Gastroretentive Technologies in Tandem with Controlled-Release Strategies: A Potent Answer to Oral Drug Bioavailability and Patient Compliance Implications
Abstract
1. Introduction
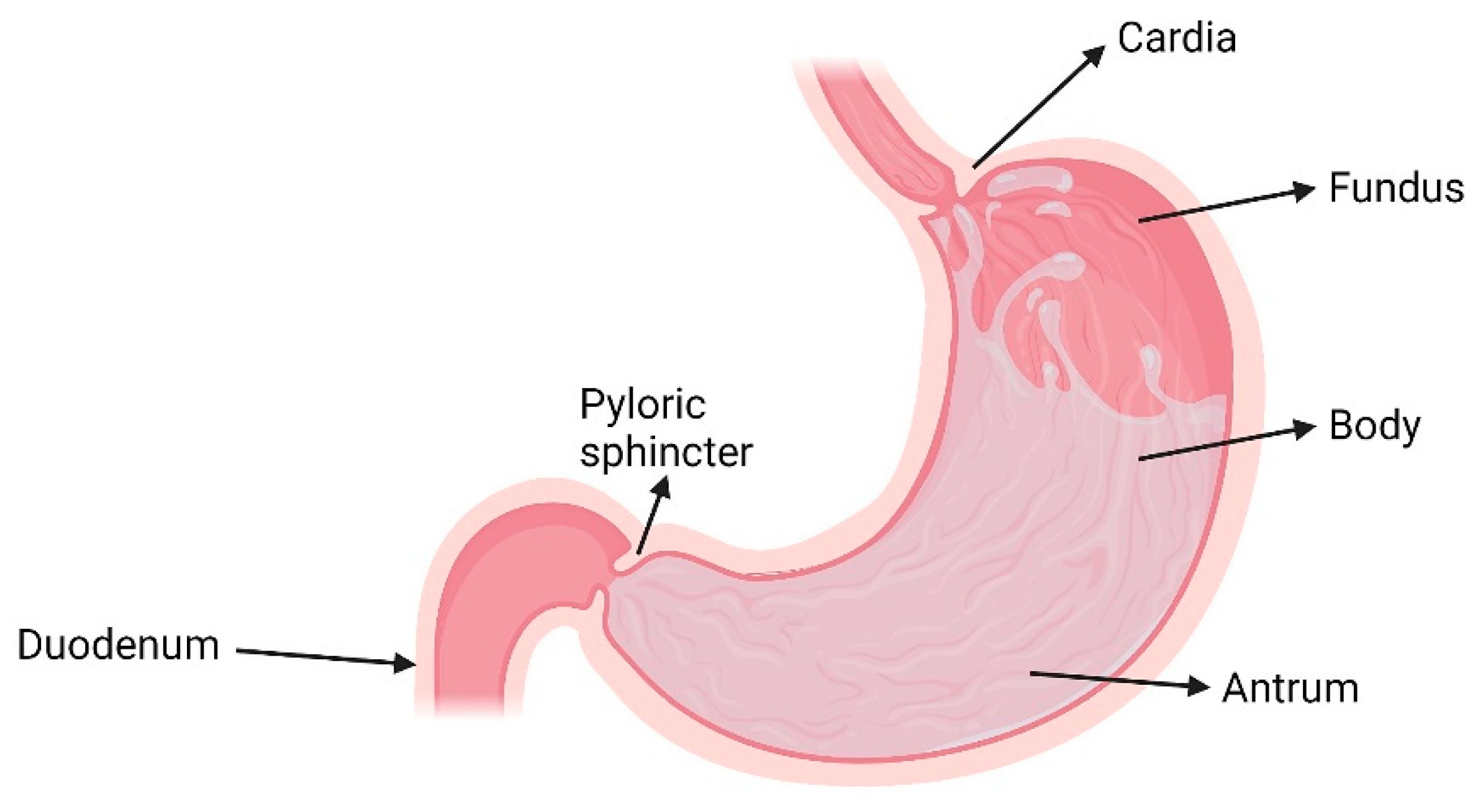
2. The Stomach
3. Factors Affecting the Efficacy of GRDDS
3.1. Formulation-Related Factors
3.2. Physiological Factors
3.3. Patient-Related Factors
4. Gastroretentive Drug Delivery Technologies and Their Controlled-Release Applications
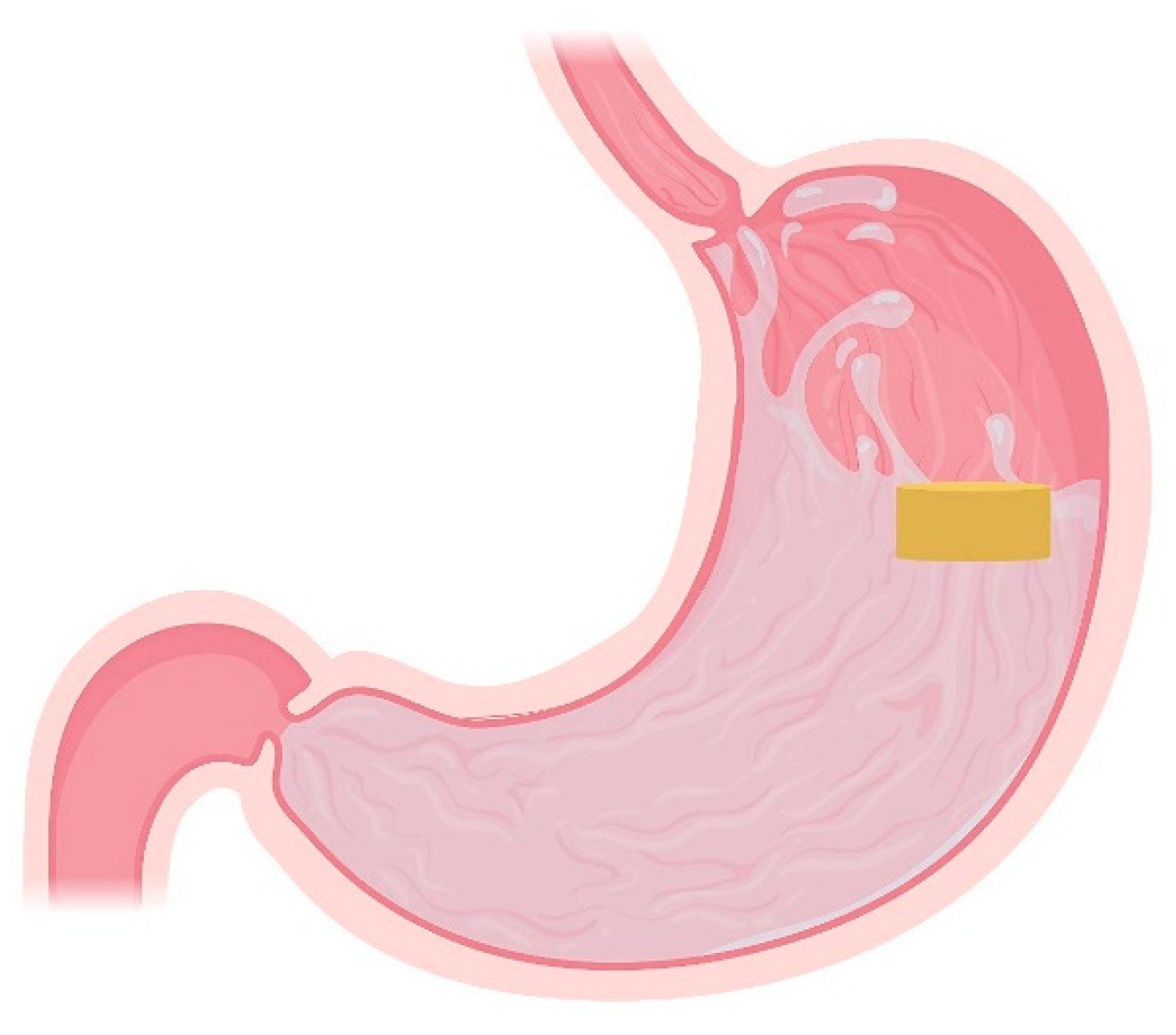
4.1. Floating Systems
4.1.1. Non-Effervescent Floating Systems
- Hydrodynamically Balanced Systems
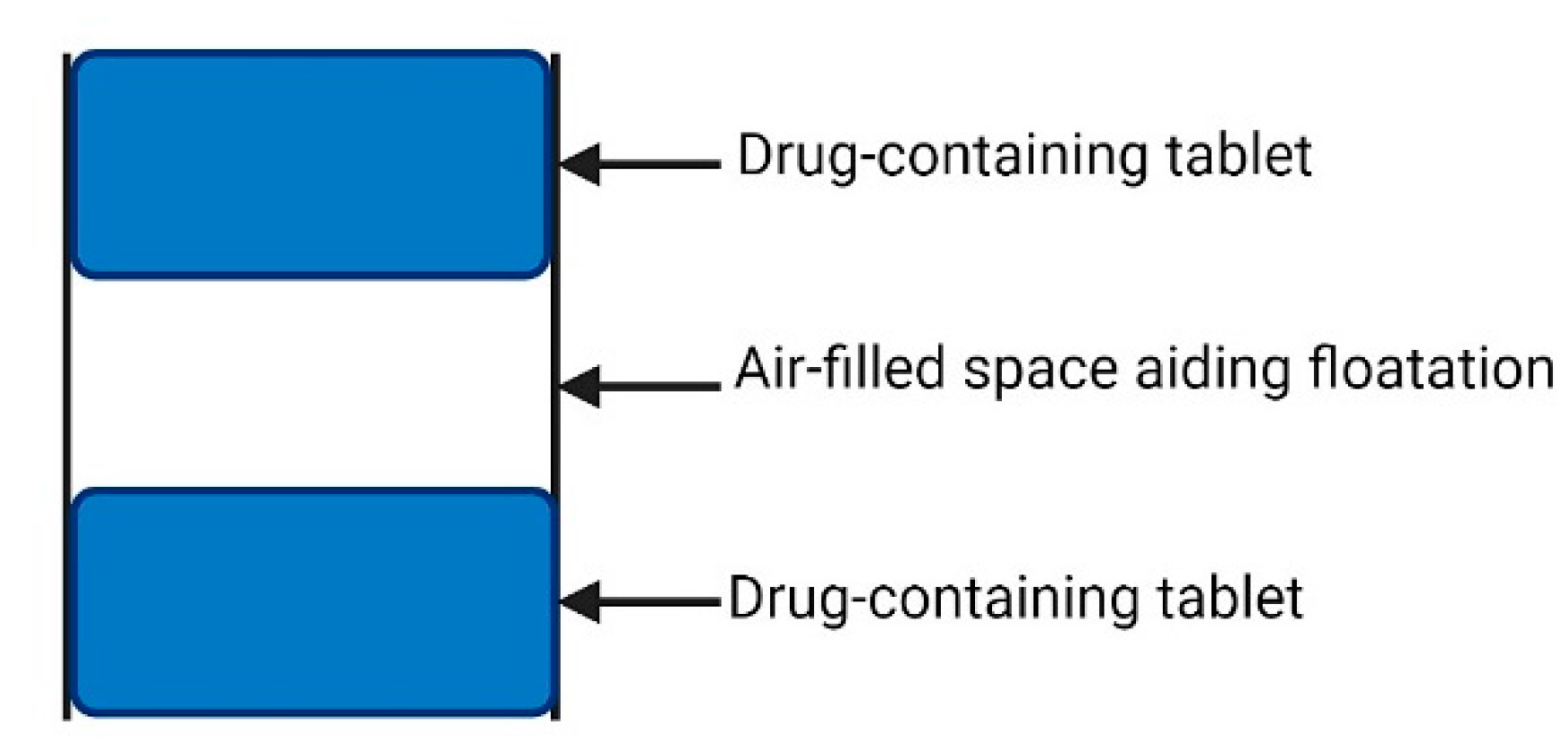
- Non-Effervescent Tablets
- Low-Density Systems
4.1.2. Effervescent Floating Systems
4.1.3. Raft-Forming Systems
4.2. High-Density Systems
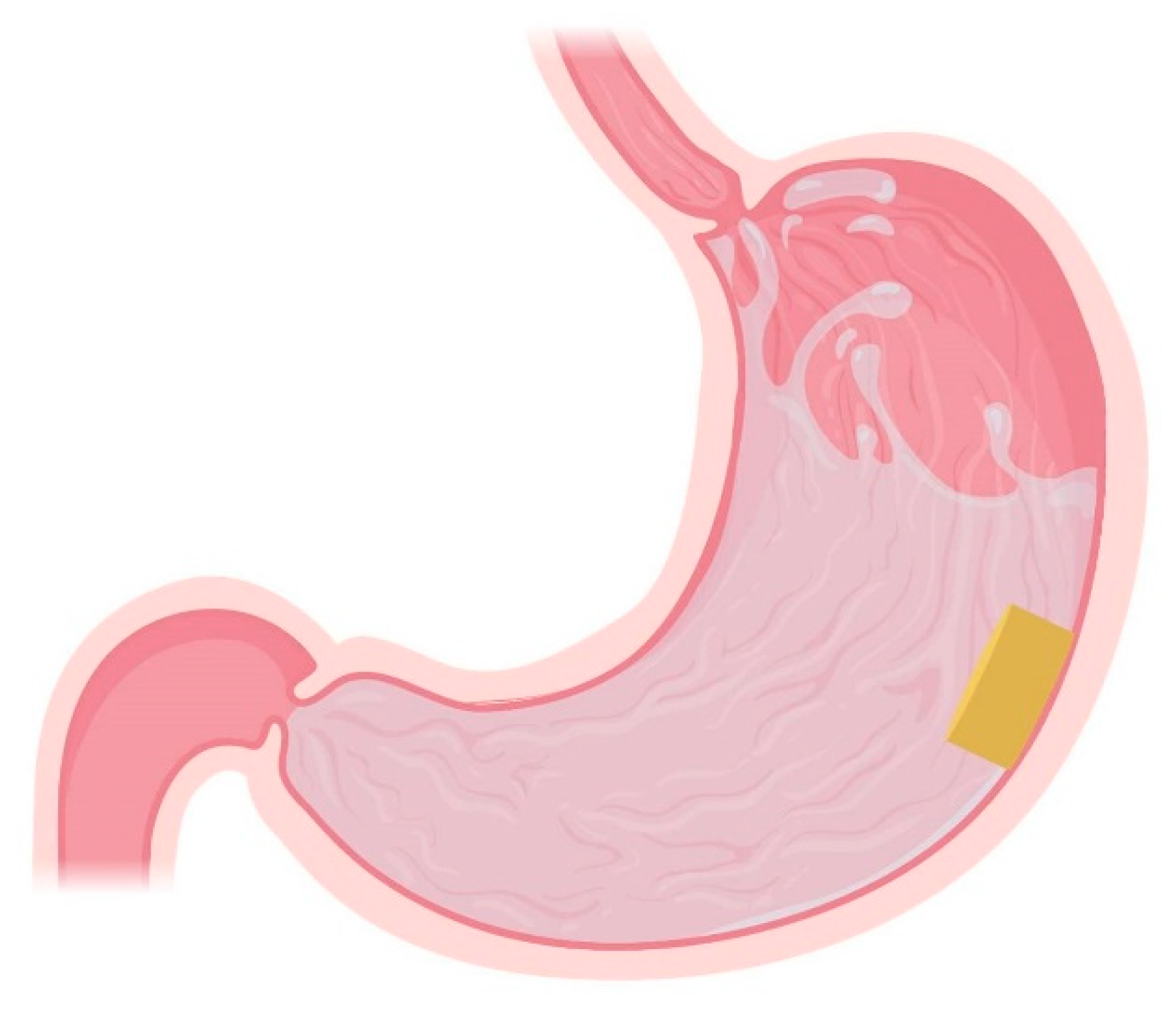
4.3. Mucoadhesive/Bioadhesive Systems
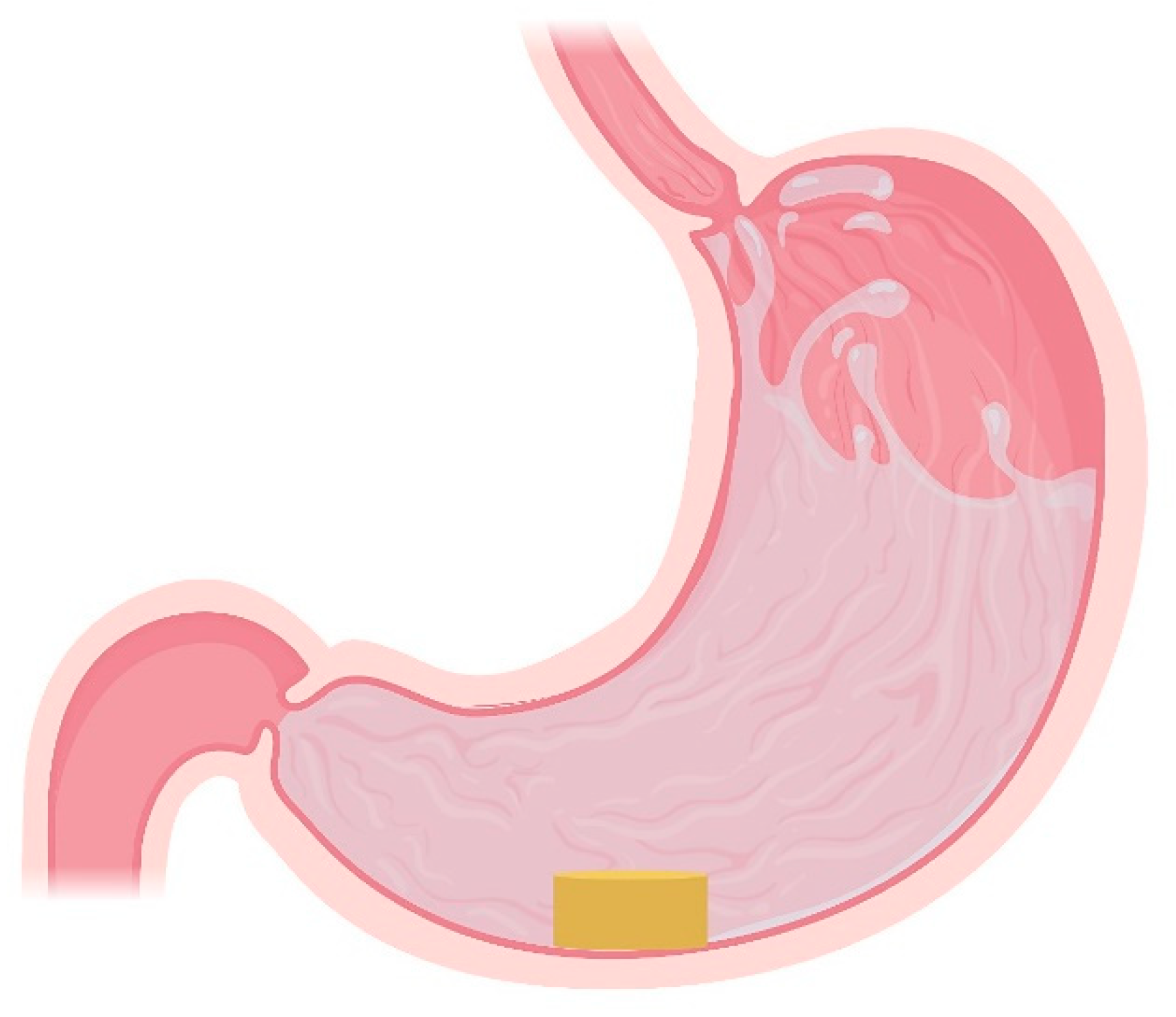
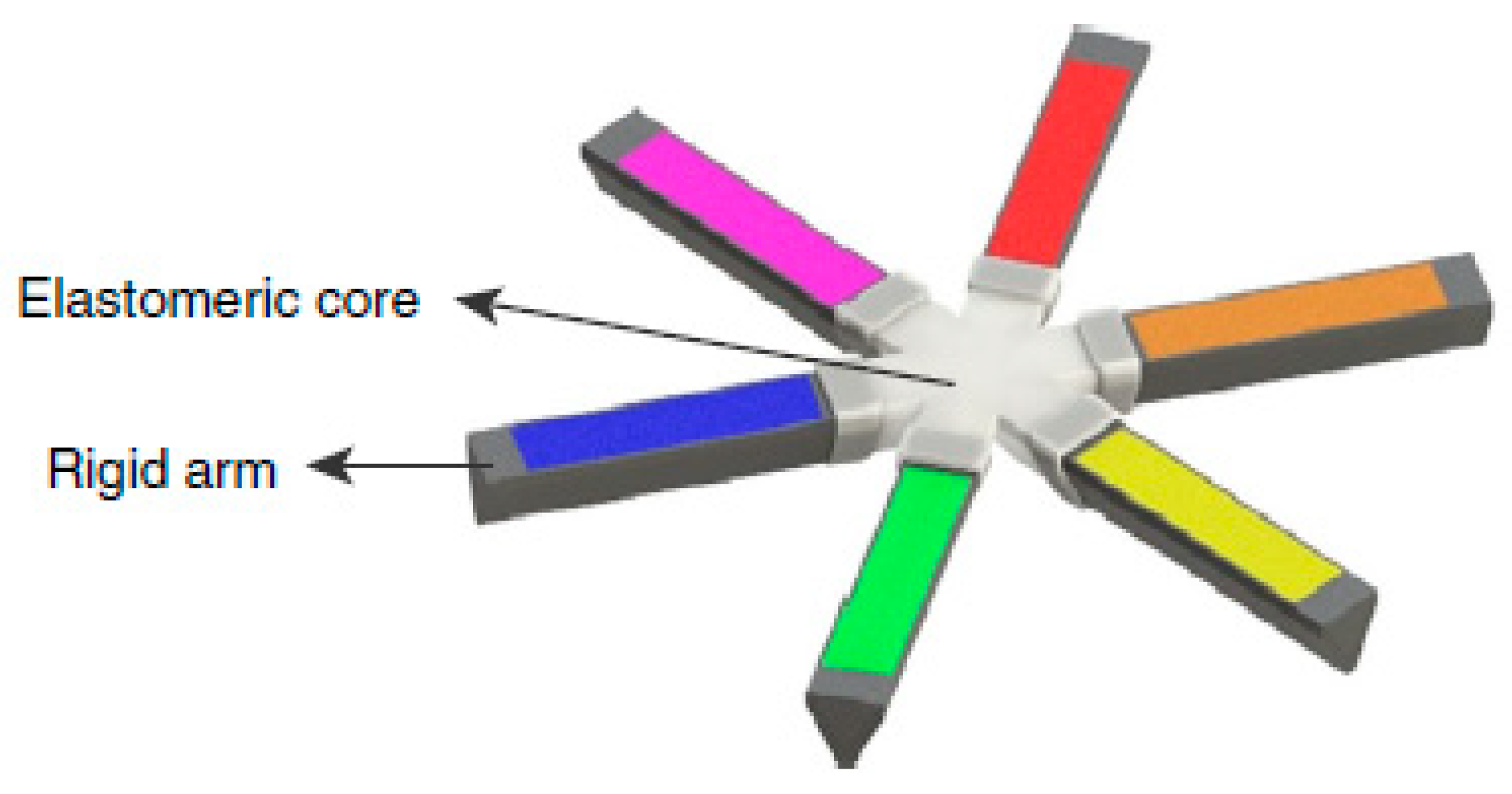
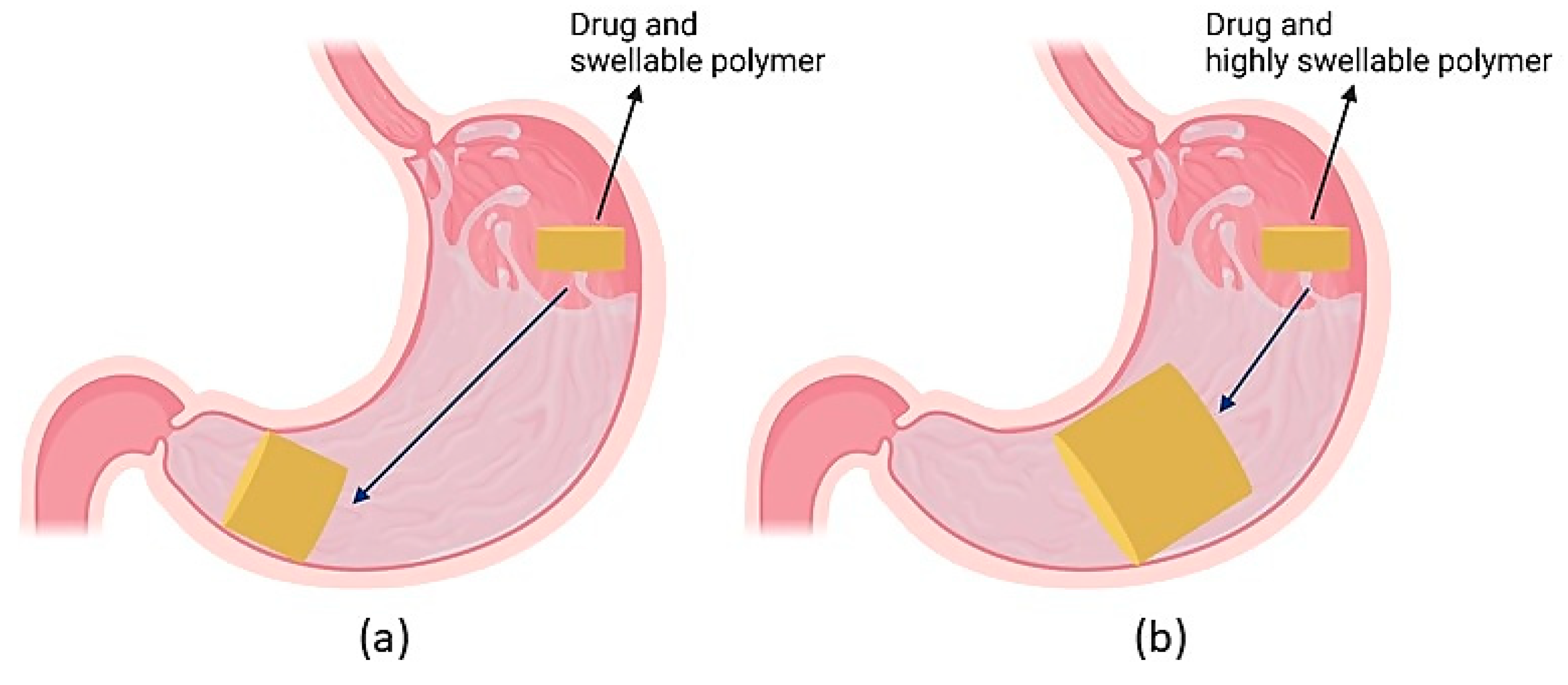
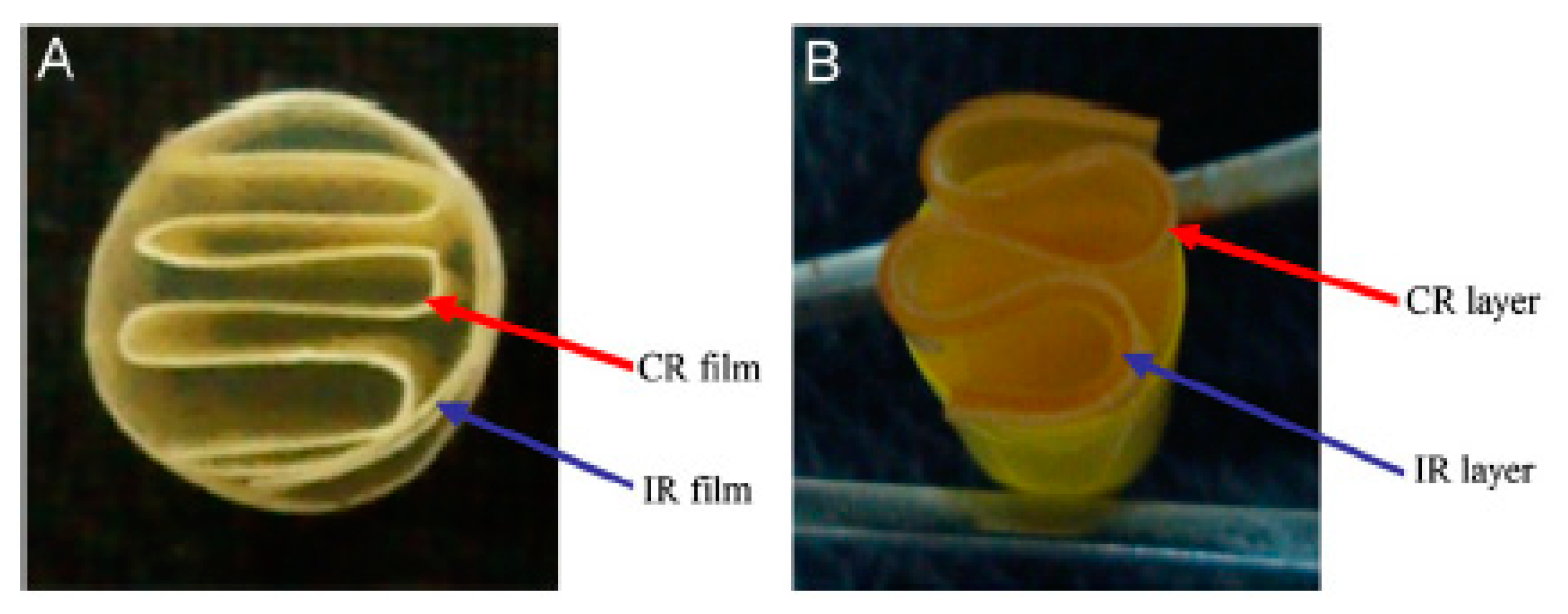
4.4. Expandable Systems
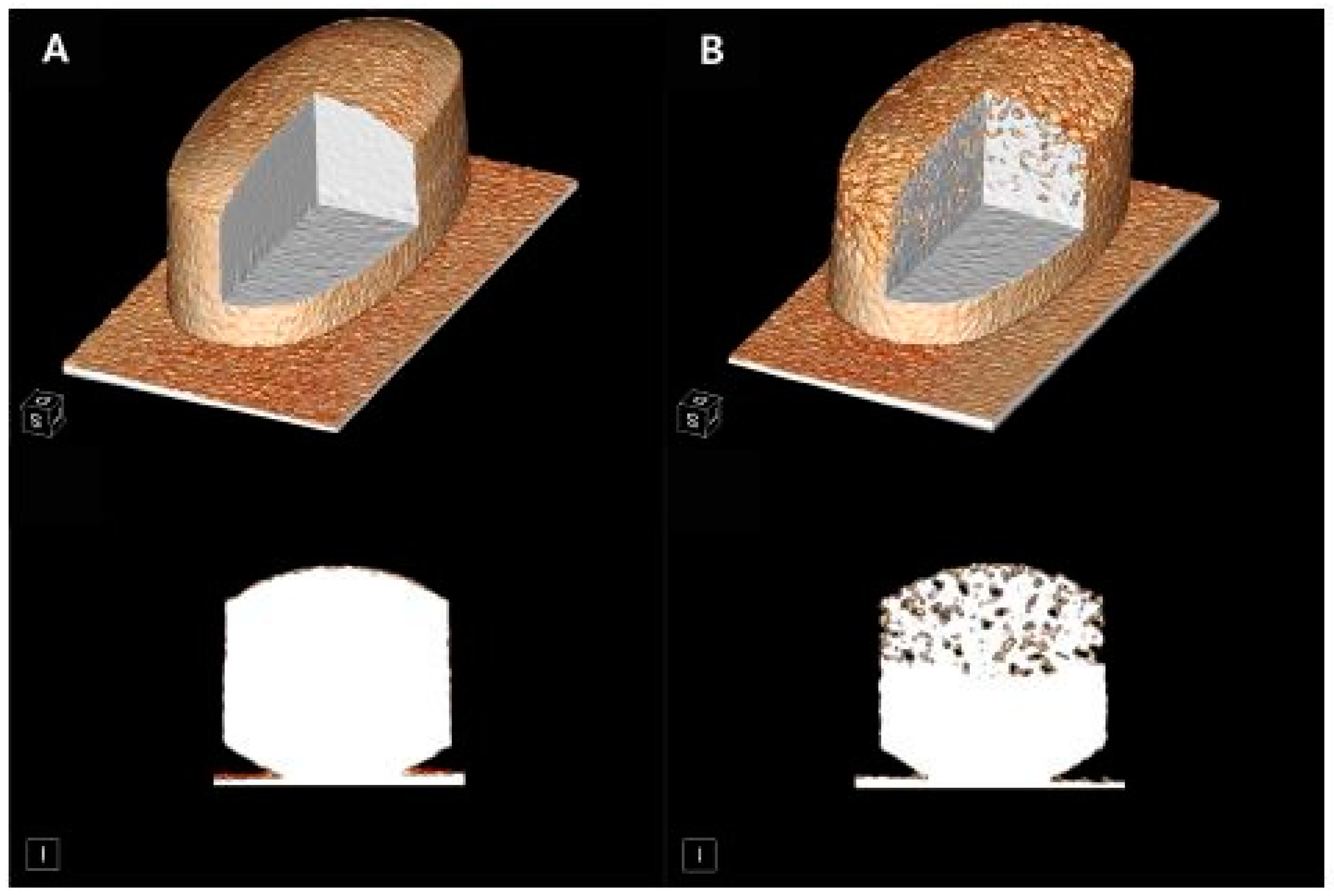
4.5. Superporous Hydrogel Systems
4.6. Osmotic Systems
4.7. Ion-Exchange Resin Systems
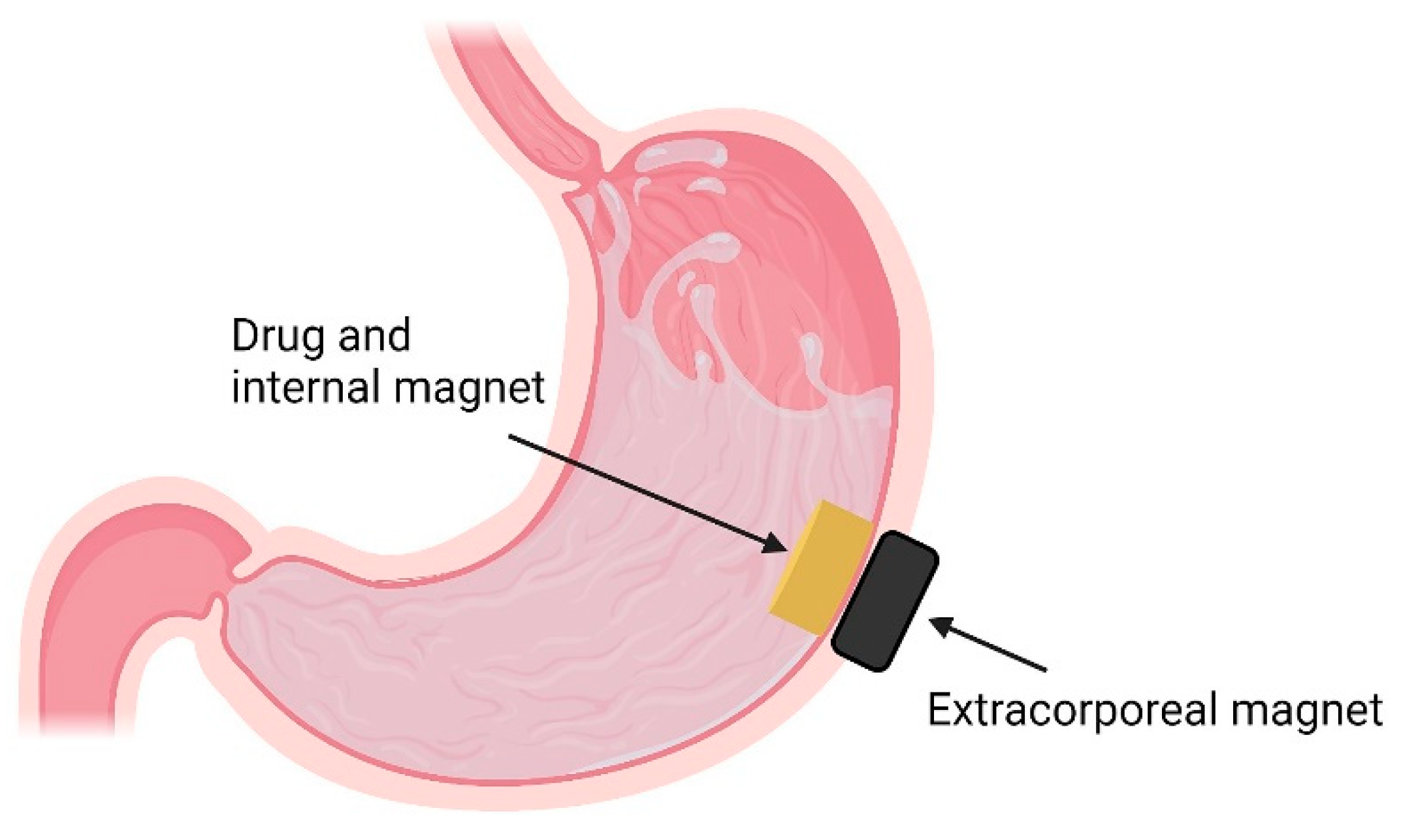
4.8. Magnetic Systems
4.9. Combinatory Approaches
4.9.1. Floating and Expandable Systems
4.9.2. Mucoadhesive and Expandable Systems
4.9.3. Floating and Mucoadhesive Systems
5. Future Considerations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, L.; Qin, C.; Chen, K.; Zhu, C.; Cao, H.; Zhou, J.; He, W.; Zhang, Q. Gastro-floating tablets of cephalexin: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2013, 452, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Ho, H.-O.; Lee, T.-Y.; Sheu, M.-T. Physical characterizations and sustained release profiling of gastroretentive drug delivery systems with improved floating and swelling capabilities. Int. J. Pharm. 2013, 441, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Amit Kumar, N.; Jadupati, M.; Kalyan Kumar, S. Gastroretentive drug delivery technologies: Current approaches and future potential. J. Pharm. Educ. Res. 2010, 1, 1. [Google Scholar]
- Gröning, R.; Cloer, C.; Georgarakis, M.; Müller, R.S. Compressed collagen sponges as gastroretentive dosage forms: In vitro and in vivo studies. Eur. J. Pharm. Sci. 2007, 30, 1–6. [Google Scholar] [CrossRef]
- Lopes, C.M.; Bettencourt, C.; Rossi, A.; Buttini, F.; Barata, P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int. J. Pharm. 2016, 510, 144–158. [Google Scholar] [CrossRef]
- Gröning, R.; Heun, G. Oral Dosage Forms with Controlled Gastrointestinal Transit. Drug Dev. Ind. Pharm. 1984, 10, 527–539. [Google Scholar] [CrossRef]
- Moës, A.J. Gastroretentive dosage forms. Crit. Rev. Ther. Drug Carr. Syst. 1993, 10, 143–195. [Google Scholar]
- Deshpande, A.A.; Rhodes, C.T.; Shah, N.H.; Malick, A.W. Controlled-Release Drug Delivery Systems for Prolonged Gastric Residence: An Overview. Drug Dev. Ind. Pharm. 1996, 22, 531–539. [Google Scholar] [CrossRef]
- Bardonnet, P.L.; Faivre, V.; Pugh, W.J.; Piffaretti, J.C.; Falson, F. Gastroretentive dosage forms: Overview and special case of Helicobacter pylori. J. Control. Release 2006, 111, 1–18. [Google Scholar] [CrossRef]
- Tripathi, J.; Thapa, P.; Maharjan, R.; Jeong, S.H. Current State and Future Perspectives on Gastroretentive Drug Delivery Systems. Pharmaceutics 2019, 11, 193. [Google Scholar] [CrossRef]
- Rouge, N.; Buri, P.; Doelker, E. Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int. J. Pharm. 1996, 136, 117–139. [Google Scholar] [CrossRef]
- Darbasizadeh, B.; Motasadizadeh, H.; Foroughi-Nia, B.; Farhadnejad, H. Tripolyphosphate-crosslinked chitosan/poly (ethylene oxide) electrospun nanofibrous mats as a floating gastro-retentive delivery system for ranitidine hydrochloride. J. Pharm. Biomed. Anal. 2018, 153, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Hwang, K.-M.; Park, Y.S.; Nguyen, T.-T.; Park, E.-S. Preparation and evaluation of non-effervescent gastroretentive tablets containing pregabalin for once-daily administration and dose proportional pharmacokinetics. Int. J. Pharm. 2018, 550, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.-M.; Cho, C.-H.; Tung, N.-T.; Kim, J.-Y.; Rhee, Y.-S.; Park, E.-S. Release kinetics of highly porous floating tablets containing cilostazol. Eur. J. Pharm. Biopharm. 2017, 115, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Klausner, E.A.; Lavy, E.; Friedman, M.; Hoffman, A. Expandable gastroretentive dosage forms. J. Control. Release 2003, 90, 143–162. [Google Scholar] [CrossRef]
- Thapa, P.; Jeong, S. Effects of Formulation and Process Variables on Gastroretentive Floating Tablets with A High-Dose Soluble Drug and Experimental Design Approach. Pharmaceutics 2018, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, I.; Quirino-Barreda, T.; Villafuerte-Robles, L. Sustained delivery of captopril from floating matrix tablets. Int. J. Pharm. 2008, 362, 37–43. [Google Scholar] [CrossRef]
- El-Zahaby, S.A.; Kassem, A.A.; El-Kamel, A.H. Design and evaluation of gastroretentive levofloxacin floating mini-tablets-in-capsule system for eradication of Helicobacter pylori. Saudi Pharm. J. 2014, 22, 570–579. [Google Scholar] [CrossRef]
- Sarkar, D.; Nandi, G.; Changder, A.; Hudati, P.; Sarkar, S.; Ghosh, L.K. Sustained release gastroretentive tablet of metformin hydrochloride based on poly (acrylic acid)-grafted-gellan. Int. J. Biol. Macromol. 2017, 96, 137–148. [Google Scholar] [CrossRef]
- Sarparanta, M.P.; Bimbo, L.M.; Mäkilä, E.M.; Salonen, J.J.; Laaksonen, P.H.; Helariutta, A.M.K.; Linder, M.B.; Hirvonen, J.T.; Laaksonen, T.J.; Santos, H.A.; et al. The mucoadhesive and gastroretentive properties of hydrophobin-coated porous silicon nanoparticle oral drug delivery systems. Biomaterials 2012, 33, 3353–3362. [Google Scholar] [CrossRef]
- He, W.; Li, Y.; Zhang, R.; Wu, Z.; Yin, L. Gastro-floating bilayer tablets for the sustained release of metformin and immediate release of pioglitazone: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2014, 476, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Michelson, E.L. Calcium Antagonists in Cardiology: Update on Sustained-release Drug Delivery Systems. Clin Cardiol. 1991, 14, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Hutton, J.T.; Morris, J.L. Long-acting carbidopa-levodopa in the management of moderate and advanced Parkinson’s disease. Neurology 1992, 42, 51–56, discussion 57–60. [Google Scholar] [PubMed]
- Wagstaff, A.J.; Goa, K.L. Once-weekly fluoxetine. Drugs 2001, 61, 2221–2228, discussion 2229–2230. [Google Scholar] [CrossRef]
- Michel, M. A Benefit-Risk Assessment of Extended-Release Oxybutynin. Drug Saf. 2002, 25, 867–876. [Google Scholar] [CrossRef]
- Pieper, J.A. Understanding niacin formulations. Am. J. Manag. Care 2002, 8, S308. [Google Scholar]
- McCarberg, B. Tramadol extended-release in the management of chronic pain. Ther. Clin. Risk Manag. 2007, 3, 401. [Google Scholar]
- Patil, H.; Tiwari, R.V.; Repka, M.A. Recent advancements in mucoadhesive floating drug delivery systems: A mini-review. J. Drug Deliv. Sci. Technol. 2016, 31, 65–71. [Google Scholar] [CrossRef]
- Shtenberg, Y.; Goldfeder, M.; Prinz, H.; Shainsky, J.; Ghantous, Y.; Abu El-Naaj, I.; Schroeder, A.; Bianco-Peled, H. Mucoadhesive alginate pastes with embedded liposomes for local oral drug delivery. Int. J. Biol. Macromol. 2018, 111, 62–69. [Google Scholar] [CrossRef]
- Andrews, G.P.; Laverty, T.P.; Jones, D.S. Mucoadhesive polymeric platforms for controlled drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Jian Wang, Y.T.Y.D.K.M.Y.T.Y.I. Positively Charged Gelatin Microspheres as Gastric Mucoadhesive Drug Delivery System for Eradication of H. pylori. Drug Deliv. 2000, 7, 237–243. [Google Scholar] [CrossRef]
- Bhalla, S.; Nagpal, M. Comparison of Various Generations of Superporous Hydrogels Based on Chitosan-Acrylamide and In Vitro Drug Release. ISRN Pharm. 2013, 2013, 624841. [Google Scholar] [CrossRef][Green Version]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in superporous hydrogels. J. Control. Release 2005, 102, 3–12. [Google Scholar] [CrossRef]
- Fujimori, J.; Machida, Y.; Tanaka, S.; Nagai, T. Effect of magnetically controlled gastric residence of sustained release tablets on bioavailability of acetaminophen. Int. J. Pharm. 1995, 119, 47–55. [Google Scholar] [CrossRef]
- Gröning, R.; Berntgen, M.; Georgarakis, M. Acyclovir serum concentrations following peroral administration of magnetic depot tablets and the influence of extracorporal magnets to control gastrointestinal transit. Eur. J. Pharm. Biopharm. 1998, 46, 285–291. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, N.; Yang, F. In situ microbubble-assisted, ultrasound-controlled release of superparamagnetic iron oxide nanoparticles from gastro-retentive tablets. Int. J. Pharm. 2020, 586, 119615. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, V. Anatomy of the stomach. Surgery 2014, 32, 571–574. [Google Scholar] [CrossRef]
- Horton-Szar, D.; Griffiths, M.; Lombard, M. Gastrointestinal System/Edited by Dan Horton-Szar, 4th ed.; Mosby: Saint Louis, MO, USA, 2012. [Google Scholar]
- Soybel, D.I. Anatomy and Physiology of the Stomach. Surg. Clin. N. Am. 2005, 85, 875–894. [Google Scholar] [CrossRef] [PubMed]
- Van Den Abeele, J.; Rubbens, J.; Brouwers, J.; Augustijns, P. The dynamic gastric environment and its impact on drug and formulation behaviour. Eur. J. Pharm. Sci. 2017, 96, 207–231. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.D.; Jani, G.K.; Khutliwala, T.A.; Zala, B.S. Raft forming system—An upcoming approach of gastroretentive drug delivery system. J. Control. Release 2013, 168, 151–165. [Google Scholar] [CrossRef]
- Zate, S.; Kothawade, P.; Mahale, G.; Kapse, K.; Anantwar, S. Gastro Retentive Bioadhesive Drug Delivery System: A Review. Int. J. PharmTech Res. 2010, 2. [Google Scholar]
- Helliwell, M. The use of bioadhesives in targeted delivery within the gastrointestinal tract. Adv. Drug Deliv. Rev. 1993, 11, 221–251. [Google Scholar] [CrossRef]
- Wickham, M.J.S.; Faulks, R.M.; Mann, J.; Mandalari, G. The Design, Operation, and Application of a Dynamic Gastric Model. Dissolution Technol. 2012, 19, 15–22. [Google Scholar] [CrossRef]
- Dorożyński, P.; Kulinowski, P.; Mendyk, A.; Jachowicz, R. Gastroretentive drug delivery systems with l-dopa based on carrageenans and hydroxypropylmethylcellulose. Int. J. Pharm. 2011, 404, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, J.; Moës, A.J. The Cutoff Size for Gastric Emptying of Dosage Forms. J. Pharm. Sci. 1993, 82, 854. [Google Scholar] [CrossRef]
- Su, C.-Y.; Ho, H.-O.; Chen, Y.-C.; Yu, Y.-T.; Liu, D.-Z.; Chao, F.-C.; Sheu, M.-T. Complex Hydrogels Composed of Chitosan with Ring-opened Polyvinyl Pyrrolidone as a Gastroretentive Drug Dosage Form to Enhance the Bioavailability of Bisphosphonates. Sci. Rep. 2018, 8, 8092. [Google Scholar] [CrossRef] [PubMed]
- Lele, B.S.; Hoffman, A.S. Mucoadhesive drug carriers based on complexes of poly(acrylic acid) and PEGylated drugs having hydrolysable PEG–anhydride–drug linkages. J. Control. Release 2000, 69, 237–248. [Google Scholar] [CrossRef]
- Dressman, J.B.; Reppas, C. Oral Drug Absorption: Prediction and Assessment/Edited by Jennifer B. Dressman, Christos Reppas, 2nd ed.; Informa Healthcare: New York, NY, USA, 2010. [Google Scholar]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Gastroretentive drug delivery systems. Expert Opin. Drug Deliv. 2006, 3, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, M.S.; Kumar, A.; Pathak, K. Osmotically regulated floating asymmetric membrane capsule for controlled site-specific delivery of ranitidine hydrochloride: Optimization by central composite design. AAPS PharmSciTech 2012, 13, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wen, H.; Yang, F.; Yu, Y.; Gai, X.; Wang, H.; Li, P.; Pan, W.; Yang, X. Study of controlled-release floating tablets of dipyridamole using the dry-coated method. Drug Dev. Ind. Pharm. 2018, 44, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Chen, H.; Rui, Y.; Yang, F.; Ma, N.; Wu, Z. Floating tablets for controlled release of ofloxacin via compression coating of hydroxypropyl cellulose combined with effervescent agent. Int. J. Pharm. 2015, 489, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.M.; Newton, J.M.; Short, M.D. Gastrointestinal transit of pellets of differing size and density. Int. J. Pharm. 1993, 100, 81–92. [Google Scholar] [CrossRef]
- Talukder, R.; Fassihi, R. Gastroretentive delivery systems: A mini review. Drug Dev. Ind. Pharm. 2004, 30, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Streubel, A.; Siepmann, J.; Bodmeier, R. Drug delivery to the upper small intestine window using gastroretentive technologies. Curr. Opin. Pharmacol. 2006, 6, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Ali, J.; Ahuja, A.; Khar, R.K.; Baboota, S. Floating drug delivery systems: A review. AAPS PharmSciTech 2005, 6, E372–E390. [Google Scholar] [CrossRef]
- Awasthi, R.; Kulkarni, G.T. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: Where do we stand? Drug Deliv. 2016, 23, 378–394. [Google Scholar] [CrossRef]
- Shaha, S.H.; Patel, J.K.; Pundarikakshudu, K. An overview of a gastro-retentive floating drug delivery system. Asian J. Pharm. 2009, 4, 65–80. [Google Scholar]
- Calbet, J.A.; MacLean, D.A. Role of caloric content on gastric emptying in humans. J. Physiol. 1997, 498 (Pt 2), 553–559. [Google Scholar] [CrossRef]
- Juvonen, K.R.; Purhonen, A.K.; Salmenkallio-Marttila, M.; Lähteenmäki, L.; Laaksonen, D.E.; Herzig, K.H.; Uusitupa, M.I.; Poutanen, K.S.; Karhunen, L.J. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J. Nutr. 2009, 139, 461–466. [Google Scholar] [CrossRef]
- Zhu, Y.; Hsu, W.H.; Hollis, J.H. The impact of food viscosity on eating rate, subjective appetite, glycemic response and gastric emptying rate. PLoS ONE 2013, 8, e67482. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, G.D. Progress in Controlled Gastroretentive Delivery Systems. Trop. J. Pharm. Res. 2008, 7. [Google Scholar] [CrossRef]
- Nguyen, N.Q.; Debreceni, T.L.; Burgstad, C.M.; Wishart, J.M.; Bellon, M.; Rayner, C.K.; Wittert, G.A.; Horowitz, M. Effects of Posture and Meal Volume on Gastric Emptying, Intestinal Transit, Oral Glucose Tolerance, Blood Pressure and Gastrointestinal Symptoms After Roux-en-Y Gastric Bypass. Obes. Surg. 2015, 25, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Mojaverian, P.; Vlasses, P.H.; Kellner, P.E.; Rocci, M.L., Jr. Effects of gender, posture, and age on gastric residence time of an indigestible solid: Pharmaceutical considerations. Pharm. Res. 1988, 5, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Mohammed, S.D.; Farmer, A.D.; Wang, D.; Zarate, N.; Hobson, A.R.; Hellström, P.M.; Semler, J.R.; Kuo, B.; Rao, S.S.; et al. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: Influence of age, gender, study country and testing protocol. Aliment. Pharm. Ther. 2015, 42, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Wald, A.; Van Thiel, D.H.; Hoechstetter, L.; Gavaler, J.S.; Egler, K.M.; Verm, R.; Scott, L.; Lester, R. Gastrointestinal transit: The effect of the menstrual cycle. Gastroenterology 1981, 80, 1497–1500. [Google Scholar] [CrossRef]
- Krygowska-Wajs, A.; Cheshire, W.P., Jr.; Wszolek, Z.K.; Hubalewska-Dydejczyk, A.; Jasinska-Myga, B.; Farrer, M.J.; Moskala, M.; Sowa-Staszczak, A. Evaluation of gastric emptying in familial and sporadic Parkinson disease. Parkinsonism Relat. Disord. 2009, 15, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, K.; Kalantzis, C.; Papadopoulos, A.A.; Apostolopoulos, P.; Rokkas, T.; Kalantzis, N.; Ladas, S.D. Video-capsule endoscopy gastric and small bowel transit time and completeness of the examination in patients with diabetes mellitus. Dig. Liver Dis. 2007, 39, 575–580. [Google Scholar] [CrossRef]
- Choi, B.Y.; Park, H.J.; Hwang, S.J.; Park, J.B. Preparation of alginate beads for floating drug delivery system: Effects of CO2 gas-forming agents. Int. J. Pharm. 2002, 239, 81–91. [Google Scholar] [CrossRef]
- Rossi, A.; Conti, C.; Colombo, G.; Castrati, L.; Scarpignato, C.; Barata, P.; Sandri, G.; Caramella, C.; Bettini, R.; Buttini, F.; et al. Floating modular drug delivery systems with buoyancy independent of release mechanisms to sustain amoxicillin and clarithromycin intra-gastric concentrations. Drug Dev. Ind. Pharm. 2016, 42, 332–339. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Hwang, K.-M.; Kim, S.-H.; Park, E.-S. Development of novel bilayer gastroretentive tablets based on hydrophobic polymers. Int. J. Pharm. 2020, 574. [Google Scholar] [CrossRef]
- Verma, A.D.J.; Verma, N.; Nayak, A.K. Chitosan-Hydroxypropyl Methylcellulose Matrices as Carriers for Hydrodynamically Balanced Capsules of Moxifloxacin HCl. Curr. Drug Deliv. 2017, 14, 83–90. [Google Scholar] [CrossRef]
- Nayak, A.K.; Das, B.; Maji, R. Gastroretentive hydrodynamically balanced systems of ofloxacin: In vitro evaluation. Saudi Pharm. J. 2013, 21, 113–117. [Google Scholar] [CrossRef]
- Reddy, L.H.V.; Murthy, R.S.R. Floating Dosage Systems in Drug Delivery. Ther. Drug Carr. Syst. 2002, 19, 36. [Google Scholar] [CrossRef]
- Hwang, S.J.; Park, H.; Park, K. Gastric retentive drug-delivery systems. Crit Rev. Ther. Drug Carr. Syst 1998, 15, 243–284. [Google Scholar]
- Nayak, A.K.; Malakar, J. Formulation and in vitro evaluation of Hydrodynamically balanced system for theophylline delivery. J. Basic Clin. Pharm. 2011, 2, 133–137. [Google Scholar] [PubMed]
- Patra, C.N.; Kumar, A.B.; Pandit, H.K.; Singh, S.P.; Devi, M.V. Design and evaluation of sustained release bilayer tablets of propranolol hydrochloride. Acta Pharm. 2007, 57, 479. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B.; Murthy, T.K.; Raveendra Pai, M.; Mehta, P.R.; Chowdary, P.B. Controlled release formulation of tramadol hydrochloride using hydrophilic and hydrophobic matrix system. AAPS PharmSciTech 2003, 4, 18–23. [Google Scholar] [CrossRef]
- Erni, W.; Held, K. The Hydrodynamically Balanced System: A Novel Principle of Controlled Drug Release. Eur. Neurol. 1987, 27 (Suppl. 1), 21–27. [Google Scholar] [CrossRef]
- Jansen, E.N.; Meerwaldtt, J.D. Madopar HBS in nocturnal symptoms of Parkinson’s disease. Adv. Neurol 1990, 53, 527–531. [Google Scholar]
- Koller, W.C.; Pahwa, R. Treating motor fluctuations with controlled-release levodopa preparations. Neurology 1994, 44, S23–S28. [Google Scholar]
- Oth, M.; Franz, M.; Timmermans, J.; Möes, A. The bilayer floating capsule: A stomach-directed drug delivery system for misoprostol. Pharm. Res. 1992, 9, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Krogel, I.; Bodmeier, R. Development of a multifunctional matrix drug delivery system surrounded by an impermeable cylinder. J. Control. Release 1999, 61, 43–50. [Google Scholar] [CrossRef]
- Qin, C.; Wu, M.; Xu, S.; Wang, X.; Shi, W.; Dong, Y.; Yang, L.; He, W.; Han, X.; Yin, L. Design and optimization of gastro-floating sustained-release tablet of pregabalin: In vitro and in vivo evaluation. Int. J. Pharm. 2018, 545, 37–44. [Google Scholar] [CrossRef]
- Mehta, D.M.; Parejiya, P.B.; Patel, H.K.; Trivedi, P.J.; Suthar, D.D.; Shelat, P.K. Design, optimization and pharmacokinetics of novel prolonged gastroretentive drug delivery system of quetiapine fumarate. J. Pharm. Investig. 2016, 46, 453–465. [Google Scholar] [CrossRef]
- Lin, H.-L.; Chen, L.-C.; Cheng, W.-T.; Cheng, W.-J.; Ho, H.-O.; Sheu, M.-T. Preparation and Characterization of a Novel Swellable and Floating Gastroretentive Drug Delivery System (sfGRDDS) for Enhanced Oral Bioavailability of Nilotinib. Pharmaceutics 2020, 12, 137. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Wang, H.-J.; Wang, J.-Y. Preparation and evaluation of captopril loaded gastro-retentive zein based porous floating tablets. Int. J. Pharm. 2020, 579, 119185. [Google Scholar] [CrossRef]
- Oh, J.-H.; Eun Lee, J.; Jeong Kim, Y.; Oh, T.-O.; Han, S.; Jeon, E.K.; Shin, K.; Kim, D.-H.; Hye Park, C.; Lee, Y.-J. Designing of the fixed-dose gastroretentive bilayer tablet for sustained release of metformin and immediate release of atorvastatin. Drug Dev. Ind. Pharm. 2016, 42, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.-O.; Kim, J.-Y.; Ha, J.-M.; Chi, S.-C.; Rhee, Y.-S.; Park, C.-W.; Park, E.-S. Preparation of highly porous gastroretentive metformin tablets using a sublimation method. Eur. J. Pharm. Biopharm. 2013, 83, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Sako, K.; Mizumoto, T.; Kajiyama, A.; Ohmura, T. Influence of physical factors in gastrointestinal tract on acetaminophen release from controlled-release tablets in fasted dogs. Int. J. Pharm. 1996, 137, 225–232. [Google Scholar] [CrossRef]
- Hajare PP, R.P. Gastroretentive microballoons: A novel approach for drug delivery. Int. J. Pharm. Sci. Res. 2020, 11, 1075–1083. [Google Scholar]
- Maghsoodi, M.; Hemati, E.; Qadermazi, B.; Yari, Z. Hollow microspheres for gastroretentive floating- pulsatile drug delivery: Preparation and in vitro evaluation. Adv. Pharm. Bull. 2011, 1, 55–61. [Google Scholar] [CrossRef]
- Kawashima, Y.; Niwa, T.; Takeuchi, H.; Hino, T.; Itoh, Y. Hollow microspheres for use as a floating controlled drug delivery system in the stomach. J. Pharm. Sci. 1992, 81, 135–140. [Google Scholar] [CrossRef]
- Jayanthi, G.; Jayaswal, S.B.; Srivastava, A.K. Formulation and evaluation of terfenadine microballoons for oral controlled release. Pharmazie 1995, 50, 769–770. [Google Scholar] [PubMed]
- Sato, Y.; Kawashima, Y.; Takeuchi, H.; Yamamoto, H. In vivo evaluation of riboflavin-containing microballoons for floating controlled drug delivery system in healthy human volunteers. J. Control. Release 2003, 93, 39–47. [Google Scholar] [CrossRef]
- Sato, Y.; Kawashima, Y.; Takeuchi, H.; Yamamoto, H. In vitro and in vivo evaluation of riboflavin-containing microballoons for a floating controlled drug delivery system in healthy humans. Int. J. Pharm. 2004, 275, 97–107. [Google Scholar] [CrossRef]
- Sato, Y.; Kawashima, Y.; Takeuchi, H.; Yamamoto, H.; Fujibayashi, Y. Pharmacoscintigraphic evaluation of riboflavin-containing microballoons for a floating controlled drug delivery system in healthy humans. J. Control. Release 2004, 98, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; Ghorab, M.; Kamel, R.; Salama, A.H. Design and optimization of gastro-retentive microballoons for enhanced bioavailability of cinnarizine. Drug Deliv. Transl. Res. 2016, 6, 210–224. [Google Scholar] [CrossRef]
- Tadros, M.I.; Fahmy, R.H. Controlled-release triple anti-inflammatory therapy based on novel gastroretentive sponges: Characterization and magnetic resonance imaging in healthy volunteers. Int. J. Pharm. 2014, 472, 27–39. [Google Scholar] [CrossRef]
- Siepmann, J.; Streubel, A.; Peppas, N.A. Understanding and Predicting Drug Delivery from Hydrophilic Matrix Tablets Using the “Sequential Layer” Model. Pharm. Res. 2002, 19, 306–314. [Google Scholar] [CrossRef]
- Rahim, S.A.; Carter, P.; Elkordy, A.A. Influence of calcium carbonate and sodium carbonate gassing agents on pentoxifylline floating tablets properties. Powder Technol. 2017, 322, 65–74. [Google Scholar] [CrossRef]
- Baumgartner, S.; Kristl, J.; Vrečer, F.; Vodopivec, P.; Zorko, B. Optimisation of floating matrix tablets and evaluation of their gastric residence time. Int. J. Pharm. 2000, 195, 125–135. [Google Scholar] [CrossRef]
- Raza, A.; Shen, N.; Li, J.; Chen, Y.; Wang, J.-Y. Formulation of zein based compression coated floating tablets for enhanced gastric retention and tunable drug release. Eur. J. Pharm. Sci. 2019, 132, 163–173. [Google Scholar] [CrossRef]
- Hwang, K.-M.; Nguyen, T.-T.; Seok, S.H.; Jo, H.-I.; Cho, C.-H.; Hwang, K.-M.; Kim, J.-Y.; Park, C.-W.; Rhee, Y.-S.; Park, E.-S. Swellable and porous bilayer tablet for gastroretentive drug delivery: Preparation and in vitro-in vivo evaluation. Int. J. Pharm. 2019, 572, 118783. [Google Scholar] [CrossRef]
- Diós, P.; Nagy, S.; Pál, S.; Pernecker, T.; Kocsis, B.; Budán, F.; Horváth, I.; Szigeti, K.; Bölcskei, K.; Máthé, D.; et al. Preformulation studies and optimization of sodium alginate based floating drug delivery system for eradication of Helicobacter pylori. Eur. J. Pharm. Biopharm. 2015, 96, 196–206. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Triaxial Electrospun Nanofiber Membranes for Controlled Dual Release of Functional Molecules. ACS Appl. Mater. Interfaces 2013, 5, 8241–8245. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Yu, X.; Chai, Q.; Ayres, N.; Steckl, A.J. Stimuli-Responsive Self-Immolative Polymer Nanofiber Membranes Formed by Coaxial Electrospinning. ACS Appl. Mater. Interfaces 2017, 9, 11858–11865. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef]
- Palo, M.; Kogermann, K.; Laidmäe, I.; Meos, A.; Preis, M.; Heinämäki, J.; Sandler, N. Development of Oromucosal Dosage Forms by Combining Electrospinning and Inkjet Printing. Mol. Pharm. 2017, 14, 808–820. [Google Scholar] [CrossRef]
- Adibkia, K.; Hamedeyazdan, S.; Javadzadeh, Y. Drug release kinetics and physicochemical characteristics of floating drug delivery systems. Expert Opin. Drug Deliv. 2011, 8, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Garg, T.; Goyal, A.K.; Rath, G. Polymeric nanofibers: Targeted gastro-retentive drug delivery systems. J. Drug Target. 2015, 23, 109–124. [Google Scholar] [CrossRef]
- Tort, S.; Han, D.; Steckl, A.J. Self-inflating floating nanofiber membranes for controlled drug delivery. Int. J. Pharm. 2020, 579, 119164. [Google Scholar] [CrossRef]
- Abou Youssef, N.A.H.; Kassem, A.A.; El-Massik, M.A.E.; Boraie, N.A. Development of gastroretentive metronidazole floating raft system for targeting Helicobacter pylori. Int. J. Pharm. 2015, 486, 297–305. [Google Scholar] [CrossRef]
- Kerdsakundee, N.; Mahattanadul, S.; Wiwattanapatapee, R. Development and evaluation of gastroretentive raft forming systems incorporating curcumin-Eudragit® EPO solid dispersions for gastric ulcer treatment. Eur. J. Pharm. Biopharm. 2015, 94, 513–520. [Google Scholar] [CrossRef]
- Lambert, J.R.; Korman, M.G.; Nicholson, L.; Chan, J.G. In-vivo anti-reflux and raft properties of alginates. Aliment. Pharm. Ther. 1990, 4, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Hampson, F.C.; Jolliffe, I.G.; Bakhtyari, A.; Taylor, G.; Sykes, J.; Johnstone, L.M.; Dettmar, P.W. Alginate-antacid combinations: Raft formation and gastric retention studies. Drug Dev. Ind. Pharm. 2010, 36, 614–623. [Google Scholar] [CrossRef]
- Abouelatta, S.M.; Aboelwafa, A.A.; El-Gazayerly, O.N. Gastroretentive raft liquid delivery system as a new approach to release extension for carrier-mediated drug. Drug Deliv. 2018, 25, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Fabregas, J.L.; Claramunt, J.; Cucala, J.; Pous, R.; Siles, A. “In-Vitro” Testing of an Antacid Formulation with Prolonged Gastric Residence Time (Almagate Flot-Coat®). Drug Dev. Ind. Pharm. 1994, 20, 1199–1212. [Google Scholar] [CrossRef]
- Nabarawi El, M.A.; Teaima, M.H.; Abd El-Monem, R.A.; El Nabarawy, N.A.; Gaber, D.A. Formulation, release characteristics, and bioavailability study of gastroretentive floating matrix tablet and floating raft system of Mebeverine HCl. Drug Des. Dev. Ther. 2017, 11, 1081–1093. [Google Scholar] [CrossRef]
- Cheng, C.L.; Koo, M.W. Effects of Centella asiatica on ethanol induced gastric mucosal lesions in rats. Life Sci. 2000, 67, 2647–2653. [Google Scholar] [CrossRef]
- Wannasarit, S.; Mahattanadul, S.; Issarachot, O.; Puttarak, P.; Wiwattanapatapee, R. Raft-forming gastro-retentive formulations based on Centella asiatica extract-solid dispersions for gastric ulcer treatment. Eur. J. Pharm. Sci. 2020, 143, 105204. [Google Scholar] [CrossRef] [PubMed]
- Hampson, F.C.; Farndale, A.; Strugala, V.; Sykes, J.; Jolliffe, I.G.; Dettmar, P.W. Alginate rafts and their characterisation. Int. J. Pharm. 2005, 294, 137–147. [Google Scholar] [CrossRef]
- Hanif, M.; Shah, S.; Rasul, A.; Abbas, G.; Zaman, M.; Amjad, M.W.; Abdul Ghafoor Raja, M.; Khan, H.U.; Ashfaq, M.; Iqbal, O. Enhancement of Oral Bioavailability of Ibandronate Through Gastroretentive Raft Forming Drug Delivery System: In Vitro and In Vivo Evaluation. Int. J. Nanomed. 2020, 15, 4847–4858. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.; Aboul-Enein, H.Y. A Validated HPLC Assay Method for the Determination of Sodium Alginate in Pharmaceutical Formulations. J. Chromatogr. Sci. 2013, 51, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Purohit, R. Development of Novel High Density Gastroretentive Multiparticulate Pulsatile Tablet of Clopidogrel Bisulfate Using Quality by Design Approach. AAPS PharmSciTech 2017, 18, 3208–3218. [Google Scholar] [CrossRef]
- Sharma, A.; Goyal, A.K.; Rath, G. Development and Characterization of Gastroretentive High-Density Pellets Lodged With Zero Valent Iron Nanoparticles. J. Pharm. Sci. 2018, 107, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, A.J. The science of adhesion. J. Mater. Sci. 1982, 17, 617–651. [Google Scholar] [CrossRef]
- Smart, J. The basics and underlying mechanisms of mucoadhesion. Adv. Drug Deliv. Rev. 2005, 57, 1556–1568. [Google Scholar] [CrossRef]
- Dodou, D.; Breedveld, P.; Wieringa, P.A. Mucoadhesives in the gastrointestinal tract: Revisiting the literature for novel applications. Eur. J. Pharm. Biopharm. 2005, 60, 1–16. [Google Scholar] [CrossRef]
- Jiménez-castellanos, M.R.; Zia, H.; Rhodes, C.T. Mucoadhesive Drug Delivery Systems. Drug Dev. Ind. Pharm. 1993, 19, 143–194. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.H.; Robinson, J.R. Bioadhesive-based dosage forms: The next generation. J. Pharm. Sci. 2000, 89, 850–866. [Google Scholar] [CrossRef]
- Ugwoke, M.I.; Agu, R.U.; Verbeke, N.; Kinget, R. Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv. Drug Deliv. Rev. 2005, 57, 1640–1665. [Google Scholar] [CrossRef]
- Ahagon, A.; Gent, A.N. Effect of interfacial bonding on the strength of adhesion. J. Polym. Sci. Polym. Phys. Ed. 1975, 13, 1285–1300. [Google Scholar] [CrossRef]
- Vasir, J.K.; Tambwekar, K.; Garg, S. Bioadhesive microspheres as a controlled drug delivery system. Int. J. Pharm. 2003, 255, 13–32. [Google Scholar] [CrossRef]
- Campion, R.P. The Influence of Structure on Autohesion (Self-Tack) and other forms of Diffusion into Polymers. J. Adhes. 1975, 7, 1–23. [Google Scholar] [CrossRef]
- Voyutskii, S.S.; Voiutskii, S.S. Autohesion and Adhesion of High Polymers; Interscience Publishers: New York, NY, USA, 1963. [Google Scholar]
- Wake, W.C. Theories of adhesion and uses of adhesives: A review. Polymer 1978, 19, 291–308. [Google Scholar] [CrossRef]
- Jabbari, E.; Peppas, N.A. A model for interdiffusion at interfaces of polymers with dissimilar physical properties. Polymer 1995, 36, 575–586. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Chen JL, C.G. Compositions producing adhesion through hydration. In Adhesion in Biological Systems; Manly, R.S., Ed.; Academic Press: New York, NY, USA, 1970; pp. 163–181. [Google Scholar]
- Illum, L. Chitosan and its use as a pharmaceutical excipient. Pharm. Res. 1998, 15, 1326–1331. [Google Scholar] [CrossRef]
- Nakatsuka, S.; Andrady, A.L. Permeability of vitamin B-12 in chitosan membranes. Effect of crosslinking and blending with poly(vinyl alcohol) on permeability. J. Appl. Polym. Sci. 1992, 44, 17–28. [Google Scholar] [CrossRef]
- Vishal Gupta, N.; Shivakumar, H.G. Preparation and characterization of superporous hydrogels as gastroretentive drug delivery system for rosiglitazone maleate. DARU 2010, 18, 200–210. [Google Scholar]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Inouye, K.; Machida, Y.; Sannan, T.; Nagai, T. Buoyant sustained release tablets based on chitosan. Drug Des. Deliv. 1988, 2, 165–175. [Google Scholar]
- Chandy, T.; Sharma, C.P. Chitosan matrix for oral sustained delivery of ampicillin. Biomaterials 1993, 14, 939–944. [Google Scholar] [CrossRef]
- Patel, V.R.; Amiji, M.M. Preparation and Characterization of Freeze-dried Chitosan-Poly(Ethylene Oxide) Hydrogels for Site-Specific Antibiotic Delivery in the Stomach. Pharm. Res. 1996, 13, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.C.; Ravi Kumar, M.N. Drug release behavior of beads and microgranules of chitosan. Biomaterials 2000, 21, 1115–1119. [Google Scholar] [CrossRef]
- Souza, M.P.C.; Sábio, R.M.; Ribeiro, T.C.; Santos, A.M.D.; Meneguin, A.B.; Chorilli, M. Highlighting the impact of chitosan on the development of gastroretentive drug delivery systems. Int. J. Biol. Macromol. 2020, 159, 804–822. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.; Zaki, N. Optimization and Evaluation of Gastroretentive Ranitidine HCl Microspheres by Using Factorial Design with Improved Bioavailability and Mucosal Integrity in Ulcer Model. AAPS PharmSciTech 2017, 18, 957–973. [Google Scholar] [CrossRef]
- Hou, J.Y.; Gao, L.N.; Meng, F.Y.; Cui, Y.L. Mucoadhesive microparticles for gastroretentive delivery: Preparation, biodistribution and targeting evaluation. Mar. Drugs 2014, 12, 5764–5787. [Google Scholar] [CrossRef]
- Ruiz-Caro, R.; Gago-Guillan, M.; Otero-Espinar, F.J.; Veiga, M.D. Mucoadhesive tablets for controlled release of acyclovir. Chem. Pharm. Bull. 2012, 60, 1249. [Google Scholar] [CrossRef]
- Salonen, J.; Kaukonen, A.M.; Hirvonen, J.; Lehto, V.P. Mesoporous silicon in drug delivery applications. J. Pharm. Sci. 2008, 97, 632–653. [Google Scholar] [CrossRef]
- Prestidge, C.A.; Barnes, T.J.; Lau, C.H.; Barnett, C.; Loni, A.; Canham, L. Mesoporous silicon: A platform for the delivery of therapeutics. Expert Opin. Drug Deliv. 2007, 4, 101–110. [Google Scholar] [CrossRef]
- El-Zahaby, S.A.; Kassem, A.A.; El-Kamel, A.H. Formulation and in vitro evaluation of size expanding gastro-retentive systems of levofloxacin hemihydrate. Int. J. Pharm. 2014, 464, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Salessiotis, N. Measurement of the diameter of the pylorus in man: Part I. Experimental project for clinical application. Am. J. Surg. 1972, 124, 331–333. [Google Scholar] [CrossRef]
- Lalloo, A.K.; McConnell, E.L.; Jin, L.; Elkes, R.; Seiler, C.; Wu, Y. Decoupling the role of image size and calorie intake on gastric retention of swelling-based gastric retentive formulations: Pre-screening in the dog model. Int. J. Pharm. 2012, 431, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Mandal, U.K.; Chatterjee, B.; Senjoti, F.G. Gastro-retentive drug delivery systems and their in vivo success: A recent update. Asian J. Pharmceutical Sci. 2016, 11, 575–584. [Google Scholar] [CrossRef]
- Sivaneswari, S.; Karthikeyan, E.; Chandana, P.J. Novel expandable gastro retentive system by unfolding mechanism of levetiracetam using simple lattice design—Formulation optimization and in vitro evaluation. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 63–72. [Google Scholar] [CrossRef]
- Rimawi, I.B.; Muqedi, R.H.; Kanaze, F.I. Development of Gabapentin Expandable Gastroretentive Controlled Drug Delivery System. Sci. Rep. 2019, 9, 11675. [Google Scholar] [CrossRef]
- Dey, S.; Chattopadhyay, S.; Mazumder, B. Formulation and Evaluation of Fixed-Dose Combination of Bilayer Gastroretentive Matrix Tablet Containing Atorvastatin as Fast-Release and Atenolol as Sustained-Release. BioMed Res. Int. 2014, 2014, 396106–396112. [Google Scholar] [CrossRef]
- Bellinger, A.M.; Jafari, M.; Grant, T.M.; Zhang, S.; Slater, H.C.; Wenger, E.A.; Mo, S.; Lee, Y.-A.L.; Mazdiyasni, H.; Kogan, L.; et al. Oral, ultra–long-lasting drug delivery: Application toward malaria elimination goals. Sci. Transl. Med. 2016, 8, 365ra157. [Google Scholar] [CrossRef]
- Traverso, G.; Langer, R. Perspective: Special delivery for the gut. Nature 2015, 519, S19. [Google Scholar] [CrossRef]
- Kirtane, A.R.; Abouzid, O.; Minahan, D.; Bensel, T.; Hill, A.L.; Selinger, C.; Bershteyn, A.; Craig, M.; Mo, S.S.; Mazdiyasni, H.; et al. Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat. Commun. 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Lyndra Therapeutics. 19 July 2021—Lyndra Therapeutics Announces Positive Outcome of End-of-Phase 2 Meeting with the FDA for Lyndra’s Weekly Risperidone (LYN-005) for the Treatment of Adults with Schizophrenia and Other Indications. Available online: www.lyndra.com (accessed on 23 July 2021).
- Lyndra Therapeutics. 21 January 2021—Lyndra Therapeutics Announces First Subjects Dosed in Phase 1 Clinical Trial of Once-Weekly Rosuvastatin Extended-Release Capsule, LYN-047. Available online: www.lyndra.com (accessed on 23 July 2021).
- Lyndra Therapeutics. 6 May 2021—Lyndra Therapeutics Receives FDA Clearance of Investigational New Drug Application for LYN-014, its Once-Weekly Oral Levomethadone Treatment in Development for Opioid Use Disorder. Available online: www.lyndra.com (accessed on 23 July 2021).
- Chen, J.; Park, H.; Park, K. Synthesis of superporous hydrogels: Hydrogels with fast swelling and superabsorbent properties. J. Biomed. Mater. Res. 1999, 44, 53–62. [Google Scholar] [CrossRef]
- Askari, F.; Nafisi, S.; Omidian, H.; Hashemi, S.A. Synthesis and characterization of acrylic-based superabsorbents. J. Appl. Polym. Sci. 1993, 50, 1851–1855. [Google Scholar] [CrossRef]
- Omidian, H.; Park, K.; Rocca, J.G. Recent developments in superporous hydrogels. J. Pharm. Pharmacol. 2007, 59, 317–327. [Google Scholar] [CrossRef]
- Omidian, H.; Rocca, J.G.; Park, K. Elastic, Superporous Hydrogel Hybrids of Polyacrylamide and Sodium Alginate. Macromol. Biosci. 2006, 6, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhou, L.; Nie, S.; Yan, T.; Tang, X.; Pan, W. A novel gastric-resident osmotic pump tablet: In vitro and in vivo evaluation. Int. J. Pharm. 2010, 383, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, J.; Zhu, S. Delivery of prazosin hydrochloride from osmotic pump system prepared by coating the core tablet with an indentation. Drug Deliv. 2007, 14, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Chueh, H.R.; Zia, H.; Rhodes, C.T. Optimization of Sotalol Floating and Bioadhesive Extended Release Tablet Formulations. Drug Dev. Ind. Pharm. 1995, 21, 1725–1747. [Google Scholar] [CrossRef]
- Iannuccelli, V.; Coppi, G.; Bernabei, M.T.; Cameroni, R. Air compartment multiple-unit system for prolonged gastric residence. Part I. Formulation study. Int. J. Pharm. 1998, 174, 47–54. [Google Scholar] [CrossRef]
- Guan, J.; Zhou, L.; Pan, Y.; Han, H.; Xu, H.; Pan, W. A Novel Gastro-Retentive Osmotic Pump Capsule Using Asymmetric Membrane Technology: In Vitro and In Vivo Evaluation. Pharm. Res. 2009, 27, 105. [Google Scholar] [CrossRef]
- POLYOX Water-Soluble Resins Dissolving Techniques; The Dow Chemical Company: Midland, MI, USA, 2003; Form No. 326-00002-0303 AMS.
- Desai, N.; Purohit, R. Design and Development of Clopidogrel Bisulfate Gastroretentive Osmotic Formulation Using Quality by Design Tools. AAPS PharmSciTech 2017, 18, 2626–2638. [Google Scholar] [CrossRef]
- Neumann, M.; Heimhardt, C.; Seidlitz, K.; Koziolek, M.; Schneider, F.; Schiller, C.; Hanke, U.; Anschütz, M.; Knopke, C.; Donath, F.; et al. Development of a furosemide-containing expandable system for gastric retention. J. Control. Release 2021, 338, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Schneider, F.; Koziolek, M.; Garbacz, G.; Weitschies, W. A novel mechanical antrum model for the prediction of the gastroretentive potential of dosage forms. Int. J. Pharm. 2017, 530, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chang, R.-K.; Hussain, M.A. Ion-exchange resins as drug delivery carriers. J. Pharm. Sci. 2009, 98, 3886–3902. [Google Scholar] [CrossRef] [PubMed]
- Sriwongjanya, M.; Bodmeier, R. Effect of ion exchange resins on the drug release from matrix tablets. Eur. J. Pharm. Biopharm. 1998, 46, 321–327. [Google Scholar] [CrossRef]
- Borodkin, S.; Sundberg, D.P. Polycarboxylic acid ion-exchange resin adsorbates for taste coverage in chewable tablets. J. Pharm. Sci. 1971, 60, 1523–1527. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Ping, Q.-N.; Xiao, B. Microencapsulation and characterization of tramadol–resin complexes. J. Control. Release 2000, 66, 107–113. [Google Scholar] [CrossRef]
- Jeong, S.H.; Park, K. Drug loading and release properties of ion-exchange resin complexes as a drug delivery matrix. Int. J. Pharm. 2008, 361, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.C.C.S.; Deora, A.S. Drug loading and release properties of ionexchange resin complexes which preparedby batch process. J. Drug Deliv. Ther. 2014, 4, 66–73. [Google Scholar]
- Umamaheshwari, R.B.; Jain, S.; Jain, N.K. A new approach in gastroretentive drug delivery system using cholestyramine. Drug Deliv. 2003, 10, 151–160. [Google Scholar] [CrossRef]
- Murphy, C.S.; Pillay, V.; Choonara, Y.E.; du Toit, L.C. Gastroretentive Drug Delivery Systems: Current Developments in Novel System Design and Evaluation. Curr. Drug Deliv. 2009, 6, 451–460. [Google Scholar] [CrossRef]
- Laurent, S.; Bridot, J.L.; Elst, L.V.; Muller, R.N. Magnetic iron oxide nanoparticles for biomedical applications. Future Med. Chem. 2010, 2, 427–449. [Google Scholar] [CrossRef]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.S. Nanotheranostics—application and further development of nanomedicine strategies for advanced theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef]
- Li, S.; Lin, S.; Daggy, B.P.; Mirchandani, H.L.; Chien, Y.W. Effect of HPMC and Carbopol on the release and floating properties of Gastric Floating Drug Delivery System using factorial design. Int. J. Pharm. 2003, 253, 13–22. [Google Scholar] [CrossRef]
- Shalaby, W.S.W.; Blevins, W.E.; Park, K. In vitro and in vivo studies of enzyme-digestible hydrogels for oral drug delivery. J. Control. Release 1992, 19, 131–144. [Google Scholar] [CrossRef]
- Klausner, E.A.; Lavy, E.; Barta, M.; Cserepes, E.; Friedman, M.; Hoffman, A. Novel gastroretentive dosage forms: Evaluation of gastroretentivity and its effect on levodopa absorption in humans. Pharm. Res. 2003, 20, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, V.; Yadav, V.; Gaikwad, M. Novel sustained release and swellable gastroretentive dosage form for ciprofloxacin hydrochloride. Int. J. Pharm. Investig. 2014, 4, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Zentner, G.; Park, J.S.; Liu, F. Polymer Blends that Swell in an Acidic Environment and Deswell in a Basic Environment. U.S. Patent 6730327, 3 May 2004. [Google Scholar]
- Sandri, S.R.; Bonferoni, M.C.; Ferrari, F.; Mori, M.; Caramella, C. The role of chitosan as a mucoadhesive agent in mucosal drug delivery. J. Drug Deliv. Sci. Technol. 2012, 22, 275–284. [Google Scholar] [CrossRef]
- Darandale, S.S.; Vavia, P.R. Design of a gastroretentive mucoadhesive dosage form of furosemide for controlled release. Acta Pharm. Sin. B 2012, 2, 509–517. [Google Scholar] [CrossRef]
- Dey, S.K.; De, P.K.; De, A.; Ojha, S.; De, R.; Mukhopadhyay, A.K.; Samanta, A. Floating mucoadhesive alginate beads of amoxicillin trihydrate: A facile approach for H. pylori eradication. Int. J. Biol. Macromol. 2016, 89, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Bera, H.; Kandukuri, S.G.; Nayak, A.K.; Boddupalli, S. Alginate–sterculia gum gel-coated oil-entrapped alginate beads for gastroretentive risperidone delivery. Carbohydr. Polym. 2015, 120, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Gao, Y.; Zhu, J. Preparation and evaluation of glyceryl monooleate-coated hollow-bioadhesive microspheres for gastroretentive drug delivery. Int. J. Pharm. 2011, 413, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Karemore, M.N.; Bali, N.R. Gellan gum based gastroretentive tablets for bioavailability enhancement of cilnidipine in human volunteers. Int. J. Biol. Macromol. 2021, 174, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.D.; Sharma, H.; Jeswani, G.; Jha, A.K. Chapter 12—Novel gels: Implications for drug delivery. In Nanostructures for Drug Delivery; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 379–412. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Ghilan, A.; Neamtu, I.; Nita, L.E.; Rusu, A.G.; Chiriac, V.M. Advancement in the Biomedical Applications of the (Nano)gel Structures Based on Particular Polysaccharides. Macromol. Biosci. 2019, 19, 1900187. [Google Scholar] [CrossRef]
- Kundu, B.; Brancato, V.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Kundu, S.C. Silk fibroin promotes mineralization of gellan gum hydrogels. Int. J. Biol. Macromol. 2020, 153, 1328–1334. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Łapa, A.; Reczyńska, K.; Pietryga, K.; Balcaen, L.; Mendes, A.C.; Schaubroeck, D.; Van Der Voort, P.; Dokupil, A.; Plis, A.; et al. Mineralization of gellan gum hydrogels with calcium and magnesium carbonates by alternate soaking in solutions of calcium/magnesium and carbonate ion solutions. J. Tissue Eng. Regen. Med. 2018, 12, 1825–1834. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.F.; Ismail, S.; Tadros, M.I.; Elnabarawi, M.A. Alfuzosin hydrochloride-loaded low-density gastroretentive sponges: Development, in vitro characterization and gastroretentive monitoring in healthy volunteers via MRI. Pharm. Dev. Technol. 2020, 25, 566–578. [Google Scholar] [CrossRef]
- Guru, P.R.; Nayak, A.K.; Sahu, R.K. Oil-entrapped sterculia gum–alginate buoyant systems of aceclofenac: Development and in vitro evaluation. Colloids Surf. B Biointerfaces 2013, 104, 268–275. [Google Scholar] [CrossRef]
- Abd El Hady, W.E.; Soliman, O.A.E.; El Sabbagh, H.M.; Mohamed, E.A. Glutaraldehyde-crosslinked chitosan-polyethylene oxide nanofibers as a potential gastroretentive delivery system of nizatidine for augmented gastroprotective activity. Drug Deliv. 2021, 28, 1795–1809. [Google Scholar] [CrossRef]
- Schneider, F.; Koziolek, M.; Weitschies, W. In Vitro and In Vivo Test Methods for the Evaluation of Gastroretentive Dosage Forms. Pharmaceutics 2019, 11, 416. [Google Scholar] [CrossRef] [PubMed]

| Delivery System | Brand Name | Active Pharmaceutical Ingredient | Phase | Manufacturing Company |
|---|---|---|---|---|
| Hydrodynamically Balanced | Madopar® | Levodopa and Benserazide | Commercial | Intec Pharma (Israel) |
| Valrelease® | Diazepam | Commercial | Roche (UK) | |
| Non-effervescent floating tablet | Glucophage® XR | Metformin | Commercial | Merck KGaA (Germany) |
| Effervescent floating | Cifran® | Ciprofloxacin | Commercial | Ranbaxy (India) |
| Raft-forming | Liquid Gaviscon® | Sodium bicarbonate and Calcium carbonate | Commercial | Reckitt Benckiser Healthcare (UK) Ltd. |
| Gaviscon® Tablets | Sodium bicarbonate and Calcium carbonate | Commercial | Reckitt Benckiser Healthcare (UK) Ltd. | |
| Topalkan® | Aluminium and Magnesium | Commercial | Pierre Fabre Medicament (France) | |
| Mucoadhesive/Bioadhesive | Xifaxan® | Rifaximin | Commercial | Lupin (India) |
| Expandable | Accordion Pill® Levodopa/Carbidopa | Levodopa and Carbidopa | Phase III Clinical Trials | Intec Pharma (Israel) |
| Requip® XL (Geomatrix® technology) | Ropinirole | Commercial | GlaxoSmithKline (UK) | |
| Glumetza® (Acuform® technology) | Metformin | Commercial | Salix (USA) | |
| Nucynta® ER (Acuform® technology) | Tapentadol | Commercial | Depomed (USA) | |
| Gralise® (Acuform® technology) | Gabapentin | Commercial | Depomed (USA) | |
| Janumet® XR | Sitagliptin and metformin | Commercial | Merck Sharp & Dohme (USA) | |
| LYN-005 (Long-acting Pill technology) | Risperidone | Phase II Clinical Trials | Lyndra® Therapeutics (USA) | |
| Osmotic | Coreg® CR | Carvedilol Phosphate | Commercial | GlaxoSmithKline (UK) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrettos, N.-N.; Roberts, C.J.; Zhu, Z. Gastroretentive Technologies in Tandem with Controlled-Release Strategies: A Potent Answer to Oral Drug Bioavailability and Patient Compliance Implications. Pharmaceutics 2021, 13, 1591. https://doi.org/10.3390/pharmaceutics13101591
Vrettos N-N, Roberts CJ, Zhu Z. Gastroretentive Technologies in Tandem with Controlled-Release Strategies: A Potent Answer to Oral Drug Bioavailability and Patient Compliance Implications. Pharmaceutics. 2021; 13(10):1591. https://doi.org/10.3390/pharmaceutics13101591
Chicago/Turabian StyleVrettos, Napoleon-Nikolaos, Clive J. Roberts, and Zheying Zhu. 2021. "Gastroretentive Technologies in Tandem with Controlled-Release Strategies: A Potent Answer to Oral Drug Bioavailability and Patient Compliance Implications" Pharmaceutics 13, no. 10: 1591. https://doi.org/10.3390/pharmaceutics13101591
APA StyleVrettos, N.-N., Roberts, C. J., & Zhu, Z. (2021). Gastroretentive Technologies in Tandem with Controlled-Release Strategies: A Potent Answer to Oral Drug Bioavailability and Patient Compliance Implications. Pharmaceutics, 13(10), 1591. https://doi.org/10.3390/pharmaceutics13101591






