Abstract
Cancer stem cells (CSCs) are characterized by intrinsic self-renewal and tumorigenic properties, and play important roles in tumor initiation, progression, and resistance to diverse forms of anticancer therapy. Accordingly, targeting signaling pathways that are critical for CSC maintenance and biofunctions, including the Wnt, Notch, Hippo, and Hedgehog signaling cascades, remains a promising therapeutic strategy in multiple cancer types. Furthermore, advances in various cancer omics approaches have largely increased our knowledge of the molecular basis of CSCs, and provided numerous novel targets for anticancer therapy. However, the majority of recently identified targets remain ‘undruggable’ through small-molecule agents, whereas the implications of exogenous RNA interference (RNAi, including siRNA and miRNA) may make it possible to translate our knowledge into therapeutics in a timely manner. With the recent advances of nanomedicine, in vivo delivery of RNAi using elaborate nanoparticles can potently overcome the intrinsic limitations of RNAi alone, as it is rapidly degraded and has unpredictable off-target side effects. Herein, we present an update on the development of RNAi-delivering nanoplatforms in CSC-targeted anticancer therapy and discuss their potential implications in clinical trials.
1. Introduction
Cancer is one of the leading causes of poor quality of life and mortality worldwide [1,2]. Currently, most cancer patients have micro- or macroscopic systemic metastases when they are initially diagnosed [1]. As such, systemic therapies, including chemotherapy, targeted therapy, and immunotherapy continue to be the main lines of treatment in antitumor strategies. Although numerous new drugs have emerged, drug resistance frequently occurs and remains a dominant obstacle to cancer treatment [3]. Initially, the combined administration of agents with distinct mechanisms of action was employed to solve the resistance of single-agent therapy. This approach, named polychemotherapy, is effective in the early stage of chemotherapy, while its efficacy plateaus over the following period. Multiple mechanisms of drug resistance to structurally and mechanistically distinct antitumor agents has emerged as a new challenge [4]. Most patients who die from cancer eventually develop resistance to multiple therapeutic modalities [5]. Although new therapeutic strategies, including targeted therapies and immunotherapy, have been proposed [6,7], cancer resistance continues to emerge by similar mechanisms [8,9,10]. As a result, several approaches aimed at combating unique pathways of drug resistance emerged fast and contributed significantly to improved prognosis [11].
It has been well established that most malignant tumors are composed of multiple phenotypically distinct subpopulations of cancer cells that promote varied responses to chemotherapy [12]. Among these, the cancer stem cell (CSC) concept posits the presence of a minor subpopulation that is highly capable of self-renewal and multidirectional differentiation and largely contributes to the formation of heterogeneous tumor masses [13]. The introduction of the CSC concept provides a framework for the understanding of intratumoral heterogeneity [14,15]. In recent years, drug resistance has been well-documented to be tightly linked with CSC phenotypes [14,16]. For example, breast cancer was found to have multiple signaling pathways that drive CSC properties along with chemoresistance [17,18,19,20]. These multiple drugs resisting CSCs lead to residual cancer cells during chemotherapy, which are deemed to be responsible for the subsequent relapse of tumors [16,21].
Nevertheless, CSC-targeted therapy has remained unsatisfactory in recent decades, with the primary issue being that most CSC prominences are unresponsive to small molecular agents [22]. One of the most documented mechanisms of CSC multidrug resistance (MDR) is the increased expression of plasma membrane located ATP-binding cassette (ABC) transporters that enhance drug efflux [21], and variants in these genes may impact individualized treatment efficacy or adverse drug reactions [23,24]. In addition, the CSC phenotype exhibits increased resistance to chemotherapy, not only by raising drug efflux but also by regulating many other stem characteristics, including upregulated antiapoptotic proteins and increased DNA damage repair ability [25]. In this context, several signaling pathways and malignant molecules are critical in stemness maintenance, such as the Wnt and Notch pathways, and the NF-E2-Related Factor 2 (NRF2), CD44, prominin 1 (PROM1 or CD133), epithelial cell adhesion molecule (EpCAM) and Twist family BHLH transcription factors [13,21,25]. As the synthesis or screening of chemical agents targeting protein structure and biofunctions presents several hurdles, few chemical agents have been found or constructed to effectively inhibit CSC drivers or effectors [26]. In contrast, genetic silencing by RNA interference (RNAi), including small interfering RNA (siRNA) and microRNA (miRNA), can be rapidly translated into implications. Moreover, it has been documented that the genetic silence of these CSC regulators resulted in decreased cell stemness and improved drug sensitivity in vitro [27,28,29]. However, there are numerous physical and physiological barriers preventing RNAi delivery from systemic administration to the tumor location in vivo, such as degradation by RNase, off-target effects before efficient loading of tumor tissue, and poor membrane permeability and intracellular retain [30,31]. Importantly, nanomedicines present a novel and promising therapeutic approach to overcome these limitations of conventional RNAi therapeutics [32,33]. The elaborate nanoparticles provide a physical barrier that prevents contact and elimination by RNase, and are equipped with a lipophilic outer surface that enhances transmembrane uptake. In addition, RNAi-delivering nanoparticles can be embellished with hyaluronic acids (HA) or other ligands that mediate tumor targeting. In this review, we will explore recent advances in recent decades and present a perspective on the potential targets and novel nanoplatforms that were developed to target CSCs.
2. CSC Modulators and Potential Targets for RNAi Therapy
CSCs were indicated to originate from nonmalignant stem or progenitor cells in several studies. The signaling pathways that are crucial for normal stem cell homeostasis and function, including the Wnt, Notch, Hedgehog and Hippo pathways, are also commonly altered and have key regulatory functions supporting the stemness maintenance, survival and drug resistance of CSCs [13,34]. Accordingly, inhibition of these signaling pathways might be a promising approach for CSC-directed therapy across multiple cancer types, which contributes to inhibition of cancer relapse and enhances the efficacy of oncological treatments. Referring to the knowledge of CSC biology, a few CSC-directed agents have been developed and even entered clinical trials [16,35]. Furthermore, some drugs currently used for the treatment of general tumors might also have a certain degree of effectiveness against CSCs, such as inhibitors of AKT and ALK in cancer therapy [36,37]. Nevertheless, the wide crosslinks and compensatory mechanisms among these pathways lead to huge limitations in therapy effectiveness. In contrast, genetic therapy using RNAi initiatives expands the number of candidates that can be targeted, achieving highly specific, widespread and possibly curative therapeutic effects [38].
2.1. Targeting Wnt Pathway with RNAi Therapeutics
The canonical Wnt pathway is mediated by the activation of a β-catenin (encoded by CTNNB1)-centered transcriptional complex with the assistance of several transcriptional cofactors [39]. Currently, at least 19 members were found in the WNT family, a series of secretory glycoproteins functioning as ligands. On the other hand, at least 10 isoforms of Frizzled family proteins act as surface receptors together with their various coreceptors, such as low-density lipoprotein receptor-related protein 5 (LRP5) and LRP6. This ligand-receptor interaction disrupts β-catenin from its degradation complex, leading to β-catenin accumulation. Subsequently, β-catenin transposes into the nucleus thus causing the activation of the T cell-specific transcription factor & lymphoid enhancer-binding factor (TCF-LEF) transcriptional complex. The activated TCF-LEF complex results in the regulation of the expression of diverse genes, especially the genes supporting CSC properties, such as MYCN, Cyclin D1 (CCND1), and CD44 [13] (Figure 1). Cancer outcomes have a close relationship with the regulation of the Wnt pathway, including postoperational local relapses and metastasis. For example, Wnt signaling is frequently upregulated and tightly linked with poor prognosis in cancers, commonly due to inactivating mutations of adenomatous polyposis coli protein (APC) that mediate the ubiquitination and degradation of β-catenin [40,41]. Besides, induction of Dickkopf-related protein 1 (DKK1), an endogenous inhibitor of LRP5 and LRP6, results in the delay of cancer progression [42,43].
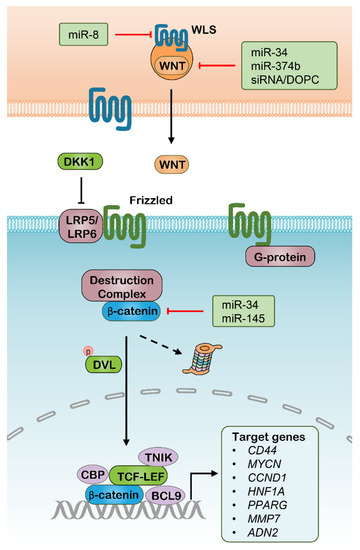
Figure 1.
The canonical Wnt signaling pathway and potential RNAi targets. WLS, WNT ligand secretion mediator; DKK1, Dickkopf–related protein 1; LRP, Lipoprotein receptor–related protein; DVL, Disheveled Segment Polarity Protein; CBP, Cyclic AMP response element–binding protein; TNIK, TRAF2 And NCK Interacting Kinase; TCF, T cell–specific transcription factor; LEF, Lymphoid enhancer–binding factor; BCL9, B–Cell Lymphoma 9 Protein.
As a result, small-molecule inhibitors and antibodies targeting these critical components of the Wnt pathway were rapidly developed, but have not yet demonstrated sufficient effectiveness or are still being investigated in small-scale clinical trials. In a phase I clinical study, for example, PRI-724, an inhibitor of the β-catenin interaction with the transcriptional coactivator cyclic AMP response element-binding protein (CBP), converted eight patients (40%) into stable disease with a median progression-free survival (PFS) of two months [44]. Another antagonist of the β-catenin-CBP complex, E7386, significantly attenuated Wnt signaling in patient-derived hepatocellular carcinoma (HCC) xenograft models, and is being tested in early phase clinical studies [45]. Besides, antagonistic antibodies of Frizzled receptors, such as Vantictumab and Lpafricept, and anti-ROR1 antibody Cirmtuzumab are still in phase I trials without convincing outcomes [46,47,48,49,50,51].
To date, many attempts have been made for interfering Wnt signaling with RNAi in vitro and in vivo (Table 1). Treatment with nanoparticle-delivered siWNT1 as a single therapy or part of combinatorial immunotherapies acted to halt tumor growth in a lung adenocarcinoma model [52]. However, in this study, DOPC liposomes loaded with siWNT1 caused no more than half reduction of the WNT1 mRNA amount, which suggests that both siWNT1 sequences and their nanocarriers can be modified, thus improving intracellular accumulation and the efficacy of genes silencing. Several miRNAs have been identified as WNT signaling inhibitors in various cancer types, which are mainly directed to β-catenin, WNTs and WNT ligand secretion mediator (WLS) (Figure 1). For example, the miR-34 was identified to directly target multiple genes involved in the Wnt pathway, including WNT1, WNT3, LRP6, CTNNB1, and LEF1, and has a variety of functions in tumor suppression [53]. In the same way, another study found that miR-145 tends to suppress Wnt signaling through targeting CTNNB1 and significantly inhibits colon cancer cell growth [54]. In addition, some upstream activators of the Wnt pathway were also demonstrated to be modulated by miRNAs with roles in antitumor effects, such as miR-8, which directly targets the WLS [55], and miR-9, which modulates the translation of C-X-C motif chemokine receptor 4 (CXCR4) [56]. Accordingly, RNAi therapeutics targeting Wnt signaling might provide promising approaches for CSC therapy. These Wnt interfering miRNAs can be delivered by specifically designed nanoparticles, but their implication in vivo requires more evidence. Moreover, a number of genes in the downstream Wnt pathways can be targeted through RNAi delivery, especially CD44, which is also known as an identity biomarker of CSCs across multiple cancer types [57]. CD44, as a cell-surface glycoprotein, is overexpressed in several types of CSCs and frequently characterized with alternative spliced variants [58]. CD44 is primarily known as a receptor of hyaluronic acid (HA), and is also reported to bind to other extracellular matrix (ECM) ligands, including matrix metalloproteinases (MMPs), osteopontin and collagens, which are deemed to mediate intercellular interactions, ECM adhesion and migration [59,60]. The pre-mRNAs for this gene undergo complex alternative splicing to produce various lengths of variants, resulting in a range of protein isoforms with distinct biofunctions. The different functional roles of these CD44 isoforms are not fully understood. however, the isoform 4 with all the variable exons spliced was indicated to have the strongest correlation with CSC properties in various cancer types, such as breast cancer [61,62], colorectal cancer [63,64,65], liver cancer [66], and bladder cancer [67]. The HA binds to and activates CD44 signaling pathways that induce enhanced cell proliferation and survival, and modulates the cytoskeleton to promote cellular motility. Mounting evidence has demonstrated that a subpopulation of cancer cells with positive CD44 and negative or low expression CD24 (CD44+/CD24−/low) are characterized by high tumorigenicity, as a few hundred of these cells were able to form solid tumors that was found to regain their parental heterogeneity into NOD/SCID mice [68]. Moreover, CD44 was also well known to be critical in stemness maintenance in various cancers, including breast cancer [61,69,70], liver cancer [71], pancreatic cancer [72] and bladder cancer [73]. Decreased CSC phenotypes were found by interfering with CD44 expression in these cancer types. As a result, several RNAi-delivered nanoparticles were designed for cancer therapy through silencing CD44 individually or combined with other antitumor drugs [74,75]. These separate studies indicated that nanoparticle implication significantly increase the work efficacy of RNAi-mediated CD44 knockdown in vivo, meanwhile, which may be further improved through the modification of these nanoparticles.

Table 1.
Investigational RNAi therapeutics targeting CSC regulators.
2.2. Targeting Notch Pathway with RNAi Therapeutics
Similar to the Wnt pathway, the Notch signaling pathway is another developing pathway that mediates intercellular communication, and has a great correlation to multiple aspects of cancer biology, especially CSC properties and tumor immunity [95,96]. This pathway functions through transmembrane ligands and receptors interaction, which comprise Delta-like ligand 1 (DLL1), DLL3 and DLL4, Jagged 1 (JAG1) and JAG2 as canonical Notch ligands, and Notch 1–4 paralogues as Notch receptors [97]. This interaction between neighboring cells induces a two-step proteolytic cleavage of the Notch receptor, with the first-step cleavage performed by disintegrin and metalloproteinase domain-containing protein (ADAM) enzymes, either ADAM10 or ADAM17, and the second-step cleavage mediated by γ-secretase. Furthermore, the cleaved Notch intracellular domain (NICD) is released and translocates into the nucleus to regulate the expression of a range of genes, especially CSC-correlated genes such as MYC, CCND3 and ERBB2, where it is combined with several other transcriptional cofactors, such as Mastermind- like 1 (MAML1), (Figure 2) [98,99,100]. Notably, the significance of the Notch signaling outputs in the context of CSCs is highlighted by the findings that Notch signaling interference has the potential to simultaneously repress tumorigenesis and drug resistance. Several Notch-pathway inhibitors with distinct targets and mechanisms have been developed or are now under clinical investigation. The γ-secretase is the first target used for designing inhibitors of Notch signaling. Inhibition of γ-secretase halts NICD release by blocking the second cleavage of Notch receptors, which was shown to have strong antitumor activity in various preclinical cancer models, such as pancreatic adenocarcinoma (PDAC) and T cell acute lymphoblastic leukemia (T-ALL) [101,102]. However, the majority of these γ-secretase inhibitors have been discontinued, most commonly owing to the unfavorable outcomes in phase I/II clinical studies [13]. In addition, their off-tumor side effects are another frequent problem typically involving the gastrointestinal system and electrolyte balance. In addition to small-molecule agents, several antagonistic monoclonal antibodies (mAbs) have been developed to target distinct domains of Notch ligands and receptors, which is another strategy to inhibit aberrant Notch signaling. For the most investigated example, Demcizumab is a humanized anti-DLL4 IgG2 mAb, whose antitumor efficacy in combination with specific first-line antitumor drugs has been tested on PDAC and non-small cell lung cancer (NSCLC) respectively in various phase I/II trials [103,104]. Brontictuzumab is designed as an antagonistic mAb of NOTCH1 mAb; however, it was revealed to have limited antitumor activity in several clinical studies focusing on hematological malignancies and solid tumors [105,106]. Similar to γ-secretase inhibitors, most clinical studies of these mAbs were suspended due to their unfavorable results in early phase studies.
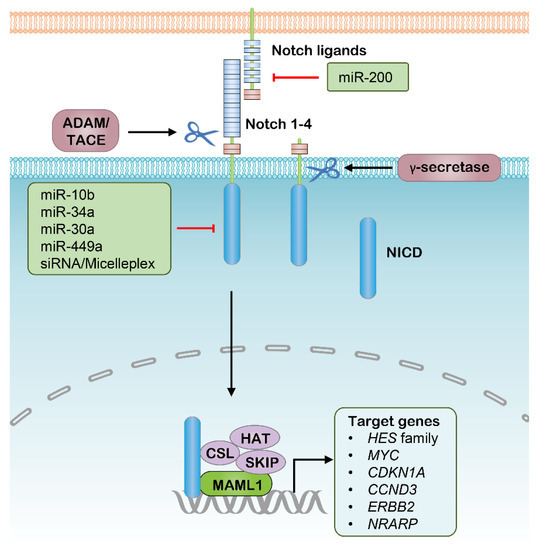
Figure 2.
The canonical Notch signaling pathway and potential RNAi targets. ADAM, Disintegrin and metalloproteinase domain–containing protein; TACE, Tumor necrosis factor–α converting enzyme; NICD, Notch intracellular domain; CSL, CBF1/Su(H)/Lag–1; HAT, Histone acetyltransferase; SKIP, Ski–interacting protein; MAML1, Mastermind–like 1.
As such, targeting Notch components with RNAi therapeutics modified with nanomedicine might be another promising strategy to inhibit aberrant Notch signaling in cancer treatment (Table 1). CSCs are considered to largely contribute to tumor relapse in hepatocellular carcinoma (HCC), accounting for poor survival, while a micellar nanoparticle that delivers siNOTCH1 was able to efficiently suppress NOTCH1 expression in HCC cells, leading to increased sensitivity to platinum and decreased CSC percentage in a xenograft model [54]. Several other studies demonstrated the feasibility of inhibiting Notch signaling by delivering siRNA targeting Notch ligands or receptors in vivo [107,108,109,110]. For example, siNOTCH1-loaded nanoparticles significantly inhibit Notch signaling, thereby attenuating rheumatoid arthritis in mouse models [107,110]. Thus, nanoparticle-aided highly effective siRNA showed promising implications in Notch-directed cancer therapy. In addition to siRNA, several miRNAs involved in the regulation of Notch signaling were used as monotherapy or codelivery with chemical drugs in various preclinical cancer models (Figure 2), whereas mounting miRNAs are involved in the regulation of the Notch signaling and cancer stemness [111]. Most notably, miR-34a was well documented in targeting and attenuating the expression of NOTCH1 and led to progression arrest in multiple cancer types [47,112]. Several subsequent studies showed that nanoparticle-carried miR-34a potently decreased the expression of NOTCH1, resulting in inhibition of cell proliferation and migration of breast cancer [48,49,51], and viability reduction of fibrosarcoma [51]. In CSC enriched glioma, exogenous miR-10b exposure led to suppression of NOTCH1, thus diminishing the invasiveness, angiogenesis and tumor growth in the brain, and significantly prolonging the survival of tumor-bearing mice [46]. Thus, with the assistance of nanoparticles, these siRNAs and miRNAs could be potent nucleic acid therapeutics of CSCs by interfering with Notch signaling.
2.3. Hippo Pathway and Potential RNAi Targets
The highly conserved Hippo signaling pathway acts to regulate the balance of cell proliferation and apoptosis [113]. The functions of the canonical Hippo signaling pathway are mediated by the transcriptional complex with coactivators Yes-associated protein 1 (YAP1) and WW domain containing transcription regulator 1 (WWTR1, usually known as TAZ), which promote the transcription of target genes involved in CSC properties, such as epithelial-to-mesenchymal transition (EMT), anti-apoptosis, and self-renewal [113]. Indeed, increased activity in YAP1 and/or TAZ led to the expansion of CSC populations and cancer progression [114]. On the other hand, the Hippo pathway is regulated by the successive activation of two kinase complexes, with the first comprising macrophage stimulating 1 (MST1) and MST2, and the second comprising large tumor suppressor kinase 1 (LATS1) and LATS2, together with the adaptors salvador family WW domain containing protein 1 (SAV1) and MOB kinase activator 1 (MOB1), respectively. In this context, the YAP1 and TAZ can be phosphorylated and driven into degradation upon the upstream signals, intercellular contact, G protein-coupled receptors and cell adhesion (Figure 3).
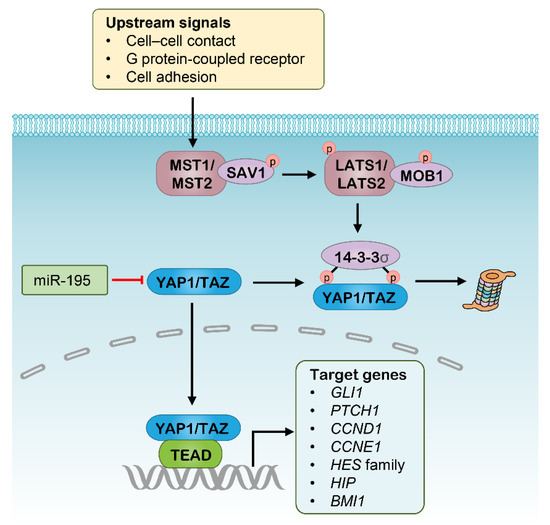
Figure 3.
The Hippo signaling pathway and potential RNAi targets. MST, Macrophage stimulating; SAV1, Salvador homologue 1; LATS, Large tumor suppresser kinase; MOB1, MOB domain kinase activator 1; YAP1, Yes-associated protein 1; TAZ, WW domain containing transcription regulator 1; TEAD, TEA domain.
Targeting CSCs through blocking Hippo signaling has been well documented and has showed promising results (Table 1) [115,116]. For example, several small-molecule inhibitors of the YAP1 transcriptional complex, including Verteporfin, CA3 and vestigial-like protein 4 (VGLL4) mimicking peptide, were shown to have potent antitumor activity in various cancer types, especially inhibiting tumorigenesis, CSCs enrichment and resistance to radiation [117,118,119]. In addition, treatment with a NEDD8-activating enzyme (NAE) inhibitor leads to rapid degradation of the YAP1/TAZ complex via suppressing the activity of the cullin-Ring subtype of ubiquitin ligases that stabilize the LATS kinase complex [120,121]. Accordingly, Hippo cascades also have great potential as RNAi targets, and some attempts have been made in this area. Nanoparticle-delivered siRNAs for MST1/2 were shown to effectively suppress the expression of MST1 and MST2, and to enhance Hippo signaling thereby leading to hepatocyte proliferation [122]. In addition, several miRNAs were found to be involved in Hippo signaling regulation, and some showed great antitumor activity as therapeutics (Figure 3). The miR-195 was identified to suppress Hippo signaling by binding to the 3′-untranslated region (3′-UTR) of the human YAP1 mRNA, whose expression was validated in a separate cohort of colorectal carcinoma (CRC) and significantly associated with poor survival of patients [55]. Subsequent experiments indicated that overexpression of miR-195-5p in CRC cell lines repressed cell growth, colony formation, invasion, and migration [55]. Another study revealed that the expression of miR-582 decreased the proportion of phosphorylated YAP1/TAZ in NSCLC cells, potentially by targeting actin regulators [56]. As such, these miRNAs are potential candidates as therapeutics targeting CSCs, although this needs further investigation. However, Hippo-directed RNAi therapeutics are currently being investigated in vitro, and still require more preclinical evidence before entry into clinical trials.
2.4. Hedgehog Pathway and Potential RNAi Targets
The Hedgehog signaling pathway has an important role in embryonic development and its aberrant activity has been linked to a variety of tumor types [123]. The Hedgehog signaling is mediated by three mature hedgehog ligands, including Sonic hedgehog (SHH), Indian hedgehog (IHH) and Desert hedgehog (DHH). The binding of Hedgehog ligands to Patched (PTCH) transmembrane receptors relieves their inhibitory effect on Smoothened (SMO), thereby leading to nuclear localization and activation of GLI transcription factors. The activated GLIs drive gene expression with roles involved in cell self-renewal, proliferation, and survival (Figure 4) [123]. This pathway provides a novel target for cancer therapy because the modulation of Hedgehog signaling is tightly correlated with CSC properties [124]. The investigation of small-molecule agents targeting the Hedgehog pathway in cancer continues to be an active research area, which is mainly directed to Hedgehog ligands, SMO or GLIs. Several SMO inhibitors, including Vismodegib, Sonidegib and Glasdegib, have been approved successively since their potent activity in repressing Hedgehog signaling and cancer progression [125,126,127]. Accordingly, these targets can also be silenced with siRNA, resulting in suppression of Hedgehog signaling, which has been tested in a range of preclinical studies (Table 1) [128,129,130]. Additionally, several miRNAs have been identified to be involved in the regulation of Hedgehog signaling (Figure 4), of which some might be used as antitumor therapeutics with the assistance of nanomedicine. The upregulated miR-326 was revealed to decrease SMO expression, resulting in an elevated rate of apoptosis in chronic myeloid leukemia (CML) cells, which could be beneficial in eradicating CD34+ CML stem cells [78]. Another SMO-directed miRNA, miR-14, was found to suppress Hedgehog signaling activity through screening the 3′ untranslated regions (3′UTRs) of the Hedgehog pathway genes against a genome-wide miRNA library, which functions by cotargeting PTCH and SMO [131]. Various GLI-directed miRNAs have also been identified in different studies. For example, separate studies found that miR-378a-3p directly targets Gli3 in activated hepatic stellate cells and leads to reduced expression of Gli3 [132,133]. Research results from another group indicated that upregulated miR-324-5p significantly inhibited GLI1 expression resulting in reduced stem cell compartment, cell growth and survival in multiple myeloma [77]. In lung adenocarcinoma cells, interference with miR-182-5p mimicked GLI2 silencing and resulted in the suppression of tumorigenesis and cisplatin resistance [80]. In addition, there are several miRNAs that have been indicated to inhibit Hedgehog signaling with unclear mechanisms, such as miR-186 and miR-338-5p [79,81]. The identification of these Hedgehog pathway-directed miRNAs provides possibilities for their implications in cancer therapy as mono-delivered or co-delivered with anti-tumor drugs using nanoplatforms, while their efficacy in vivo for targeting Hedgehog signaling requires additional research.
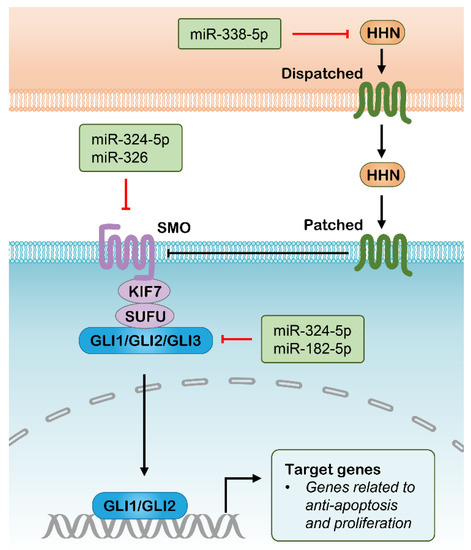
Figure 4.
Hedgehog signaling pathway and potential RNAi targets. HHN, Hedgehog N–terminal fragment; SMO, Smoothened; KIF7, Kinesin family member 7; SUFU, suppressor of fused homologue; GLI, Glioma-Associated Oncogene Homolog 1.
2.5. Other CSCs Targets for RNAi Therapy
In addition to CD44, another well-documented CSC marker is CD133, also known as prominin 1 (PROM1), which functions to suppress stem cell differentiation. CD133 was first identified in tumor initial cells (TICs) of glioma, since injection of as few as one hundred CD133+ glioma cells produced a new mass with similar phenotypes to the original tumor, whereas injection of one hundred thousand CD133-glioma cells could not even produce a tumor. Afterwards, CD133 was validated to be a CSC marker in HCC, colorectal carcinoma, and ovarian cancers. However, the pathophysiological mechanisms of CD133 in cancer stemness maintenance remain unknown. The finding on CSC lines showed that CSCs in the G1/G0 phase have reduced CD133 activity compared to those in the G2/M phase, suggesting a tight link to the cell cycle of CD133 [134]. In addition, it was suggested that CD133 may play a function in cellular glucose metabolism. In this context, high glucose stimulation induced the upregulation of CD133 with concomitant downregulation of its phosphorylation [135]. As a result, silencing CD33 through nanoparticle-delivered RNAi is deemed to be a promising method for CSC-targeted cancer therapy. Several other CSC biomarkers & effectors, such as TWIST1, ALDH, EpCAM, glucose, and transporters (GLUTs), were also used as CSC targets for cancer therapy, which largely depend on the specific cancer types. Moreover, the number of ABC transporters was found to be correlated with the maturation state that the most primitive cells exhibit the greatest efflux activity. For example, subfamily B member 1 (ABCB1), also known as MDR-1 or p-glycoprotein (P-gp), was first identified and cloned, and was subsequently shown to be responsible for clinical MDR in many cancers, such as colorectal cancer, breast cancer, lung cancer, etc.. Afterwards, C subfamily member 1 (ABCC1) and G subfamily member 2 (ABCG2) were identified successively and found to mediate clinical MDR across cancer types. There are 48 members of the human ABC family, with some exhibiting exceptional pharmacological specificity. The most well-known reactive oxidative species (ROS) scavenger NRF2 is shown to be highly expressed in CSCs, and NRF2 silencing returns the high levels of ROS and the sensitivity to chemotherapy. A wide spectrum of agents exert antitumor activity through the production of excessive ROS, but the CSCs have an enhanced ROS elimination system to reduce ROS-mediated DNA damage and cell apoptosis. Mounting exploratory work was carried out, but no highly specific and efficient compounds targeting these CSC factors were found or synthesized. As such, numerous studies have been performed to verify the feasibility of small-molecule RNAi delivery for the abrogation of CSC-associated factors, which should be largely progressed as the rapid advance of nano-material.
3. Nanoplatforms for RNAi Delivery
As discussed above, RNAi-based therapy has recently come to be utilized as a novel attractive strategy for cancer treatment. However, RNAi technology presents many limitations in its potential clinical applications, including rapid clearance by the renal system, target tissue uptake selectivity, the efficiency of cellular uptake, and long-term efficacy. To overcome these obstacles, researchers have introduced the use of nonviral carriers for the delivery of RNAi molecules [136,137]. Nanocarriers as a type of nonviral carrier have attracted more considerable attention, which is capable of promoting drug administration and drug accumulation in tumor tissues through elaborate drug encapsulation, thereby maximizing therapeutic efficacy and minimizing the undesirable side effects. Thus, nanocarriers are emerging as an outstanding delivery system for RNAi molecules [137,138,139]. The extensively investigated nanocarriers applied to RNAi molecule delivery can be generally classified into four major groups: polymer-based nanoparticles, lipid-based nanoparticles, inorganic nanoparticles and bio-inspired nanoparticles (Figure 5) [140,141,142,143]. To deepen our understanding of the potential of these various nanoparticles in the delivery of RNAi molecules for cancer therapy, the following section briefly reviews the different nanocarriers for delivered RNAi molecules and the recent progress of RNAi-based therapy using nanocarriers for targeting cancer stem cells.
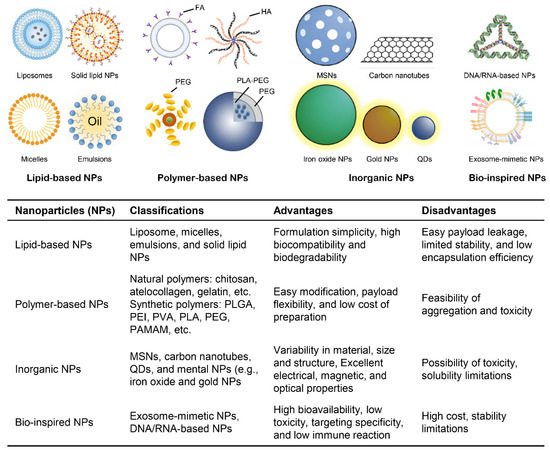
Figure 5.
Classes of RNAi–delivered nanoparticles. FA, Folate; HA, Hyaluronic acid; PEG, Polyethylene glycol; PLA, Polylactic acid; MSNs, Mesoporous silica nanomaterials; PLGA, Polylactic–co–glycolic acid; PVA, Polyvinyl alcohol; PAMAM, Polyamidoamine; QDs, Quantum dots.
3.1. Lipid-Based Nanoparticles
Generally, lipid-based nanoparticles are artificially manufactured drug delivery vehicles in which the inner core is completely covered by an outer lipid bilayer coating. Lipid-based nanoparticles are widely used for satisfying biocompatibility, good stability, controlled drug release and targeting properties. Furthermore, for successful drug delivery, the physicochemical parameters of lipid-based nanoparticles can be modified by changing the lipid components, drug-lipid ratio, and fabrication process. In recent decades, a wide variety of lipid-based nanoparticles have been reported, including solid lipid nanoparticles, liposomes, micelles, and emulsions [144,145,146]. Among these various lipid-based nanoparticles, liposomes are the most commonly used because of their excellent performance indicators such as high stability, good bioavailability, controlled release, low toxicity, long-term circulation, and tumor-targeted specificity. Liposomes have been reported to be used as drug delivery vehicles that have attracted increasing interest in both the basic and clinical biomedical sciences. According to their surface charge distribution, liposomes are divided into three types: cationic liposomes, neutral liposomes, and anionic liposomes. Cationic liposomes are the most broadly used as RNAi delivery carriers because of their high affinity for negatively charged nucleic acids. The lipids of cationic liposomes are made up of cationic lipids and neutral auxiliary lipids, cationic lipids include DOTMA, DOTAP, DOSPA, DMRIE, and DC-Chol; and neutral auxiliary lipids include DOPE, DOPC, PE, phosphatidylcholine, and cholesterol. With the rapid progress of liposome-based technology, liposomes have evolved to multifunctional pharmaceutical nanocarriers combing several specific properties, such as long-circulating liposomes, pH-sensitive liposomes, and targeted liposomes [147,148,149,150]. Currently, solid lipid nanoparticles have also been applied for the systemic delivery of RNAi because they can be disinfected and lyophilized owing to their exceptional stability in humans [151]. Fortunately, these recent advances have improved the use of lipid-based nanoparticles for gene-based therapy for targeting cancer stem cells. For example, Li and colleagues developed novel GLI1-targeted siRNA nanoparticles that are functionally modified by a DSPE-HA conjugate, as a specific ligand of the CD44 receptor. In this study, the GLI1-targeted siRNA nanoparticles selectively eliminated gastric CSCs for dual-targeting CD44 and GLI1, and consequently exhibited impressive therapeutic efficacy in gastric cancer [74]. A separate study demonstrated that the drug resistance of hepatocellular carcinoma can be overcome through eliminating HCC CSCs by codelivering Bmi1 siRNA with cisplatin in cationic nanoparticles [152].
3.2. Polymer-Based Nanoparticles
Polymer-based nanoparticles are well-exploited carriers for RNAi delivery. In terms of origin, they are classified into two major groups: natural and synthetic polymer-based nanoparticles [153,154]. The natural polymers used for gene-based therapy include chitosan, atelocollagen, folate (FA), HA, and gelatin, and are biocompatible, biodegradable, and generally nontoxic, even at high concentrations. Chitosan has been successfully used in gene delivery systems [155,156,157]. Novel chitosan nanoparticles were developed to deliver functional miRNA mimics to macrophages through regulating ABCA1 expression and cholesterol efflux to target atherosclerotic lesions [158]. Apart from natural polymer-based nanoparticles, synthetic polymer-based nanoparticles have also been used in the delivery of RNAi molecules. Synthetic polymer-based nanoparticles dominate the majority of gene delivery systems, which mainly consist of chitosan derivatives, PLGA, PEI, PVA, PLA, PEG, and PAMAM, etc. Similar to natural polymers, synthetic polymers are characterized by good stability, high drug-loading capacity, and biodegradability [159,160,161]. Noteworthily, synthetic polymers are relatively effortless to be modified with ligand bindings and stimuli-responsive units for controlled release and targeted delivery. However, some synthetic polymers could not be directly utilized for RNAi molecule delivery owing to a lack of cationic motifs, thereby leading to low electrostatic interactions between polymers and RNAi molecules. To resolve this issue, nanoparticles need to be modified with various cationic motifs or cationic polymers [162]. The 6 (G6) TEA-core PAMAM dendrimer forms stable dendriplexes that were synthesized with a p70S6K siRNA, and showed significant tumor suppression by inhibiting stemness and metastasis of ovarian cancer [163]. To effectively enhance the therapy of ovarian cancer, an impressive delivery system was designed, which includes a PPI dendrimer, a synthetic analog of LHRH peptide, paclitaxel, and siRNA molecules targeted to CD44 mRNA, together to be a specific CD44+ ovarian cancer cell death inducer. Consequently, treatment with the designed nanoparticles led to efficient ovarian cancer suppression [75]. In addition, a novel aptamer-PEI-siRNA nanoparticle was utilized for targeting the putative cancer stem cell marker EpCAM, leading to inhibition of the cancer cell proliferation. Another group demonstrated that NPsiPLK1with LY364947 pretreatment cooperatively promotes remarkable antitumor effects on breast cancer [164]. In particular, a novel synthetic siRNA nanoparticle composed of a cationic oligomer (PEI1200), a hydrophilic polymer (polyethylene glycol) and a biodegradable lipid-based crosslinking moiety was developed. This nanoparticle with siMDR1 could significantly downregulate the expression of MDR1 in human colon CSCs, resulting in effectively increasing the chemosensitivity of human colon CSCs to paclitaxel [165]. Likewise, the reduction of MALAT1 by delivering targeted nanoparticles carrying MALAT1 siRNA improved the sensitivity of glioblastoma to temozolomide [166]. It is worth noting that targeting glucose uptake by systemic delivery of NPsiGLUT3, a cationic lipid-associated PFG-PLA nanoparticle that can efficiently deliver specific siRNA targeting GLUT3, is a successful strategy for inhibiting the growth of glioma cells [167]. Taken together, although substantial progress has been achieved in the field of polymer-based nanoparticles over the past decades, there remain many concerns about the ultimate fate of synthetic polymers and their degradation products.
3.3. Inorganic Nanoparticles
In the past few years, inorganic nanoparticles have attracted increasing attention as potential diagnostic and therapeutic applications due to their nanoscale size and unique physicochemical characteristics compared to lipid- and polymer-based nanoparticles. In particular, inorganic nanoparticles possess excellent electrical, optical and magnetic properties, making inorganic nanoparticles applicable for the imaging and ablation of malignant tissue. Numerous inorganic nanoparticles have been reported, including mesoporous silica nanomaterials (MSNs), carbon nanotubes (CNTs), quantum dots (QDs), and metal nanoparticles (e.g., iron oxide and gold nanoparticles) [168,169,170]. Among inorganic nanoparticles, MSNs are most commonly applied due to the following critical physicochemical properties: ordered porous structure, large surface area and pore volume, high tunable particle size, two functional surfaces, and good biocompatibility. MSNs are usually modified to transform into positive charge-functionalized MSNs by appropriate approaches including amination-modification, metal cations codelivered vector and coassembly cationic polymer, because unmodified MSNs often exhibit negative charges which would reduce interactions with negatively charged nucleic acids. Therefore, in addition to surface charge modification of MSNs to enhance gene loading capacity, MSNs have been modified with multiple targeting agents to achieve better applications [171,172,173]. For example, codelivery of siTWIST-MSN-HA and cisplatin showed significant advantages in targeting specificity and targeting efficacy. These nanoparticles have potential applications for overcoming clinical challenges in ovarian and other TWIST overexpressing cancers [174]. Despite the exciting progress in the development of MSN-based nanoparticles for gene delivery, there are still many challenges that need to be addressed to facilitate their further development. In particular, the benefits and disadvantages of MSN-based carriers in vivo should be systematically investigated. Carbon nanotubes exhibit specific physical properties (structural, electronic, optical, and magnetic properties) that render them innovative materials for the delivery of therapeutic molecules. They can be either composed of single-walled carbon nanotubes (SWNT) or multi-walled carbon nanotubes (MWNT). Although SWNT and MWNT have been used to form stable complexes with siRNA to silence tumor-related gene expression in tumor cells [175,176], the applications of siRNA delivery with functionalized carbon nanotubes in targeted treatment of CSCs have not yet been demonstrated. Therefore, this attractive approach based on carbon nanotubes presents a potential therapeutic strategy by targeting CSCs using RNAi delivery across multiple different tumor types. Quantum dots are fluorescent semiconductor materials. Recent advances in new approaches to QD synthesis and covering enable quantum dots to be used as ideal candidates for imaging, diagnostics, and therapeutic delivery. In the field of therapeutic delivery, QDs have been used to promote gene therapy through delivery and imaging of treatment with RNAi [177,178,179,180,181]. However, little is known about the effect of QDs/RNAi complexes in CSCs. The application of QDs/RNAi nanoparticles for gene silencing in CSCs needs to be further explored. Metal nanoparticles are another highly exploited material for inorganic nanoparticles synthesis. Gold nanoparticles (AuNPs), a group of metal nanoparticles, have been widely used in imaging, diagnostics and therapy biomedical applications. In particular, the design of AuNPs-based covalent and noncovalent RNAi nanoparticles provides a promising therapeutic option for cancer and a number of other diseases for humans [182,183]. For instance, a glucose-installed sub-50-nm unimer polyion complex-assembled gold nanoparticle (Glu-NP) was developed for systemic delivery of siRNA to GLUT1-overexpressing breast cancer stem-like cells. Subsequent results suggested that multifunctional modified gold nanoparticles could be a promising nanoparticle for CSC-targeted cancer treatment [184]. It is worth noting that the potential toxicity of metal nanoparticles needs to be carefully and precisely studied in gene therapy applications.
3.4. Bio-Inspired Nanoparticles
In addition to the nanoparticles briefly mentioned above, researchers have extensively exploited new bio-inspired nanoparticles for gene delivery, such as exosome-mimetic nanoparticles. Exosomes are nanosized extracellular vesicles naturally secreted by cells, whose function is triggering intercellular communication by transferring biological information between cells. However, cell-derived exosomes are relatively finite, and their purification is also difficult. Thus, the generation of exosome-mimetic nanoparticles based on the knowledge of exosome surface structure and physiology is an attractive concept for the development of future favorable nanoparticles for the delivery of RNAi therapeutics. The exosome-mimetic nanoparticles display eminent physiochemical properties compared to the exosomes that originate from cells. For example, exosome-derived siRNA against RAD51- and RAD52 could decrease fibrosarcoma cell viability and proliferation [185]. In a similar attempt, exosome-mimetic nanoplatforms were designed for targeted cancer drug delivery. Fuente et al. designed a multifunctional nanoplatform mimicking exosomes, F-EMNs loaded with therapeutic RNAs (miR145), that could efficiently transport therapeutic RNAs to targeted cells. In another study, bioengineered exosome-mimetic nanoparticles were designed to deliver chemotherapeutic drugs. The results suggested that the antitumor effect of exosome-mimetic nanoparticles Raw264.7NVDox was significantly greater than conventional chemotherapeutic-loaded nanoparticles [186,187]. Research on exosome-based cancer therapies is not limited to experimental models. Several clinical studies have been completed or remain ongoing. In a phase I study, autologous dendritic cell (DC)-derived exosomes (Dex) were directly loaded with MAGE 3 antigens and tested against metastatic melanoma [188]. All of these interesting studies suggest that exosome-based RNAi delivery systems may have advantages in anti-CSC targeted cancer therapy. Moreover, some studies have indicated that DNA/RNA-based nanoparticles, as bio-inspired nanoparticles, are suitable for drug delivery and tissue engineering [141,142,143]. RNA nanotechnology was applied to design RNA nanoparticles containing anti-miR21 and the CD133 aptamer payloads for targeting TNBC. These RNA nanoparticles displayed not only high tumor-targeting specificity but also high efficacy for tumor growth inhibition in TNBC, which further highlighted the potential application of DNA/RNA-based nanoparticles for cancer therapy [189]. Similarly, hTERT promoter-driven VISA nanoparticle-delivered miR-34a (TV-miR-34a) was utilized in BCSCs and presented a great therapeutic effect. In this context, the VISA vectors represent essentially a VP16-GAL4-WPRE integrated systemic amplifier. In brief, TV-miR-34a can significantly inhibit breast cancer cell growth, which has great application potential in breast cancer therapy [190]. Meanwhile, bio-inspired functional lipoprotein-like nanoparticles have been studied for gene delivery [191]. For example, CXCR4 receptor-stimulated lipoprotein-like nanoparticles carrying miR-34a achieved efficient accumulation in glioma initiating cells and subsequently availably restrained glioma initiating cell stemness and chemoresistance [192]. Accordingly, there are still many challenges and opportunities for bio-inspired nanoparticles, and they will certainly play a critical role in the realization of multifunctional nanoparticles for RNAi delivery.
4. Conclusions
The CSC hypothesis posits that CSCs are greatly responsible for tumor heterogeneity, tumorigenesis, and therapy resistance, having an important role in cancer initiation and progression [14,25]. In particular, CSCs and their fueling heterogeneous mass are widely recognized to facilitate cancer resistance to various therapy approaches, which are directly correlated with poor clinical outcomes [193,194]. In this contexts are characterized by low proliferative ability but a high rate of asymmetric divisions that produce two cell populations, with one cell population succeeding instemness and the other cell population obtaining high proliferative capacity [195]. Therefore, effective treatment strategies must focus on inactively proliferative CSCs, while most traditional therapeutics are directed to highly growing non-CSCs [3,14]. In recent years, substantial advances have been achieved in various areas of cancer gene therapy, especially with the assistance of rapidly developing delivery materials that greatly improve the stability and targeting capacity of nucleic acids in vivo. Moreover, achievements in genomics research largely increased our understanding of the genetic basis of cancers and provided a range of new targets for therapy [196]. However, the majority of recently identified targets remain ‘undruggable’ by chemical agents. As such, the potential of exogenous RNAi may make it possible to translate our knowledge into therapeutics in a timely manner. More than a decade after the initial implication of RNAi in cancer treatment, several RNAi-based therapeutics have acquired regulatory approvals to be tested in early phase clinical trials [32]. In addition, a range of new miRNAs that have potential in CSC regulation have been revealed with the progress in epigenomics studies, which provide emerging candidates as CSC-directed RNAi therapeutics. With the recent advances of nanomedicine, in vivo delivery of RNAi using elaborate nanoparticles can potently overcome the intrinsic limitations of RNAi alone being rapidly degraded and having unpredictable off-target outcomes; however, their broad application will require continued efforts, especially on the RNAi stability, interfering efficiency and targeting ability. It is important to strictly audit the performance of these recombination agents as they are considered to be prompted into later stage trials; however, a group of RNAi therapeutics directing cancer has shown promising clinical efficacy through subcutaneous administration [32]. This highlights the possibility of siRNA therapeutics in clinical applications, and suggests that RNAi has broad potential in cancer therapy in humans.
As discussed before, there are mounting siRNAs and miRNAs that are involved in CSC suppression. It should be believed that many of them can be delivered in vivo for CSC-directed therapy. In addition, a large number of nanoparticles endowed with stability and targeting capacity showed promising results as RNAi cargoes. Therefore, their distinct combinations provide mounting possibilities for potential implications for in vivo investigation.
Author Contributions
Conceptualization, H.C. and Y.T.; writing-original draft preparation, Y.T. and Y.C.; writing-review and editing, Z.Z. (Zhe Zhang)and B.T.; supervision, H.C. and Z.Z. (Zongguang Zhou). All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (82073246 and 82003113), China Postdoctoral Science Foundation (220M673208), Post-Doctor Research Project, West China Hospital, Sichuan University (2020HXBH039 and 2020HXBH061), The Fundamental Research Funds for the Central Universities (2020SCU12025), 1.3.5 Project for disciplines of excellence, West China Hospital, Sichuan University (2016105 and ZYGD20006) and Sichuan Science and Technology Program (2020YFS0068).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A.; Fawell, S.; Floc’h, N.; Flemington, V.; McKerrecher, D.; Smith, P.D. Challenges and Opportunities in Cancer Drug Resistance. Chem. Rev. 2021, 121, 3297–3351. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Conte, E. Targeting monocytes/macrophages in fibrosis and cancer diseases: Therapeutic approaches. Pharmacy 2021, 11, 108031. [Google Scholar] [CrossRef]
- Raguraman, R.; Srivastava, A.; Munshi, A.; Ramesh, R. Therapeutic approaches targeting molecular signaling pathways common to diabetes, lung diseases and cancer. Adv. Drug Deliv. Rev. 2021, 178, 113918. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, Z.; Lee, P.L.; Guan, P.; Aau, M.Y.; Lee, S.T.; Feng, M.; Lim, C.Z.; Lee, E.Y.; Wee, Z.N.; et al. PDK1 signaling toward PLK1-MYC activation confers oncogenic transformation, tumor-initiating cell activation, and resistance to mTOR-targeted therapy. Cancer Discov. 2013, 3, 1156–1171. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.M.; Lee, D.G.; Lee, J.; Park, J.G.; Han, T.S.; Cho, H.S.; Cho, Y.L.; Bae, K.H.; Park, Y.J.; et al. Loss of desmoglein-2 promotes gallbladder carcinoma progression and resistance to EGFR-targeted therapy through Src kinase activation. Cell Death Differ. 2021, 28, 968–984. [Google Scholar] [CrossRef]
- Horn, L.A.; Fousek, K.; Palena, C. Tumor Plasticity and Resistance to Immunotherapy. Trends Cancer 2020, 6, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.A.; Burke, K.P.; Van Allen, E.M. Genomic Approaches to Understanding Response and Resistance to Immunotherapy. Clin. Cancer Res. 2016, 22, 5642–5650. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Clara, J.A.; Monge, C.; Yang, Y.; Takebe, N. Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat. Rev. Clin. Oncol. 2020, 17, 204–232. [Google Scholar] [CrossRef]
- Qin, S.; Jiang, J.; Lu, Y.; Nice, E.C.; Huang, C.; Zhang, J.; He, W. Emerging role of tumor cell plasticity in modifying therapeutic response. Signal Transduct. Target. Ther. 2020, 5, 228. [Google Scholar] [CrossRef]
- Prager, B.C.; Xie, Q.; Bao, S.; Rich, J.N. Cancer Stem Cells: The Architects of the Tumor Ecosystem. Cell. Stem Cell 2019, 24, 41–53. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Wang, T.; Fahrmann, J.F.; Lee, H.; Li, Y.J.; Tripathi, S.C.; Yue, C.; Zhang, C.; Lifshitz, V.; Song, J.; Yuan, Y.; et al. JAK/STAT3-Regulated Fatty Acid beta-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018, 27, 136–150.e135. [Google Scholar] [CrossRef]
- Ryoo, I.G.; Choi, B.H.; Ku, S.K.; Kwak, M.K. High CD44 expression mediates p62-associated NFE2L2/NRF2 activation in breast cancer stem cell-like cells: Implications for cancer stem cell resistance. Redox Biol. 2018, 17, 246–258. [Google Scholar] [CrossRef]
- Cazet, A.S.; Hui, M.N.; Elsworth, B.L.; Wu, S.Z.; Roden, D.; Chan, C.L.; Skhinas, J.N.; Collot, R.; Yang, J.; Harvey, K.; et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun. 2018, 9, 2897. [Google Scholar] [CrossRef]
- Ye, S.; Ding, Y.F.; Jia, W.H.; Liu, X.L.; Feng, J.Y.; Zhu, Q.; Cai, S.L.; Yang, Y.S.; Lu, Q.Y.; Huang, X.T.; et al. SET Domain-Containing Protein 4 Epigenetically Controls Breast Cancer Stem Cell Quiescence. Cancer Res. 2019, 79, 4729–4743. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mayea, Y.; Mir, C.; Masson, F.; Paciucci, R.; ME, L.L. Insights into new mechanisms and models of cancer stem cell multidrug resistance. Semin. Cancer Biol. 2020, 60, 166–180. [Google Scholar] [CrossRef]
- Shen, S.; Xia, J.X.; Wang, J. Nanomedicine-mediated cancer stem cell therapy. Biomaterials 2016, 74, 1–18. [Google Scholar] [CrossRef]
- Relling, M.V.; Evans, W.E. Pharmacogenomics in the clinic. Nature 2015, 526, 343–350. [Google Scholar] [CrossRef]
- Gandara-Mireles, J.A.; Lares-Asseff, I.; Reyes Espinoza, E.A.; Blanco, J.G.; Gonzalez Font, A.E.; Cordova Hurtado, L.P.; Castaneda, V.L.; Fierro, I.V.; Romero, L.P.; Reyes, H.A. Association of genetic polymorphisms NCF4 rs1883112, CBR3 rs1056892, and ABCC1 rs3743527 with the cardiotoxic effects of doxorubicin in children with acute lymphoblastic leukemia. Pharm. Genom. 2021, 31, 108–115. [Google Scholar] [CrossRef]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef]
- Han, J.; Won, M.; Kim, J.H.; Jung, E.; Min, K.; Jangili, P.; Kim, J.S. Cancer stem cell-targeted bio-imaging and chemotherapeutic perspective. Chem. Soc. Rev. 2020, 49, 7856–7878. [Google Scholar] [CrossRef]
- Arfaoui, A.; Rioualen, C.; Azzoni, V.; Pinna, G.; Finetti, P.; Wicinski, J.; Josselin, E.; Macario, M.; Castellano, R.; Léonard-Stumpf, C.; et al. A genome-wide RNAi screen reveals essential therapeutic targets of breast cancer stem cells. EMBO Mol. Med. 2019, 11, e9930. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Ahmad, A.; Azmi, A.S.; Kong, D.; Banerjee, S.; Sarkar, F.H. Targeting miRNAs involved in cancer stem cell and EMT regulation: An emerging concept in overcoming drug resistance. Drug Resist. Updates 2010, 13, 109–118. [Google Scholar] [CrossRef]
- Takahashi, R.U.; Miyazaki, H.; Takeshita, F.; Yamamoto, Y.; Minoura, K.; Ono, M.; Kodaira, M.; Tamura, K.; Mori, M.; Ochiya, T. Loss of microRNA-27b contributes to breast cancer stem cell generation by activating ENPP1. Nat. Commun. 2015, 6, 7318. [Google Scholar] [CrossRef]
- Dong, Y.; Siegwart, D.J.; Anderson, D.G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019, 144, 133–147. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, A.; Miyata, K.; Kataoka, K. Recent progress in development of siRNA delivery vehicles for cancer therapy. Adv. Drug Deliv. Rev. 2016, 104, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Tao, W.; Zou, Y.; Farokhzad, O.C.; Shi, B. Nanotechnology-Based Strategies for siRNA Brain Delivery for Disease Therapy. Trends Biotechnol. 2018, 36, 562–575. [Google Scholar] [CrossRef]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef] [PubMed]
- LaBarge, M.A. The difficulty of targeting cancer stem cell niches. Clin. Cancer Res. 2010, 16, 3121–3129. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.V.; Allen, J.E.; Dicker, D.T.; El-Deiry, W.S. Small-Molecule ONC201/TIC10 Targets Chemotherapy-Resistant Colorectal Cancer Stem-like Cells in an Akt/Foxo3a/TRAIL-Dependent Manner. Cancer Res. 2015, 75, 1423–1432. [Google Scholar] [CrossRef]
- Xu, B.S.; Chen, H.Y.; Que, Y.; Xiao, W.; Zeng, M.S.; Zhang, X. ALK(ATI) interacts with c-Myc and promotes cancer stem cell-like properties in sarcoma. Oncogene 2020, 39, 151–163. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.H.; Sailor, M.J. Rekindling RNAi Therapy: Materials Design Requirements for In Vivo siRNA Delivery. Adv. Mater. 2019, 31, e1903637. [Google Scholar] [CrossRef]
- Anastas, J.N.; Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 2013, 13, 11–26. [Google Scholar] [CrossRef]
- Caspi, M.; Wittenstein, A.; Kazelnik, M.; Shor-Nareznoy, Y.; Rosin-Arbesfeld, R. Therapeutic targeting of the oncogenic Wnt signaling pathway for treating colorectal cancer and other colonic disorders. Adv. Drug Deliv. Rev. 2021, 169, 118–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, 4562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Morris, J.P.t.; Yan, W.; Schofield, H.K.; Gurney, A.; Simeone, D.M.; Millar, S.E.; Hoey, T.; Hebrok, M.; Pasca di Magliano, M. Canonical wnt signaling is required for pancreatic carcinogenesis. Cancer Res. 2013, 73, 4909–4922. [Google Scholar] [CrossRef]
- Goyal, L.; Sirard, C.; Schrag, M.; Kagey, M.H.; Eads, J.R.; Stein, S.; El-Khoueiry, A.B.; Manji, G.A.; Abrams, T.A.; Khorana, A.A.; et al. Phase I and Biomarker Study of the Wnt Pathway Modulator DKN-01 in Combination with Gemcitabine/Cisplatin in Advanced Biliary Tract Cancer. Clin. Cancer Res. 2020, 26, 6158–6167. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Ikoma, A.; Shibakawa, M.; Shimoda, S.; Harada, K.; Saio, M.; Imamura, J.; Osawa, Y.; Kimura, M.; Nishikawa, K.; et al. Safety, Tolerability, and Preliminary Efficacy of the Anti-Fibrotic Small Molecule PRI-724, a CBP/β-Catenin Inhibitor, in Patients with Hepatitis C Virus-related Cirrhosis: A Single-Center, Open-Label, Dose Escalation Phase 1 Trial. EBioMedicine 2017, 23, 79–87. [Google Scholar] [CrossRef]
- Yamada, K.; Hori, Y.; Inoue, S.; Yamamoto, Y.; Iso, K.; Kamiyama, H.; Yamaguchi, A.; Kimura, T.; Uesugi, M.; Ito, J.; et al. E7386, a Selective Inhibitor of the Interaction between β-Catenin and CBP, Exerts Antitumor Activity in Tumor Models with Activated Canonical Wnt Signaling. Cancer Res. 2021, 81, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.R.; Becerra, C.; Richards, D.; Mita, A.; Osborne, C.; O'Shaughnessy, J.; Zhang, C.; Henner, R.; Kapoun, A.M.; Xu, L.; et al. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Res. Treat. 2020, 184, 53–62. [Google Scholar] [CrossRef]
- Choi, M.Y.; Widhopf, G.F., 2nd; Ghia, E.M.; Kidwell, R.L.; Hasan, M.K.; Yu, J.; Rassenti, L.Z.; Chen, L.; Chen, Y.; Pittman, E.; et al. Phase I Trial: Cirmtuzumab Inhibits ROR1 Signaling and Stemness Signatures in Patients with Chronic Lymphocytic Leukemia. Cell Stem Cell 2018, 22, 951–959.e953. [Google Scholar] [CrossRef] [PubMed]
- Dotan, E.; Cardin, D.B.; Lenz, H.J.; Messersmith, W.; O'Neil, B.; Cohen, S.J.; Denlinger, C.S.; Shahda, S.; Astsaturov, I.; Kapoun, A.M.; et al. Phase Ib Study of Wnt Inhibitor Ipafricept with Gemcitabine and nab-paclitaxel in Patients with Previously Untreated Stage IV Pancreatic Cancer. Clin. Cancer Res. 2020, 26, 5348–5357. [Google Scholar] [CrossRef] [PubMed]
- Jimeno, A.; Gordon, M.; Chugh, R.; Messersmith, W.; Mendelson, D.; Dupont, J.; Stagg, R.; Kapoun, A.M.; Xu, L.; Uttamsingh, S.; et al. A First-in-Human Phase I Study of the Anticancer Stem Cell Agent Ipafricept (OMP-54F28), a Decoy Receptor for Wnt Ligands, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2017, 23, 7490–7497. [Google Scholar] [CrossRef]
- Davis, S.L.; Cardin, D.B.; Shahda, S.; Lenz, H.J.; Dotan, E.; O'Neil, B.H.; Kapoun, A.M.; Stagg, R.J.; Berlin, J.; Messersmith, W.A.; et al. A phase 1b dose escalation study of Wnt pathway inhibitor vantictumab in combination with nab-paclitaxel and gemcitabine in patients with previously untreated metastatic pancreatic cancer. Investig. New Drugs 2020, 38, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, L.; Cui, B.; Wu, C.; Choi, M.Y.; Chen, Y.; Zhang, L.; Rassenti, L.Z.; Widhopf Ii, G.F.; Kipps, T.J. Cirmtuzumab inhibits Wnt5a-induced Rac1 activation in chronic lymphocytic leukemia treated with ibrutinib. Leukemia 2017, 31, 1333–1339. [Google Scholar] [CrossRef]
- Kerdidani, D.; Chouvardas, P.; Arjo, A.R.; Giopanou, I.; Ntaliarda, G.; Guo, Y.A.; Tsikitis, M.; Kazamias, G.; Potaris, K.; Stathopoulos, G.T.; et al. Wnt1 silences chemokine genes in dendritic cells and induces adaptive immune resistance in lung adenocarcinoma. Nat. Commun. 2019, 10, 1405. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, H.S.; Kim, N.G.; Lee, I.; Choi, H.S.; Li, X.Y.; Kang, S.E.; Cha, S.Y.; Ryu, J.K.; Na, J.M.; et al. p53 and microRNA-34 are suppressors of canonical Wnt signaling. Sci. Signal. 2011, 4, ra71. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Noguchi, S.; Mori, T.; Naoe, T.; Maruo, K.; Akao, Y. Tumor-suppressive microRNA-145 targets catenin δ-1 to regulate Wnt/β-catenin signaling in human colon cancer cells. Cancer Lett. 2013, 335, 332–342. [Google Scholar] [CrossRef]
- Kennell, J.A.; Gerin, I.; MacDougald, O.A.; Cadigan, K.M. The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proc. Natl. Acad. Sci. USA Am. 2008, 105, 15417–15422. [Google Scholar] [CrossRef]
- Yu, T.; Liu, K.; Wu, Y.; Fan, J.; Chen, J.; Li, C.; Yang, Q.; Wang, Z. MicroRNA-9 inhibits the proliferation of oral squamous cell carcinoma cells by suppressing expression of CXCR4 via the Wnt/β-catenin signaling pathway. Oncogene 2014, 33, 5017–5027. [Google Scholar] [CrossRef]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic Acid-Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Adv. Mater. 2019, 31, e1803549. [Google Scholar] [CrossRef] [PubMed]
- Ponta, H.; Sherman, L.; Herrlich, P.A. CD44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef]
- Roedig, H.; Damiescu, R.; Zeng-Brouwers, J.; Kutija, I.; Trebicka, J.; Wygrecka, M.; Schaefer, L. Danger matrix molecules orchestrate CD14/CD44 signaling in cancer development. Semin. Cancer Niology 2020, 62, 31–47. [Google Scholar] [CrossRef]
- Govindaraju, P.; Todd, L.; Shetye, S.; Monslow, J.; Puré, E. CD44-dependent inflammation, fibrogenesis, and collagenolysis regulates extracellular matrix remodeling and tensile strength during cutaneous wound healing. Matrix. Biol. 2019, 75, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.L.; Cao, J.L.; Xie, H.Y.; Sun, R.; Yang, L.F.; Shao, Z.M.; Li, D.Q. Cancer-Associated MORC2-Mutant M276I Regulates an hnRNPM-Mediated CD44 Splicing Switch to Promote Invasion and Metastasis in Triple-Negative Breast Cancer. Cancer Res. 2018, 78, 5780–5792. [Google Scholar] [CrossRef]
- Tripathi, V.; Sixt, K.M.; Gao, S.; Xu, X.; Huang, J.; Weigert, R.; Zhou, M.; Zhang, Y.E. Direct Regulation of Alternative Splicing by SMAD3 through PCBP1 Is Essential to the Tumor-Promoting Role of TGF-beta. Mol. Cell 2016, 64, 549–564. [Google Scholar] [CrossRef]
- Puppo, M.; Bucci, G.; Rossi, M.; Giovarelli, M.; Bordo, D.; Moshiri, A.; Gorlero, F.; Gherzi, R.; Briata, P. miRNA-Mediated KHSRP Silencing Rewires Distinct Post-transcriptional Programs during TGF-beta-Induced Epithelial-to-Mesenchymal Transition. Cell Rep. 2016, 16, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Sasaki, E.; Kimura, K.; Komori, K.; Shimizu, Y.; Yatabe, Y.; Aoki, M. HNRNPLL, a newly identified colorectal cancer metastasis suppressor, modulates alternative splicing of CD44 during epithelial-mesenchymal transition. Gut 2018, 67, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Nishimura, J.; Kagawa, Y.; Kano, Y.; Takahashi, Y.; Wu, X.; Hiraki, M.; Hamabe, A.; Konno, M.; Haraguchi, N.; et al. Significance of Polypyrimidine Tract-Binding Protein 1 Expression in Colorectal Cancer. Mol. Cancer Ther. 2015, 14, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huang, X.H.; Dong, L.; Hu, D.; Ge, C.; Zhan, Y.Q.; Xu, W.X.; Yu, M.; Li, W.; Wang, X.; et al. PCBP-1 regulates alternative splicing of the CD44 gene and inhibits invasion in human hepatoma cell line HepG2 cells. Mol. Cancer 2010, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, G.; Hua, X.; Li, Y.; Yan, H.; Che, X.; Tian, Z.; Liufu, H.; Huang, C.; Li, J.; et al. CD44s is a crucial ATG7 downstream regulator for stem-like property, invasion, and lung metastasis of human bladder cancer (BC) cells. Oncogene 2019, 38, 3301–3315. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Kim, D.H.; Lim, J.M.; Lee, J.W.; Jeong, S.J.; Kim, K.P.; Surh, Y.J. Breast Cancer Cell-Derived Soluble CD44 Promotes Tumor Progression by Triggering Macrophage IL1β Production. Cancer Res. 2020, 80, 1342–1356. [Google Scholar]
- Liu, X.; Taftaf, R.; Kawaguchi, M.; Chang, Y.F.; Chen, W.; Entenberg, D.; Zhang, Y.; Gerratana, L.; Huang, S.; Patel, D.B.; et al. Homophilic CD44 Interactions Mediate Tumor Cell Aggregation and Polyclonal Metastasis in Patient-Derived Breast Cancer Models. Cancer Discov. 2019, 9, 96–113. [Google Scholar] [CrossRef]
- Dhar, D.; Antonucci, L.; Nakagawa, H.; Kim, J.Y.; Glitzner, E.; Caruso, S.; Shalapour, S.; Yang, L.; Valasek, M.A.; Lee, S.; et al. Liver Cancer Initiation Requires p53 Inhibition by CD44-Enhanced Growth Factor Signaling. Cancer Cell 2018, 33, 1061–1077.e1066. [Google Scholar] [CrossRef]
- Nallasamy, P.; Nimmakayala, R.K.; Karmakar, S.; Leon, F.; Seshacharyulu, P.; Lakshmanan, I.; Rachagani, S.; Mallya, K.; Zhang, C.; Ly, Q.P.; et al. Pancreatic Tumor Microenvironment Factor Promotes Cancer Stemness via SPP1-CD44 Axis. Gastroenterology 2021, 161, 1998–2013.e7. [Google Scholar] [CrossRef] [PubMed]
- Sottnik, J.L.; Vanderlinden, L.; Joshi, M.; Chauca-Diaz, A.; Owens, C.; Hansel, D.E.; Sempeck, C.; Ghosh, D.; Theodorescu, D. Androgen Receptor Regulates CD44 Expression in Bladder Cancer. Cancer Res. 2021, 81, 2833–2846. [Google Scholar] [CrossRef]
- Yao, H.; Sun, L.; Li, J.; Zhou, X.; Li, R.; Shao, R.; Zhang, Y.; Li, L. A Novel Therapeutic siRNA Nanoparticle Designed for Dual-Targeting CD44 and Gli1 of Gastric Cancer Stem Cells. Int. J. Nanomed. 2020, 15, 7013–7034. [Google Scholar] [CrossRef]
- Shah, V.; Taratula, O.; Garbuzenko, O.B.; Taratula, O.R.; Rodriguez-Rodriguez, L.; Minko, T. Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: An optimal delivery of siRNA and anticancer drug. Clin. Cancer Res. 2013, 19, 6193–6204. [Google Scholar] [CrossRef] [PubMed]
- Qian, D.; Chen, K.; Deng, H.; Rao, H.; Huang, H.; Liao, Y.; Sun, X.; Lu, S.; Yuan, Z.; Xie, D.; et al. MicroRNA-374b Suppresses Proliferation and Promotes Apoptosis in T-cell Lymphoblastic Lymphoma by Repressing AKT1 and Wnt-16. Clin. Cancer Res. 2015, 21, 4881–4891. [Google Scholar] [CrossRef]
- Vallejo, D.M.; Caparros, E.; Dominguez, M. Targeting Notch signalling by the conserved miR-8/200 microRNA family in development and cancer cells. EMBO J. 2011, 30, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Teo, S.; Lam, D.H.; Jeyaseelan, K.; Wang, S. MicroRNA-10b pleiotropically regulates invasion, angiogenicity and apoptosis of tumor cells resembling mesenchymal subtype of glioblastoma multiforme. Cell Death Dis. 2012, 3, e398. [Google Scholar] [CrossRef]
- Bu, P.; Chen, K.Y.; Chen, J.H.; Wang, L.; Walters, J.; Shin, Y.J.; Goerger, J.P.; Sun, J.; Witherspoon, M.; Rakhilin, N.; et al. A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells. Cell. Stem Cell 2013, 12, 602–615. [Google Scholar] [CrossRef]
- Deng, X.; Cao, M.; Zhang, J.; Hu, K.; Yin, Z.; Zhou, Z.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of MiR-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef]
- Wang, S.; Cao, M.; Deng, X.; Xiao, X.; Yin, Z.; Hu, Q.; Zhou, Z.; Zhang, F.; Zhang, R.; Wu, Y.; et al. Degradable hyaluronic acid/protamine sulfate interpolyelectrolyte complexes as miRNA-delivery nanocapsules for triple-negative breast cancer therapy. Adv. Healthc. Mater. 2015, 4, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, D.M.; Day, E.S. Dual Regulation of miR-34a and Notch Signaling in Triple-Negative Breast Cancer by Antibody/miRNA Nanocarriers. Mol. Ther. Nucleic Acids 2020, 21, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Costa, D.F.; Sarisozen, C.; Luther, E.; Mattheolabakis, G.; Dhargalkar, P.P.; Torchilin, V.P. Mixed Nanosized Polymeric Micelles as Promoter of Doxorubicin and miRNA-34a Co-Delivery Triggered by Dual Stimuli in Tumor Tissue. Small 2016, 12, 4837–4848. [Google Scholar] [CrossRef]
- Ortega, M.; Bhatnagar, H.; Lin, A.P.; Wang, L.; Aster, J.C.; Sill, H.; Aguiar, R.C. A microRNA-mediated regulatory loop modulates NOTCH and MYC oncogenic signals in B- and T-cell malignancies. Leukemia 2015, 29, 968–976. [Google Scholar] [CrossRef]
- Kawasaki, H.; Takeuchi, T.; Ricciardiello, F.; Lombardi, A.; Biganzoli, E.; Fornili, M.; De Bortoli, D.; Mesolella, M.; Cossu, A.M.; Scrima, M.; et al. Definition of miRNA Signatures of Nodal Metastasis in LCa: miR-449a Targets Notch Genes and Suppresses Cell Migration and Invasion. Mol. Ther. Nucleic Acids 2020, 20, 711–724. [Google Scholar] [CrossRef]
- Shen, S.; Sun, C.Y.; Du, X.J.; Li, H.J.; Liu, Y.; Xia, J.X.; Zhu, Y.H.; Wang, J. Co-delivery of platinum drug and siNotch1 with micelleplex for enhanced hepatocellular carcinoma therapy. Biomaterials 2015, 70, 71–83. [Google Scholar] [CrossRef]
- Sun, M.; Song, H.; Wang, S.; Zhang, C.; Zheng, L.; Chen, F.; Shi, D.; Chen, Y.; Yang, C.; Xiang, Z.; et al. Integrated analysis identifies microRNA-195 as a suppressor of Hippo-YAP pathway in colorectal cancer. J. Hematol. Oncol. 2017, 10, 79. [Google Scholar] [CrossRef]
- Zhu, B.; Finch-Edmondson, M.; Lee, Y.; Wan, Y.; Sudol, M.; DasGupta, R. miR-582-5p Is a Tumor Suppressor microRNA Targeting the Hippo-YAP/TAZ Signaling Pathway in Non-Small Cell Lung Cancer. Cancers 2021, 13, 756. [Google Scholar] [CrossRef]
- Ferretti, E.; De Smaele, E.; Miele, E.; Laneve, P.; Po, A.; Pelloni, M.; Paganelli, A.; Di Marcotullio, L.; Caffarelli, E.; Screpanti, I.; et al. Concerted microRNA control of Hedgehog signalling in cerebellar neuronal progenitor and tumour cells. EMBO J. 2008, 27, 2616–2627. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Xu, A.; Xu, J.; Huang, H.; Chen, L.; Su, Y.; Zhang, L.; Li, J.; Fan, F.; Deng, J.; et al. MicroRNA-324-5p regulates stemness, pathogenesis and sensitivity to bortezomib in multiple myeloma cells by targeting hedgehog signaling. Int. J. Cancer 2018, 142, 109–120. [Google Scholar] [CrossRef]
- Babashah, S.; Sadeghizadeh, M.; Hajifathali, A.; Tavirani, M.R.; Zomorod, M.S.; Ghadiani, M.; Soleimani, M. Targeting of the signal transducer Smo links microRNA-326 to the oncogenic Hedgehog pathway in CD34+ CML stem/progenitor cells. Int. J. Cancer 2013, 133, 579–589. [Google Scholar] [CrossRef]
- Wu, S.; Han, M.; Zhang, C. Overexpression of microRNA-186 inhibits angiogenesis in retinoblastoma via the Hedgehog signaling pathway by targeting ATAD2. J. Cell. Physiol. 2019, 234, 19059–19072. [Google Scholar] [CrossRef] [PubMed]
- Seidl, C.; Panzitt, K.; Bertsch, A.; Brcic, L.; Schein, S.; Mack, M.; Leithner, K.; Prinz, F.; Olschewski, H.; Kornmueller, K.; et al. MicroRNA-182-5p regulates hedgehog signaling pathway and chemosensitivity of cisplatin-resistant lung adenocarcinoma cells via targeting GLI2. Cancer Lett. 2020, 469, 266–276. [Google Scholar] [CrossRef]
- Zhou, H.; Han, L.; Wang, H.; Wei, J.; Guo, Z.; Li, Z. Chidamide Inhibits Glioma Cells by Increasing Oxidative Stress via the miRNA-338-5p Regulation of Hedgehog Signaling. Oxidative Med. Cell. Longev. 2020, 2020, 7126976. [Google Scholar] [CrossRef]
- Majumder, S.; Crabtree, J.S.; Golde, T.E.; Minter, L.M.; Osborne, B.A.; Miele, L. Targeting Notch in oncology: The path forward. Nat. Rev. Drug Discov. 2021, 20, 125–144. [Google Scholar] [CrossRef]
- Meurette, O.; Mehlen, P. Notch Signaling in the Tumor Microenvironment. Cancer Cell 2018, 34, 536–548. [Google Scholar] [CrossRef]
- Bray, S.J. Notch signalling in context. Nat. Rev. Mol. Cell Biol. 2016, 17, 722–735. [Google Scholar] [CrossRef]
- Demitrack, E.S.; Samuelson, L.C. Notch regulation of gastrointestinal stem cells. J. Physiol. 2016, 594, 4791–4803. [Google Scholar] [CrossRef] [PubMed]
- Koch, U.; Lehal, R.; Radtke, F. Stem cells living with a Notch. Development 2013, 140, 689–704. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Naureen, H.; Attar, R. Regulation of cell signaling pathways by circular RNAs and microRNAs in different cancers: Spotlight on Wnt/β-catenin, JAK/STAT, TGF/SMAD, SHH/GLI, NOTCH and Hippo pathways. Semin. Cell Dev. Biol. 2021, 13, S1084. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.; Basu, B.; Smith, D.M.; Gopinathan, A.; Evans, J.; Steward, W.P.; Palmer, D.; Propper, D.; Venugopal, B.; Hategan, M.; et al. A phase I trial of the γ-secretase inhibitor MK-0752 in combination with gemcitabine in patients with pancreatic ductal adenocarcinoma. Br. J. Cancer 2018, 118, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Papayannidis, C.; DeAngelo, D.J.; Stock, W.; Huang, B.; Shaik, M.N.; Cesari, R.; Zheng, X.; Reynolds, J.M.; English, P.A.; Ozeck, M.; et al. A Phase 1 study of the novel gamma-secretase inhibitor PF-03084014 in patients with T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma. Blood Cancer J. 2015, 5, e350. [Google Scholar] [CrossRef]
- Cubillo, G.A.; Dean, A.; Mu, O.A.; Hidalgo, M.; Pazo-Cid, R.; Martin, M.; Macarulla, M.T.; Lipton, L.; Harris, M.; Manzano-Mozo, J.L. 620PDYOSEMITE: A 3 arm double-blind randomized phase 2 study of gemcitabine, paclitaxel protein-bound particles for injectable suspension, and placebo (GAP) versus gemcitabine, paclitaxel protein-bound particles for injectable suspension and either 1 or 2 truncated courses of demcizumab (GAD). Ann. Oncol. 2017, 28, 211. [Google Scholar]
- Mckeage, M.J.; Hughes, B.; Markman, B.; Hidalgo, M.; Kotasek, D.J. A Phase 1b Study of the Anticancer Stem Cell Agent Demcizumab (DEM), Pemetrexed (PEM), and Carboplatin (CARBO) in pts with First-Line Nonsquamous NSCLC. J. Clin. Oncol. 2016, 32, 2544. [Google Scholar] [CrossRef]
- Ferrarotto, R.; Eckhardt, G.; Patnaik, A.; LoRusso, P.; Faoro, L.; Heymach, J.V.; Kapoun, A.M.; Xu, L.; Munster, P. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann. Oncol. 2018, 29, 1561–1568. [Google Scholar] [CrossRef]
- Casulo, C.; Jia, R.; Dang, N.H.; Gore, L.; Diefenbach, C.; Beaven, A.W.; Castro, J.E.; Porcu, P.; Faoro, L.; Dupont, J. Safety and Preliminary Efficacy Results of a Phase I First-in-Human Study of the Novel Notch-1 Targeting Antibody Brontictuzumab (OMP-52M51) Administered Intravenously to Patients with Hematologic Malignancies. Blood 2016, 128, 5108. [Google Scholar] [CrossRef]
- Kofoed Andersen, C.; Khatri, S.; Hansen, J.; Slott, S.; Pavan Parvathaneni, R.; Mendes, A.C.; Chronakis, I.S.; Hung, S.C.; Rajasekaran, N.; Ma, Z.; et al. Carbon Nanotubes-Potent Carriers for Targeted Drug Delivery in Rheumatoid Arthritis. Pharmaceutics 2021, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Cai, Q.; West, M.B.; Youm, I.; Huang, X.; Li, W.; Cheng, W.; Nakmali, D.; Ewert, D.L.; Kopke, R.D. Regeneration of Cochlear Hair Cells and Hearing Recovery through Hes1 Modulation with siRNA Nanoparticles in Adult Guinea Pigs. Mol. Ther. 2018, 26, 1313–1326. [Google Scholar] [CrossRef]
- Koga, J.I.; Nakano, T.; Dahlman, J.E.; Figueiredo, J.L.; Zhang, H.; Decano, J.; Khan, O.F.; Niida, T.; Iwata, H.; Aster, J.C.; et al. Macrophage Notch Ligand Delta-Like 4 Promotes Vein Graft Lesion Development: Implications for the Treatment of Vein Graft Failure. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2343–2353. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, J.S.; Lee, S.J.; Jang, J.; Park, J.S.; Back, S.H.; Bahn, G.; Park, J.H.; Kang, Y.M.; Kim, S.H.; et al. Notch1 targeting siRNA delivery nanoparticles for rheumatoid arthritis therapy. J. Control Release 2015, 216, 140–148. [Google Scholar] [CrossRef]
- Kao, S.H.; Cheng, W.C.; Wang, Y.T.; Wu, H.T.; Yeh, H.Y.; Chen, Y.J.; Tsai, M.H.; Wu, K.J. Regulation of miRNA Biogenesis and Histone Modification by K63-Polyubiquitinated DDX17 Controls Cancer Stem-like Features. Cancer Res. 2019, 79, 2549–2563. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wei, X.; He, J.; Cao, Q.; Du, D.; Zhan, X.; Zeng, Y.; Yuan, S.; Sun, L. The comprehensive landscape of miR-34a in cancer research. Cancer Metastasis Rev. 2021, 11, 234. [Google Scholar] [CrossRef]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef]
- Cordenonsi, M.; Zanconato, F.; Azzolin, L.; Forcato, M.; Rosato, A.; Frasson, C.; Inui, M.; Montagner, M.; Parenti, A.R.; Poletti, A.; et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011, 147, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. New route to targeting the Hippo pathway. Nat. Rev. Drug Discov. 2021, 20, 344. [Google Scholar] [CrossRef]
- Dey, A.; Varelas, X.; Guan, K.L. Targeting the Hippo pathway in cancer, fibrosis, wound healing and regenerative medicine. Nat. Rev. Drug Discov. 2020, 19, 480–494. [Google Scholar] [CrossRef]
- Al-Moujahed, A.; Brodowska, K.; Stryjewski, T.P.; Efstathiou, N.E.; Vasilikos, I.; Cichy, J.; Miller, J.W.; Gragoudas, E.; Vavvas, D.G. Verteporfin inhibits growth of human glioma in vitro without light activation. Sci. Rep. 2017, 7, 7602. [Google Scholar] [CrossRef]
- Morales, F.; Giordano, A. Overview of CDK9 as a target in cancer research. Cell Cycle Georget. Tex. 2016, 15, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Wang, H.; Shi, Z.; Dong, A.; Zhang, W.; Song, X.; He, F.; Wang, Y.; Zhang, Z.; Wang, W.; et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 2014, 25, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, A.C.; Bauer, T.M.; Aggarwal, C.; Lee, C.B.; Harvey, R.D.; Cohen, R.B.; Sedarati, F.; Nip, T.K.; Faessel, H.; Dash, A.B.; et al. Phase Ib study of pevonedistat, a NEDD8-activating enzyme inhibitor, in combination with docetaxel, carboplatin and paclitaxel, or gemcitabine, in patients with advanced solid tumors. Investig. New Drugs 2019, 37, 87–97. [Google Scholar] [CrossRef]
- Swords, R.T.; Erba, H.P.; DeAngelo, D.J.; Bixby, D.L.; Altman, J.K.; Maris, M.; Hua, Z.; Blakemore, S.J.; Faessel, H.; Sedarati, F.; et al. Pevonedistat (MLN4924), a First-in-Class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: A phase 1 study. Br. J. Haematol. 2015, 169, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Bogorad, R.L.; Barnes, C.; Walsh, S.; Zhuang, I.; Nonaka, H.; Ruda, V.; Kuchimanchi, S.; Nechev, L.; Akinc, A.; et al. RNAi-nanoparticulate manipulation of gene expression as a new functional genomics tool in the liver. J. Hepatol. 2016, 64, 899–907. [Google Scholar] [CrossRef]
- Petrov, K.; Wierbowski, B.M.; Salic, A. Sending and Receiving Hedgehog Signals. Annu. Rev. Cell Dev. Biol. 2017, 33, 145–168. [Google Scholar] [CrossRef]
- Justilien, V.; Fields, A.P. Molecular pathways: Novel approaches for improved therapeutic targeting of Hedgehog signaling in cancer stem cells. Clin. Cancer Res. 2015, 21, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Norsworthy, K.J.; By, K.; Subramaniam, S.; Zhuang, L.; Del Valle, P.L.; Przepiorka, D.; Shen, Y.L.; Sheth, C.M.; Liu, C.; Leong, R.; et al. FDA Approval Summary: Glasdegib for Newly Diagnosed Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 6021–6025. [Google Scholar] [CrossRef]
- Shord, S.S.; Casey, D.; Zhao, H.; Demko, S.; Keegan, P.; Pazdur, R. FDA Approval Summary: Sonidegib-Response. Clin. Cancer Res. 2017, 23, 5994. [Google Scholar] [CrossRef]
- Axelson, M.; Liu, K.; Jiang, X.; He, K.; Wang, J.; Zhao, H.; Kufrin, D.; Palmby, T.; Dong, Z.; Russell, A.M.; et al. U.S. Food and Drug Administration approval: Vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin. Cancer Res. 2013, 19, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, P.; Singh, M.; Triche, T.J.; Guzman, M.; Merchant, A.A. GLI3 repressor determines Hedgehog pathway activation and is required for response to SMO antagonist glasdegib in AML. Blood 2017, 129, 3465–3475. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Z.; Caviglia, J.M.; Corey, K.E.; Herfel, T.M.; Cai, B.; Masia, R.; Chung, R.T.; Lefkowitch, J.H.; Schwabe, R.F.; et al. Hepatocyte TAZ/WWTR1 Promotes Inflammation and Fibrosis in Nonalcoholic Steatohepatitis. Cell Metab. 2016, 24, 848–862. [Google Scholar] [CrossRef]
- Gao, J.; Graves, S.; Koch, U.; Liu, S.; Jankovic, V.; Buonamici, S.; El Andaloussi, A.; Nimer, S.D.; Kee, B.L.; Taichman, R.; et al. Hedgehog signaling is dispensable for adult hematopoietic stem cell function. Cell. Stem Cell 2009, 4, 548–558. [Google Scholar] [CrossRef]
- Kim, K.; Vinayagam, A.; Perrimon, N. A rapid genome-wide microRNA screen identifies miR-14 as a modulator of Hedgehog signaling. Cell Rep. 2014, 7, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Wang, S.; Kim, J.; Rao, K.M.; Park, S.Y.; Chung, I.; Ha, C.S.; Kim, S.W.; Yun, Y.H.; Jung, Y. MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat. Commun. 2016, 7, 10993. [Google Scholar] [CrossRef]
- Kim, J.; Hyun, J.; Wang, S.; Lee, C.; Jung, Y. MicroRNA-378 is involved in hedgehog-driven epithelial-to-mesenchymal transition in hepatocytes of regenerating liver. Cell Death Dis. 2018, 9, 721. [Google Scholar] [CrossRef]
- Glumac, P.M.; LeBeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Y.; Gupta, N.; Liu, X.; He, A.; Liu, L.; Zuo, J.; Chang, Y.; Fang, F. Pentaspan membrane glycoprotein, prominin-1, is involved in glucose metabolism and cytoskeleton alteration. Biochem. Mosc. 2007, 72, 854–862. [Google Scholar] [CrossRef]
- Picanço-Castro, V.; Pereira, C.G.; Covas, D.T.; Porto, G.S.; Athanassiadou, A.; Figueiredo, M.L. Emerging patent landscape for non-viral vectors used for gene therapy. Nat. Biotechnol. 2020, 38, 151–157. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef]
- Chou, L.Y.; Ming, K.; Chan, W.C. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C.; Sleiman, H.F. DNA nanotechnology. Nat. Rev. Mater. 2018, 3, 196. [Google Scholar] [CrossRef]
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Yingchoncharoen, P.; Kalinowski, D.S.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef]
- Namiki, Y.; Fuchigami, T.; Tada, N.; Kawamura, R.; Matsunuma, S.; Kitamoto, Y.; Nakagawa, M. Nanomedicine for cancer: Lipid-based nanostructures for drug delivery and monitoring. Acc. Chem. Res. 2011, 44, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, S.; Koide, H.; Asai, T. Recent advances in siRNA delivery mediated by lipid-based nanoparticles. Adv. Drug Deliv. Rev. 2020, 154–155, 64–78. [Google Scholar] [CrossRef]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chen, Y.; Zhao, P.; Xue, H.; You, J.; Li, B.; Liu, Y.; He, C.; Zhang, X.; Fan, L.; et al. Enhancing the therapeutic effect via elimination of hepatocellular carcinoma stem cells using Bmi1 siRNA delivered by cationic cisplatin nanocapsules. Nanomedicine 2018, 14, 2009–2021. [Google Scholar] [CrossRef]
- Tanner, P.; Baumann, P.; Enea, R.; Onaca, O.; Palivan, C.; Meier, W. Polymeric vesicles: From drug carriers to nanoreactors and artificial organelles. Acc. Chem. Res. 2011, 44, 1039–1049. [Google Scholar] [CrossRef]
- Singha, K.; Namgung, R.; Kim, W.J. Polymers in small-interfering RNA delivery. Nucleic Acid Ther. 2011, 21, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.M.; Leong, K.W. Natural polymers for gene delivery and tissue engineering. Adv. Drug Deliv. Rev. 2006, 58, 487–499. [Google Scholar] [CrossRef]
- Gai, C.; Liu, C.; Wu, X.; Yu, M.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. MT1DP loaded by folate-modified liposomes sensitizes erastin-induced ferroptosis via regulating miR-365a-3p/NRF2 axis in non-small cell lung cancer cells. Cell Death Dis. 2020, 11, 751. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, X.; Wang, H.; Fan, R.; Hu, Y.; Hu, X.; Li, J. Dasatinib self-assembled nanoparticles decorated with hyaluronic acid for targeted treatment of tumors to overcome multidrug resistance. Drug Deliv. 2021, 28, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of MicroRNAs by Chitosan Nanoparticles to Functionally Alter Macrophage Cholesterol Efflux in Vitro and in Vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef]
- Schaffert, D.; Wagner, E. Gene therapy progress and prospects: Synthetic polymer-based systems. Gene Ther. 2008, 15, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Huang, T.; Xiao, J.; Liao, Z.; Ouyang, J.; Dong, J.; Xian, C.J.; Hu, J.; Wang, L.; Ke, Y.; et al. The immuno-reactivity of polypseudorotaxane functionalized magnetic CDMNP-PEG-CD nanoparticles. J. Cell. Mol. Med. 2021, 25, 561–574. [Google Scholar] [CrossRef]
- Chen, J.; Cao, L.; Cui, Y.; Tu, K.; Wang, H.; Wang, L.Q. The exploration of endocytic mechanisms of PLA-PEG nanoparticles prepared by coaxialtri-capillary electrospray-template removal method. Colloids Surf. B Biointerfaces 2018, 161, 10–17. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef]
- Ma, J.; Kala, S.; Yung, S.; Chan, T.M.; Cao, Y.; Jiang, Y.; Liu, X.; Giorgio, S.; Peng, L.; Wong, A.S.T. Blocking Stemness and Metastatic Properties of Ovarian Cancer Cells by Targeting p70(S6K) with Dendrimer Nanovector-Based siRNA Delivery. Mol. Ther. 2018, 26, 70–83. [Google Scholar] [CrossRef]
- Zuo, Z.Q.; Chen, K.G.; Yu, X.Y.; Zhao, G.; Shen, S.; Cao, Z.T.; Luo, Y.L.; Wang, Y.C.; Wang, J. Promoting tumor penetration of nanoparticles for cancer stem cell therapy by TGF-β signaling pathway inhibition. Biomaterials 2016, 82, 48–59. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, G.; Liu, J.; Ma, N.; Chivukula, P.; Perelman, L.; Okada, K.; Chen, Z.; Gough, D.; Yu, L. Novel biodegradable lipid nano complex for siRNA delivery significantly improving the chemosensitivity of human colon cancer stem cells to paclitaxel. J. Control Release 2009, 140, 277–283. [Google Scholar] [CrossRef]
- Kim, S.S.; Harford, J.B.; Moghe, M.; Rait, A.; Pirollo, K.F.; Chang, E.H. Targeted nanocomplex carrying siRNA against MALAT1 sensitizes glioblastoma to temozolomide. Nucleic Acids Res. 2018, 46, 1424–1440. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.F.; Liu, Y.; Shen, S.; Zhu, Y.H.; Wang, J. Targeting glucose uptake with siRNA-based nanomedicine for cancer therapy. Biomaterials 2015, 51, 1–11. [Google Scholar] [CrossRef]
- Heuer-Jungemann, A.; Feliu, N.; Bakaimi, I.; Hamaly, M.; Alkilany, A.; Chakraborty, I.; Masood, A.; Casula, M.F.; Kostopoulou, A.; Oh, E.; et al. The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev. 2019, 119, 4819–4880. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Petukhova, A.; Kumacheva, E. Properties and emerging applications of self-assembled structures made from inorganic nanoparticles. Nat. Nanotechnol. 2010, 5, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Yang, Y.; Liu, B.; He, J.; Nie, Z. Polymer-guided assembly of inorganic nanoparticles. Chem. Soc. Rev. 2020, 49, 465–508. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous silica nanoparticles for drug and gene delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem Soc Rev 2012, 41, 2590–2605. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Glackin, C.A.; Horwitz, M.A.; Zink, J.I. Nanomachines and Other Caps on Mesoporous Silica Nanoparticles for Drug Delivery. Acc. Chem. Res. 2019, 52, 1531–1542. [Google Scholar] [CrossRef] [PubMed]
- Shahin, S.A.; Wang, R.; Simargi, S.I.; Contreras, A.; Parra Echavarria, L.; Qu, L.; Wen, W.; Dellinger, T.; Unternaehrer, J.; Tamanoi, F.; et al. Hyaluronic acid conjugated nanoparticle delivery of siRNA against TWIST reduces tumor burden and enhances sensitivity to cisplatin in ovarian cancer. Nanomedicine 2018, 14, 1381–1394. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Zhang, Y.; Zeng, B.; Wang, S.; Zhu, T.; Roden, R.B.; Chen, Y.; Yang, R. Delivery of telomerase reverse transcriptase small interfering RNA in complex with positively charged single-walled carbon nanotubes suppresses tumor growth. Clin. Cancer Res. 2006, 12, 4933–4939. [Google Scholar] [CrossRef]
- Wen, Z.; Feng, Y.; Hu, Y.; Lian, L.; Huang, H.; Guo, L.; Chen, S.; Yang, Q.; Zhang, M.; Wan, L.; et al. Multiwalled carbon nanotubes co-delivering sorafenib and epidermal growth factor receptor siRNA enhanced tumor-suppressing effect on liver cancer. Aging 2021, 13, 1872–1882. [Google Scholar] [CrossRef] [PubMed]
- Olerile, L.D.; Liu, Y.; Zhang, B.; Wang, T.; Mu, S.; Zhang, J.; Selotlegeng, L.; Zhang, N. Near-infrared mediated quantum dots and paclitaxel co-loaded nanostructured lipid carriers for cancer theragnostic. Colloids Surf. B-Biointerfaces 2017, 150, 121–130. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Wang, M.; Zhang, Y.; Chen, G.; Li, L.; Wu, D.; Wang, Q. In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window. Biomaterials 2014, 35, 393–400. [Google Scholar] [CrossRef]
- Tada, H.; Higuchi, H.; Wanatabe, T.M.; Ohuchi, N. In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res. 2007, 67, 1138–1144. [Google Scholar] [CrossRef]
- Chen, A.A.; Derfus, A.M.; Khetani, S.R.; Bhatia, S.N. Quantum dots to monitor RNAi delivery and improve gene silencing. Nucleic Acids Res. 2005, 33, e190. [Google Scholar] [CrossRef]
- Li, J.M.; Zhao, M.X.; Su, H.; Wang, Y.Y.; Tan, C.P.; Ji, L.N.; Mao, Z.W. Multifunctional quantum-dot-based siRNA delivery for HPV18 E6 gene silence and intracellular imaging. Biomaterials 2011, 32, 7978–7987. [Google Scholar] [CrossRef] [PubMed]
- Seferos, D.S.; Prigodich, A.E.; Giljohann, D.A.; Patel, P.C.; Mirkin, C.A. Polyvalent DNA nanoparticle conjugates stabilize nucleic acids. Nano Lett. 2009, 9, 308–311. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Yu, X.; Zha, X.; Fu, Q.; Liu, B.; Wang, X.; Chen, Y.; Chen, Y.; Shan, Y.; et al. The effect of conjugation to gold nanoparticles on the ability of low molecular weight chitosan to transfer DNA vaccine. Biomaterials 2008, 29, 111–117. [Google Scholar] [CrossRef]
- Yi, Y.; Kim, H.J.; Zheng, M.; Mi, P.; Naito, M.; Kim, B.S.; Min, H.S.; Hayashi, K.; Perche, F.; Toh, K.; et al. Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells. J. Control. Release 2019, 295, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Shtam, T.A.; Kovalev, R.A.; Varfolomeeva, E.Y.; Makarov, E.M.; Kil, Y.V.; Filatov, M.V. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun. Signal. 2013, 11, 88. [Google Scholar] [CrossRef]
- Vazquez-Rios, A.J.; Molina-Crespo, A.; Bouzo, B.L.; Lopez-Lopez, R.; Moreno-Bueno, G.; de la Fuente, M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J. Nanobiotechnol. 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.S.; Roh, T.Y.; Park, J.; Nilsson, J.; Lotvall, J.; Kim, Y.K.; Gho, Y.S. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Dorval, T.; Chaput, N.; Andre, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xiong, G.; Guo, S.; Xu, C.; Xu, R.; Guo, P.; Shu, D. Delivery of Anti-miRNA for Triple-Negative Breast Cancer Therapy Using RNA Nanoparticles Targeting Stem Cell Marker CD133. Mol. Ther. 2019, 27, 1252–1261. [Google Scholar] [CrossRef]
- Lin, X.; Chen, W.; Wei, F.; Zhou, B.P.; Hung, M.C.; Xie, X. Nanoparticle Delivery of miR-34a Eradicates Long-term-cultured Breast Cancer Stem Cells via Targeting C22ORF28 Directly. Theranostics 2017, 7, 4805–4824. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Vakilzadeh, H.; Ghassami, E. Lipoprotein Like Nanoparticles Used in Drug and Gene Delivery. Curr. Pharm. Des. 2016, 22, 3466–3485. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Chen, H.; Huang, J.; Song, Q.; Chen, Y.; Gu, X.; Jiang, Z.; Huang, Y.; Lin, Y.; Feng, J.; et al. Tailored Lipoprotein-Like miRNA Delivery Nanostructure Suppresses Glioma Stemness and Drug Resistance through Receptor-Stimulated Macropinocytosis. Adv. Sci. 2020, 7, 1903290. [Google Scholar] [CrossRef] [PubMed]
- Saygin, C.; Matei, D.; Majeti, R.; Reizes, O.; Lathia, J.D. Targeting Cancer Stemness in the Clinic: From Hype to Hope. Cell. Stem Cell 2019, 24, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Eyler, C.E.; Rich, J.N. Survival of the fittest: Cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 2008, 26, 2839–2845. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Duan, J.J.; Wang, J.; Li, L.; Wang, D.; Liu, X.Z.; Yang, J.; Zhang, H.R.; Lv, J.; Yang, Y.J.; et al. Inhibition of the ALDH18A1-MYCN positive feedback loop attenuates MYCN-amplified neuroblastoma growth. Sci. Transl. Med. 2020, 12, 186. [Google Scholar] [CrossRef]
- Chen, H.N.; Shu, Y.; Liao, F.; Liao, X.; Zhang, H.; Qin, Y.; Wang, Z.; Luo, M.; Liu, Q.; Xue, Z.; et al. Genomic evolution and diverse models of systemic metastases in colorectal cancer. Gut 2021, 11, 142. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).