Pharmaceutical Aspects of Nanocarriers for Smart Anticancer Therapy
Abstract
1. Introduction
2. Why Is a “Smart” Nanocarrier Needed for the Treatment of Cancer?
3. Organic Nanocarriers for Anticancer Therapy
3.1. Polymer-Based Nanocarriers
3.1.1. Polymeric Nanoparticles
3.1.2. Micelles
3.1.3. Dendrimers
3.2. Lipid-Based Nanoformulations
3.2.1. Liposomes
3.2.2. Solid Lipid Nanoparticles
3.3. Virus-Based Nanoparticles
4. Inorganic Nanocarriers and Hybrid Nanoplatforms for Anticancer Therapy
- Inorganic nanoparticles derived from metals, such as gold, silver, iridium, and platinum, which show phenomenal resistance towards oxidation;
- Magnetic nanoparticles (MNPs) are mainly derived from 3d and 4f metals, such as Fe3O4 and Gd2O3;
Superparamagnetic Iron Oxide Nanoparticles (SPIONs)
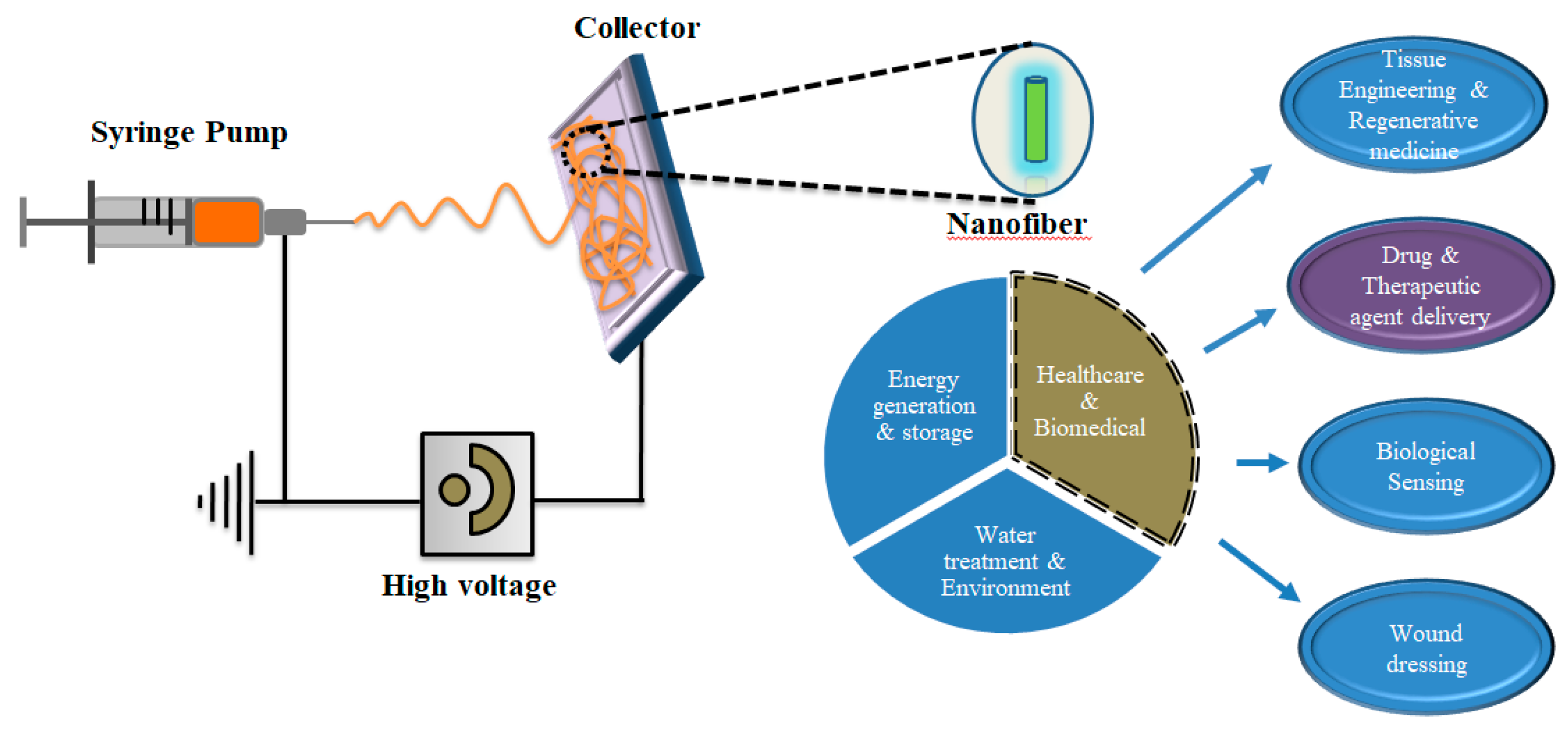
5. Electrospinning for Production of Nanofibers in Bulk
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, J.; Yang, M.; Duan, Y. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: New insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef] [PubMed]
- Awade, M.K.L. Emerging trends of nanotechnology in biomedical engineering. Int. J. Electron. Comm. Eng. Technol. 2010, 1, 25–32. [Google Scholar]
- Khalid, K.; Tan, X.; Mohd Zaid, H.F.; Tao, Y.; Lye Chew, C.; Chu, D.T.; Lam, M.K.; Ho, Y.C.; Lim, J.W.; Chin Wei, L. Advanced in developmental organic and inorganic nanomaterial: A review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef]
- Xin, Y.; Yin, M.; Zhao, L.; Meng, F.; Luo, L. Recent progress on nanoparticle-based drug delivery systems for cancer therapy. Cancer Biol. Med. 2017, 14, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Greish, K. Enhanced permeability and retention of macromolecular drugs in solid tumors: A royal gate for targeted anticancer nanomedicines. J. Drug Target 2007, 15, 457–464. [Google Scholar] [PubMed]
- Nevozhay, D.; Kanska, U.; Budzynska, R.; Boratynski, J. Current status of research on conjugates and related drug delivery systems in the treatment of cancer and other diseases. Postepy Hig. Med. Dosw. 2007, 61, 350–360. [Google Scholar]
- Huang, H.; Feng, W.; Chen, Y.; Shi, J. Inorganic nanoparticles in clinical trials and translations. Nano Today 2020, 35, 100972. [Google Scholar]
- Alphandery, E. Iron oxide nanoparticles for therapeutic applications. Drug Discov. Today 2020, 25, 141–149. [Google Scholar] [CrossRef]
- Tzogani, K.; Penttila, K.; Lapvetelainen, T.; Hemmings, R.; Koenig, J.; Freire, J.; Marcia, S.; Cole, S.; Coppola, P.; Flores, B.; et al. EMA Review of Daunorubicin and Cytarabine Encapsulated in Liposomes (Vyxeos, CPX-351) for the Treatment of Adults with Newly Diagnosed, Therapy-Related Acute Myeloid Leukemia or Acute Myeloid Leukemia with Myelodysplasia-Related Changes. Oncologist 2020, 25, e1414–e1420. [Google Scholar]
- Rosenthal, E.; Poizot-Martin, I.; Saint-Marc, T.; Spano, J.P.; Cacoub, P.; Group, D.N.X.S. Phase IV study of liposomal daunorubicin (DaunoXome) in AIDS-related Kaposi sarcoma. Am. J. Clin. Oncol 2002, 25, 57–59. [Google Scholar]
- Swenson, C.; Perkins, W.; Roberts, P.; Janoff, A. Liposome technology and the development of Myocet™(liposomal doxorubicin citrate). Breast 2001, 10, 1–7. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Hatoum, H.; Dy, G.K. First line treatment of advanced non-small-cell lung cancer-specific focus on albumin bound paclitaxel. Int. J. Nanomedicine 2014, 9, 209–221. [Google Scholar] [PubMed]
- Silverman, J.A.; Deitcher, S.R. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother. Pharmacol. 2013, 71, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.E.; Levien, T.L. Irinotecan Liposome Injection. Hosp. Pharm. 2017, 52, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kager, L.; Potschger, U.; Bielack, S. Review of mifamurtide in the treatment of patients with osteosarcoma. Ther. Clin. Risk Manag. 2010, 6, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A.C.; Szwed, E.; Spears, C.D.; Thaper, S.; Dang, L.H.; Dang, N.H. Denileukin diftitox (Ontak) as maintenance therapy for peripheral T-cell lymphomas: Three cases with sustained remission. Case Rep. Oncol. Med. 2015, 2015, 123756. [Google Scholar] [CrossRef] [PubMed]
- Enayati, M.; Mobedi, H.; Hojjati-Emami, S.; Mirzadeh, H.; Jafari-Nodoushan, M. In situ forming PLGA implant for 90 days controlled release of leuprolide acetate for treatment of prostate cancer. Polym Adv. Technol 2017, 28, 867–875. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control Release 2014, 190, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Stirland, D.L.; Nichols, J.W.; Miura, S.; Bae, Y.H. Mind the gap: A survey of how cancer drug carriers are susceptible to the gap between research and practice. J. Control Release 2013, 172, 1045–1064. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of nanoparticles. Nat. Biotechnol. 2007, 25, 1165. [Google Scholar] [PubMed]
- Oroojalian, F.; Charbgoo, F.; Hashemi, M.; Amani, A.; Yazdian-Robati, R.; Mokhtarzadeh, A.; Ramezani, M.; Hamblin, M.R. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control Release 2020, 321, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, D.; Goepferich, A. General Sites of Nanoparticle Biodistribution As a Novel Opportunity for Nanomedicine. Eur. J. Pharm. Biopharm. 2021, 166, 44–60. [Google Scholar] [CrossRef]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.G.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.X.; Yu, Q. Intra-tumor heterogeneity of cancer cells and its implications for cancer treatment. Acta Pharmacol. Sin. 2015, 36, 1219–1227. [Google Scholar] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Deliv.ering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Alfarouk, K.O.; Muddathir, A.K.; Shayoub, M.E. Tumor acidity as evolutionary spite. Cancers 2011, 3, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Lau, A.N.; Vander Heiden, M.G. Metabolism in the tumor microenvironment. Annu. Rev. Cancer Biol. 2020, 4, 17–40. [Google Scholar] [CrossRef]
- DVORAK, H. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1657. [Google Scholar]
- Yin, Q.; Shen, J.; Zhang, Z.; Yu, H.; Li, Y. Reversal of multidrug resistance by stimuli-responsive drug delivery systems for therapy of tumor. Adv. Drug Deliv. Rev. 2013, 65, 1699–1715. [Google Scholar] [PubMed]
- Damia, G.; Garattini, S. The pharmacological point of view of resistance to therapy in tumors. Cancer Treat. Rev. 2014, 40, 909–916. [Google Scholar] [CrossRef]
- Singh, Y.; Palombo, M.; Sinko, P.J. Recent trends in targeted anticancer prodrug and conjugate design. Curr. Med. Chem. 2008, 15, 1802–1826. [Google Scholar]
- Liu, J.P.; Wang, T.T.; Wang, D.G.; Dong, A.J.; Li, Y.P.; Yu, H.J. Smart nanoparticles improve therapy for drug-resistant tumors by overcoming pathophysiological barriers. Acta Pharmacol Sin. 2017, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of Long-Circulating Zwitterionic Cross-Linked Micelles for Active-Targeted Drug Deliv.ery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef]
- Barve, A.; Jain, A.; Liu, H.; Zhao, Z.; Cheng, K. Enzyme-responsive polymeric micelles of cabazitaxel for prostate cancer targeted therapy. Acta Biomater. 2020, 113, 501–511. [Google Scholar] [PubMed]
- Curcio, M.; Mauro, L.; Naimo, G.D.; Amantea, D.; Cirillo, G.; Tavano, L.; Casaburi, I.; Nicoletta, F.P.; Alvarez-Lorenzo, C.; Iemma, F. Facile synthesis of pH-responsive polymersomes based on lipidized PEG for intracellular co-delivery of curcumin and methotrexate. Colloids Surf. B Biointerfaces 2018, 167, 568–576. [Google Scholar] [CrossRef]
- Jiang, K.; Chi, T.; Li, T.; Zheng, G.; Fan, L.; Liu, Y.; Chen, X.; Chen, S.; Jia, L.; Shao, J. A smart pH-responsive nano-carrier as a drug delivery system for the targeted delivery of ursolic acid: Suppresses cancer growth and metastasis by modulating P53/MMP-9/PTEN/CD44 mediated multiple signaling pathways. Nanoscale 2017, 9, 9428–9439. [Google Scholar] [CrossRef]
- Obiweluozor, F.O.; Emechebe, G.A.; Tiwari, A.P.; Kim, J.Y.; Park, C.H.; Kim, C.S. Short duration cancer treatment: Inspired by a fast bio-resorbable smart nano-fiber device containing NIR lethal polydopamine nanospheres for effective chemo-photothermal cancer therapy. Int. J. Nanomed. 2018, 13, 6375–6390. [Google Scholar] [CrossRef]
- Martinez-Carmona, M.; Lozano, D.; Colilla, M.; Vallet-Regi, M. Lectin-conjugated pH-responsive mesoporous silica nanoparticles for targeted bone cancer treatment. Acta Biomater. 2018, 65, 393–404. [Google Scholar] [PubMed]
- Wang, Q.; Lei, D.; Chen, F.; Chen, Y.; Luo, X. Tracing Difference: In Vitro and in Vivo Antitumor Property Comparison of pH-Sensitive Biomimetic Phosphorylcholine Micelles with Insensitive Micelles. ACS Biomater. Sci. Eng. 2019, 5, 2258–2270. [Google Scholar] [CrossRef]
- Emami, J.; Kazemi, M.; Hasanzadeh, F.; Minaiyan, M.; Mirian, M.; Lavasanifar, A. Novel pH-triggered biocompatible polymeric micelles based on heparin-alpha-tocopherol conjugate for intracellular delivery of docetaxel in breast cancer. Pharm. Dev. Technol. 2020, 25, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-W.; Yeh, C.-W.; Kuan, C.-H.; Wang, L.-W.; Chen, L.-H.; Wu, H.-C.; Sun, J.-S. Tailored design of multifunctional and programmable pH-responsive self-assembling polypeptides as drug delivery nanocarrier for cancer therapy. Acta Biomater. 2017, 58, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Xu, Z.; Huang, H.; Wang, Y.; Zhao, J.; Guo, X.; Zhou, S. A NIR light triggered disintegratable nanoplatform for enhanced penetration and chemotherapy in deep tumor tissues. Biomaterials 2020, 245, 119840. [Google Scholar] [CrossRef]
- Yuan, Z.; Qu, S.; He, Y.; Xu, Y.; Liang, L.; Zhou, X.; Gui, L.; Gu, Y.; Chen, H. Thermosensitive drug-loading system based on copper sulfide nanoparticles for combined photothermal therapy and chemotherapy in vivo. Biomater. Sci. 2018, 6, 3219–3230. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.K.; Soe, Z.C.; Ruttala, H.B.; Gupta, B.; Ramasamy, T.; Thapa, R.K.; Gautam, M.; Ou, W.; Nguyen, H.T.; Jeong, J.-H. In situ fabrication of mesoporous silica-coated silver-gold hollow nanoshell for remotely controllable chemo-photothermal therapy via phase-change molecule as gatekeepers. Int. J. Pharm. 2018, 548, 92–103. [Google Scholar] [CrossRef]
- Wang, X.; Xuan, Z.; Zhu, X.; Sun, H.; Li, J.; Xie, Z. Near-infrared photoresponsive drug delivery nanosystems for cancer photo-chemotherapy. J. Nanobiotechnol. 2020, 18, 108. [Google Scholar] [CrossRef]
- Yan, X.; Li, S.; Qu, Y.; Wang, W.; Chen, B.; Liu, S.; Ma, X.; Yu, X. Redox-responsive multifunctional polypeptides conjugated with au nanoparticles for tumor-targeting gene therapy and their 1+ 1> 2 synergistic effects. ACS Biomater. Sci. Eng. 2019, 6, 463–473. [Google Scholar] [CrossRef]
- Wang, Z.; Ling, L.; Du, Y.; Yao, C.; Li, X. Reduction responsive liposomes based on paclitaxel-ss-lysophospholipid with high drug loading for intracellular delivery. Int. J. Pharm. 2019, 564, 244–255. [Google Scholar] [PubMed]
- Ling, L.; Ismail, M.; Du, Y.; Yao, C.; Li, X. Lipoic acid-derived cross-linked liposomes for reduction-responsive delivery of anticancer drug. Int. J. Pharm 2019, 560, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, X.; Zhao, Y.; Zhi, D.; Cui, S.; Zhang, E.; Lan, H.; Du, J.; Zhang, Z.; Zhang, S. Stimuli-responsive polysaccharide enveloped liposome for targeting and penetrating delivery of survivin-shRNA into breast tumor. ACS Appl. Mater. Interfaces 2020, 12, 22074–22087. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.; Dong, L.; Zhang, P.; Mu, Y.; Cui, X.; Zhou, J.; Huo, M.; Yin, T. Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int. J. Nanomed. 2019, 14, 4649–4666. [Google Scholar] [CrossRef] [PubMed]
- Behroozi, F.; Abdkhodaie, M.-J.; Abandansari, H.S.; Satarian, L.; Molazem, M.; Al-Jamal, K.T.; Baharvand, H. Engineering folate-targeting diselenide-containing triblock copolymer as a redox-responsive shell-sheddable micelle for antitumor therapy in vivo. Acta Biomater. 2018, 76, 239–256. [Google Scholar] [CrossRef]
- Li, M.; Guo, J.W.; Wen, W.Q.; Chen, J.K. Biodegradable Redox-Sensitive Star Polymer Nanomicelles for Enhancing Doxorubicin Delivery. Nanomaterials 2019, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Han, J.; Zhao, F.; Pan, X.; Tian, B.; Ding, X.; Zhang, J. Redox-sensitive micelles based on retinoic acid modified chitosan conjugate for intracellular drug delivery and smart drug release in cancer therapy. Carbohydr. Polym. 2019, 215, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, S.; Wang, Y.; Liu, X.; Hu, F.; Sun, J.; Yuan, H. Lipase-triggered water-responsive “Pandora’s Box” for cancer therapy: Toward induced neighboring effect and enhanced drug penetration. Adv. Mater. 2018, 30, 1706407. [Google Scholar] [CrossRef]
- Yu, X.; Gou, X.; Wu, P.; Han, L.; Tian, D.; Du, F.; Chen, Z.; Liu, F.; Deng, G.; Chen, A.T.; et al. Activatable Protein Nanoparticles for Targeted Deliv.ery of Therapeutic Peptides. Adv. Mater. 2018, 30, 1705383. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Mackiewicz, M.; Yazdimamaghani, M. Recent advances in the redox-responsive drug delivery nanoplatforms: A chemical structure and physical property perspective. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111536. [Google Scholar]
- He, W.; Du, Y.; Zhou, W.; Yao, C.; Li, X. Redox-sensitive dimeric camptothecin phosphatidylcholines-based liposomes for improved anticancer efficacy. Nanomedicine 2019, 14, 3057–3074. [Google Scholar] [CrossRef] [PubMed]
- Qi, A.; Deng, L.; Liu, X.; Wang, S.; Zhang, X.; Wang, B.; Li, L. Gelatin-Encapsulated Magnetic Nanoparticles for pH, Redox, and Enzyme Multiple Stimuli-Responsive Drug Deliv.ery and Magnetic Resonance Imaging. J. Biomed. Nanotechnol. 2017, 13, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
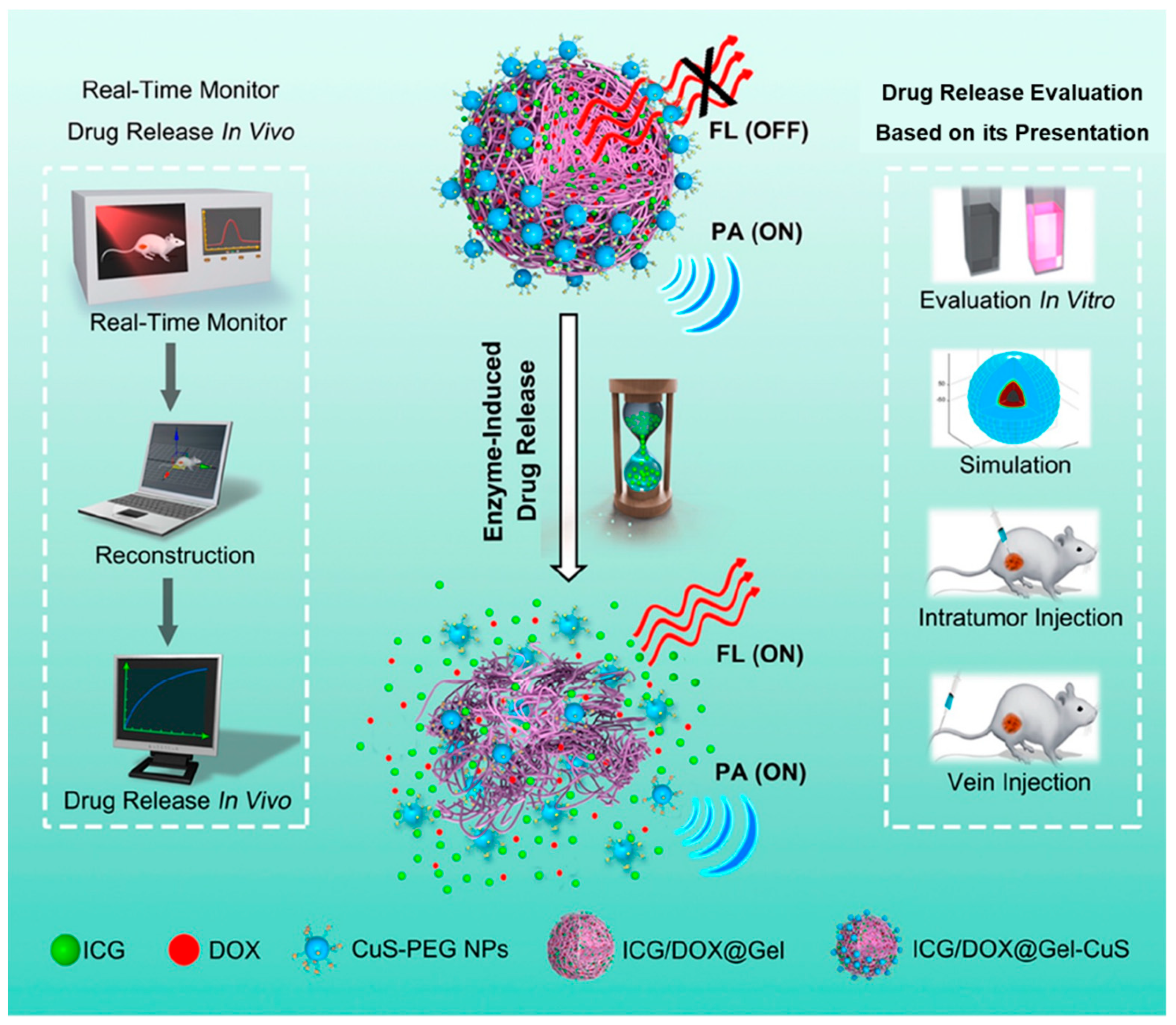
- Li, X.; Bottini, M.; Zhang, L.; Zhang, S.; Chen, J.; Zhang, T.; Liu, L.; Rosato, N.; Ma, X.; Shi, X.; et al. Core-Satellite Nanomedicines for in Vivo Real-Time Monitoring of Enzyme-Activatable Drug Release by Fluorescence and Photoacoustic Dual-Modal Imaging. ACS Nano 2019, 13, 176–186. [Google Scholar] [CrossRef]
- Jones, D. Pharmaceutical Applications of Polymers for Drug Delivery; Smithers Rapra Technology: Shawbury, UK, 2004. [Google Scholar]
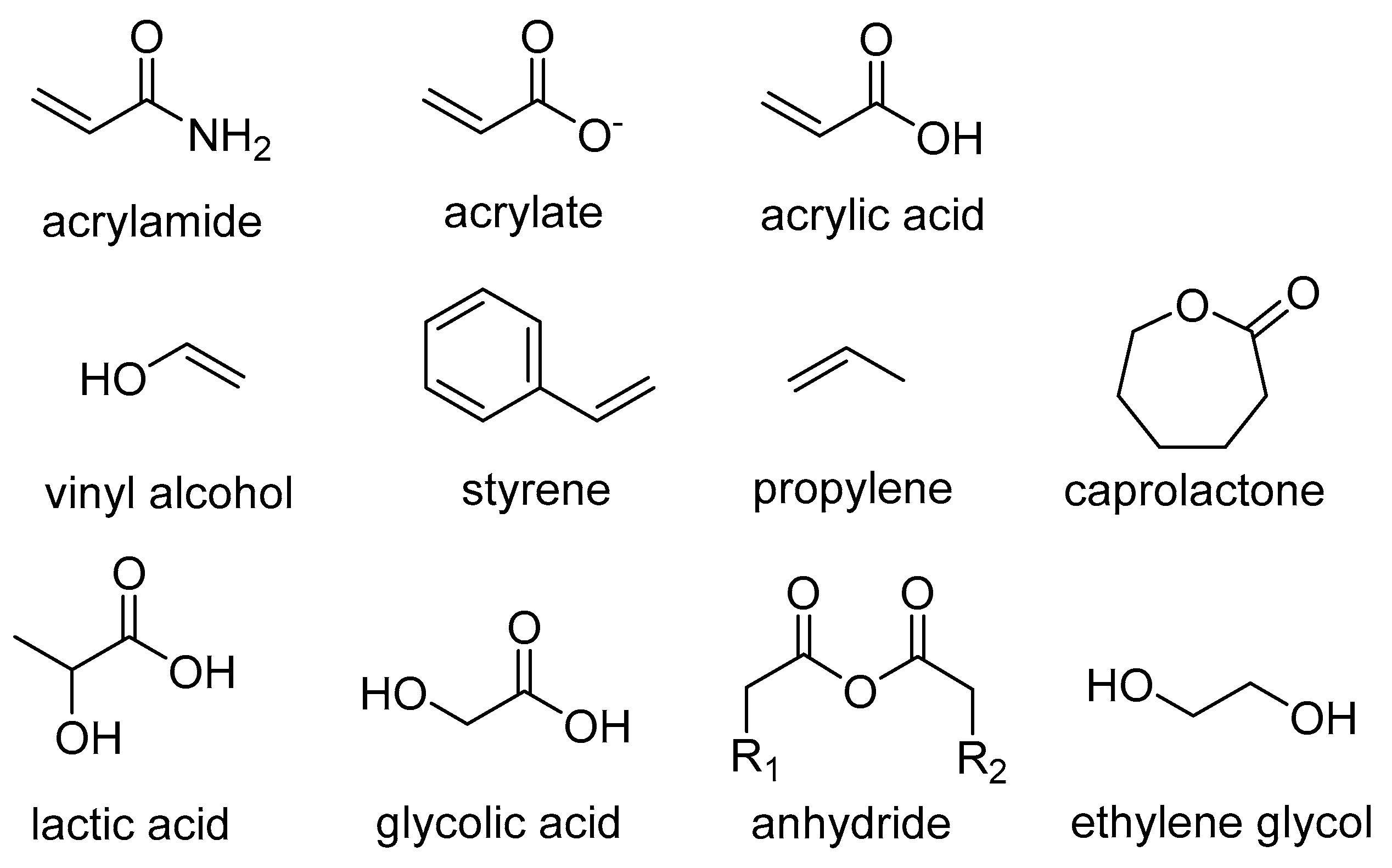
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Pandey, P.; Dureja, H. Recent Patents on Polymeric Nanoparticles for Cancer Therapy. Recent Pat. Nanotechnol. 2018, 12, 155–169. [Google Scholar]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Deliv.ery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Mohapatra, A.; Uthaman, S.; Park, I.-K. Polyethylene glycol nanoparticles as promising tools for anticancer therapeutics. In Polymeric Nanoparticles as a Promising Tool for Anti-Cancer Therapeutics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–231. [Google Scholar]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Chung, H.C.; Im, S.A.; Park, Y.H.; Kim, C.S.; Kim, S.B.; Rha, S.Y.; Lee, M.Y.; Ro, J. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2008, 108, 241–250. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, S.Y.; Kim, H.K.; Kim, S.W.; Shin, S.W.; Kim, J.S.; Park, K.; Lee, M.Y.; Heo, D.S. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann. Oncol. 2007, 18, 2009–2014. [Google Scholar] [CrossRef]
- Cavalcante, C.H.; Fernandes, R.S.; de Oliveira Silva, J.; Ramos Oda, C.M.; Leite, E.A.; Cassali, G.D.; Charlie-Silva, I.; Ventura Fernandes, B.H.; Miranda Ferreira, L.A.; de Barros, A.L.B. Doxorubicin-loaded pH-sensitive micelles: A promising alternative to enhance antitumor activity and reduce toxicity. Biomed. Pharmacother. 2021, 134, 111076. [Google Scholar] [CrossRef]
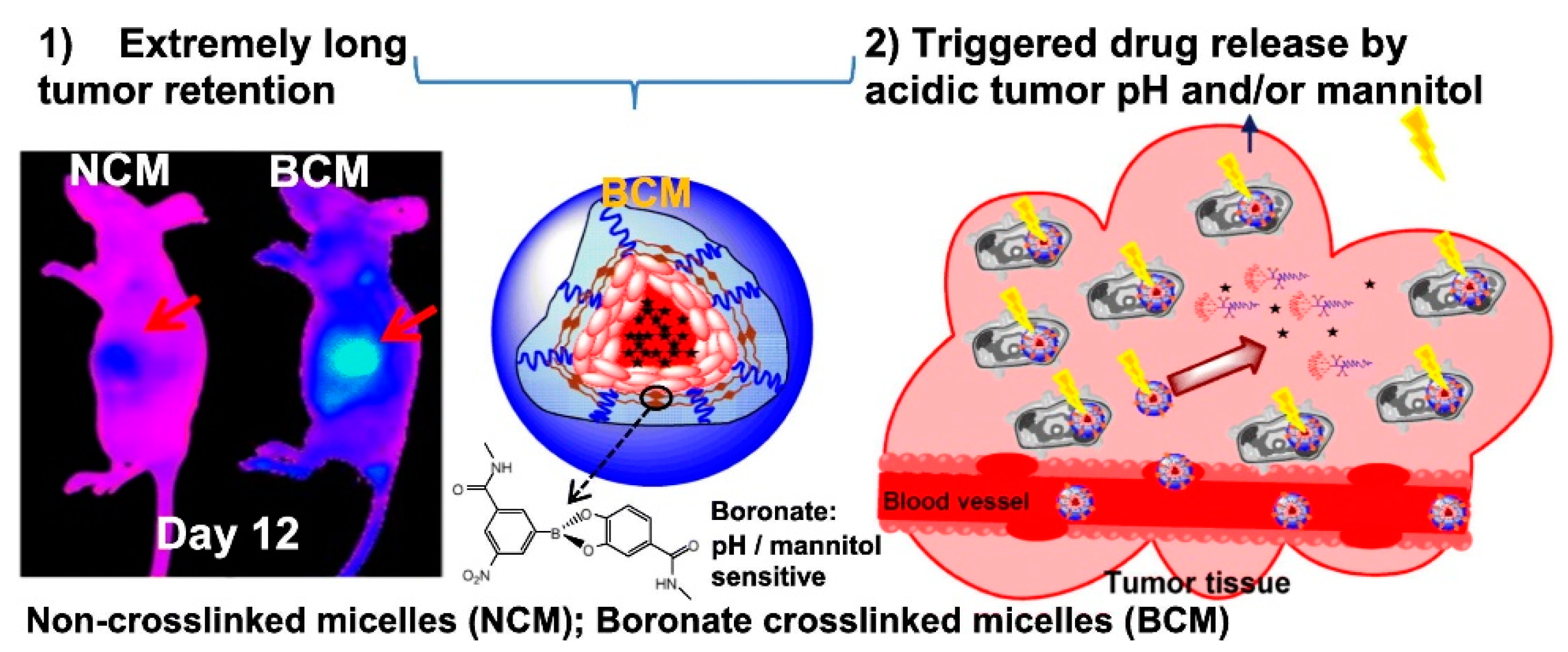
- Xiao, W.; Suby, N.; Xiao, K.; Lin, T.Y.; Al Awwad, N.; Lam, K.S.; Li, Y. Extremely long tumor retention, multi-responsive boronate crosslinked micelles with superior therapeutic efficacy for ovarian cancer. J. Control Release 2017, 264, 169–179. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers designed for functions: From physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev. 2010, 110, 1857–1959. [Google Scholar] [PubMed]
- Ihre, H.; Padilla De Jesus, O.L.; Frechet, J.M. Fast and convenient divergent synthesis of aliphatic ester dendrimers by anhydride coupling. J. Am. Chem. Soc. 2001, 123, 5908–5917. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J.; Frechet, J.M. Preparation of polymers with controlled molecular architecture. A new convergent approach to dendritic macromolecules. J. Am. Chem. Soc. 1990, 112, 7638–7647. [Google Scholar] [CrossRef]
- Franc, G.; Kakkar, A.K. Diels-Alder "click" chemistry in designing dendritic macromolecules. Chemistry 2009, 15, 5630–5639. [Google Scholar] [CrossRef] [PubMed]
- Killops, K.L.; Campos, L.M.; Hawker, C.J. Robust, efficient, and orthogonal synthesis of dendrimers via thiol-ene "click" chemistry. J. Am. Chem. Soc. 2008, 130, 5062–5064. [Google Scholar]
- Franc, G.; Kakkar, A. Dendrimer design using Cu I-catalyzed alkyne–azide “click-chemistry”. Chem. Commun. 2008, 42, 5267–5276. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef]
- Patri, A.K.; Kukowska-Latallo, J.F.; Baker, J.R., Jr. Targeted drug delivery with dendrimers: Comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex. Adv Drug Deliv. Rev. 2005, 57, 2203–2214. [Google Scholar] [CrossRef]
- Nam, J.-P.; Nam, K.; Jung, S.; Nah, J.-W.; Kim, S.W.E. Evaluation of dendrimer type bio-reducible polymer as a siRNA delivery carrier for cancer therapy. J. Control Release 2015, 209, 179–185. [Google Scholar] [CrossRef]
- Xin, Y.; Huang, M.; Guo, W.W.; Huang, Q.; Zhang, L.Z.; Jiang, G. Nano-based delivery of RNAi in cancer therapy. Mol. Cancer 2017, 16, 134. [Google Scholar] [CrossRef] [PubMed]
- Sitohy, B.; Nagy, J.A.; Dvorak, H.F. Anti-VEGF/VEGFR therapy for cancer: Reassessing the target. Cancer Res. 2012, 72, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Otis, J.B.; Zong, H.; Kotylar, A.; Yin, A.; Bhattacharjee, S.; Wang, H.; Baker, J.R., Jr.; Wang, S.H. Dendrimer antibody conjugate to target and image HER-2 overexpressing cancer cells. Oncotarget 2016, 7, 36002–36013. [Google Scholar] [PubMed]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control Release 2019, 303, 130–150. [Google Scholar]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Balazs, D.A.; Godbey, W. Liposomes for use in gene delivery. J. Drug Deliv. 2011, 2011, 326497. [Google Scholar]
- Swaminathan, J.; Ehrhardt, C. Liposomal delivery of proteins and peptides. Expert Opin. Drug Deliv. 2012, 9, 1489–1503. [Google Scholar] [CrossRef] [PubMed]
- Jahn, A.; Stavis, S.M.; Hong, J.S.; Vreeland, W.N.; DeVoe, D.L.; Gaitan, M. Microfluidic mixing and the formation of nanoscale lipid vesicles. ACS Nano 2010, 4, 2077–2087. [Google Scholar]
- Elsana, H.; Olusanya, T.O.B.; Carr-Wilkinson, J.; Darby, S.; Faheem, A.; Elkordy, A.A. Evaluation of novel cationic gene based liposomes with cyclodextrin prepared by thin film hydration and microfluidic systems. Sci. Rep. 2019, 9, 15120. [Google Scholar] [CrossRef] [PubMed]
- Lapinski, M.M.; Castro-Forero, A.; Greiner, A.J.; Ofoli, R.Y.; Blanchard, G.J. Comparison of liposomes formed by sonication and extrusion: Rotational and translational diffusion of an embedded chromophore. Langmuir 2007, 23, 11677–11683. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional nanocarriers. Adv. Drug Deliv. Rev. 2006, 58, 1532–1555. [Google Scholar] [CrossRef]
- Kim, J.H.; Shin, D.H.; Kim, J.S. Preparation, characterization, and pharmacokinetics of liposomal docetaxel for oral administration. Arch. Pharm. Res. 2018, 41, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Bayon-Cordero, L.; Alkorta, I.; Arana, L. Application of Solid Lipid Nanoparticles to Improve the Efficiency of Anticancer Drugs. Nanomaterials 2019, 9, 474. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery-A review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef]
- Smith, T.; Affram, K.; Nottingham, E.L.; Han, B.; Amissah, F.; Krishnan, S.; Trevino, J.; Agyare, E. Application of smart solid lipid nanoparticles to enhance the efficacy of 5-fluorouracil in the treatment of colorectal cancer. Sci. Rep. 2020, 10, 16989. [Google Scholar] [CrossRef]
- Hermiston, T.W.; Kuhn, I. Armed therapeutic viruses: Strategies and challenges to arming oncolytic viruses with therapeutic genes. Cancer Gene Ther. 2002, 9, 1022–1035. [Google Scholar] [CrossRef]
- Chen, X.; Gonçalves, M.A. Engineered viruses as genome editing devices. Mol. Ther. 2016, 24, 447–457. [Google Scholar] [PubMed]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Luo, S.T.; Shi, H.S.; Li, M.; Zhang, H.L.; He, S.S.; Liu, Y.; Pan, Y.; Yang, L. AAV2-mediated gene transfer of VEGF-Trap with potent suppression of primary breast tumor growth and spontaneous pulmonary metastases by long-term expression. Oncol. Rep. 2012, 28, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.; Silva, G.; Ribeiro, A.S.; Oliveira, M.; Garrido, M.; Bandeira, V.S.; Nascimento, A.; Coroadinha, A.S.; Peixoto, C.; Barbas, A.; et al. Evaluation of AAV-mediated delivery of shRNA to target basal-like breast cancer genetic vulnerabilities. J. Biotechnol. 2019, 300, 70–77. [Google Scholar] [PubMed]
- Koudelka, K.J.; Pitek, A.S.; Manchester, M.; Steinmetz, N.F. Virus-based nanoparticles as versatile nanomachines. Annu. Rev. Virol. 2015, 2, 379–401. [Google Scholar] [CrossRef]
- Li, C.; Samulski, R.J. Engineering adeno-associated virus vectors for gene therapy. Nat. Rev. Genet. 2020, 21, 255–272. [Google Scholar]
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Morozova, S.; Alikina, M.; Vinogradov, A.; Pagliaro, M. Silicon Quantum Dots: Synthesis, Encapsulation, and Application in Light-Emitting Diodes. Front. Chem. 2020, 8, 191. [Google Scholar] [CrossRef]
- Wisser, M.D.; Chea, M.; Lin, Y.; Wu, D.M.; Mao, W.L.; Salleo, A.; Dionne, J.A. Strain-induced modification of optical selection rules in lanthanide-based upconverting nanoparticles. Nano Lett. 2015, 15, 1891–1897. [Google Scholar]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Banobre-Lopez, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Singer, S.J. Preparation of an electron-dense antibody conjugate. Nature 1959, 183, 1523–1524. [Google Scholar]
- Yasuda, K.; Yamamoto, N. Metal labelled antibody method indirect label of chloromercuriferrocene to antibody. Acta Histochem. Cytochem. 1975, 8, 215–219. [Google Scholar] [CrossRef][Green Version]
- Sternberger, L.A. Electron microscopic immunocytochemistry: A review. J. Histochem. Cytochem. 1967, 15, 139–159. [Google Scholar]
- Faulk, W.P.; Taylor, G.M. Communication to the editors: An immunocolloid method for the electron microscope. Immunochemistry 1971, 8, 1081–1083. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Prato, M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 674–679. [Google Scholar] [CrossRef]
- Kumar, M.; Raza, K. C60-fullerenes as drug delivery carriers for anticancer agents: Promises and hurdles. Pharm. Nanotechnol. 2017, 5, 169–179. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Advances in drug delivery nanosystems using graphene-based materials and carbon nanotubes. Materials 2021, 14, 1059. [Google Scholar] [CrossRef] [PubMed]
- Chikan, V.; McLaurin, E.J. Rapid Nanoparticle Synthesis by Magnetic and Microwave Heating. Nanomaterials 2016, 6, 85. [Google Scholar] [CrossRef]
- Ebadi, M.; Bullo, S.; Buskara, K.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Release of a liver anticancer drug, sorafenib from its PVA/LDH- and PEG/LDH-coated iron oxide nanoparticles for drug delivery applications. Sci. Rep. 2020, 10, 21521. [Google Scholar] [PubMed]
- Ayubi, M.; Karimi, M.; Abdpour, S.; Rostamizadeh, K.; Parsa, M.; Zamani, M.; Saedi, A. Magnetic nanoparticles decorated with PEGylated curcumin as dual targeted drug delivery: Synthesis, toxicity and biocompatibility study. Mater. Sci. Eng. C 2019, 104, 109810. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Annabi, N.; Mostafavi, E.; Anzabi, M.; Khalilov, R.; Saghfi, S.; Mehrizadeh, M.; Akbarzadeh, A. Synthesis, characterization and in vitro evaluation of magnetic nanoparticles modified with PCL-PEG-PCL for controlled delivery of 5FU. Artif. Cells Nanomed. Biotechnol. 2018, 46, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Bekaroğlu, M.G.; Alemdar, A.; İşçi, S. Comparison of ionic polymers in the targeted drug delivery applications as the coating materials on the Fe3O4 nanoparticles. Mater. Sci. Eng. C 2019, 103, 109838. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kohestanian, M.; Streb, C. pH and thermal dual-responsive poly(NIPAM-co-GMA)-coated magnetic nanoparticles via surface-initiated RAFT polymerization for controlled drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110418. [Google Scholar] [CrossRef]
- Reczyńska, K.; Marszałek, M.; Zarzycki, A.; Reczyński, W.; Kornaus, K.; Pamuła, E.; Chrzanowski, W. Superparamagnetic iron oxide nanoparticles modified with silica layers as potential agents for lung cancer treatment. Nanomaterials 2020, 10, 1076. [Google Scholar] [CrossRef]
- Gholami, A.; Rasoul-amini, S.; Ebrahiminezhad, A.; Seradj, S.H.; Ghasemi, Y. Lipoamino acid coated superparamagnetic iron oxide nanoparticles concentration and time dependently enhanced growth of human hepatocarcinoma cell line (Hep-G2). J. Nanomater. 2015, 2015, 150. [Google Scholar] [CrossRef]
- Barahuie, F.; Dorniani, D.; Saifullah, B.; Gothai, S.; Hussein, M.Z.; Pandurangan, A.K.; Arulselvan, P.; Norhaizan, M.E. Sustained release of anticancer agent phytic acid from its chitosan-coated magnetic nanoparticles for drug-delivery system. Int. J. Nanomed. 2017, 12, 2361–2372. [Google Scholar] [CrossRef] [PubMed]
- Akal, Z.; Alpsoy, L.; Baykal, A. Biomedical applications of SPION@ APTES@ PEG-folic acid@ carboxylated quercetin nanodrug on various cancer cells. Appl. Surf. Sci. 2016, 378, 572–581. [Google Scholar] [CrossRef]
- Liu, D.; Hong, Y.; Li, Y.; Hu, C.; Yip, T.-C.; Yu, W.-K.; Zhu, Y.; Fong, C.-C.; Wang, W.; Au, S.-K. Targeted destruction of cancer stem cells using multifunctional magnetic nanoparticles that enable combined hyperthermia and chemotherapy. Theranostics 2020, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.R.; Carriao, M.S.; Pacheco, M.T.; Branquinho, L.C.; de Souza, A.L.R.; Bakuzis, A.F.; Lima, E.M. Triggered release of paclitaxel from magnetic solid lipid nanoparticles by magnetic hyperthermia. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 547–553. [Google Scholar] [CrossRef]
- Cui, Y.N.; Xu, Q.X.; Davoodi, P.; Wang, D.P.; Wang, C.H. Enhanced intracellular delivery and controlled drug release of magnetic PLGA nanoparticles modified with transferrin. Acta Pharmacol. Sin. 2017, 38, 943–953. [Google Scholar] [CrossRef]
- Hoare, T.; Santamaria, J.; Goya, G.F.; Irusta, S.; Lin, D.; Lau, S.; Padera, R.; Langer, R.; Kohane, D.S. A magnetically triggered composite membrane for on-demand drug delivery. Nano Lett. 2009, 9, 3651–3657. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Fernández-Pacheco, R.; Ibarra, M.R.; Santamaría, J. Magnetic nanoparticles for drug delivery. Nano Today 2007, 2, 22–32. [Google Scholar] [CrossRef]
- Rodzinski, A.; Guduru, R.; Liang, P.; Hadjikhani, A.; Stewart, T.; Stimphil, E.; Runowicz, C.; Cote, R.; Altman, N.; Datar, R.; et al. Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci. Rep. 2016, 6, 20867. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Lim, C.T. Synthesis, optical properties, and chemical–biological sensing applications of one-dimensional inorganic semiconductor nanowires. Prog. Mater. Sci. 2013, 58, 705–748. [Google Scholar]
- Hassanzadeh, P.; Kharaziha, M.; Nikkhah, M.; Shin, S.R.; Jin, J.; He, S.; Sun, W.; Zhong, C.; Dokmeci, M.R.; Khademhosseini, A. Chitin nanofiber micropatterned flexible substrates for tissue engineering. J. Mater. Chem. B 2013, 1, 4217–4224. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Yin, P.T.; Uehara, T.M.; Chueng, S.T.D.; Yang, L.; Lee, K.B. Guiding stem cell differentiation into oligodendrocytes using graphene-nanofiber hybrid scaffolds. Adv. Mater. 2014, 26, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zou, W.; Su, Y.; Zhu, Y.; Jiang, H.; Shen, J.; Li, C. Activated nitrogen-doped carbon nanofibers with hierarchical pore as efficient oxygen reduction reaction catalyst for microbial fuel cells. J. Power Sour. 2014, 266, 36–42. [Google Scholar] [CrossRef]
- Shang, M.; Wang, W.; Sun, S.; Gao, E.; Zhang, Z.; Zhang, L.; O’Hayre, R. The design and realization of a large-area flexible nanofiber-based mat for pollutant degradation: An application in photocatalysis. Nanoscale 2013, 5, 5036–5042. [Google Scholar] [PubMed]
- Shahriar, S.M.S.; Mondal, J.; Hasan, M.N.; Revuri, V.; Lee, D.Y.; Lee, Y.K. Electrospinning Nanofibers for Therapeutics Delivery. Nanomaterials 2019, 9, 532. [Google Scholar] [CrossRef]
- Azfarniam, L.; Norouzi, M. Multifunctional polyester fabric using a multicomponent treatment. Fibers Polym. 2016, 17, 298–304. [Google Scholar] [CrossRef]
- Niu, C.; Meng, J.; Wang, X.; Han, C.; Yan, M.; Zhao, K.; Xu, X.; Ren, W.; Zhao, Y.; Xu, L.; et al. General synthesis of complex nanotubes by gradient electrospinning and controlled pyrolysis. Nat. Commun. 2015, 6, 7402. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, L.; Hu, Y.; Srinivasan, M.; Cheng, F.; Chen, J.; Ramakrishna, S. Fabrication of spinel one-dimensional architectures by single-spinneret electrospinning for energy storage applications. ACS Nano 2015, 9, 1945–1954. [Google Scholar] [CrossRef]
- Muerza-Cascante, M.L.; Haylock, D.; Hutmacher, D.W.; Dalton, P.D. Melt electrospinning and its technologization in tissue engineering. Tissue Eng. Part. B Rev. 2015, 21, 187–202. [Google Scholar] [CrossRef]
- Xu, H.; Lu, X.; Li, J.; Ding, D.; Wang, H.; Li, X.; Xie, W. Superior antitumor effect of extremely high drug loading self-assembled paclitaxel nanofibers. Int. J. Pharm. 2017, 526, 217–224. [Google Scholar] [CrossRef]
- Lv, H.; Wu, C.; Liu, X.; Bai, A.; Cao, Y.; Shang, W.; Hu, L.; Liu, Y. Folate-functionalized mesoporous hollow SnO2 nanofibers as a targeting drug carrier to improve the antitumor effect of paclitaxel for liver cancer therapy. BioMed Res. Int. 2018, 2018, 8526190. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Abdali, Z.; Liu, S.; Miller, D.W. Salinomycin-loaded Nanofibers for Glioblastoma Therapy. Sci. Rep. 2018, 8, 9377. [Google Scholar] [CrossRef]
- Rinoldi, C.; Zargarian, S.S.; Nakielski, P.; Li, X.; Liguori, A.; Petronella, F.; Presutti, D.; Wang, Q.; Costantini, M.; De Sio, L.; et al. Nanotechnology-Assisted RNA Deliv.ery: From Nucleic Acid Therapeutics to COVID-19 Vaccines. Small Methods 2021, 5, 2100402. [Google Scholar] [CrossRef]
- Bonam, S.R.; Kotla, N.G.; Bohara, R.A.; Rochev, Y.; Webster, T.J.; Bayry, J. Potential immuno-nanomedicine strategies to fight COVID-19 like pulmonary infections. Nano Today 2021, 36, 101051. [Google Scholar] [CrossRef] [PubMed]

| Name (API) | Approved Indication | Formulation and Administration Route | References |
|---|---|---|---|
| Doxil/Caelyx (doxorubicin) | Ovarian cancer, multiple myeloma | PEGylated liposome and intravenous infusion | [13] |
| DaunoXome (daunorubicin) | Kaposi’s sarcoma | Liposome and intravenous infusion | [11] |
| Ontak (Engineered fusion protein combining diphtheria toxin with interleukin-2) | Cutaneous T-cell lymphoma | Proteinaceous nanoparticle and intravenous infusion | [18] |
| Myocet (doxorubicin) | Metastatic breast cancer | Liposome and intravenous infusion | [12] |
| Eligard (Leuprolide acetate) | Advanced prostate cancer | Polymeric nanoparticle and subcutaneous injection | [19] |
| Abraxane (paclitaxel) | Non-small cell lung cancer, metastatic breast cancer, metastatic pancreatic cancer | Albumin-bound nanoparticle and intravenous infusion | [14] |
| Marqibo (vincristine) | Acute lymphoblastic leukemia | Liposome and intravenous infusion | [15] |
| MEPACT (mifamurtide) | Osteosarcoma | Liposome and intravenous infusion | [17] |
| Onivyde/MM-398 (irinotecan) | Metastatic pancreatic cancer | PEGylated liposome and intravenous infusion | [16] |
| VYXEOS/CPX-351 (cytarabine and daunorubicin) | Acute myeloid leukemia | Liposome and intravenous infusion | [10] |
| NBTXR3/Hensify (radiotherapy) | Squamous cell carcinoma | Hafnium oxide nanoparticle and intratumoral injection | [8] |
| NanoTherm (Iron oxide) | Brain tumor | Magnetic nanoparticle and intratumoral injection | [9] |
| Stimulus | Formulation | References |
|---|---|---|
| pH | Polymersome by self-assembling of a carboxyl-terminated polyethylene glycol amphiphile | [40] |
| pH | Lectin-conjugated mesoporous silica nanoparticle | [43] |
| pH | Phosphorylcholine polymer micelle | [44] |
| pH | Polymeric micelle based on heparin-α-tocopherol conjugate | [45] |
| pH | Self-assembling polypeptide and calcium phosphate | [46] |
| Photothermal | Dipalmitoyl phosphatidylcholine liposome | [47] |
| Photothermal | Copper sulfide nanoparticle | [48] |
| Photothermal | Silica-coated silver-gold nanoshell | [49] |
| Redox | Zwitterionic cross-linked micelle based on a penta-block copolymer | [38] |
| Redox | Inorganic nanoparticle functionalized by organic group, polysaccharide, or peptide | [51] |
| Redox | Liposome with disulfide-phospholipid conjugate | [52,53,62] |
| Redox | Polymeric nanomicelle | [55,56,57,58] |
| Enzyme | Micelle formed from two amphiphilic block copolymers | [39] |
| Enzyme | Monostearin/amorphous calcium carbonate nanoparticle | [59] |
| Enzyme | Self-assembled protein nanoparticle | [60] |
| pH, redox, and enzyme | Gelatin-encapsulated magnetic nanoparticle | [63] |
| Parameter | DTX (20 mg·kg−1, Mean ± SE) | Eudragit-Coated Liposomal DTX (10 mg·kg−1, Mean ± SE) |
|---|---|---|
| tmax (min) | 110 ± 10.0 | 90.0 ± 9.49 |
| Cmax (μg·mL−1) | 0.0112 ± 0.00193 | 0.00981 ± 0.00169 |
| Ka (min−1) | 0.0609 ± 0.0257 | 0.0349 ± 0.0165 |
| K (min−1) | 0.00168 ± 0.000726 | 0.000995 ± 0.000181 |
| t1/2 (min) | 567 ± 181 | 818 ± 182 |
| AUC (μg·min·mL−1) | 6.98 ± 0.846 | 10.8 ± 2.39 |
| Vβ (mL·kg−1) | 44,745 ± 14,275 | 64,605 ± 14,381 |
| BA (%) | 1.91 ± 0.232 | 5.92 ± 1.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.R.; Chakraborty, K.; An, J.M.; Mondal, J.; Yoon, H.Y.; Lee, Y.-k. Pharmaceutical Aspects of Nanocarriers for Smart Anticancer Therapy. Pharmaceutics 2021, 13, 1875. https://doi.org/10.3390/pharmaceutics13111875
Hwang SR, Chakraborty K, An JM, Mondal J, Yoon HY, Lee Y-k. Pharmaceutical Aspects of Nanocarriers for Smart Anticancer Therapy. Pharmaceutics. 2021; 13(11):1875. https://doi.org/10.3390/pharmaceutics13111875
Chicago/Turabian StyleHwang, Seung Rim, Kushal Chakraborty, Jeong Man An, Jagannath Mondal, Hong Yeol Yoon, and Yong-kyu Lee. 2021. "Pharmaceutical Aspects of Nanocarriers for Smart Anticancer Therapy" Pharmaceutics 13, no. 11: 1875. https://doi.org/10.3390/pharmaceutics13111875
APA StyleHwang, S. R., Chakraborty, K., An, J. M., Mondal, J., Yoon, H. Y., & Lee, Y.-k. (2021). Pharmaceutical Aspects of Nanocarriers for Smart Anticancer Therapy. Pharmaceutics, 13(11), 1875. https://doi.org/10.3390/pharmaceutics13111875