Protein-Based Nanoparticles for the Imaging and Treatment of Solid Tumors: The Case of Ferritin Nanocages, a Narrative Review
Abstract
:1. Introduction
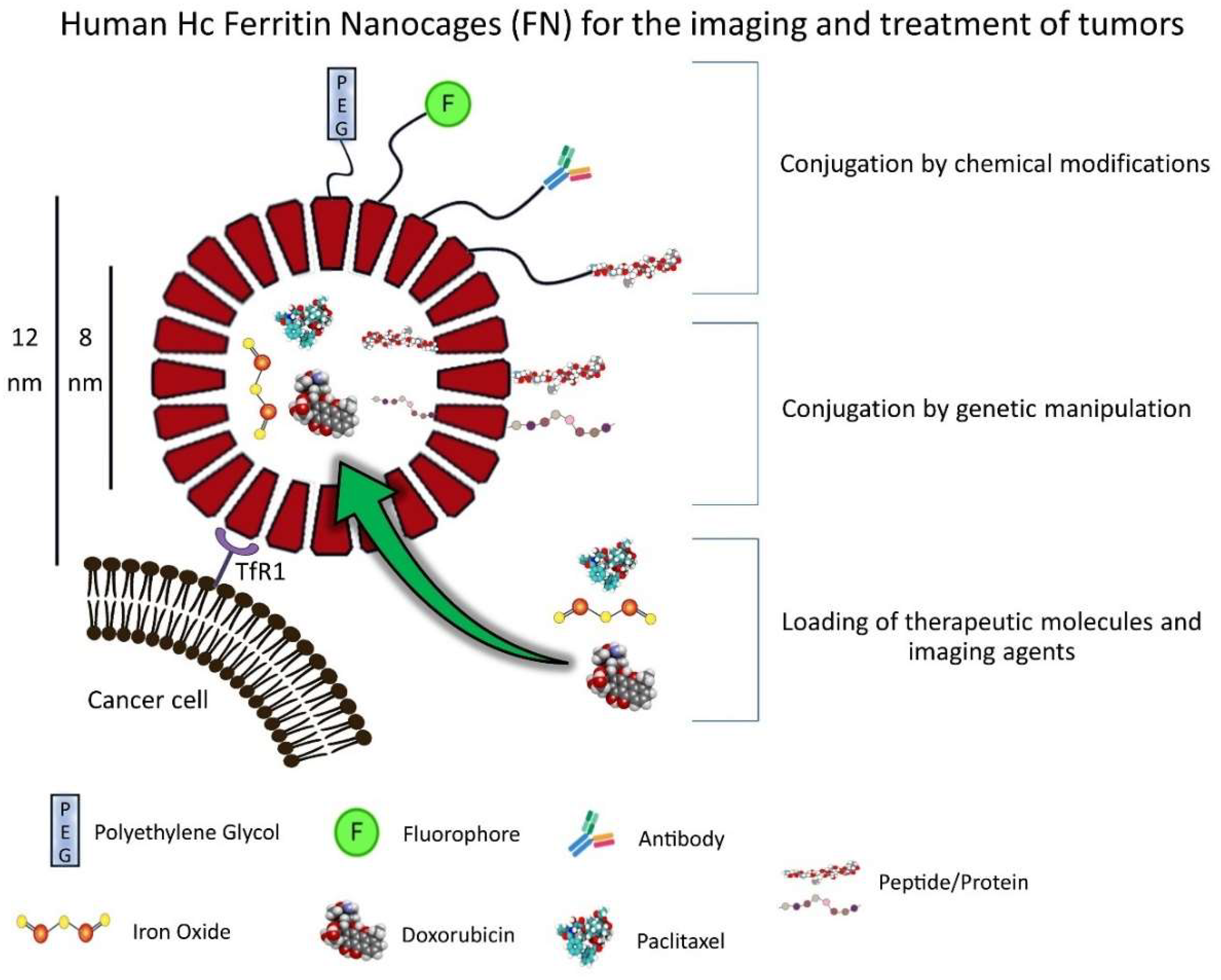
2. FN Structure and Properties
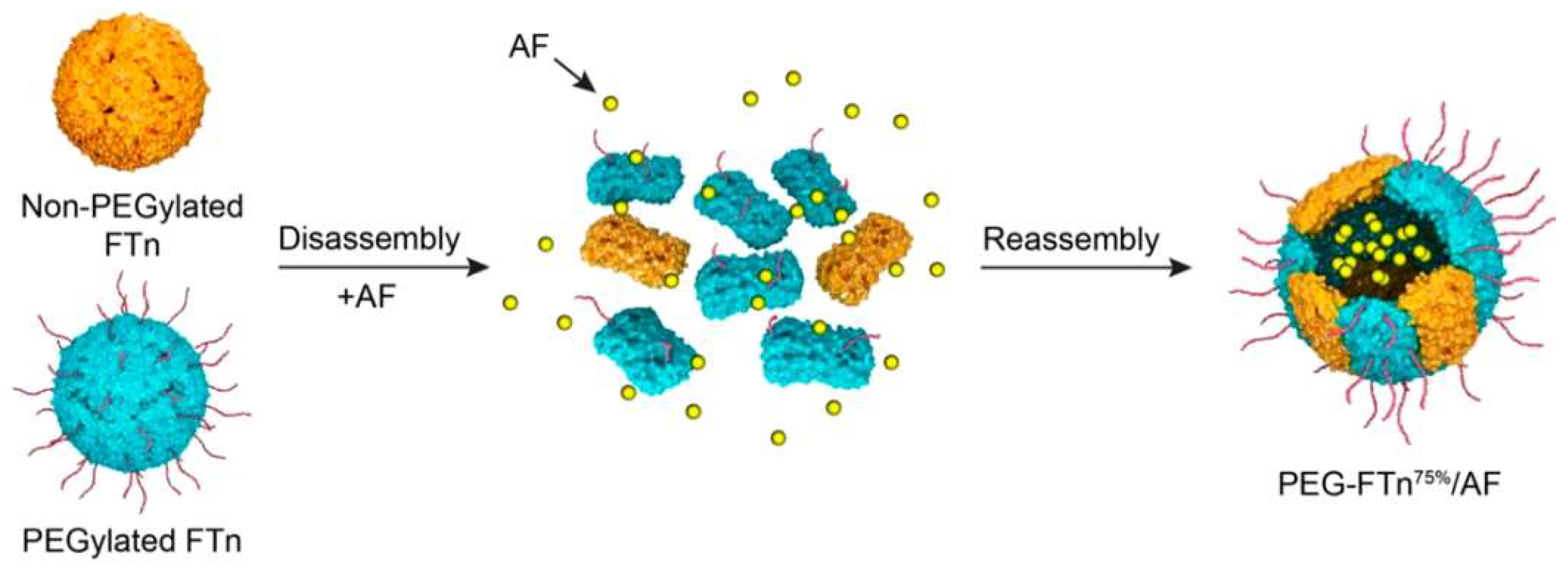
Strategies for Loading FN
3. Production and Modifications of FN
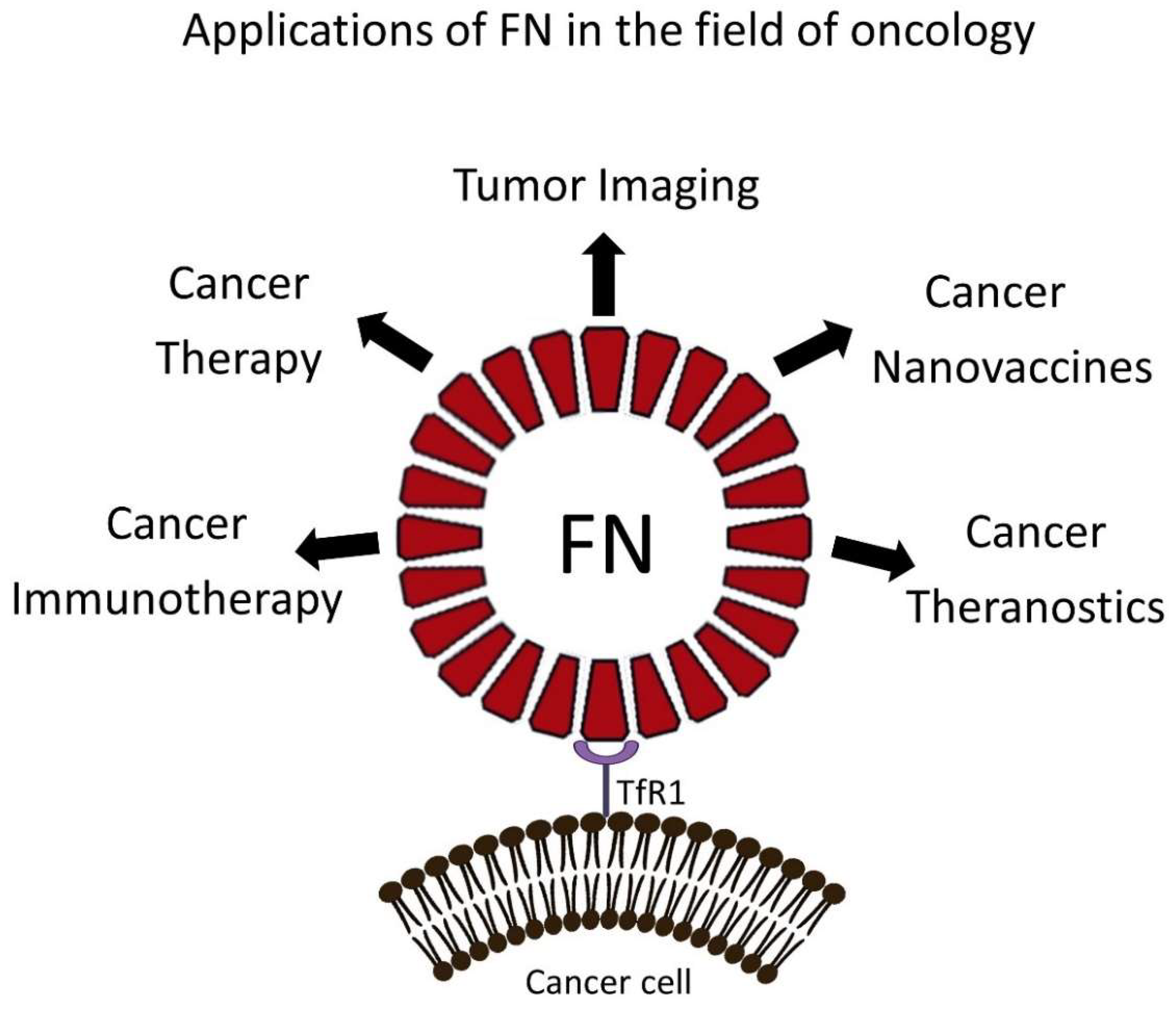
4. FN-Based NPs for Cancer Treatment in Preclinical Models
4.1. FN-Based NPs for Immunomodulation and Immunotherapy
4.2. FN-Based NPs for the Treatment of Brain Tumors
5. Preclinical Exploitation of FN-Based NPs for Tumor Imaging
6. Drawbacks and Future Perspective of FN
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, Y.; Nie, G. Multifunctional biomolecule nanostructures for cancer therapy. Natl. Rev. Mater. 2021, 6, 766–783. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Wang, Y.; Yang, G.; Wang, Y.; Ju, R. Recent progress in nanomedicine for enhanced cancer chemotherapy. Theranostics 2021, 11, 6370–6392. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, H.H.; Holt-Casper, D.; Grainger, D.W.; Ghandehari, H. Nanoparticle uptake: The phagocyte problem. Nano Today 2015, 10, 487–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-Like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnol. 2021, 19, 59. [Google Scholar] [CrossRef]
- Bhaskar, S.; Lim, S. Engineering protein nanocages as carriers for biomedical applications. NPG Asia Mater. 2017, 9, e371. [Google Scholar] [CrossRef]
- Truffi, M.; Fiandra, L.; Sorrentino, L.; Monieri, M.; Corsi, F.; Mazzucchelli, S. Ferritin nanocages: A biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol. Res. 2016, 107, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 589–599. [Google Scholar] [CrossRef]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta Bioenerg. 1996, 1275, 161–203. [Google Scholar] [CrossRef] [Green Version]
- Chasteen, N.D.; Harrison, P.M. Mineralization in ferritin: An efficient means of iron storage. J. Struct. Biol. 1999, 126, 182–194. [Google Scholar] [CrossRef] [Green Version]
- Cohen, L.A.; Gutierrez, L.; Weiss, A.; Leichtmann-Bardoogo, Y.; Zhang, D.L.; Crooks, D.R.; Sougrat, R.; Morgenstern, A.; Galy, B.; Hentze, M.W.; et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 2010, 116, 1574–1584. [Google Scholar] [CrossRef] [Green Version]
- Moreira, A.C.; Mesquita, G.; Gomes, M.S. Ferritin: An inflammatory player keeping iron at the core of pathogen-host interactions. Microorganisms 2020, 8, 589. [Google Scholar] [CrossRef] [Green Version]
- Cullis, J.O.; Fitzsimons, E.J.; Griffiths, W.J.H.; Tsochatzis, E.; Thomas, D.W. Investigation and management of a raised serum ferritin. Br. J. Haematol. 2018, 181, 331–340. [Google Scholar] [CrossRef]
- Camaschella, C.; Girelli, D. The changing landscape of iron deficiency. Mol. Asp. Med. 2020, 75, 100861. [Google Scholar] [CrossRef]
- Asano, T.; Komatsu, M.; Yamaguchi-Iwai, Y.; Ishikawa, F.; Mizushima, N.; Iwai, K. Distinct Mechanisms of Ferritin Delivery to Lysosomes in Iron-Depleted and Iron-Replete Cells. Mol. Cell. Biol. 2011, 31, 2040–2052. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Ching, A.; Lagace, C.; Linsenmayer, T. Nuclear ferritin-mediated protection of corneal epithelial cells from oxidative damage to DNA. Dev. Dyn. 2008, 237, 2676–2683. [Google Scholar] [CrossRef]
- Ahmad, S.; Moriconi, F.; Naz, N.; Sultan, S.; Sheikh, N.; Ramadori, G.; Malik, I.A. Ferritin L and ferritin H are differentially located within hepatic and extra hepatic organs under physiological and acute phase conditions. Int. J. Clin. Exp. Pathol. 2013, 6, 622–629. [Google Scholar]
- Darshan, D.; Vanoaica, L.; Richman, L.; Beermann, F.; Kühn, L.C. Conditional deletion of ferritin H in mice induces loss of iron storage and liver damage. Hepatology 2009, 50, 852–860. [Google Scholar] [CrossRef]
- Ryu, M.S.; Zhang, D.; Protchenko, O.; Shakoury-Elizeh, M.; Philpott, C.C. PCBP1 and NCOA4 regulate erythroid iron storage and heme biosynthesis. J. Clin. Investig. 2017, 127, 1786–1797. [Google Scholar] [CrossRef] [Green Version]
- Surguladze, N.; Patton, S.; Cozzi, A.; Fried, M.G.; Connor, J.R. Characterization of nuclear ferritin and mechanism of translocation. Biochem. J. 2005, 388, 731–740. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, L.; Di Penta, A.; Carmona, U.; Yang, F.; Schöps, R.; Brandsch, M.; Zugaza, J.L.; Knez, M. H-Chain Ferritin: A Natural Nuclei Targeting and Bioactive Delivery Nanovector. Adv. Healthc. Mater. 2015, 4, 1305–1310. [Google Scholar] [CrossRef]
- Bellini, M.; Mazzucchelli, S.; Galbiati, E.; Sommaruga, S.; Fiandra, L.; Truffi, M.; Rizzuto, M.A.; Colombo, M.; Tortora, P.; Corsi, F.; et al. Protein nanocages for self-triggered nuclear delivery of DNA-targeted chemotherapeutics in Cancer Cells. J. Control. Release 2014, 196, 184–196. [Google Scholar] [CrossRef]
- Kurzątkowska, K.; Pazos, M.A., II; Herschkowitz, J.I.; Hepel, M. Cancer-Targeted Controlled Delivery of Chemotherapeutic Anthracycline Derivatives Using Apoferritin Nanocage Carriers. Int. J. Mol. Sci. 2021, 22, 1362. [Google Scholar] [CrossRef]
- Santambrogio, P.; Levi, S.; Arosio, P.; Palagi, L.; Vecchio, G.; Lawson, D.M.; Yewdall, S.J.; Artymiuk, P.J.; Harrison, P.M.; Jappelli, R.; et al. Evidence that a salt bridge in the light chain contributes to the physical stability difference between heavy and light human ferritins. J. Biol. Chem. 1992, 267, 14077–14083. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Meng, D.; Wang, D.; Blanchard, C.L.; Zhou, Z. Effect of atmospheric cold plasma on structure, activity, and reversible assembly of the phytoferritin. Food Chem. 2018, 264, 41–48. [Google Scholar] [CrossRef]
- Sitia, L.; Sevieri, M.; Bonizzi, A.; Allevi, R.; Morasso, C.; Foschi, D.; Corsi, F.; Mazzucchelli, S. Development of Tumor-Targeted Indocyanine Green-Loaded Ferritin Nanoparticles for Intraoperative Detection of Cancers. ACS Omega 2020, 5, 12035–12045. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Chen, X.; Sun, G.; Chen, X.; Yin, Y.; Jin, Y.; Mi, Q.; Ma, L.; Yang, Y.; Yan, X.; et al. A natural drug entry channel in the ferritin nanocage. Nano Today 2020, 35, 100948. [Google Scholar] [CrossRef]
- Zhen, Z.; Tang, W.; Chen, H.; Lin, X.; Todd, T.; Wang, G.; Cowger, T.; Chen, X.; Xie, J. RGD-Modified Apoferritin Nanoparticles for Efficient Drug Delivery to Tumors. ACS Nano 2013, 7, 4830–4837. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Fan, K.; Yan, X. Ferritin drug carrier (FDC) for tumor targeting therapy. J. Control. Release 2019, 311–312, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, D.; He, J.; Hong, J.; Yuan, C.; Liang, M. Cargo loading within ferritin nanocages in preparation for tumor-targeted delivery. Nat. Protoc. 2021, 16, 4878–4896. [Google Scholar] [CrossRef]
- Jin, P.; Sha, R.; Zhang, Y.; Liu, L.; Bian, Y.; Qian, J.; Qian, J.; Lin, J.; Ishimwe, N.; Hu, Y.; et al. Blood Circulation-Prolonging Peptides for Engineered Nanoparticles Identified via Phage Display. Nano Lett. 2019, 19, 1467–1478. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, Y.; Wang, Z.; Jacobson, O.; Zhang, F.; Yung, B.C.; Zhang, P.; Gao, H.; Niu, G.; Liu, G.; et al. Metal ion assisted interface re-engineering of a ferritin nanocage for enhanced biofunctions and cancer therapy. Nanoscale 2018, 10, 1135–1144. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, S.; Ma, Z.; Ji, P.; Ma, X.; Wu, Z.; Qi, X. Genetic recombination of poly(l-lysine) functionalized apoferritin nanocages that resemble viral capsid nanometer-sized platforms for gene therapy. Biomater. Sci. 2020, 8, 1759–1770. [Google Scholar] [CrossRef]
- Jeon, I.S.; Yoo, J.D.; Gurung, S.; Kim, M.; Lee, C.; Park, E.J.; Park, R.W.; Lee, B.; Kim, S. Anticancer nanocage platforms for combined immunotherapy designed to harness immune checkpoints and deliver anticancer drugs. Biomaterials 2021, 270, 120685. [Google Scholar] [CrossRef]
- Ma, Y.; Li, R.; Dong, Y.; You, C.; Huang, S.; Li, X.; Wang, F.; Zhang, Y. tLyP-1 peptide functionalized human H chain ferritin for targeted delivery of paclitaxel. Int. J. Nanomed. 2021, 16, 789–802. [Google Scholar] [CrossRef]
- Andreata, F.; Bonizzi, A.; Sevieri, M.; Truffi, M.; Monieri, M.; Sitia, L.; Silva, F.; Sorrentino, L.; Allevi, R.; Zerbi, P.; et al. Co-Administration of H-ferritin-doxorubicin and Trastuzumab in neoadjuvant setting improves efficacy and prevents cardiotoxicity in HER2 + murine breast cancer model. Sci. Rep. 2020, 10, 11425. [Google Scholar] [CrossRef]
- Huang, X.; Chisholm, J.; Zhuang, J.; Xiao, Y.; Duncan, G.; Chen, X.; Suk, J.S.; Hanes, J. Protein nanocages that penetrate airway mucus and tumor tissue. Proc. Natl. Acad. Sci. USA 2017, 114, E6595–E6602. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zhuang, J.; Chung, S.W.; Huang, B.; Halpert, G.; Negron, K.; Sun, X.; Yang, J.; Oh, Y.; Hwang, P.M.; et al. Hypoxia-tropic Protein Nanocages for Modulation of Tumor- and Chemotherapy-Associated Hypoxia. ACS Nano 2019, 13, 236–247. [Google Scholar] [CrossRef]
- Mazzucchelli, S.; Truffi, M.; Baccarini, F.; Beretta, M.; Sorrentino, L.; Bellini, M.; Rizzuto, M.A.; Ottria, R.; Ravelli, A.; Ciuffreda, P.; et al. H-Ferritin-nanocaged olaparib: A promising choice for both BRCA-mutated and sporadic triple negative breast cancer. Sci. Rep. 2017, 7, 7505. [Google Scholar] [CrossRef]
- Bonizzi, A.; Truffi, M.; Sevieri, M.; Allevi, R.; Sitia, L.; Ottria, R.; Sorrentino, L.; Sottani, C.; Negri, S.; Grignani, E.; et al. Everolimus nanoformulation in biological nanoparticles increases drug responsiveness in resistant and low-responsive breast cancer cell lines. Pharmaceutics 2019, 11, 384. [Google Scholar] [CrossRef] [Green Version]
- Pandolfi, L.; Bellini, M.; Vanna, R.; Morasso, C.; Zago, A.; Carcano, S.; Avvakumova, S.; Bertolini, J.; Rizzuto, M.; Colombo, M.; et al. H-Ferritin Enriches the Curcumin Uptake and Improves the Therapeutic Efficacy in Triple Negative Breast Cancer Cells. Biomacromolecules 2017, 18, 3318–3330. [Google Scholar] [CrossRef]
- Sitia, L.; Bonizzi, A.; Mazzucchelli, S.; Negri, S.; Sottani, C.; Grignani, E.; Rizzuto, M.A.; Prosperi, D.; Sorrentino, L.; Morasso, C.; et al. Selective Targeting of Cancer-Associated Fibroblasts by Engineered H-Ferritin Nanocages Loaded with Navitoclax. Cells 2021, 10, 328. [Google Scholar] [CrossRef]
- Fan, K.; Jia, X.; Zhou, M.; Wang, K.; Conde, J.; He, J.; Tian, J.; Yan, X. Ferritin Nanocarrier Traverses the Blood Brain Barrier and Kills Glioma. ACS Nano 2018, 12, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Chuang, C.-P.; Chen, Y.-J.; Wang, H.-Y.; Lin, J.-J.; Huang, C.-Y.; Wei, K.-C.; Huang, F.-T. Integrin α2β1-targeting ferritin nanocarrier traverses the blood–brain barrier for effective glioma chemotherapy. J. Nanobiotechnol. 2021, 19, 180. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.; Fu, Y.; Huang, S.; Guo, C.; Li, L.; Wang, L.; Zhang, Z.; Zhang, L. Target delivering paclitaxel by ferritin heavy chain nanocages for glioma treatment. J. Control. Release 2020, 323, 191–202. [Google Scholar] [CrossRef]
- Rizzuto, M.A.; Magro, R.D.; Barbieri, L.; Pandolfi, L.; Sguazzini-Viscontini, A.; Truffi, M.; Salvioni, L.; Corsi, F.; Colombo, M.; Re, F.; et al. H-Ferritin nanoparticle-mediated delivery of antibodies across a BBB in vitro model for treatment of brain malignancies. Biomater. Sci. 2021, 9, 2032–2042. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Zhang, R.; Chen, X.; Sun, G.; Zhou, M.; Han, Q.; Zhang, B.; Zhao, Y.; Jiang, B.; et al. Bioengineered Dual-Targeting Protein Nanocage for Stereoscopical Loading of Synergistic Hydrophilic/Hydrophobic Drugs to Enhance Anticancer Efficacy. Adv. Funct. Mater. 2021, 31, 2102004. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, R.; Zhang, J.; Hou, Y.; Chen, X.; Zhou, M.; Tian, X.; Hao, C.; Fan, K.; Yan, X. GRP78-targeted ferritin nanocaged ultra-high dose of doxorubicin for hepatocellular carcinoma therapy. Theranostics 2019, 9, 2167–2182. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Wang, Y.; Wang, H.; Cao, H.; Wang, Z.; Wang, J.; Li, J.; Li, Y.; Zhang, Z.; Wang, S. Apoferritin nanocages loading mertansine enable effective eradiation of cancer stem-like cells in vitro. Int. J. Pharm. 2018, 553, 201–209. [Google Scholar] [CrossRef]
- Ferraro, G.; Pratesi, A.; Cirri, D.; Imbimbo, P.; Monti, D.M.; Messori, L.; Merlino, A. Arsenoplatin-Ferritin nanocage: Structure and cytotoxicity. Int. J. Mol. Sci. 2021, 22, 1874. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Yan, J.; Liu, Y.; Yang, R.; Pan, D.; Wang, L.; Xu, Y.; Li, X.; Yang, M. Nanoparticle ferritin-bound erastin and rapamycin: A nanodrug combining autophagy and ferroptosis for anticancer therapy. Biomater. Sci. 2019, 7, 3779–3787. [Google Scholar] [CrossRef]
- Zhai, M.; Wang, Y.; Zhang, L.; Liang, M.; Fu, S.; Cui, L.; Yang, M.; Gong, W.; Li, Z.; Yu, L.; et al. Glioma targeting peptide modified apoferritin nanocage. Drug Deliv. 2018, 25, 1013–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.Y.; Yang, S.J.; Peng, C.L.; Shieh, M.J. Panitumumab-Conjugated and Platinum-Cored pH-Sensitive Apoferritin Nanocages for Colorectal Cancer-Targeted Therapy. ACS Appl. Mater. Interfaces 2018, 10, 6096–6106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Cheng, W.; Zhang, X.; Shao, R.; Li, Z. A pH-Induced Reversible Assembly System with Resveratrol-Controllable Loading and Release for Enhanced Tumor-Targeting Chemotherapy. Nanoscale Res. Lett. 2019, 14, 305. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, Z.; Jiang, M.; Li, S.; Yuan, H.; Sun, H.; Yang, F.; Liang, H. Developing a Novel Gold(III) Agent to Treat Glioma Based on the Unique Properties of Apoferritin Nanoparticles: Inducing Lethal Autophagy and Apoptosis. J. Med. Chem. 2020, 63, 13695–13708. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Z.; Zhou, X.; Guo, Z.; Zhang, J.; Zhu, P.; Yao, S.; Zhu, M. Ferritin nanoparticle-based SpyTag/SpyCatcher-enabled click vaccine for tumor immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2019, 16, 69–78. [Google Scholar] [CrossRef]
- Shan, H.; Dou, W.; Zhang, Y.; Qi, M. Targeted ferritin nanoparticle encapsulating CpG oligodeoxynucleotides induces tumor-associated macrophage M2 phenotype polarization into M1 phenotype and inhibits tumor growth. Nanoscale 2020, 12, 22268–22280. [Google Scholar] [CrossRef]
- Jiang, B.; Jia, X.; Ji, T.; Zhou, M.; He, J.; Wang, K.; Tian, J.; Yan, X.; Fan, K. Ferritin nanocages for early theranostics of tumors via inflammation-enhanced active targeting. Sci. China Life Sci. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Song, R.; Ruan, M.; Dai, J.; Xue, W. Biomimetic magnetofluorescent ferritin nanoclusters for magnetic resonance and fluorescence-dual modal imaging and targeted tumor therapy. J. Mater. Chem. B 2021, 9, 2494–2504. [Google Scholar] [CrossRef]
- Aslan, T.N.; Aşık, E.; Güray, N.T.; Volkan, M. The potential application of gold-apoferritin nanocages conjugated with 2-amino-2-deoxy-glucose for imaging of breast cancer cells. JBIC J. Biol. Inorg. Chem. 2020, 25, 1139–1152. [Google Scholar] [CrossRef]
- Bitonto, V.; Alberti, D.; Ruiu, R.; Aime, S.; Geninatti Crich, S.; Cutrin, J.C. L-ferritin: A theranostic agent of natural origin for MRI visualization and treatment of breast cancer. J. Control. Release 2020, 319, 300–310. [Google Scholar] [CrossRef]
- Li, J.; Ji, H.; Jing, Y.; Wang, S. pH- and acoustic-responsive platforms based on perfluoropentane-loaded protein nanoparticles for ovarian tumor-targeted ultrasound imaging and therapy. Nanoscale Res. Lett. 2020, 15, 31. [Google Scholar] [CrossRef]
- Sevieri, M.; Sitia, L.; Bonizzi, A.; Truffi, M.; Mazzucchelli, S.; Corsi, F. Tumor Accumulation and Off-Target Biodistribution of an Indocyanine-Green Fluorescent Nanotracer: An Ex Vivo Study on an Orthotopic Murine Model of Breast Cancer. Int. J. Mol. Sci. 2021, 22, 1601. [Google Scholar] [CrossRef]
- Kim, J.-W.; Lee, K.-K.; Park, K.-W.; Kim, M.; Lee, C.-S. Genetically Modified Ferritin Nanoparticles with Bone-Targeting Peptides for Bone Imaging. Int. J. Mol. Sci. 2021, 22, 4854. [Google Scholar] [CrossRef]
- Cao, C.; Wang, X.; Cai, Y.; Sun, L.; Tian, L.; Wu, H.; He, X.; Lei, H.; Liu, W.; Chen, G.; et al. Targeted In Vivo Imaging of Microscopic Tumors with Ferritin-based Nanoprobes Across Biological Barriers. Adv. Mater. 2014, 26, 2566–2571. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Zhao, G. Ferritin nanocage: A versatile nanocarrier utilized in the field of food, nutrition, and medicine. Nanomaterials 2020, 10, 1894. [Google Scholar] [CrossRef]
- Plath, L.D.; Ozdemir, A.; Aksenov, A.A.; Bier, M.E. Determination of Iron Content and Dispersity of Intact Ferritin by Superconducting Tunnel Junction Cryodetection Mass Spectrometry. Anal. Chem. 2015, 87, 8985–8993. [Google Scholar] [CrossRef]
- Silva, F.; Sitia, L.; Allevi, R.; Bonizzi, A.; Sevieri, M.; Morasso, C.; Truffi, M.; Corsi, F.; Mazzucchelli, S. Combined method to remove endotoxins from protein nanocages for drug delivery applications: The case of human ferritin. Pharmaceutics 2021, 13, 229. [Google Scholar] [CrossRef]
- Jin, Y.; He, J.; Fan, K.; Yan, X. Ferritin variants: Inspirations for rationally designing protein nanocarriers. Nanoscale 2019, 11, 12449–12459. [Google Scholar] [CrossRef]
- Zhang, S.; Zang, J.; Zhang, X.; Chen, H.; Mikami, B.; Zhao, G. “Silent” Amino Acid Residues at Key Subunit Interfaces Regulate the Geometry of Protein Nanocages. ACS Nano 2016, 10, 10382–10388. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Chen, H.; Zang, J.; Zhao, X.; Zhao, G.; Wang, H. Selective Elimination of the Key Subunit Interfaces Facilitates Conversion of Native 24-mer Protein Nanocage into 8-mer Nanorings. J. Am. Chem. Soc. 2018, 140, 14078–14081. [Google Scholar] [CrossRef]
- Choi, S.H.; Choi, K.; Chan Kwon, I.; Ahn, H.J. The incorporation of GALA peptide into a protein cage for an acid-inducible molecular switch. Biomaterials 2010, 31, 5191–5198. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Li, G.; Zhao, G.; Zhao, X.; Wang, H. AB loop engineered ferritin nanocages for drug loading under benign experimental conditions. Chem. Commun. 2019, 55, 12344–12347. [Google Scholar] [CrossRef]
- Zang, J.; Chen, H.; Zhang, X.; Zhang, C.; Guo, J.; Du, M.; Zhao, G. Disulfide-Mediated conversion of 8-mer bowl-like protein architecture into three different nanocages. Nat. Commun. 2019, 10, 778. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Zhang, T.; Lv, C.; Liu, Y.; Wang, Y.; Zhao, G. His-Mediated Reversible Self-Assembly of Ferritin Nanocages through Two Different Switches for Encapsulation of Cargo Molecules. ACS Nano 2020, 14, 17080–17090. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, C.; Li, Z.; Yin, S.; Wang, Q.; Guo, F.; Zhang, Y.; Yu, R.; Liu, Y.; Su, Z. Extending Half Life of H-Ferritin Nanoparticle by Fusing Albumin Binding Domain for Doxorubicin Encapsulation. Biomacromolecules 2018, 19, 773–781. [Google Scholar] [CrossRef]
- Falvo, E.; Tremante, E.; Arcovito, A.; Papi, M.; Elad, N.; Boffi, A.; Morea, V.; Conti, G.; Toffoli, G.; Fracasso, G.; et al. Improved Doxorubicin Encapsulation and Pharmacokinetics of Ferritin-Fusion Protein Nanocarriers Bearing Proline, Serine, and Alanine Elements. Biomacromolecules 2016, 17, 514–522. [Google Scholar] [CrossRef]
- Yin, S.; Wang, Y.; Zhang, B.; Qu, Y.; Liu, Y.; Dai, S.; Zhang, Y.; Wang, Y.; Bi, J. Engineered human heavy-chain ferritin with half-life extension and tumor targeting by PAS and RGDK peptide functionalization. Pharmaceutics 2021, 13, 521. [Google Scholar] [CrossRef]
- Falvo, E.; Malagrinò, F.; Arcovito, A.; Fazi, F.; Colotti, G.; Tremante, E.; Di Micco, P.; Braca, A.; Opri, R.; Giuffrè, A.; et al. The presence of glutamate residues on the PAS sequence of the stimuli-sensitive nano-ferritin improves in vivo biodistribution and mitoxantrone encapsulation homogeneity. J. Control. Release 2018, 275, 177–185. [Google Scholar] [CrossRef]
- Zheng, L.; Hu, X.; Wu, H.; Mo, L.; Xie, S.; Li, J.; Peng, C.; Xu, S.; Qiu, L.; Tan, W. In Vivo Monocyte/Macrophage-Hitchhiked Intratumoral Accumulation of Nanomedicines for Enhanced Tumor Therapy. J. Am. Chem. Soc. 2020, 142, 382–391. [Google Scholar] [CrossRef]
- Zelepukin, I.V.; Yaremenko, A.V.; Shipunova, V.O.; Babenyshev, A.V.; Balalaeva, I.V.; Nikitin, P.I.; Deyev, S.M.; Nikitin, M.P. Nanoparticle-Based drug delivery via RBC-hitchhiking for the inhibition of lung metastases growth. Nanoscale 2019, 11, 1636–1646. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.; Lee, S.G.; Yoon, H.R.; Lee, J.M.; Oh, H.J.; Kim, H.M.; Jung, Y. Four-Fold Channel-Nicked Human Ferritin Nanocages for Active Drug Loading and pH-Responsive Drug Release. Angew. Chem. Int. Ed. 2018, 57, 2909–2913. [Google Scholar] [CrossRef]
- Men, D.; Zhang, T.-T.; Hou, L.-W.; Zhou, J.; Zhang, Z.-P.; Shi, Y.-Y.; Zhang, J.-L.; Cui, Z.-Q.; Deng, J.-Y.; Wang, D.-B.; et al. Self-Assembly of Ferritin Nanoparticles into an Enzyme Nanocomposite with Tunable Size for Ultrasensitive Immunoassay. ACS Nano 2015, 9, 10852–10860. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Lee, S.J.; Kang, Y.S.; Ryu, J.H.; Kwon, K.C.; Jo, E.; Yhee, J.Y.; Kwon, I.C.; Kim, K.; Lee, J. Engineered proteinticles for targeted delivery of siRNA to cancer cells. Adv. Funct. Mater. 2015, 25, 1279–1286. [Google Scholar] [CrossRef]
- Li, L.; Muñoz-Culla, M.; Carmona, U.; Lopez, M.P.; Yang, F.; Trigueros, C.; Otaegui, D.; Zhang, L.; Knez, M. Ferritin-Mediated siRNA delivery and gene silencing in human tumor and primary cells. Biomaterials 2016, 98, 143–151. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Wei, C.J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Whittle, J.R.R.; Rao, S.S.; Kong, W.P.; Wang, L.; Nabel, G.J. Self-Assembling influenza nanoparticle vaccines elicit broadly neutralizing H1N1 antibodies. Nature 2013, 499, 102–106. [Google Scholar] [CrossRef]
- Lee, B.-R.; Ko, H.K.; Ryu, J.H.; Ahn, K.Y.; Lee, Y.-H.; Oh, S.J.; Na, J.H.; Kim, T.W.; Byun, Y.; Kwon, I.C.; et al. Engineered Human Ferritin Nanoparticles for Direct Delivery of Tumor Antigens to Lymph Node and Cancer Immunotherapy. Sci. Rep. 2016, 6, 35182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.A.; Kang, Y.J.; Shin, C.; Ra, J.S.; Shin, H.H.; Hong, S.Y.; Do, Y.; Kang, S. Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell (DC)-based vaccine development. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 561–569. [Google Scholar] [CrossRef]
- Jiang, B.; Yan, L.; Zhang, J.; Zhou, M.; Shi, G.; Tian, X.; Fan, K.; Hao, C.; Yan, X. Biomineralization Synthesis of the Cobalt Nanozyme in SP94-Ferritin Nanocages for Prognostic Diagnosis of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2019, 11, 9747–9755. [Google Scholar] [CrossRef]
- Chuckran, C.A.; Liu, C.; Bruno, T.C.; Workman, C.J.; Vignali, D.A. Neuropilin-1: A checkpoint target with unique implications for cancer immunology and immunotherapy. J. Immunother. Cancer 2020, 8, e000967. [Google Scholar] [CrossRef]
- Khoshnejad, M.; Greineder, C.F.; Pulsipher, K.W.; Villa, C.H.; Altun, B.; Pan, D.C.; Tsourkas, A.; Dmochowski, I.J.; Muzykantov, V.R. Ferritin Nanocages with Biologically Orthogonal Conjugation for Vascular Targeting and Imaging. Bioconjug. Chem. 2018, 29, 1209–1218. [Google Scholar] [CrossRef]
- Palombarini, F.; Masciarelli, S.; Incocciati, A.; Liccardo, F.; Di Fabio, E.; Iazzetti, A.; Fabrizi, G.; Fazi, F.; Macone, A.; Bonamore, A.; et al. Self-Assembling ferritin-dendrimer nanoparticles for targeted delivery of nucleic acids to myeloid leukemia cells. J. Nanobiotechnol. 2021, 19, 172. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tosi, G.; Belletti, D.; Pederzoli, F.; Ruozi, B. Apoferritin nanocage as drug reservoir: Is it a reliable drug delivery system? Expert Opin. Drug Deliv. 2016, 13, 1341–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadori, G.; Cameron, S. Effects of systemic chemotherapy on the liver. Ann. Hepatol. 2010, 9, 133–143. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalt. Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Carrino, D.; Gulisano, M.; Ghelardini, C.; Di Cesare Mannelli, L.; Pacini, A. Oxaliplatin-Induced Neuropathy: Genetic and Epigenetic Profile to Better Understand How to Ameliorate This Side Effect. Front. Mol. Biosci. 2021, 8, 643824. [Google Scholar] [CrossRef]
- Jagieła, J.; Bartnicki, P.; Rysz, J. Nephrotoxicity as a Complication of Chemotherapy and Immunotherapy in the Treatment of Colorectal Cancer, Melanoma and Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 4618. [Google Scholar] [CrossRef]
- Mazzucchelli, S.; Bellini, M.; Fiandra, L.; Truffi, M.; Rizzuto, M.A.; Sorrentino, L.; Longhi, E.; Nebuloni, M.; Prosperi, D.; Corsi, F.; et al. Nanometronomic treatment of 4T1 breast cancer with nanocaged doxorubicin prevents drug resistance and circumvents cardiotoxicity. Oncotarget 2016, 8, 8383–8396. [Google Scholar] [CrossRef]
- Liang, M.; Fan, K.; Zhou, M.; Duan, D.; Zheng, J.; Yang, D.; Feng, J.; Yan, X. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc. Natl. Acad. Sci. USA 2014, 111, 14900–14905. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wang, X.; Diao, H.; Zhang, J.; Li, H.; Sun, H.; Guo, Z. Encapsulation of platinum anticancer drugs by apoferritin. Chem. Commun. 2007, 33, 3453–3455. [Google Scholar] [CrossRef]
- Ferraro, G.; Pica, A.; Petruk, G.; Pane, F.; Amoresano, A.; Cilibrizzi, A.; Vilar, R.; Monti, D.M.; Merlino, A. Preparation, structure, cytotoxicity and mechanism of action of ferritin-Pt(II) terpyridine compound nanocomposites. Nanomedicine 2018, 13, 2995–3007. [Google Scholar] [CrossRef] [Green Version]
- Comerford, K.M.; Wallace, T.J.; Karhausen, J.; Louis, N.A.; Montalto, M.C.; Colgan, S.P. Hypoxia-Inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002, 62, 3387–3394. [Google Scholar]
- Tacchini, L.; Bianchi, L.; Bernelli-Zazzera, A.; Cairo, G. Transferrin Receptor Induction by Hypoxia: HIF-1-mediated transcriptional activation and cell-specific post-transcriptional regulation. J. Biol. Chem. 1999, 274, 24142–24146. [Google Scholar] [CrossRef] [Green Version]
- Vitale, I.; Manic, G.; Coussens, L.M.; Kroemer, G.; Galluzzi, L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019, 30, 36–50. [Google Scholar] [CrossRef]
- Allavena, P.; Anfray, C.; Ummarino, A.; Andón, F.T. Therapeutic manipulation of tumor-associated macrophages: Facts and hopes from a clinical and translational perspective. Clin. Cancer Res. 2021, 27, 3291–3297. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Pisanti, S.; Napolitano, F.; Di Somma, S.; Martinelli, R.; Portella, G. Tumor-Associated macrophage status in cancer treatment. Cancers 2020, 12, 1987. [Google Scholar] [CrossRef]
- Corna, G.; Campana, L.; Pignatti, E.; Castiglioni, A.; Tagliafico, E.; Bosurgi, L.; Campanella, A.; Brunelli, S.; Manfredi, A.A.; Apostoli, P.; et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica 2010, 95, 1814. [Google Scholar] [CrossRef] [Green Version]
- Marques, O.; Porto, G.; Rêma, A.; Faria, F.; Paula, A.C.; Gomez-Lazaro, M.; Silva, P.; da Silva, B.M.; Lopes, C. Local iron homeostasis in the breast ductal carcinoma microenvironment. BMC Cancer 2016, 16, 187. [Google Scholar] [CrossRef] [Green Version]
- Cairo, G.; Recalcati, S.; Mantovani, A.; Locati, M. Iron trafficking and metabolism in macrophages: Contribution to the polarized phenotype. Trends Immunol. 2011, 32, 241–247. [Google Scholar] [CrossRef]
- Alkhateeb, A.A.; Han, B.; Connor, J.R. Ferritin stimulates breast cancer cells through an iron-independent mechanism and is localized within tumor-associated macrophages. Breast Cancer Res. Treat. 2013, 137, 733–744. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef] [Green Version]
- Sofias, A.M.; Combes, F.; Koschmieder, S.; Storm, G.; Lammers, T. A paradigm shift in cancer nanomedicine: From traditional tumor targeting to leveraging the immune system. Drug Discov. Today 2021, 26, 1482–1489. [Google Scholar] [CrossRef]
- Mainini, F.; De Santis, F.; Fucà, G.; Di Nicola, M.; Rivoltini, L.; Eccles, M. Nanobiotechnology and Immunotherapy: Two Powerful and Cooperative Allies against Cancer. Cancers 2021, 13, 3765. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Moos, T.; Morgan, E.H. Transferrin and Transferrin Receptor Function in Brain Barrier Systems. Cell. Mol. Neurobiol. 2000, 20, 77–95. [Google Scholar] [CrossRef]
- Rosager, A.M.; Sørensen, M.D.; Dahlrot, R.H.; Hansen, S.; Schonberg, D.L.; Rich, J.N.; Lathia, J.D.; Kristensen, B.W. Transferrin receptor-1 and ferritin heavy and light chains in astrocytic brain tumors: Expression and prognostic value. PLoS ONE 2017, 12, e0182954. [Google Scholar] [CrossRef]
- Fiandra, L.; Mazzucchelli, S.; Truffi, M.; Bellini, M.; Sorrentino, L.; Corsi, F. In Vitro Permeation of FITC-loaded Ferritins Across a Rat Blood-brain Barrier: A Model to Study the Delivery of Nanoformulated Molecules. J. Vis. Exp. 2016, 2016, 54279. [Google Scholar] [CrossRef]
- Phan, T.G.; Croucher, P.I. The dormant cancer cell life cycle. Nat. Rev. Cancer 2020, 20, 398–411. [Google Scholar] [CrossRef]
- Pienta, K.J.; Hammarlund, E.U.; Axelrod, R.; Amend, S.R.; Brown, J.S. Convergent Evolution, Evolving Evolvability, and the Origins of Lethal Cancer. Mol. Cancer Res. 2020, 18, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.; Hwang, H.S.; Na, K. Theranostics and contrast agents for magnetic resonance imaging. Biomater. Res. 2018, 22, 20. [Google Scholar] [CrossRef]
- Millet, I.; Pages, E.; Hoa, D.; Merigeaud, S.; Doyon, F.C.; Prat, X.; Taourel, P. Pearls and pitfalls in breast MRI. Br. J. Radiol. 2012, 85, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Farwell, M.D.; Pryma, D.A.; Mankoff, D.A. PET/CT imaging in cancer: Current applications and future directions. Cancer 2014, 120, 3433–3445. [Google Scholar] [CrossRef] [PubMed]
- Kikano, E.G.; Avril, S.; Marshall, H.; Jones, R.S.; Montero, A.J.; Avril, N. PET/CT Variants and Pitfalls in Breast Cancers. Semin. Nucl. Med. 2021, 51, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhang, P.; Hou, Y.; Ning, H.; Wang, Z.; Huang, J.; Gao, M. “Smart” Nanoprobes for Visualization of Tumor Microenvironments. Adv. Healthc. Mater. 2018, 7, e1800391. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Yue, X.; Dai, Z. Cyanine Conjugate-Based Biomedical Imaging Probes. Adv. Healthc. Mater. 2020, 9, e2001327. [Google Scholar] [CrossRef] [PubMed]
- Morato, Y.L.; Paredes, K.O.; Chamizo, L.L.; Marciello, M.; Filice, M. Recent Advances in Multimodal Molecular Imaging of Cancer Mediated by Hybrid Magnetic Nanoparticles. Polymers 2021, 13, 2989. [Google Scholar] [CrossRef] [PubMed]
- Thammineedi, S.R.; Saksena, A.R.; Nusrath, S.; Iyer, R.R.; Shukla, S.; Patnaik, S.C.; Reddy, R.P.; Boleneni, N.; Sharma, R.M.; Smith, L.; et al. Fluorescence-Guided cancer surgery—A new paradigm. J. Surg. Oncol. 2021, 123, 1679–1698. [Google Scholar] [CrossRef] [PubMed]
- Son, G.M.; Ahn, H.-M.; Lee, I.Y.; Ha, G.W. Multifunctional Indocyanine Green Applications for Fluorescence-Guided Laparoscopic Colorectal Surgery. Ann. Coloproctol. 2021, 37, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Egloff-Juras, C.; Bezdetnaya, L.; Dolivet, G.; Lassalle, H.-P. NIR fluorescence-guided tumor surgery: New strategies for the use of indocyanine green. Int. J. Nanomed. 2019, 14, 7823–7838. [Google Scholar] [CrossRef] [Green Version]
- Sofias, A.M.; Bjørkøy, G.; Ochando, J.; Sønstevold, L.; Hegvik, M.; Davies, C.dL.; Haraldseth, O.; Lammers, T.; Mulder, W.J.M.; Hak, S. Cyclic Arginine–Glycine–Aspartate-Decorated Lipid Nanoparticle Targeting toward Inflammatory Lesions Involves Hitchhiking with Phagocytes. Adv. Sci. 2021, 8, 2100370. [Google Scholar] [CrossRef]
- Sofias, A.M.; Toner, Y.C.; Meerwaldt, A.E.; van Leent, M.M.T.; Soultanidis, G.; Elschot, M.; Gonai, H.; Grendstad, K.; Flobak, Å.; Neckmann, U.; et al. Tumor Targeting by α v β 3-Integrin-Specific Lipid Nanoparticles Occurs via Phagocyte Hitchhiking. ACS Nano 2020, 14, 7832–7846. [Google Scholar] [CrossRef]
- Dong, X.; Chu, D.; Wang, Z. Leukocyte-Mediated Delivery of Nanotherapeutics in Inflammatory and Tumor Sites. Theranostics 2017, 7, 751–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, T.L.; Hauser, D.; Gruber, T.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A.; Lyck, R. Cellular Shuttles: Monocytes/Macrophages Exhibit Transendothelial Transport of Nanoparticles under Physiological Flow. ACS Appl. Mater. Interfaces 2017, 9, 18501–18511. [Google Scholar] [CrossRef]


| FN Origin | Purpose | Modifications | Loaded with | In Vivo Tested? | Reference |
|---|---|---|---|---|---|
| Human Hc FN | Cancer therapy | BCP1 peptide | DOX | Yes | [31] |
| Human Hc FN | Cancer therapy | Mutations to enhance the binding of Cu2+ | DOX | Yes | [32] |
| Human Hc FN | Cancer therapy | 4 Lysines (C-terminus) | siRNA (EGFR) | Yes | [33] |
| Human Hc FN | Cancer therapy | PD-L1 binding peptide | DOX | Yes | [34] |
| Human Hc FN | Cancer therapy | tLyP-1 peptide | PTX | Yes | [35] |
| Human Hc FN | Cancer therapy | Trastuzumab | DOX | Yes | [36] |
| Human Hc FN | Cancer therapy | PEGylation (50% subunits) | DOX | Yes | [37] |
| Human Hc FN | Cancer therapy | PEGylation (75% subunits) | Acriflavine | Yes | [38] |
| Human Hc FN | Cancer therapy | None | Olaparib | No | [39] |
| Human Hc FN | Cancer therapy | None | Everolimus | No | [40] |
| Human Hc FN | Cancer therapy | None | Curcumin | No | [41] |
| Human Hc FN | Cancer therapy | Anti FAP antibody | Navitoclax | Yes | [42] |
| Human Hc FN | Cancer therapy | None | DOX | Yes | [43] |
| Human Hc FN | Cancer therapy | α2β1 targeting peptide | DOX | Yes | [44] |
| Human Hc FN | Cancer therapy | None | PTX | Yes | [45] |
| Human Hc FN | Cancer therapy | Trastuzumab or Cetuximab | Empty | No | [46] |
| Human Hc FN | Cancer therapy | Pout peptide (C terminus) | EPI, Camptothecin | Yes | [47] |
| Pyrococcus furiosus FN | Cancer therapy | SP94 peptide | DOX | Yes | [48] |
| Horse spleen FN | Cancer therapy | None | Mertansine | No | [49] |
| Horse spleen FN | Cancer therapy | None | Arsenoplatin-1 | No | [50] |
| Horse spleen FN | Cancer therapy | Emulsified FN (size 78nm) | Rapamycin and Erastin | Yes | [51] |
| Horse spleen FN | Cancer therapy | GKRK peptide | Vincristine | Yes | [52] |
| Unspecified | Cancer therapy | PEG–Panitumumab | Oxaliplatin | Yes | [53] |
| Unspecified | Cancer therapy | RGD peptide | Resveratrol | Yes | [54] |
| Unspecified | Cancer therapy | None | Au(III) thiosemicarbazone | Yes | [55] |
| Pyrococcus furiosus FN | Cancer nanovaccine | SpyCatcher | SpyTagged peptides | Yes | [56] |
| Human Hc FN | Cancer Immunotherapy | M2pep peptide (N-terminus), cationic peptide (C-terminus) | CpG | Yes | [57] |
| Human Hc FN | Cancer Theranostic | None | Iron Oxide (core) and IRdye800 or DOX | Yes | [58] |
| Human Hc FN | Cancer Theranostic | Coated with RBC (functionalized with FA) | Iron Oxide, Cy5.5 | Yes | [59] |
| Horse spleen FN | Cancer Theranostic | 2-amino-2-deoxy-glucose | Gold NP | No | [60] |
| Horse spleen FN | Cancer Theranostic | None | Endogenous Iron | Yes | [61] |
| Unspecified | Cancer Theranostic | PEG–FA | Perfluoropentane | Yes (imaging only) | [62] |
| Human Hc FN | Tumor Imaging | None | ICG | Yes | [26,63] |
| Human Hc FN | Tumor Imaging | SDSSD peptide or hydroxyapatite binding peptide | Cy5 | Yes | [64] |
| Human Hc FN | Tumor Imaging | None | Iron Oxide or Cy5.5 | Yes | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mainini, F.; Bonizzi, A.; Sevieri, M.; Sitia, L.; Truffi, M.; Corsi, F.; Mazzucchelli, S. Protein-Based Nanoparticles for the Imaging and Treatment of Solid Tumors: The Case of Ferritin Nanocages, a Narrative Review. Pharmaceutics 2021, 13, 2000. https://doi.org/10.3390/pharmaceutics13122000
Mainini F, Bonizzi A, Sevieri M, Sitia L, Truffi M, Corsi F, Mazzucchelli S. Protein-Based Nanoparticles for the Imaging and Treatment of Solid Tumors: The Case of Ferritin Nanocages, a Narrative Review. Pharmaceutics. 2021; 13(12):2000. https://doi.org/10.3390/pharmaceutics13122000
Chicago/Turabian StyleMainini, Francesco, Arianna Bonizzi, Marta Sevieri, Leopoldo Sitia, Marta Truffi, Fabio Corsi, and Serena Mazzucchelli. 2021. "Protein-Based Nanoparticles for the Imaging and Treatment of Solid Tumors: The Case of Ferritin Nanocages, a Narrative Review" Pharmaceutics 13, no. 12: 2000. https://doi.org/10.3390/pharmaceutics13122000
APA StyleMainini, F., Bonizzi, A., Sevieri, M., Sitia, L., Truffi, M., Corsi, F., & Mazzucchelli, S. (2021). Protein-Based Nanoparticles for the Imaging and Treatment of Solid Tumors: The Case of Ferritin Nanocages, a Narrative Review. Pharmaceutics, 13(12), 2000. https://doi.org/10.3390/pharmaceutics13122000








