Development and Validation of a Questionnaire to Measure Medication Adherence to Direct-Acting Agents in Patients with Hepatitis C
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation of the HCV-AD Items
2.2. HCV-AD Evaluation
2.3. Sample
2.4. Statistical Analysis
3. Results
3.1. Exploratory Factor Analysis
3.2. Internal Consistency
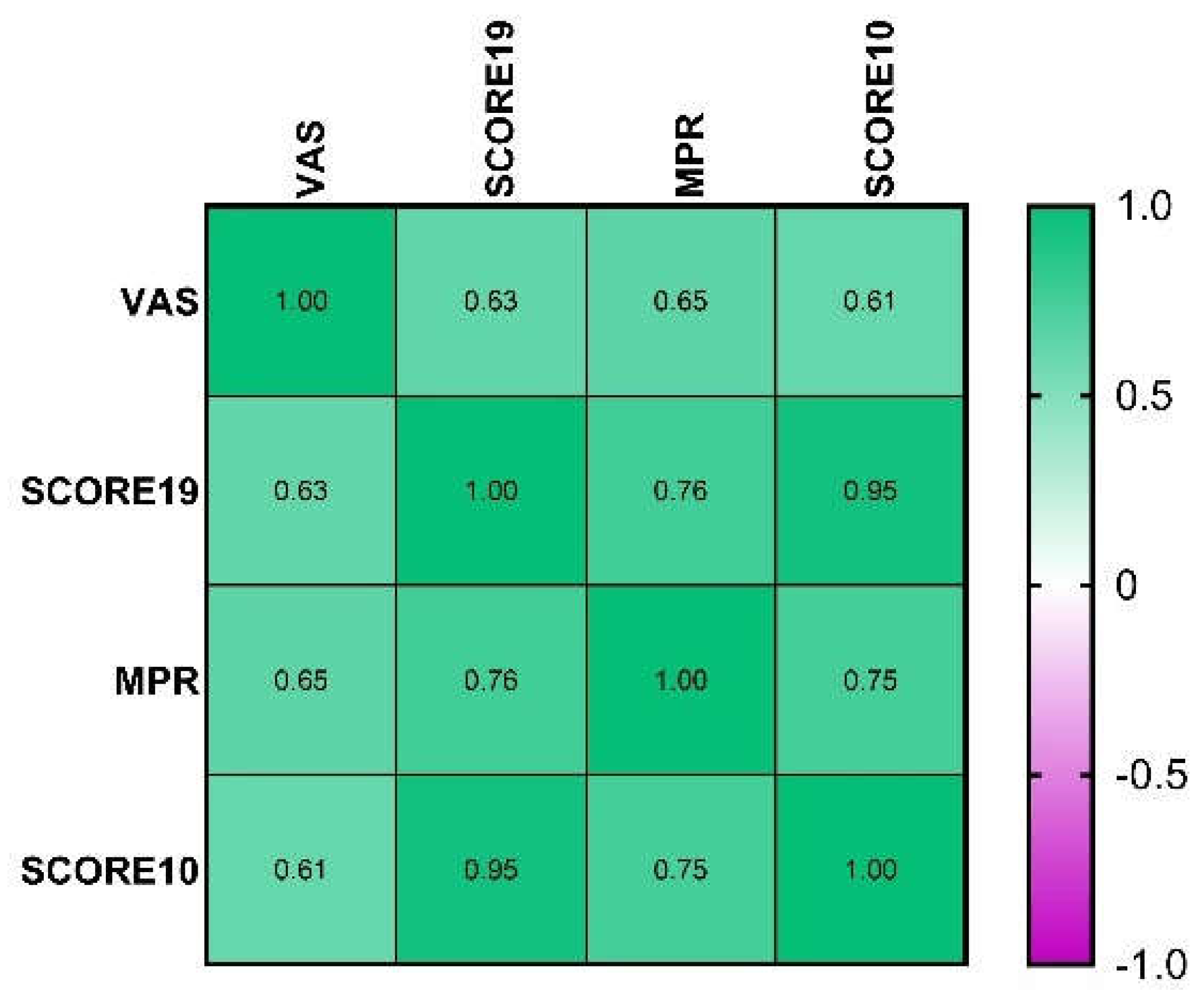
3.3. Construct Validity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahid, I.; Ibrahim, M.M. All Oral Interferon-free Direct-acting Antivirals as Combination Therapies to Cure Hepatitis C. Curr. Mol. Med. 2018, 18, 409–435. [Google Scholar] [CrossRef] [PubMed]
- Houghton, M. Hepatitis C Virus: 30 Years after Its Discovery. Cold Spring Harb. Perspect Med. 2019, 9, a037069. [Google Scholar] [CrossRef] [PubMed]
- Popa, P.; Streba, C.T.; Calita, M.; Iovanescu, V.F.; Florescu, D.N.; Ungureanu, B.S.; Stanculescu, A.D.; Ciurea, R.N.; Oancea, C.N.; Georgescu, D.; et al. Value of endoscopy with narrow-band imaging and probe-based confocal laser endomicroscopy in the diagnosis of preneoplastic lesions of gastrointestinal tract. Rom. J. Morphol. Embryol. 2020, 61, 759–767. [Google Scholar] [CrossRef]
- Dore, G.J.; Bajis, S. Hepatitis C Virus elimination: Laying the foundation for achieving 2030 targets. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 91–92. [Google Scholar] [CrossRef]
- Butaru, A.E.; Doica, I.P.; Gheonea, D.I.; Rogoveanu, I.; Diculescu, M.; Oancea, C.N. Preliminary Results of the Micro-Elimination Project of Hepatitis C in a Disadvantaged Town in South-West of Romania-Orsova. Curr. Health Sci. J. 2020, 46, 217–221. [Google Scholar]
- Halfon, P.; Locarnini, S. Hepatitis C virus resistance to protease inhibitors. J. Hepatol. 2011, 55, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Ing Lorenzini, K.; Girardin, F. Direct-acting antiviral interactions with opioids, alcohol or illicit drugs of abuse in HCV-infected patients. Liver Int. 2020, 40, 32–44. [Google Scholar] [CrossRef]
- Bowry, A.D.; Shrank, W.H.; Lee, J.L.; Stedman, M.; Choudhry, N.K. A systematic review of adherence to cardiovascular medications in resource-limited settings. J. Gen. Intern. Med. 2011, 26, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.P.; Moghimi, Y.; Marcus, R.; Lim, J.K.; Litwin, A.H.; Altice, F.L. Evidence-based interventions to enhance assessment, treatment, and adherence in the chronic Hepatitis C care continuum. Int. J. Drug Policy 2015, 26, 922–935. [Google Scholar] [CrossRef][Green Version]
- Haynes, R.B. A Critical Review of the “Determinants” of Patient Compliance with Therapeutic Regimens; Johns Hopkins University Press: Baltimore, MD, USA, 1976. [Google Scholar]
- Burnier, M. Is There a Threshold for Medication Adherence? Lessons Learnt from Electronic Monitoring of Drug Adherence. Front. Pharmacol. 2019, 9, 1540. [Google Scholar] [CrossRef]
- Baumgartner, P.C.; Haynes, R.B.; Hersberger, K.E.; Arnet, I. A Systematic Review of Medication Adherence Thresholds Dependent of Clinical Outcomes. Front. Pharmacol. 2018, 9, 1290. [Google Scholar] [CrossRef]
- Bolduc, C.; McCall III, K.; Stickney, K.; Gelinas, A.; Levesques, E. Applicability of a new specialty pharmacy-reported measure describing completion of therapy for hepatitis C. J. Manag. Care Spec. Pharm. 2021, 27, 263–267. [Google Scholar]
- Petersen, T.; Townsend, K.; Gordon, L.A.; Sidharthan, S.; Silk, R.; Nelson, A.; Gross, C.; Calderón, M.; Proschan, M.; Osinusi, A.; et al. High adherence to all-oral directly acting antiviral HCV therapy among an inner-city patient population in a phase 2a study. Hepatol. Int. 2016, 10, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Burton, M.J.; Voluse, A.C.; Patel, A.B.; Konkle-Parker, D. Measuring adherence to hepatitis C direct-acting antiviral medications: Using the VAS in an HCV treatment clinic. South Med. J. 2018, 111, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.R.; Juday, T.R.; Manthena, S.R.; Jing, Y.; Sood, V. The impact of ribavirin on real-world adherence rates in hepatitis C patientstreated with sofosbuvir plus simeprevir. Clin. Outcomes Res. 2015, 7, 637–642. [Google Scholar]
- Doica, I.P.; Florescu, D.N.; Oancea, C.N.; Turcu-Stiolica, A.; Subtirelu, M.S.; Dumitra, G.; Rogoveanu, I.; Gheonea, D.I.; Ungureanu, B.S. Telemedicine Chronic Viral Hepatitis C Treatment during the Lockdown Period in Romania: A pilot study. Int. J. Environ. Res. Public Health. 2021, 18, 3694. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.M.; Castillo-Mancilla, J.R.; Morrow, M.; MaWhinney, S.; Rowan, S.E.; Wyles, D.; Blum, J.; Huntley, R.; Salah, L.M.; Tehrani, A.; et al. Adherence to Direct-Acting Antiviral Therapy in People Actively Using Drugs and Alcohol: The INCLUD Study. Open Forum Infect. Dis. 2020, 8, ofaa564. [Google Scholar] [CrossRef] [PubMed]
- Taerel, A.E.; Turcu-Stiolica, A. Study on the range of drugs authorized in Romania—A determinant element for the accessibility and availability of drugs. Farmacia 2009, 57, 254–259. [Google Scholar]
- Pednekar, P.; Agh, T.; Melmenas, M.; Raval, A.; Bennett, B.; Borah, B.; Hutchins, D.; Manias, E.; Williams, A.; Hiligsmann, M.; et al. Methods for measuring multiple medication adherence: A systematic review—Report of the ISPOR medication adherence and persistence special interest group. Value Health 2019, 22, 139–156. [Google Scholar] [CrossRef]
- Kishore, K.; Jaswal, V.; Kulkarni, V.; De, D. Practical guidelines to develop and evaluate a questionnaire. Indian Dermatol. Online J. 2021, 12, 266–275. [Google Scholar] [CrossRef]
- Boateng, G.; Neilands, T.; Frongillo, E.; Melgar-Quinonez, H.; Young, S. Best Practices for Developing and Validating Scales for Health, Social, and Behavioral Research: A Primer. Front. Public Health 2018, 6, 149. [Google Scholar] [CrossRef] [PubMed]
- Hair, J.; Anderson, R.E.; Tatham, R.L.; Black, W.C. Multivariate Data Analysis, 4th ed.; Prentice-Hall Inc.: New Jersey, NJ, USA, 1995. [Google Scholar]
- McDonald, R.P. Test Theory: A Unified Treatment; Lawrence Erlbaum Associates, Inc.: New Jersey, NJ, USA, 1999. [Google Scholar]
- Hayes, A.F.; Coutts, J.J. Use Omega rather than Cronbach’s Alpha for Estimating Reliability. But…. Commun. Methods Meas. 2020, 14, 1–24. [Google Scholar] [CrossRef]
- Kelley, K. Methods for the behavioral, educational, and social sciences: An R package. Behav. Res. Methods 2007, 39, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Serper, M.; Evon, D.M.; Stewart, P.W.; Lok, A.S.; Amador, J.; Reeve, B.B.; Golin, C.E.; Fried, M.W.; Reddy, K.R.; Sterling, R.K.; et al. Medication Non-adherence in a Prospective, Multi-center Cohort Treated with Hepatitis C Direct-Acting Antivirals. J. Gen. Intern. Med. 2020, 35, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.A.; Yan, P.; Shaikh, O.S.; Chung, B.T.; Sherman, K.E. Treatment adherence and virological response rates in hepatitis C virus infected persons treated with sofosbuvir-based regimens: Results from ERCHIVES. Liver Int. 2016, 36, 1275–1283. [Google Scholar] [CrossRef]
- Dima, A.L.; van Ganse, E.; Laforest, L.; Texier, N.; de Bruin, M. & the ASTRO-LAB group. Measuring medication adherence in asthma: Development of a novel self-report tool. Psychol. Health 2017, 32, 1288–1307. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | Patients (n = 222) |
|---|---|
| Age in years, mean (SD) | 60.8 (12.1) |
| Gender–Male, n (%) | 56 (25.2%) |
| Education | |
|
Elementary school High school Faculty | 114 (51.4%) 88 (39.6%) 20 (9.0%) |
| Marital status | |
|
Married Single, never married Widowed Divorced | 162 (73%) 12 (5.4%) 40 (18%) 8 (3.6%) |
| Environmental | |
|
Urban Rural | 92 (41.4%) 130 (58.6%) |
| Employment status | |
|
Employed Unemployed Social help Retired | 64 (28.8%) 14 (6.3%) 6 (2.7%) 138 (62.2%) |
| Income | |
|
<2000 RON 2000–4000 RON 4000–6000 RON >6000 RON | 186 (94.6%) 34 (15.3%) 2 (0.90%) 0 |
| Smoking | |
|
Former smoker Smoker Never smoker | 44 (19.8%) 24 (10.8%) 154 (69.4%) |
| HCV genotype | |
| 3b | 222 (100%) |
| HCV duration in years, mean (SD) | 6.59 (6.9) |
| Treatment regimen | |
|
Ledipasvir/sofosbuvir Dasabuvir/ombitasvir/paritaprevir/ritonavir | 152 (68.5%) 70 (31.5%) |
| Treatment duration, n (%) | |
|
8 weeks 12 weeks | 112 (50.5%) 110 (49.5%) |
| SVR, sustained virologic response | 222 (100%) |
| Fibrosis stage, n (%) | |
|
F0 F0–F1 F1 F1–F2 F2 F2–F3 F3 F3–F4 F4 | 28 (12.6%) 10 (4.50%) 18 (8.1%) 36 (16.2%) 26 (11.7%) 2 (0.9%) 18 (8.1%) 10 (4.5%) 74 (33.3%) |
| Questions of Initial Questionnaire HCV-AD | Reasons |
|---|---|
| 1. In the last week, I forgot to take the prescribed medicines. | |
| 2. In the last week, I was too busy to take the prescribed medicines. | Deleted (variance = 0) |
| 3. The medicines caused side effects and I did not take them as doctor prescribed them. | |
| 4. In the last week, I did not have my medicines with me when I had to take them. | |
| 5. I am not sure the medicine will make me feel better. | |
| 6. I take too many medicines. | |
| 7. In the last week, I have modified the dose of my medicines. | Deleted (variance = 0) |
| 8. When I feel better, I do not take my medicines for a while. | |
| 9. In the last week, I skipped a dose of my medicines. | |
| 10. I avoid taking my medicines because I do not know how they work. | |
| 11. I take my medicines every day. | |
| 12. I am incredibly careful not to forget to take my medicines. | |
| 13. I do not take my medicines when I feel too sick. | |
| 14. I am afraid not to become addicted on my medicines. | |
| 15. I forget to take my prescription when I was scheduled for it. | |
| 16. I do not like to take medications. If I can work without taking them, then I do not take them. | |
| 17. I do not expect miracles after starting my treatment. | |
| 18. I strictly respect what the doctor tells me. | |
| 19. I know how to take my medicines. |
| Factor | Initial Eigenvalues | Rotation Sums of Squared Loadings | Means from Parallel Analysis | ||||
|---|---|---|---|---|---|---|---|
| Total | % of Variance | Cumulative % | Total | % of Variance | Cumulative % | ||
| 1 | 3.94 | 23.17 | 23.17 | 3.17 | 18.64 | 18.64 | 1.5218 |
| 2 | 3.00 | 17.67 | 40.73 | 2.3 | 13.55 | 32.19 | 1.4069 |
| 3 | 1.81 | 10.63 | 51.46 | 2.17 | 12.78 | 44.97 | 1.3319 |
| 4 | 1.5 | 8.8 | 60.26 | 2.09 | 12.3 | 57.27 | 1.2555 |
| 5 | 1.22 | 7.15 | 67.41 | 0.72 | 10.14 | 67.41 | 1.1949 |
| 6 | 0.98 | 5.79 | 73.2 | 1.1346 | |||
| 7 | 0.91 | 5.36 | 78.56 | 1.0779 | |||
| 8 | 0.84 | 4.96 | 83.53 | 1.0261 | |||
| 9 | 0.77 | 4.54 | 88.07 | 0.9757 | |||
| 10 | 0.55 | 3.21 | 91.28 | 0.9253 | |||
| 11 | 0.48 | 2.8 | 94.09 | 0.8789 | |||
| 12 | 0.37 | 2.19 | 96.27 | 0.8345 | |||
| 13 | 0.31 | 1.79 | 98.07 | 0.7889 | |||
| 14 | 0.22 | 1.27 | 99.34 | 0.744 | |||
| 15 | 0.11 | 0.66 | 100 | 0.6918 | |||
| 16 | 4.8*10−33 | 2.8*10−32 | 100 | 0.6418 | |||
| 17 | −9.6*10−17 | −5.7*10−16 | 100 | 0.5692 | |||
| Factor | Initial Eigenvalues | Rotation Sums of Squared Loadings | ||||
|---|---|---|---|---|---|---|
| Total | % of Variance | Cumulative % | Total | % of Variance | Cumulative % | |
| 1 | 3.28 | 32.77 | 32.77 | 3.16 | 31.55 | 31.55 |
| 2 | 2.47 | 24.73 | 57.51 | 2.48 | 24.8 | 56.35 |
| 3 | 1.29 | 12.95 | 70.45 | 1.41 | 14.10 | 70.45 |
| 4 | 0.94 | 9.44 | 79.89 | |||
| 5 | 0.86 | 8.55 | 88.44 | |||
| 6 | 0.56 | 5.63 | 94.07 | |||
| 7 | 0.46 | 4.59 | 98.66 | |||
| 8 | 0.13 | 1.34 | 100 | |||
| 9 | 1.4*10−33 | 1.4*10−32 | 100 | |||
| 10 | −7.8*10−17 | −7.8*10−16 | 100 | |||
| Survey Item | Subscale (Factors) | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| 1. In the last week, I forgot to take the prescribed medicines. | 0.887 | ||
| 3. The medicines caused side effects and I did not take them as the doctor prescribed them. | 0.887 | ||
| 4. In the last week, I did not have my medicines with me when I had to take them. | 0.883 | ||
| 9. In the last week, I skipped a dose of my medicines. | 0.883 | ||
| 5. I am not sure the medicine will make me feel better. | 0.773 | ||
| 17. I do not expect miracles after starting my treatment. | 0.712 | ||
| 6. I take too many medicines. | 0.853 | ||
| 14. I am afraid not to become addicted to my medicines. | 0.787 | ||
| 8. When I feel better, I do not take my medicines for a while. | 0.846 | ||
| 13. I do not take my medicines when I feel too sick. | 0.813 | ||
| Adherence Scales | Adherence Scores | ||
|---|---|---|---|
| Mean | SD | Range | |
| HCV-AD19 | 93.99 | 5.63 | 80.26–100 |
| HCV-AD10 | 91.53 | 8.42 | 72.5–100 |
| VAS | 9.78 | 0.49 | 8–10 |
| MPR | 96.7 | 4.93 | 87.5–100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turcu-Stiolica, A.; Doica, I.P.; Ungureanu, B.S.; Rogoveanu, I.; Florescu, D.N.; Subtirelu, M.-S.; Gheonea, D.I. Development and Validation of a Questionnaire to Measure Medication Adherence to Direct-Acting Agents in Patients with Hepatitis C. Pharmaceutics 2021, 13, 1683. https://doi.org/10.3390/pharmaceutics13101683
Turcu-Stiolica A, Doica IP, Ungureanu BS, Rogoveanu I, Florescu DN, Subtirelu M-S, Gheonea DI. Development and Validation of a Questionnaire to Measure Medication Adherence to Direct-Acting Agents in Patients with Hepatitis C. Pharmaceutics. 2021; 13(10):1683. https://doi.org/10.3390/pharmaceutics13101683
Chicago/Turabian StyleTurcu-Stiolica, Adina, Irina Paula Doica, Bogdan Silviu Ungureanu, Ion Rogoveanu, Dan Nicolae Florescu, Mihaela-Simona Subtirelu, and Dan Ionut Gheonea. 2021. "Development and Validation of a Questionnaire to Measure Medication Adherence to Direct-Acting Agents in Patients with Hepatitis C" Pharmaceutics 13, no. 10: 1683. https://doi.org/10.3390/pharmaceutics13101683
APA StyleTurcu-Stiolica, A., Doica, I. P., Ungureanu, B. S., Rogoveanu, I., Florescu, D. N., Subtirelu, M.-S., & Gheonea, D. I. (2021). Development and Validation of a Questionnaire to Measure Medication Adherence to Direct-Acting Agents in Patients with Hepatitis C. Pharmaceutics, 13(10), 1683. https://doi.org/10.3390/pharmaceutics13101683








