N-3 PUFA and Pregnancy Preserve C-Peptide in Women with Type 1 Diabetes Mellitus
Abstract
:1. Introduction
- preclinical autoimmunity towards beta cells with progressive decline in insulin production;
- the onset of clinical diabetes;
- transient remission; and
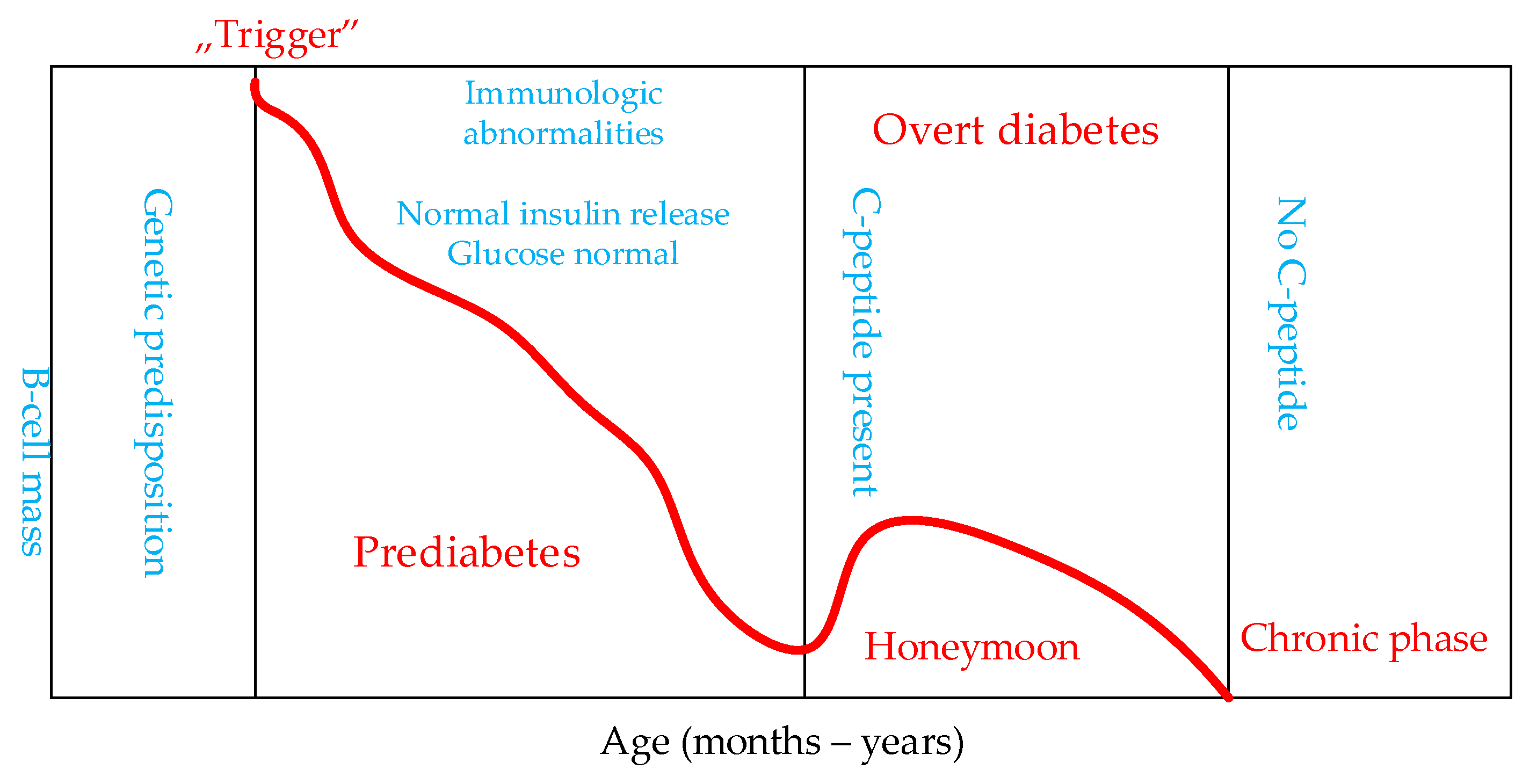
- clinically pronounced diabetes with acute and chronic complications (Figure 1).
2. Materials and Methods
Exclusion Criteria
3. Hypoglycemia in Pregnant Women with T1DM
3.1. Insulin and C-Peptide
3.2. The Immune System in Pregnancy
4. Fatty Acids
- saturated fatty acids (SFA), which do not contain double (unsaturated) bonds;
- monounsaturated fatty acids (MUFAs) in which there is one double bond between carbon atoms; and
- polyunsaturated fatty acids (PUFA) in which there are several double bonds.
Inflammatory Processes and n-3 Polyunsaturated Fatty Acids
5. n-3 PUFA and C-Peptide Preservation
5.1. n-3 PUFA and C-Peptide Preservation in Pregnant Women with T1DM
5.2. n-3 PUFA and C-Peptide Preservation in Nonpregnant Patient with T1DM
6. Vitamin D Has a Protective Effect in Preventing T1DM
7. C-peptide, Insulin Doses, and Glycemic Control
8. Conclusions and Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Holt, R.I.G.; DeVries, J.H.; Hess-Fischl, A.; Hirsch, I.B.; Kirkman, M.S.; Klupa, T.; Ludwig, B.; Nørgaard, K.; Pettus, J.; Renard, E.; et al. The Management of Type 1 Diabetes in Adults. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2021, 44, 2589–2625. [Google Scholar] [CrossRef]
- Brown, R.J.; Rother, K.I. Effects of beta-cell rest on beta-cell function: A review of clinical and preclinical data. Pediatric Diabetes 2008, 9 Pt 2, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.R. Glycemic treatment during type 1 diabetes pregnancy: A story of (mostly) sweet success! Diabetes Care 2018, 41, 1563–1571. [Google Scholar] [CrossRef] [Green Version]
- Persson, M.; Norman, M.; Hanson, U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes Care 2009, 32, 2005–2009. [Google Scholar] [CrossRef] [Green Version]
- Djelmis, J. Clinical management of pregnancy complicated with type1/type2 diabetes mellitus. In Diabetology of Pregnancy; Djelmis, J., Desoye, G., Ivanisevic, M., Eds.; Karger: Basel, Switzerland, 2005; Volume 17, pp. 161–173. [Google Scholar]
- Alexopoulos, A.S.; Blair, R.; Peters, A.L. Management of Preexisting Diabetes in Pregnancy: A Review. JAMA 2019, 321, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.S.; Donovan, L.E.; Corcoy, R.; Murphy, K.E.; Amiel, S.A.; Hunt, K.F.; Asztalos, E.; Barrett, J.F.R.; Sanchez, J.J.; de Leiva, A.; et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): A multicentre international randomised controlled trial. Lancet 2017, 390, 2347–2359. [Google Scholar] [CrossRef] [Green Version]
- Ringholm, L.; Pedersen-Bjergaard, U.; Thorsteinsson, B.; Damm, P.; Mathiesen, E.R. Hypoglycaemia during pregnancy in women with type 1 diabetes. Diabet. Med. 2012, 29, 558–566. [Google Scholar] [CrossRef]
- Kinsley, B. Achieving better outcomes in pregnancies complicated by type 1 and type 2 diabetes mellitus. Clin. Ther. 2007, 29 (Suppl. SD), S153–S160. [Google Scholar] [CrossRef]
- Leinonen, P.J.; Hiilesmaa, V.K.; Kaaja, R.J.; Teramo, K.A. Maternal Mortality in Type 1 Diabetes. Diabetes Care 2001, 24, 1501–1502. [Google Scholar] [CrossRef] [Green Version]
- Rosenn, B.M.; Miodovnik, M. Glycemic control in the diabetic pregnancy: Is tighter always better? J. Matern. Fetal Med. 2000, 9, 29–34. [Google Scholar]
- Ng, D.; Noor, N.M.; Yong, S.L. Prevalence of Hypoglycaemia among Insulin-Treated Pregnant Women with Diabetes Who Achieved Tight Glycaemic Control. J. ASEAN Fed. Endocr. Soc. 2019, 34, 29–35. [Google Scholar] [CrossRef] [Green Version]
- International Hypoglycaemia Study Group. Glucose Concentrations of Less Than 3.0 mmol/L (54 mg/dL) Should Be Reported in Clinical Trials: A Joint Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017, 40, 155–157. [Google Scholar] [CrossRef] [Green Version]
- Mayer, J.P.; Zhang, F.; DiMarchi, R.D. Insulin structure and function. Biopolymers 2007, 88, 687–713. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, A.; Hannan, A.; Uddin, J.J.; Pang, M.-D. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Wahren, J.; Ekberg, K.; Johansson, J.; Henriksson, M.; Pramanik, A.; Johansson, B.-L.; Rigler, R.; Rnvall, H.J. Role of C-peptide in human physiology. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E759–E768. [Google Scholar] [CrossRef]
- Palmer, J.P.; Fleming, G.A.; Greenbaum, C.J.; Herold, K.C.; Jansa, L.D.; Kolb, H.; Lachin, J.M.; Polonsky, K.S.; Pozzilli, P.; Skyler, J.S. ADA Workshop Report: C-Peptide. Is the Appropriate Outcome Measure for Type 1 Diabetes Clinical Trials to Preserve β-Cell Function. Diabetes 2004, 53, 250–264. [Google Scholar] [CrossRef] [Green Version]
- Steffes, M.W.; Sibley, S.; Jackson, M.; Thomas, W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003, 26, 832–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenbaum, C.J.; Anderson, A.M.; Dolan, L.M.; Mayer-Davis, E.J.; Dabelea, D.; Imperatore, G.; Marcovina, S.; Pihoker, C.; SEARCH Study Group. Preservation of beta-cell Function in auto antibody-positive youth with diabetes. Diabetes Care 2009, 32, 1839–1844. [Google Scholar] [CrossRef] [Green Version]
- Evans-Molina, C.; Sims, E.K.; DiMeglio, L.A.; Ismail, H.M.; Steck, A.K.; Palmer, J.P.; Krischer, J.P.; Geyer, S.; Xu, P.; Sosenko, J.M. Type 1 Diabetes TrialNet Study Group. β Cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight 2018, 3, e120877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbetti, F.; Simeon, I.; Taylor, S.I. Insulin: Still a miracle after all these years. J. Clin. Investig. 2019, 129, 3045–3047. [Google Scholar] [CrossRef]
- Poudel, A.; Savari, O.; Striegel, D.A.; Periwal, V.; Taxy, J.; Millis, J.M.; Witkowski, P.; Atkinson, M.A.; Hara, M. Beta-cell destruction and preservation in childhood and adult-onset type 1 diabetes. Endocrine 2015, 49, 693–702. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.J.; Jacobson, D.R.; Rankin, M.M.; Cox, A.R.; Kushner, J.A. β Cells Persist in T1D Pancreata without Evidence of Ongoing β-Cell Turnover or Neogenesis. J. Clin. Endocrinol. Metab. 2017, 102, 2647–2659. [Google Scholar] [CrossRef]
- Ziegler, A.-G.; Pflueger, M.; Winkler, C.; Achenbach, P.; Akolkar, B.; Krischer, J.P.; Bonifacio, E. Accelerated progression from islet autoimmunity to diabetes is causing the escalating incidence of type 1 diabetes in young children. J. Autoimmun. 2011, 37, 3–7. [Google Scholar] [CrossRef] [Green Version]
- Poole, J.A.; Claman, H.N. Immunology of pregnancy: Implications for the mother. Clin. Rev. Allergy Immunol. 2004, 26, 161–170. [Google Scholar] [CrossRef]
- Nalla, A.; Ringholm, L.; Sørensen, S.N.; Damm, P.; Mathiesen, E.R.; Nielsen, J.H. Possible mechanisms involved in improved beta cell function in pregnant women with type 1 diabetes. Heliyon 2020, 19, e04569. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, L.; Hindi, S.; Sorenson, R.L.; German, M.S. β-Cell adaptation in pregnancy. Diabetes Obes. Metab. 2016, 18 (Suppl. S1), 63–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieck, S.; Kaestner, K.H. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol. Metab. 2010, 21, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Amouyal, C.; Klatzmann, D.; Tibi, E.; Salem, J.-E.; Halbron, M.; Popelier, M.; Jacqueminet, S.C.; Ciangura, C.O.; Bourron, O.; Andreelli, F.A.; et al. Pregnant type 1 diabetes women with rises in C-peptide display higher levels of regulatory T cells: A pilot study. Diabetes Metab. 2021, 47, 101188. [Google Scholar] [CrossRef]
- Nielsen, L.R.; Rehfeld, J.F.; Pedersen-Bjergaard, U.; Damm, P.; Mathiesen, E.R. Pregnancy-induced rise in serum C-peptide concentrations in women with type 1 diabetes. Diabetes Care 2009, 32, 1052–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meek, C.L.; Oram, R.A.; McDonald, T.J.; Feig, D.S.; Hattersley, A.T.; Murphy, H.R.; CONCEPTT Collaborative Group. Reappearance of C-Peptide During the Third Trimester of Pregnancy in Type 1 Diabetes: Pancreatic Regeneration or Fetal Hyperinsulinism? Diabetes Care 2021, 44, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.R.; Elleri, D.; Allen, J.M.; Simmons, D.; Nodale, M.; Hovorka, R. Plasma C-peptide concentration in women with Type 1 diabetes during early and late pregnancy. Diabet. Med. 2012, 29, e361–e364. [Google Scholar] [CrossRef]
- Burdge, G.C.; Wootton, S.A. Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br. J. Nutr. 2002, 88, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.M.; Burdge, G. Long-chain n-3 PUFA: Plant v. marine sources. Proc. Nutr. Soc. 2006, 65, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvaticek, M.; Djelmis, J.; Ivanisevic, M.; Oreskovic, S.; Herman, M. Effect of eicosapentaenoic acid and docosahexaenoic acid supplementation on C-peptide preservation in pregnant women with type-1 diabetes: Randomized placebo controlled clinical trial. Eur. J. Clin. Nutr. 2017, 71, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Davis, E.J.; Dabelea, D.; Crandell, J.L.; Crume, T.; D’Agostino, R.B., Jr.; Dolan, L.; King, I.B.; Lawrence, J.M.; Norris, J.M.; Pihoker, C.; et al. Nutritional factors and preservation of C-peptide in youth with recently diagnosed type 1 diabetes: SEARCH Nutrition Ancillary Study. Diabetes Care 2013, 36, 1842–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, K.C.; Chu, A.; Go, V.L.; Saad, M.F. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am. J. Clin. Nutr. 2004, 79, 820–825. [Google Scholar] [CrossRef] [Green Version]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Ilic, S.; Jovanovic, L.; Wolitzer, A.O. Is the paradoxical first trimester drop in insulin requirement due to an increase in C-peptide concentration in pregnant Type 1 diabetic women? Diabetologia 2000, 43, 1329–1336. [Google Scholar]
- Marren, S.M.; Hammersley, S.; McDonald, B.M.; Shields, T.J.; xKnight, B.A.; Hill, A.; Bolt, R.; Tree, T.I.; Roep, B.O.; Hattersley, A.T.; et al. Persistent C-peptide is associated with reduced hypoglycaemia but not HbA1c in adults with longstanding Type 1 diabetes: Evidence for lack of intensive treatment in UK clinical practice? Diabet. Med. 2019, 36, 1092–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellens, M.J.; Vollenbrock, C.; Dekker, P.; Boesten, L.S.M.; Geelhoed-Duijvestijn, P.H.; Martine, M.C.; de Vries-Velraeds, M.M.C.; Nefs, G.; Wolffenbuttel, B.H.R.; Aanstoot, H.-J.; et al. Residual C-peptide secretion and hypoglycemia awareness in people with type 1 diabetes. BMJ Open Diabetes Res. Care 2021, 9, e002288. [Google Scholar] [CrossRef]
- Feig, D.S.; Murphy, H.R. Continuous glucose monitoring in pregnant women with Type 1 diabetes: Benefits for mothers, using pumps or pens, and their babies. Diabet. Med. 2018, 35, 430–435. [Google Scholar] [CrossRef]
- Szajewska, H.; Horvath, A.; Koletzko, B. Effect of n-3 long-chain polyunsaturated fatty acid supplementation of women with low-risk pregnancies on pregnancy outcomes and growth measures at birth: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2006, 83, 1337–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innis, S.M. Dietary (n-3) fatty acids and brain development. J. Nutr. 2007, 137, 855–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helland, I.B.; Smith, L.; Saarem, K.; Saugstad, O.D.; Drevon, C.A. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics 2003, 111, e39–e44. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA). Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar]
| Reference | Study Population | No of Participants | Evaluated Parameters | Results/Conclusions |
|---|---|---|---|---|
| Ilic, S. [41] | Pregnant women T1DM At 10 w of gestation | 10 | Fasting C-peptide | C-peptide non-detectable before pregnancy to detectable at 10 weeks of gestation. |
| Nielsen L. [30] | Pregnant women T1DM Early and late pregnancy, postpartum | 108; two groups based on serum glucose, >5.0 or <5.0 mmol/L | C-peptide, glucose, placental GH, IGF-I. | C-peptide in women with long-term T1DM C-peptide in early pregnancy: raised up to 97% by 33 weeks gestation. |
| Murphy, HR [32] | Pregnant women T1DM Early and late pregnancy | 10 | Fasting or meal- stimulated C-peptide concentration. | No change in fasting or meal-stimulated plasma C-peptide from early to late pregnancy. |
| Mayer-Davis, EJ. [38] | Young people (up to 20 years)/T1DM | 1316 | EPA and DHA, vitamins D E. Intake of leucine | Intake of n-3 PUFA sustained β-cell function, higher DHA and EPA were associated with higher fasting C-peptide. |
| Horvaticek, M. [37] | Pregnant women T1DM Three trimesters of pregnancy | 90; two groups, n-3 supplementation or placebo | Fasting C-peptide, FPG EPA and DHA in maternal and cord blood serum. | n-3 PUFAs and pregnancy stimulates the production of endogenous insulin in women with T1DM Rise in FC-peptide during pregnancy was significant in exposed group |
| Amouyal, C. [29] | Pregnant women T1DM Three trimesters of pregnancy and post-partum | 13 | Clinical, immunological and diabetes parameters. | One group (n = 7) rise in C-peptide between the 2nd and 3rd trimesters, while second group had no β-cell function improvement during pregnancy. |
| Meek, L. [31] | Pregnant women T1DM Three trimesters of pregnancy | 127; three groups based on detection of maternal C-peptide | Serum C-peptide concentration in a maternal and cord blood samples | Most women had undetectable C-peptide throughout pregnancy. Second group with detectable C-peptide characterized by lower BMI, later onset, shorter duration of T1DM improved glycemic control. A third group had the first appearance of C-peptide in maternal serum at 34 weeks gestation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delmis, J.; Ivanisevic, M.; Horvaticek, M. N-3 PUFA and Pregnancy Preserve C-Peptide in Women with Type 1 Diabetes Mellitus. Pharmaceutics 2021, 13, 2082. https://doi.org/10.3390/pharmaceutics13122082
Delmis J, Ivanisevic M, Horvaticek M. N-3 PUFA and Pregnancy Preserve C-Peptide in Women with Type 1 Diabetes Mellitus. Pharmaceutics. 2021; 13(12):2082. https://doi.org/10.3390/pharmaceutics13122082
Chicago/Turabian StyleDelmis, Josip, Marina Ivanisevic, and Marina Horvaticek. 2021. "N-3 PUFA and Pregnancy Preserve C-Peptide in Women with Type 1 Diabetes Mellitus" Pharmaceutics 13, no. 12: 2082. https://doi.org/10.3390/pharmaceutics13122082
APA StyleDelmis, J., Ivanisevic, M., & Horvaticek, M. (2021). N-3 PUFA and Pregnancy Preserve C-Peptide in Women with Type 1 Diabetes Mellitus. Pharmaceutics, 13(12), 2082. https://doi.org/10.3390/pharmaceutics13122082






