Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery
Abstract
1. Introduction
2. Chitosan Cell Interactions
2.1. Effect of pH and Zeta Potential on Cell Membrane
2.2. Effect of Chitosan on Cell Adhesion
2.3. Effect on Tight Junctions
2.4. Chitosan and Transepithelial Electrical Resistance (TEER)
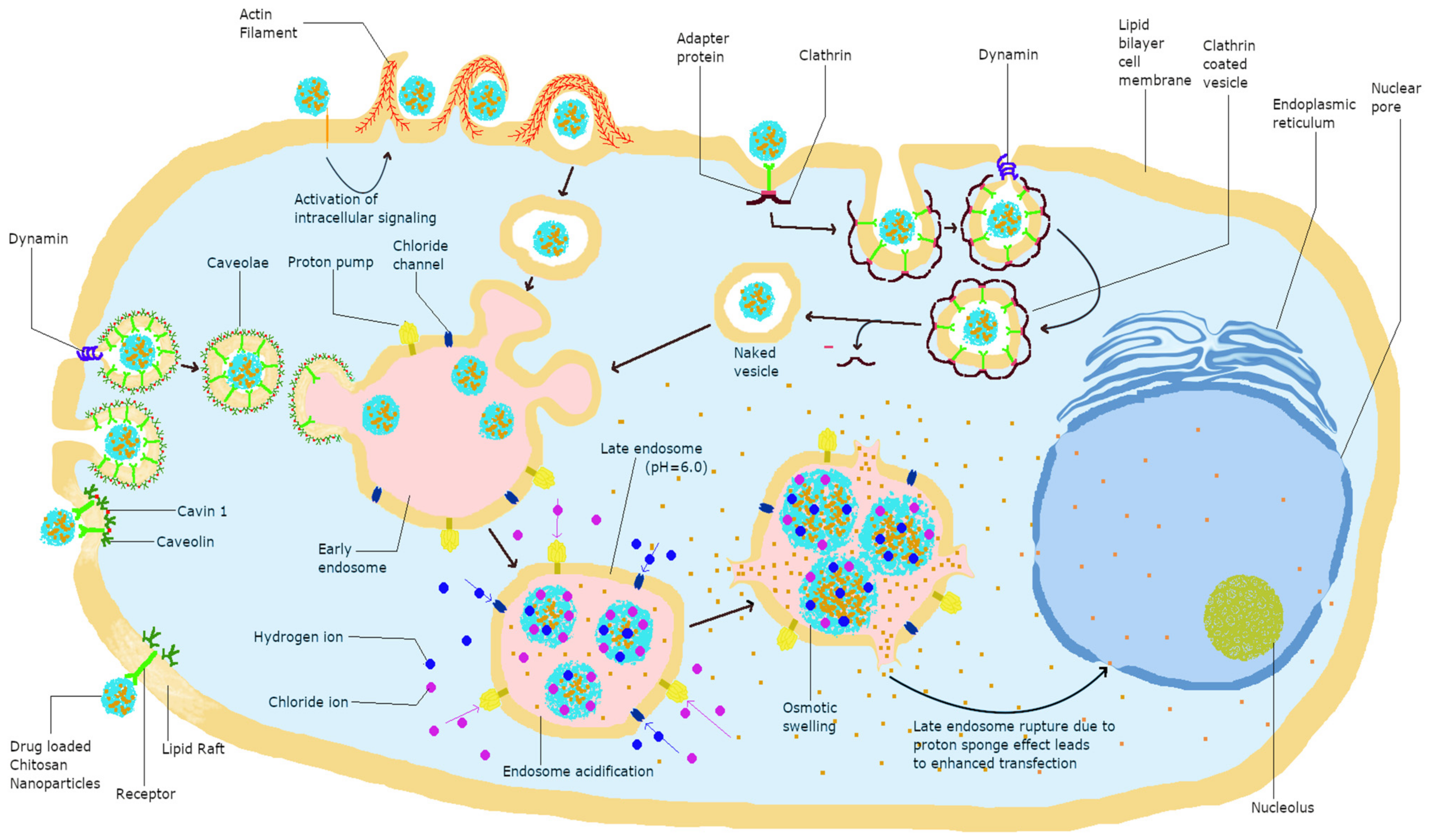
3. Pathways of Cellular Uptake
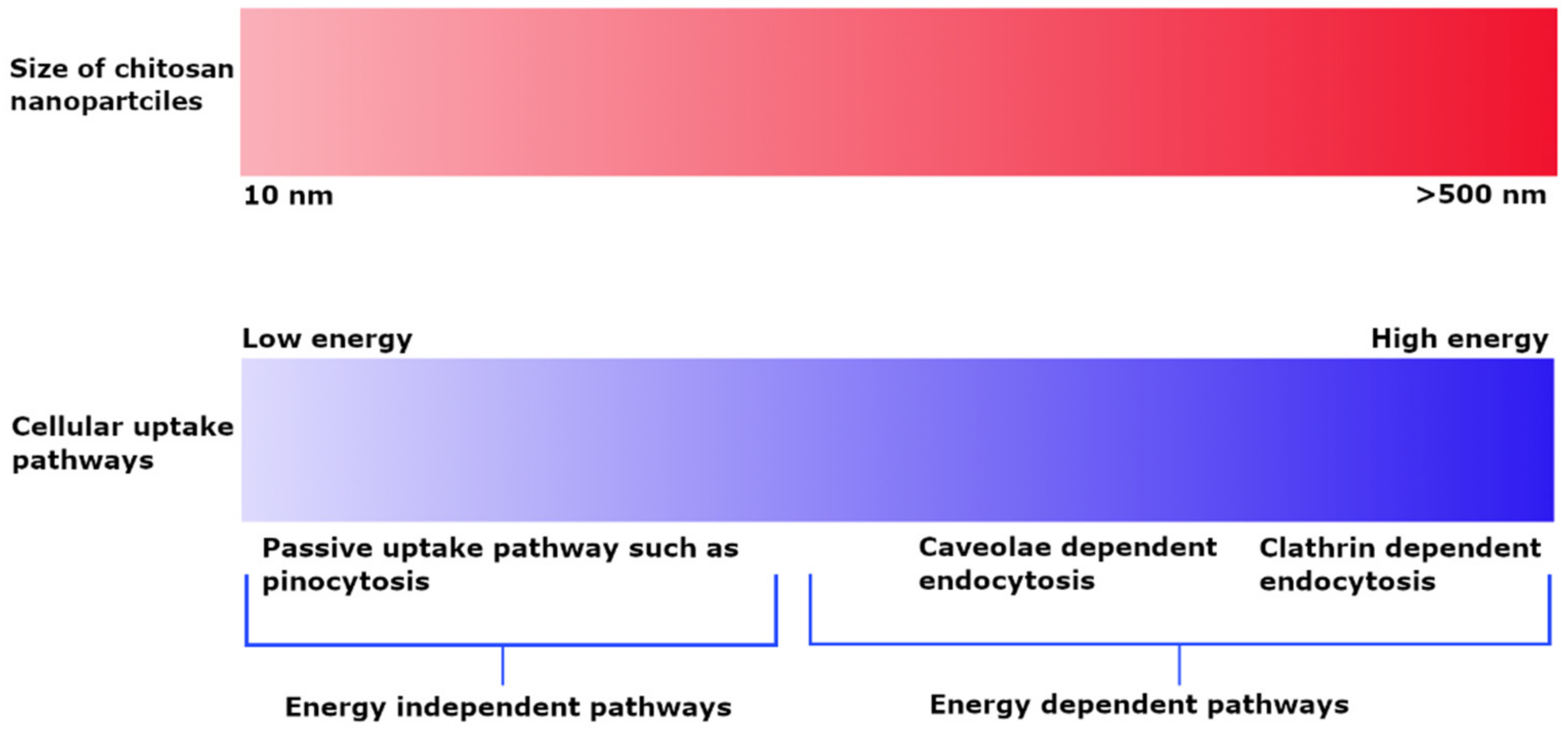
3.1. Effect of Size and Charge on Uptake Pathway
3.2. Effect of Hydrophobicity/Hydrophilicity on Uptake Pathway
3.3. Covalent Modifications
4. Intracellular Disposition of Chitosan
4.1. Endosomal Escape
4.2. Co-Localization with Lysosomes
4.3. Nuclear and Perinuclear Localization
4.4. Mitochondrial Metabolism
4.5. Exocytosis of Chitosan Nanoparticles
4.6. Cytotoxicity upon Cell Internalization
5. In Vivo Tissue Distribution and Bioavailability of Chitosan Nanoparticles
5.1. Effect of Protein Corona
5.2. Effect of Mucosal Routes of Administration
5.3. Effect of Surface Modifications
5.4. Effect of Physical Properties on Biodistribution
6. Systemic Toxicity and Elimination of Chitosan Nanoparticles
7. Current Clinical Investigations and Challenges
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Calvo, P.; Vila-Jato, J.L.; Alonso, M.J. Evaluation of Cationic Polymer-Coated Nanocapsules as Ocular Drug Carriers. Int. J. Pharm. 1997, 153, 41–50. [Google Scholar] [CrossRef]
- Berthold, A.; Cremer, K.; Kreuter, J. Preparation and Characterization of Chitosan Microspheres as Drug Carrier for Prednisolone Sodium Phosphate as Model for Anti-Inflammatory Drugs. J. Control Release 1996, 39, 17–25. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef] [PubMed]
- Moraru, C.; Mincea, M.; Menghiu, G.; Ostafe, V. Understanding the Factors Influencing Chitosan-Based Nanoparticles-Protein Corona Interaction and Drug Delivery Applications. Molecules 2020, 25, 4758. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. Cellular Screening Methods for the Study of Nanoparticle- Induced Lysosomal Damage. In Lysosomes—Associated Diseases and Methods to Study Their Function; Sharma, P.D., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3507-4. [Google Scholar]
- Jiang, L.Q.; Wang, T.Y.; Webster, T.J.; Duan, H.-J.; Qiu, J.Y.; Zhao, Z.M.; Yin, X.-X.; Zheng, C.L. Intracellular Disposition of Chitosan Nanoparticles in Macrophages: Intracellular Uptake, Exocytosis, and Intercellular Transport. Int. J. Nanomed. 2017, 12, 6383–6398. [Google Scholar] [CrossRef]
- Pilipenko, I.; Korzhikov-Vlakh, V.; Sharoyko, V.; Zhang, N.; Schäfer-Korting, M.; Rühl, E.; Zoschke, C.; Tennikova, T. PH-Sensitive Chitosan–Heparin Nanoparticles for Effective Delivery of Genetic Drugs into Epithelial Cells. Pharmaceutics 2019, 11, 317. [Google Scholar] [CrossRef]
- Nam, J.-P.; Nah, J.-W. Target Gene Delivery from Targeting Ligand Conjugated Chitosan–PEI Copolymer for Cancer Therapy. Carbohydr. Polym. 2016, 135, 153–161. [Google Scholar] [CrossRef]
- Chen, W.; Li, F.; Tang, Y.; Yang, S.; Li, J.; Yuan, Z.; Liu, Y.; Zhou, X.; Liu, C.; Zhang, X. Stepwise PH-Responsive Nanoparticles for Enhanced Cellular Uptake and on-Demand Intracellular Release of Doxorubicin. Int. J. Nanomed. 2017, 12, 4241–4256. [Google Scholar] [CrossRef]
- Chen, G.; Svirskis, D.; Lu, W.; Ying, M.; Huang, Y.; Wen, J. N-Trimethyl Chitosan Nanoparticles and CSKSSDYQC Peptide: N-Trimethyl Chitosan Conjugates Enhance the Oral Bioavailability of Gemcitabine to Treat Breast Cancer. J. Control Release 2018, 277, 142–153. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef]
- Yang, N.J.; Hinner, M.J. Getting Across the Cell Membrane: An Overview for Small Molecules, Peptides, and Proteins. Site-Specif. Protein Labeling 2015, 1266, 29–53. [Google Scholar] [CrossRef]
- Magalhaes, M.A.O.; Glogauer, M. Pivotal Advance: Phospholipids Determine Net Membrane Surface Charge Resulting in Differential Localization of Active Rac1 and Rac2. J. Leukoc. Biol. 2010, 87, 545–555. [Google Scholar] [CrossRef]
- Schipper, N.G.M.; Vårum, K.M.; Artursson, P. Chitosans as Absorption Enhancers for Poorly Absorbable Drugs. 1: Influence of Molecular Weight and Degree of Acetylation on Drug Transport Across Human Intestinal Epithelial (Caco-2) Cells. Pharm. Res. 1996, 13, 1686–1692. [Google Scholar] [CrossRef]
- Kotzé, A.F.; de Leeuw, B.J.; Lueßen, H.L.; de Boer, A.G.; Verhoef, J.C.; Junginger, H.E. Chitosans for Enhanced Delivery of Therapeutic Peptides across Intestinal Epithelia: In Vitro Evaluation in Caco-2 Cell Monolayers. Int. J. Pharm. 1997, 159, 243–253. [Google Scholar] [CrossRef]
- Yue, Z.-G.; Wei, W.; Lv, P.-P.; Yue, H.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. Surface Charge Affects Cellular Uptake and Intracellular Trafficking of Chitosan-Based Nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- Boyles, M.S.P.; Kristl, T.; Andosch, A.; Zimmermann, M.; Tran, N.; Casals, E.; Himly, M.; Puntes, V.; Huber, C.G.; Lütz-Meindl, U.; et al. Chitosan Functionalisation of Gold Nanoparticles Encourages Particle Uptake and Induces Cytotoxicity and Pro-Inflammatory Conditions in Phagocytic Cells, as Well as Enhancing Particle Interactions with Serum Components. J. Nanobiotechnol. 2015, 13, 84. [Google Scholar] [CrossRef]
- Hu, C.; Chiang, C.; Hong, P.; Yeh, M. Influence of Charge on FITC-BSA-Loaded Chondroitin Sulfate-Chitosan Nanoparticles upon Cell Uptake in Human Caco-2 Cell Monolayers. Int. J. Nanomed. 2012, 7, 4861–4872. [Google Scholar] [CrossRef][Green Version]
- Kalliola, S.; Repo, E.; Srivastava, V.; Zhao, F.; Heiskanen, J.P.; Sirviö, J.A.; Liimatainen, H.; Sillanpää, M. Carboxymethyl Chitosan and Its Hydrophobically Modified Derivative as PH-Switchable Emulsifiers. Langmuir 2018, 34, 2800–2806. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, D.R.; Tavano, L.; Mitjans, M.; Pérez, L.; Infante, M.R.; Vinardell, M.P. In Vitro Antitumor Activity of Methotrexate via PH-Sensitive Chitosan Nanoparticles. Biomaterials 2013, 34, 2758–2772. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, Z.; Park, J.; Lin, Q.; Yeo, Y. Low Molecular-Weight Chitosan as a PH-Sensitive Stealth Coating for Tumor-Specific Drug Delivery. Mol. Pharm. 2012, 9, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Khalili, A.; Ahmad, M. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chung, Y.-C.; Wang, I.-J.; Young, T.-H. Control of Cell Attachment on PH-Responsive Chitosan Surface by Precise Adjustment of Medium PH. Biomaterials 2012, 33, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Rocha Neto, J.B.M.; Taketa, T.B.; Bataglioli, R.A.; Pimentel, S.B.; Santos, D.M.; Fiamingo, A.; Costa, C.A.R.; Campana-Filho, S.P.; Carvalho, H.F.; Beppu, M.M. Tailored Chitosan/Hyaluronan Coatings for Tumor Cell Adhesion: Effects of Topography, Charge Density and Surface Composition. Appl. Surf. Sci. 2019, 486, 508–518. [Google Scholar] [CrossRef]
- Noriega, S.E.; Subramanian, A. Consequences of Neutralization on the Proliferation and Cytoskeletal Organization of Chondrocytes on Chitosan-Based Matrices. Int. J. Carbohydr. Chem. 2011, 2011, 1–13. [Google Scholar] [CrossRef]
- Lü, X.; Zhang, H.; Huang, Y.; Zhang, Y. A Proteomics Study to Explore the Role of Adsorbed Serum Proteins for PC12 Cell Adhesion and Growth on Chitosan and Collagen/Chitosan Surfaces. Regen. Biomater. 2018, 5, 261–273. [Google Scholar] [CrossRef]
- Lim, S.M.; Song, D.K.; Cho, K.J.; Oh, S.H.; Lee-Yoon, D.S.; Bae, E.H.; Lee, J.H. Cell Adhesion and Degradation Behaviors of Acetylated Chitosan Films. In Proceedings of the 3rd Kuala Lumpur International Conference on Biomedical Engineering 2006, Kuala Lumpur, Malaysia, 11–14 December 2006; Ibrahim, F., Osman, N.A.A., Usman, J., Kadri, N.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 94–97. [Google Scholar]
- Foster, L.J.R.; Ho, S.; Hook, J.; Basuki, M.; Marçal, H. Chitosan as a Biomaterial: Influence of Degree of Deacetylation on Its Physiochemical, Material and Biological Properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef]
- Dixit, S.; Baganizi, D.R.; Sahu, R.; Dosunmu, E.; Chaudhari, A.; Vig, K.; Pillai, S.R.; Singh, S.R.; Dennis, V.A. Immunological Challenges Associated with Artificial Skin Grafts: Available Solutions and Stem Cells in Future Design of Synthetic Skin. J. Biol. Eng. 2017, 11, 49. [Google Scholar] [CrossRef]
- Salati, A.; Keshvari, H.; Karkhaneh, A.; Taranejoo, S. Design and Fabrication of Artificial Skin: Chitosan and Gelatin Immobilization on Silicone by Poly Acrylic Acid Graft Using a Plasma Surface Modification Method. J. Macromol. Sci. Part B 2011, 50, 1972–1982. [Google Scholar] [CrossRef]
- Vijayan, A.; Sabareeswaran, A.; Kumar, G.S.V. PEG Grafted Chitosan Scaffold for Dual Growth Factor Delivery for Enhanced Wound Healing. Sci. Rep. 2019, 9, 19165. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A Functional Chitosan-Based Hydrogel as a Wound Dressing and Drug Delivery System in the Treatment of Wound Healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Cho, M.O.; Li, Z.; Shim, H.-E.; Cho, I.-S.; Nurunnabi, M.; Park, H.; Lee, K.Y.; Moon, S.-H.; Kim, K.-S.; Kang, S.-W.; et al. Bioinspired Tuning of Glycol Chitosan for 3D Cell Culture. NPG Asia Mater. 2016, 8, e309. [Google Scholar] [CrossRef]
- Kafi, M.A.; Aktar, K.; Todo, M.; Dahiya, R. Engineered Chitosan for Improved 3D Tissue Growth through Paxillin-FAK-ERK Activation. Regen. Biomater. 2020, 7, 141–151. [Google Scholar] [CrossRef]
- Yeh, T.-H.; Hsu, L.-W.; Tseng, M.T.; Lee, P.-L.; Sonjae, K.; Ho, Y.-C.; Sung, H.-W. Mechanism and Consequence of Chitosan-Mediated Reversible Epithelial Tight Junction Opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef]
- Sonaje, K.; Lin, K.-J.; Tseng, M.T.; Wey, S.-P.; Su, F.-Y.; Chuang, E.-Y.; Hsu, C.-W.; Chen, C.-T.; Sung, H.-W. Effects of Chitosan-Nanoparticle-Mediated Tight Junction Opening on the Oral Absorption of Endotoxins. Biomaterials 2011, 32, 8712–8721. [Google Scholar] [CrossRef]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Bhat, A.A.; Uppada, S.; Achkar, I.W.; Hashem, S.; Yadav, S.K.; Shanmugakonar, M.; Al-Naemi, H.A.; Haris, M.; Uddin, S. Tight Junction Proteins and Signaling Pathways in Cancer and Inflammation: A Functional Crosstalk. Front. Physiol. 2019, 9, 1942. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight Junctions: From Simple Barriers to Multifunctional Molecular Gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kong, M.; Zhou, Z.; Yan, D.; Yu, X.; Cheng, X.; Feng, C.; Liu, Y.; Chen, X. Mechanism of Surface Charge Triggered Intestinal Epithelial Tight Junction Opening upon Chitosan Nanoparticles for Insulin Oral Delivery. Carbohydr. Polym. 2017, 157, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for In Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Wood, E.; Dornish, M. Effect of Chitosan on Epithelial Cell Tight Junctions. Pharm. Res. 2004, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Vllasaliu, D.; Exposito-Harris, R.; Heras, A.; Casettari, L.; Garnett, M.; Illum, L.; Stolnik, S. Tight Junction Modulation by Chitosan Nanoparticles: Comparison with Chitosan Solution. Int. J. Pharm. 2010, 400, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhu, D.; Li, S.; Yu, X.; Zhao, Y.; Ouyang, X.; Xie, Z.; Li, L. Transporting Carriers for Intracellular Targeting Delivery via Non-Endocytic Uptake Pathways. Drug Deliv. 2017, 24, 45–55. [Google Scholar] [CrossRef]
- Juliano, R.L.; Carver, K. Cellular Uptake and Intracellular Trafficking of Oligonucleotides. Adv. Drug Deliv. Rev. 2015, 87, 35–45. [Google Scholar] [CrossRef]
- Malatesta, M.; Grecchi, S.; Chiesa, E.; Cisterna, B.; Costanzo, M.; Zancanaro, C. Internalized Chitosan Nanoparticles Persist for Long Time in Cultured Cells. Eur. J. Histochem. 2015, 59, 2492. [Google Scholar] [CrossRef]
- Huang, M.; Ma, Z.; Khor, E.; Lim, L.-Y. Uptake of FITC-Chitosan Nanoparticles by A549 Cells. Pharm. Res. 2002, 19, 1488–1494. [Google Scholar] [CrossRef]
- Park, J.S.; Cho, Y.W. In Vitro Cellular Uptake and Cytotoxicity of Paclitaxel-Loaded Glycol Chitosan Self-Assembled Nanoparticles. Macromol. Res. 2007, 15, 513–519. [Google Scholar] [CrossRef]
- Tahara, K.; Sakai, T.; Yamamoto, H.; Takeuchi, H.; Hirashima, N.; Kawashima, Y. Improved Cellular Uptake of Chitosan-Modified PLGA Nanospheres by A549 Cells. Int. J. Pharm. 2009, 382, 198–204. [Google Scholar] [CrossRef]
- Koppolu, B.; Zaharoff, D.A. The Effect of Antigen Encapsulation in Chitosan Particles on Uptake, Activation and Presentation by Antigen Presenting Cells. Biomaterials 2013, 34, 2359–2369. [Google Scholar] [CrossRef]
- Tammam, S.N.; Azzazy, H.M.E.; Breitinger, H.G.; Lamprecht, A. Chitosan Nanoparticles for Nuclear Targeting: The Effect of Nanoparticle Size and Nuclear Localization Sequence Density. Mol. Pharm. 2015, 12, 4277–4289. [Google Scholar] [CrossRef]
- Fröhlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Zhou, C.; Jiao, Y. Hydrophobic Modifications of Cationic Polymers for Gene Delivery. Prog. Polym. Sci. 2010, 35, 1144–1162. [Google Scholar] [CrossRef]
- Nam, H.Y.; Kwon, S.M.; Chung, H.; Lee, S.-Y.; Kwon, S.-H.; Jeon, H.; Kim, Y.; Park, J.H.; Kim, J.; Her, S.; et al. Cellular Uptake Mechanism and Intracellular Fate of Hydrophobically Modified Glycol Chitosan Nanoparticles. J. Control. Release 2009, 135, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-L.; Ho, Y.-C.; Chen, Y.-M.; Peng, S.-F.; Ke, C.-J.; Chen, K.-J.; Mi, F.-L.; Sung, H.-W. The Characteristics, Cellular Uptake and Intracellular Trafficking of Nanoparticles Made of Hydrophobically-Modified Chitosan. J. Control. Release 2010, 146, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Haldar, M.K.; Sharma, G.; Lipp, L.; Mallik, S.; Singh, J. Hexanoic Acid and Polyethylene Glycol Double Grafted Amphiphilic Chitosan for Enhanced Gene Delivery: Influence of Hydrophobic and Hydrophilic Substitution Degree. Mol. Pharm. 2014, 11, 982–994. [Google Scholar] [CrossRef]
- Wang, B.; He, C.; Tang, C.; Yin, C. Effects of Hydrophobic and Hydrophilic Modifications on Gene Delivery of Amphiphilic Chitosan Based Nanocarriers. Biomaterials 2011, 32, 4630–4638. [Google Scholar] [CrossRef]
- Zhan, J.; Ting, X.L.; Zhu, J. The Research Progress of Targeted Drug Delivery Systems. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012017. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, L.; Hu, W.; Hu, Z.-H.; Bei, Y.-Y.; Xu, J.-Y.; Wang, W.-J.; Zhang, X.-N.; Zhang, Q. Norcantharidin-Associated Galactosylated Chitosan Nanoparticles for Hepatocyte-Targeted Delivery. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 371–381. [Google Scholar] [CrossRef]
- Liu, L.; Dong, X.; Zhu, D.; Song, L.; Zhang, H.; Leng, X.G. TAT-LHRH Conjugated Low Molecular Weight Chitosan as a Gene Carrier Specific for Hepatocellular Carcinoma Cells. Int. J. Nanomed. 2014, 9, 2879–2889. [Google Scholar] [CrossRef]
- Han, L.; Tang, C.; Yin, C. Dual-Targeting and PH/Redox-Responsive Multi-Layered Nanocomplexes for Smart Co-Delivery of Doxorubicin and SiRNA. Biomaterials 2015, 60, 42–52. [Google Scholar] [CrossRef]
- Shi, G.-N.; Zhang, C.-N.; Xu, R.; Niu, J.-F.; Song, H.-J.; Zhang, X.-Y.; Wang, W.-W.; Wang, Y.-M.; Li, C.; Wei, X.-Q.; et al. Enhanced Antitumor Immunity by Targeting Dendritic Cells with Tumor Cell Lysate-Loaded Chitosan Nanoparticles Vaccine. Biomaterials 2017, 113, 191–202. [Google Scholar] [CrossRef]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Overcoming Cisplatin Resistance in Non-Small Cell Lung Cancer with Mad2 Silencing SiRNA Delivered Systemically Using EGFR-Targeted Chitosan Nanoparticles. Acta Biomater. 2017, 47, 71–80. [Google Scholar] [CrossRef]
- Yang, H.; Tang, C.; Yin, C. Estrone-Modified PH-Sensitive Glycol Chitosan Nanoparticles for Drug Delivery in Breast Cancer. Acta Biomater. 2018, 73, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chi, T.; Li, T.; Zheng, G.; Fan, L.; Liu, Y.; Chen, X.; Chen, S.; Jia, L.; Shao, J. A Smart PH-Responsive Nano-Carrier as a Drug Delivery System for the Targeted Delivery of Ursolic Acid: Suppresses Cancer Growth and Metastasis by Modulating P53/MMP-9/PTEN/CD44 Mediated Multiple Signaling Pathways. Nanoscale 2017, 9, 9428–9439. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, A.; Khalil, I.H.; Arafa, K.; Emara, M.; El-Sherbiny, I.M. Dual-Ligand Functionalized Core-Shell Chitosan-Based Nanocarrier for Hepatocellular Carcinoma-Targeted Drug Delivery. Int. J. Nanomed. 2020, 15, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, F.; Guo, J.; Xue, J.; Qian, Z.; Gu, Y. Folate-Modified Chitosan Micelles with Enhanced Tumor Targeting Evaluated by near Infrared Imaging System. Carbohydr. Polym. 2011, 86, 1118–1129. [Google Scholar] [CrossRef]
- Ramya, A.N.; Joseph, M.M.; Maniganda, S.; Karunakaran, V.; Sreelekha, T.T.; Maiti, K.K. Emergence of Gold-Mesoporous Silica Hybrid Nanotheranostics: Dox-Encoded, Folate Targeted Chemotherapy with Modulation of SERS Fingerprinting for Apoptosis Toward Tumor Eradication. Small 2017, 13, 1700819. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Sharma, S.; Gupta, P.K.; Singh, A.; Teja, B.V.; Dwivedi, P.; Gupta, G.K.; Trivedi, R.; Mishra, P.R. Vitamin B12 Functionalized Layer by Layer Calcium Phosphate Nanoparticles: A Mucoadhesive and PH Responsive Carrier for Improved Oral Delivery of Insulin. Acta Biomater. 2016, 31, 288–300. [Google Scholar] [CrossRef]
- Rekha, M.R.; Sharma, C.P. Synthesis and Evaluation of Lauryl Succinyl Chitosan Particles towards Oral Insulin Delivery and Absorption. J. Control. Release 2009, 135, 144–151. [Google Scholar] [CrossRef]
- Zhao, R.; Li, T.; Zheng, G.; Jiang, K.; Fan, L.; Shao, J. Simultaneous Inhibition of Growth and Metastasis of Hepatocellular Carcinoma by Co-Delivery of Ursolic Acid and Sorafenib Using Lactobionic Acid Modified and PH-Sensitive Chitosan-Conjugated Mesoporous Silica Nanocomplex. Biomaterials 2017, 143, 1–16. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, C.; Liu, Y.; Yuan, Z.; Chen, W.; Yang, S.; Li, J.; Zhu, W.; Zhou, X.; You, B.; et al. CD147 Monoclonal Antibody Mediated by Chitosan Nanoparticles Loaded with α-Hederin Enhances Antineoplastic Activity and Cellular Uptake in Liver Cancer Cells. Sci. Rep. 2015, 5, 17904. [Google Scholar] [CrossRef]
- Shen, J.-M.; Tang, W.-J.; Zhang, X.-L.; Chen, T.; Zhang, H.-X. A Novel Carboxymethyl Chitosan-Based Folate/Fe3O4/CdTe Nanoparticle for Targeted Drug Delivery and Cell Imaging. Carbohydr. Polym. 2012, 88, 239–249. [Google Scholar] [CrossRef]
- Mathew, M.E.; Mohan, J.C.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Folate Conjugated Carboxymethyl Chitosan–Manganese Doped Zinc Sulphide Nanoparticles for Targeted Drug Delivery and Imaging of Cancer Cells. Carbohydr. Polym. 2010, 80, 442–448. [Google Scholar] [CrossRef]
- Guan, M.; Zhou, Y.; Zhu, Q.-L.; Liu, Y.; Bei, Y.-Y.; Zhang, X.-N.; Zhang, Q. N-Trimethyl Chitosan Nanoparticle-Encapsulated Lactosyl-Norcantharidin for Liver Cancer Therapy with High Targeting Efficacy. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1172–1181. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, Y.; Guan, M.; Zhou, X.; Yang, S.; Liu, Y.; Chen, W.; Zhang, C.; Yuan, Z.; Liu, C.; et al. Low-Density Lipoprotein-Coupled N-Succinyl Chitosan Nanoparticles Co-Delivering SiRNA and Doxorubicin for Hepatocyte-Targeted Therapy. Biomaterials 2014, 35, 5965–5976. [Google Scholar] [CrossRef]
- Chou, L.Y.T.; Ming, K.; Chan, W.C.W. Strategies for the Intracellular Delivery of Nanoparticles. Chem. Soc. Rev. 2011, 40, 233–245. [Google Scholar] [CrossRef]
- Cao, Y.; Tan, Y.F.; Wong, Y.S.; Liew, M.W.J.; Venkatraman, S. Recent Advances in Chitosan-Based Carriers for Gene Delivery. Mar. Drugs 2019, 17, 381. [Google Scholar] [CrossRef]
- Lai, W.-F.; Wong, W.-T. Design of Polymeric Gene Carriers for Effective Intracellular Delivery. Trends Biotechnol. 2018, 36, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Ma, J.; Wang, X.; Yuan, Z.; Wang, W. Convenient Preparation of Charge-Adaptive Chitosan Nanomedicines for Extended Blood Circulation and Accelerated Endosomal Escape. Nano Res. 2018, 11, 4278–4292. [Google Scholar] [CrossRef]
- Moreira, C.; Oliveira, H.; Pires, L.R.; Simões, S.; Barbosa, M.A.; Pêgo, A.P. Improving Chitosan-Mediated Gene Transfer by the Introduction of Intracellular Buffering Moieties into the Chitosan Backbone. Acta Biomater. 2009, 5, 2995–3006. [Google Scholar] [CrossRef]
- Chang, K.-L.; Higuchi, Y.; Kawakami, S.; Yamashita, F.; Hashida, M. Efficient Gene Transfection by Histidine-Modified Chitosan through Enhancement of Endosomal Escape. Bioconj. Chem. 2010, 21, 1087–1095. [Google Scholar] [CrossRef]
- Huang, G.; Chen, Q.; Wu, W.; Wang, J.; Chu, P.K.; Bai, H.; Tang, G. Reconstructed Chitosan with Alkylamine for Enhanced Gene Delivery by Promoting Endosomal Escape. Carbohydr. Polym. 2020, 227, 115339. [Google Scholar] [CrossRef]
- Rathore, B.; Sunwoo, K.; Jangili, P.; Kim, J.; Kim, J.H.; Huang, M.; Xiong, J.; Sharma, A.; Yang, Z.; Qu, J.; et al. Nanomaterial Designing Strategies Related to Cell Lysosome and Their Biomedical Applications: A Review. Biomaterials 2019, 211, 25–47. [Google Scholar] [CrossRef]
- Manshian, B.B.; Pokhrel, S.; Mädler, L.; Soenen, S.J. The Impact of Nanoparticle-Driven Lysosomal Alkalinization on Cellular Functionality. J. Nanobiotechnol. 2018, 16, 85. [Google Scholar] [CrossRef]
- Fong, D.; Grégoire-Gélinas, P.; Cheng, A.P.; Mezheritsky, T.; Lavertu, M.; Sato, S.; Hoemann, C.D. Lysosomal Rupture Induced by Structurally Distinct Chitosans Either Promotes a Type 1 IFN Response or Activates the Inflammasome in Macrophages. Biomaterials 2017, 129, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Thibault, M.; Astolfi, M.; Tran-Khanh, N.; Lavertu, M.; Darras, V.; Merzouki, A.; Buschmann, M.D. Excess Polycation Mediates Efficient Chitosan-Based Gene Transfer by Promoting Lysosomal Release of the Polyplexes. Biomaterials 2011, 32, 4639–4646. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ruiz, L.; de la Fuente, M.; Párraga, J.E.; López-García, A.; Fernández, I.; Seijo, B.; Sánchez, A.; Calonge, M.; Diebold, Y. Intracellular Trafficking of Hyaluronic Acid-Chitosan Oligomer-Based Nanoparticles in Cultured Human Ocular Surface Cells. Mol. Vis. 2011, 17, 279–290. [Google Scholar] [PubMed]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into Nanoparticle Cellular Uptake and Intracellular Targeting. J. Control Release 2014, 190, 485–499. [Google Scholar] [CrossRef]
- Tammam, S.N.; Azzazy, H.M.E.; Lamprecht, A. How Successful Is Nuclear Targeting by Nanocarriers? J. Control Release 2016, 229, 140–153. [Google Scholar] [CrossRef]
- Opanasopit, P.; Rojanarata, T.; Apirakaramwong, A.; Ngawhirunpat, T.; Ruktanonchai, U. Nuclear Localization Signal Peptides Enhance Transfection Efficiency of Chitosan/DNA Complexes. Int. J. Pharm. 2009, 382, 291–295. [Google Scholar] [CrossRef]
- Lien, C.-F.; Molnár, É.; Toman, P.; Tsibouklis, J.; Pilkington, G.J.; Górecki, D.C.; Barbu, E. In Vitro Assessment of Alkylglyceryl-Functionalized Chitosan Nanoparticles as Permeating Vectors for the Blood–Brain Barrier. Biomacromolecules 2012, 13, 1067–1073. [Google Scholar] [CrossRef]
- Barbieri, S.; Sonvico, F.; Como, C.; Colombo, G.; Zani, F.; Buttini, F.; Bettini, R.; Rossi, A.; Colombo, P. Lecithin/Chitosan Controlled Release Nanopreparations of Tamoxifen Citrate: Loading, Enzyme-Trigger Release and Cell Uptake. J. Control Release 2013, 167, 276–283. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, K.; Oh, Y.-K.; Kwon, S.-H.; Her, S.; Kim, I.-S.; Choi, K.; Lee, S.J.; Kim, H.; Lee, S.G. Tumor Specificity and Therapeutic Efficacy of Photosensitizer-Encapsulated Glycol Chitosan-Based Nanoparticles in Tumor-Bearing Mice. Biomaterials 2009, 30, 2929–2939. [Google Scholar] [CrossRef]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.J.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Zorov, S.D.; Babenko, V.A.; Zorov, D.B. Functional Significance of the Mitochondrial Membrane Potential. Biochem. Mosc. Suppl. Ser. Membr. Cell Biol. 2018, 12, 20–26. [Google Scholar] [CrossRef]
- Qi, L.-F.; Xu, Z.-R.; Li, Y.; Jiang, X.; Han, X.-Y. In Vitro Effects of Chitosan Nanoparticles on Proliferation of Human Gastric Carcinoma Cell Line MGC803 Cells. World J. Gastroenterol. 2005, 11, 5136–5141. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, X.; Su, C.; Zhao, L.; Shi, Y. Chitosan Nanoparticles Induced the Antitumor Effect in Hepatocellular Carcinoma Cells by Regulating ROS-Mediated Mitochondrial Damage and Endoplasmic Reticulum Stress. Artif. Cells Nanomed. Biotechnol. 2019, 47, 747–756. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, L.; Song, Y.; He, J.; Wu, L.; Zhao, C.; Xiao, Y.; Li, W.; Cai, B.; Cheng, H.; et al. Hierarchical Targeted Hepatocyte Mitochondrial Multifunctional Chitosan Nanoparticles for Anticancer Drug Delivery. Biomaterials 2015, 52, 240–250. [Google Scholar] [CrossRef]
- Hou, J.; Yu, X.; Shen, Y.; Shi, Y.; Su, C.; Zhao, L. Triphenyl Phosphine-Functionalized Chitosan Nanoparticles Enhanced Antitumor Efficiency Through Targeted Delivery of Doxorubicin to Mitochondria. Nanoscale Res. Lett. 2017, 12, 158. [Google Scholar] [CrossRef]
- Wang, T.; Hou, J.; Su, C.; Zhao, L.; Shi, Y. Hyaluronic Acid-Coated Chitosan Nanoparticles Induce ROS-Mediated Tumor Cell Apoptosis and Enhance Antitumor Efficiency by Targeted Drug Delivery via CD44. J. Nanobiotechnol. 2017, 15, 7. [Google Scholar] [CrossRef]
- Mallick, S.; Song, S.J.; Bae, Y.; Choi, J.S. Self-Assembled Nanoparticles Composed of Glycol Chitosan-Dequalinium for Mitochondria-Targeted Drug Delivery. Int. J. Biol. Macromol. 2019, 132, 451–460. [Google Scholar] [CrossRef]
- He, B.; Wu, F.; Fan, L.; Li, X.-H.; Liu, Y.; Liu, Y.-J.; Ding, W.-J.; Deng, M.; Zhou, Y. Carboxymethylated Chitosan Protects Schwann Cells against Hydrogen Peroxide-Induced Apoptosis by Inhibiting Oxidative Stress and Mitochondria Dependent Pathway. Eur. J. Pharmacol. 2018, 825, 48–56. [Google Scholar] [CrossRef]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In Vitro Toxicity Assessment of Chitosan Oligosaccharide Coated Iron Oxide Nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef]
- Park, J.H.; Oh, N. Endocytosis and Exocytosis of Nanoparticles in Mammalian Cells. Int. J. Nanomed. 2014, 9, 51. [Google Scholar] [CrossRef]
- Sakhtianchi, R.; Minchin, R.F.; Lee, K.-B.; Alkilany, A.M.; Serpooshan, V.; Mahmoudi, M. Exocytosis of Nanoparticles from Cells: Role in Cellular Retention and Toxicity. Adv. Colloid Interface Sci. 2013, 201–202, 18–29. [Google Scholar] [CrossRef]
- Park, J.S.; Han, T.H.; Lee, K.Y.; Han, S.S.; Hwang, J.J.; Moon, D.H.; Kim, S.Y.; Cho, Y.W. N-Acetyl Histidine-Conjugated Glycol Chitosan Self-Assembled Nanoparticles for Intracytoplasmic Delivery of Drugs: Endocytosis, Exocytosis and Drug Release. J. Control Release 2006, 115, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Tadokoro, S.; Yamamoto, H.; Kawashima, Y.; Hirashima, N. The Suppression of IgE-Mediated Histamine Release from Mast Cells Following Exocytic Exclusion of Biodegradable Polymeric Nanoparticles. Biomaterials 2012, 33, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Bakhru, S.H.; Altiok, E.; Highley, C.; Delubac, D.; Suhan, J.; Hitchens, T.K.; Ho, C.; Zappe, S. Enhanced Cellular Uptake and Long-Term Retention of Chitosan-Modified Iron-Oxide Nanoparticles for MRI-Based Cell Tracking. Int. J. Nanomed. 2012, 7, 4613. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-H.; Hu, H.-Y.; Qiao, M.-X.; Zhu, J.; Qi, J.-W.; Hu, C.-J.; Zhang, Q.; Chen, D.-W. PH-Sensitive Chitosan-Derived Nanoparticles as Doxorubicin Carriers for Effective Anti-Tumor Activity: Preparation and in Vitro Evaluation. Colloids Surf. B Biointerfaces 2012, 94, 184–191. [Google Scholar] [CrossRef]
- Trif, M.; Florian, P.E.; Roseanu, A.; Moisei, M.; Craciunescu, O.; Astete, C.E.; Sabliov, C.M. Cytotoxicity and Intracellular Fate of PLGA and Chitosan-Coated PLGA Nanoparticles in Madin-Darby Bovine Kidney (MDBK) and Human Colorectal Adenocarcinoma (Colo 205) Cells. J. Biomed. Mater. Res. A 2015, 103, 3599–3611. [Google Scholar] [CrossRef]
- Durán, V.; Yasar, H.; Becker, J.; Thiyagarajan, D.; Loretz, B.; Kalinke, U.; Lehr, C.-M. Preferential Uptake of Chitosan-Coated PLGA Nanoparticles by Primary Human Antigen Presenting Cells. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102073. [Google Scholar] [CrossRef]
- Grenha, A.; Grainger, C.I.; Dailey, L.A.; Seijo, B.; Martin, G.P.; Remuñán-López, C.; Forbes, B. Chitosan Nanoparticles Are Compatible with Respiratory Epithelial Cells in Vitro. Eur. J. Pharm. Sci. 2007, 31, 73–84. [Google Scholar] [CrossRef]
- Rajam, M.; Pulavendran, S.; Rose, C.; Mandal, A.B. Chitosan Nanoparticles as a Dual Growth Factor Delivery System for Tissue Engineering Applications. Int. J. Pharm. 2011, 410, 145–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Xi, X.; Shrestha, S.; Jiang, P.; Zhang, W.; Gao, C. Amino Acid-Modified Chitosan Nanoparticles for Cu2+ Chelation to Suppress CuO Nanoparticle Cytotoxicity. J. Mater. Chem. B 2017, 5, 3521–3530. [Google Scholar] [CrossRef]
- Nafee, N.; Schneider, M.; Schaefer, U.F.; Lehr, C.-M. Relevance of the Colloidal Stability of Chitosan/PLGA Nanoparticles on Their Cytotoxicity Profile. Int. J. Pharm. 2009, 381, 130–139. [Google Scholar] [CrossRef]
- Sarangapani, S.; Patil, A.; Ngeow, Y.K.; Mohan, R.E.; Asundi, A.; Lang, M.J. Chitosan Nanoparticles’ Functionality as Redox Active Drugs through Cytotoxicity, Radical Scavenging and Cellular Behaviour. Integr. Biol. 2018, 10, 313–324. [Google Scholar] [CrossRef]
- Lin, L.; He, J.; Li, J.; Xu, Y.; Li, J.; Wu, Y. Chitosan Nanoparticles Strengthen Vγ9Vδ2 T-Cell Cytotoxicity through Upregulation of Killing Molecules and Cytoskeleton Polarization. Int. J. Nanomed. 2019, 14, 9325–9336. [Google Scholar] [CrossRef]
- Richardson, S.C.W.; Kolbe, H.V.J.; Duncan, R. Potential of Low Molecular Mass Chitosan as a DNA Delivery System: Biocompatibility, Body Distribution and Ability to Complex and Protect DNA. Int. J. Pharm. 1999, 178, 231–243. [Google Scholar] [CrossRef]
- Suzuki, Y.S.; Momose, Y.; Higashi, N.; Shigematsu, A.; Park, K.B.; Kim, Y.M.; Kim, J.R.; Ryu, J.M. Biodistribution and Kinetics of Holmium-166-Chitosan Complex (DW-166HC) in Rats and Mice. J. Nucl. Med. 1998, 39, 2161–2166. [Google Scholar]
- Onishi, H.; Machida, Y. Biodegradation and Distribution of Water-Soluble Chitosan in Mice. Biomaterials 1999, 20, 175–182. [Google Scholar] [CrossRef]
- Banerjee, T.; Mitra, S.; Kumar Singh, A.; Kumar Sharma, R.; Maitra, A. Preparation, Characterization and Biodistribution of Ultrafine Chitosan Nanoparticles. Int. J. Pharm. 2002, 243, 93–105. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, Biodistribution and Toxicity of Chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Kadam, R.S.; Bourne, D.W.A.; Kompella, U.B. Nano-Advantage in Enhanced Drug Delivery with Biodegradable Nanoparticles: Contribution of Reduced Clearance. Drug Metab. Dispos. 2012, 40, 1380–1388. [Google Scholar] [CrossRef]
- Termsarasab, U.; Yoon, I.-S.; Park, J.-H.; Moon, H.T.; Cho, H.-J.; Kim, D.-D. Polyethylene Glycol-Modified Arachidyl Chitosan-Based Nanoparticles for Prolonged Blood Circulation of Doxorubicin. Int. J. Pharm. 2014, 464, 127–134. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Cao, J.; Zhang, Y.; Xu, Z.; Qin, X.; Wang, W.; Yuan, Z. Facile Fabrication of Poly(Acrylic Acid) Coated Chitosan Nanoparticles with Improved Stability in Biological Environments. Eur. J. Pharm. Biopharm. 2017, 112, 148–154. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, P.; Zhao, X.; Wang, J.; Wang, J.; Wang, C.; Ren, L.; Zhang, Q. O-Carboxymethyl-Chitosan/Organosilica Hybrid Nanoparticles as Non-Viral Vectors for Gene Delivery. Mater. Sci. Eng. C 2009, 29, 2045–2049. [Google Scholar] [CrossRef]
- Katas, H.; Alpar, H.O. Development and Characterisation of Chitosan Nanoparticles for SiRNA Delivery. J. Control Release 2006, 115, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhang, Z.; Luo, Z.; Yu, J.; Liang, R.; Li, X.; Chen, H. Chitosan Grafted Methoxy Poly(Ethylene Glycol)-Poly(ε-Caprolactone) Nanosuspension for Ocular Delivery of Hydrophobic Diclofenac. Sci. Rep. 2015, 5, 11337. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.-C.; Na, J.H.; Jeong, S.Y.; Kim, D.-E.; Kwon, I.C.; Choi, K.; Ahn, C.-H.; Kim, K. Biocompatible Glycol Chitosan-Coated Gold Nanoparticles for Tumor-Targeting CT Imaging. Pharm. Res. 2014, 31, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Duan, R.; Liu, R.; Guan, M.; Chen, W.; Ma, J.; Chen, M.; Du, B.; Zhang, Q. Chitosan-Stabilized Self-Assembled Fluorescent Gold Nanoclusters for Cell Imaging and Biodistribution In Vivo. ACS Biomater. Sci. Eng. 2018, 4, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.-M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic Understanding of In Vivo Protein Corona Formation on Polymeric Nanoparticles and Impact on Pharmacokinetics. Nat. Commun. 2017, 8, 777. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Bertrand, N.; Zope, H.; Farokhzad, O.C. Emerging Understanding of the Protein Corona at the Nano-Bio Interfaces. Nano Today 2016, 11, 817–832. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Preferential Binding of Positive Nanoparticles on Cell Membranes Is Due to Electrostatic Interactions: A Too Simplistic Explanation That Does Not Take into Account the Nanoparticle Protein Corona. Mater. Sci. Eng. C 2017, 70, 889–896. [Google Scholar] [CrossRef]
- Almalik, A.; Donno, R.; Cadman, C.J.; Cellesi, F.; Day, P.J.; Tirelli, N. Hyaluronic Acid-Coated Chitosan Nanoparticles: Molecular Weight-Dependent Effects on Morphology and Hyaluronic Acid Presentation. J. Control Release 2013, 172, 1142–1150. [Google Scholar] [CrossRef]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N.; et al. Hyaluronic Acid Coated Chitosan Nanoparticles Reduced the Immunogenicity of the Formed Protein Corona. Sci. Rep. 2017, 7, 10542. [Google Scholar] [CrossRef]
- Basu, A.; Kundu, S.; Basu, C.; Ghosh, S.K.; Sur, R.; Mukherjee, A. Biopolymer Nanoparticle Surface Chemistry Dictates the Nature and Extent of Protein Hard Corona. J. Mol. Liq. 2019, 282, 169–176. [Google Scholar] [CrossRef]
- Abouelmagd, S.A.; Ku, Y.J.; Yeo, Y. Low Molecular Weight Chitosan-Coated Polymeric Nanoparticles for Sustained and PH-Sensitive Delivery of Paclitaxel. J. Drug Target. 2015, 23, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Tekie, F.S.M.; Hajiramezanali, M.; Geramifar, P.; Raoufi, M.; Dinarvand, R.; Soleimani, M.; Atyabi, F. Controlling Evolution of Protein Corona: A Prosperous Approach to Improve Chitosan-Based Nanoparticle Biodistribution and Half-Life. Sci. Rep. 2020, 10, 9664. [Google Scholar] [CrossRef]
- Chen, M.-C.; Wong, H.-S.; Lin, K.-J.; Chen, H.-L.; Wey, S.-P.; Sonaje, K.; Lin, Y.-H.; Chu, C.-Y.; Sung, H.-W. The Characteristics, Biodistribution and Bioavailability of a Chitosan-Based Nanoparticulate System for the Oral Delivery of Heparin. Biomaterials 2009, 30, 6629–6637. [Google Scholar] [CrossRef]
- Raj, P.M.; Raj, R.; Kaul, A.; Mishra, A.K.; Ram, A. Biodistribution and Targeting Potential Assessment of Mucoadhesive Chitosan Nanoparticles Designed for Ulcerative Colitis via Scintigraphy. RSC Adv. 2018, 8, 20809–20821. [Google Scholar] [CrossRef]
- Navarro, S.M.; Darensbourg, C.; Cross, L.; Stout, R.; Coulon, D.; Astete, C.E.; Morgan, T.; Sabliov, C.M. Biodistribution of PLGA and PLGA/Chitosan Nanoparticles after Repeat-Dose Oral Delivery in F344 Rats for 7 Days. Ther. Deliv. 2014, 5, 1191–1201. [Google Scholar] [CrossRef]
- Gartziandia, O.; Herran, E.; Pedraz, J.L.; Carro, E.; Igartua, M.; Hernandez, R.M. Chitosan Coated Nanostructured Lipid Carriers for Brain Delivery of Proteins by Intranasal Administration. Colloids Surf. B Biointerfaces 2015, 134, 304–313. [Google Scholar] [CrossRef]
- Yousfan, A.; Rubio, N.; Hakim Natouf, A.; Daher, A.; Al-Kafry, N.; Venner, K.; Kafa, H. Preparation and Characterisation of PHT-Loaded Chitosan Lecithin Nanoparticles for Intranasal Drug Delivery to the Brain. RSC Adv. 2020, 10, 28992–29009. [Google Scholar] [CrossRef]
- Franca, J.R.; Foureaux, G.; Fuscaldi, L.L.; Ribeiro, T.G.; Rodrigues, L.B.; Bravo, R.; Castilho, R.O.; Yoshida, M.I.; Cardoso, V.N.; Fernandes, S.O.; et al. Bimatoprost-Loaded Ocular Inserts as Sustained Release Drug Delivery Systems for Glaucoma Treatment: In Vitro and In Vivo Evaluation. PLoS ONE 2014, 9, e95461. [Google Scholar] [CrossRef]
- Franca, J.R.; Fuscaldi, L.L.; Ribeiro, T.G.; Foureaux, G.; Cesar, A.L.A.; Castilho, R.O.; Cronemberger, S.; Ferreira, A.J.; Fernandes, S.O.A.; Cardoso, V.N.; et al. Use of Chitosan as Pharmaceutical Excipient in Ocular Drug Delivery Systems: Sterilization and Pharmacokinetics. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2227–2237. [Google Scholar] [CrossRef]
- Kamiyama, K.; Onishi, H.; Machida, Y. Biodisposition Characteristics of N-Succinyl-Chitosan and Glycol-Chitosan in Normal and Tumor-Bearing Mice. Biol. Pharm. Bull. 1999, 22, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Jiang, S.; Gao, Y.; Zhang, W.; Hu, S.; Cheng, X.; Zhang, C.; Sun, P.; Ke, W.; et al. Biodistribution and Biocompatibility of Glycyrrhetinic Acid and Galactose-Modified Chitosan Nanoparticles as a Novel Targeting Vehicle for Hepatocellular Carcinoma. Nanomedicine 2020, 15, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Williams, G.R.; Wu, J.; Wu, J.; Zhang, X.; Chen, X.; Li, S.; Jiao, J.; Zhu, L.-M. A Chitosan-Based Cascade-Responsive Drug Delivery System for Triple-Negative Breast Cancer Therapy. J. Nanobiotechnol. 2019, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.S.; Farsakoglu, Y.; Crecente-Campo, J.; de la Fuente, M.; González, S.F.; Alonso, M.J. Carboxymethyl-β-Glucan/Chitosan Nanoparticles: New Thermostable and Efficient Carriers for Antigen Delivery. Drug Deliv. Transl. Res. 2021, 11, 1689–1702. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Wu, Z.; Wang, Z.; Niu, H.; Li, C. Nasal Absorption Enhancement of Insulin Using PEG-Grafted Chitosan Nanoparticles. Eur. J. Pharm. Biopharm. 2008, 68, 526–534. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and In Vitro/In Vivo Evaluation of Resveratrol-Loaded Carboxymethyl Chitosan Nanoparticles. Drug Deliv. 2016, 23, 971–981. [Google Scholar] [CrossRef]
- Wang, L.; Li, L.; Sun, Y.; Ding, J.; Li, J.; Duan, X.; Li, Y.; Junyaprasert, V.B.; Mao, S. In Vitro and In Vivo Evaluation of Chitosan Graft Glyceryl Monooleate as Peroral Delivery Carrier of Enoxaparin. Int. J. Pharm. 2014, 471, 391–399. [Google Scholar] [CrossRef]
- Asasutjarit, R.; Theerachayanan, T.; Kewsuwan, P.; Veeranodha, S.; Fuongfuchat, A.; Ritthidej, G.C. Development and Evaluation of Diclofenac Sodium Loaded-N-Trimethyl Chitosan Nanoparticles for Ophthalmic Use. AAPS PharmSciTech 2015, 16, 1013–1024. [Google Scholar] [CrossRef]
- Narayanan, D.; Anitha, A.; Jayakumar, R.; Chennazhi, K.P. In Vitro and In Vivo Evaluation of Osteoporosis Therapeutic Peptide PTH 1–34 Loaded PEGylated Chitosan Nanoparticles. Mol. Pharm. 2013, 10, 4159–4167. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/o-Carboxymethyl Chitosan Nanoparticles for Efficient and Safe Oral Anticancer Drug Delivery: In Vitro and In Vivo Evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Chen, D.; Jin, X.; Zhao, X.; Zhang, C.; Ping, Q. In Vivo Evaluation of Novel Chitosan Graft Polymeric Micelles for Delivery of Paclitaxel. Drug Deliv. 2011, 18, 181–189. [Google Scholar] [CrossRef]
- Braz, L.; Grenha, A.; Ferreira, D.; Rosa da Costa, A.M.; Gamazo, C.; Sarmento, B. Chitosan/Sulfated Locust Bean Gum Nanoparticles: In Vitro and In Vivo Evaluation towards an Application in Oral Immunization. Int. J. Biol. Macromol. 2017, 96, 786–797. [Google Scholar] [CrossRef]
- Yu, J.-M.; Li, Y.-J.; Qiu, L.-Y.; Jin, Y. Polymeric Nanoparticles of Cholesterol-Modified Glycol Chitosan for Doxorubicin Delivery: Preparation and in-Vitro and in-Vivo Characterization. J. Pharm. Pharmacol. 2009, 61, 713–719. [Google Scholar] [CrossRef]
- Zhang, C.; Qu, G.; Sun, Y.; Wu, X.; Yao, Z.; Guo, Q.; Ding, Q.; Yuan, S.; Shen, Z.; Ping, Q.; et al. Pharmacokinetics, Biodistribution, Efficacy and Safety of N-Octyl-O-Sulfate Chitosan Micelles Loaded with Paclitaxel. Biomaterials 2008, 29, 1233–1241. [Google Scholar] [CrossRef]
- Bachir, Z.A.; Huang, Y.; He, M.; Huang, L.; Hou, X.; Chen, R.; Gao, F. Effects of PEG Surface Density and Chain Length on the Pharmacokinetics and Biodistribution of Methotrexate-Loaded Chitosan Nanoparticles. Int. J. Nanomed. 2018, 13, 5657–5671. [Google Scholar] [CrossRef]
- Dong, W.; Han, B.; Feng, Y.; Song, F.; Chang, J.; Jiang, H.; Tang, Y.; Liu, W. Pharmacokinetics and Biodegradation Mechanisms of a Versatile Carboxymethyl Derivative of Chitosan in Rats: In Vivo and In Vitro Evaluation. Biomacromolecules 2010, 11, 1527–1533. [Google Scholar] [CrossRef]
- Sonin, D.; Pochkaeva, E.; Zhuravskii, S.; Postnov, V.; Korolev, D.; Vasina, L.; Kostina, D.; Mukhametdinova, D.; Zelinskaya, I.; Skorik, Y.; et al. Biological Safety and Biodistribution of Chitosan Nanoparticles. Nanomaterials 2020, 10, 810. [Google Scholar] [CrossRef]
- Nadesh, R.; Narayanan, D.; Sreerekha, P.R.; Vadakumpully, S.; Mony, U.; Koyakkutty, M.; Nair, S.V.; Menon, D. Hematotoxicological Analysis of Surface-Modified and -Unmodified Chitosan Nanoparticles. J. Biomed. Mater. Res. A 2013, 101, 2957–2966. [Google Scholar] [CrossRef]
- Sonaje, K.; Lin, Y.-H.; Juang, J.-H.; Wey, S.-P.; Chen, C.-T.; Sung, H.-W. In Vivo Evaluation of Safety and Efficacy of Self-Assembled Nanoparticles for Oral Insulin Delivery. Biomaterials 2009, 30, 2329–2339. [Google Scholar] [CrossRef]
- Shan, X.; Xu, T.; Liu, Z.; Hu, X.; Zhang, Y.-D.; Wang, B. Safety and Toxicology of the Intravenous Administration of Ang2-SiRNA Plasmid Chitosan Magnetic Nanoparticles. Mol. Med. Rep. 2017, 15, 736–742. [Google Scholar] [CrossRef]
- Islam, N.; Dmour, I.; Taha, M.O. Degradability of Chitosan Micro/Nanoparticles for Pulmonary Drug Delivery. Heliyon 2019, 5, e01684. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Marques, C.; Som, C.; Schmutz, M.; Borges, O.; Borchard, G. How the Lack of Chitosan Characterization Precludes Implementation of the Safe-by-Design Concept. Front. Bioeng. Biotechnol. 2020, 8, 165. [Google Scholar] [CrossRef]
- El-Kamary, S.S.; Pasetti, M.F.; Mendelman, P.M.; Frey, S.E.; Bernstein, D.I.; Treanor, J.J.; Ferreira, J.; Chen, W.H.; Sublett, R.; Richardson, C.; et al. Adjuvanted Intranasal Norwalk Virus-Like Particle Vaccine Elicits Antibodies and Antibody-Secreting Cells That Express Homing Receptors for Mucosal and Peripheral Lymphoid Tissues. J. Infect. Dis. 2010, 202, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.J.; Noulin, N.; Catchpole, A.; Stittelaar, K.J.; de Waal, L.; Kroeze, E.J.B.V.; Hinchcliffe, M.; Smith, A.; Montomoli, E.; Piccirella, S.; et al. Intranasal H5N1 Vaccines, Adjuvanted with Chitosan Derivatives, Protect Ferrets against Highly Pathogenic Influenza Intranasal and Intratracheal Challenge. PLoS ONE 2014, 9, e93761. [Google Scholar] [CrossRef]

| Properties of Chitosan | Cell Lines | Effect on Cell Interaction | Ref |
|---|---|---|---|
| Positive surface charge | A549, HKC, MRC-5, CCC-HSF-1, HUVEC, CRL-2472, UT-7, and K562 | Promoted the internalization rate and increased the cellular uptake | [19] |
| THP-1 | Rapid internalization | [20] | |
| Caco-2 | Rapid uptake in cells | [21] | |
| PC-3, PC12 | Better adhesion of cell line to hyaluronic acid–chitosan films | [27] | |
| pKa (6.5) | ARPE-19 | Charge reversal and effective uptake | [10] |
| SKOV-3, NCI/ADR-RES | pH-sensitive stealth coating and better association with cells | [24] | |
| HeLa, HaCaT, H1299 and NIH-3T3 | Deprotonation at higher pH leads to desorption of fibronectin increasing detachment of cells | [26] | |
| Hydrophilicity (deacetylation)/ Hydrophobicity (acetylation) | Fibroblast and chondrocytic cells | Higher acetylation resulting in increased hydrophobicity and lower surface charge leading to decreased adhesion of cells | [30] |
| Olfactory epithelial cells | Higher degree of deacetylation beneficial for cell growth and adhesion | [30] | |
| Permeability | Intestinal epithelium | Transiently open the epithelial tight junction by redistributing claudin-4 and facilitate penetration of insulin | [40,41,45] |
| Caco-2 | Dose-dependent transepithelial electrical resistance and increase permeability | [47] | |
| Calu-3 | Displacement of zonula occludens-1 protein results in enhance permeability | [48] |
| No. | Chitosan/Modifications | Ligands | Receptors | Cell Lines | Process of Uptake | Ref |
|---|---|---|---|---|---|---|
| 1 | Chitosan | Mannose | Mannose receptor | B16 melanoma tumor cells | Mannose receptor-mediated endocytosis | [69] |
| Lactobionic acid bearing galactose | Asialoglycoprotein receptor | HeLa, CT-26, and Hep G2 cells | Asialoglycoprotein receptor-mediated endocytosis | [66] | ||
| TAT-LHRH | LHRH receptor | BEL-7402 cells | LHRH receptor-mediated endocytosis | [67] | ||
| Folic acid | Folate receptor | HeLa human cervical and SKOV3 ovarian cancer cells | Folate receptor-mediated endocytosis | [75] | ||
| Folic acid | Folate receptor | HepG2 and HeLa cells | Folate receptor-mediated endocytosis | [72] | ||
| Vitamin B12 | Intrinsic factor receptor | Caco-2 cells | Passive diffusion and intrinsic factor receptor-mediated endocytosis | [76] | ||
| Lauryl and succinyl moieties | Mucoadhesion | Caco-2 cells | Paracellular uptake | [77] | ||
| Lactobionic acid | Asialoglycoprotein receptor | SMMC-7721 liver cancer cells | Asialoglycoprotein receptor-mediated endocytosis | [78] | ||
| CD147 antibody | Asialoglycoprotein receptor | HepG2 liver cancer and SMMC-7721 cells | Caveolae-dependent pathway | [79] | ||
| 2 | Carboxy-methyl chitosan | HER-2/neu binding peptide | Human epidermal growth factor receptor 2 | HEK 293 cells | Human epidermal growth factor receptor-mediated endocytosis | [11] |
| Folic acid | Folate receptor | HepG2 cells | Folate receptor-mediated endocytosis | [80] | ||
| Folic acid | Folate receptor | MCF-7 breast cancer cells | Folate receptor-mediated endocytosis | [81] | ||
| 3 | N-trimethyl chitosan | Galactose | Galactose receptors | QGY-7703 cells | Galactose receptor-mediated endocytosis | [68] |
| Galactose | Asialoglycoprotein receptor | HepG2 human liver cancer cells | Galactose receptor-mediated endocytosis | [82] | ||
| CSK peptide | HT29-MTX-E12 intestinal goblet cells | HT29-MTX-E12 cells | Clathrin- and caveolae-mediated endocytosis | [13] | ||
| 4 | PEGylated chitosan | EGFR targeting peptide | Asialoglycoprotein receptor | A549 human lung adeno carcinoma cells | Epidermal growth factor receptor-mediated endocytosis | [70] |
| 5 | GC-PDPA co-polymers | Estrogen | Estrogen receptor | MCF-7 cells | Estrogen receptor- mediated endocytosis | [71] |
| 6 | N-succinyl chitosan | ApoB100 | LDL receptor | HepG2/ADM cells | Low density lipoprotein receptor-mediated endocytosis | [83] |
| 7 | N-succinyl-N -octyl chitosan | Folic acid | Folate receptor | Bel-7402 and A549 cells | Folate receptor-mediated endocytosis | [74] |
| No. | Causes of Premature Elimination | Chitosan/Modifications | Significance | Drug Loaded | Effect on Drug Distribution and Elimination | Ref |
|---|---|---|---|---|---|---|
| 1 | Non-specific uptake by spleen and liver | Chitosan | Positive surface charge | Cyclosporine | Lower apparent clearance and elimination rate constants; hence, longer circulation half-life and higher plasma AUC. | [130] |
| Poly(methacrylic acid) functionalized chitosan | Negatively charged coating of PMAA | 10-Hydroxy camptothecin | Significantly elongated blood circulation time from 12 to 24 h and reduced blood clearance (Cl) from 30.57 to 6.72 mL/h in vivo. | [87] | ||
| Polyethylene glycol-conjugated chitosan oligosaccharide-arachidic acid | Stealth effect | Doxorubicin | Slower in vivo clearance rate subsequently extending the circulation time. | [131] | ||
| 2 | Opsonization | Chitosan funtionalized with poly(acrylic acid) | Colloidal stability and decreased protein adsorption capacity | None | Excellent stability in plasma and a remarkable buffering capacity. | [132] |
| 3 | Enzymatic degradation of biological drugs | O-carboxymethyl-chitosan/organosilica | Protection against DNase I and serum degradation | DNA complexes | Preventing pre-elimination of DNA and avoiding the dissociation of DNA in aqueous solution. | [133] |
| Chitosan glutamate | Protection against enzymatic degradation | siRNA | Prevention of rapid degradation and better biological effect than naked siRNA. | [134] | ||
| 4 | Corneal clearance by metabolic enzymes | Methoxy poly(ethylene glycol)-poly(ε-caprolactone) and chitosan block polymer | Bioadhesion and prevents degradation | Diclofenac | Enhanced pre-corneal retention and penetration of the nanosuspension. | [135] |
| 5 | Physiological instability or aggregation | Glycol chitosan | Biocompatibility | Gold nanoparticles | Excellent stability and biocompatibility | [136] |
| 6 | Degradation by reactive oxygen species | Chitosan grafted with N-Acetyl-L-cysteine | Resistant to reactive oxygen species | Gold nanocluster | Reductant and stabilizer. | [137] |
| No. | Chitosan Modification | Drug | Size (nm) | Zeta Potential (mV) | Targeting Site | Routes of Administration | References |
|---|---|---|---|---|---|---|---|
| 1. | Polyethylene glycol-grafted chitosan | Insulin | 150–300 | +16 to +30 | Mucosal absorption | Intranasal | [157] |
| 2. | Carboxymethyl chitosan | Resveratrol | 155.3 ± 15.2 | 10.28 ± 6.4 | GIT | Oral | [158] |
| 3. | Chitosan graft glyceryl mono-oleate | Enoxaparin | 230.7 ± 7.3 | 21.6 ± 0.3 | GIT | Intragastric | [159] |
| 4. | N-trimethyl chitosan | Diclofenac Sodium | 130–190 | +4 to +9 | Ocular | Ophthalmic | [160] |
| 5. | PEGylated chitosan | Human parathyroid hormone 1-34 | 200–250 | +35 | Systemic circulation | Oral | [161] |
| 6. | O-carboxymeymethy chitosan | Doxorubicin hydrochloride | 250–300 | −33.8 ± 1.6 | pH responsive oral chemotherapy | Oral | [162] |
| 7. | N-octyl-N-(2-carboxyl-cyclohexamethenyl) chitosan | Paclitaxel | 145.9 ± 8.4 | −14.8 ± 0.6 | Tumor targeting | Intravenous | [163] |
| 8. | Locus bean gum sulfate derivative-conjugated chitosan | Ovalbumin | 180–200 | +9 to +14 | Immune reaction | Oral/Subcutaneous | [164] |
| 9. | Cholesterol-modified glycol chitosan | Doxorubicin | 237–336 | -- | Tumor targeting | Intravenous administration | [165] |
| No. | Clinical Trial Phase | Year | Composition | Type of Formulation |
|---|---|---|---|---|
| 1 | Phase 1 | 2010 | Chitosan + mannitol + sucrose + monophosphoryl lipid adjuvant | Intranasal vaccine |
| 2 | Phase 1b/2 | 2021 | Chitosan | Oral supplement |
| 3 | Phase 2/3 | 2019 | Chitosan NPs | Oral irrigation solution |
| 4 | Phase 4 | 2014 | Chitosan | Solution (12 mg/mL) |
| 5 | Not listed | 2018 | Chitosan nanoparticle gel | Oral irrigation solution |
| 6 | Phase 1 | 2011 (recently published in 2019) | Chitosan-N-acetylcysteine (Lacrimera®) | Eye drops |
| 7 | Phase 2 | 2007 | HEP-40 chitosan (enzymatic polychitosamine hydrolysate) (Libracol®) | Oral |
| 8 | Phase 3 | 2016 | Chitosan + isosorbide dinitrate versus either alone | Gel spray |
| 9 | Phase 3 | 2017 | Chitosan + ketamine | Intranasal spray |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics 2021, 13, 1686. https://doi.org/10.3390/pharmaceutics13101686
Aibani N, Rai R, Patel P, Cuddihy G, Wasan EK. Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics. 2021; 13(10):1686. https://doi.org/10.3390/pharmaceutics13101686
Chicago/Turabian StyleAibani, Noorjahan, Raj Rai, Parth Patel, Grace Cuddihy, and Ellen K. Wasan. 2021. "Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery" Pharmaceutics 13, no. 10: 1686. https://doi.org/10.3390/pharmaceutics13101686
APA StyleAibani, N., Rai, R., Patel, P., Cuddihy, G., & Wasan, E. K. (2021). Chitosan Nanoparticles at the Biological Interface: Implications for Drug Delivery. Pharmaceutics, 13(10), 1686. https://doi.org/10.3390/pharmaceutics13101686






