Stimuli Responsive Nitric Oxide-Based Nanomedicine for Synergistic Therapy
Abstract
:1. Introduction
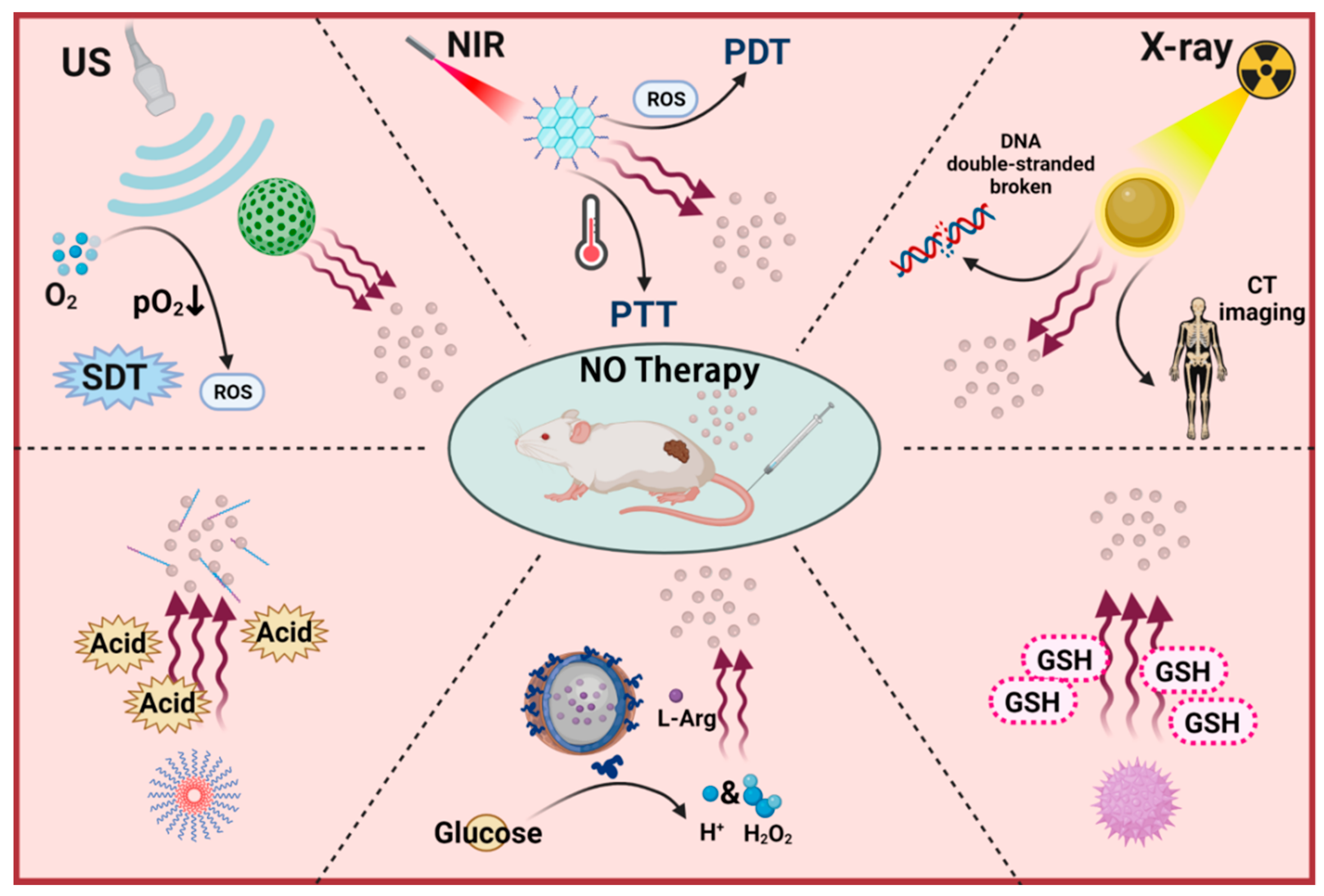
2. Exogenous Stimuli-Responsive NO Nanomedicines
2.1. Light-Triggered NO Nanomedicines
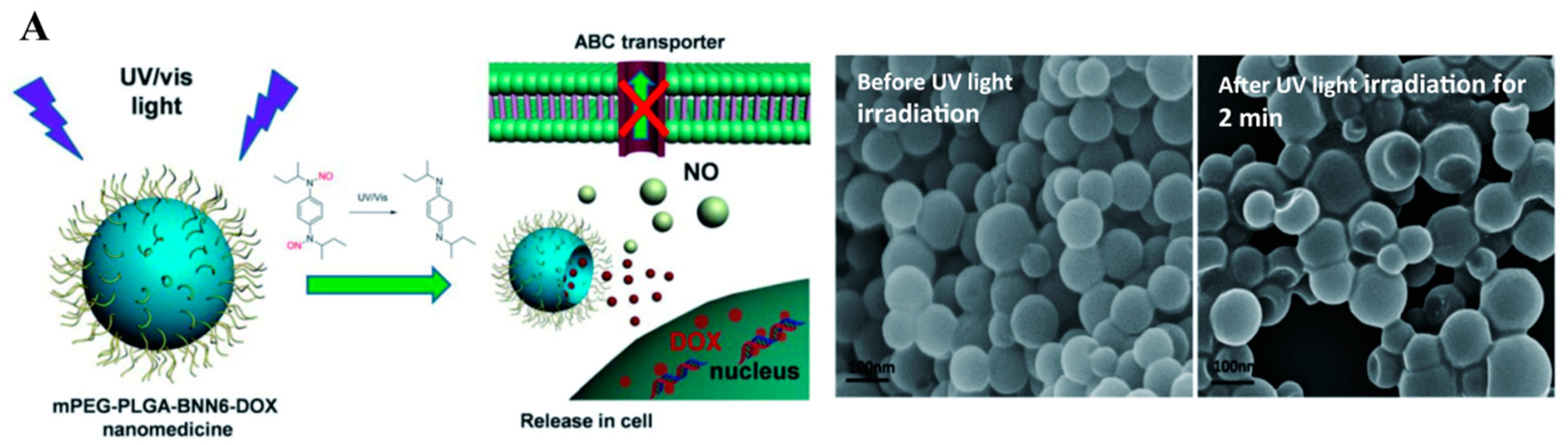
2.1.1. Ultraviolet–Visible Triggered NO Nanomedicines
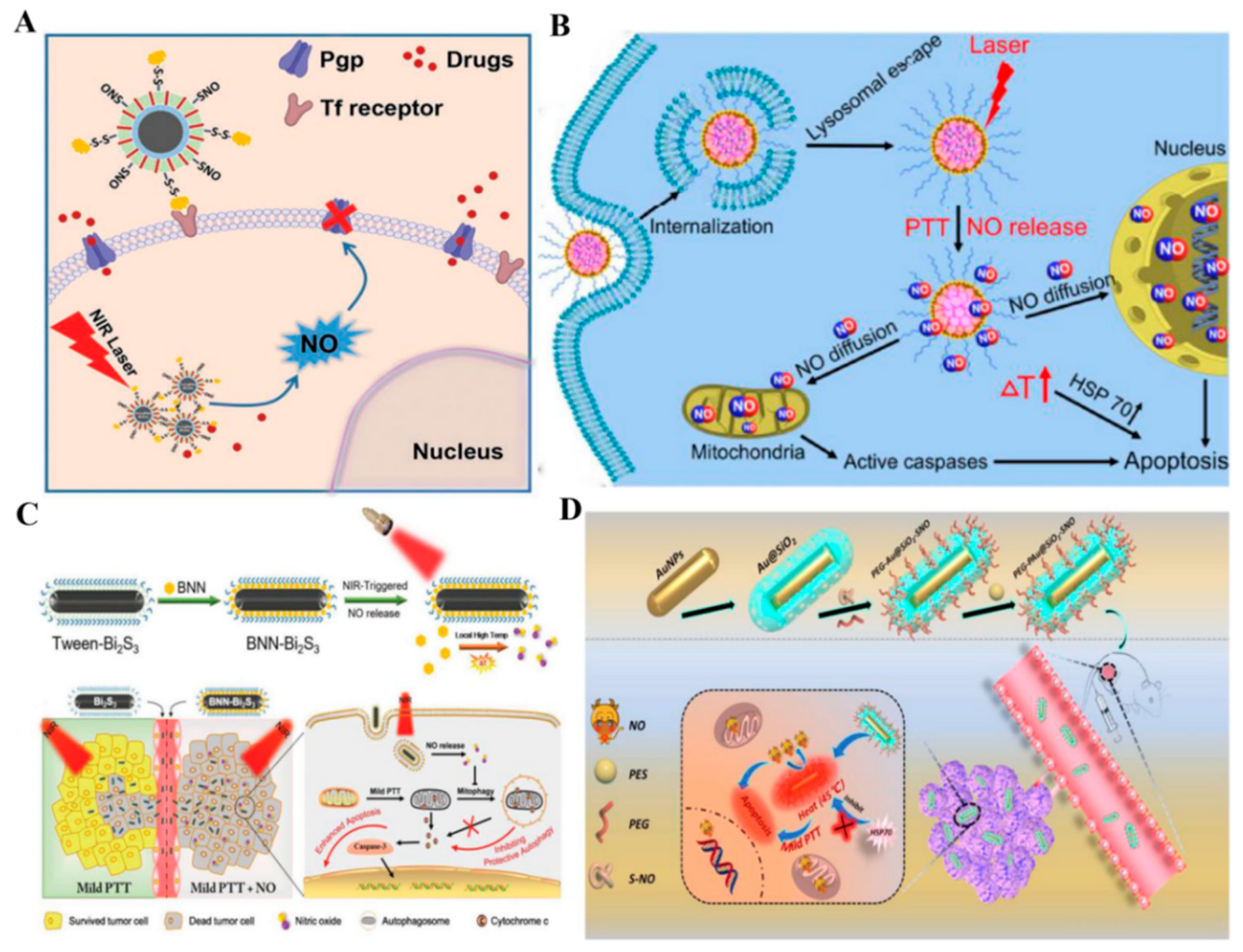
2.1.2. First Near-Infrared (NIR-I) Photothermal Triggered NO Nanomedicines
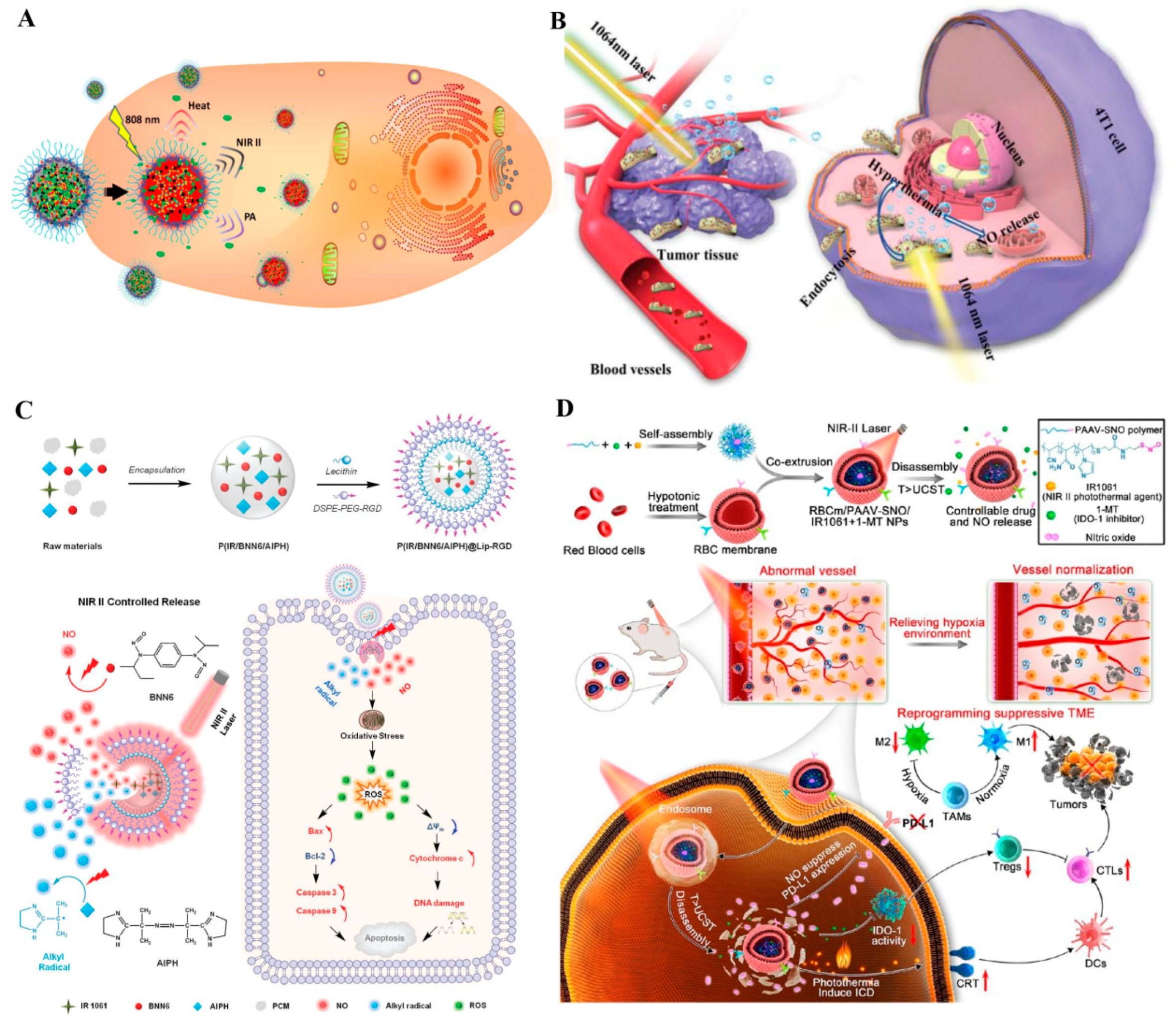
2.1.3. Second NIR (NIR-II) Photothermal Triggered NO Nanomedicines
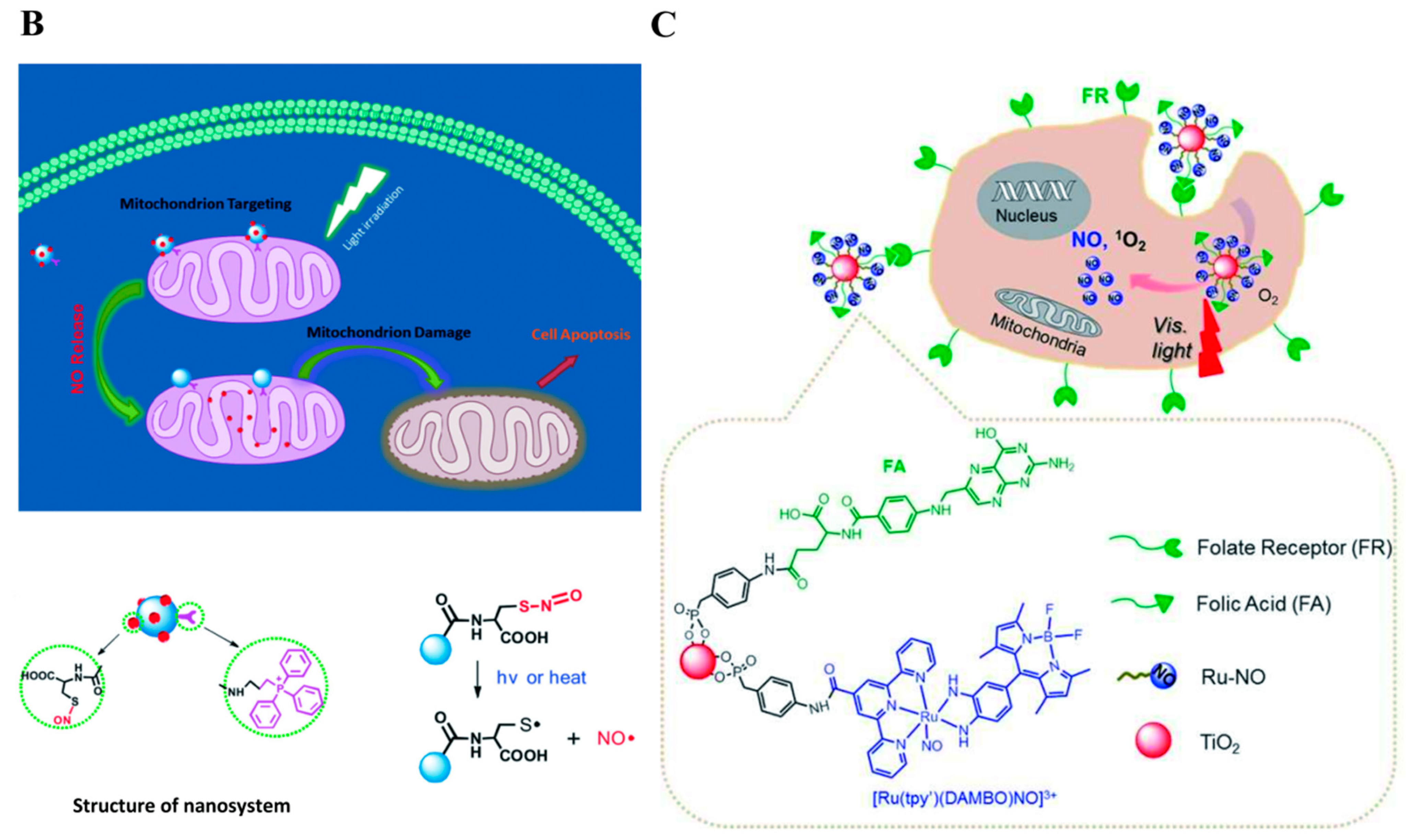
2.1.4. NIR Photodynamic Triggered NO Nanomedicines
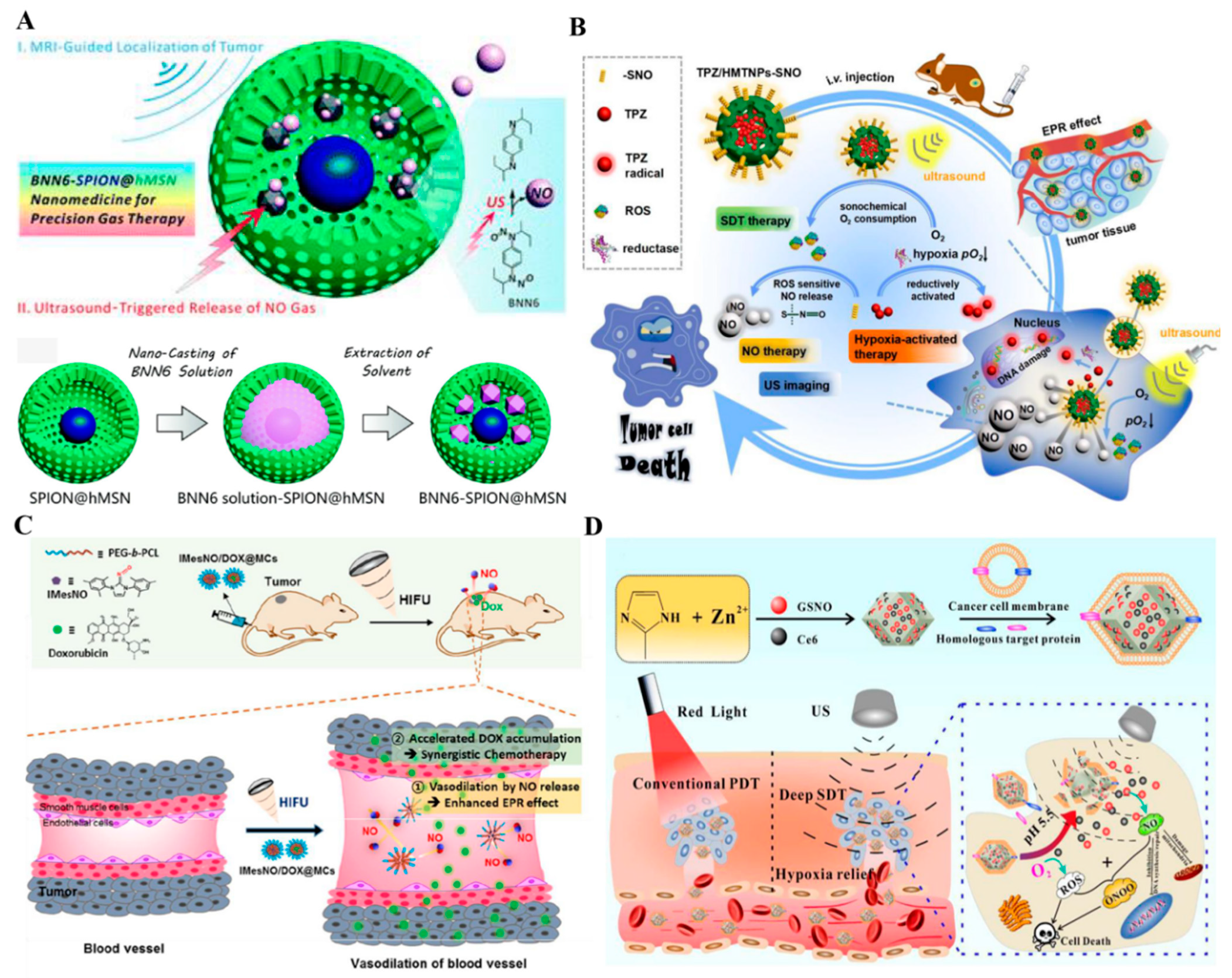
2.2. Ultrasound Triggered NO Nanomedicines
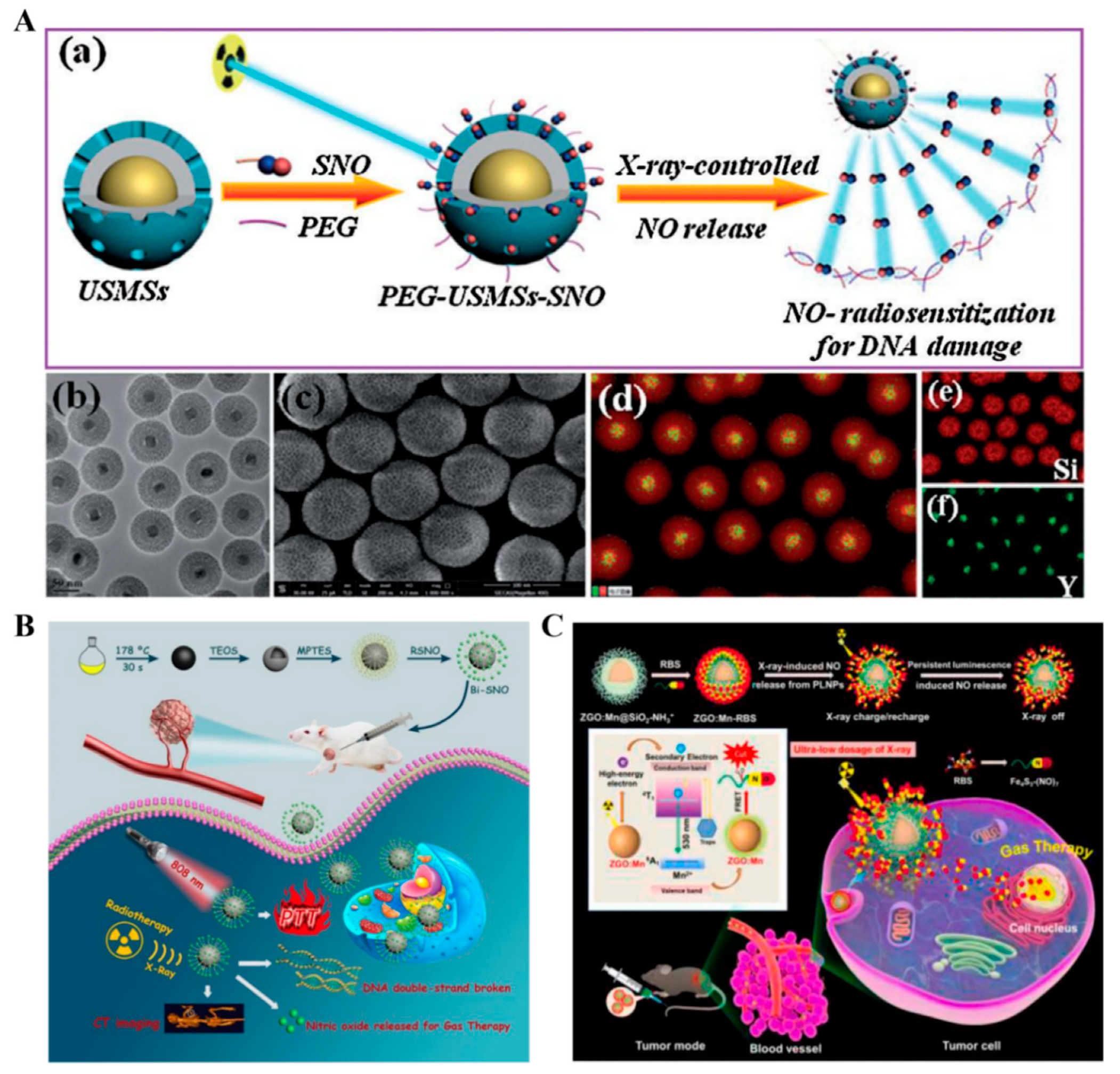
2.3. X-ray-Triggered NO Nanomedicines
3. Endogenous Stimuli-Responsive NO Nanomedicines
3.1. Glutathione-Triggered NO Nanomedicines
3.2. pH-Triggered NO Nanomedicines
3.3. Glucose-Triggered NO Nanomedicines
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gu, Z.; Dong, Y.; Xu, S.; Wang, L.; Liu, Z. Molecularly Imprinted Polymer-Based Smart Prodrug Delivery System for Specific Targeting, Prolonged Retention, and Tumor Microenvironment-Triggered Release. Angew. Chem. Int. Ed. Engl. 2020, 60, 2663–2667. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Y.; Fu, H.; Huang, H.; Wu, Z.; Zhao, M.; Yang, X.; Guo, Q.; Duan, Y.; Sun, Y. Multifunctional tumor-targeted PLGA nanoparticles delivering Pt(IV)/siBIRC5 for US/MRI imaging and overcoming ovarian cancer resistance. Biomaterials 2020, 269, 120478. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, S.; Gao, W. What Went Wrong with Anticancer Nanomedicine Design and How to Make It Right. ACS Nano 2020, 14, 12281–12290. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B.; Chen, S.; Wang, Y.; Zhang, Y.; Wang, Y.; Wei, D.; Zhang, L.; Rong, G.; Weng, Y.; et al. Near-Infrared Light Irradiation Induced Mild Hyperthermia Enhances Glutathione Depletion and DNA Interstrand Cross-Link Formation for Efficient Chemotherapy. ACS Nano 2020, 14, 14831–14845. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, T.; He, Q. Strategies for engineering advanced nanomedicines for gas therapy of cancer. Nat. Sci. Rev. 2020, 7, 1485–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, R.; Chen, Z.; Dai, C.; Guo, X.; Feng, W.; Liu, Z.; Lin, H.; Chen, Y.; Wu, R. Engineering two-dimensional silicene composite nanosheets for dual-sensitized and photonic hyperthermia-augmented cancer radiotherapy. Biomaterials 2020, 269, 120455. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Liu, P.; Xu, S.; Luo, X. Core-Shell Multifunctional Nanomaterial-Based All-in-One Nanoplatform for Simultaneous Multilayer Imaging of Dual Types of Tumor Biomarkers and Photothermal Therapy. Anal. Chem. 2020, 92, 15169–15178. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Zhang, L.; Zhao, G.; Xu, S.; Li, L.; Su, Z.; Liu, R.; Wang, C. An EPR-independent therapeutic strategy: Cancer cell-mediated dual-drug delivery depot for diagnostics and prevention of hepatocellular carcinoma metastasis. Biomaterials 2020, 268, 120541. [Google Scholar] [CrossRef]
- Lin, H.; Chen, Y.; Shi, J. Nanoparticle-triggered in situ catalytic chemical reactions for tumour-specific therapy. Chem. Soc. Rev. 2018, 47, 1938–1958. [Google Scholar] [CrossRef]
- Fu, J.; Li, T.; Yang, Y.; Jiang, L.; Wang, W.; Fu, L.; Zhu, Y.; Hao, Y. Activatable nanomedicine for overcoming hypoxia-induced resistance to chemotherapy and inhibiting tumor growth by inducing collaborative apoptosis and ferroptosis in solid tumors. Biomaterials 2020, 268, 120537. [Google Scholar] [CrossRef]
- Guo, X.; Liu, F.; Deng, J.; Dai, P.; Qin, Y.; Li, Z.; Wang, B.; Fan, A.; Wang, Z.; Zhao, Y. Electron-Accepting Micelles Deplete Reduced Nicotinamide Adenine Dinucleotide Phosphate and Impair Two Antioxidant Cascades for Ferroptosis-Induced Tumor Eradication. ACS Nano 2020, 14, 14715–14730. [Google Scholar] [CrossRef]
- Zhou, S.; Zhen, Z.; Paschall, A.V.; Xue, L.; Yang, X.; Bebin Blackwell, A.-G.; Cao, Z.; Zhang, W.; Wang, M.; Teng, Y.; et al. FAP-Targeted Photodynamic Therapy Mediated by Ferritin Nanoparticles Elicits an Immune Response against Cancer Cells and Cancer Associated Fibroblasts. Adv. Funct. Mater. 2020, 31, 2007017. [Google Scholar] [CrossRef]
- Jiang, M.; Mu, J.; Jacobson, O.; Wang, Z.; He, L.; Zhang, F.; Yang, W.; Lin, Q.; Zhou, Z.; Ma, Y.; et al. Reactive Oxygen Species Activatable Heterodimeric Prodrug as Tumor-Selective Nanotheranostics. ACS Nano 2020, 14, 16875–16886. [Google Scholar] [CrossRef]
- Li, Y.; Xie, J.; Um, W.; You, D.G.; Kwon, S.; Zhang, L.; Zhu, J.; Park, J.H. Sono/Photodynamic Nanomedicine-Elicited Cancer Immunotherapy. Adv. Funct. Mater. 2020, 31, 2008061. [Google Scholar] [CrossRef]
- Zhuang, Q.; Xu, J.; Deng, D.; Chao, T.; Li, J.; Zhang, R.; Peng, R.; Liu, Z. Bacteria-derived membrane vesicles to advance targeted photothermal tumor ablation. Biomaterials 2020, 268, 120550. [Google Scholar] [CrossRef]
- Głowacka, U.; Brzozowski, T.; Magierowski, M. Synergisms, Discrepancies and Interactions between Hydrogen Sulfide and Carbon Monoxide in the Gastrointestinal and Digestive System Physiology, Pathophysiology and Pharmacology. Biomolecules 2020, 10, 445. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Duan, X.H.; Guan, Q.Q.; Liu, J.; Yang, X.; Zhang, F.; Huang, P.; Shen, J.; Shuai, X.T.; Cao, Z. Mesoporous Polydopamine Carrying Manganese Carbonyl Responds to Tumor Microenvironment for Multimodal Imaging-Guided Cancer Therapy. Adv. Funct. Mater. 2019, 29, 11. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, S.F.; Su, L.; Song, J. Gas-Mediated Cancer Bioimaging and Therapy. ACS Nano 2019, 13, 10887–10917. [Google Scholar] [CrossRef]
- Szabo, C. Gasotransmitters in cancer: From pathophysiology to experimental therapy. Nat. Rev. Drug Discov. 2016, 15, 185–203. [Google Scholar] [CrossRef] [Green Version]
- He, Q. Precision gas therapy using intelligent nanomedicine. Biomater. Sci. 2017, 5, 2226–2230. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Mori, Y.; Feng, H.; Phan, K.Q.; Kishimura, A.; Kang, J.H.; Mori, T.; Katayama, Y. Rapid and continuous accumulation of nitric oxide-releasing liposomes in tumors to augment the enhanced permeability and retention (EPR) effect. Int. J. Pharm. 2019, 565, 481–487. [Google Scholar] [CrossRef]
- Yao, X.; Ma, S.; Peng, S.; Zhou, G.; Xie, R.; Jiang, Q.; Guo, S.; He, Q.; Yang, W. Zwitterionic Polymer Coating of Sulfur Dioxide-Releasing Nanosystem Augments Tumor Accumulation and Treatment Efficacy. Adv. Healthc. Mater. 2020, 9, e1901582. [Google Scholar] [CrossRef]
- Li, S.; Song, X.; Zhu, W.; Chen, Y.; Zhu, R.; Wang, L.; Chen, X.; Song, J.; Yang, H. Light-Switchable Yolk-Mesoporous Shell UCNPs@MgSiO3 for Nitric Oxide-Evoked Multidrug Resistance Reversal in Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 30066–30076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deng, M.; Shi, X.; Zhang, C.; Qu, X.; Hu, X.; Wang, W.; Kong, D.; Huang, P. Cascaded amplification of intracellular oxidative stress and reversion of multidrug resistance by nitric oxide prodrug based-supramolecular hydrogel for synergistic cancer chemotherapy. Bioact. Mater. 2021, 6, 3300–3313. [Google Scholar] [CrossRef]
- Zhao, P.; Jin, Z.; Chen, Q.; Yang, T.; Chen, D.; Meng, J.; Lu, X.; Gu, Z.; He, Q. Local generation of hydrogen for enhanced photothermal therapy. Nat. Commun. 2018, 9, 4241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S.; Wang, H.; Xin, Y.; Zhao, W.; Zhan, M.; Li, J.; Cai, R.; Lu, L. Second near-infrared photoactivatable hydrogen selenide nanogenerators for metastasis-inhibited cancer therapy. Nano Today 2021, 40, 101240. [Google Scholar] [CrossRef]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Torchilin, V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yung, B.C.; Kim, W.J.; Chen, X. Combination of nitric oxide and drug delivery systems: Tools for overcoming drug resistance in chemotherapy. J. Control. Release 2017, 263, 223–230. [Google Scholar] [CrossRef]
- Folkes, L.K.; O’Neill, P. Modification of DNA damage mechanisms by nitric oxide during ionizing radiation. Free Radic. Biol. Med. 2013, 58, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Matson, J.B. Gasotransmitter delivery via self-assembling peptides: Treating diseases with natural signaling gases. Adv. Drug Deliv. Rev. 2017, 110–111, 137–156. [Google Scholar] [CrossRef]
- Yin, H.; Guan, X.; Lin, H.; Pu, Y.; Fang, Y.; Yue, W.; Zhou, B.; Wang, Q.; Chen, Y.; Xu, H. Nanomedicine-Enabled Photonic Thermogaseous Cancer Therapy. Adv. Sci. 2020, 7, 1901954. [Google Scholar] [CrossRef]
- Yang, T.; Zelikin, A.N.; Chandrawati, R. Progress and Promise of Nitric Oxide-Releasing Platforms. Adv. Sci. 2018, 5, 1701043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Delivering nitric oxide with nanoparticles. J. Control. Release 2015, 205, 190–205. [Google Scholar] [CrossRef]
- SoRelle, R. Nobel prize awarded to scientists for nitric oxide discoveries. Circulation 1998, 98, 2365–2366. [Google Scholar] [CrossRef] [Green Version]
- Schulz, R.; Rassaf, T.; Massion, P.B.; Kelm, M.; Balligand, J.L. Recent advances in the understanding of the role of nitric oxide in cardiovascular homeostasis. Pharm. Ther. 2005, 108, 225–256. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wan, J.; Li, P.; Ran, H.; Chen, H.; Wang, Z.; Zhang, L. A novel NIR-controlled NO release of sodium nitroprusside-doped Prussian blue nanoparticle for synergistic tumor treatment. Biomaterials 2019, 214, 119213. [Google Scholar] [CrossRef]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: Part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef]
- Somasundaram, V.; Basudhar, D.; Bharadwaj, G.; No, J.H.; Ridnour, L.A.; Cheng, R.Y.S.; Fujita, M.; Thomas, D.D.; Anderson, S.K.; McVicar, D.W.; et al. Molecular Mechanisms of Nitric Oxide in Cancer Progression, Signal Transduction, and Metabolism. Antioxid. Redox Signal. 2019, 30, 1124–1143. [Google Scholar] [CrossRef]
- Holotiuk, V.V.; Kryzhanivska, A.Y.; Churpiy, I.K.; Tataryn, B.B.; Ivasiutyn, D.Y. Role of nitric oxide in pathogenesis of tumor growth and its possible application in cancer treatment. Exp. Oncol. 2019, 41, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ji, P.; Peng, S.Y.; Pan, P.; Zheng, D.W.; Li, C.X.; Sun, Y.X.; Zhang, X.Z. Nitric Oxide Release Device for Remote-Controlled Cancer Therapy by Wireless Charging. Adv. Mater. 2020, 32, e2000376. [Google Scholar] [CrossRef]
- Singh, N.; Patel, K.; Sahoo, S.K.; Kumar, R. Human nitric oxide biomarker as potential NO donor in conjunction with superparamagnetic iron oxide @ gold core shell nanoparticles for cancer therapeutics. Colloids Surf. B Biointerfaces 2018, 163, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Bronte, V.; Nitti, D. Nitric oxide, a double edged sword in cancer biology: Searching for therapeutic opportunities. Med. Res. Rev. 2007, 27, 317–352. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Fang, J.; Maeda, H. Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application. Cancer Sci. 2009, 100, 2426–2430. [Google Scholar] [CrossRef]
- Farah, C.; Michel, L.Y.M.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Gao, Z.; Liu, Y.; Zhao, J.; Ou, H.; Xu, F.; Ding, D. Polymeric Nitric Oxide Delivery Nanoplatforms for Treating Cancer, Cardiovascular Diseases, and Infection. Adv. Healthc. Mater. 2021, 10, e2001550. [Google Scholar] [CrossRef]
- Chung, M.F.; Liu, H.Y.; Lin, K.J.; Chia, W.T.; Sung, H.W. A pH-Responsive Carrier System that Generates NO Bubbles to Trigger Drug Release and Reverse P-Glycoprotein-Mediated Multidrug Resistance. Angew. Chem. Int. Ed. Engl. 2015, 54, 9890–9893. [Google Scholar] [CrossRef]
- Scicinski, J.; Oronsky, B.; Ning, S.; Knox, S.; Peehl, D.; Kim, M.M.; Langecker, P.; Fanger, G. NO to cancer: The complex and multifaceted role of nitric oxide and the epigenetic nitric oxide donor, RRx-001. Redox Biol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- De Ridder, M.; Verellen, D.; Verovski, V.; Storme, G. Hypoxic tumor cell radiosensitization through nitric oxide. Nitric Oxide 2008, 19, 164–169. [Google Scholar] [CrossRef]
- Sun, F.; Wang, Y.; Luo, X.; Ma, Z.; Xu, Y.; Zhang, X.; Lv, T.; Zhang, Y.; Wang, M.; Huang, Z.; et al. Anti-CD24 Antibody-Nitric Oxide Conjugate Selectively and Potently Suppresses Hepatic Carcinoma. Cancer Res. 2019, 79, 3395–3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Liu, Y.; Wang, Z.; Yang, M.; Gu, Y. 808 nm-light-excited upconversion nanoprobe based on LRET for the ratiometric detection of nitric oxide in living cancer cells. Nanoscale 2018, 10, 10641–10649. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.Z.; Loizidou, M.; Ahmed, M.; Charles, I.G. The role of nitric oxide in cancer. Cell Res. 2002, 12, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Ridnour, L.A.; Thomas, D.D.; Donzelli, S.; Espey, M.G.; Roberts, D.D.; Wink, D.A.; Isenberg, J.S. The biphasic nature of nitric oxide responses in tumor biology. Antioxid. Redox Signal. 2006, 8, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yung, B.C.; Chen, X. Stimuli-Responsive NO Release for On-Demand Gas-Sensitized Synergistic Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2018, 57, 8383–8394. [Google Scholar] [CrossRef]
- Xu, J.; Zeng, F.; Wu, H.; Wu, S. A mitochondrial-targeting and NO-based anticancer nanosystem with enhanced photo-controllability and low dark-toxicity. J. Mater. Chem. B 2015, 3, 4904–4912. [Google Scholar] [CrossRef] [PubMed]
- Keshet, R.; Erez, A. Arginine and the metabolic regulation of nitric oxide synthesis in cancer. Dis. Model. Mech. 2018, 11, dmm033332. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Ren, H.; Liu, J.; Wang, Y.; MEng, Z.; He, Z.; Miao, W.; Chen, G.; Li, X. A switchable NO-releasing nanomedicine for enhanced cancer therapy and inhibition of metastasis. Nanoscale 2019, 11, 5474–5488. [Google Scholar] [CrossRef]
- Raju, G.S.R.; Dariya, B.; Mungamuri, S.K.; Chalikonda, G.; Kang, S.M.; Khan, I.N.; Sushma, P.S.; Nagaraju, G.P.; Pavitra, E.; Han, Y.K. Nanomaterials multifunctional behavior for enlightened cancer therapeutics. Semin. Cancer Biol. 2021, 69, 178–189. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Tang, Y.; Zou, J.; Wang, P.; Zhang, Y.; Si, W.; Huang, W.; Dong, X. A light-induced nitric oxide controllable release nano-platform based on diketopyrrolopyrrole derivatives for pH-responsive photodynamic/photothermal synergistic cancer therapy. Chem. Sci. 2018, 9, 8103–8109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FEng, L.; Liu, B.; Xie, R.; Wang, D.; Qian, C.; Zhou, W.; Liu, J.; Jana, D.; Yang, P.; Zhao, Y. An Ultrasmall SnFe2O4 Nanozyme with Endogenous Oxygen Generation and Glutathione Depletion for Synergistic Cancer Therapy. Adv. Funct. Mater. 2020, 31, 2006216. [Google Scholar] [CrossRef]
- Quader, S.; Liu, X.; Toh, K.; Su, Y.L.; Maity, A.R.; Tao, A.; Paraiso, W.K.D.; Mochida, Y.; Kinoh, H.; Cabral, H.; et al. Supramolecularly enabled pH- triggered drug action at tumor microenvironment potentiates nanomedicine efficacy against glioblastoma. Biomaterials 2021, 267, 120463. [Google Scholar] [CrossRef]
- Yu, W.; Liu, T.; Zhang, M.; Wang, Z.; Ye, J.; Li, C.X.; Liu, W.; Li, R.; FEng, J.; Zhang, X.Z. O2 Economizer for Inhibiting Cell Respiration to Combat the Hypoxia Obstacle in Tumor Treatments. ACS Nano 2019, 13, 1784–1794. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Q.; Tang, Z.; Liu, C.; Li, Z.; Wu, A.; Lin, H. Tumor Microenvironment Stimuli-Responsive Fluorescence Imaging and Synergistic Cancer Therapy by Carbon-Dot-Cu2+ Nanoassemblies. Angew. Chem. Int. Ed. Engl. 2020, 59, 21041–21048. [Google Scholar] [CrossRef]
- Cheng, J.; He, K.; Shen, Z.; Zhang, G.; Yu, Y.; Hu, J. Nitric Oxide (NO)-Releasing Macromolecules: Rational Design and Biomedical Applications. Front. Chem. 2019, 7, 530. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.R.; Megson, I.L. Recent developments in nitric oxide donor drugs. Br. J. Pharm. 2007, 151, 305–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; He, Q.; Liu, Y.; Zhang, F.; Yang, X.; Wang, Z.; Lu, N.; Fan, W.; Lin, L.; Niu, G.; et al. Light-Responsive Biodegradable Nanomedicine Overcomes Multidrug Resistance via NO-Enhanced Chemosensitization. ACS Appl. Mater. Interfaces 2016, 8, 13804–13811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zeng, F.; Wu, H.; Hu, C.; Yu, C.; Wu, S. Preparation of a mitochondria-targeted and NO-releasing nanoplatform and its enhanced pro-apoptotic effect on cancer cells. Small 2014, 10, 3750–3760. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.J.; An, L.; Tang, W.W.; Yang, S.P.; Liu, J.G. Photo-controlled targeted intracellular delivery of both nitric oxide and singlet oxygen using a fluorescence-trackable ruthenium nitrosyl functional nanoplatform. Chem. Commun. 2015, 51, 2555–2558. [Google Scholar] [CrossRef]
- Guo, R.R.; Tian, Y.; Wang, Y.J.; Yang, W.L. Near-Infrared Laser-Triggered Nitric Oxide Nanogenerators for the Reversal of Multidrug Resistance in Cancer. Adv. Funct. Mater. 2017, 27, 1606398. [Google Scholar] [CrossRef]
- Huang, X.; Xu, F.; Hou, H.; Hou, J.; Wang, Y.; Zhou, S. Stimuli-responsive nitric oxide generator for light-triggered synergistic cancer photothermal/gas therapy. Nano Res. 2019, 12, 1361–1370. [Google Scholar] [CrossRef]
- Yu, Y.T.; Shi, S.W.; Wang, Y.; Zhang, Q.L.; Gao, S.H.; Yang, S.P.; Liu, J.G. A Ruthenium Nitrosyl-Functionalized Magnetic Nanoplatform with Near-Infrared Light-Controlled Nitric Oxide Delivery and Photothermal Effect for Enhanced Antitumor and Antibacterial Therapy. ACS Appl. Mater. Interfaces 2020, 12, 312–321. [Google Scholar] [CrossRef]
- Zhang, X.; Du, J.; Guo, Z.; Yu, J.; Gao, Q.; Yin, W.; Zhu, S.; Gu, Z.; Zhao, Y. Efficient Near Infrared Light Triggered Nitric Oxide Release Nanocomposites for Sensitizing Mild Photothermal Therapy. Adv. Sci. 2019, 6, 1801122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Jiang, R.; Wang, Q.; Li, X.; Hu, X.; Yuan, Y.; Lu, X.; Wang, W.; Huang, W.; Fan, Q. Semiconducting polymer nanotheranostics for NIR-II/Photoacoustic imaging-guided photothermal initiated nitric oxide/photothermal therapy. Biomaterials 2019, 217, 119304. [Google Scholar] [CrossRef]
- You, C.; Li, Y.; Dong, Y.; Ning, L.; Zhang, Y.; Yao, L.; Wang, F. Low-Temperature Trigger Nitric Oxide Nanogenerators for Enhanced Mild Photothermal Therapy. ACS Biomater. Sci. Eng. 2020, 6, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Du, C.; Qian, J.; Dong, C.M. NIR-Responsive Polypeptide Nanocomposite Generates NO Gas, Mild Photothermia, and Chemotherapy to Reverse Multidrug-Resistant Cancer. Nano Lett. 2019, 19, 4362–4370. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, Y.; Liang, Z.; Song, X.; Huang, Y.; Qiu, L.; Qiu, X.; Yu, S.; Xue, W. Near infrared II laser controlled free radical releasing nanogenerator for synergistic nitric oxide and alkyl radical therapy of breast cancer. Nanoscale 2021, 13, 11169–11187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, D.; Guo, X.; Jin, L.; ZhEng, J.; Wang, Y.; Chen, S.; ZhEng, X.; ZEng, L.; Guo, M.; et al. Fighting Immune Cold and Reprogramming Immunosuppressive Tumor Microenvironment with Red Blood Cell Membrane-Camouflaged Nanobullets. ACS Nano 2020, 14, 17442–17457. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, Y.X.; Du, C.; Wang, C.W.; Chen, T.T.; Wang, Y.; Wang, J.; Yao, Y.; Dong, C.M. NO-releasing polypeptide nanocomposites reverse cancer multidrug resistance via triple therapies. Acta Biomater. 2021, 123, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, Q.; Lei, M.; Xiong, L.; Shi, K.; Tan, L.; Jin, Z.; Wang, T.; Qian, Z. Facile Coordination-Precipitation Route to Insoluble Metal Roussin’s Black Salts for NIR-Responsive Release of NO for Anti-Metastasis. ACS Appl. Mater. Interfaces 2017, 9, 36473–36477. [Google Scholar] [CrossRef]
- Fan, J.; He, N.; He, Q.; Liu, Y.; Ma, Y.; Fu, X.; Liu, Y.; Huang, P.; Chen, X. A novel self-assembled sandwich nanomedicine for NIR-responsive release of NO. Nanoscale 2015, 7, 20055–20062. [Google Scholar] [CrossRef]
- Wan, S.S.; ZEng, J.Y.; Cheng, H.; Zhang, X.Z. ROS-induced NO generation for gas therapy and sensitizing photodynamic therapy of tumor. Biomaterials 2018, 185, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhan, Q.; Li, Y.; Zhou, L.; Wei, S. Multiple Functions Integrated inside a Single Molecule for Amplification of Photodynamic Therapy Activity. Mol. Pharm. 2020, 17, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chang, Y.; FEng, Y.; Li, X.; ChEng, Y.; Jian, H.; Ma, X.; ZhEng, R.; Wu, X.; Xu, K.; et al. Nitric Oxide Stimulated Programmable Drug Release of Nanosystem for Multidrug Resistance Cancer Therapy. Nano Lett. 2019, 19, 6800–6811. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Wang, X.; Liu, J.; Wang, Z.; Wang, W.; Kong, D.; Leng, X. ICG/l-Arginine Encapsulated PLGA Nanoparticle-Thermosensitive Hydrogel Hybrid Delivery System for Cascade Cancer Photodynamic-NO Therapy with Promoted Collagen Depletion in Tumor Tissues. Mol. Pharm. 2021, 18, 928–939. [Google Scholar] [CrossRef]
- Xiang, H.J.; DEng, Q.; An, L.; Guo, M.; Yang, S.P.; Liu, J.G. Tumor cell specific and lysosome-targeted delivery of nitric oxide for enhanced photodynamic therapy triggered by 808 nm near-infrared light. Chem. Commun. 2016, 52, 148–151. [Google Scholar] [CrossRef]
- Jin, Z.; Wen, Y.; Hu, Y.; Chen, W.; ZhEng, X.; Guo, W.; Wang, T.; Qian, Z.; Su, B.L.; He, Q. MRI-guided and ultrasound-triggered release of NO by advanced nanomedicine. Nanoscale 2017, 9, 3637–3645. [Google Scholar] [CrossRef]
- FEng, Q.; Li, Y.; Yang, X.; Zhang, W.; Hao, Y.; Zhang, H.; Hou, L.; Zhang, Z. Hypoxia-specific therapeutic agents delivery nanotheranostics: A sequential strategy for ultrasound mediated on-demand tritherapies and imaging of cancer. J. Control. Release 2018, 275, 192–200. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, J.; Park, J.; Lee, Y.M.; Saravanakumar, G.; Park, K.M.; Choi, W.; Kim, K.; Lee, E.; Kim, C.; et al. Tumor vasodilation by N-Heterocyclic carbene-based nitric oxide delivery triggered by high-intensity focused ultrasound and enhanced drug homing to tumor sites for anti-cancer therapy. Biomaterials 2019, 217, 119297. [Google Scholar] [CrossRef]
- An, J.; Hu, Y.G.; Li, C.; Hou, X.L.; ChEng, K.; Zhang, B.; Zhang, R.Y.; Li, D.Y.; Liu, S.J.; Liu, B.; et al. A pH/Ultrasound dual-response biomimetic nanoplatform for nitric oxide gas-sonodynamic combined therapy and repeated ultrasound for relieving hypoxia. Biomaterials 2020, 230, 119636. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; Liu, Z.; Chen, G.; Li, X.; Ren, H. Damaging Tumor Vessels with an Ultrasound-Triggered NO Release Nanosystem to Enhance Drug Accumulation and T Cells Infiltration. Int. J. Nanomed. 2021, 16, 2597–2613. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, H.; Jia, X.; Chen, Y.; Ma, M.; Sun, L.; Chen, H. Ultrasound-Triggered Nitric Oxide Release Platform Based on Energy Transformation for Targeted Inhibition of Pancreatic Tumor. ACS Nano 2016, 10, 10816–10828. [Google Scholar] [CrossRef]
- Fan, W.; Bu, W.; Zhang, Z.; Shen, B.; Zhang, H.; He, Q.; Ni, D.; Cui, Z.; Zhao, K.; Bu, J.; et al. X-ray Radiation-Controlled NO-Release for On-Demand Depth-Independent Hypoxic Radiosensitization. Angew. Chem. Int. Ed. Engl. 2015, 54, 14026–14030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, S.; Zhang, N.; Kuang, Y.; Li, W.; Gai, S.; He, F.; Gulzar, A.; Yang, P. X-ray-triggered NO-released Bi-SNO nanoparticles: All-in-one nano-radiosensitizer with photothermal/gas therapy for enhanced radiotherapy. Nanoscale 2020, 12, 19293–19307. [Google Scholar] [CrossRef]
- Xue, Z.; Jiang, M.; Liu, H.; Zeng, S.; Hao, J. Low dose soft X-ray-controlled deep-tissue long-lasting NO release of persistent luminescence nanoplatform for gas-sensitized anticancer therapy. Biomaterials 2020, 263, 120384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Song, H.; Ji, S.; Wang, X.; Huang, P.; Zhang, C.; Wang, W.; Kong, D. NO prodrug-conjugated, self-assembled, pH-responsive and galactose receptor targeted nanoparticles for co-delivery of nitric oxide and doxorubicin. Nanoscale 2018, 10, 4179–4188. [Google Scholar] [CrossRef] [PubMed]
- Deepagan, V.G.; Ko, H.; Kwon, S.; Rao, N.V.; Kim, S.K.; Um, W.; Lee, S.; Min, J.; Lee, J.; Choi, K.Y.; et al. Intracellularly Activatable Nanovasodilators to Enhance Passive Cancer Targeting Regime. Nano Lett. 2018, 18, 2637–2644. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Cao, J.; Zhang, Y.; Gao, X.; ChEng, M.; Liu, Y.; Wang, W.; Yuan, Z. A glutathione responsive nitric oxide release system based on charge-reversal chitosan nanoparticles for enhancing synergistic effect against multidrug resistance tumor. Nanomedicine 2019, 20, 102015. [Google Scholar] [CrossRef]
- Deng, Y.; Jia, F.; Chen, X.; Jin, Q.; Ji, J. ATP Suppression by pH-Activated Mitochondria-Targeted Delivery of Nitric Oxide Nanoplatform for Drug Resistance Reversal and Metastasis Inhibition. Small 2020, 16, e2001747. [Google Scholar] [CrossRef]
- Song, Q.; Tan, S.; Zhuang, X.; Guo, Y.; Zhao, Y.; Wu, T.; Ye, Q.; Si, L.; Zhang, Z. Nitric oxide releasing d-α-tocopheryl polyethylene glycol succinate for enhancing antitumor activity of doxorubicin. Mol. Pharm. 2014, 11, 4118–4129. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, D.J.; Choi, G.H.; Yang, D.N.; Heo, J.S.; Lee, S.C. pH-Responsive mineralized nanoparticles as stable nanocarriers for intracellular nitric oxide delivery. Colloids Surf. B Biointerfaces 2016, 146, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhan, M.; Li, W.; Chu, C.; Xing, D.; Lu, S.; Hu, X. Photoacoustic Cavitation-Ignited Reactive Oxygen Species to Amplify Peroxynitrite Burst by Photosensitization-Free Polymeric Nanocapsules. Angew. Chem. Int. Ed. Engl. 2021, 60, 4720–4731. [Google Scholar] [CrossRef]
- Fan, W.; Lu, N.; Huang, P.; Liu, Y.; Yang, Z.; Wang, S.; Yu, G.; Liu, Y.; Hu, J.; He, Q.; et al. Glucose-Responsive Sequential Generation of Hydrogen Peroxide and Nitric Oxide for Synergistic Cancer Starving-Like/Gas Therapy. Angew. Chem. Int. Ed. Engl. 2017, 56, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, X.; Lei, L.; Liu, B.; Wu, S.; Shen, J. Tumor Microenvironment-Activatable Cyclic Cascade Reaction to Reinforce Multimodal Combination Therapy by Destroying the Extracellular Matrix. ACS Appl. Mater. Interfaces 2021, 13, 12960–12971. [Google Scholar] [CrossRef]
- Hou, J.; Pan, Y.; Zhu, D.; Fan, Y.; FEng, G.; Wei, Y.; Wang, H.; Qin, K.; Zhao, T.; Yang, Q.; et al. Targeted delivery of nitric oxide via a ‘bump-and-hole’-based enzyme–prodrug pair. Nat. Chem. Biol. 2018, 15, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Xiang, H.J.; Wang, Y.; Zhang, Q.L.; An, L.; Yang, S.P.; Ma, Y.; Wang, Y.; Liu, J.G. Ruthenium nitrosyl functionalized graphene quantum dots as an efficient nanoplatform for NIR-light-controlled and mitochondria-targeted delivery of nitric oxide combined with photothermal therapy. Chem. Commun. 2017, 53, 3253–3256. [Google Scholar] [CrossRef]
- Su, C.H.; Li, W.P.; Tsao, L.C.; Wang, L.C.; Hsu, Y.P.; Wang, W.J.; Liao, M.C.; Lee, C.L.; Yeh, C.S. Enhancing Microcirculation on Multitriggering Manner Facilitates Angiogenesis and Collagen Deposition on Wound Healing by Photoreleased NO from Hemin-Derivatized Colloids. ACS Nano 2019, 13, 4290–4301. [Google Scholar] [CrossRef]
- Tessaro, A.L.; Fraix, A.; Pedrozo da Silva, A.C.; Gazzano, E.; Riganti, C.; Sortino, S. “Three-Bullets” Loaded Mesoporous Silica Nanoparticles for Combined Photo/Chemotherapy. Nanomaterials 2019, 9, 823. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Chen, C.; Qiu, Y.; Xu, C.; Yao, J. Paying attention to tumor blood vessels: Cancer phototherapy assisted with nano delivery strategies. Biomaterials 2020, 268, 120562. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Lin, Q. Light-Triggered Click Chemistry. Chem. Rev. 2021, 121, 6991–7031. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Park, S.J.; Jung, W.H.; Cho, Y.; Ahn, D.J.; Lee, Y.S.; Kim, S. Injectable Single-Component Peptide Depot: Autonomously Rechargeable Tumor Photosensitization for Repeated Photodynamic Therapy. ACS Nano 2020, 14, 15793–15805. [Google Scholar] [CrossRef]
- Karaki, F.; Kabasawa, Y.; Yanagimoto, T.; Umeda, N.; Firman Urano, Y.; Nagano, T.; Otani, Y.; Ohwada, T. Visible-light-triggered release of nitric oxide from N-pyramidal nitrosamines. Chemistry 2012, 18, 1127–1141. [Google Scholar] [CrossRef]
- Fraix, A.; Marino, N.; Sortino, S. Phototherapeutic Release of Nitric Oxide with Engineered Nanoconstructs. Top. Curr. Chem. 2016, 370, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Chegaev, K.; Fraix, A.; Gazzano, E.; Abd-Ellatef, G.E.; Blangetti, M.; Rolando, B.; Conoci, S.; Riganti, C.; Fruttero, R.; Gasco, A.; et al. Light-Regulated NO Release as a Novel Strategy To Overcome Doxorubicin Multidrug Resistance. ACS Med. Chem. Lett. 2017, 8, 361–365. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, K.; Chen, B.; Zhou, S.; Li, N.; Liu, C.; Yang, J.; Lin, R.; Zhang, T.; He, W. A polyethylenimine-based diazeniumdiolate nitric oxide donor accelerates wound healing. Biomater. Sci. 2019, 7, 1607–1616. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Wang, Y.; Li, X.; Zhang, G.; Zhang, G.; Hu, J. Light-triggered nitric oxide (NO) release from photoresponsive polymersomes for corneal wound healing. Chem. Sci. 2020, 11, 186–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Tian, G.; Yin, W.Y.; Wang, L.M.; ZhEng, X.P.; Yan, L.; Li, J.X.; Su, H.R.; Chen, C.Y.; Gu, Z.J.; et al. Controllable Generation of Nitric Oxide by Near-Infrared-Sensitized Upconversion Nanoparticles for Tumor Therapy. Adv. Funct. Mater. 2015, 25, 3049–3056. [Google Scholar] [CrossRef]
- Pu, K.; Huang, J.; Jiang, Y.; Li, J.; Huang, J. Molecular Chemiluminescent Probes with a Record Long Near-infrared Turn-on Wavelength for In vivo Imaging. Angew. Chem. Int. Ed. Engl. 2020, 60, 3999–4003. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Lee, J.; Park, H.; Park, Y.; Jung, S.; Lim, J.; Choi, H.C.; Kim, W.J. In vivo self-degradable graphene nanomedicine operated by DNAzyme and photo-switch for controlled anticancer therapy. Biomaterials 2020, 263, 120402. [Google Scholar] [CrossRef]
- Liu, J.S.; PEng, S.J.; Li, G.F.; Zhao, Y.X.; MEng, X.Y.; Yu, X.R.; Li, Z.H.; Chen, J.M. Polydopamine Nanoparticles for Deep Brain Ablation via Near-Infrared Irradiation. ACS Biomater. Sci. Eng. 2020, 6, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, Q.; Zhao, Y.; Liu, H.; Song, S.; Zhao, Y.; Lin, Q.; Chang, Y. Near-infrared light-mediated and nitric oxide-supplied nanospheres for enhanced synergistic thermo-chemotherapy. J. Mater. Chem. B 2019, 7, 548–555. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, Y.H.; Ma, Z.Q.; He, C.J.; Wu, Y.L.; An, Q. Designing cancer nanodrugs that are highly loaded, pH-responsive, photothermal, and possess a favored morphology: A hierarchical assembly of DOX and layer-by-layer modified rGO. Chin. Chem. Lett. 2019, 30, 489–493. [Google Scholar] [CrossRef]
- Zhang, A.M.; Hai, L.; Wang, T.Z.; ChEng, H.; Li, M.; He, X.X.; Wang, K.M. NIR-triggered drug delivery system based on phospholipid coated ordered mesoporous carbon for synergistic chemo-photothermal therapy of cancer cells. Chin. Chem. Lett. 2020, 31, 3158–3162. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, J.; Kim, J.; Kim, Y.; Song, H.B.; Kim, J.H.; Kim, K.; Kim, W.J. Light-Induced Acid Generation on a Gatekeeper for Smart Nitric Oxide Delivery. ACS Nano 2016, 10, 4199–4208. [Google Scholar] [CrossRef]
- Sung, Y.C.; Jin, P.R.; Chu, L.A.; Hsu, F.F.; Wang, M.R.; Chang, C.C.; Chiou, S.J.; Qiu, J.T.; Gao, D.Y.; Lin, C.C.; et al. Delivery of nitric oxide with a nanocarrier promotes tumour vessel normalization and potentiates anti-cancer therapies. Nat. Nanotechnol. 2019, 14, 1160–1169. [Google Scholar] [CrossRef]
- Luo, D.; Wang, X.; Walker, E.; Wang, J.; Springer, S.; Lou, J.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Nanoparticles Yield Increased Drug Uptake and Therapeutic Efficacy upon Sequential Near-Infrared Irradiation. ACS Nano 2020, 14, 15193–15203. [Google Scholar] [CrossRef]
- Han, R.; Xiao, Y.; Yang, Q.; Pan, M.; Hao, Y.; He, X.; PEng, J.; Qian, Z. Ag2S nanoparticle-mediated multiple ablations reinvigorates the immune response for enhanced cancer photo-immunotherapy. Biomaterials 2021, 264, 120451. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Yang, G.; Wei, B.; Wang, Y.; Zhou, S. Near-infrared light switching nitric oxide nanoemitter for triple-combination therapy of multidrug resistant cancer. Acta Biomater. 2019, 100, 365–377. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, X.T.; Shang, Y.; Li, Y.H.; Yin, X.B. Theranostic Mn-Porphyrin Metal-Organic Frameworks for Magnetic Resonance Imaging-Guided Nitric Oxide and Photothermal Synergistic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 28390–28398. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, W.; Dong, Z.; Chao, Y.; Xu, L.; Chen, M.; Liu, Z. 1D Coordination Polymer Nanofibers for Low-Temperature Photothermal Therapy. Adv. Mater. 2017, 29, 3588. [Google Scholar] [CrossRef]
- Zhang, K.; MEng, X.D.; Cao, Y.; Yang, Z.; Dong, H.F.; Zhang, Y.D.; Lu, H.T.; Shi, Z.J.; Zhang, X.J. Metal-Organic Framework Nanoshuttle for Synergistic Photodynamic and Low-Temperature Photothermal Therapy. Adv. Funct. Mater. 2018, 28, 10. [Google Scholar] [CrossRef]
- Zhao, J.; Zhong, D.; Zhou, S. NIR-I-to-NIR-II fluorescent nanomaterials for biomedical imaging and cancer therapy. J. Mater. Chem. B 2018, 6, 349–365. [Google Scholar] [CrossRef]
- Wang, J.; Wu, C.C.; Qin, X.R.; Huang, Y.Y.; Zhang, J.N.; Chen, T.T.; Wang, Y.; Ding, Y.; Yao, Y. NIR-II light triggered nitric oxide release nanoplatform combined chemo-photothermal therapy for overcoming multidrug resistant cancer. J. Mater. Chem. B 2021, 9, 1698–1706. [Google Scholar] [CrossRef]
- Sun, H.T.; Zhang, Q.; Li, J.C.; PEng, S.J.; Wang, X.L.; Cai, R. Near-infrared photoactivated nanomedicines for photothermal synergistic cancer therapy. Nano Today 2021, 37, 29. [Google Scholar] [CrossRef]
- PEng, S.J.; Ouyang, B.S.; Men, Y.Z.; Du, Y.; Cao, Y.B.; Xie, R.H.; Pang, Z.Q.; Shen, S.; Yang, W.L. Biodegradable zwitterionic polymer membrane coating endowing nanoparticles with ultra-long circulation and enhanced tumor photothermal therapy. Biomaterials 2020, 231, 13. [Google Scholar] [CrossRef]
- Wang, C.; Dai, C.; Hu, Z.; Li, H.; Yu, L.; Lin, H.; Bai, J.; Chen, Y. Photonic cancer nanomedicine using the near infrared-II biowindow enabled by biocompatible titanium nitride nanoplatforms. Nanoscale Horiz 2019, 4, 415–425. [Google Scholar] [CrossRef] [PubMed]
- She, D.J.; PEng, S.J.; Liu, L.; Huang, H.H.; ZhEng, Y.Y.; Lu, Y.P.; GEng, D.Y.; Yin, B. Biomimic FeS2 nanodrug with hypothermal photothermal effect by clinical approved NIR-II light for augmented chemodynamic therapy. Chem. Eng. J. 2020, 400, 13. [Google Scholar] [CrossRef]
- Lin, S.; Lin, H.; Yang, M.; Ge, M.; Chen, Y.; Zhu, Y. A two-dimensional MXene potentiates a therapeutic microneedle patch for photonic implantable medicine in the second NIR biowindow. Nanoscale 2020, 12, 10265–10276. [Google Scholar] [CrossRef]
- Han, X.; Jing, X.; Yang, D.; Lin, H.; Wang, Z.; Ran, H.; Li, P.; Chen, Y. Therapeutic mesopore construction on 2D Nb2C MXenes for targeted and enhanced chemo-photothermal cancer therapy in NIR-II biowindow. Theranostics 2018, 8, 4491–4508. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Gao, S.; Dai, C.; Chen, Y.; Shi, J. A Two-Dimensional Biodegradable Niobium Carbide (MXene) for Photothermal Tumor Eradication in NIR-I and NIR-II Biowindows. J. Am. Chem. Soc. 2017, 139, 16235–16247. [Google Scholar] [CrossRef]
- Wen, S.F.; Zhang, K.; Li, Y.; Fan, J.Q.; Chen, Z.M.; Zhang, J.P.; Wang, H.; Wang, L. A self-assembling peptide targeting VEGF receptors to inhibit angiogenesis. Chin. Chem. Lett. 2020, 31, 3153–3157. [Google Scholar] [CrossRef]
- Chen, J.; Lin, S.; Zhao, D.; Guan, L.; Hu, Y.; Wang, Y.; Lin, K.; Zhu, Y. Palladium Nanocrystals-Engineered Metal-Organic Frameworks for Enhanced Tumor Inhibition by Synergistic Hydrogen/Photodynamic Therapy. Adv. Funct. Mater. 2020, 31, 2006853. [Google Scholar] [CrossRef]
- Tan, L.; Huang, R.; Li, X.; Liu, S.; Shen, Y.M. Controllable release of nitric oxide and doxorubicin from engineered nanospheres for synergistic tumor therapy. Acta Biomater. 2017, 57, 498–510. [Google Scholar] [CrossRef]
- Shen, L.J.; Zhou, T.J.; Fan, Y.T.; Chang, X.; Wang, Y.; Sun, J.G.; Xing, L.; Jiang, H.L. Recent progress in tumor photodynamic immunotherapy. Chin. Chem. Lett. 2020, 31, 1709–1716. [Google Scholar] [CrossRef]
- Li, S.Y.; ChEng, H.; Qiu, W.X.; Zhang, L.; Wan, S.S.; ZEng, J.Y.; Zhang, X.Z. Cancer cell membrane-coated biomimetic platform for tumor targeted photodynamic therapy and hypoxia-amplified bioreductive therapy. Biomaterials 2017, 142, 149–161. [Google Scholar] [CrossRef]
- Fang, R.H.; Hu, C.M.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef]
- Li, S.Y.; ChEng, H.; Xie, B.R.; Qiu, W.X.; ZEng, J.Y.; Li, C.X.; Wan, S.S.; Zhang, L.; Liu, W.L.; Zhang, X.Z. Cancer Cell Membrane Camouflaged Cascade Bioreactor for Cancer Targeted Starvation and Photodynamic Therapy. ACS Nano 2017, 11, 7006–7018. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Ding, Y.; Xue, X.L.; Zhou, S.S.; Li, C.; Zhang, X.K.; Jiang, X.Q. Entrapping multifunctional dendritic nanoparticles into a hydrogel for local therapeutic delivery and synergetic immunochemotherapy. Nano Res. 2018, 11, 6062–6073. [Google Scholar] [CrossRef]
- Wang, T.; Wang, D.; Liu, J.; FEng, B.; Zhou, F.; Zhang, H.; Zhou, L.; Yin, Q.; Zhang, Z.; Cao, Z.; et al. Acidity-Triggered Ligand-Presenting Nanoparticles To Overcome Sequential Drug Delivery Barriers to Tumors. Nano Lett. 2017, 17, 5429–5436. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Yuan, H.; Yang, Z.; von Roemeling, C.A.; Qie, Y.; Zhao, H.; Wang, Y.; Jiang, W.; Kim, B.Y.S. Therapeutic Remodeling of the Tumor Microenvironment Enhances Nanoparticle Delivery. Adv. Sci. 2019, 6, 1802070. [Google Scholar] [CrossRef] [Green Version]
- Zinger, A.; Koren, L.; Adir, O.; Poley, M.; Alyan, M.; Yaari, Z.; Noor, N.; Krinsky, N.; Simon, A.; Gibori, H.; et al. Collagenase Nanoparticles Enhance the Penetration of Drugs into Pancreatic Tumors. ACS Nano 2019, 13, 11008–11021. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Jiang, J.; Zhou, Z.; WEng, Z.; Xu, Y.; Liu, L.; Zhang, W.; Yang, Y.; Luo, J.; Wang, X. Near-Infrared Light and Upconversion Nanoparticle Defined Nitric Oxide-Based Osteoporosis Targeting Therapy. ACS Nano 2021, 15, 13692–13702. [Google Scholar] [CrossRef]
- Zaccagna, F.; Anzidei, M.; Sandolo, F.; Marincola, B.C.; Palla, C.; Leonardi, A.; Caliolo, G.; Andreani, F.; De Soccio, V.; Catalano, C.; et al. MRgFUS for liver and pancreas cancer treatments: The Umberto I hospital experience. Transl. Cancer Res. 2014, 3, 430–441. [Google Scholar] [CrossRef]
- Min, H.S.; You, D.G.; Son, S.; Jeon, S.; Park, J.H.; Lee, S.; Kwon, I.C.; Kim, K. Echogenic Glycol Chitosan Nanoparticles for Ultrasound-Triggered Cancer Theranostics. Theranostics 2015, 5, 1402–1418. [Google Scholar] [CrossRef]
- Min, H.S.; Son, S.; You, D.G.; Lee, T.W.; Lee, J.; Lee, S.; Yhee, J.Y.; Lee, J.; Han, M.H.; Park, J.H.; et al. Chemical gas-generating nanoparticles for tumor-targeted ultrasound imaging and ultrasound-triggered drug delivery. Biomaterials 2016, 108, 57–70. [Google Scholar] [CrossRef]
- Liang, S.; DEng, X.; Ma, P.; Cheng, Z.; Lin, J. Recent Advances in Nanomaterial-Assisted Combinational Sonodynamic Cancer Therapy. Adv. Mater. 2020, 32, e2003214. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.J.; Guo, M.; An, L.; Yang, S.P.; Zhang, Q.L.; Liu, J.G. A multifunctional nanoplatform for lysosome targeted delivery of nitric oxide and photothermal therapy under 808 nm near-infrared light. J. Mater. Chem. B 2016, 4, 4667–4674. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhang, X.; Guo, Z.; Xie, J.; Dong, X.; Zhu, S.; Du, J.; Gu, Z.; Zhao, Y. X-Ray-Controlled Generation of Peroxynitrite Based on Nanosized LiLuF4: Ce3+ Scintillators and their Applications for Radiosensitization. Adv. Mater. 2018, 30, e1804046. [Google Scholar] [CrossRef]
- Nosrati, H.; Charmi, J.; Salehiabar, M.; Abhari, F.; Danafar, H. Tumor Targeted Albumin Coated Bismuth Sulfide Nanoparticles (Bi2S3) as Radiosensitizers and Carriers of Curcumin for Enhanced Chemoradiation Therapy. ACS Biomater. Sci. Eng. 2019, 5, 4416–4424. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Bednarski, M.; Oronsky, B.; Scicinski, J.; Knox, S.J. Novel nitric oxide generating compound glycidyl nitrate enhances the therapeutic efficacy of chemotherapy and radiotherapy. Biochem. Biophys. Res. Commun. 2014, 447, 537–542. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, W.; Wang, R.; Hopkins, S.P.; Spagnoli, J.C.; Racin, M.; Bai, L.; Li, L.; Jiang, W.; Yang, X.; et al. Nanoparticles Encapsulating Nitrosylated Maytansine to Enhance Radiation Therapy. ACS Nano 2020, 14, 1468–1481. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Liu, Y.; Zhao, F.; Guo, Y.; Li, X.; Wu, M.; Chang, J.; Yu, C. Radiation-responsive scintillating nanotheranostics for reduced hypoxic radioresistance under ROS/NO-mediated tumor microenvironment regulation. Theranostics 2018, 8, 5870–5889. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Tu, K.; Xu, H.; Wang, L.; Yuan, X.; Qin, X.; Kong, L.; Chu, Q.; Zhang, Z. Improving tumor hypoxia and radiotherapy resistance via in situ nitric oxide release strategy. Eur. J. Pharm. BioPharm. 2020, 150, 96–107. [Google Scholar] [CrossRef]
- Wilson, A.; Menon, V.; Khan, Z.; Alam, A.; Litovchick, L.; Yakovlev, V. Nitric oxide-donor/PARP-inhibitor combination: A new approach for sensitization to ionizing radiation. Redox Biol. 2019, 24, 101169. [Google Scholar] [CrossRef]
- Fu, J.; Liu, L.; Huang, Z.; Lai, Y.; Ji, H.; PEng, S.; Tian, J.; Zhang, Y. Hybrid molecule from O2-(2,4-dinitrophenyl)diazeniumdiolate and oleanolic acid: A glutathione S-transferase π-activated nitric oxide prodrug with selective anti-human hepatocellular carcinoma activity and improved stability. J. Med. Chem. 2013, 56, 4641–4655. [Google Scholar] [CrossRef]
- Sun, Z.; Yi, Z.; Cui, X.; Chen, X.; Su, W.; Ren, X.; Li, X. Tumor-targeted and nitric oxide-generated nanogels of keratin and hyaluronan for enhanced cancer therapy. Nanoscale 2018, 10, 12109–12122. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, Y.; Zou, Y.; Wang, Y.; Niu, D.; He, Q.; Huang, Z.; Zhu, W.; Tian, H.; Shi, J.; et al. Dual Intratumoral Redox/Enzyme-Responsive NO-Releasing Nanomedicine for the Specific, High-Efficacy, and Low-Toxic Cancer Therapy. Adv. Mater. 2018, 30, e1704490. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, Y.; Yang, X.; Tian, C.; Yan, Y.; Zhang, H.; Shi, J.; Zhang, H.; Zhang, Z. Intracellular NO-Generator Based on Enzyme Trigger for Localized Tumor-Cytoplasm Rapid Drug Release and Synergetic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 255–268. [Google Scholar] [CrossRef]
- Sun, B.; DEng, C.; MEng, F.; Zhang, J.; Zhong, Z. Robust, active tumor-targeting and fast bioresponsive anticancer nanotherapeutics based on natural endogenous materials. Acta Biomater. 2016, 45, 223–233. [Google Scholar] [CrossRef]
- Feng, X.; Xu, W.; Li, Z.; Song, W.; Ding, J.; Chen, X. Immunomodulatory Nanosystems. Adv. Sci. 2019, 6, 1900101. [Google Scholar] [CrossRef]
- Hu, Y.; Lv, T.; Ma, Y.; Xu, J.; Zhang, Y.; Hou, Y.; Huang, Z.; Ding, Y. Nanoscale Coordination Polymers for Synergistic NO and Chemodynamic Therapy of Liver Cancer. Nano Lett. 2019, 19, 2731–2738. [Google Scholar] [CrossRef]
- Li, K.; Lin, C.; He, Y.; Lu, L.; Xu, K.; Tao, B.; Xia, Z.; ZEng, R.; Mao, Y.; Luo, Z.; et al. Engineering of Cascade-Responsive Nanoplatform to Inhibit Lactate Efflux for Enhanced Tumor Chemo-Immunotherapy. ACS Nano 2020, 14, 14164–14180. [Google Scholar] [CrossRef]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial pH and pO2 gradients in solid tumors in vivo: High-resolution measurements reveal a lack of correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, M.; ChEng, J.; Yin, J.; Huang, C.; Cui, H.; Zhang, X.; Zhao, G. Acidity-Triggered Tumor-Targeted Nanosystem for Synergistic Therapy via a Cascade of ROS Generation and NO Release. ACS Appl. Mater. Interfaces 2020, 12, 28975–28984. [Google Scholar] [CrossRef]
- Li, Y.; Lin, J.; Zhi, X.; Li, P.; Jiang, X.; Yuan, J. Triple stimuli-responsive keratin nanoparticles as carriers for drug and potential nitric oxide release. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bi, Y.; Ruan, H.; Sun, G.; Cui, X.; Yang, X.; Qin, C. Hollow S-nitrosothiols nanoparticle with polymeric brushes for nitric oxide (NO)-releasing as tumor targeted chemotherapy. J. Biomater. Sci. Polym Ed. 2019, 30, 122–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, Y.; Ding, X.; Shen, W.; Li, M.; Wagner, E.; Xiao, C.; Chen, X. A Multistage Cooperative Nanoplatform Enables Intracellular Co-Delivery of Proteins and Chemotherapeutics for Cancer Therapy. Adv. Mater. 2020, 32, e2000013. [Google Scholar] [CrossRef] [PubMed]
- Ling, P.; Qian, C.; Gao, F.; Lei, J. Enzyme-immobilized metal-organic framework nanosheets as tandem catalysts for the generation of nitric oxide. Chem. Commun. 2018, 54, 11176–11179. [Google Scholar] [CrossRef]
- Yang, F.; Li, M.; Liu, Y.; Wang, T.; Feng, Z.; Cui, H.; Gu, N. Glucose and magnetic-responsive approach toward in situ nitric oxide bubbles controlled generation for hyperglycemia theranostics. J. Control. Release 2016, 228, 87–95. [Google Scholar] [CrossRef] [PubMed]

| Trigger for NO Release | Nanomedicine Formulation | NO Donor | NO Release Mechanism | Theranostic Method | Reference |
|---|---|---|---|---|---|
| UV–Vis | mPEG–PLGA–BNN6–DOX | BNN6 | The breaking of bonds | NO therapy–chemotherapy | [68] |
| Cdot–TPP–SNO | R-SNO | The breaking of bonds | NO therapy | [69] | |
| Ru-NO@TiO2 | Ru-NO | The breaking of bonds | NO therapy–PDT | [70] | |
| NIR | PTNG | R-SNO | The breaking of bonds | NO therapy–PTT–chemotherapy | [71] |
| PpRE@PEG–PpIX | PpRE | The breaking of bonds | NO therapy–PTT | [72] | |
| Fe3O4@PDA@Ru-NO@FA | Ru-NO | The breaking of bonds | NO therapy–PTT–MRI | [73] | |
| BNN–Bi2S3 | BNN6 | The breaking of bonds | NO therapy–mild PTT | [74] | |
| PNOC–PDA | R-SNO | The breaking of bonds | NO therapy–mild PTT–chemotherapy | [75] | |
| PEG–PAu@SiO2–SNO | R-SNO | The breaking of bonds | NO therapy–mild PTT | [76] | |
| PFTDPP–SNAP | SNAP | The breaking of bonds | NO therapy–PTT– NIR II/PA imaging | [77] | |
| Nb2C–MSNs–SNO | R-SNO | The breaking of bonds | NO therapy–PTT– PA imaging | [33] | |
| P(IR/BNN6/AIPH)@Lip–RGD | BNN6 | The breaking of bonds | NO therapy–alkyl radicals–PTT | [78] | |
| RBCm/PAAV–SNO | R-SNO | The breaking of bonds | NO therapy–PTT–reprogramming tumor immunosuppressive microenvironment | [79] | |
| PDA–PLC/DOX | R-SNO | The breaking of bonds | NO therapy–PTT–chemotherapy | [80] | |
| Me–RBSs | RBS | The breaking of bonds | NO therapy | [81] | |
| GO–BNN6 | BNN6 | The breaking of bonds | NO therapy | [82] | |
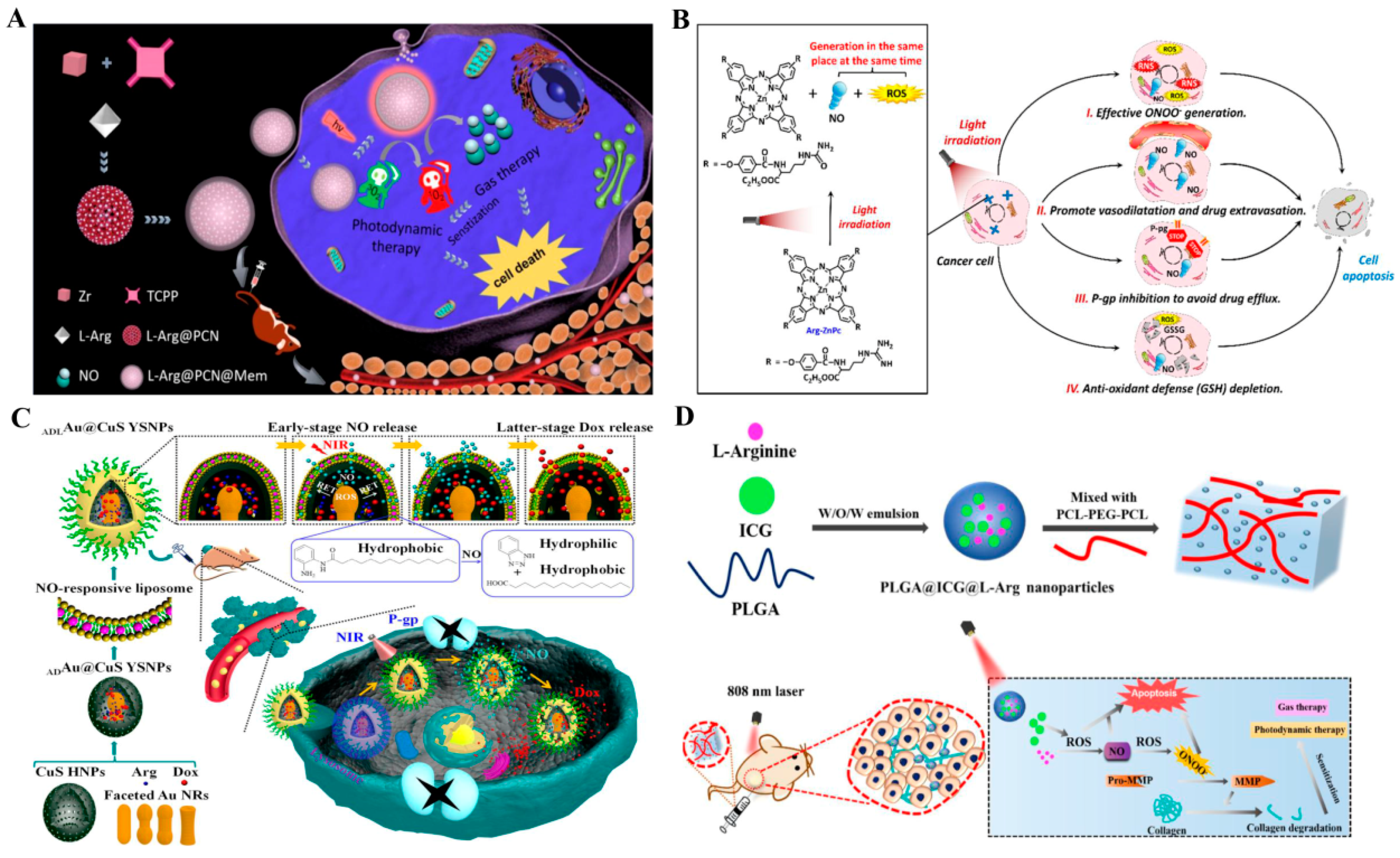
| L-Arg@PCN@Mem | L-Arg | Oxidation–reduction reaction | NO therapy–PDT | [83] | |
| Arg–ZnPc | L-Arg | Oxidation–reduction reaction | NO therapy–PDT | [84] | |
| ADLAu@CuS YSNPs | L-Arg | Oxidation–reduction reaction | NO therapy–PDT–chemotherapy | [85] | |
| PLGA@ICG@L-Arg | L-Arg | Oxidation–reduction reaction | NO therapy–PDT | [86] | |
| Lyso–Ru-NO@FA@C–TiO2 | Ru-NO | The breaking of bonds | NO therapy–PDT | [87] | |
| US | BNN6–SPION@hMSN | BNN6 | The breaking of bonds | NO therapy–MRI | [88] |
| TPZ/HMTNPs–SNO | R-SNO | The breaking of bonds | NO therapy–SDT–USI | [89] | |
| IMesNO/DOX@MCs | IMesNO | The breaking of bonds | NO therapy–chemotherapy | [90] | |
| GSNO/Ce6@ZIF–8@Cytomembrane (GCZ@M) | GSNO | Oxidation–reduction reaction | NO therapy–SDT | [91] | |
| SNO–HSA–PTX | R-SNO | The breaking of bonds | NO therapy–chemotherapy–immunotherapy | [92] | |
| peptide−HMSN−LA | L-Arg | Oxidation–reduction reaction | NO therapy–SDT | [93] | |
| X-ray | PEG–USMSs–SNO | R-SNO | The breaking of bonds | NO therapy–radiotherapy | [94] |
| Bi–SNO | R-SNO | The breaking of bonds | NO therapy–radiotherapy–CT imaging–PTT | [95] | |
| ZGO:Mn–RBS | RBS | The breaking of bonds | NO therapy–radiotherapy | [96] | |
| GSH | p(Gd–Az–JSK) | alkynyl-JSK | The nucleophilic attacking | NO therapy–chemotherapy | [97] |
| PEG–b–NO-Dex–DOX | NO-Dex | Oxidation–reduction reaction | NO therapy–chemotherapy | [98] | |
| HCPT/CTS–NO–DMMA | PSF | Oxidation–reduction reaction | NO therapy | [99] | |
| α–CD–DOX–NO–DA | R-SNO | / | NO therapy–chemotherapy | [100] | |
| TNO3–DOX | TNO3 | Oxidation–reduction reaction | NO therapy–chemotherapy | [101] | |
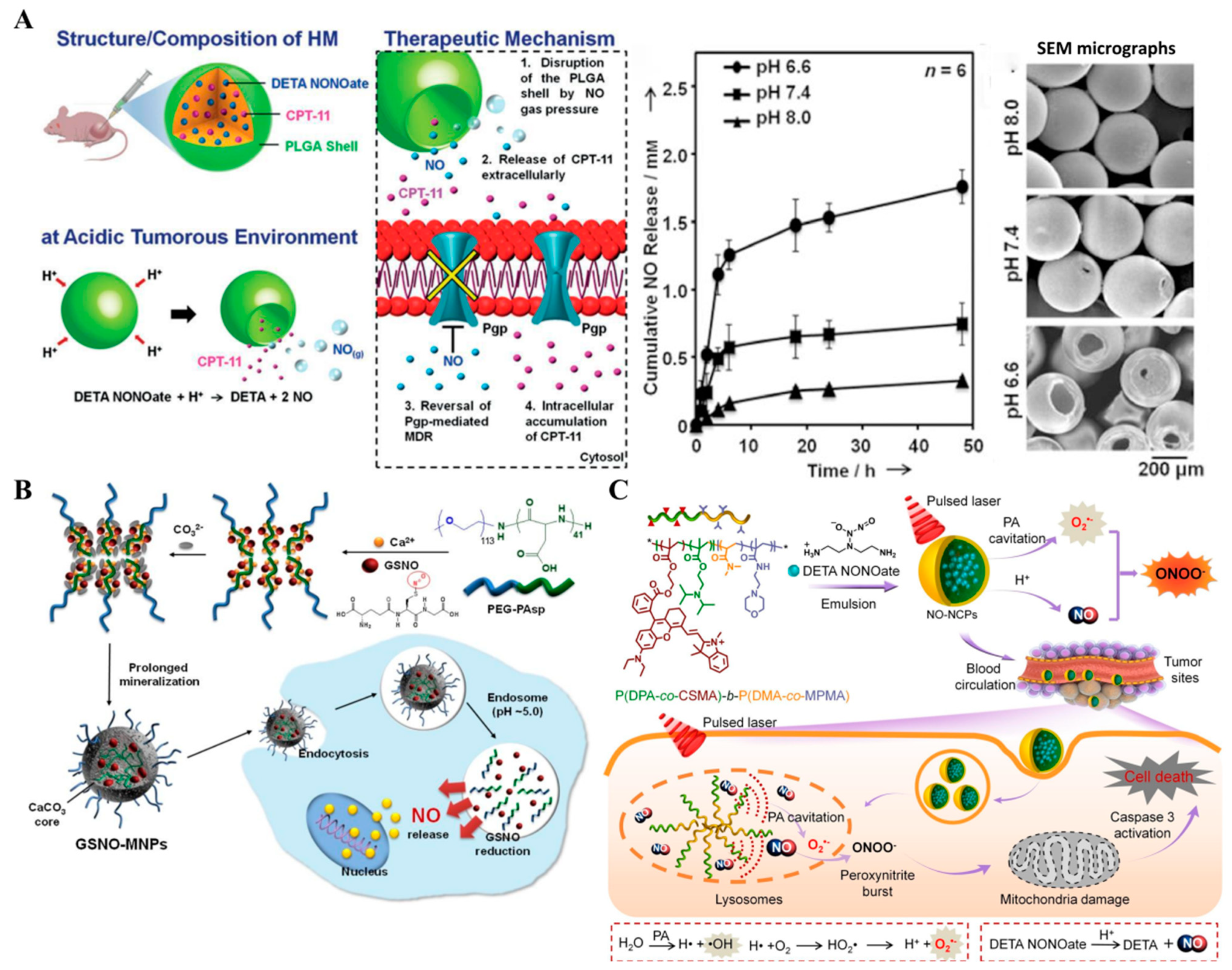
| pH | hollow microsphere (HM) | DETA NONOate | Hydrolysis reaction | NO therapy–chemotherapy | [49] |
| GSNO-MNPs | GSNO | Hydrolysis reaction–oxidation–reduction reaction | NO therapy–chemotherapy | [102] | |
| NO–NCPs | DETA NONOate | Hydrolysis reaction | NO therapy–PDT–PA imaging | [103] | |
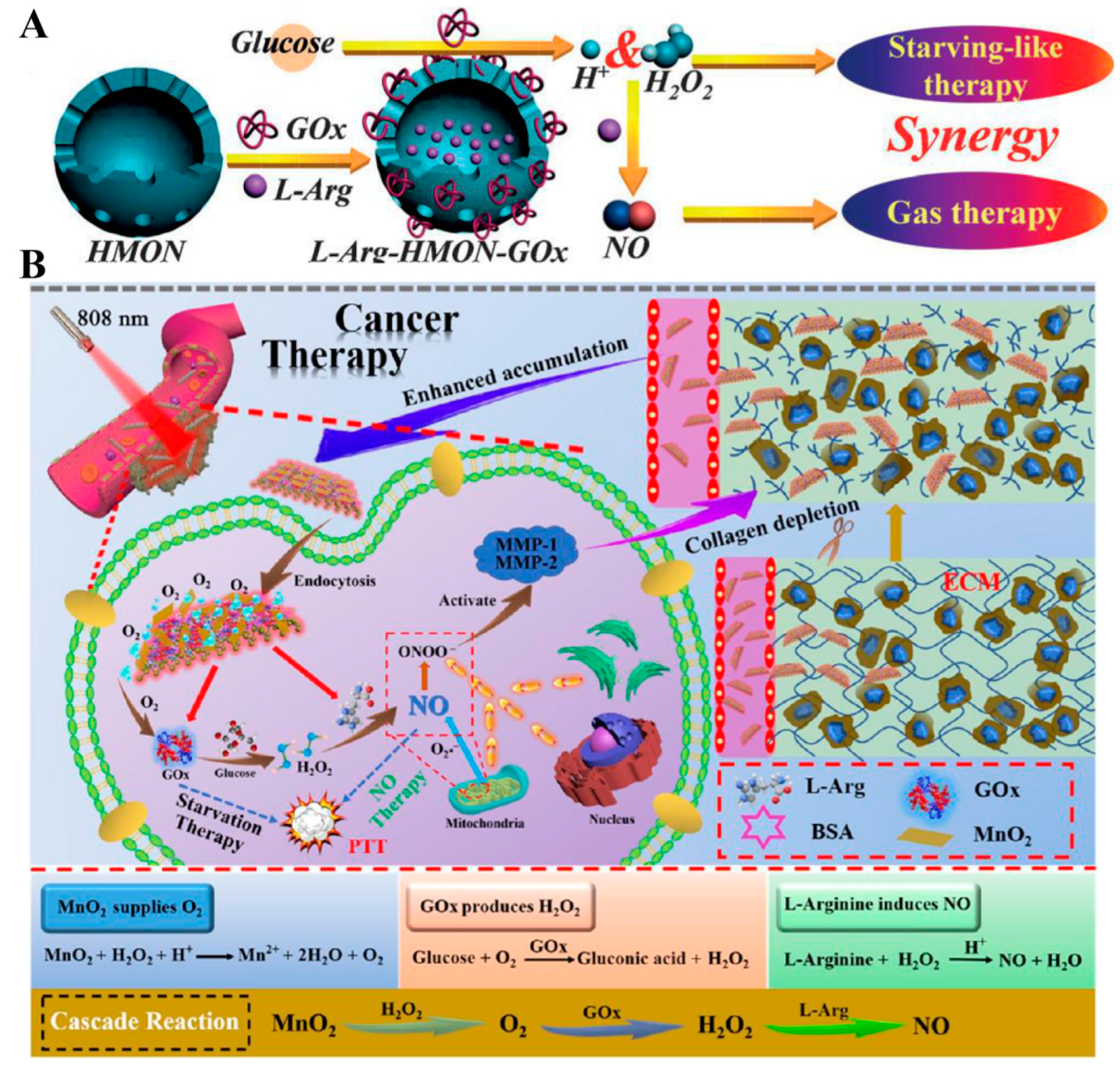
| Glucose | L-Arg–HMON–GOx | L-Arg | Oxidation–reduction reaction | NO therapy–starving therapy–USI | [104] |
| BPNs–Arg–GOx@MnO2(BAGM) | L-Arg | Oxidation–reduction reaction | NO therapy–starving therapy–PTT | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Ouyang, X.; Peng, Y.; Peng, S. Stimuli Responsive Nitric Oxide-Based Nanomedicine for Synergistic Therapy. Pharmaceutics 2021, 13, 1917. https://doi.org/10.3390/pharmaceutics13111917
Zhao Y, Ouyang X, Peng Y, Peng S. Stimuli Responsive Nitric Oxide-Based Nanomedicine for Synergistic Therapy. Pharmaceutics. 2021; 13(11):1917. https://doi.org/10.3390/pharmaceutics13111917
Chicago/Turabian StyleZhao, Yijun, Xumei Ouyang, Yongjun Peng, and Shaojun Peng. 2021. "Stimuli Responsive Nitric Oxide-Based Nanomedicine for Synergistic Therapy" Pharmaceutics 13, no. 11: 1917. https://doi.org/10.3390/pharmaceutics13111917
APA StyleZhao, Y., Ouyang, X., Peng, Y., & Peng, S. (2021). Stimuli Responsive Nitric Oxide-Based Nanomedicine for Synergistic Therapy. Pharmaceutics, 13(11), 1917. https://doi.org/10.3390/pharmaceutics13111917







