Delta T, a Useful Indicator for Pharmacy Dispensing Data to Monitor Medication Adherence
Abstract
1. Introduction
2. Methods
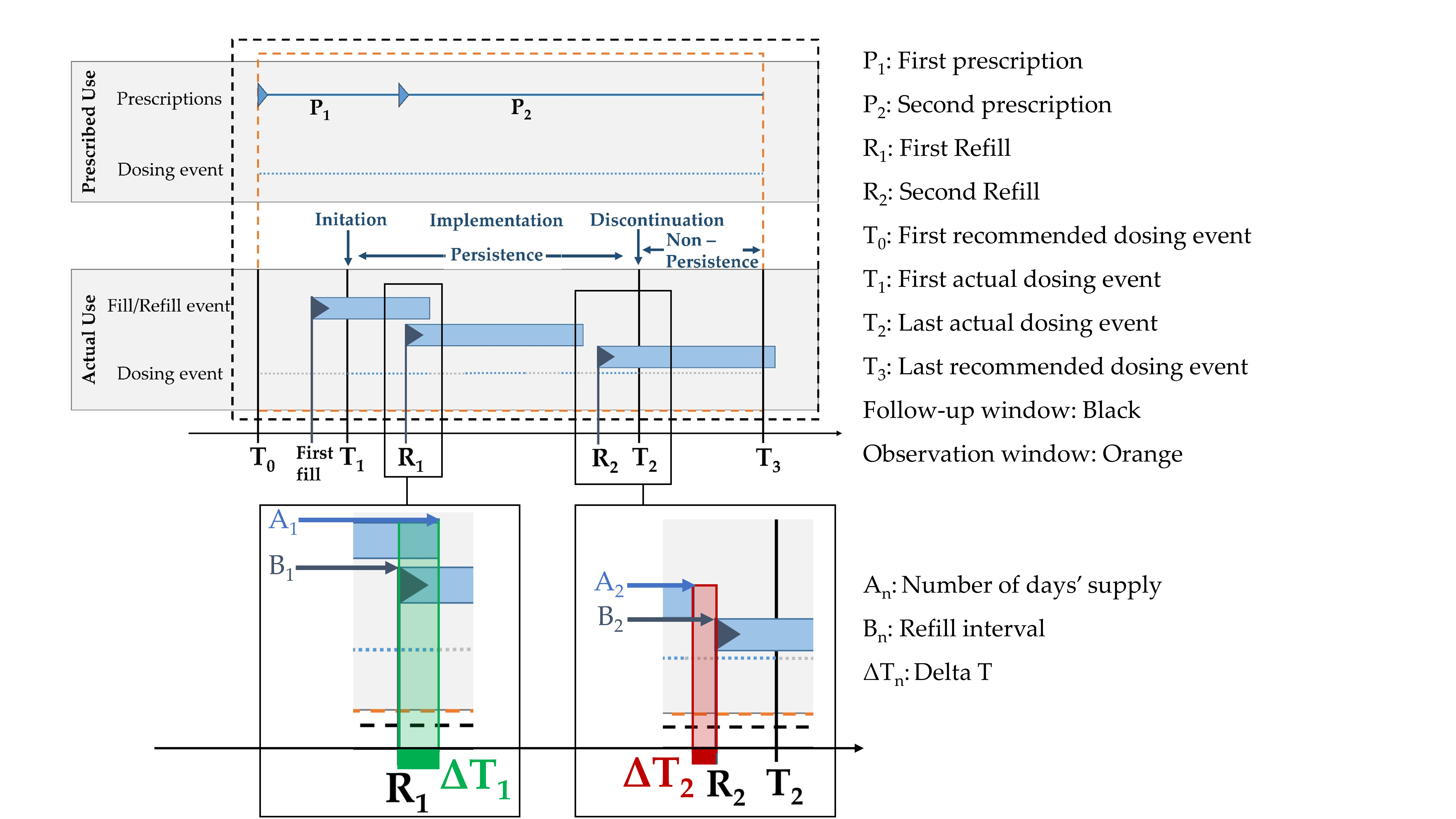
2.1. Development of the New Estimate Delta T (ΔT)
2.2. Data Source
2.3. Analytical Procedure and Statistical Analysis
3. Results
3.1. Study Population
3.2. Mean Delta T and Dichotomized Delta T
3.3. Comparison with the Medication Possession Ratio
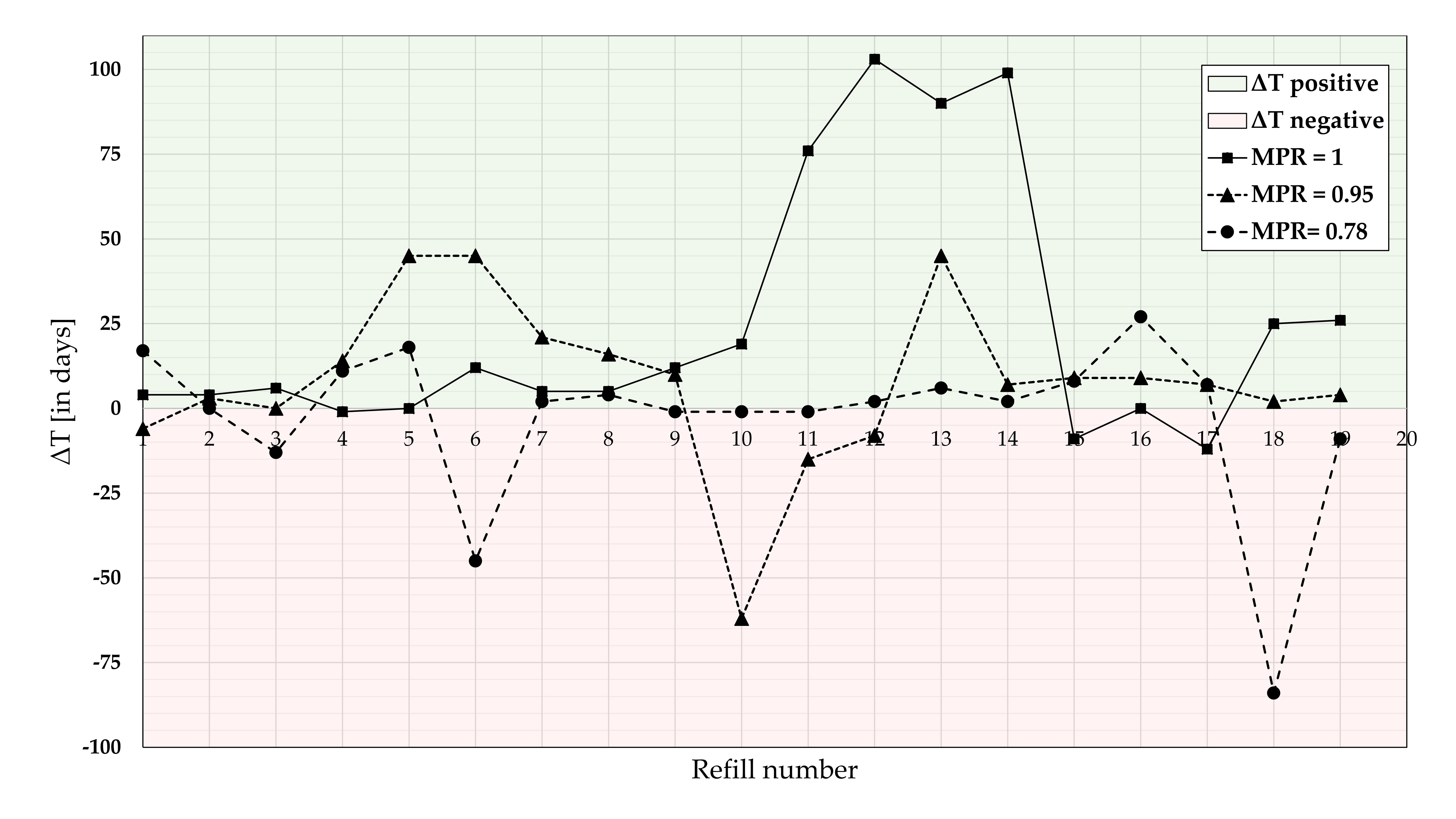
3.4. Individual Refill Pattern of Three Illustrative Patients
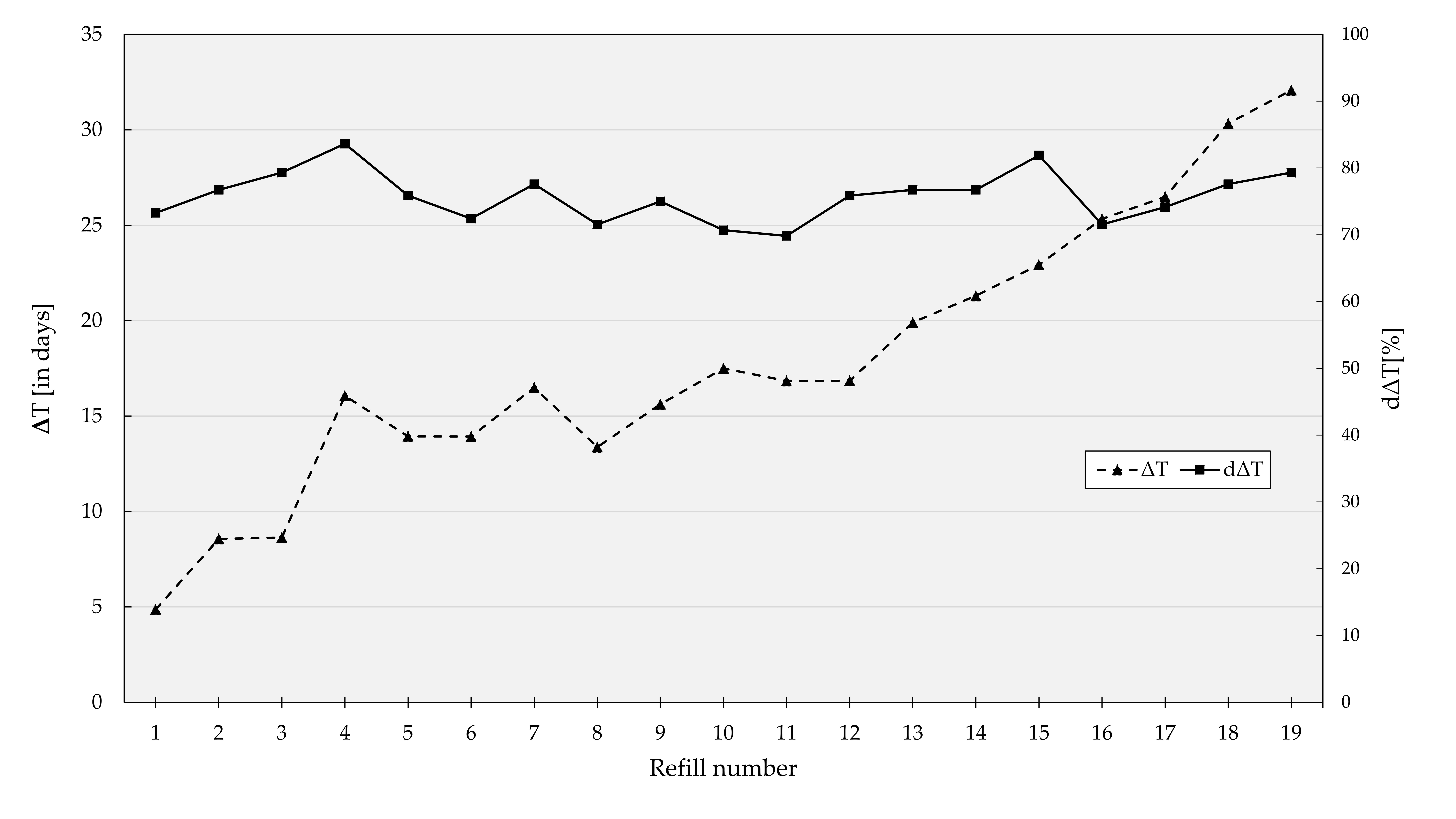
3.5. Refill Trend in the Population
3.6. Refill Groups within the Population
4. Discussion
4.1. Estimating the Refill Behavior with Mean ΔT
4.2. Documenting the Changing Refill Behavior with ΔT
4.3. Potential Applications for ΔT in Research
4.4. Potential Applications for ΔT in Practice
4.5. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Elements for Calculating ΔT
| Element | Definition | Standard and Calculation |
| Medication event [19] | Prescribing or dispensing record of a given medication with a given strength. | M |
| Refill event | Prescribing or dispensing record of a given medication with the exclusion of the first medication event | R = M − 1 |
| Start and endpoints of the observation period [20] | The period starts at t0 and ends at tn or ta | t0 = date of first medication event tn = date of last refill ta = arbitrary date |
| Observation period [20] | Number of days in the entire period | tn − t0 or ta − t0 |
| Quantity dispensed [20] | Number of dispensed medication units (e.g., tablets, pills, etc.) | [quant_disp]n |
| Prescribed daily dosage (PDD) [20] | Amounts of units to be consumed per day according to the dosing instructions | PDDn = number of units per dose x number of doses per day |
| Number of days’ supply (An) with oversupply | Number of days medication available with oversupply | ([quant_disp]n/ PDDn) + Cn − 1 |
| Refill interval (Bn) [20] | Number of days between two dispensations | tn − tn − 1 |
| Delta T (ΔT) | Difference between number of days’ supply and refill interval | ΔTn = An − Bn |
| dDelta T (dΔT) | Dichotomized Delta T | dΔTn = An − Bn If ΔTn ≥ 0 = 1 If ΔTn < 0 = 0 |
| Oversupply (Cn) [20] | Number of days’ supply accumulated from previous dispensing (stockpile) | If ΔT > 0 |
| Gap (Dn) [20] | Number of days without medication supply | If ΔT < 0 |
| Medication adherence measures for comparison: Medication possession ratio (MPR) [23] | CMA1 = number of days dispensed, excluding the last refill/first to last dispensing | )/(tn − t0) |
References
- Lehmann, A.; Aslani, P.; Ahmed, R.; Celio, J.; Gauchet, A.; Bedouch, P.; Bugnon, O.; Allenet, B.; Schneider, M.P. Assessing medication adherence: Options to consider. Int. J. Clin. Pharm. 2014, 36, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.B.; Amico, K.R.; Bova, C.; Womack, J.A. A proposal for quality standards for measuring medication adherence in research. AIDS Behav. 2013, 17, 284–297. [Google Scholar] [CrossRef]
- Pillittere-Dugan, D.; Nau, D.P.; Mcdonough, K.; Pierre, Z. Development and testing of performance measures for pharmacy services. J. Am. Pharm. Assoc. 2009, 49, 212–219. [Google Scholar] [CrossRef] [PubMed]
- PharmacyQualityAlliance. PQA Measures. Available online: https://www.pqaalliance.org/measures-overview (accessed on 26 May 2021).
- Steiner, J.F.; Prochazka, A.V. The assessment of refill compliance using pharmacy records: Methods, validity, and applications. J. Clin. Epidemiol. 1997, 50, 105–116. [Google Scholar] [CrossRef]
- Sattler, E.L.P.; Lee, J.S.; Perri, M. Medication (Re)fill Adherence Measures Derived from Pharmacy Claims Data in Older Americans: A Review of the Literature. Drugs Aging 2013, 30, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Dima, A.L.; Dediu, D. Computation of adherence to medication and visualization of medication histories in R with AdhereR: Towards transparent and reproducible use of electronic healthcare data. PLoS ONE 2017, 12, e0174426. [Google Scholar] [CrossRef]
- Kozma, C.; Dickson, M.; Phillips, A.L.; Meletiche, D.M. Medication possession ratio: Implications of using fixed and variable observation periods in assessing adherence with disease-modifying drugs in patients with multiple sclerosis. Patient Prefer. Adherence 2013, 7, 509. [Google Scholar] [CrossRef]
- Declercq, J.; Choi, L. Statistical considerations for medication adherence research. Curr. Med. Res. Opin. 2020, 36, 1549–1557. [Google Scholar] [CrossRef]
- Sperber, C.; Samarasinghe, S.R.; Lomax, G.P. An upper and lower bound of the Medication Possession Ratio. Patient Prefer. Adherence 2017, 11, 1469–1478. [Google Scholar] [CrossRef]
- Baumgartner, P.C.; Haynes, R.B.; Hersberger, K.E.; Arnet, I. A systematic review of medication adherence thresholds dependent of clinical outcomes. Front. Pharmacol. 2018, 9, 1290. [Google Scholar] [CrossRef]
- Altice, F.; Evuarherhe, O.; Shina, S.; Carter, G.; Beaubrun, A.C. Adherence to HIV treatment regimens: Systematic literature review and meta-analysis. Patient Prefer. Adherence 2019, 13, 475–490. [Google Scholar] [CrossRef]
- Souverein, P.C.; Koster, E.S.; Colice, G.; Van Ganse, E.; Chisholm, A.; Price, D.; Dima, A.L. Inhaled Corticosteroid Adherence Patterns in a Longitudinal Asthma Cohort. J. Allergy Clin. Immunol. Pract. 2017, 5, 448–456.e2. [Google Scholar] [CrossRef]
- Haag, M.; Lehmann, A.; Hersberger, K.E.; Schneider, M.P.; Gauchet, A.; Vrijens, B.; Arnet, I.; Allenet, B. The ABC taxonomy for medication adherence translated into French and German. Br. J. Clin. Pharmacol. 2019, 86, 734–744. [Google Scholar] [CrossRef]
- Vrijens, B.; De Geest, S.; Hughes, D.A.; Przemyslaw, K.; Demonceau, J.; Ruppar, T.; Dobbels, F.; Fargher, E.; Morrison, V.; Lewek, P.; et al. A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 2012, 73, 691–705. [Google Scholar] [CrossRef]
- De Geest, S.; Zullig, L.L.; Dunbar-Jacob, J.; Helmy, R.; Hughes, D.A.; Wilson, I.B.; Vrijens, B. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann. Intern. Med. 2018, 169, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bijlsma, M.J.; Janssen, F.; Hak, E. Estimating time-varying drug adherence using electronic records: Extending the proportion of days covered (PDC) method. Pharmacoepidemiol. Drug Saf. 2016, 25, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Allemann, S.S.; Dediu, D.; Dima, A.L. Beyond Adherence Thresholds: A Simulation Study of the Optimal Classification of Longitudinal Adherence Trajectories from Medication Refill Histories. Front. Pharmacol. 2019, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Dima, A.L.; Allemann, S.S.; Dunbar-Jacob, J.; Hughes, D.A.; Vrijens, B.; Wilson, I.B. TEOS: A framework for constructing operational definitions of medication adherence based on Timelines—Events—Objectives—Sources. Br. J. Clin. Pharmacol. 2020, 87, 2521–2533. [Google Scholar] [CrossRef]
- Arnet, I.; Kooij, M.J.; Messerli, M.; Hersberger, K.E.; Heerdink, E.R.; Bouvy, M. Proposal of Standardization to Assess Adherence with Medication Records: Methodology Matters. Ann. Pharmacother. 2016, 50, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.C.; Wiley-Exley, E.K.; Richards, S.; Domino, M.E.; Carey, T.S.; Sleath, B.L. Contrasting Measures of Adherence with Simple Drug Use, Medication Switching, and Therapeutic Duplication. Ann. Pharmacother. 2009, 43, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Arnet, I.; Abraham, I.; Messerli, M.; Hersberger, K.E. A method for calculating adherence to polypharmacy from dispensing data records. Int. J. Clin. Pharm. 2014, 36, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.M.; Xu, M.; Feldstein, A.; Smith, D.; Waterbury, A.; Rand, C. Comparison of pharmacy-based measures of medication adherence. BMC Health Serv. Res. 2012, 12, 155. [Google Scholar] [CrossRef]
- Doro, P.; Benko, R.; Kosik, E.; Matuz, M.; Toth, K.; Soos, G. Utilization of oral antihyperglycemic drugs over a 7-year period (1998–2004) in a Hungarian population and adherence to drug therapy. Eur. J. Clin. Pharmacol. 2005, 61, 893–897. [Google Scholar] [CrossRef]
- Caro, J.J.; Ishak, K.J.; Huybrechts, K.F.; Raggio, G.; Naujoks, C. The impact of compliance with osteoporosis therapy on fracture rates in actual practice. Osteoporos. Int. 2004, 15, 1003–1008. [Google Scholar] [CrossRef]
- Hansen, R.A.; Farley, J.F.; Droege, M.; Maciejewski, M.L. A retrospective cohort study of economic outcomes and adherence to monotherapy with metformin, pioglitazone, or a sulfonylurea among patients with type 2 diabetes mellitus in the United States from 2003 to 2005. Clin. Ther. 2010, 32, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Borne, R.T.; O’Donnell, C.; Turakhia, M.P.; Varosy, P.D.; Jackevicius, C.A.; Marzec, L.N.; Masoudi, F.A.; Hess, P.L.; Maddox, T.M.; Ho, P.M. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: Findings from the veterans health administration. BMC Cardiovasc. Disord. 2017, 17, 236. [Google Scholar] [CrossRef]
- Stroupe, K.T.; Teal, E.Y.; Weiner, M.; Gradus-Pizlo, I.; Brater, D.C.; Murray, M.D. Health care and medication costs and use among older adults with heart failure. Am. J. Med. 2004, 116, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Stroupe, K.T.; Teal, E.Y.; Tu, W.; Weiner, M.; Murray, M.D. Association of Refill Adherence and Health Care Use Among Adults with Hypertension in an Urban Health Care System. Pharmacotherapy 2006, 26, 779–789. [Google Scholar] [CrossRef]
- Dilokthornsakul, P.; Chaiyakunapruk, N.; Nimpitakpong, P.; Jeanpeerapong, N.; Jampachaisri, K.; Lee, T.A. Understanding medication oversupply and its predictors in the outpatient departments in Thailand. BMC Health Serv. Res. 2014, 14, 408. [Google Scholar] [CrossRef]
- Franklin, J.M.; Shrank, W.H.; Pakes, J.; Sanfelix-Gimeno, G.; Matlin, O.S.; Brennan, T.A.; Choudhry, N.K. Group-based trajectory models: A new approach to classifying and predicting long-term medication adherence. Med. Care 2013, 51, 789–796. [Google Scholar] [CrossRef]
- Hickson, R.P.; Annis, I.E.; Killeya-Jones, L.A.; Fang, G. Opening the black box of the group-based trajectory modeling process to analyze medication adherence patterns: An example using real-world statin adherence data. Pharmacoepidemiol. Drug Saf. 2019, 29, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Messerli, M.; Blozik, E.; Vriends, N.; Hersberger, K.E. Impact of a community pharmacist-led medication review on medicines use in patients on polypharmacy—A prospective randomised controlled trial. BMC Health Serv. Res. 2016, 16, 145. [Google Scholar] [CrossRef]
- Nsiah, I.; Imeri, H.; Jones, A.C.; Bentley, J.P.; Barnard, M.; Kang, M. The impact of medication synchronization programs on medication adherence: A meta-analysis. J. Am. Pharm. Assoc. 2021, 61, e202–e211. [Google Scholar] [CrossRef]
- Torres-Robles, A.; Wiecek, E.; Cutler, R.; Drake, B.; Benrimoj, S.I.; Fernandez-Llimos, F.; Garcia-Cardenas, V. Using dispensing data to evaluate adherence implementation rates in community pharmacy. Front. Pharmacol. 2019, 10, 130. [Google Scholar] [CrossRef]
- Gellad, W.F.; Thorpe, C.T.; Steiner, J.F.; Voils, C.I. The myths of medication adherence. Pharmacoepidemiol. Drug Saf. 2017, 26, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Grace, K.A.; Foster, T.G.; Crawley, M.J.; Erowele, G.I.; Sun, H.J.; Turner, P.T.; Sullenberger, L.E.; Taylor, A.J. How should we measure medication adherence in clinical trials and practice? Ther. Clin. Risk Manag. 2007, 3, 685–690. [Google Scholar] [CrossRef][Green Version]
- Anghel, L.A.; Farcas, A.M.; Oprean, R.N. An overview of the common methods used to measure treatment adherence. Med. Pharm. Rep. 2019, 92, 117–122. [Google Scholar] [CrossRef]
- Fénélon-Dimanche, R.; Guénette, L.; Trudel-Bourgault, F.; Yousif, A.; Lalonde, G.; Beauchesne, M.-F.; Collin, J.; Blais, L. Development of an electronic tool (e-AdPharm) to address unmet needs and barriers of community pharmacists to provide medication adherence support to patients. Res. Soc. Adm. Pharm. 2021, 17, 506–513. [Google Scholar] [CrossRef]
- Anderson, L.J.; Nuckols, T.K.; Coles, C.; Le, M.M.; Schnipper, J.L.; Shane, R.; Jackevicius, C.; Lee, J.; Pevnick, J.M.; Choudhry, N.K.; et al. A systematic overview of systematic reviews evaluating medication adherence interventions. Am. J. Health-Syst. Pharm. 2020, 77, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Nieuwlaat, R.; Wilczynski, N.; Navarro, T.; Hobson, N.; Jeffery, R.; Keepanasseril, A.; Agoritsas, T.; Mistry, N.; Iorio, A.; Jack, S.; et al. Interventions for enhancing medication adherence. Cochrane Database Syst. Rev. 2014, 11, CD000011. [Google Scholar] [CrossRef]
- Pednekar, P.P.; Ágh, T.; Malmenäs, M.; Raval, A.D.; Bennett, B.M.; Borah, B.J.; Hutchins, D.S.; Manias, E.; Williams, A.F.; Hiligsmann, M.; et al. Methods for Measuring Multiple Medication Adherence: A Systematic Review—Report of the ISPOR Medication Adherence and Persistence Special Interest Group. Value Health 2019, 22, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Arnet, I.; Greenland, M.; Knuiman, M.W.; Rankin, J.M.; Hung, J.; Nedkoff, L.; Briffa, T.; Sanfilippo, F. Operationalization and validation of a novel method to calculate adherence to polypharmacy with refill data from the Australian pharmaceutical benefits scheme (PBS) database. Clin. Epidemiol. 2018, 10, 1181–1194. [Google Scholar] [CrossRef] [PubMed]
- Karve, S.; Cleves, M.A.; Helm, M.; Hudson, T.J.; West, D.S.; Martin, B.C. An Empirical Basis for Standardizing Adherence Measures Derived from Administrative Claims Data among Diabetic Patients. Med. Care 2008, 46, 1125–1133. [Google Scholar] [CrossRef] [PubMed]


| Patient | Median ΔT (IQR) [In Days] | Mean ΔT ± SD [In Days] | Max ΔT [In Days] | Min ΔT [In Days] | Range (=Max ΔT–Min ΔT) [In Days] | dΔT [%] | Sum of Days without Supply (=Sum of Negative ΔT) [In Days] |
|---|---|---|---|---|---|---|---|
| MPR = 1 | 6 (26) | 24.4 ± 36.5.3 | 103 | −12 | 115 | 84.2 | 22 |
| MPR = 0.95 | 7 (16) | 7.7 ± 23.4 | 45 | −62 | 107 | 78.9 | 91 |
| MPR = 0.78 | 2 (9) | −2.6 ± 23.9 | 27 | −84 | 111 | 63.2 | 154 |
| Cluster Number | Characterization of the Clusters | Number of Patients (%) | Mean Age ± SD [In Years] | Percentage of Women [%] | ΔT ± SD [In Days] | dΔT ± SD [%] | MPR ± SD |
|---|---|---|---|---|---|---|---|
| 1 | Refills “on time” | 71 (61.2) | 70.8 ± 10.3 | 54.9 | 25.4 ± 27.7 | 87.3 ± 10.2 | 1.01 ± 0.09 |
| 2 | Erratic refills | 29 (25.0) | 75.1 ± 12.3 | 54.7 | 19.0 ± 29.7 | 72.1 ± 13.3 | 0.99 ± 0.10 |
| 3 | Gaps at the end of refill period | 8 (6.9) | 70.1 ± 13.7 | 37.5% | −6.6 ± 7.8 | 39.5 ± 8.9 | 0.79 ± 0.13 |
| 4 | Gaps in the middle of refill period | 8 (6.9) | 71.1 ± 12.3 | 62.5% | −12.0 ± 8.9 | 34.9 ± 15.1 | 0.75 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumgartner, P.C.; Vrijens, B.; Allemann, S.; Hersberger, K.E.; Arnet, I. Delta T, a Useful Indicator for Pharmacy Dispensing Data to Monitor Medication Adherence. Pharmaceutics 2022, 14, 103. https://doi.org/10.3390/pharmaceutics14010103
Baumgartner PC, Vrijens B, Allemann S, Hersberger KE, Arnet I. Delta T, a Useful Indicator for Pharmacy Dispensing Data to Monitor Medication Adherence. Pharmaceutics. 2022; 14(1):103. https://doi.org/10.3390/pharmaceutics14010103
Chicago/Turabian StyleBaumgartner, Pascal C., Bernard Vrijens, Samuel Allemann, Kurt E. Hersberger, and Isabelle Arnet. 2022. "Delta T, a Useful Indicator for Pharmacy Dispensing Data to Monitor Medication Adherence" Pharmaceutics 14, no. 1: 103. https://doi.org/10.3390/pharmaceutics14010103
APA StyleBaumgartner, P. C., Vrijens, B., Allemann, S., Hersberger, K. E., & Arnet, I. (2022). Delta T, a Useful Indicator for Pharmacy Dispensing Data to Monitor Medication Adherence. Pharmaceutics, 14(1), 103. https://doi.org/10.3390/pharmaceutics14010103








