Abdominoplasty Skin-Based Dressing for Deep Wound Treatment—Evaluation of Different Methods of Preparation on Therapeutic Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Skin Collection and Biobanking
2.2. Decellularization Procedures
2.3. Histochemical and Immunohistochemical Staining
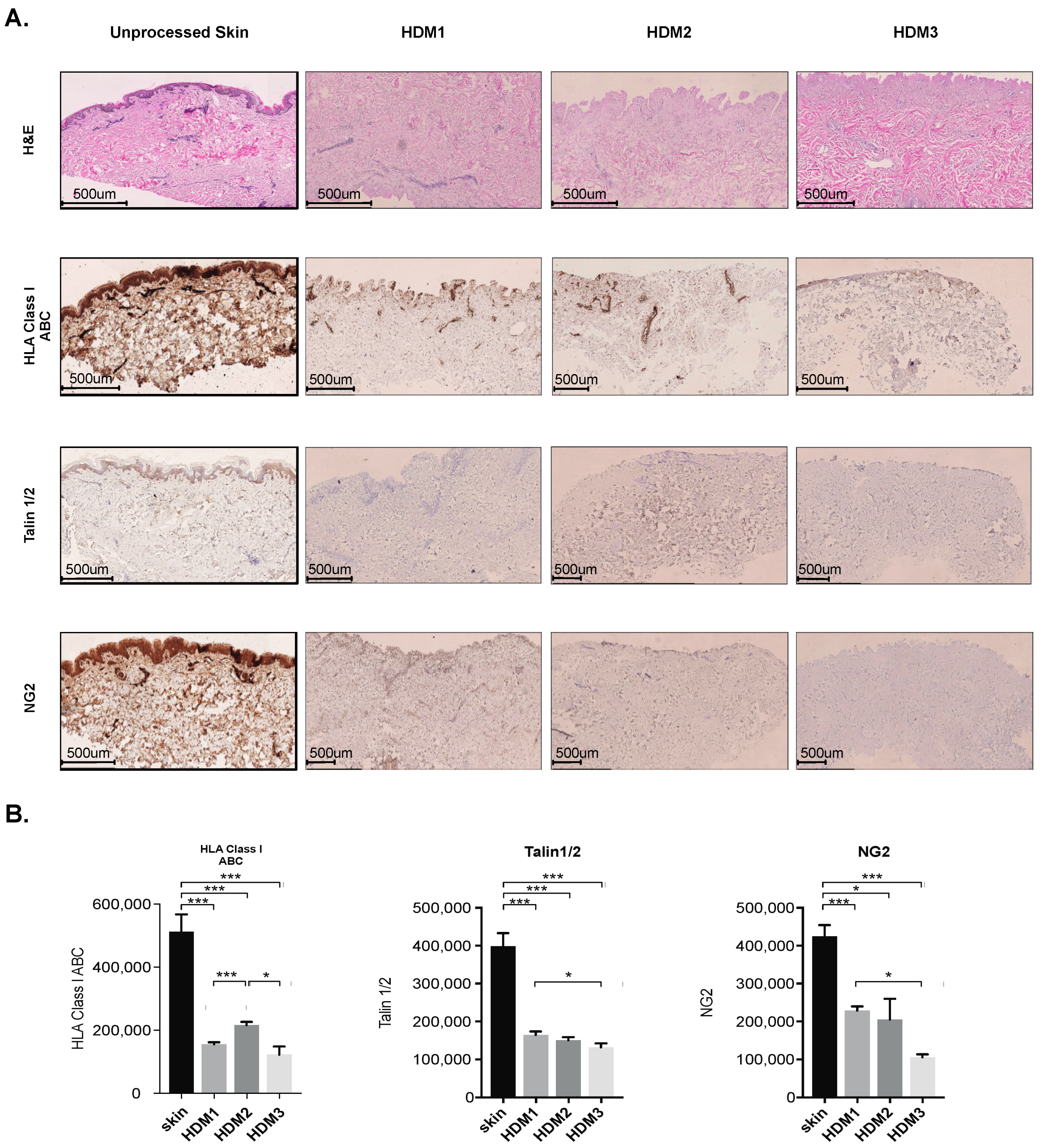
2.4. Quantification of Immunohistochemical Staining
2.5. Peripheral Blood Mononuclear Cells Isolation
2.6. Proliferation Assay
2.7. Murine Model of Wound Healing
2.8. RNA Isolation and Quantitative PCR Assay
2.9. Statistics
3. Results
3.1. Abdominoplasty Skin Is Suitable for Human Acellular Dermal Matrix Preparation
3.2. Decellularization of Abdominoplasty Skin Preserves Collagen III and IV Architecture
3.3. Abdominoplasty Skin-Derived ADM Accelerates Wound Closure
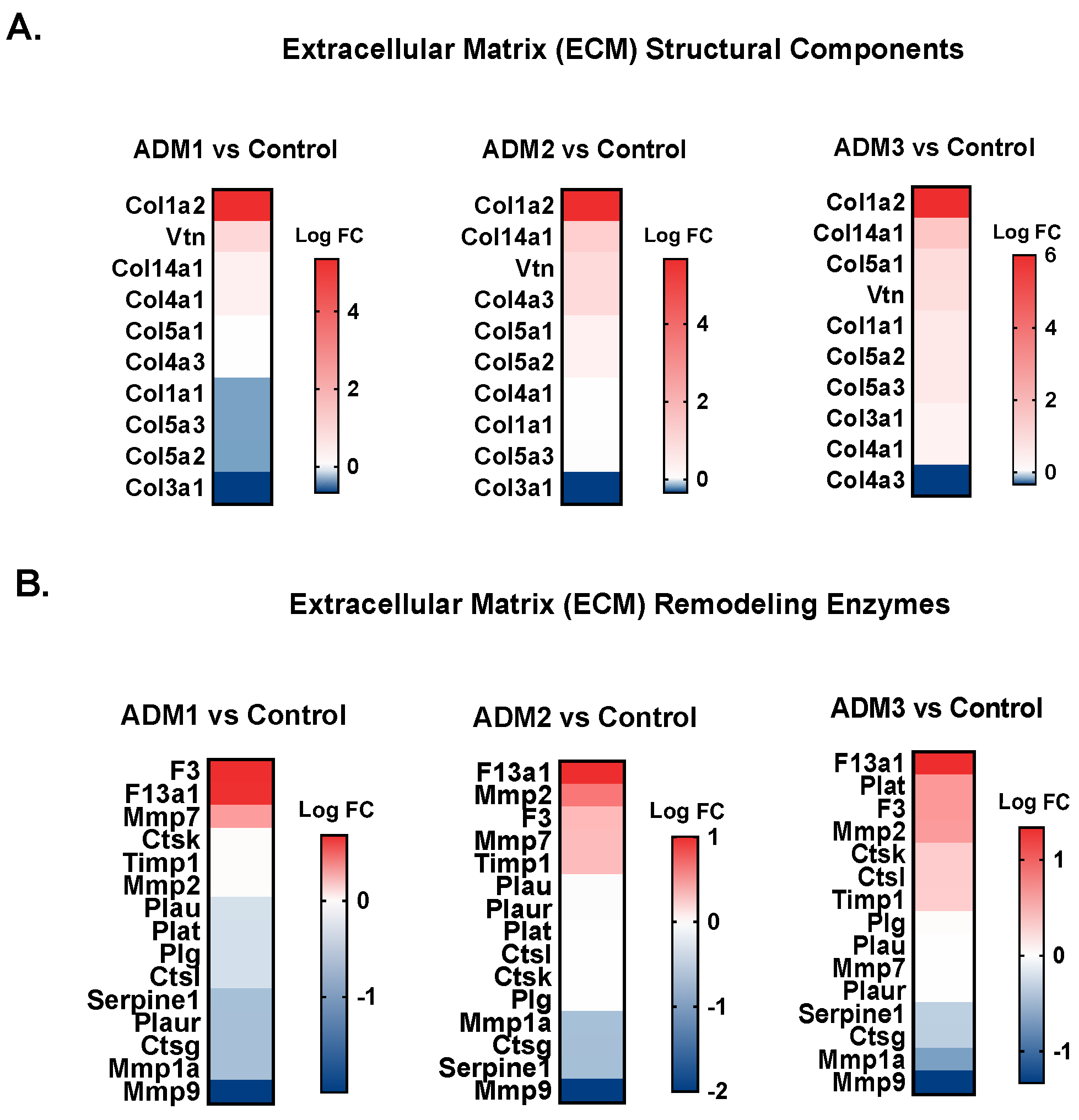
3.4. ADM1-Treated Wounds Show Slightly Modified Transcriptomic Profiles When Compared to ADM2- and ADM3-Treated Counterparts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivera, A.E.; Spencer, J.M. Clinical aspects of full-thickness wound healing. Clin. Dermatol. 2007, 25, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.; Pham, L.; Henderson, J.; Stalnaker, K.J.; Anderson, R.R.; Tam, J. Multi-faceted enhancement of full-thickness skin wound healing by treatment with autologous micro skin tissue columns. Sci. Rep. 2021, 11, 1688. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Mohammadi, A.A.; Kabiri, H.; Hashempoor, M.R.; Mahmoodi, M.; Amini, M.; Mehrabani, D. The healing effect of Wharton’s jelly stem cells seeded on biological scaffold in chronic skin ulcers: A randomized clinical trial. J. Cosmet. Dermatol. 2019, 18, 1961–1967. [Google Scholar] [CrossRef] [PubMed]
- Percival, N.J. Classification of Wounds and their Management. Surgery 2002, 20, 114–117. [Google Scholar] [CrossRef]
- Wanner, M.; Adams, C.; Ratner, D. Chapter 9—Skin Grafts. In Flaps and Grafts in Dermatologic Surgery; Rohrer, T.E., Cook, J.L., Migden, M.R., Nguyen, T.H., Mellette, J.R., Eds.; W.B. Saunders: Edinburgh, UK, 2007; pp. 107–116. [Google Scholar]
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic Diabetic Wounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655. [Google Scholar] [CrossRef]
- Cazzell, S.; Moyer, P.M.; Samsell, B.; Dorsch, K.; McLean, J.; Moore, M.A. A Prospective, Multicenter, Single-Arm Clinical Trial for Treatment of Complex Diabetic Foot Ulcers with Deep Exposure Using Acellular Dermal Matrix. Adv. Skin Wound Care 2019, 32, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Truong, A.T.; Kowal-Vern, A.; Latenser, B.A.; Wiley, D.E.; Walter, R.J. Comparison of dermal substitutes in wound healing utilizing a nude mouse model. J. Burn. Wounds 2005, 4, e4. [Google Scholar]
- Boháč, M.; Danišovič, Ľ.; Koller, J.; Dragúňová, J.; Varga, I. What happens to an acellular dermal matrix after implantation in the human body? A histological and electron microscopic study. Eur. J. Histochem. 2018, 62, 2873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, D.J.; Khan, F.; Belch, J.J.; Mitchell, M.R.; Leese, G.P. Blood flow changes in diabetic foot ulcers treated with dermal replacement therapy. J. Foot Ankle Surg. 2002, 41, 233–237. [Google Scholar] [CrossRef]
- Easterbrook, C.; Maddern, G. Porcine and bovine surgical products: Jewish, Muslim, and Hindu perspectives. Arch. Surg. 2008, 143, 366–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.J.; Ha, J.H.; Jin, U.S. Comparison of irradiated and non-irradiated acellular dermal matrices in breast reconstruction under radiotherapy. Arch. Plast. Surg. 2021, 48, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Matarasso, A.; Matarasso, D.M.; Matarasso, E.J. Abdominoplasty: Classic principles and technique. Clin. Plast. Surg. 2014, 41, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.; Prosser, H.C.; Tan, J.T.; Vanags, L.Z.; Ng, M.K.; Bursill, C.A. Murine model of wound healing. J. Vis. Exp. 2013, 75, e50265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Ge, J.; Tredget, E.E.; Wu, Y. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat. Protoc. 2013, 8, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.E.; Farhat, W.A.; Ming, J.M.; McCammon, K.A. Review of clinical experience on biomaterials and tissue engineering of urinary bladder. World J. Urol. 2020, 38, 2081–2093. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Mauck, R.L.; Gorman, J.H.; Gorman, R.C. Acellular biomaterials: An evolving alternative to cell-based therapies. Sci. Transl. Med. 2013, 5, 176ps174. [Google Scholar] [CrossRef] [Green Version]
- Zenn, M.; Venturi, M.; Pittman, T.; Spear, S.; Gurtner, G.; Robb, G.; Mesbahi, A.; Dayan, J. Optimizing Outcomes of Postmastectomy Breast Reconstruction with Acellular Dermal Matrix: A Review of Recent Clinical Data. Eplasty 2017, 17, e18. [Google Scholar]
- Hussey, G.S.; Dziki, J.L.; Badylak, S.F. Extracellular matrix-based materials for regenerative medicine. Nat. Rev. Mater. 2018, 3, 159–173. [Google Scholar] [CrossRef]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef]
- Pliszczyński, J.; Nita, M.; Kowalewski, C.; Woźniak, K.; Eljaszewicz, A.; Moniuszko, M.; Kamiński, A.; Śladowski, D.; Zimek, Z.; Majewski, S.; et al. Transplantation of a New Biological Product in Rare Diseases, Such as Epidermolysis Bullosa: Response and Clinical Outcome. Transplant. Proc. 2020, 52, 2239–2243. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.G.; Tzeng, Y.S.; Wang, C.H. Treatment of severe burn with DermACELL(®), an acellular dermal matrix. Int. J. Burn. Trauma 2012, 2, 105–109. [Google Scholar]
- Wollina, U. One-stage Reconstruction of Soft Tissue Defects with the Sandwich Technique: Collagen-elastin Dermal Template and Skin Grafts. J. Cutan. Aesthet. Surg. 2011, 4, 176–182. [Google Scholar] [CrossRef]
- Nita, M.; Pliszczyński, J.; Eljaszewicz, A.; Moniuszko, M.; Ołdak, T.; Woźniak, K.; Majewski, S.; Kowalewski, C.; Kamiński, A.; Śladowski, D.; et al. Surgical Treatment of Wounds Using Stem Cells in Epidermolysis Bullosa (EB); IntechOpen: London, UK, 2021. [Google Scholar]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.Y.; Smith, T.D.; Meli, V.S.; Tran, T.N.; Botvinick, E.L.; Liu, W.F. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater. 2017, 47, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.N.; Valentin, J.E.; Stewart-Akers, A.M.; McCabe, G.P.; Badylak, S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009, 30, 1482–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, S.R.; Chiu, B.; Churchill, T.A.; Zhu, L.; Lakey, J.R.T.; Ross, D.B. Comparison of aortic valve allograft decellularization techniques in the rat. J. Biomed. Mater. Res. A 2006, 79, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Kasimir, M.T.; Rieder, E.; Seebacher, G.; Silberhumer, G.; Wolner, E.; Weigel, G.; Simon, P. Comparison of different decellularization procedures of porcine heart valves. Int. J. Artif. Organs 2003, 26, 421–427. [Google Scholar] [CrossRef]
- Prasertsung, I.; Kanokpanont, S.; Bunaprasert, T.; Thanakit, V.; Damrongsakkul, S. Development of acellular dermis from porcine skin using periodic pressurized technique. J. Biomed. Mater. Res. B 2008, 85, 210–219. [Google Scholar] [CrossRef]
- Grauss, R.W.; Hazekamp, M.G.; Oppenhuizen, F.; van Munsteren, C.J.; Gittenberger-de Groot, A.C.; DeRuiter, M.C. Histological evaluation of decellularised porcine aortic valves: Matrix changes due to different decellularisation methods. Eur. J. Cardiothorac. Surg. 2005, 27, 566–571. [Google Scholar] [CrossRef]
- Syed, O.; Walters, N.J.; Day, R.M.; Kim, H.-W.; Knowles, J.C. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater. 2014, 10, 5043–5054. [Google Scholar] [CrossRef] [Green Version]
- Woods, T.; Gratzer, P.F. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials 2005, 26, 7339–7349. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, R.; Zavazava, N.; Steinmann, J.; Müller-Ruchholtz, W. Interaction of papain-digested HLA class I molecules with human alloreactive cytotoxic T lymphocytes (CTL). Clin. Exp. Immunol. 1993, 91, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Zavazava, N.; Krönke, M. Soluble HLA class I molecules induce apoptosis in alloreactive cytotoxic T lymphocytes. Nat. Med. 1996, 2, 1005–1010. [Google Scholar] [CrossRef]
- Sayegh, M.H.; Carpenter, C.B. Role of indirect allorecognition in allograft rejection. Int. Rev. Immunol. 1996, 13, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Spaggiari, G.M.; Contini, P.; Dondero, A.; Carosio, R.; Puppo, F.; Indiveri, F.; Zocchi, M.R.; Poggi, A. Soluble HLA class I induces NK cell apoptosis upon the engagement of killer-activating HLA class I receptors through FasL-Fas interaction. Blood 2002, 100, 4098–4107. [Google Scholar] [CrossRef]
- Mayrand, D.; Laforce-Lavoie, A.; Larochelle, S.; Langlois, A.; Genest, H.; Roy, M.; Moulin, V.J. Angiogenic properties of myofibroblasts isolated from normal human skin wounds. Angiogenesis 2012, 15, 199–212. [Google Scholar] [CrossRef]
- Sumiyoshi, H.; Kitamura, H.; Matsuo, N.; Tatsukawa, S.; Ishikawa, K.; Okamoto, O.; Fujikura, Y.; Fujiwara, S.; Yoshioka, H. Transient expression of mouse pro-α3(V) collagen gene (Col5a3) in wound healing. Connect. Tissue Res. 2012, 53, 313–317. [Google Scholar] [CrossRef]
- Birk, D.E. Type V collagen: Heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron 2001, 32, 223–237. [Google Scholar] [CrossRef]
- Avraamides, C.J.; Garmy-Susini, B.; Varner, J.A. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 2008, 8, 604–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokinen, J.; Dadu, E.; Nykvist, P.; Käpylä, J.; White, D.J.; Ivaska, J.; Vehviläinen, P.; Reunanen, H.; Larjava, H.; Häkkinen, L.; et al. Integrin-mediated cell adhesion to type I collagen fibrils. J. Biol. Chem. 2004, 279, 31956–31963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, D.M.; Han, J.; Ginsberg, M.H. Alpha4 integrins and the immune response. Immunol. Rev. 2002, 186, 118–124. [Google Scholar] [CrossRef] [PubMed]



| Decellularization Protocol | Phase 1 | Phase 2 | Washing | Storage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reagents | Time (h) | Temp. (°C) | RPM | Epidermis Removal | Reagents | Time (h) | Temp. (°C) | RPM | Washing/Quantity | Time (h) | (RPM) | Lyophilization/Temp (°C) | |
| ADM1 | 1M NaCl + antibiotic mix | 24 | 37 | 40 | Mechanical | SDS 0.1% + Antibiotic Mix | 24 | 37 | 40 | H2O/5 | 24 | 60 | YES/−70 |
| ADM2 | 2M NaCl | 24 | 37 | 40 | Mechanical | SDS 0.1% + Antibiotic Mix | 24 | 37 | 40 | H2O/5 | 24 | 60 | YES/−70 |
| ADM3 | TrypLE Select + antibiotic mix | 24 | 37 | 40 | Not Applicable | 3% Triton X-100 in PBS + Antibiotic Mix | 24 | 37 | 40 | H2O/5 | 24 | 60 | YES/−70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groth, D.; Poplawska, I.; Tynecka, M.; Grubczak, K.; Holl, J.; Starosz, A.; Janucik, A.; Borkowska, K.; Juchniewicz, D.; Hady, H.R.; et al. Abdominoplasty Skin-Based Dressing for Deep Wound Treatment—Evaluation of Different Methods of Preparation on Therapeutic Potential. Pharmaceutics 2021, 13, 2118. https://doi.org/10.3390/pharmaceutics13122118
Groth D, Poplawska I, Tynecka M, Grubczak K, Holl J, Starosz A, Janucik A, Borkowska K, Juchniewicz D, Hady HR, et al. Abdominoplasty Skin-Based Dressing for Deep Wound Treatment—Evaluation of Different Methods of Preparation on Therapeutic Potential. Pharmaceutics. 2021; 13(12):2118. https://doi.org/10.3390/pharmaceutics13122118
Chicago/Turabian StyleGroth, Dawid, Izabela Poplawska, Marlena Tynecka, Kamil Grubczak, Jordan Holl, Aleksandra Starosz, Adrian Janucik, Klaudia Borkowska, Dorota Juchniewicz, Hady Razak Hady, and et al. 2021. "Abdominoplasty Skin-Based Dressing for Deep Wound Treatment—Evaluation of Different Methods of Preparation on Therapeutic Potential" Pharmaceutics 13, no. 12: 2118. https://doi.org/10.3390/pharmaceutics13122118
APA StyleGroth, D., Poplawska, I., Tynecka, M., Grubczak, K., Holl, J., Starosz, A., Janucik, A., Borkowska, K., Juchniewicz, D., Hady, H. R., Czaban, S., Reszec, J., Kaminski, A., Czech, T., Kowalewski, C., Fiedor, P., Zimek, Z., Lewandowska, H., Oldak, T., ... Eljaszewicz, A. (2021). Abdominoplasty Skin-Based Dressing for Deep Wound Treatment—Evaluation of Different Methods of Preparation on Therapeutic Potential. Pharmaceutics, 13(12), 2118. https://doi.org/10.3390/pharmaceutics13122118







