Intratracheal Administration of Chloroquine-Loaded Niosomes Minimize Systemic Drug Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Niosomes
2.3. Characterizations of Niosomes
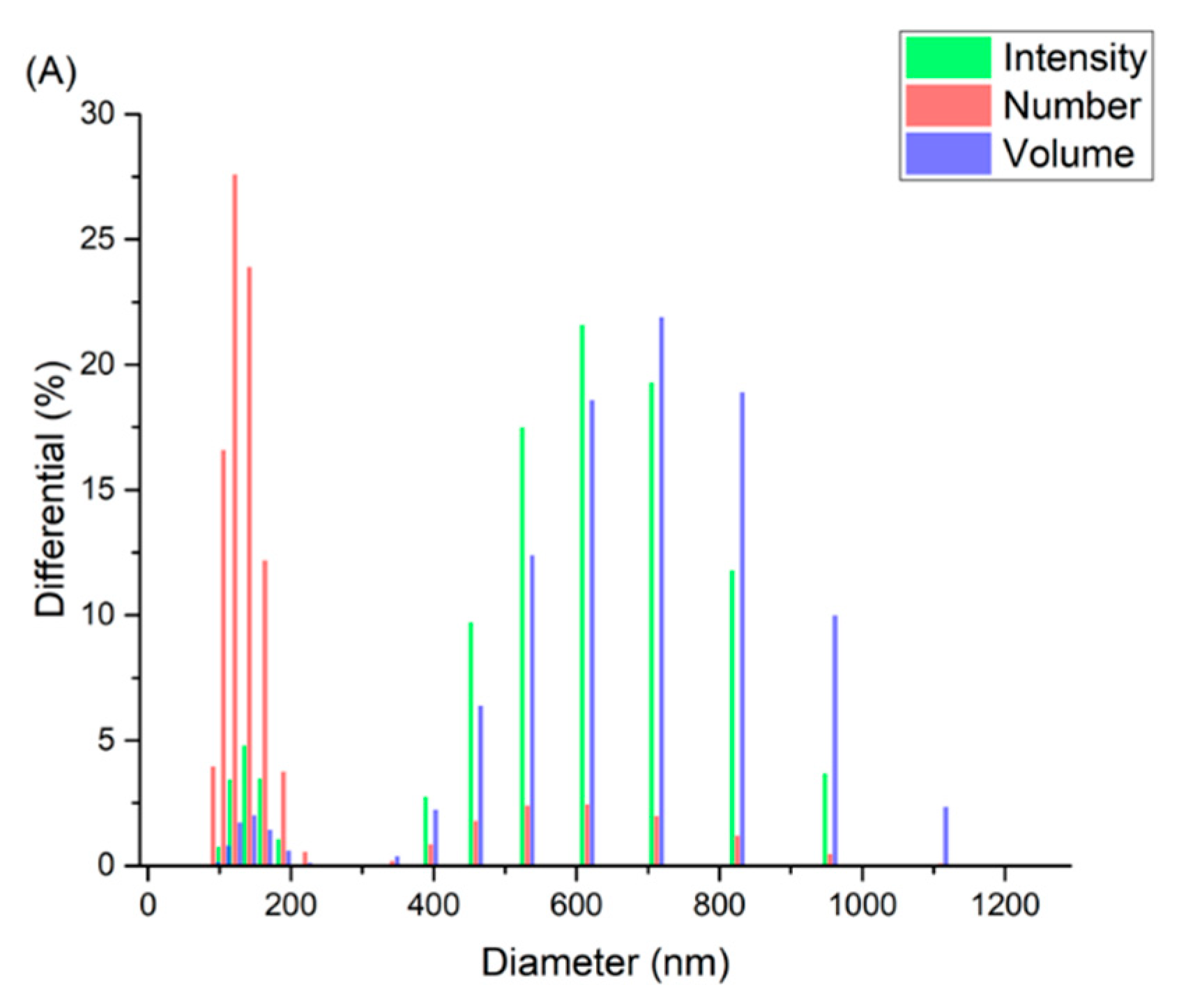
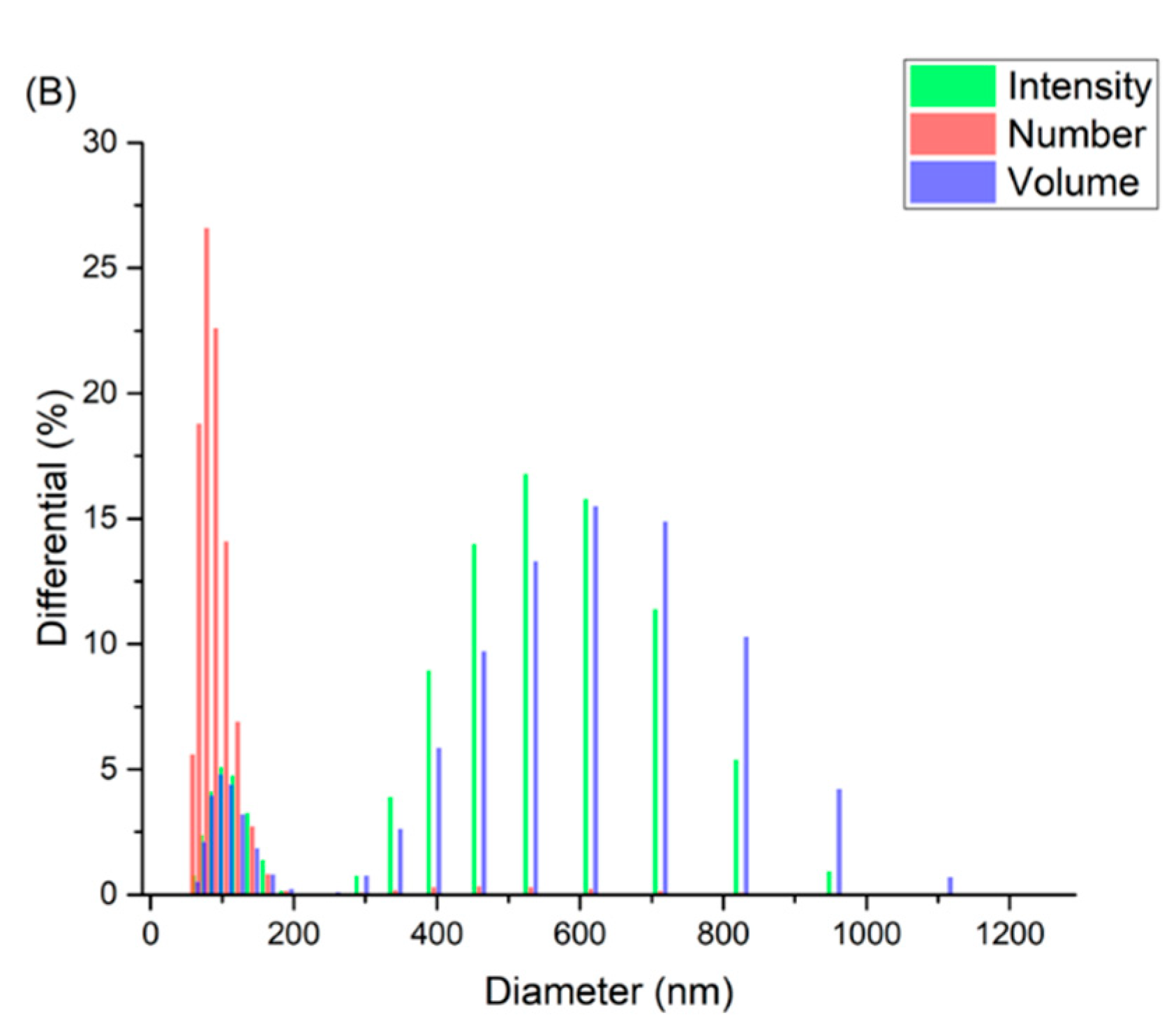
2.3.1. Dynamic Light Scattering
2.3.2. Transmission Electron Microscopy
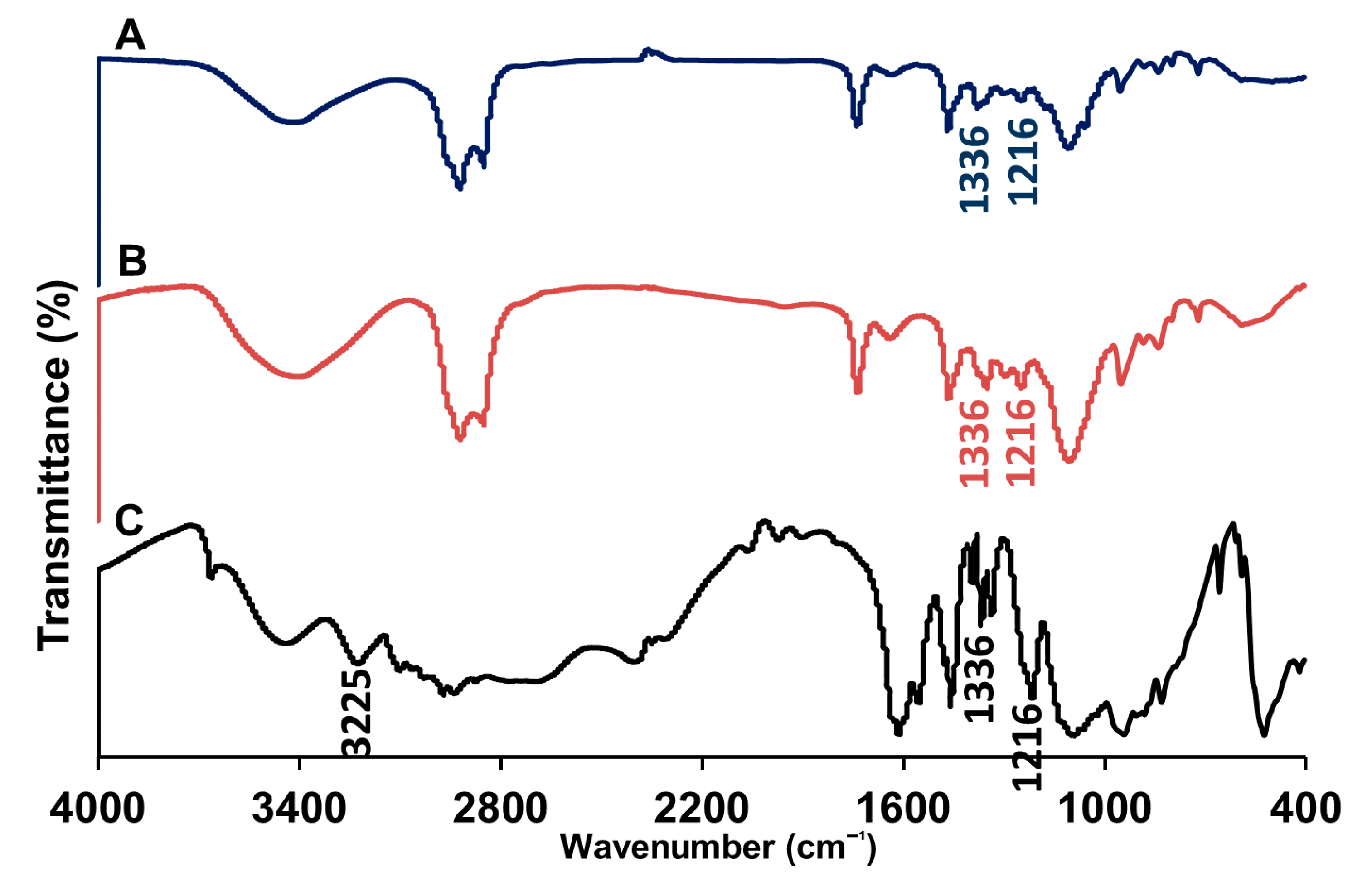
2.3.3. Fourier Transform-Infrared Spectroscopy
2.3.4. Thermogravimetric Analysis
2.4. Evaluation of the Entrapment Efficiency
2.5. In Vitro Release Study
2.6. Evaluation of Micromeritic Properties of Lyophilized Drug-Loaded Niosomes/Lactose Blends
2.6.1. Angle of Repose
2.6.2. Bulk and Tapped Density
2.6.3. Carr’s Compressibility Index and Hausner Ratio
2.7. In Vivo Evaluation of the Developed Nanomaterials
2.7.1. Assessment of Chloroquine Concentration in Blood Samples
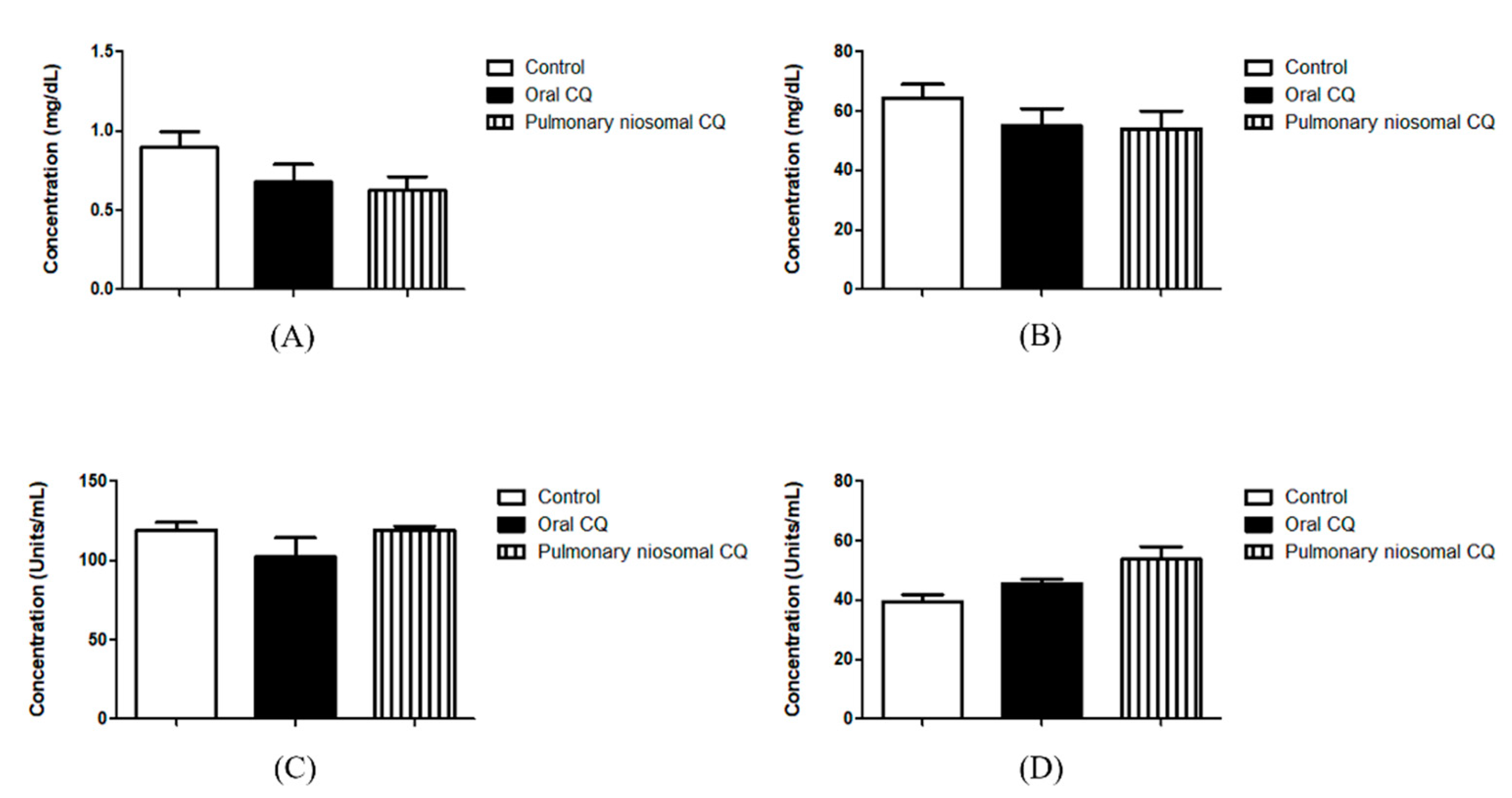
2.7.2. Assessment of Nephrotoxicity Indices
Assessment of Creatinine Level
Assessment of Urea Level
2.7.3. Assessment of Hepatotoxicity Indices
Assessment of AST Level
Assessment of ALT Level
2.8. Statistical Analyses
3. Results and Discussion
3.1. Preparation and Characterizations of the Niosomal Dispersion
3.2. In Vitro Release Studies
3.3. Evaluation of Powder Flowing Properties (Micromeritic Properties)
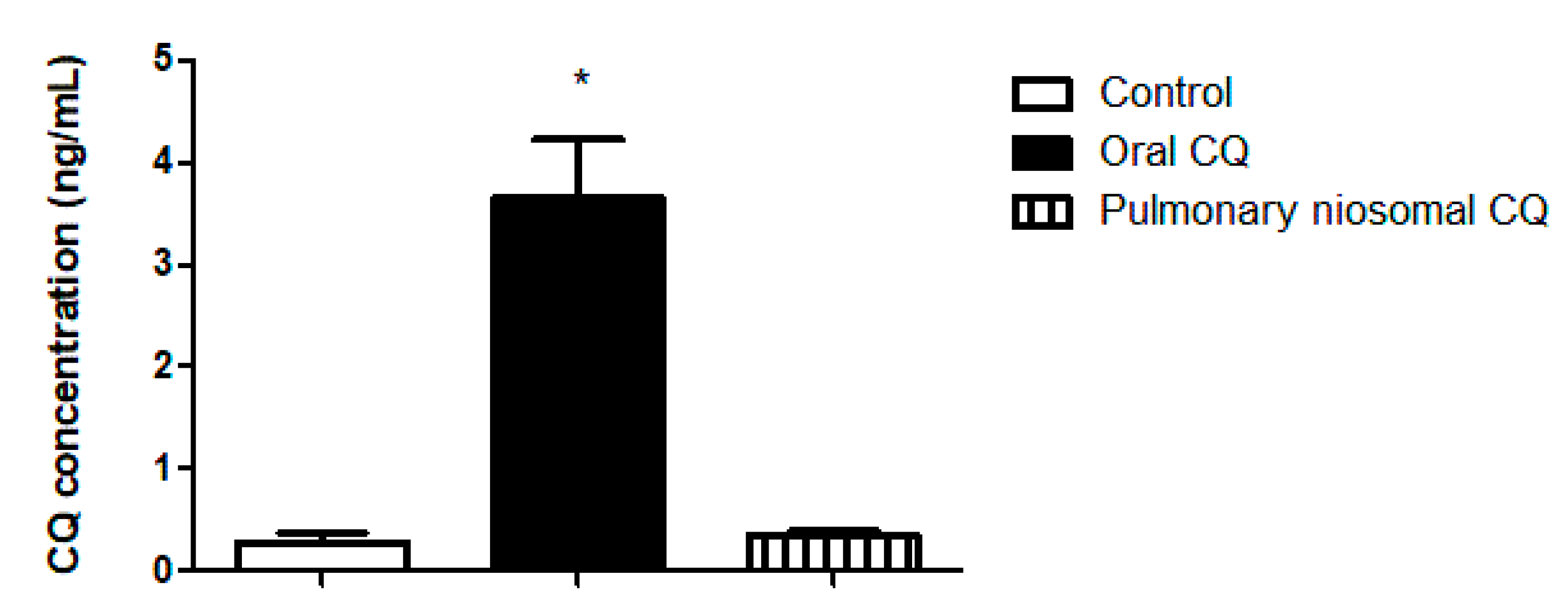
3.4. In Vivo Valuation of the Chloroquine-Loaded Niosomes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pardeshi, C.V.; Agnihotri, V.V.; Patil, K.Y.; Pardeshi, S.R.; Surana, S.J. Mannose-anchored N,N,N-trimethyl chitosan nanoparticles for pulmonary administration of etofylline. Int. J. Biol. Macromol. 2020, 165, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Sankhe, K.; Khan, T.; Bhavsar, C.; Momin, M.; Omri, A. Selective drug deposition in lungs through pulmonary drug delivery system for effective management of drug-resistant TB. Expert Opin. Drug Deliv. 2019, 16, 525–538. [Google Scholar] [CrossRef]
- Jin, X.; Song, L.; Ma, C.C.; Zhang, Y.C.; Yu, S. Pulmonary route of administration is instrumental in developing therapeutic interventions against respiratory diseases. Saudi Pharm. J. 2020, 28, 1655–1665. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, A.A.; Al-Mahallawi, A.M.; Abdelrahim, M.E.; Ali, A.M.A. Preparation, optimization, and in vitro simulated inhalation delivery of carvedilol nanoparticles loaded on a coarse carrier intended for pulmonary administration. Int. J. Nanomed. 2015, 10, 6339–6353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Jha, A.; DR, M.; Mishra, B. Targeted drug nanocrystals for pulmonary delivery: A potential strategy for lung cancer therapy. Expert Opin. Drug Deliv. 2020, 17, 1459–1472. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Pollack, S.F.; Li, R.; Gonzalez, A.M.; Fan, J.; Zou, J.; Leininger, S.E.; Pavía-Sanders, A.; Johnson, R.; et al. Improving paclitaxel delivery: In vitro and in vivo characterization of PEGylated polyphosphoester-based nanocarriers. J. Am. Chem. Soc. 2015, 137, 2056–2066. [Google Scholar] [CrossRef] [PubMed]
- Thabet, Y.; Elsabahy, M.; Eissa, N.G. Methods for preparation of niosomes: A focus on thin-film hydration method. Methods 2021, 1–7. [Google Scholar] [CrossRef]
- Mohamed, H.B.; El-Shanawany, S.M.; Hamad, M.A.; Elsabahy, M. Niosomes: A Strategy toward Prevention of Clinically Significant Drug Incompatibilities. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-mahallawi, A.M.; Fares, A.R.; Abd-Elsalam, W.H. Enhanced Permeation of Methotrexate via Loading into Ultra-permeable Niosomal Vesicles: Fabrication, Statistical Optimization, Ex Vivo Studies, and In Vivo Skin Deposition and Tolerability. AAPS PharmSciTech 2019, 20, 1–10. [Google Scholar] [CrossRef]
- Ghafelehbashi, R.; Akbarzadeh, I.; Tavakkoli Yaraki, M.; Lajevardi, A.; Fatemizadeh, M.; Heidarpoor Saremi, L. Preparation, physicochemical properties, in vitro evaluation and release behavior of cephalexin-loaded niosomes. Int. J. Pharm. 2019, 569, 118580. [Google Scholar] [CrossRef]
- Gugleva, V.; Titeva, S.; Rangelov, S.; Momekova, D. Design and in vitro evaluation of doxycycline hyclate niosomes as a potential ocular delivery system. Int. J. Pharm. 2019, 567, 118431. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Pereira, B.B. Challenges and cares to promote rational use of chloroquine and hydroxychloroquine in the management of coronavirus disease 2019 (COVID-19) pandemic: A timely review. J. Toxicol. Environ. Heal. Part B Crit. Rev. 2020, 23, 177–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.C.; Wang, C.B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Abdelhamid, S.G.; Metwally, A.K.A. Chloroquine and hydroxychloroquine for combating COVID-19: Investigating efficacy and hypothesizing new formulations using Bio/chemoinformatics tools. Informatics Med. Unlocked 2020, 21, 100446. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.Y.; Frieman, M.; Wolfram, J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat. Nanotechnol. 2020, 15, 247–249. [Google Scholar] [CrossRef] [Green Version]
- WHO. Antiviral Drugs That Are Approved or under Evaluation for the Treatment of COVID-19. 2020, 19, 47–88. Available online: https://files.covid19treatmentguidelines.nih.gov/guidelines/section/section_64.pdf (accessed on 8 July 2021).
- Abdel-Rashid, R.S.; Abd Allah, F.I.; Hassan, A.A.; Hashim, F.M. Design, optimization, and in-vivo hypoglycemic effect of nanosized Glibenclamide for inhalation delivery. J. Liposome Res. 2020, 1–35. [Google Scholar] [CrossRef]
- Fathi, H.A.; Abdelkader, A.; AbdelKarim, M.S.; Abdelaziz, A.A.; El Mokhtar, M.A.; Allam, A.; Fetih, G.; El Badry, M.; Elsabahy, M. Electrospun vancomycin-loaded nanofibers for management of methicillin-resistant Staphylococcus aureus-induced skin infections. Int. J. Pharm. 2020, 586, 119620. [Google Scholar] [CrossRef] [PubMed]
- Auda, S.H.; Fathalla, D.; Fetih, G.; El-Badry, M.; Shakeel, F. Niosomes as transdermal drug delivery system for celecoxib: In vitro and in vivo studies. Polym. Bull. 2016, 73, 1229–1245. [Google Scholar] [CrossRef]
- Nasr, A.; Gardouh, A.; Ghorab, M. Novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of olmesartan medoxomil: Design, formulation, pharmacokinetic and bioavailability evaluation. Pharmaceutics 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.M.S.; Martins, R.M.; Freitas, L.A.P. Characteristics of piroxicam granules prepared by fluidized bed hot melt granulation. Adv. Powder Technol. 2018, 29, 934–940. [Google Scholar] [CrossRef]
- Kho, K.; Hadinoto, K. Dry powder inhaler delivery of amorphous drug nanoparticles: Effects of the lactose carrier particle shape and size. Powder Technol. 2013, 233, 303–311. [Google Scholar] [CrossRef]
- Allam, A.; El-Mokhtar, M.A.; Elsabahy, M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J. Pharm. Pharmacol. 2019, 71, 1209–1221. [Google Scholar] [CrossRef]
- Kashef, M.T.; Saleh, N.M.; Assar, N.H.; Ramadan, M.A. The Antimicrobial Activity of Ciprofloxacin-Loaded Niosomes against Ciprofloxacin-Resistant and Biofilm-Forming Staphylococcus aureus. Infect. Drug Resist. 2020, 13, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.A.; Eladawy, S.A.; El-Enin, A.S.M.A.; Hussein, Z.M. Development and investigation of timolol maleate niosomal formulations for the treatment of glaucoma. J. Pharm. Investig. 2020, 50, 59–70. [Google Scholar] [CrossRef]
- Mohsen, A.M.; AbouSamra, M.M.; ElShebiney, S.A. Enhanced oral bioavailability and sustained delivery of glimepiride via niosomal encapsulation: In-vitro characterization and in-vivo evaluation. Drug Dev. Ind. Pharm. 2017, 43, 1254–1264. [Google Scholar] [CrossRef]
- Teaima, M.H.; El Mohamady, A.M.; El-Nabarawi, M.A.; Mohamed, A.I. Formulation and evaluation of niosomal vesicles containing ondansetron HCL for trans-mucosal nasal drug delivery. Drug Dev. Ind. Pharm. 2020, 46, 751–761. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef] [Green Version]
- Akbari, V.; Abedi, D.; Pardakhty, A.; Sadeghi-Aliabadi, H. Ciprofloxacin nano-niosomes for targeting intracellular infections: An in vitro evaluation. J. Nanoparticle Res. 2013, 15, 1556. [Google Scholar] [CrossRef]
- Manosroi, A.; Khanrin, P.; Lohcharoenkal, W.; Werner, R.G.; Götz, F.; Manosroi, W.; Manosroi, J. Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int. J. Pharm. 2010, 392, 304–310. [Google Scholar] [CrossRef]
- Otroj, M.; Taymouri, S.; Varshosaz, J.; Mirian, M. Preparation and characterization of dry powder containing sunitinib loaded PHBV nanoparticles for enhanced pulmonary delivery. J. Drug Deliv. Sci. Technol. 2020, 56, 101570. [Google Scholar] [CrossRef]
- Nemati, E.; Mokhtarzadeh, A.; Panahi-Azar, V.; Mohammadi, A.; Hamishehkar, H.; Mesgari-Abbasi, M.; Ezzati Nazhad Dolatabadi, J.; de la Guardia, M. Ethambutol-Loaded Solid Lipid Nanoparticles as Dry Powder Inhalable Formulation for Tuberculosis Therapy. AAPS PharmSciTech 2019, 20, 1–9. [Google Scholar] [CrossRef]
- Gamal, A.; Saeed, H.; Sayed, O.M.; Kharshoum, R.M.; Salem, H.F. Proniosomal Microcarriers: Impact of Constituents on the Physicochemical Properties of Proniosomes as a New Approach to Enhance Inhalation Efficiency of Dry Powder Inhalers. AAPS PharmSciTech 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Mishra, S.M.; Rohera, B.D. An integrated, quality by design (QbD) approach for design, development and optimization of orally disintegrating tablet formulation of carbamazepine. Pharm. Dev. Technol. 2017, 22, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Katakam, P.; Reddy, M.B.V.; Hwisa, N.T.; Assaleh, F.H.; Dey, B.; Murthy, S.V.; Chandu, B.R.; Adiki, S.K. Formulation and evaluation of sustained release venlafaxine tablets using hydrophilic-hydrophobic polymer combinations by melt granulation. J. Sci. Innov. Res. 2014, 3, 49–59. [Google Scholar]
- Date, A.A.; Desai, N.; Dixit, R.; Nagarsenker, M. Self-nanoemulsifying drug delivery systems: Formulation insights, applications and advances. Nanomedicine 2010, 5, 1595–1616. [Google Scholar] [CrossRef] [PubMed]
- Salústio, P.J.; Machado, M.; Nunes, T.; Sousa e Silva, J.P.; Costa, P.C. Lactose monohydrate flow characterization using shear cell method. Pharm. Dev. Technol. 2020, 25, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.M.; Amaro, M.I.; Paluch, K.J.; Tajber, L. Dry powders for oral inhalation free of lactose carrier particles. Adv. Drug Deliv. Rev. 2014, 75, 32–52. [Google Scholar] [CrossRef] [PubMed]
- Mosgoeller, W.; Prassl, R.; Zimmer, A. Nanoparticle-Mediated Treatment of Pulmonary Arterial Hypertension, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; Volume 508, ISBN 9780123918604. [Google Scholar]
- Al-Hashemi, H.M.B.; Al-Amoudi, O.S.B. A review on the angle of repose of granular materials. Powder Technol. 2018, 330, 397–417. [Google Scholar] [CrossRef]
- Debnath, S.K.; Saisivam, S.; Debanth, M.; Omri, A. Development and evaluation of Chitosan nanoparticles based dry powder inhalation formulations of Prothionamide. PLoS ONE 2018, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Chloroquine or hydroxychloroquine for prophylaxis of COVID-19. Lancet Infect. Dis. 2020, 20, 1118. [Google Scholar] [CrossRef]
- Muller, R. Systemic toxicity of chloroquine and hydroxychloroquine: Prevalence, mechanisms, risk factors, prognostic and screening possibilities. Rheumatol. Int. 2021, 41, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Mubagwa, K. Cardiac effects and toxicity of chloroquine: A short update. Int. J. Antimicrob. Agents 2020, 56, 106057. [Google Scholar] [CrossRef] [PubMed]


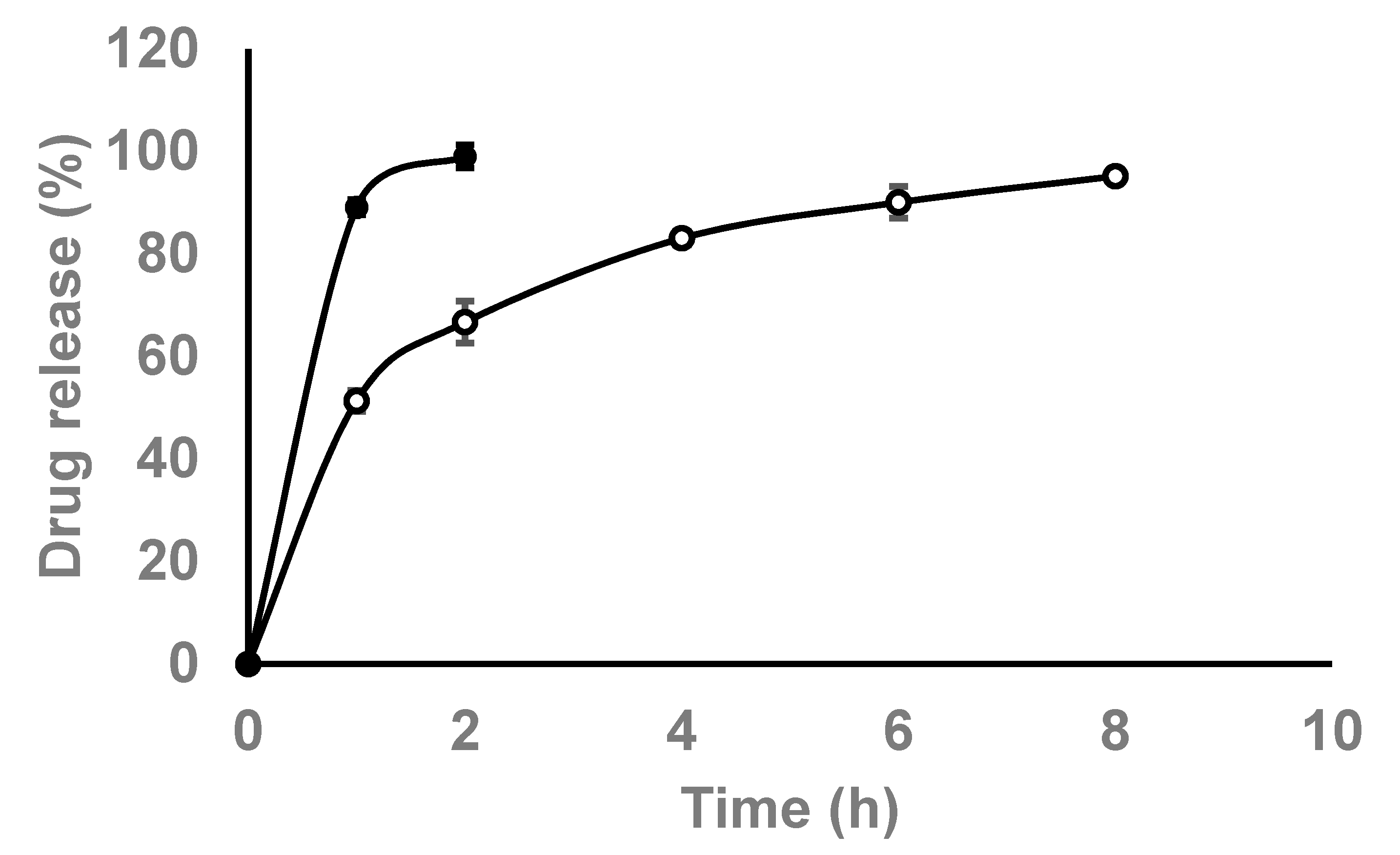
| Kinetic Model | Correlation Coefficient (r2) |
|---|---|
| Zero-order | 0.7302 |
| First-order | 0.9814 |
| Higuchi | 0.9395 |
| Korsmeyer–Peppas | 0.9564 |
| Formulation | Niosomes: Lactose Ratio | Angle of Repose | Carr’s Index | Hausner Ratio |
|---|---|---|---|---|
| Empty Niosomes | 1:1 | N/A | N/A | N/A |
| 1:2 | N/A | 27.5 ± 1.2 | 1.4 ± 0.0 | |
| 1:3 | 26.0 ± 0.3 | 25.4 ± 0.5 | 1.3 ± 0.0 | |
| 1:4 | 31.8 ± 1.2 | 31.6 ± 0.6 | 1.5 ± 0.0 | |
| Chloroquine-loaded Niosomes | 1:1 | N/A | N/A | N/A |
| 1:2 | N/A | N/A | N/A | |
| 1:3 | N/A | 36.8 ± 0.0 | 1.6 ± 0.0 | |
| 1:4 | 28.7 ± 0.3 | 36.5 ± 0.3 | 1.6 ± 0.0 | |
| Chloroquine-loaded Niosomes (1% mannitol) | 1:1 | 26.0 ± 1.1 | 29.1 ± 1.0 | 1.4 ± 0.0 |
| 1:2 | 33.68 ± 0.3 | 27.9 ± 3.1 | 1.4 ± 0.1 | |
| 1:3 | 39.2 ± 0.9 | 32.9 ± 2.0 | 1.5 ± 0.1 | |
| 1:4 | 39.7 ± 4.1 | 40.3 ± 0.7 | 1.7 ± 0.0 | |
| Chloroquine-loaded Niosomes (1% trehalose) | 1:1 | N/A | N/A | N/A |
| 1:2 | N/A | N/A | N/A | |
| 1:3 | 33.7 ± 0.5 | 33.3 ± 1.4 | 1.5 ± 0.0 | |
| 1:4 | 34.3 ± 0.8 | 38.2 ± 3.4 | 1.6 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saafan, H.A.; Ibrahim, K.M.; Thabet, Y.; Elbeltagy, S.M.; Eissa, R.A.; Ghaleb, A.H.; Ibrahim, F.; Elsabahy, M.; Eissa, N.G. Intratracheal Administration of Chloroquine-Loaded Niosomes Minimize Systemic Drug Exposure. Pharmaceutics 2021, 13, 1677. https://doi.org/10.3390/pharmaceutics13101677
Saafan HA, Ibrahim KM, Thabet Y, Elbeltagy SM, Eissa RA, Ghaleb AH, Ibrahim F, Elsabahy M, Eissa NG. Intratracheal Administration of Chloroquine-Loaded Niosomes Minimize Systemic Drug Exposure. Pharmaceutics. 2021; 13(10):1677. https://doi.org/10.3390/pharmaceutics13101677
Chicago/Turabian StyleSaafan, Hesham A., Kamilia M. Ibrahim, Yasmeena Thabet, Sara M. Elbeltagy, Rana A. Eissa, Ashraf H. Ghaleb, Fathy Ibrahim, Mahmoud Elsabahy, and Noura G. Eissa. 2021. "Intratracheal Administration of Chloroquine-Loaded Niosomes Minimize Systemic Drug Exposure" Pharmaceutics 13, no. 10: 1677. https://doi.org/10.3390/pharmaceutics13101677
APA StyleSaafan, H. A., Ibrahim, K. M., Thabet, Y., Elbeltagy, S. M., Eissa, R. A., Ghaleb, A. H., Ibrahim, F., Elsabahy, M., & Eissa, N. G. (2021). Intratracheal Administration of Chloroquine-Loaded Niosomes Minimize Systemic Drug Exposure. Pharmaceutics, 13(10), 1677. https://doi.org/10.3390/pharmaceutics13101677






