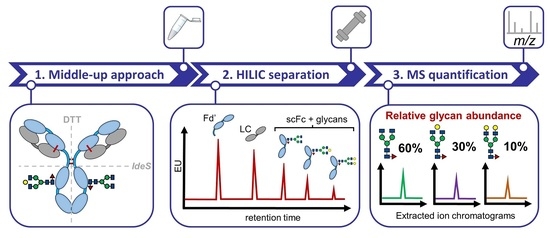
Quantitative N-Glycan Profiling of Therapeutic Monoclonal Antibodies Performed by Middle-Up Level HILIC-HRMS Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Sample Preparation
2.2.1. 2-AB Glycan Labelling
2.2.2. RFMS Glycan Labelling
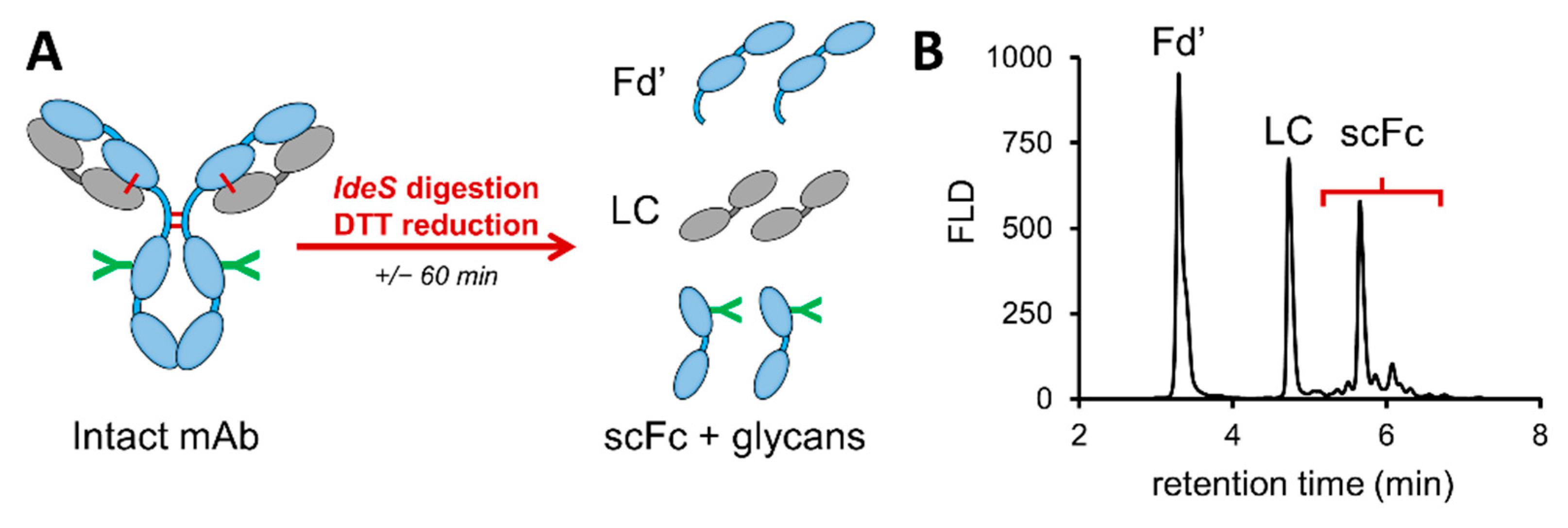
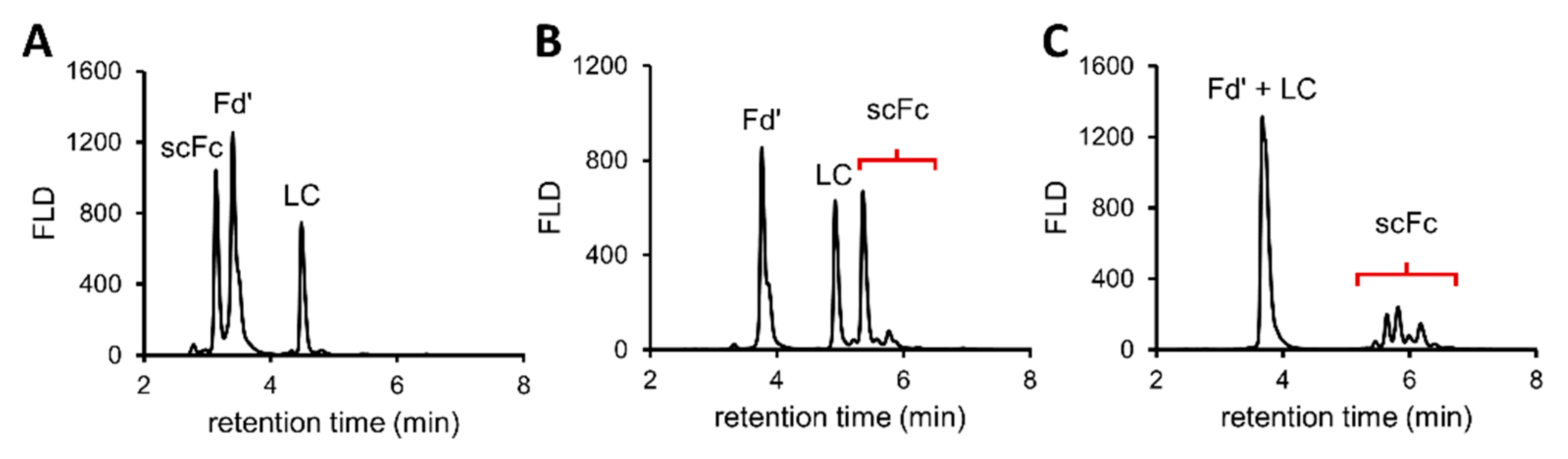
2.2.3. Protein Subunit Generation
2.3. Instrumentation and Columns
2.4. Chromatographic Conditions
3. Results and Discussion
3.1. Comparison of Quantitative N-Glycoprofiling Methods
3.1.1. Released N-Glycan Analysis
3.1.2. Middle-Up HILIC-HRMS Glycan Profiling
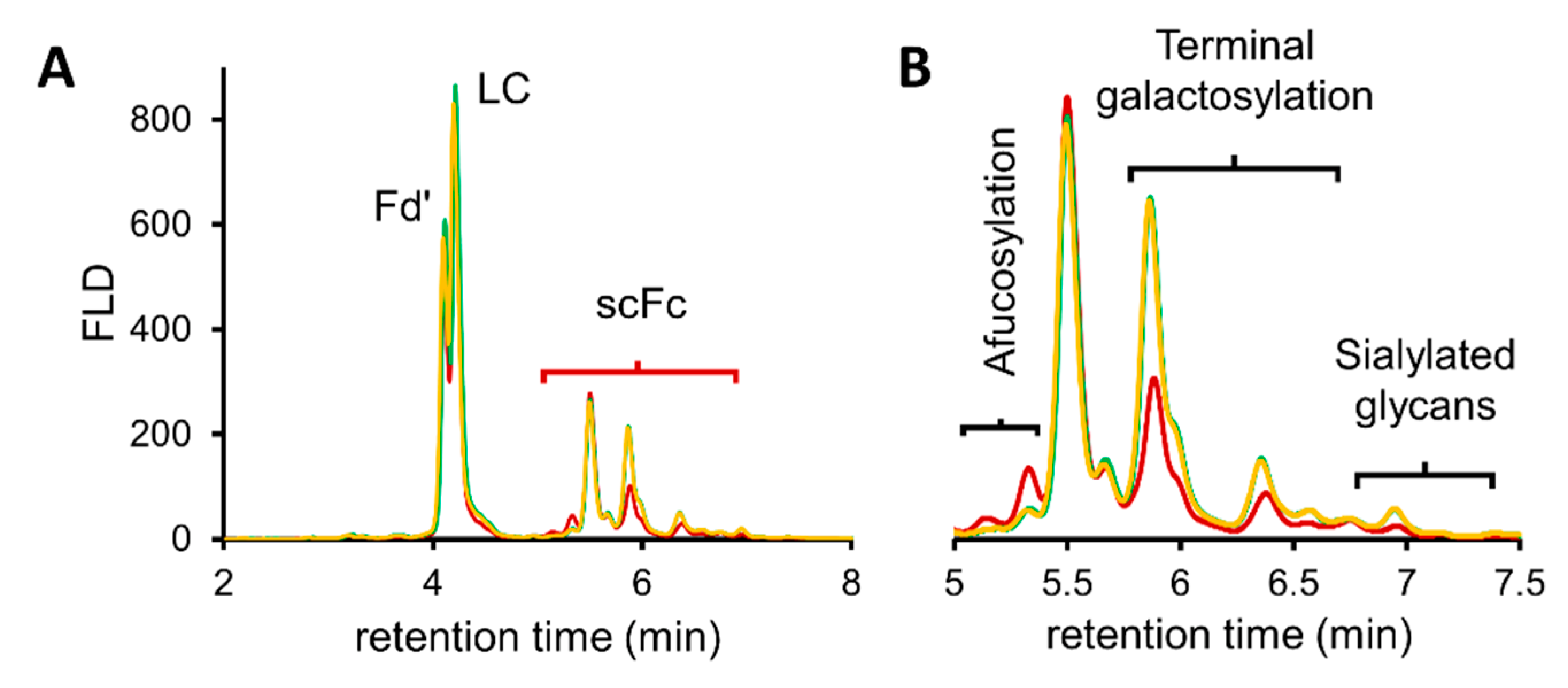
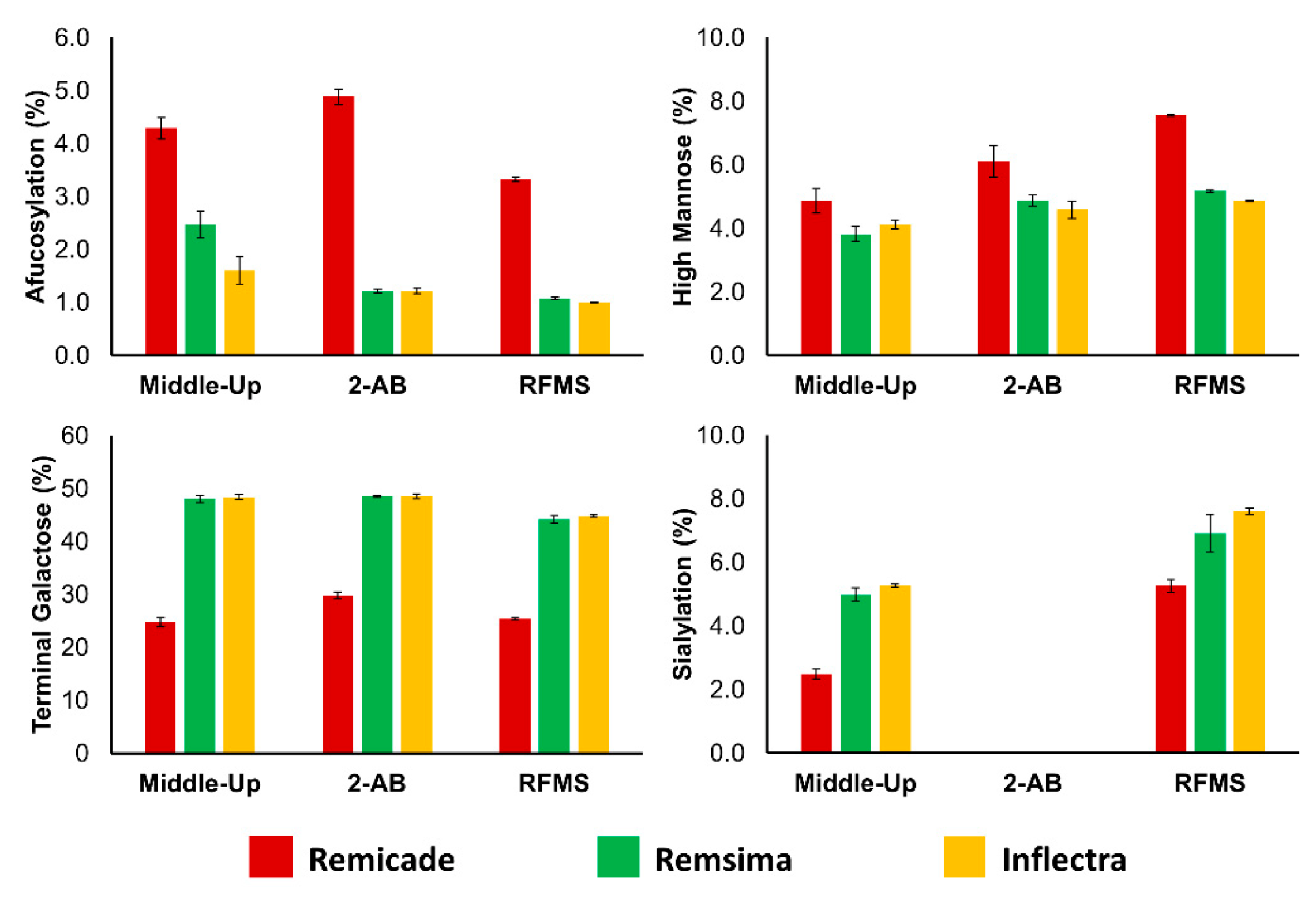
3.2. Application of Quantitative Middle-Up Analysis to Diverse mAb Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mullard, A. FDA approves 100th monoclonal antibody product. Nat. Rev. Drug Discov. 2021, 20, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Wagner-Rousset, E.; Ayoub, D.; Van Dorsselaer, A.; Sanglier-Cianférani, S. Characterization of Therapeutic Antibodies and Related Products. Anal. Chem. 2013, 85, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-R.; Song, A.; Bergelson, S.; Arroll, T.; Parekh, B.; May, K.; Chung, S.; Strouse, R.; Mire-Sluis, A.; Schenerman, M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat. Rev. Drug Discov. 2011, 10, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Cymer, F.; Beck, H.; Rohde, A.; Reusch, D. Therapeutic monoclonal antibody N-glycosylation—Structure, function and therapeutic potential. Biologicals 2018, 52, 1–11. [Google Scholar] [CrossRef]
- Higel, F.; Seidl, A.; Sörgel, F.; Friess, W. N-glycosylation heterogeneity and the influence on structure, function and pharmacokinetics of monoclonal antibodies and Fc fusion proteins. Eur. J. Pharm. Biopharm. 2016, 100, 94–100. [Google Scholar] [CrossRef]
- Aich, U.; Lakbub, J.; Liu, A. State-of-the-art technologies for rapid and high-throughput sample preparation and analysis of N -glycans from antibodies. Electrophoresis 2016, 37, 1468–1488. [Google Scholar] [CrossRef] [PubMed]
- Reusch, D.; Tejada, M.L. Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology 2015, 25, 1325–1334. [Google Scholar] [CrossRef]
- Duivelshof, B.L.; Jiskoot, W.; Beck, A.; Veuthey, J.-L.; Guillarme, D.; D’Atri, V. Glycosylation of biosimilars: Recent advances in analytical characterization and clinical implications. Anal. Chim. Acta 2019, 1089, 1–18. [Google Scholar] [CrossRef]
- Mastrangeli, R.; Palinsky, W.; Bierau, H. Glycoengineered antibodies: Towards the next-generation of immunotherapeutics. Glycobiology 2019, 29, 199–210. [Google Scholar] [CrossRef]
- Szekrényes, Á.; Park, S.S.; Cosgrave, E.; Jones, A.; Haxo, T.; Kimzey, M.; Pourkaveh, S.; Szabó, Z.; Sosic, Z.; Feng, P.; et al. Multi-site N-Glycan mapping study 2: UHPLC. Electrophoresis 2018, 39, 998–1005. [Google Scholar] [CrossRef]
- Reusch, D.; Haberger, M.; Maier, B.; Maier, M.; Kloseck, R.; Zimmermann, B.; Hook, M.; Szabo, Z.; Tep, S.; Wegstein, J.; et al. Comparison of methods for the analysis of therapeutic immunoglobulin G Fc-glycosylation profiles—Part 1: Separation-based methods. MAbs 2015, 7, 167–179. [Google Scholar] [CrossRef]
- Zhou, S.; Veillon, L.; Dong, X.; Huang, Y.; Mechref, Y. Direct comparison of derivatization strategies for LC-MS/MS analysis of N -glycans. Analyst 2017, 142, 4446–4455. [Google Scholar] [CrossRef]
- Lauber, M.A.; Thomson, J.; Connor, S.O.; Brousmiche, D.W.; Hua, Z.; Mccarthy, S.M.; Koza, S.M.; Guthrie, E.; Magnelli, P.; Taron, C.H.; et al. Rapid Preparation of Released N-Glycans for Hilic Analysis Using a Novel Fluorescence and Ms-Active Labeling Reagent. Anal. Chem. 2015, 87, 5401–5409. [Google Scholar] [CrossRef]
- Kimzey, M.; Szabo, Z.; Sharma, V.; Gyenes, A.; Tep, S.; Taylor, A.; Jones, A.; Hyche, J.; Haxo, T.; Vlasenko, S. Development of an Instant Glycan Labeling Dye for High Throughput Analysis by Mass Spectrometry. Available online: https://www.europa-bioproducts.com/pdf/IPC_Glycan-Labeling-Dye-2015_handout_v3_r2.pdf (accessed on 25 August 2021).
- Keser, T.; Pavić, T.; Lauc, G.; Gornik, O. Comparison of 2-Aminobenzamide, Procainamide and RapiFluor-MS as Derivatizing Agents for High-Throughput HILIC-UPLC-FLR-MS N-glycan Analysis. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, V.; Nováková, L.; Fekete, S.; Stoll, D.; Lauber, M.; Beck, A.; Guillarme, D. Orthogonal Middle-up Approaches for Characterization of the Glycan Heterogeneity of Etanercept by Hydrophilic Interaction Chromatography Coupled to High-Resolution Mass Spectrometry. Anal. Chem. 2019, 91, 873–880. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, V.; Fekete, S.; Beck, A.; Lauber, M.; Guillarme, D. Hydrophilic Interaction Chromatography Hyphenated with Mass Spectrometry: A Powerful Analytical Tool for the Comparison of Originator and Biosimilar Therapeutic Monoclonal Antibodies at the Middle-up Level of Analysis. Anal. Chem. 2017, 89, 2086–2092. [Google Scholar] [CrossRef]
- D’Atri, V.; Fekete, S.; Stoll, D.; Lauber, M.; Beck, A.; Guillarme, D. Characterization of an antibody-drug conjugate by hydrophilic interaction chromatography coupled to mass spectrometry. J. Chromatogr. B 2018, 1080, 37–41. [Google Scholar] [CrossRef]
- D’Atri, V.; Dumont, E.; Vandenheede, I.; Guillarme, D.; Sandra, P.; Sandra, K. Hydrophilic interaction chromatography for the characterization of therapeutic monoclonal antibodies at protein, peptide, and glycan levels. LC-GC Eur. 2017, 30, 424–434. [Google Scholar]
- Bobály, B.; D’Atri, V.; Beck, A.; Guillarme, D.; Fekete, S. Analysis of recombinant monoclonal antibodies in hydrophilic interaction chromatography: A generic method development approach. J. Pharm. Biomed. Anal. 2017, 145, 24–32. [Google Scholar] [CrossRef]
- D’Atri, V.; Goyon, A.; Bobaly, B.; Beck, A.; Fekete, S.; Guillarme, D. Protocols for the analytical characterization of therapeutic monoclonal antibodies. III—Denaturing chromatographic techniques hyphenated to mass spectrometry. J. Chromatogr. B 2018, 1096, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Duivelshof, B.L.; Murisier, A.; Camperi, J.; Fekete, S.; Beck, A.; Guillarme, D.; D’Atri, V. Therapeutic Fc-fusion proteins: Current analytical strategies. J. Sep. Sci. 2020, 1–28. [Google Scholar] [CrossRef] [PubMed]
- D’Atri, V.; Guillarme, D. Characterization of Glycosylated Proteins at Subunit Level by HILIC/MS. In Mass Spectrometry of Glycoproteins. Methods in Molecular Biology; Delobel, A., Ed.; Humana: New York, NY, USA, 2021; pp. 85–95. [Google Scholar]
- Camperi, J.; Dai, L.; Guillarme, D.; Stella, C. Development of a 3D-LC/MS Workflow for Fast, Automated, and Effective Characterization of Glycosylation Patterns of Biotherapeutic Products. Anal. Chem. 2020, 92, 4357–4363. [Google Scholar] [CrossRef] [PubMed]
- Bobaly, B.; D’Atri, V.; Goyon, A.; Colas, O.; Beck, A.; Fekete, S.; Guillarme, D. Protocols for the analytical characterization of therapeutic monoclonal antibodies. II—Enzymatic and chemical sample preparation. J. Chromatogr. B 2017, 1060, 325–335. [Google Scholar] [CrossRef]
- Sokolowska, I.; Mo, J.; Pirkolachahi, F.R.; Mcvean, C.; Meijer, L.A.T.; Switzar, L.; Balog, C.; Lewis, M.J.; Hu, P. Implementation of a High-Resolution Liquid Chromatography—Mass Spectrometry Method in Quality Control Laboratories for Release and Stability Testing of a Commercial Antibody Product. Anal. Chem. 2020, 92, 2369–2373. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Migliore, N.; Mehrman, S.J.; Cunningham, J.; Lewis, M.J.; Hu, P. High-Throughput, Automated Protein A Purification Platform with Multiattribute LC–MS Analysis for Advanced Cell Culture Process Monitoring. Anal. Chem. 2016, 88, 8673–8679. [Google Scholar] [CrossRef]
- Waters Care and Use Manual 715004903—GlycoWorks RapiFluor-MS N-Glycan Kit—24 Samples. Available online: https://www.waters.com/webassets/cms/support/docs/715004903.pdf (accessed on 25 August 2021).
- McCall, S.A.; Lauber, M.A.; Koza, S.M.; Chambers, E.E. Waters Application Note 720005516EN—Profiling Released High Mannose and Complex N-Glycan Structures from Monoclonal Antibodies Using RapiFluor-MS Labeling and Optimized Hydrophilic Interaction Chromatography. Available online: https://www.waters.com/webassets/cms/library/docs/720005516en.pdf (accessed on 25 August 2021).
- Wang, Q.; Lauber, M.A. Waters Application Note 720005850EN—Optimizing HILIC-based Analyses of RapidFluor-MS Labeled Sialylated N-Glycans. Available online: https://www.waters.com/webassets/cms/library/docs/720005850en.pdf (accessed on 25 August 2021).
- Raju, T.S. Glycosylation Variations with Expression Systems and Their Impact on Biological Activity of Therapeutic Immunoglobulins. Available online: https://www.qa-bio.com/docs/T.Raju_Glycosylation_Variation_in_Expression_Systems.pdf (accessed on 25 August 2021).
- Tebbey, P.W.; Varga, A.; Naill, M.; Clewell, J.; Venema, J. Consistency of quality attributes for the glycosylated monoclonal antibody Humira® (adalimumab). MAbs 2015, 7, 805–811. [Google Scholar] [CrossRef]
- Giorgetti, J.; D’Atri, V.; Canonge, J.; Lechner, A.; Guillarme, D.; Colas, O.; Wagner-Rousset, E.; Beck, A.; Leize-Wagner, E.; François, Y.-N. Monoclonal antibody N-glycosylation profiling using capillary electrophoresis—Mass spectrometry: Assessment and method validation. Talanta 2018, 178, 530–537. [Google Scholar] [CrossRef]
- Pisupati, K.; Tian, Y.; Okbazghi, S.; Benet, A.; Ackermann, R.; Ford, M.; Saveliev, S.; Hosfield, C.M.; Urh, M.; Carlson, E.; et al. A Multidimensional Analytical Comparison of Remicade and the Biosimilar Remsima. Anal. Chem. 2017, 89, 4838–4846. [Google Scholar] [CrossRef]
- Lee, C.; Jeong, M.; Lee, J.J.; Seo, S.; Cho, S.C.; Zhang, W.; Jaquez, O. Glycosylation profile and biological activity of Remicade® compared with Flixabi® and Remsima®. MAbs 2017, 9, 968–977. [Google Scholar] [CrossRef]
- Fang, J.; Doneanu, C.; Alley, W.R.; Yu, Y.Q.; Beck, A.; Chen, W. Advanced assessment of the physicochemical characteristics of Remicade® and Inflectra® by sensitive LC/MS techniques. MAbs 2016, 8, 1021–1034. [Google Scholar] [CrossRef][Green Version]
- Wagner-Rousset, E.; Colas, O.; Chenu, S.; François, Y.-N.; Guillarme, D.; Cianferani, S.; Tsybin, Y.O.; Sjögren, J.; Delobel, A.; Beck, A. Fast Afucosylation Profiling of Glycoengineered Antibody Subunits by Middle-Up Mass Spectrometry. In Mass Spectrometry of Glycoproteins. Methods in Molecular Biology; Delobel, A., Ed.; Humana: New York, NY, USA, 2021; pp. 73–83. [Google Scholar]
- Beck, A.; Reichert, J.M. Marketing approval of mogamulizumab. MAbs 2012, 4, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, J.; Aich, U.; Hurum, D.; Basumallick, L.; Pohl, C.; Kandzia, S. Thermo Scientific Application Note PN71420-EN—2-Amino Benzamide Labeling of Oligosaccharides: How Mich Sialic Acid Is Lost? Available online: https://assets.thermofisher.com/TFS-Assets/CMD/posters/PN-71420-IC-LC-MS-Amino-Benzamide-Oligosaccharides-PN71420-EN.pdf (accessed on 25 August 2021).
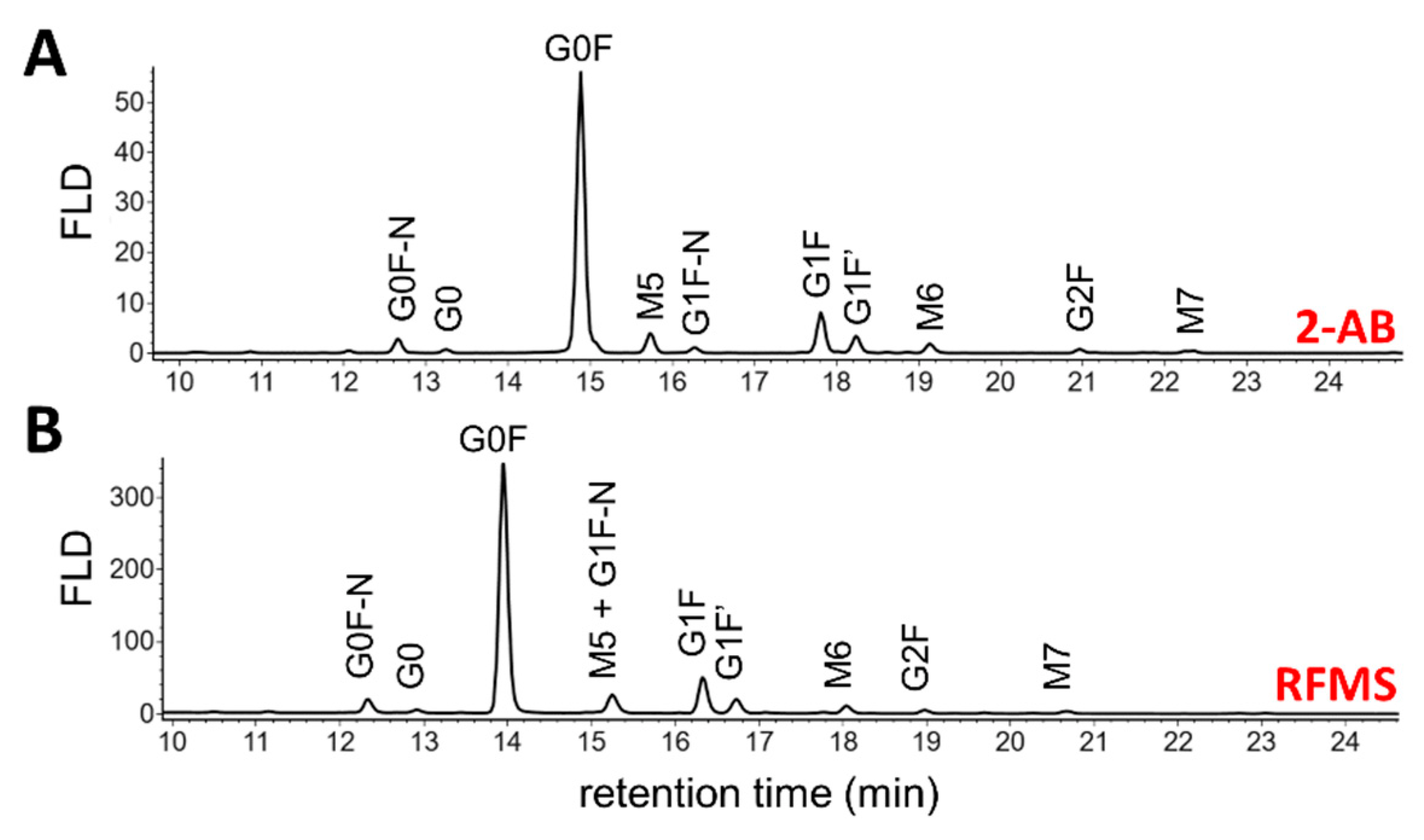

| Glycan Species | 2-AB | RFMS | Middle-Up | |||
|---|---|---|---|---|---|---|
| FLR | MS | FLR | MS | FLR | MS (XIC) | |
| G0F | 71.5 (0.4) | n.q. | 70.3 (0.3) | 69.8 (1.3) | 64.7 (2.3) | 71.5 (0.4) |
| G1F | 10.3 (0.1) | n.q. | 10.3 (0.1) | 9.9 (0.4) | 12.7 (0.2) | 15.2 (0.4) |
| G1F’ | 4.3 (<0.1) | n.q. | 4.3 (0.1) | 4.3 (0.3) | 3.5 (0.1) | n.q. |
| M5 | 4.6 (0.4) | n.q. | 6.1 (<0.1) | 6.8 (<0.1) | 8.3 (0.3) | 4.7 (0.3) |
| G0F-N | 3.3 (0.1) | n.q. | 3.8 (<0.1) | 4.2 (0.2) | 4.6 (0.6) | 2.8 (<0.1) |
| M6 | 2.3 (0.2) | n.d. | 2.3 (<0.1) | 2.3 (0.1) | 3.3 (0.4) | 2.2 (0.2) |
| G1F-N | 1.3 (<0.1) | n.d. | 0.2 (<0.1) | n.d. | n.d. | 1 (0.2) |
| G2F | 1.0 (<0.1) | n.d. | 1.0 (<0.1) | 1.0 (0.1) | 1.5 (0.4) | 0.7 (0.1) |
| G0 | 0.9 (<0.1) | n.d. | 0.9 (<0.1) | 0.9 (<0.1) | n.d. | 0.9 (<0.1) |
| M7 | 0.5 (<0.1) | n.d. | 0.9 (<0.1) | 1.0 (0.3) | 1.5 (0.4) | 1.0 (0.1) |
| Name (scFc-K + Glycan) | Mass | RT | Relative Abundance | Charge States | |||
|---|---|---|---|---|---|---|---|
| (min) | (%, n = 3) | Stdev | 12+ | 13+ | 14+ | ||
| G0F | 25,199.88 | 5.69 | 71.5 | 0.4 | 2101.00 | 1939.46 | 1801.00 |
| G1F | 25,362.02 | 6.11 | 15.2 | 0.4 | 2114.51 | 1951.93 | 1812.58 |
| M5 | 24,971.63 | 5.88 | 4.7 | 0.3 | 2081.98 | 1921.90 | 1784.70 |
| G0F-N | 24,996.68 | 5.53 | 2.8 | 0.0 | 2084.06 | 1923.83 | 1786.48 |
| M6 | 25,133.77 | 6.34 | 2.2 | 0.2 | 2095.49 | 1934.37 | 1796.28 |
| G1F-N | 25,158.83 | 6.01 | 1.0 | 0.2 | 2097.58 | 1936.30 | 1798.07 |
| G2F | 25,524.16 | 6.59 | 0.7 | 0.1 | 2128.02 | 1964.40 | 1824.16 |
| G0 | 25,053.74 | 5.54 | 0.9 | 0.1 | 2088.82 | 1928.22 | 1790.56 |
| M7 | 25,295.91 | 6.78 | 1.0 | 0.1 | 2109.00 | 1946.85 | 1807.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duivelshof, B.L.; Denorme, S.; Sandra, K.; Liu, X.; Beck, A.; Lauber, M.A.; Guillarme, D.; D’Atri, V. Quantitative N-Glycan Profiling of Therapeutic Monoclonal Antibodies Performed by Middle-Up Level HILIC-HRMS Analysis. Pharmaceutics 2021, 13, 1744. https://doi.org/10.3390/pharmaceutics13111744
Duivelshof BL, Denorme S, Sandra K, Liu X, Beck A, Lauber MA, Guillarme D, D’Atri V. Quantitative N-Glycan Profiling of Therapeutic Monoclonal Antibodies Performed by Middle-Up Level HILIC-HRMS Analysis. Pharmaceutics. 2021; 13(11):1744. https://doi.org/10.3390/pharmaceutics13111744
Chicago/Turabian StyleDuivelshof, Bastiaan L., Steffy Denorme, Koen Sandra, Xiaoxiao Liu, Alain Beck, Matthew A. Lauber, Davy Guillarme, and Valentina D’Atri. 2021. "Quantitative N-Glycan Profiling of Therapeutic Monoclonal Antibodies Performed by Middle-Up Level HILIC-HRMS Analysis" Pharmaceutics 13, no. 11: 1744. https://doi.org/10.3390/pharmaceutics13111744
APA StyleDuivelshof, B. L., Denorme, S., Sandra, K., Liu, X., Beck, A., Lauber, M. A., Guillarme, D., & D’Atri, V. (2021). Quantitative N-Glycan Profiling of Therapeutic Monoclonal Antibodies Performed by Middle-Up Level HILIC-HRMS Analysis. Pharmaceutics, 13(11), 1744. https://doi.org/10.3390/pharmaceutics13111744