotaxanes for Increasing Cellular Uptake Efficiency in Macrophages through Receptor-Mediated Endocytosis. Molecules 2019, 24, 439. [Google Scholar] [CrossRef]
- Tamura, A.; Yui, N. Polyrotaxane-based systemic delivery of β-cyclodextrins for potentiating therapeutic efficacy in a mouse model of Niemann-Pick type C disease. J. Control. Release 2018, 269, 148–158. [Google Scholar] [CrossRef]
- Nishida, K.; Tamura, A.; Yui, N. ER stress-mediated autophagic cell death induction through methylated β-cyclodextrins-threaded acid-labile polyrotaxanes. J. Control. Release 2018, 275, 20–31. [Google Scholar] [CrossRef]
- Arisaka, Y.; Yui, N. Polyrotaxane-based biointerfaces with dynamic biomaterial functions. J. Mater. Chem. B 2019, 7, 2123–2129. [Google Scholar] [CrossRef]
- Ooya, T.; Eguchi, M.; Yui, N. Supramolecular Design for Multivalent Interaction: Maltose Mobility along Polyrotaxane Enhanced Binding with Concanavalin A. J. Am. Chem. Soc. 2003, 125, 13016–13017. [Google Scholar] [CrossRef]
- Hyun, H.; Yui, N. Ligand Accessibility to Receptor Binding Sites Enhanced by Movable Polyrotaxanes. Macromol. Biosci. 2011, 11, 765–771. [Google Scholar] [CrossRef]
- Eguchi, M.; Ooya, T.; Yui, N. Controlling the mechanism of trypsin inhibition by the numbers of α-cyclodextrins and carboxyl groups in carboxyethylester-polyrotaxanes. J. Control. Release 2004, 96, 301–307. [Google Scholar] [CrossRef]
- Tamura, A.; Ikeda, G.; Seo, J.-H.; Tsuchiya, K.; Yajima, H.; Sasaki, Y.; Akiyoshi, K.; Yui, N. Molecular logistics using cytocleavable polyrotaxanes for the reactivation of enzymes delivered in living cells. Sci. Rep. 2013, 3, 2252. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Ikeda, G.; Nishida, K.; Yui, N. Cationic Polyrotaxanes as a Feasible Framework for the Intracellular Delivery and Sustainable Activity of Anionic Enzymes: A Comparison Study with Methacrylate-Based Polycations. Macromol. Biosci. 2015, 15, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Yui, N. Cellular internalization and gene silencing of siRNA polyplexes by cytocleavable cationic polyrotaxanes with tailored rigid backbones. Biomaterials 2013, 34, 2480–2491. [Google Scholar] [CrossRef] [PubMed]
- Inada, T.; Tamura, A.; Terauchi, M.; Yamaguchi, S.; Yui, N. Silencing-mediated enhancement of osteogenic differentiation by supramolecular ternary siRNA polyplexes comprising biocleavable cationic polyrotaxanes and anionic fusogenic peptides. Biomater. Sci. 2018, 6, 440–450. [Google Scholar] [CrossRef]
- Terauchi, M.; Ikeda, G.; Nishida, K.; Tamura, A.; Yamaguchi, S.; Harada, K.; Yui, N. Supramolecular Polyelectrolyte Complexes of Bone Morphogenetic Protein-2 with Sulfonated Polyrotaxanes to Induce Enhanced Osteogenic Differentiation. Macromol. Biosci. 2015, 15, 953–964. [Google Scholar] [CrossRef]
- Terauchi, M.; Inada, T.; Kanemaru, T.; Ikeda, G.; Tonegawa, A.; Nishida, K.; Arisaka, Y.; Tamura, A.; Yamaguchi, S.; Yui, N. Potentiating bioactivity of BMP-2 by polyelectrolyte complexation with sulfonated polyrotaxanes to induce rapid bone regeneration in a mouse calvarial defect. J. Biomed. Mater. Res. Part A 2017, 105, 1355–1363. [Google Scholar] [CrossRef]
- Rawadi, G.; Vayssière, B.; Dunn, F.; Baron, R.; Roman-Roman, S. BMP-2 Controls Alkaline Phosphatase Expression and Osteoblast Mineralization by a Wnt Autocrine Loop. J. Bone Miner. Res. 2003, 18, 1842–1853. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B. Molecular Mechanisms Mediating Proliferation/Differentiation Interrelationships during Progressive Development of the Osteoblast Phenotype. Endocr. Rev. 1993, 14, 424–442. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Kleinschmidt, J.C. The Critical Size Defect as an Experimental Model to Test Bone Repair Materials. J. Craniofacial Surg. 1990, 1, 60–68. [Google Scholar] [CrossRef]

| Anionic Polymer | Growth Factor | Reference |
|---|---|---|
| Heparin | BMP-2 | [90,91] |
| Dextran sulfate | BMP-2 | [90] |
| Dermatan sulfate | BMP-2 | [102] |
| 2-N,6-O-sulfated chitosan | BMP-2 | [103] |
| Cellulose sulfate | BMP-2 | [104] |
| Chitosan sulfate | BMP-2 | [104] |
| Poly(vinyl sulfonate) | FGF-2 | [105] |
| Poly(glutamic acid) | BMP-2 | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terauchi, M.; Tamura, A.; Arisaka, Y.; Masuda, H.; Yoda, T.; Yui, N. Cyclodextrin-Based Supramolecular Complexes of Osteoinductive Agents for Dental Tissue Regeneration. Pharmaceutics 2021, 13, 136. https://doi.org/10.3390/pharmaceutics13020136
Terauchi M, Tamura A, Arisaka Y, Masuda H, Yoda T, Yui N. Cyclodextrin-Based Supramolecular Complexes of Osteoinductive Agents for Dental Tissue Regeneration. Pharmaceutics. 2021; 13(2):136. https://doi.org/10.3390/pharmaceutics13020136
Chicago/Turabian StyleTerauchi, Masahiko, Atsushi Tamura, Yoshinori Arisaka, Hiroki Masuda, Tetsuya Yoda, and Nobuhiko Yui. 2021. "Cyclodextrin-Based Supramolecular Complexes of Osteoinductive Agents for Dental Tissue Regeneration" Pharmaceutics 13, no. 2: 136. https://doi.org/10.3390/pharmaceutics13020136
APA StyleTerauchi, M., Tamura, A., Arisaka, Y., Masuda, H., Yoda, T., & Yui, N. (2021). Cyclodextrin-Based Supramolecular Complexes of Osteoinductive Agents for Dental Tissue Regeneration. Pharmaceutics, 13(2), 136. https://doi.org/10.3390/pharmaceutics13020136






