PMP(Porphyrin–Micelle–PSMA) Nanoparticles for Photoacoustic and Ultrasound Signal Amplification in Mouse Prostate Cancer Xenografts
Abstract
:1. Introduction
2. Results
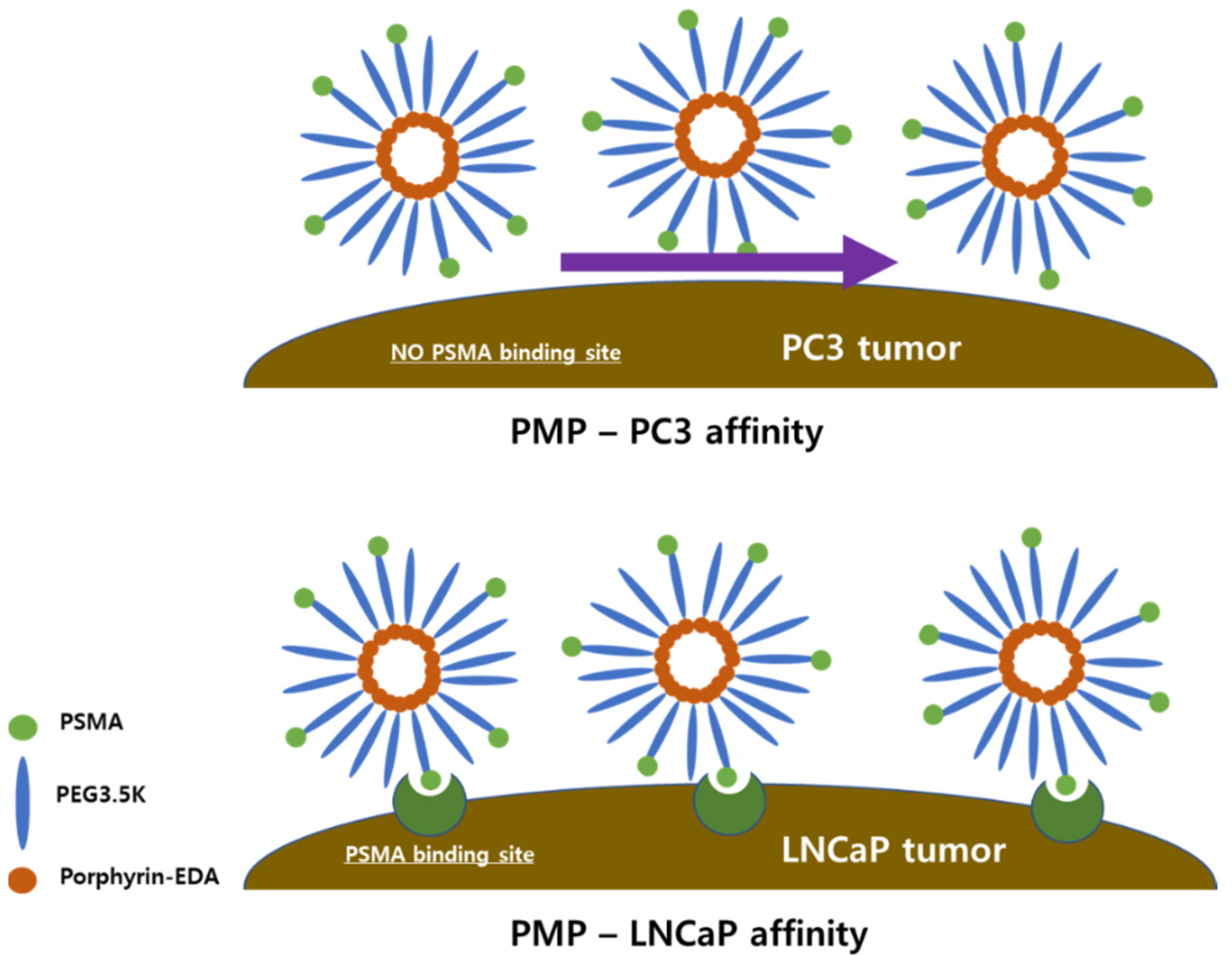
2.1. Schematic of Porphyrin-Micelle-PSMA (PMP) Tumor Binding Phenomenon
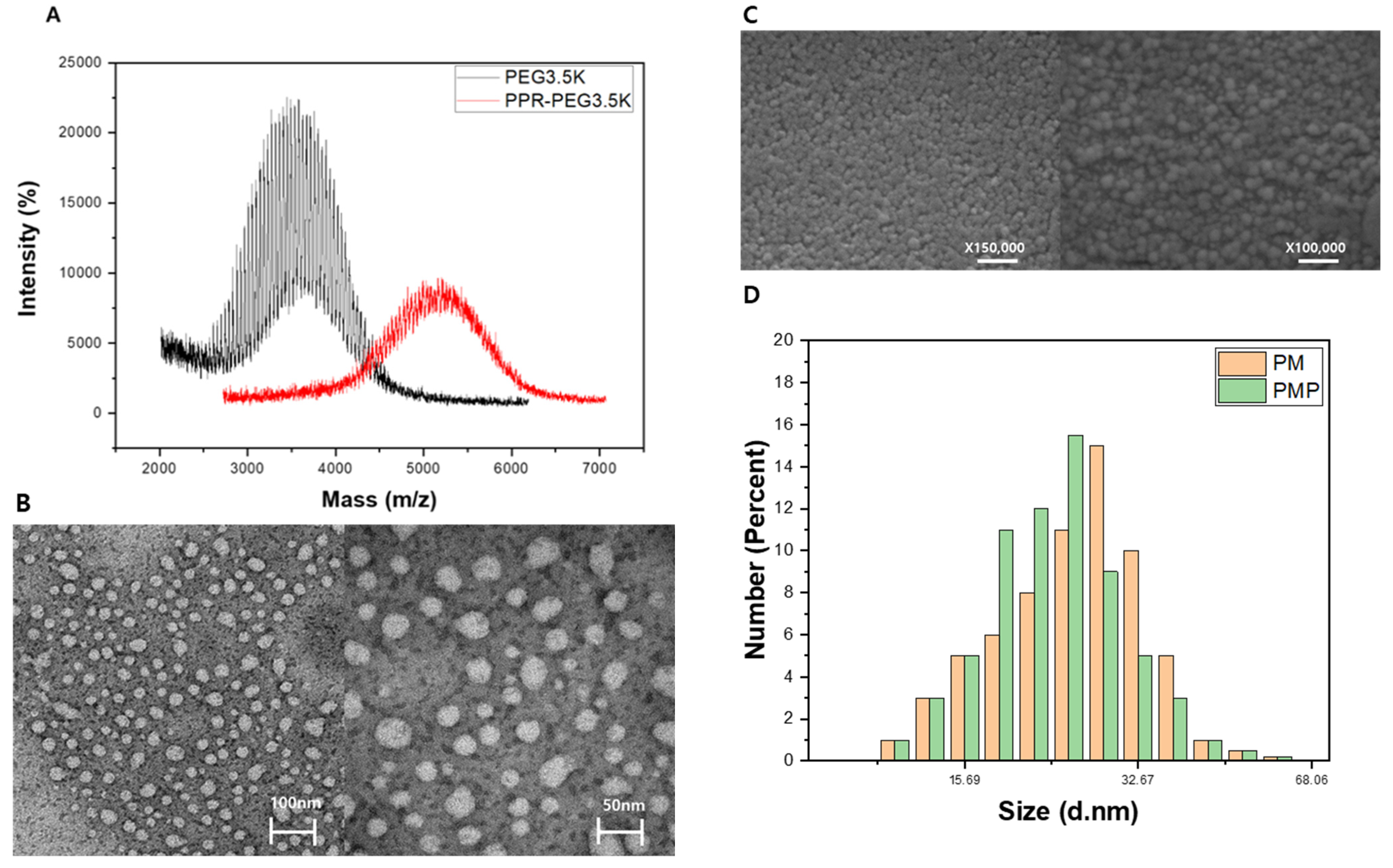
2.2. Characterization of PM and PMP
2.3. In Vitro Cell Viability Study and Confocal Microscopy
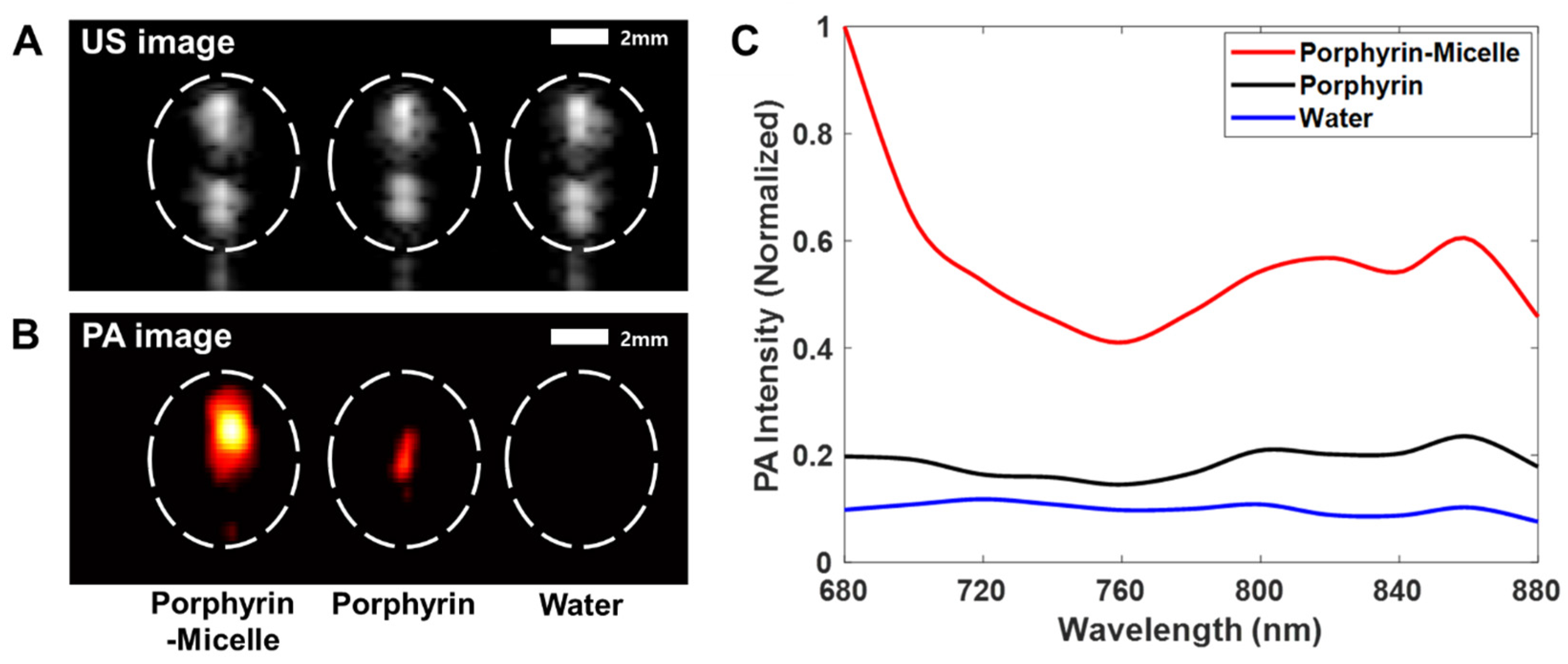
2.4. Selection of Optimal Laser Wavelength
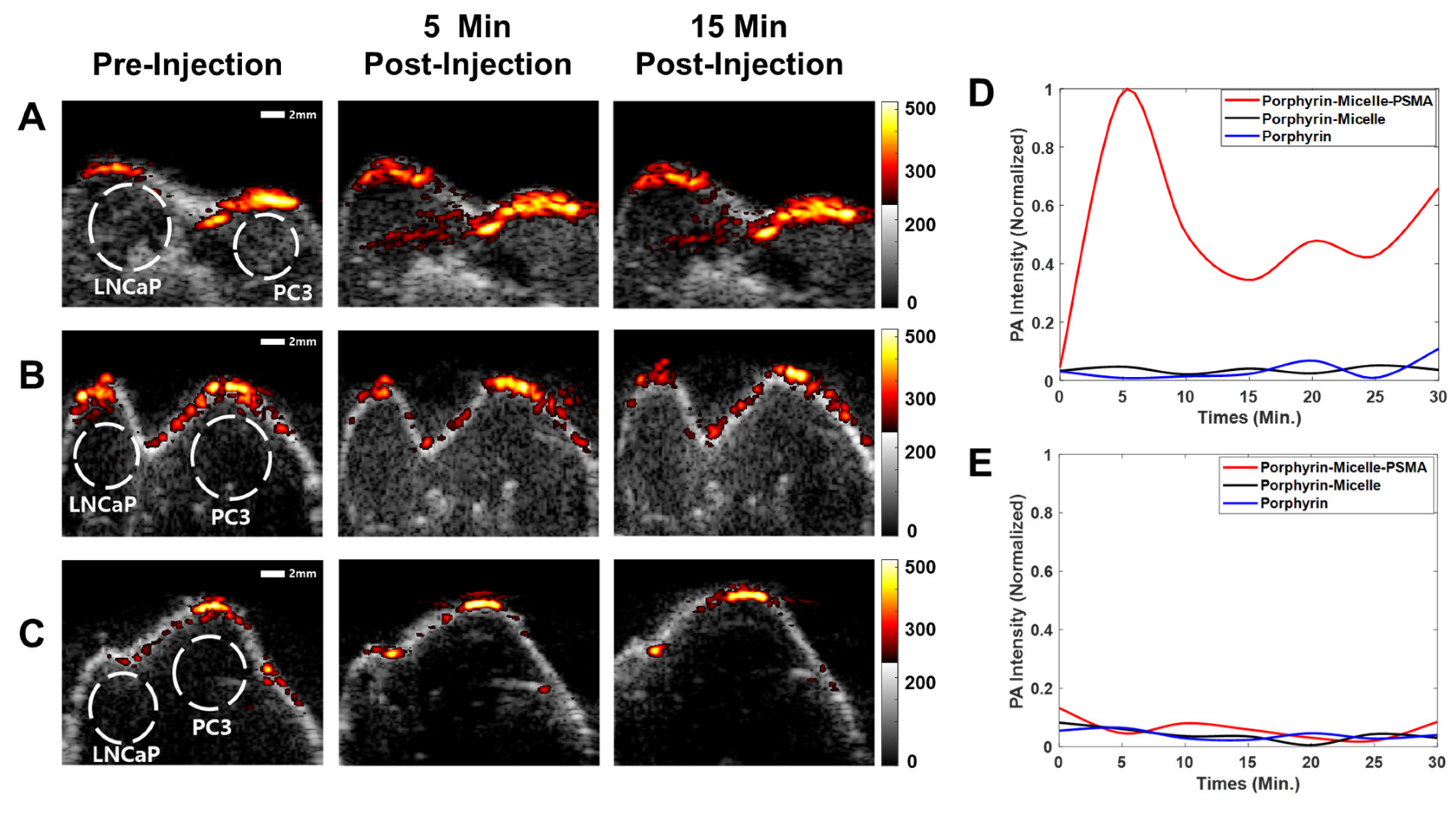
2.5. Photoacoustic Imaging of the Tumor In Vivo
3. Discussion
4. Materials and Methods
4.1. Reagents and Equipment
4.2. Preparation of PMP
4.3. Characterization of PMP
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. Confocal Laser Scanning Microscopy
4.7. In Vivo Study
4.8. Photoacoustic Protocols Ex-Vivo
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelen, V. Epidemiology of prostate cancer. In Prostate Cancer; Springer: New York, NY, USA, 2007; pp. 1–8. [Google Scholar]
- Brawley, O.W. Trends in prostate cancer in the United States. J. Natl. Cancer Inst. 2012, 2012, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, P.; Asselin, E. Resistance to chemotherapy and hormone therapy in endometrial cancer. Endocr. Relat. Cancer 2009, 16, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Mannuel, H.D.; Hussain, A. Evolving role of surgery, radiation, hormone therapy, and chemotherapy in high-risk locally advanced prostate cancer. Clin. Genitourin. Cancer 2006, 5, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.P.; Khoo, V.S.; Norman, A.R.; Meyer, L.; Nahum, A.; Tait, D.; Yarnold, J.; Horwich, A. Comparison of radiation side-effects of conformal and conventional radiotherapy in prostate cancer: A randomised trial. Lancet 1999, 353, 267–272. [Google Scholar] [CrossRef]
- Korfage, I.J.; Hak, T.; de Koning, H.J.; Essink-Bot, M.-L. Patients’ perceptions of the side-effects of prostate cancer treatment—A qualitative interview study. Soc. Sci. Med. 2006, 63, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, D.; Ash, D.; Al-Qaisieh, B.; Carey, B.; Joseph, J.; Clair, S.S.; Gould, K. Side effects of permanent I125 prostate seed implants in 667 patients treated in Leeds. Radiother. Oncol. 2007, 82, 46–49. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Van der Leest, M.; Cornel, E.; Israël, B.; Hendriks, R.; Padhani, A.R.; Hoogenboom, M.; Zamecnik, P.; Bakker, D.; Yanti Setiasti, A.; Veltman, J.; et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naive men with elevated prostate-specific antigen: A large prospective multicenter clinical study. Eur. Urol. 2019, 75, 570–578. [Google Scholar]
- Hoeks, C.M.; Schouten, M.G.; Bomers, J.G.; Hoogendoorn, S.P.; de Kaa, C.A.H.-V.; Hambrock, T.; Vergunst, H.; Sedelaar, J.M.; Fütterer, J.J.; Barentsz, J.O. Three-tesla magnetic resonance–guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: Detection of clinically significant prostate cancers. Eur. Urol. 2012, 62, 902–909. [Google Scholar] [CrossRef]
- Fascelli, M.; Rais-Bahrami, S.; Sankineni, S.; Brown, A.M.; George, A.K.; Ho, R.; Frye, T.; Kilchevsky, A.; Chelluri, R.; Abboud, S.; et al. Combined biparametric prostate magnetic resonance imaging and prostate-specific antigen in the detection of prostate cancer: A validation study in a biopsy-naive patient population. Urol. 2016, 88, 125–134. [Google Scholar] [CrossRef]
- Park, B.K.; Park, J.W.; Park, S.Y.; Kim, C.K.; Lee, H.M.; Jeon, S.S.; Seo, S.I.; Jeong, B.C.; Choi, H.Y. Prospective evaluation of 3-T MRI performed before initial transrectal ultrasound–guided prostate biopsy in patients with high prostate-specific antigen and no previous biopsy. Am. J. Roentgenol. 2011, 197, W876–W881. [Google Scholar] [CrossRef]
- Rais-Bahrami, S.; Siddiqui, M.M.; Vourganti, S.; Turkbey, B.; Rastinehad, A.R.; Stamatakis, L.; Truong, H.; Walton-Diaz, A.; Hoang, A.N.; Nix, J.W.; et al. Diagnostic value of biparametric magnetic resonance imaging (MRI) as an adjunct to prostate-specific antigen (PSA)-based detection of prostate cancer in men without prior biopsies. BJU Int. 2014, 115, 381–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in biomedical imaging and cancer therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Siddique, S.; Chow, J.C.L. Gold nanoparticles for drug delivery and cancer therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Labrie, F.; Dupont, A.; Suburu, R.; Cusan, L.; Tremblay, M.; Gomez, J.-L.; Emond, J. Serum prostate specific antigen as pre-screening test for prostate cancer. J. Urol. 1992, 147, 846–851. [Google Scholar] [CrossRef]
- Mohler, J.; Bahnson, R.R.; Boston, B.; Busby, J.E.; D’Amico, A.; Eastham, J.; Enke, C.A.; George, D.; Horwitz, E.M.; Huben, R.P.; et al. Prostate cancer. J. Natl. Compr. Cancer Netw. 2010, 8, 162–200. [Google Scholar] [CrossRef] [PubMed]
- Govers, T.M.; Hessels, D.; Vlaeminck-Guillem, V.; Schmitz-Dräger, B.J.; Stief, C.G.; Martinez-Ballesteros, C.; Ferro, M.; Borque-Fernando, A.; Rubio-Briones, J.; Sedelaar, J.P.M.; et al. Cost-effectiveness of SelectMDx for prostate cancer in four European countries: A comparative modeling study. Prostate Cancer Prostatic Dis. 2018, 22, 101–109. [Google Scholar] [CrossRef]
- Evans, N.; Jackson, A.; Matlin, S.; Towill, R. High-performance liquid chromatographic analysis of porphyrins in clinical materials. J. Chromatogr. A 1976, 125, 345–355. [Google Scholar] [CrossRef]
- Mang, T.S.; Dougherty, T.J.; Potter, W.R.; Boyle, D.G.; Somer, S.; Moan, J. Photobleaching of porphyrins used in photodynamic therapy and implications for therapy. Photochem. Photobiol. 1987, 45, 501–506. [Google Scholar] [CrossRef]
- Kessel, D. Methods in Porphyrin Photosensitization; Springer: New York, NY, USA, 1985. [Google Scholar]
- Akbarzadeh, I.; Yaraki, M.T.; Bourbour, M.; Noorbazargan, H.; Lajevardi, A.; Shilsar, S.M.S.; Heidari, F.; Mousavian, S.M. Optimized doxycycline-loaded niosomal formulation for treatment of infection-associated prostate cancer: An in-vitro investigation. J. Drug Deliv. Sci. Technol. 2020, 57, 101715. [Google Scholar] [CrossRef]
- Yaraki, M.T.; Pan, Y.; Hu, F.; Yu, Y.; Liu, B.; Tan, Y.N. Nanosilver-enhanced AIE photosensitizer for simultaneous bioimaging and photodynamic therapy. Mater. Chem. Front. 2020, 4, 3074–3085. [Google Scholar] [CrossRef]
- Bressler, N.M.; Bressler, S.B. Photodynamic therapy with verteporfin (Visudyne): Impact on ophthalmology and visual sciences. Investig. Ophthalmol. Vis. Sci. 2000, 41, 624–628. [Google Scholar]
- Bressler, N.M. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two-year results of 2 randomized clinical trials-tap report. Arch. Ophthalmol. 2001, 119, 198–207. [Google Scholar]
- Khurana, M.; Collins, H.A.; Karotki, A.; Anderson, H.L.; Cramb, D.T.; Wilson, B.C. Quantitative in vitro demonstration of two-photon photodynamic therapy using Photofrin® and Visudyne®. Photochem. Photobiol. 2007, 83, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liang, X.; Dai, Z. Porphyrin-loaded nanoparticles for cancer theranostics. Nanoscale 2016, 8, 12394–12405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, J.; Ma, F.; Ling, P.; Liu, J.; Ju, H. A porphyrin photosensitized metal–organic framework for cancer cell apoptosis and caspase responsive theranostics. Chem. Commun. 2015, 51, 10831–10834. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, E.D.; Dolphin, D.; Brückner, C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar] [CrossRef]
- Bonnett, R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Maurer, T.; Eiber, M.; Schwaiger, M.E.M.; Gschwend, T.M.J.E. Current use of PSMA–PET in prostate cancer management. Nat. Rev. Urol. 2016, 13, 226–235. [Google Scholar] [CrossRef]
- Ghosh, A.; Heston, W.D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell. Biochem. 2004, 91, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Afshar-Oromieh, A.; Babich, J.W.; Kratochwil, C.; Giesel, F.L.; Eisenhut, M.; Kopka, K.; Haberkorn, U. The rise of PSMA ligands for diagnosis and therapy of prostate cancer. J. Nucl. Med. 2016, 57, 79S–89S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulrich, H.; Wrenger, C. Disease-specific biomarker discovery by aptamers. Cytom. Part A 2009, 75, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Dowling, P.; Clynes, M. Conditioned media from cell lines: A complementary model to clinical specimens for the discovery of disease-specific biomarkers. Proteomics 2011, 11, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Lolli, F.; Mulinacci, B.; Carotenuto, A.; Bonetti, B.; Sabatino, G.; Mazzanti, B.; D’Ursi, A.M.; Novellino, E.; Pazzagli, M.; Lovato, L.; et al. An N-glucosylated peptide detecting disease-specific autoantibodies, biomarkers of multiple sclerosis. Proc. Natl. Acad. Sci. USA 2005, 102, 10273–10278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmuck, S.; von Klot, C.A.; Henkenberens, C.; Sohns, J.M.; Christiansen, H.; Wester, H.-J.; Ross, T.L.; Bengel, F.M.; Derlin, T. Initial experience with volumetric 68Ga-PSMA I&T PET/CT for Assessment of whole-body tumor burden as a quantitative imaging biomarker in patients with prostate cancer. J. Nucl. Med. 2017, 58, 1962–1968. [Google Scholar] [CrossRef] [Green Version]
- Bravaccini, S.; Puccetti, M.; Bocchini, M.; Ravaioli, S.; Celli, M.; Scarpi, E.; De Giorgi, U.; Tumedei, M.M.; Raulli, G.; Cardinale, L.; et al. PSMA expression: A potential ally for the pathologist in prostate cancer diagnosis. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Kim, E.-K.; Kwak, J.Y.; Yoo, Y.; Song, T.-K.; Chang, J.H. Optimal laser wavelength for photoacoustic imaging of breast microcalcifications. Appl. Phys. Lett. 2011, 99, 153702. [Google Scholar] [CrossRef]
- Kim, H.; Jo, G.; Chang, J.H. Ultrasound-assisted photothermal therapy and real-time treatment monitoring. Biomed. Opt. Express 2018, 9, 4472–4480. [Google Scholar] [CrossRef]

| Porphyrin Micelle | Size (nm) | Zeta Potential (mV) |
|---|---|---|
| PM | 23 ± 4.5 | −11.3 ± 2.1 |
| PMP | 26 ± 6.2 | −14.4 ± 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Han, W.; Chang, J.H.; Lee, H.J. PMP(Porphyrin–Micelle–PSMA) Nanoparticles for Photoacoustic and Ultrasound Signal Amplification in Mouse Prostate Cancer Xenografts. Pharmaceutics 2021, 13, 1636. https://doi.org/10.3390/pharmaceutics13101636
Kim D, Han W, Chang JH, Lee HJ. PMP(Porphyrin–Micelle–PSMA) Nanoparticles for Photoacoustic and Ultrasound Signal Amplification in Mouse Prostate Cancer Xenografts. Pharmaceutics. 2021; 13(10):1636. https://doi.org/10.3390/pharmaceutics13101636
Chicago/Turabian StyleKim, Daehyun, Wonkook Han, Jin Ho Chang, and Hak Jong Lee. 2021. "PMP(Porphyrin–Micelle–PSMA) Nanoparticles for Photoacoustic and Ultrasound Signal Amplification in Mouse Prostate Cancer Xenografts" Pharmaceutics 13, no. 10: 1636. https://doi.org/10.3390/pharmaceutics13101636
APA StyleKim, D., Han, W., Chang, J. H., & Lee, H. J. (2021). PMP(Porphyrin–Micelle–PSMA) Nanoparticles for Photoacoustic and Ultrasound Signal Amplification in Mouse Prostate Cancer Xenografts. Pharmaceutics, 13(10), 1636. https://doi.org/10.3390/pharmaceutics13101636








