Data-Enriched Edible Pharmaceuticals (DEEP) with Bespoke Design, Dose and Drug Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ink and Substrate Preparation
2.3. Inkjet Printing of DEEP
2.4. Readability of DEEP
2.5. Coating of DEEP
2.6. Drug Content Analysis
2.7. Drug Release Studies
2.8. Long-Term Stability
2.9. Statistical Analysis
3. Results and Discussion
3.1. Fabrication of DEEP
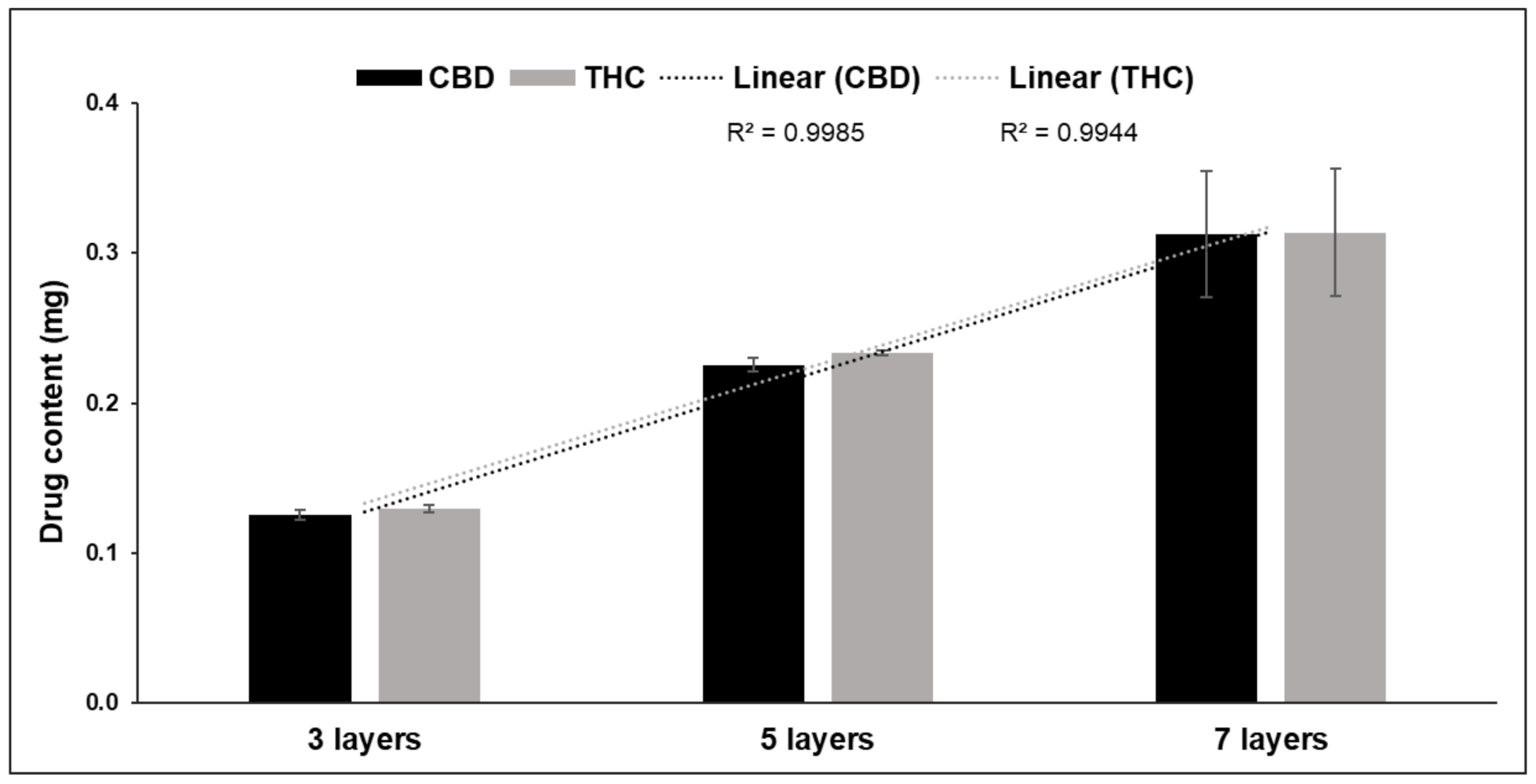
3.2. Drug Content
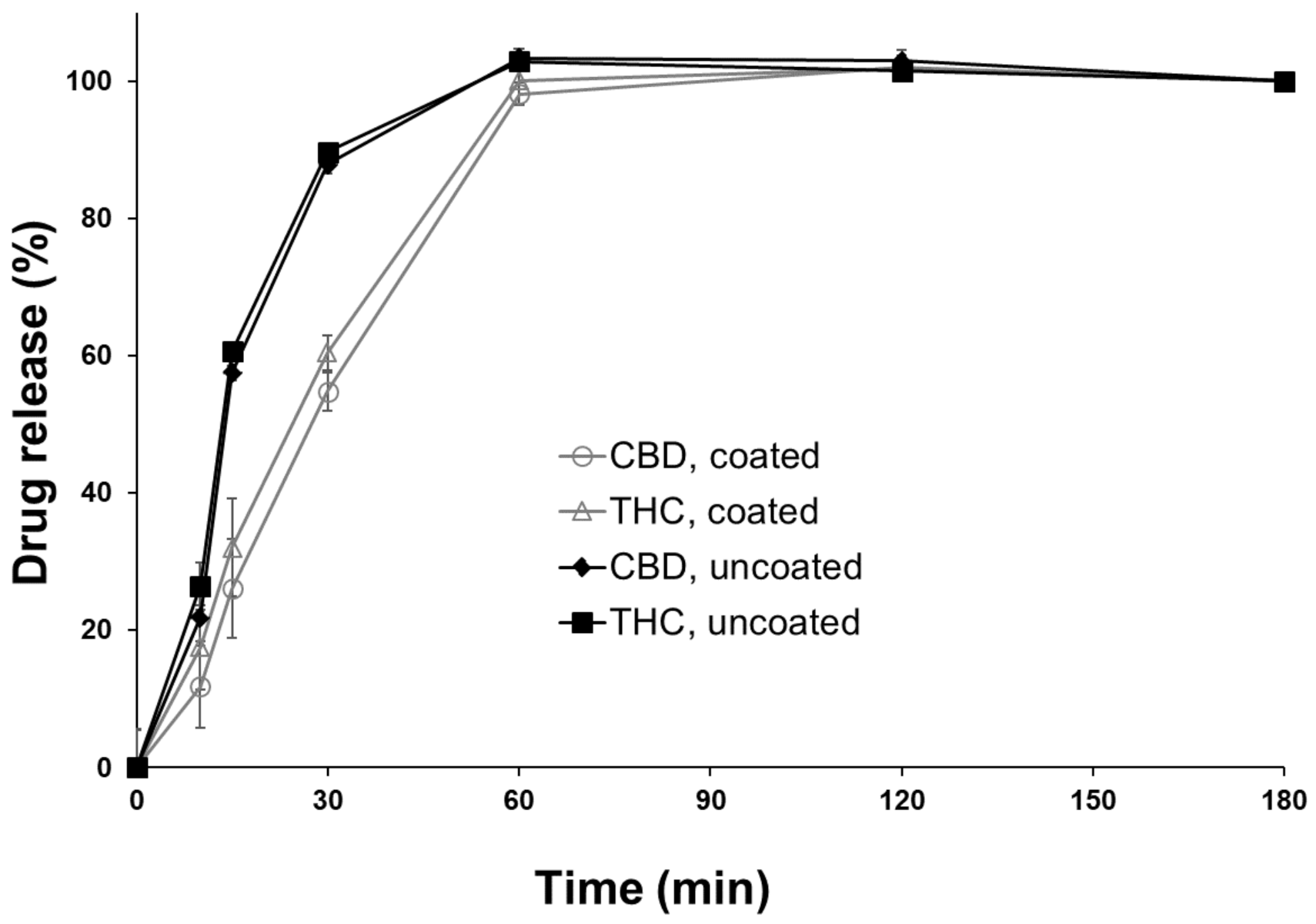
3.3. Drug Release
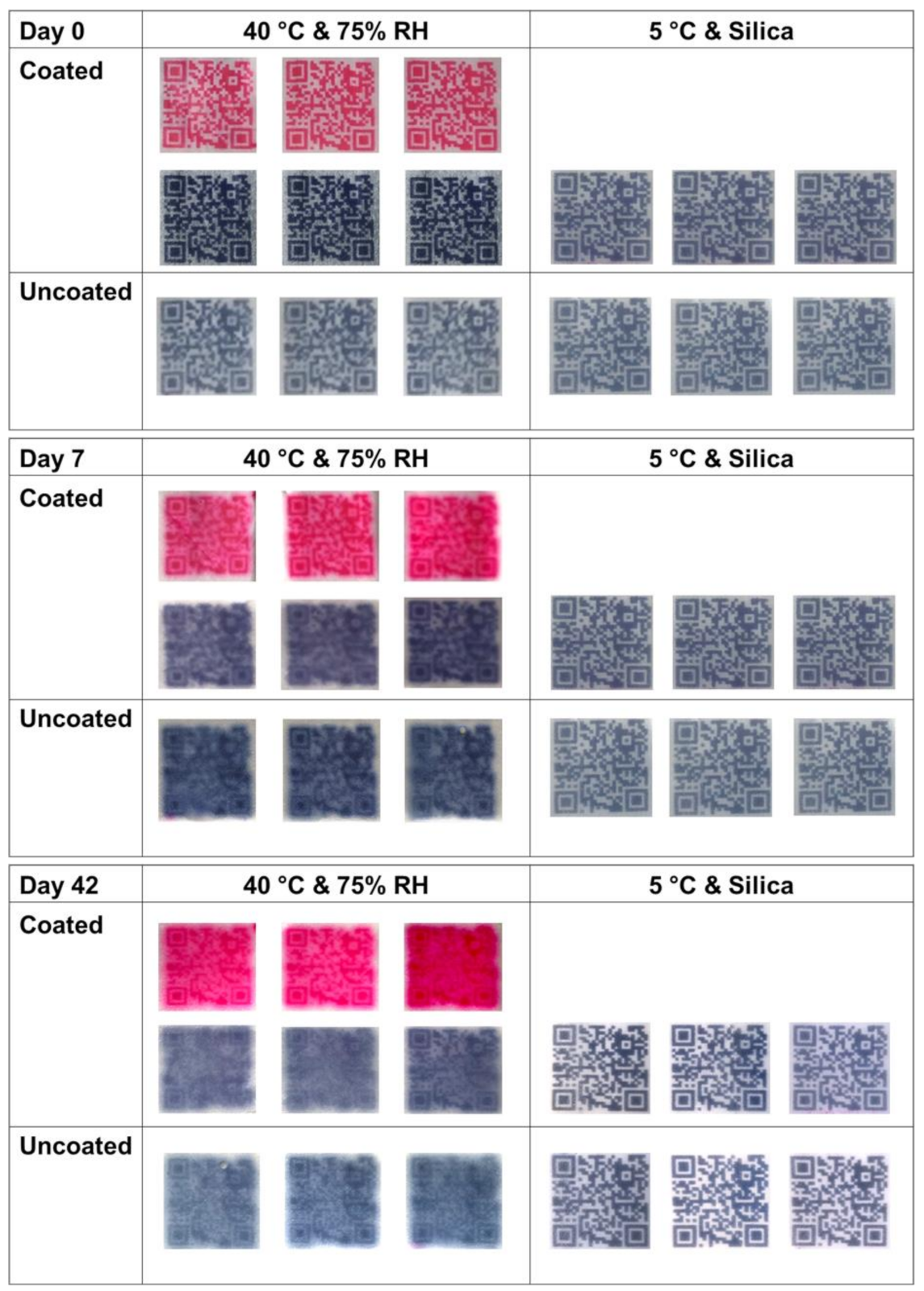
3.4. Long-Term Physical Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Lind, J.; Kälvemark Sporrong, S.; Kaae, S.; Rantanen, J.; Genina, N. Social aspects in additive manufacturing of pharmaceutical products. Expert Opin. Drug Deliv. 2017, 14, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Spear, B.B.; Heath-Chiozzi, M.; Huff, J. Clinical application of pharmacogenetics. Trends Mol. Med. 2001, 7, 201–204. [Google Scholar] [CrossRef]
- Personalized Medicine: Motivation, Challenges, and Progress. Available online: https://reference.medscape.com/medline/abstract/29935653 (accessed on 14 June 2021).
- Pirmohamed, M. Personalized pharmacogenomics: Predicting efficacy and adverse drug reactions. Annu. Rev. Genom. Hum. Genet. 2014, 15, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, J.; Boos, J. Paediatric and geriatric drug delivery. Expert Opin. Drug Deliv. 2007, 4, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Raijada, D.; Wac, K.; Greisen, E.; Rantanen, J.; Genina, N. Integration of personalized drug delivery systems into digital health. Adv. Drug Deliv. Rev. 2021, 176, 113857. [Google Scholar] [CrossRef] [PubMed]
- Ursino, M.; Zohar, S.; Lentz, F.; Alberti, C.; Friede, T.; Stallard, N.; Comets, E. Dose-finding methods for Phase I clinical trials using pharmacokinetics in small populations. Biom. J. 2017, 59, 804–825. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ranmal, S.; Batchelor, H.K.; Orlu-Gul, M.; Ernest, T.B.; Thomas, I.W.; Flanagan, T.; Tuleu, C. Patient-centred pharmaceutical design to improve acceptability of medicines: Similarities and differences in paediatric and geriatric populations. Drugs 2014, 74, 1871–1889. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Jacobsen, L.; Jhaveri, R.; Bradford, K.K. Effectiveness of pediatric pill swallowing interventions: A systematic review. Pediatrics 2015, 135, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Polaha, J.; Dalton, W.T.; Lancaster, B.M. Parental report of medication acceptance among youth: Implications for everyday practice. South Med. J. 2008, 101, 1106–1112. [Google Scholar] [CrossRef]
- Schirm, E.; Tobi, H.; De Vries, T.W.; Choonara, I.; De Jong-Van Den Berg, L.T.W. Lack of appropriate formulations of medicines for children in the community. Acta Paediatr. Int. J. Paediatr. 2003, 92, 1486–1489. [Google Scholar] [CrossRef]
- Hajjar, E.R.; Cafiero, A.C.; Hanlon, J.T. Polypharmacy in elderly patients. Am. J. Geriatr. Pharmacother. 2007, 5, 345–351. [Google Scholar] [CrossRef]
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergence of 3D printed dosage forms: Opportunities and challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Fastø, M.M.; Genina, N.; Kaae, S.; Kälvemark Sporrong, S. Perceptions, preferences and acceptability of patient designed 3D printed medicine by polypharmacy patients: A pilot study. Int. J. Clin. Pharm. 2019, 41, 1290–1298. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.A.; Douroumis, D. 3D Printed “Starmix” Drug Loaded Dosage Forms for Paediatric Applications. Pharm. Res. 2018, 35, 34. [Google Scholar] [CrossRef]
- Goyanes, A.; Scarpa, M.; Kamlow, M.; Gaisford, S.; Basit, A.W.; Orlu, M. Patient acceptability of 3D printed medicines. Int. J. Pharm. 2017, 530, 71–78. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Fatouros, D.G. Haptic Evaluation of 3D-printed Braille-encoded Intraoral Films. Eur. J. Pharm. Sci. 2021, 157, 105605. [Google Scholar] [CrossRef]
- Edinger, M.; Bar-Shalom, D.; Sandler, N.; Rantanen, J.; Genina, N. QR encoded smart oral dosage forms by inkjet printing. Int. J. Pharm. 2018, 536, 138–145. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Xian Tan, H.; Awad, A.; Buanz, A.; Gaisford, S.; Basit, A.W.; Goyanes, A. Track-and-trace: Novel anti-counterfeit measures for 3D printed personalized drug products using smart material inks. Int. J. Pharm. 2019, 567, 118443. [Google Scholar] [CrossRef]
- Öblom, H.; Sjöholm, E.; Rautamo, M.; Sandler, N. Towards Printed Pediatric Medicines in Hospital Pharmacies: Comparison of 2D and 3D-Printed Orodispersible Warfarin Films with Conventional Oral Powders in Unit Dose Sachets. Pharmaceutics 2019, 11, 334. [Google Scholar] [CrossRef] [Green Version]
- Öblom, H.; Cornett, C.; Bøtker, J.; Frokjaer, S.; Hansen, H.; Rades, T.; Rantanen, J.; Genina, N. Data-enriched edible pharmaceuticals (DEEP) of medical cannabis by inkjet printing. Int. J. Pharm. 2020, 589, 119866. [Google Scholar] [CrossRef]
- Raijada, D.; Genina, N.; Fors, D.; Wisaeus, E.; Peltonen, J.; Rantanen, J.; Sandler, N. A step toward development of printable dosage forms for poorly soluble drugs. J. Pharm. Sci. 2013, 102, 3694–3704. [Google Scholar] [CrossRef]
- Chao, M.; Genina, N.; Beer, N.; Kälvemark Sporrong, S. Data-Enriched Edible Pharmaceuticals (DEEP): Patients’ preferences, perceptions and acceptability of new dosage forms and their digital aspects–An interview study. Adv. Drug Deliv. Rev. 2021, 176, 113857. [Google Scholar]
- Bastaki, M.; Farrell, T.; Bhusari, S.; Bi, X.; Scrafford, C. Estimated daily intake and safety of FD&C food-colour additives in the US population. Food Addit. Contam.-Part A Chem. Anal. Control Exp. Risk Assess. 2017, 34, 891–904. [Google Scholar] [CrossRef]
- Scientific Opinion on the re-evaluation of Erythrosine (E 127) as a food additive. EFSA J. 2011, 9, 1854. [CrossRef]
- Scientific Opinion on the re-evaluation of Brilliant Black BN (E 151) as a food additive. EFSA J. 2010, 8, 1540. [CrossRef]
- Iftimi, L.-D.; Edinger, M.; Bar-Shalom, D.; Rantanen, J.; Genina, N. Edible solid foams as porous substrates for inkjet-printable pharmaceuticals. Eur. J. Pharm. Biopharm. 2019, 136, 38–47. [Google Scholar] [CrossRef]
- Edinger, M.; Iftimi, L.-D.; Markl, D.; Al-Sharabi, M.; Bar-Shalom, D.; Rantanen, J.; Genina, N. Quantification of Inkjet-Printed Pharmaceuticals on Porous Substrates Using Raman Spectroscopy and Near-Infrared Spectroscopy. AAPS PharmSciTech 2019, 20, 207. [Google Scholar] [CrossRef]
- Genina, N.; Fors, D.; Palo, M.; Peltonen, J.; Sandler, N. Behavior of printable formulations of loperamide and caffeine on different substrates-Effect of print density in inkjet printing. Int. J. Pharm. 2013, 453, 488–497. [Google Scholar] [CrossRef]
- Buanz, A.B.M.; Saunders, M.H.; Basit, A.W.; Gaisford, S. Preparation of personalized-dose salbutamol sulphate oral films with thermal ink-jet printing. Pharm. Res. 2011, 28, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.; Alomari, M.; Dodoo, C.; Trenfield, S.; Velaga, S.; Basit, A.; Gaisford, S. Personalisation of warfarin therapy using thermal ink-jet printing. Eur. J. Pharm. Sci. 2018, 117, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.C.; Wibier, L.; Kiefer, O.; Orlu, M.; Breitkreutz, J.; Woerdenbag, H.J.; Taxis, K. A Pediatrics Utilization Study in The Netherlands to Identify Active Pharmaceutical Ingredients Suitable for Inkjet Printing on Orodispersible Films. Pharmaceutics 2020, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Printable Medicines: A Microdosing Device For Producing Personalized Medicines. Available online: https://www.pharmtech.com/view/printable-medicines-microdosing-device-producing-personalized-medicines (accessed on 18 August 2021).
- Kabir, M.A.; Reo, J.P. Hydroxypropyl cellulose. In Handbook of Pharmaceutical Excipients; Rowe, C., Sheskey, P., Quinn, M., Eds.; Pharmaceutical Press and American Pharmacists Association: London, UK; Chicago, IL, USA, 2009; pp. 317–322. [Google Scholar]
- Genina, N.; Fors, D.; Vakili, H.; Ihalainen, P.; Pohjala, L.; Ehlers, H.; Kassamakov, I.; Haeggström, E.; Vuorela, P.; Peltonen, J.; et al. Tailoring controlled-release oral dosage forms by combining inkjet and flexographic printing techniques. Eur. J. Pharm. Sci. 2012, 47, 615–623. [Google Scholar] [CrossRef]
- Yang, Q.; Yuan, F.; Xu, L.; Yan, Q.; Yang, Y.; Wu, D.; Guo, F.; Yang, G. An Update of Moisture Barrier Coating for Drug Delivery. Pharmaceutics 2019, 11, 436. [Google Scholar] [CrossRef] [Green Version]

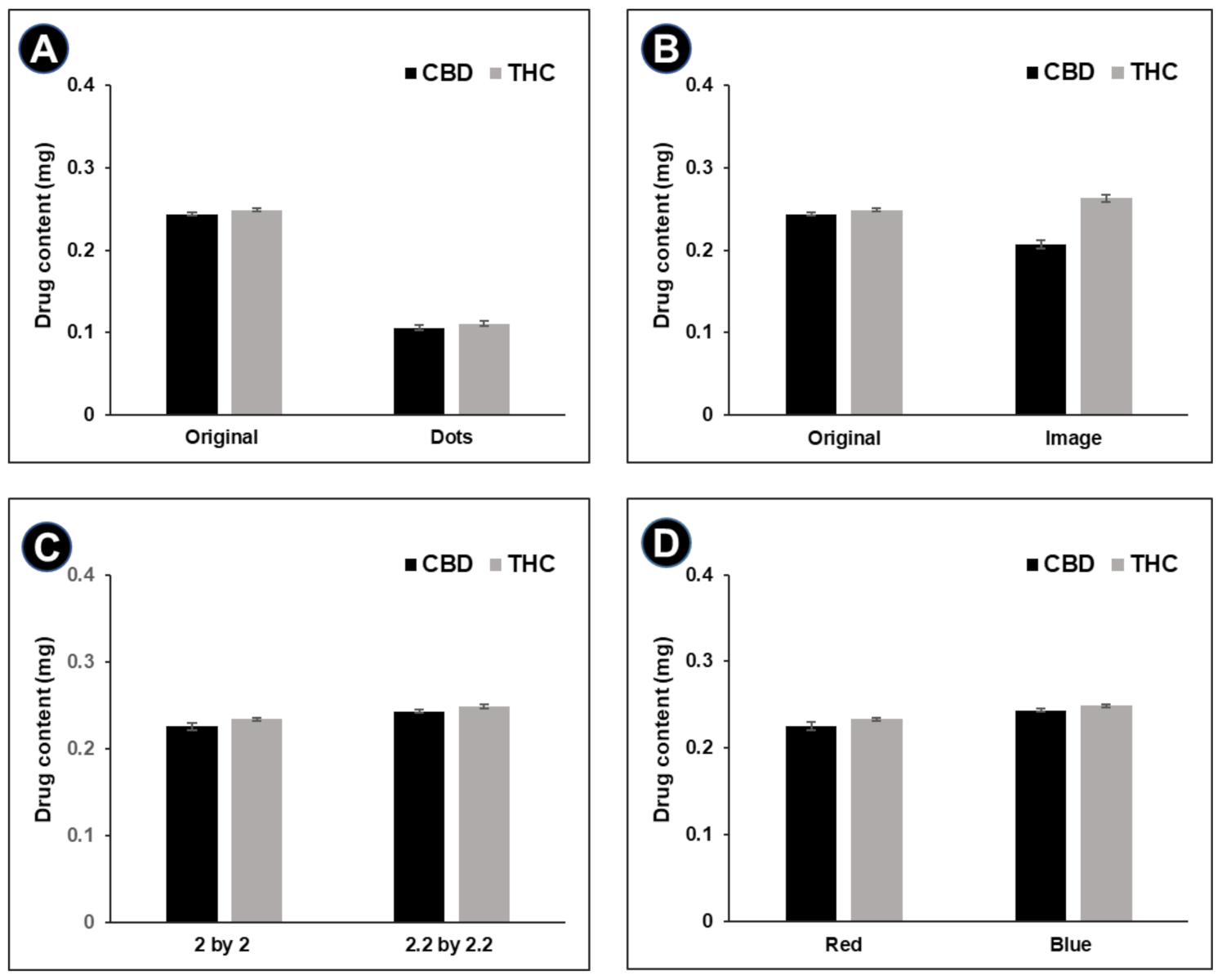
| Image of Digital Design | Digital Design | Total Pixel Count | Colored Pixel Count | Pixel Ratio to Original QR Code Pattern | Ratio in Content of CBD/THC to Original QR Code Pattern, n = 3 |
|---|---|---|---|---|---|
 | Original QR code pattern, 2 × 2 cm2 | 12,769 | 6480 | 1.00 | 1.00/1.00 |
 | Dotted QR code pattern, 2 × 2 cm2 | 12,656 | 3053 | 0.47 | 0.43/0.44 |
 | QR code pattern with embedded ‘heart’ image | 12,656 | 6697 | 1.03 | 0.85/1.06 |
 | QR code pattern, 2.2 × 2.2 cm2 | 15,376 | 7656 | 1.18 | 1.08/1.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, M.; Öblom, H.; Cornett, C.; Bøtker, J.; Rantanen, J.; Sporrong, S.K.; Genina, N. Data-Enriched Edible Pharmaceuticals (DEEP) with Bespoke Design, Dose and Drug Release. Pharmaceutics 2021, 13, 1866. https://doi.org/10.3390/pharmaceutics13111866
Chao M, Öblom H, Cornett C, Bøtker J, Rantanen J, Sporrong SK, Genina N. Data-Enriched Edible Pharmaceuticals (DEEP) with Bespoke Design, Dose and Drug Release. Pharmaceutics. 2021; 13(11):1866. https://doi.org/10.3390/pharmaceutics13111866
Chicago/Turabian StyleChao, Meie, Heidi Öblom, Claus Cornett, Johan Bøtker, Jukka Rantanen, Sofia Kälvemark Sporrong, and Natalja Genina. 2021. "Data-Enriched Edible Pharmaceuticals (DEEP) with Bespoke Design, Dose and Drug Release" Pharmaceutics 13, no. 11: 1866. https://doi.org/10.3390/pharmaceutics13111866
APA StyleChao, M., Öblom, H., Cornett, C., Bøtker, J., Rantanen, J., Sporrong, S. K., & Genina, N. (2021). Data-Enriched Edible Pharmaceuticals (DEEP) with Bespoke Design, Dose and Drug Release. Pharmaceutics, 13(11), 1866. https://doi.org/10.3390/pharmaceutics13111866







