A Squalene-Based Nanoemulsion for Therapeutic Delivery of Resiquimod
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
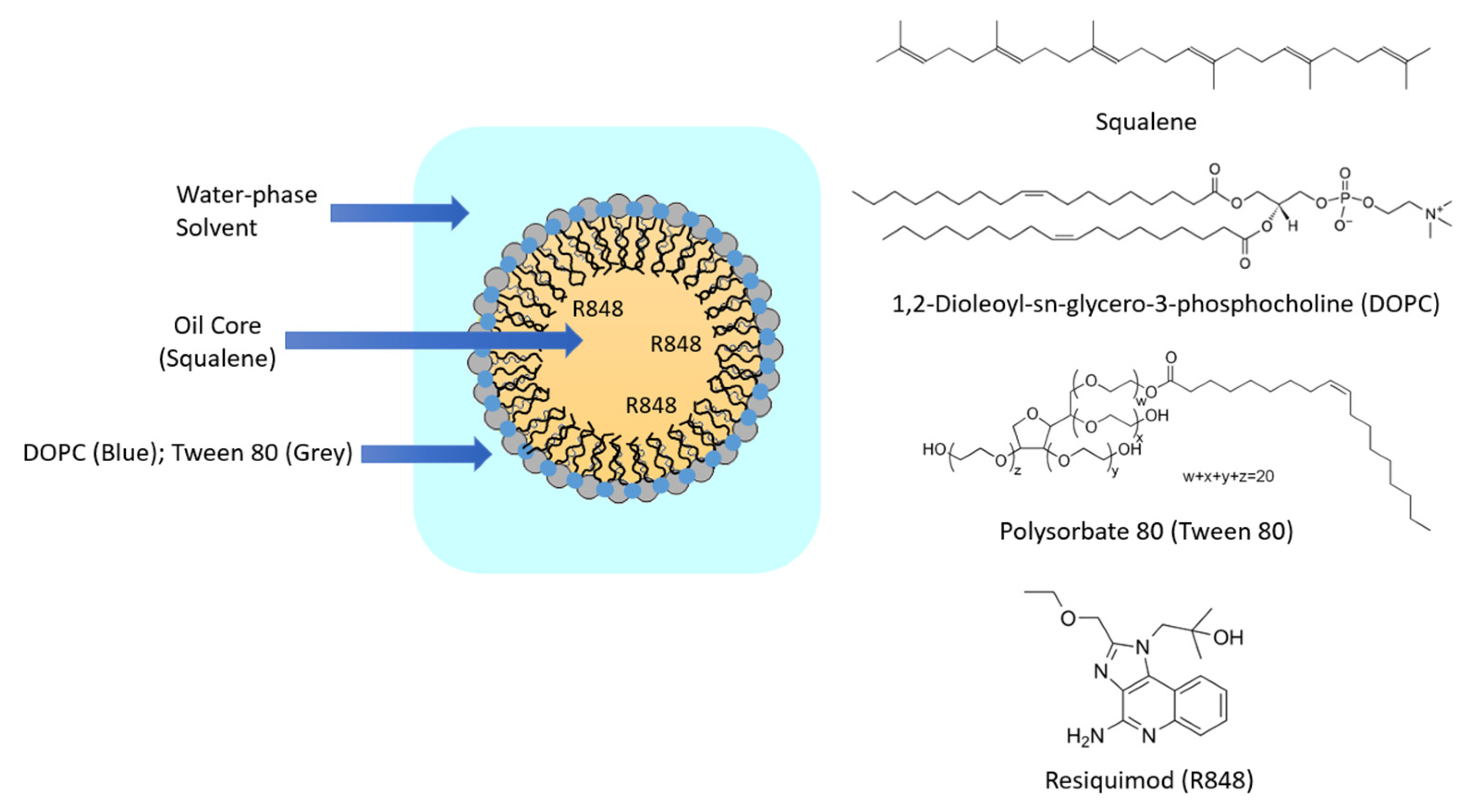
2.2. R848-NE Formulation and Characterization
2.3. Cell Culture
2.4. In Vitro Macrophage Stimulation Imaging
2.5. In Vitro Cytokine Induction Evaluation by Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. In Vivo Antitumor Efficacy
2.7. In Vivo Cytokine Measurement
2.8. In Vivo Gene Regulation by Real-Time qPCR
2.9. Statistical Analysis
3. Results and Discussion
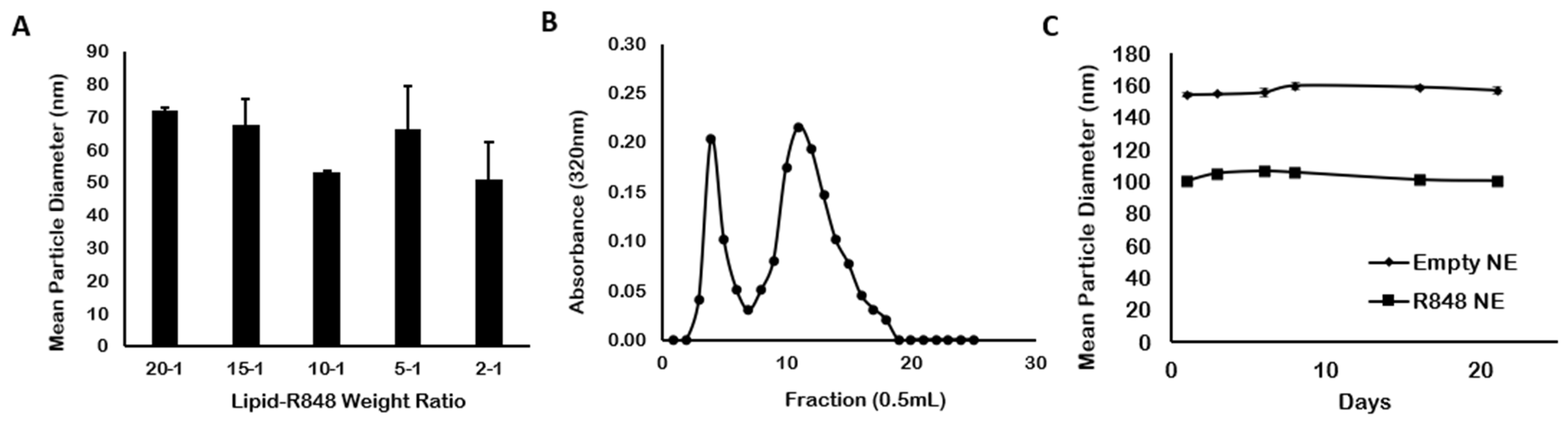
3.1. Particle Characterization
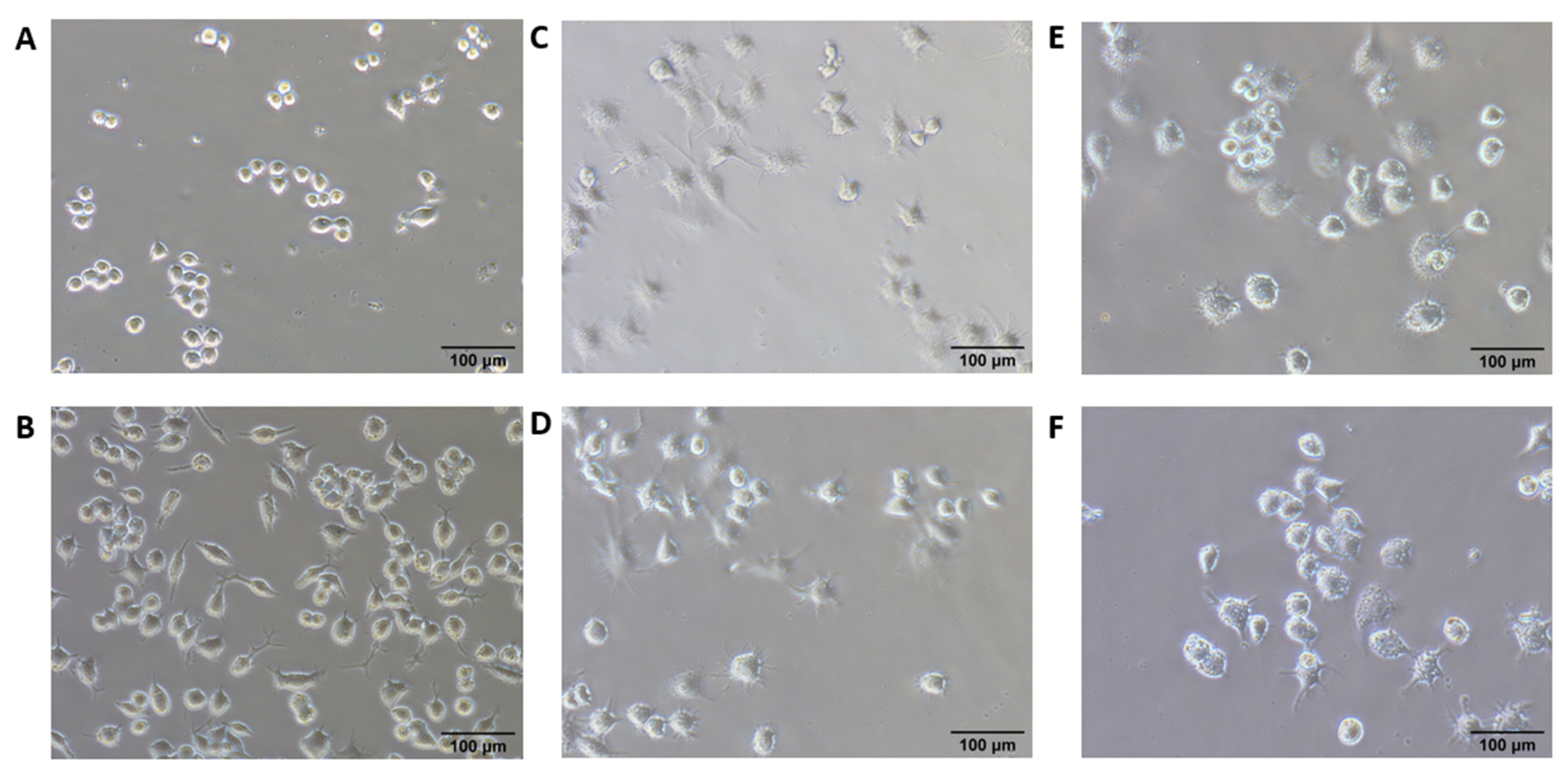
3.2. In Vitro Macrophage Stimulation
3.3. In Vivo Antitumor Efficacy
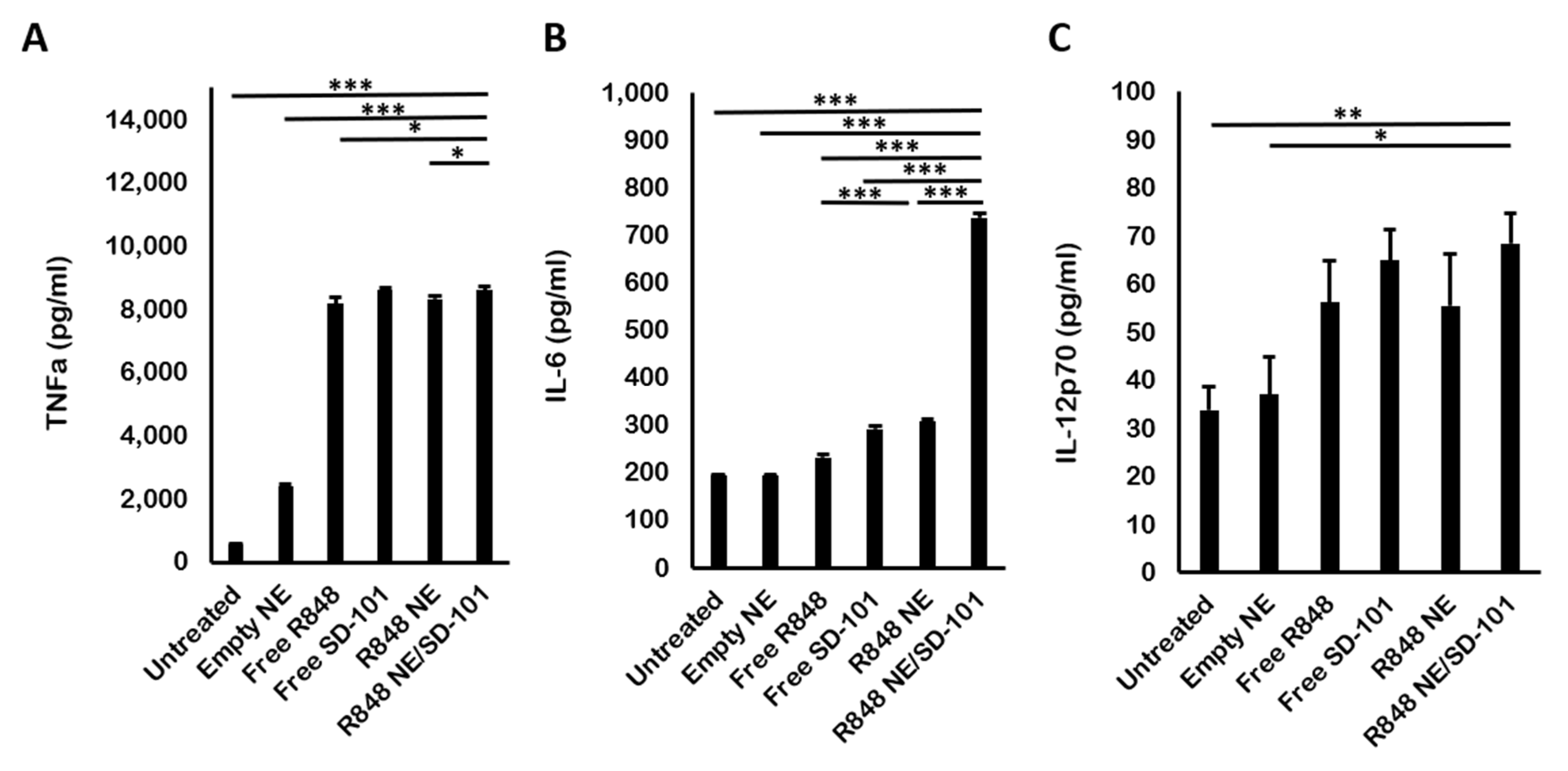
3.4. In Vivo Cytokine Production
3.5. In Vivo Gene Regulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urban-Wojciuk, Z.; Khan, M.M.; Oyler, B.L.; Fåhraeus, R.; Marek-Trzonkowska, N.; Nita-Lazar, A.; Hupp, T.R.; Goodlett, D.R. The role of tlrs in anti-cancer immunity and tumor rejection. Front. Immunol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Banday, A.H.; Jeelani, S.; Hruby, V.J. Cancer vaccine adjuvants - Recent clinical progress and future perspectives. Immunopharmacol. Immunotoxicol. 2015, 37, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, K.; Bloy, N.; Buqué, A.; Cremer, I.; Eggermont, A.; Fridman, W.H.; Fucikova, J.; Galon, J.; Špíšek, R.; Zitvogel, L.; et al. Trial Watch: Immunostimulation with Toll-like receptor agonists in cancer therapy. Oncoimmunology 2016, 5, e1088631. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.G.; Vasilakos, J.P.; Tross, D.; Smirnov, D.; Klinman, D.M. Combination therapy targeting toll like receptors 7, 8 and 9 eliminates large established tumors. J. Immunother. Cancer 2014, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Dong, L.; Ni, H.; Zhou, S.; Xu, Z.; Hoellwarth, J.S.; Chen, X.; Zhang, R.; Chen, Q.; Liu, F.; et al. Combined TLR7/8 and TLR9 Ligands Potentiate the Activity of a Schistosoma japonicum DNA Vaccine. PLoS Negl. Trop. Dis. 2013, 7, e2164. [Google Scholar] [CrossRef] [Green Version]
- Moody, M.A.; Santra, S.; Vandergrift, N.A.; Sutherland, L.L.; Gurley, T.C.; Drinker, M.S.; Allen, A.A.; Xia, S.-M.; Meyerhoff, R.R.; Parks, R.; et al. Toll-Like Receptor 7/8 (TLR7/8) and TLR9 Agonists Cooperate To Enhance HIV-1 Envelope Antibody Responses in Rhesus Macaques. J. Virol. 2014, 88, 3329–3339. [Google Scholar] [CrossRef] [Green Version]
- YIN, T.; HE, S.; WANG, Y. Toll-like receptor 7/8 agonist, R848, exhibits antitumoral effects in a breast cancer model. Mol. Med. Rep. 2015, 12, 3515–3520. [Google Scholar] [CrossRef] [Green Version]
- Rook, A.H.; Gelfand, J.M.; Wysocka, M.; Troxel, A.B.; Benoit, B.; Surber, C.; Elenitsas, R.; Buchanan, M.A.; Leahy, D.S.; Watanabe, R.; et al. Topical resiquimod can induce disease regression and enhance T-cell effector functions in cutaneous T-cell lymphoma. Blood 2015, 126, 1452–1461. [Google Scholar] [CrossRef] [Green Version]
- Smits, E.L.J.M.; Cools, N.; Lion, E.; Van Camp, K.; Ponsaerts, P.; Berneman, Z.N.; Van Tendeloo, V.F.I. The Toll-like receptor 7/8 agonist resiquimod greatly increases the immunostimulatory capacity of human acute myeloid leukemia cells. Cancer Immunol. Immunother. 2010, 59, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Melis, M.H.M.; Wilkinson, R.W.; Adlard, A.L.; Stratford, I.J.; Honeychurch, J.; Illidge, T.M. Systemic delivery of a TLR7 agonist in combination with radiation primes durable antitumor immune responses in mouse models of lymphoma. Blood 2013, 121, 251–259. [Google Scholar] [CrossRef]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Thauvin, C.; Widmer, J.; Mottas, I.; Hocevar, S.; Allémann, E.; Bourquin, C.; Delie, F. Development of resiquimod-loaded modified PLA-based nanoparticles for cancer immunotherapy: A kinetic study. Eur. J. Pharm. Biopharm. 2019, 139, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Yang, J.; Huang, L.; Liu, D. Effect of non-ionic surfactants on the formation of DNA/emulsion complexes and emulsion-mediated gene transfer. Pharm. Res. 1996, 13, 1642–1646. [Google Scholar] [CrossRef]
- Hara, T.; Tan, Y.; Huang, L. In vivo gene delivery to the liver using reconstituted chylomicron remnants as a novel nonviral vector. Proc. Natl. Acad. Sci. USA 1997, 94, 14547–14552. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, H.; Dubernet, C.; Puisieux, F.; Benita, S.; Couvreur, P. Submicron cationic emulsions as a new delivery system for oligonucleotides. Pharm. Res. 1999, 16, 30–36. [Google Scholar] [CrossRef]
- Yao, S.; Aykas, D.P.; Rodriguez-Saona, L. Rapid Authentication of Potato Chip Oil by Vibrational Spectroscopy Combined with Pattern Recognition Analysis. Foods 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, H.F.; Bruxel, F.; Fraga, M.; Schuh, R.S.; Zorzi, G.K.; Matte, U.; Fattal, E. Cationic nanoemulsions as nucleic acids delivery systems. Int. J. Pharm. 2017, 534, 356–367. [Google Scholar] [CrossRef]
- Kim, E.H.; Woodruff, M.C.; Grigoryan, L.; Maier, B.; Lee, S.H.; Mandal, P.; Cortese, M.; Natrajan, M.S.; Ravindran, R.; Ma, H.; et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. Elife 2020, 9, e52687. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.K.; Kim, J.H. Effects of squalene on the immune responses in mice(II): Cellular and non-specific immune response and antitumor activity of squalene. Arch. Pharm. Res. 1992, 15, 20–29. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Szeimies, R.-M.; Bichel, J.; Ortonne, J.-P.; Stockfleth, E.; Lee, J.; Meng, T.-C. A phase II dose-ranging study of topical resiquimod to treat actinic keratosis. Br. J. Dermatol. 2008, 159, 205–210. [Google Scholar] [CrossRef]
- Ribas, A.; Medina, T.; Kummar, S.; Amin, A.; Kalbasi, A.; Drabick, J.J.; Barve, M.; Daniels, G.A.; Wong, D.J.; Schmidt, E.V.; et al. SD-101 in Combination with Pembrolizumab in Advanced Melanoma: Results of a Phase Ib, Multicenter Study. Cancer Discov. 2018, 8, 1250–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, R.; Reagan, P.M.; Friedberg, J.W.; Bartlett, N.L.; Gordon, L.I.; Leung, A.; Peterkin, J.; Xing, B.; Coffman, R.; Janssen, R.; et al. SD-101, a Novel Class C CpG-Oligodeoxynucleotide (ODN) Toll-like Receptor 9 (TLR9) Agonist, Given with Low Dose Radiation for Untreated Low Grade B-Cell Lymphoma: Interim Results of a Phase 1/2 Trial. Blood 2016, 128, 2974. [Google Scholar] [CrossRef]
- Milhem, M.M.; Long, G.V.; Hoimes, C.J.; Amin, A.; Lao, C.D.; Conry, R.M.; Hunt, J.; Daniels, G.A.; Almubarak, M.; Shaheen, M.F.; et al. Phase 1b/2, open label, multicenter, study of the combination of SD-101 and pembrolizumab in patients with advanced melanoma who are naïve to anti-PD-1 therapy. J. Clin. Oncol. 2019, 37, 9534. [Google Scholar] [CrossRef]
- Kitaoka, M.; Naritomi, A.; Kawabe, Y.; Kamihira, M.; Kamiya, N.; Goto, M. Transcutaneous pollinosis immunotherapy using a solid-in-oil nanodispersion system carrying T cell epitope peptide and R848. Bioeng. Transl. Med. 2017, 2, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Engel, A.L.; Holt, G.E.; Lu, H. The pharmacokinetics of Toll-like receptor agonists and the impact on the immune system. Expert Rev. Clin. Pharmacol. 2011, 4, 275–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Peine, K.J.; Gupta, G.; Brackman, D.J.; Papenfuss, T.L.; Ainslie, K.M.; Satoskar, A.R.; Bachelder, E.M. Liposomal resiquimod for the treatment of Leishmania donovani infection. J. Antimicrob. Chemother. 2014, 69, 168–175. [Google Scholar] [CrossRef] [Green Version]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Baranov, M.V.; Kumar, M.; Sacanna, S.; Thutupalli, S.; van den Bogaart, G. Modulation of Immune Responses by Particle Size and Shape. Front. Immunol. 2021, 11, 607945. [Google Scholar] [CrossRef]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [Green Version]
- Bartosh, T.; Ylostalo, J. Macrophage Inflammatory Assay. Bio-Protoc. 2014, 4, e1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celhar, T.; Pereira-Lopes, S.; Thornhill, S.I.; Lee, H.Y.; Dhillon, M.K.; Poidinger, M.; Connolly, J.E.; Lim, L.H.K.; Biswas, S.K.; Fairhurst, A.-M. TLR7 and TLR9 ligands regulate antigen presentation by macrophages. Int. Immunol. 2016, 28, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervantes, J.L.; Weinerman, B.; Basole, C.; Salazar, J.C. TLR8: The forgotten relative revindicated. Cell. Mol. Immunol. 2012, 9, 434–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, K.; Chung, Y.M.; Endoh, Y.; Geczy, C.L. TLR9 Ligands Induce S100A8 in Macrophages via a STAT3-Dependent Pathway which Requires IL-10 and PGE2. PLoS One 2014, 9, e103629. [Google Scholar] [CrossRef] [PubMed]
- Ivory, C.P.A.; Prystajecky, M.; Jobin, C.; Chadee, K. Toll-like receptor 9-dependent macrophage activation by Entamoeba histolytica DNA. Infect. Immun. 2008, 76, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef]
- Martinez, F.O. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Liu, X.; Liu, Y.; Zhang, Q.; Yao, Z.; Huang, B.; Zhang, P.; Li, N.; Cao, X. Zinc Finger Protein 64 Promotes Toll-like Receptor-triggered Proinflammatory and Type I Interferon Production in Macrophages by Enhancing p65 Subunit Activation*. J. Biol. Chem. 2013, 288, 24600–24608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amcheslavsky, A.; Zou, W.; Bar-Shavit, Z. Toll-like Receptor 9 Regulates Tumor Necrosis Factor-α Expression by Different Mechanisms. J. Biol. Chem. 2004, 279, 54039–54045. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Tian, Y.; Chan, S.T.; Kim, J.Y.; Cho, C.; Ou, J.J. TNF-α Induced by Hepatitis C Virus via TLR7 and TLR8 in Hepatocytes Supports Interferon Signaling via an Autocrine Mechanism. PLOS Pathog. 2015, 11, e1004937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.; Osório, N.S.; Saraiva, M.; Cunha, C.; Almeida, A.J.; Teixeira-Coelho, M.; Ludovico, P.; Pedrosa, J.; Pitzurra, L.; Aversa, F.; et al. The C Allele of rs5743836 Polymorphism in the Human TLR9 Promoter Links IL-6 and TLR9 Up-Regulation and Confers Increased B-Cell Proliferation. PLoS One 2011, 6, e28256. [Google Scholar] [CrossRef] [Green Version]
- Rao, H.; Zeng, Q.; Liang, Y.; Xiao, C.; Xie, S.; Xu, X. Correlation between TLR9 Expression and Cytokine Secretion in the Clinical Diagnosis of Systemic Lupus Erythematosus. Mediators Inflamm. 2015, 2015, 710720. [Google Scholar] [CrossRef]
- Asami, T.; Ishii, M.; Fujii, H.; Namkoong, H.; Tasaka, S.; Matsushita, K.; Ishii, K.; Yagi, K.; Fujiwara, H.; Funatsu, Y.; et al. Modulation of Murine Macrophage TLR7/8-Mediated Cytokine Expression by Mesenchymal Stem Cell-Conditioned Medium. Mediators Inflamm. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Cant, R.; Dalgleish, A.G.; Allen, R.L. Naltrexone Inhibits IL-6 and TNFα Production in Human Immune Cell Subsets following Stimulation with Ligands for Intracellular Toll-Like Receptors. Front. Immunol. 2017, 8, 809. [Google Scholar] [CrossRef] [Green Version]
- Macatonia, S.E.; Hosken, N.A.; Litton, M.; Vieira, P.; Hsieh, C.S.; Culpepper, J.A.; Wysocka, M.; Trinchieri, G.; Murphy, K.M.; O’Garra, A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J. Immunol. 1995, 154, 5071–5079. [Google Scholar]
- O’Garra, A.; Murphy, K.M. From IL-10 to IL-12: How pathogens and their products stimulate APCs to induce TH1 development. Nat. Immunol. 2009, 10, 929–932. [Google Scholar] [CrossRef]
- Short, K.K.; Miller, S.M.; Walsh, L.; Cybulski, V.; Bazin, H.; Evans, J.T.; Burkhart, D. Co-encapsulation of synthetic lipidated TLR4 and TLR7/8 agonists in the liposomal bilayer results in a rapid, synergistic enhancement of vaccine-mediated humoral immunity. J. Control. Release 2019, 315, 186–196. [Google Scholar] [CrossRef]
- Fox, C.B.; Sivananthan, S.J.; Duthie, M.S.; Vergara, J.; Guderian, J.A.; Moon, E.; Coblentz, D.; Reed, S.G.; Carter, D. A nanoliposome delivery system to synergistically trigger TLR4 AND TLR7. J. Nanobiotechnol. 2014, 12, 17. [Google Scholar] [CrossRef] [Green Version]
- Goff, P.H.; Hayashi, T.; Martínez-Gil, L.; Corr, M.; Crain, B.; Yao, S.; Cottam, H.B.; Chan, M.; Ramos, I.; Eggink, D.; et al. Synthetic Toll-Like Receptor 4 (TLR4) and TLR7 Ligands as Influenza Virus Vaccine Adjuvants Induce Rapid, Sustained, and Broadly Protective Responses. J. Virol. 2015, 89, 3221–3235. [Google Scholar] [CrossRef] [Green Version]
- Goff, P.H.; Hayashi, T.; He, W.; Yao, S.; Cottam, H.B.; Tan, G.S.; Crain, B.; Krammer, F.; Messer, K.; Pu, M.; et al. Synthetic Toll-Like Receptor 4 (TLR4) and TLR7 Ligands Work Additively via MyD88 To Induce Protective Antiviral Immunity in Mice. J. Virol. 2017, 91, e01050-17. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-J.; Moon, D.; Kong, S.J.; Lee, Y.S.; Yoo, Y.; Kim, S.; Kim, C.; Chon, H.J.; Kim, J.-H.; Choi, K.-J. Antitumor effects of IL-12 and GM-CSF co-expressed in an engineered oncolytic HSV-1. Gene Ther. 2021, 28, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Oreja-Guevara, C.; Ramos-Cejudo, J.; Aroeira, L.S.; Chamorro, B.; Diez-Tejedor, E. TH1/TH2 Cytokine profile in relapsing-remitting multiple sclerosis patients treated with Glatiramer acetate or Natalizumab. BMC Neurol. 2012, 12, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Liao, R.; Lv, J.; Li, S.; Zheng, D.; Qin, L.; Wu, D.; Chen, S.; Long, Y.; Wu, Q.; et al. IL-6 trans-signaling promotes the expansion and anti-tumor activity of CAR T cells. Leukemia 2021, 35, 1380–1391. [Google Scholar] [CrossRef] [PubMed]
- Hjelm, B.E.; Kilbourne, J.; Herbst-Kralovetz, M.M. TLR7 and 9 agonists are highly effective mucosal adjuvants for norovirus virus-like particle vaccines. Hum. Vaccines Immunother. 2014, 10, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Kakwere, H.; Zhang, H.; Ingham, E.S.; Nura-Raie, M.; Tumbale, S.K.; Allen, R.; Tam, S.M.; Wu, B.; Liu, C.; Kheirolomoom, A.; et al. Systemic Immunotherapy with Micellar Resiquimod–Polymer Conjugates Triggers a Robust Antitumor Response in a Breast Cancer Model. Adv. Healthc. Mater. 2021, 10, 2100008. [Google Scholar] [CrossRef] [PubMed]
- Leong, W.I.; Ames, R.Y.; Haverkamp, J.M.; Torres, L.; Kline, J.; Bans, A.; Rocha, L.; Gallotta, M.; Guiducci, C.; Coffman, R.L.; et al. Low-dose metronomic cyclophosphamide complements the actions of an intratumoral C-class CpG TLR9 agonist to potentiate innate immunity and drive potent T cell-mediated anti-tumor responses. Oncotarget 2019, 10, 7220–7237. [Google Scholar] [CrossRef] [Green Version]
- Martins-Filho, O.; Mello, J.; Correa-Oliveira, R. The spleen is an important site of T cell activation during human hepatosplenic schistosomiasis. Mem. Inst. Oswaldo Cruz 1998, 93, 159–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; Bester, J.; Pretorius, E. The inflammatory effects of TNF-α and complement component 3 on coagulation. Sci. Rep. 2018, 8, 1812. [Google Scholar] [CrossRef] [Green Version]
- Chi, H.; Li, C.; Zhao, F.S.; Zhang, L.; Ng, T.B.; Jin, G.; Sha, O. Anti-tumor Activity of Toll-Like Receptor 7 Agonists. Front. Pharmacol. 2017, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Montfort, A.; Colacios, C.; Levade, T.; Andrieu-Abadie, N.; Meyer, N.; Ségui, B. The TNF Paradox in Cancer Progression and Immunotherapy. Front. Immunol. 2019, 10, 1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kearney, C.J.; Vervoort, S.J.; Hogg, S.J.; Ramsbottom, K.M.; Freeman, A.J.; Lalaoui, N.; Pijpers, L.; Michie, J.; Brown, K.K.; Knight, D.A.; et al. Tumor immune evasion arises through loss of TNF sensitivity. Sci. Immunol. 2018, 3, eaar3451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.-L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Zhou, L.; Feng, X.; Cheng, J.; Yu, Y.; Gong, Y.; Zhu, Y.; Li, C.; Tian, L.; et al. Increased HMGB1 and cleaved caspase-3 stimulate the proliferation of tumor cells and are correlated with the poor prognosis in colorectal cancer. J. Exp. Clin. Cancer Res. 2015, 34, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, C.; Wang, Y.; Qiu, M.K.; Wang, S.Q.; Liu, Y.B.; Quan, Z.W.; Ou, J.M. Knockdown of HMGB1 inhibits cell proliferation and induces apoptosis in hemangioma via downregulation of AKT pathway. J. Biol. Regul. Homeost. Agents 2017, 31, 41–49. [Google Scholar]
- Tel, J.; Hato, S.V.; Torensma, R.; Buschow, S.I.; Figdor, C.G.; Lesterhuis, W.J.; de Vries, I.J.M. The chemotherapeutic drug oxaliplatin differentially affects blood DC function dependent on environmental cues. Cancer Immunol. Immunother. 2012, 61, 1101–1111. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.A.A.; Gale, E.C.; Roth, G.A.; Maikawa, C.L.; Correa, S.; Yu, A.C.; Appel, E.A. Nanoparticles Presenting Potent TLR7/8 Agonists Enhance Anti-PD-L1 Immunotherapy in Cancer Treatment. Biomacromolecules 2020, 21, 3704–3712. [Google Scholar] [CrossRef]
- Mullins, S.R.; Vasilakos, J.P.; Deschler, K.; Grigsby, I.; Gillis, P.; John, J.; Elder, M.J.; Swales, J.; Timosenko, E.; Cooper, Z.; et al. Intratumoral immunotherapy with TLR7/8 agonist MEDI9197 modulates the tumor microenvironment leading to enhanced activity when combined with other immunotherapies. J. Immunother. Cancer 2019, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Diskin, B.; Adam, S.; Cassini, M.F.; Sanchez, G.; Liria, M.; Aykut, B.; Buttar, C.; Li, E.; Sundberg, B.; Salas, R.D.; et al. PD-L1 engagement on T cells promotes self-tolerance and suppression of neighboring macrophages and effector T cells in cancer. Nat. Immunol. 2020, 21, 442–454. [Google Scholar] [CrossRef]
- Gou, Q.; Dong, C.; Xu, H.; Khan, B.; Jin, J.; Liu, Q.; Shi, J.; Hou, Y. PD-L1 degradation pathway and immunotherapy for cancer. Cell Death Dis. 2020, 11, 955. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Zhang, C.; Wang, Y.; Cheng, T.; Duan, L.; Tong, Z.; Tan, S.; Zhang, H.; Saw, P.E.; et al. Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 6640–6650. [Google Scholar] [CrossRef]
- Liu, Y.; Zugazagoitia, J.; Ahmed, F.S.; Henick, B.S.; Gettinger, S.N.; Herbst, R.S.; Schalper, K.A.; Rimm, D.L. Immune Cell PD-L1 Colocalizes with Macrophages and Is Associated with Outcome in PD-1 Pathway Blockade Therapy. Clin. Cancer Res. 2020, 26, 970–977. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.-X.; et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 2020, 11, 4835. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
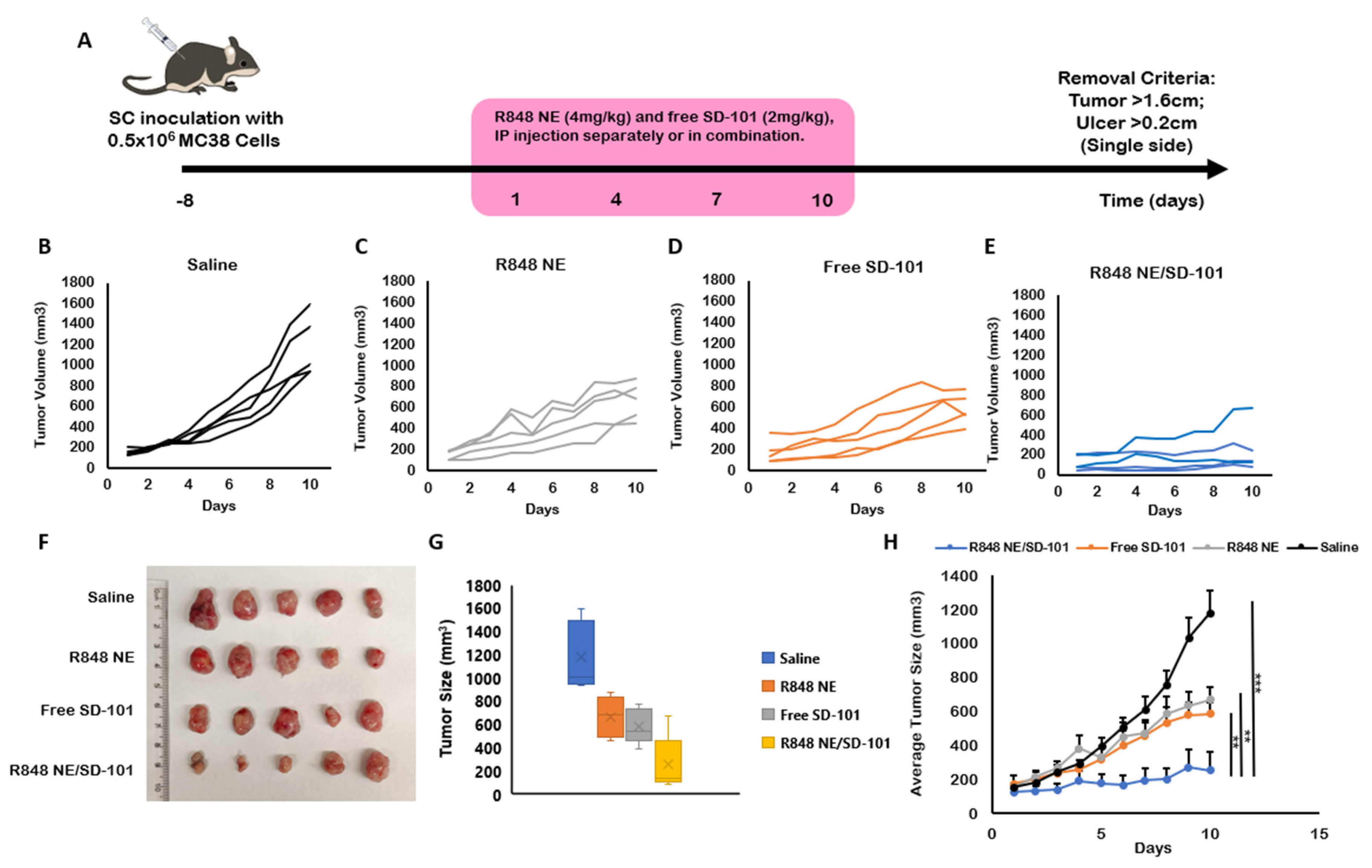
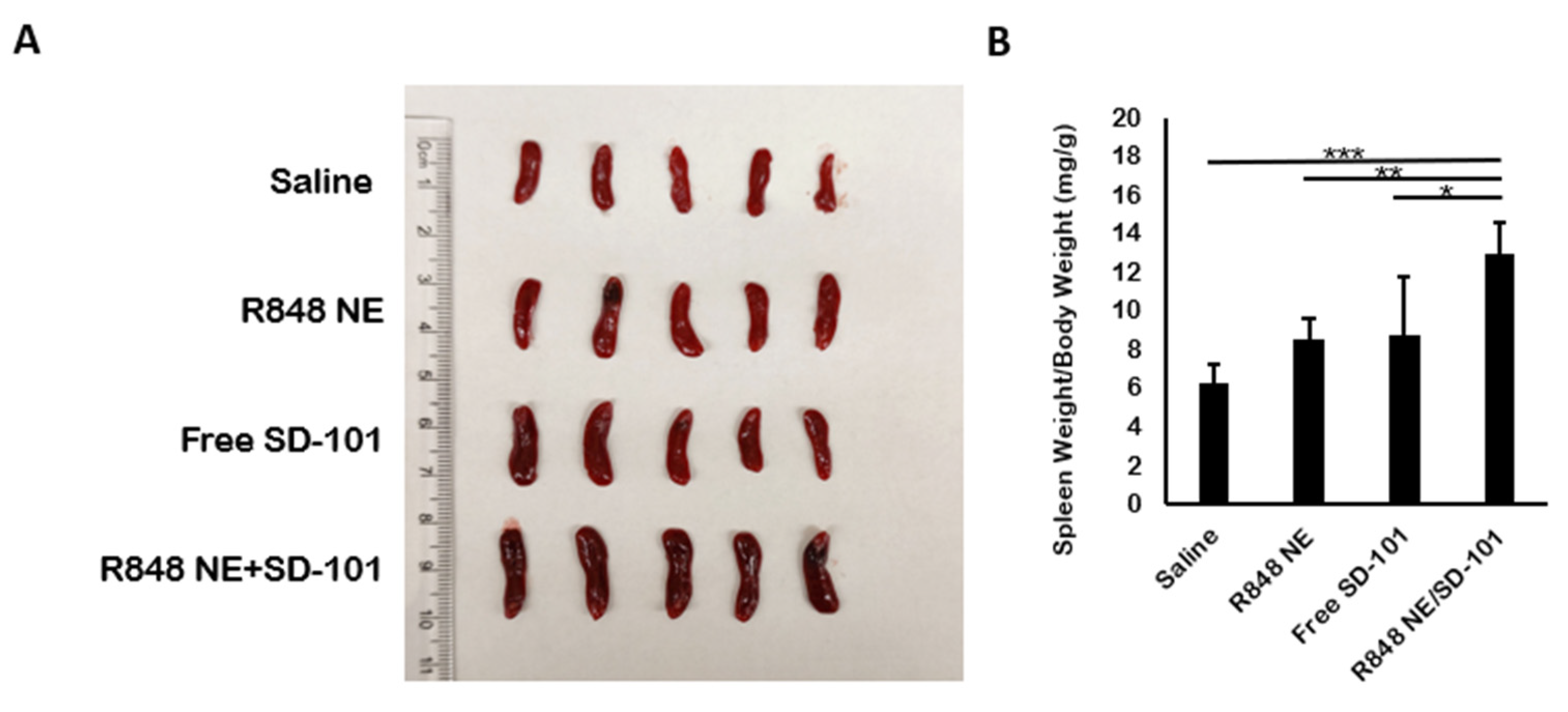
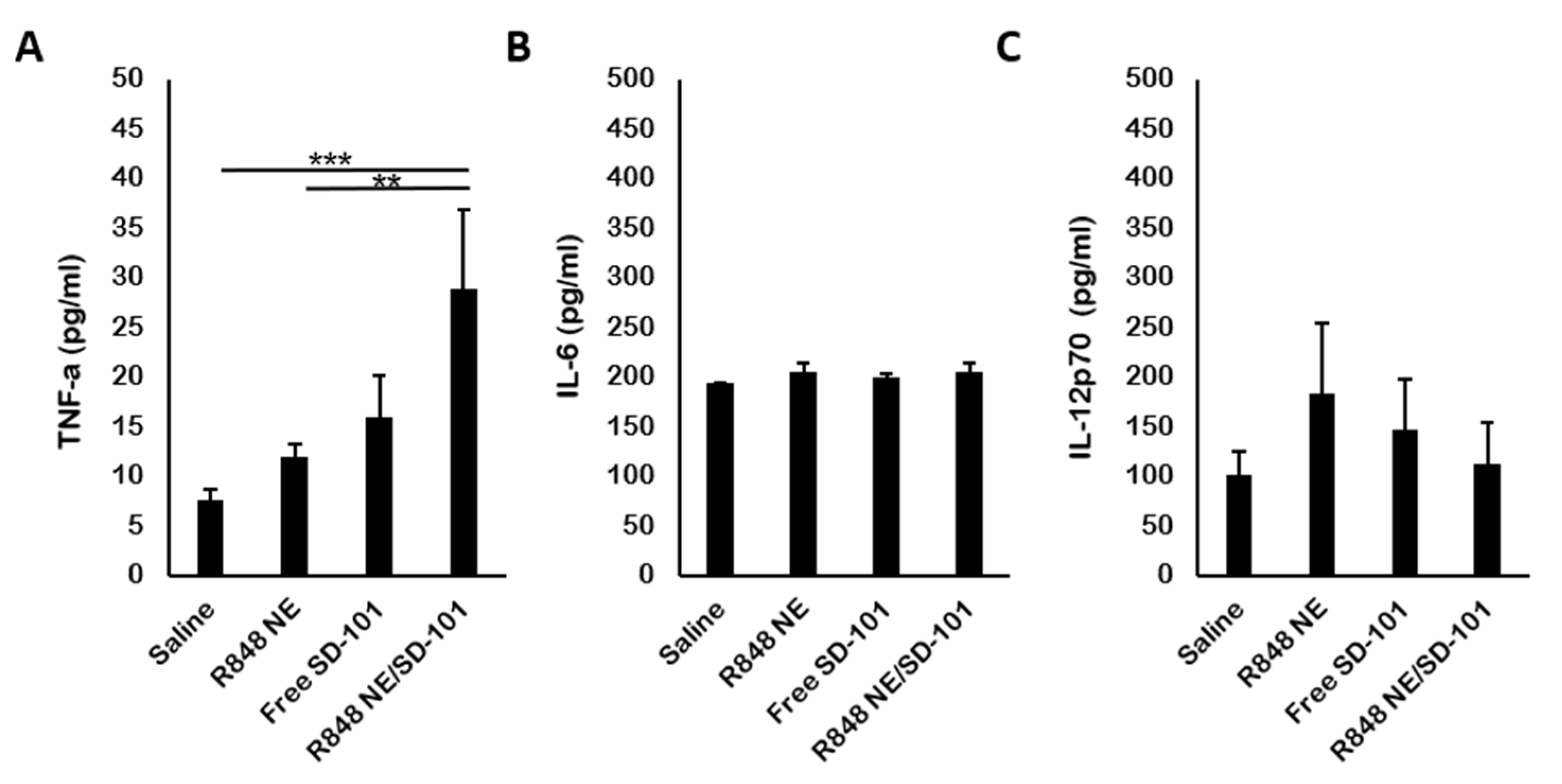

| Treatment Group | Mean TGI% | Standard Deviation |
|---|---|---|
| R848 NE | 50.72 | 16.83 |
| SD-101 | 65.65 | 12.88 |
| R848 NE/SD-101 | 84.62 | 28.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Kuo, J.C.-T.; Zhang, C.; Huang, Y.; Zhou, Z.; Lee, R.J. A Squalene-Based Nanoemulsion for Therapeutic Delivery of Resiquimod. Pharmaceutics 2021, 13, 2060. https://doi.org/10.3390/pharmaceutics13122060
Zhang Z, Kuo JC-T, Zhang C, Huang Y, Zhou Z, Lee RJ. A Squalene-Based Nanoemulsion for Therapeutic Delivery of Resiquimod. Pharmaceutics. 2021; 13(12):2060. https://doi.org/10.3390/pharmaceutics13122060
Chicago/Turabian StyleZhang, Zhongkun, Jimmy Chun-Tien Kuo, Chi Zhang, Yirui Huang, Zerui Zhou, and Robert J. Lee. 2021. "A Squalene-Based Nanoemulsion for Therapeutic Delivery of Resiquimod" Pharmaceutics 13, no. 12: 2060. https://doi.org/10.3390/pharmaceutics13122060
APA StyleZhang, Z., Kuo, J. C.-T., Zhang, C., Huang, Y., Zhou, Z., & Lee, R. J. (2021). A Squalene-Based Nanoemulsion for Therapeutic Delivery of Resiquimod. Pharmaceutics, 13(12), 2060. https://doi.org/10.3390/pharmaceutics13122060






