Administration and Therapeutic Drug Monitoring of β-lactams and Vancomycin in Critical Care Units in Colombia: The ANTIBIOCOL Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population, Recruitment and Sample
2.2. Statistical Analysis
3. Results
3.1. Sociodemographic Information
3.2. Clinical Approach
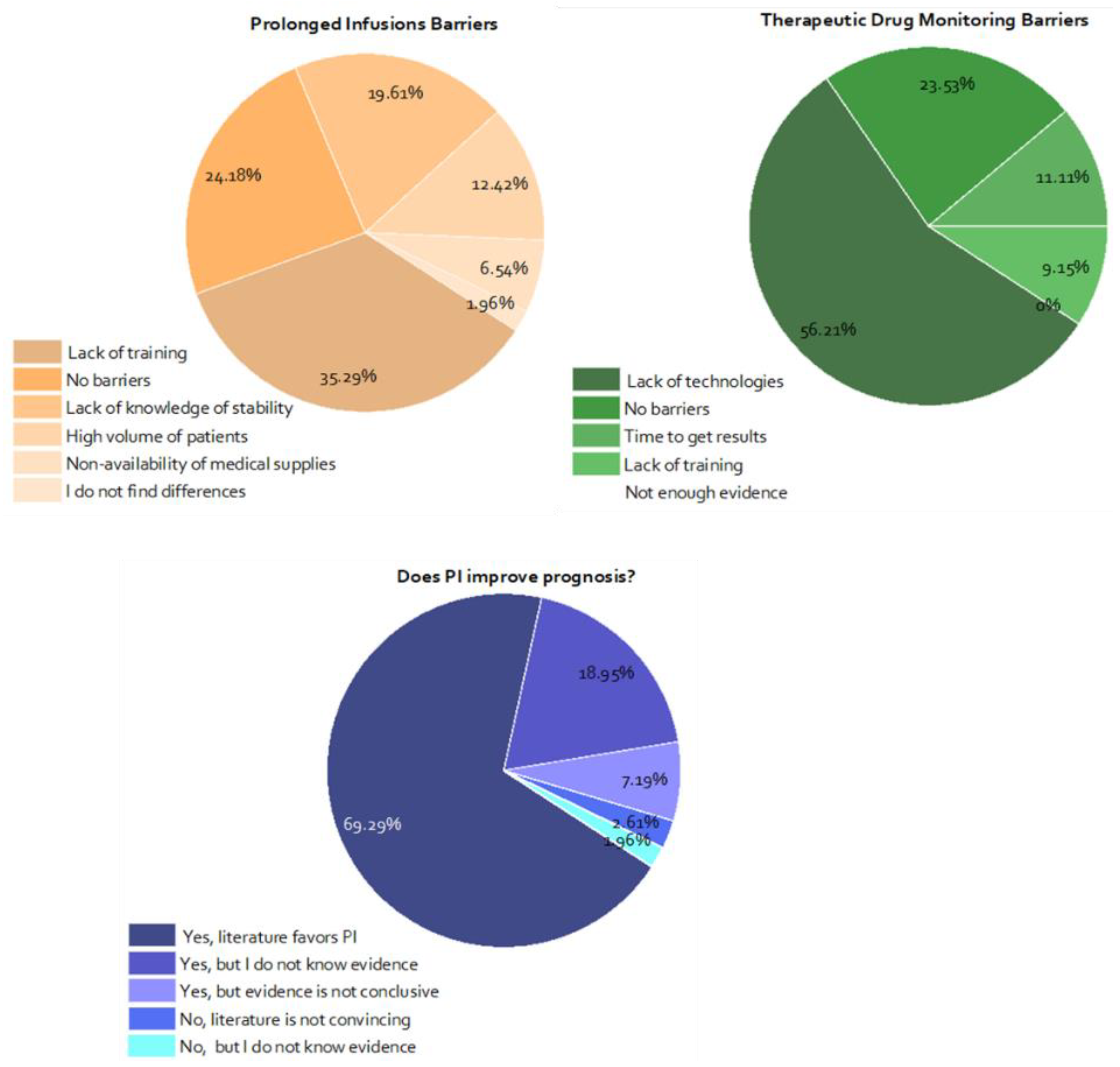
3.3. Attitudes and Barriers towards Prolonged/Continuous Infusions and TDM
3.4. Factors Associated with the Use of Prolonged/Continuous Infusions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Garzon, V.; Bustos, R.H.; Pinacho, D.G. Personalized Medicine for Antibiotics: The Role of Nanobiosensors in Therapeutic Drug Monitoring. J. Pers. Med. 2020, 10, 147. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, M.H. Overview of therapeutic drug monitoring. Korean J. Intern. Med. 2009, 24, 1–10. [Google Scholar] [CrossRef]
- Osorio, C.; Garzon, L.; Jaimes, D.; Silva, E.; Bustos, R.H. Impact on Antibiotic Resistance, Therapeutic Success, and Control of Side Effects in Therapeutic Drug Monitoring (TDM) of Daptomycin: A Scoping Review. Antibiotics 2021, 10, 263. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Norris, R.; Paterson, D.L.; Martin, J.H. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 2012, 73, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udy, A.A.; Roberts, J.A.; Lipman, J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013, 39, 2070–2082. [Google Scholar] [CrossRef] [PubMed]
- Fratoni, A.J.; Nicolau, D.P.; Kuti, J.L. A guide to therapeutic drug monitoring of beta-lactam antibiotics. Pharmacotherapy 2021, 41, 220–233. [Google Scholar] [CrossRef]
- Park, S.J.; Lim, N.R.; Park, H.J.; Yang, J.W.; Kim, M.J.; Kim, K.; In, Y.W.; Lee, Y.M. Evaluation of risk factors for vancomycin-induced nephrotoxicity. Int. J. Clin. Pharm. 2018, 40, 1328–1334. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health-Syst. Pharm. 2020, 77, 835–864. [Google Scholar]
- Wysocki, M.; Delatour, F.; Faurisson, F.; Rauss, A.; Pean, Y.; Misset, B.; Thomas, F.; Timsit, J.F.; Similowski, T.; Mentec, H.; et al. Continuous versus intermittent infusion of vancomycin in severe Staphylococcal infections: Prospective multicenter randomized study. Antimicrob. Agents Chemother. 2001, 45, 2460–2467. [Google Scholar] [CrossRef] [Green Version]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic drug monitoring-based dose optimisation of piperacillin and meropenem: A randomised controlled trial. Intensive Care Med. 2014, 40, 380–387. [Google Scholar] [CrossRef]
- Economou, C.J.P.; Wong, G.; McWhinney, B.; Ungerer, J.P.J.; Lipman, J.; Roberts, J.A. Impact of β-lactam antibiotic therapeutic drug monitoring on dose adjustments in critically ill patients undergoing continuous renal replacement therapy. Int. J. Antimicrob. Agents 2017, 49, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Fournier, A.; Eggimann, P.; Pagani, J.L.; Revelly, J.P.; Decosterd, L.A.; Marchetti, O.; Pannatier, A.; Voirol, P.; Que, Y.A. Impact of the introduction of real-time therapeutic drug monitoring on empirical doses of carbapenems in critically ill burn patients. Burn. J. Int. Soc. Burn. Injuries 2015, 41, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Lechtig-Wasserman, S.; Liebisch-Rey, H.; Diaz-Pinilla, N.; Blanco, J.; Fuentes-Barreiro, Y.V.; Bustos, R.H. Carbapenem Therapeutic Drug Monitoring in Critically Ill Adult Patients and Clinical Outcomes: A Systematic Review with Meta-Analysis. Antibiotics 2021, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Cotta, M.O.; Little, P.J.; McWhinney, B.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Is high-dose β-lactam therapy associated with excessive drug toxicity in critically ill patients? Minerva Anestesiol. 2016, 82, 957–965. [Google Scholar]
- Richter, D.C.; Dietrich, M.; Lalev, L.D.; Schmitt, F.C.F.; Fiedler, M.O.; Bruckner, T.; Stoerzinger, D.; Chiriac, U.; Klein, S.; Hackert, T.; et al. Prolonged Infusion of β-lactams Decreases Mortality in Patients with Septic Shock: A Retrospective before-and-after Study. Antibiotics 2021, 10, 687. [Google Scholar] [CrossRef]
- Kondo, Y.; Ota, K.; Imura, H.; Hara, N.; Shime, N. Prolonged versus intermittent beta-lactam antibiotics intravenous infusion strategy in sepsis or septic shock patients: A systematic review with meta-analysis and trial sequential analysis of randomized trials. J. Intensive Care 2020, 8, 77. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR). Crit. Car. 2019, 23, 104. [Google Scholar]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic Monitoring of Vancomycin for Serious Methicillin-resistant Staphylococcus aureus Infections: A Revised Consensus Guideline and Review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2020, 71, 1361–1364. [Google Scholar] [PubMed]
- Ye, Z.K.; Li, C.; Zhai, S.D. Guidelines for therapeutic drug monitoring of vancomycin: A systematic review. PLoS ONE 2014, 9, e99044. [Google Scholar] [CrossRef] [PubMed]
- Felton, T.W.; Hope, W.W.; Roberts, J.A. How severe is antibiotic pharmacokinetic variability in critically ill patients and what can be done about it? Diagn Microbiol. Infect. Dis. 2014, 79, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, A.; Csajka, C.; Thoma, Y.; Buclin, T.; Widmer, N. Benchmarking therapeutic drug monitoring software: A review of available computer tools. Clin. Pharmacokinet. 2013, 52, 9–22. [Google Scholar] [CrossRef]
- Veiga, R.P.; Paiva, J.A. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef] [Green Version]
- McQuillen, D.P.; MacIntyre, A.T. The Value That Infectious Diseases Physicians Bring to the Healthcare System. J. Infect. Dis. 2017, 216, S588–S593. [Google Scholar] [CrossRef] [Green Version]
- Macheda, G.; Luc, A.; Beraud, G.; Castan, B.; Gauzit, R.; Lesprit, P.; Tattevin, P.; Thilly, N.; Pulcini, C. Impact of the French Infectious Diseases Society’s (SPILF) proposals for shorter antibiotic therapies. Med. Mal. Infect. 2019, 49, 456–462. [Google Scholar] [CrossRef]
- Marrie, T.J.; Lau, C.Y.; Wheeler, S.L.; Wong, C.J.; Vandervoort, M.K.; Feagan, B.G. A controlled trial of a critical pathway for treatment of community-acquired pneumonia. CAPITAL Study Investigators. Community-Acquired Pneumonia Intervention Trial Assessing Levofloxacin. JAMA 2000, 283, 749–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roger, P.M.; Michelangeli, C.; Girard, D.; Etienne, P.; Borredon, G.; Dautezac, V.; Keita-Perse, O.; Del Giudice, P. Streamlined guidelines for antibiotic therapies are required for greater efficacy. Med. Mal. Infect. 2019, 49, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Spivak, E.S.; Cosgrove, S.E.; Srinivasan, A. Measuring Appropriate Antimicrobial Use: Attempts at Opening the Black Box. Clin. Infect. Dis. 2016, 63, 1639–1644. [Google Scholar] [PubMed] [Green Version]
- Buyle, F.M.; Decruyenaere, J.; De Waele, J.; Tulkens, P.M.; Van Audenrode, T.; Depuydt, P.; Claeys, G.; Robays, H.; Vogelaers, D. A survey of beta-lactam antibiotics and vancomycin dosing strategies in intensive care units and general wards in Belgian hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Charmillon, A.; Novy, E.; Agrinier, N.; Leone, M.; Kimmoun, A.; Levy, B.; Demore, B.; Dellamonica, J.; Pulcini, C. The ANTIBIOPERF study: A nationwide cross-sectional survey about practices for beta-lactam administration and therapeutic drug monitoring among critically ill patients in France. Clin. Microbiol. Infect. 2016, 22, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liebchen, U.; Paal, M.; Scharf, C.; Schroeder, I.; Grabein, B.; Zander, J.; Siebers, C.; Zoller, M. The ONTAI study-a survey on antimicrobial dosing and the practice of therapeutic drug monitoring in German intensive care units. J. Crit. Care 2020, 60, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Tabah, A.; De Waele, J.; Lipman, J.; Zahar, J.R.; Cotta, M.O.; Barton, G.; Timsit, J.F.; Roberts, J.A.; Working Group for Antimicrobial Use in the ICU within the Infection Section of the European Society of Intensive Care Medicine (ESICM). The ADMIN-ICU survey: A survey on antimicrobial dosing and monitoring in ICUs. J. Antimicrob. Chemother. 2015, 70, 2671–2677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, G.; Brinkman, A.; Benefield, R.J.; Carlier, M.; De Waele, J.J.; El Helali, N.; Frey, O.; Harbarth, S.; Huttner, A.; McWhinney, B.; et al. An international, multicentre survey of beta-lactam antibiotic therapeutic drug monitoring practice in intensive care units. J. Antimicrob. Chemother. 2014, 69, 1416–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longuet, P.; Lecapitaine, A.L.; Cassard, B.; Batista, R.; Gauzit, R.; Lesprit, P.; Haddad, R.; Vanjak, D.; Diamantis, S. Preparing and administering injectable antibiotics: How to avoid playing God. Med. Mal. Infect. 2016, 46, 242–268. [Google Scholar] [CrossRef]
- Porta, M. A Dictionary of Epidemiology, 6th ed.; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Barton, G.J.; Morecroft, C.W.; Henney, N.C. A survey of antibiotic administration practices involving patients with sepsis in UK critical care units. Int J. Clin. Pharm. 2020, 42, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Flannery, A.H.; Bissell, B.D.; Bastin, M.T.; Morris, P.E.; Neyra, J.A. Continuous Versus Intermittent Infusion of Vancomycin and the Risk of Acute Kidney Injury in Critically Ill Adults: A Systematic Review and Meta-Analysis. Crit Care Med. 2020, 48, 912–918. [Google Scholar] [CrossRef]
- Kim, B.; Lee, M.J.; Moon, S.M.; Park, S.Y.; Song, K.H.; Lee, H.; Park, J.S.; Lee, M.S.; Choi, S.M.; Yeom, J.S.; et al. Current status of antimicrobial stewardship programmes in Korean hospitals: Results of a 2018 nationwide survey. J. Hosp. Infect. 2020, 104, 172–180. [Google Scholar] [CrossRef]

| Descriptive Analysis | ||||
|---|---|---|---|---|
| Demographic | % | n/N | Median | IQR |
| Age (years) | 41.50 | (34.0−50.9) | ||
| ≤30 | 12.42 | 19/153 | ||
| 31−40 | 34.64 | 53/153 | ||
| 41−50 | 28.76 | 44/153 | ||
| 51−60 | 22.88 | 35/153 | ||
| ≥60 | 1.31 | 2/153 | ||
| Sex | ||||
| Men | 67.97 | 104/153 | ||
| Women | 32.03 | 49/153 | ||
| University Hospital | ||||
| Yes | 53.59 | 82/153 | ||
| No | 46.41 | 71/153 | ||
| Type of ICU | ||||
| Medical | 59.48 | 91/153 | ||
| Surgical | 9.15 | 14/153 | ||
| Mixed | 31.37 | 48/153 | ||
| Membership of the infection committee | ||||
| Yes | 30.72 | 47/153 | ||
| No | 69.28 | 106/153 | ||
| Experience time (years) | 10 | (4−17) | ||
| <5 | 30.07 | 46/153 | ||
| 5−10 | 24.84 | 38/153 | ||
| >10 | 45.10 | 69/153 | ||
| Monthly dedication in ICU (Hours) | 200 | (160−250) | ||
| <60 | 9.15 | 14/153 | ||
| 60−120 | 9.8 | 15/153 | ||
| 121−200 | 32.68 | 50/153 | ||
| >200 | 48.37 | 74/153 | ||
| Antimicrobial education | ||||
| Formal | 30.72 | 47/153 | ||
| Informal | 49.67 | 76/153 | ||
| None of the Above | 19.61 | 30/153 | ||
| Clinical practice | % | n/N | Median | IQR |
| Infectologist Weekly visits | 3 | (1−5) | ||
| 0 | 7.19 | 11/153 | ||
| 1–4 | 56.86 | 87/153 | ||
| 5–7 | 35.95 | 55/153 | ||
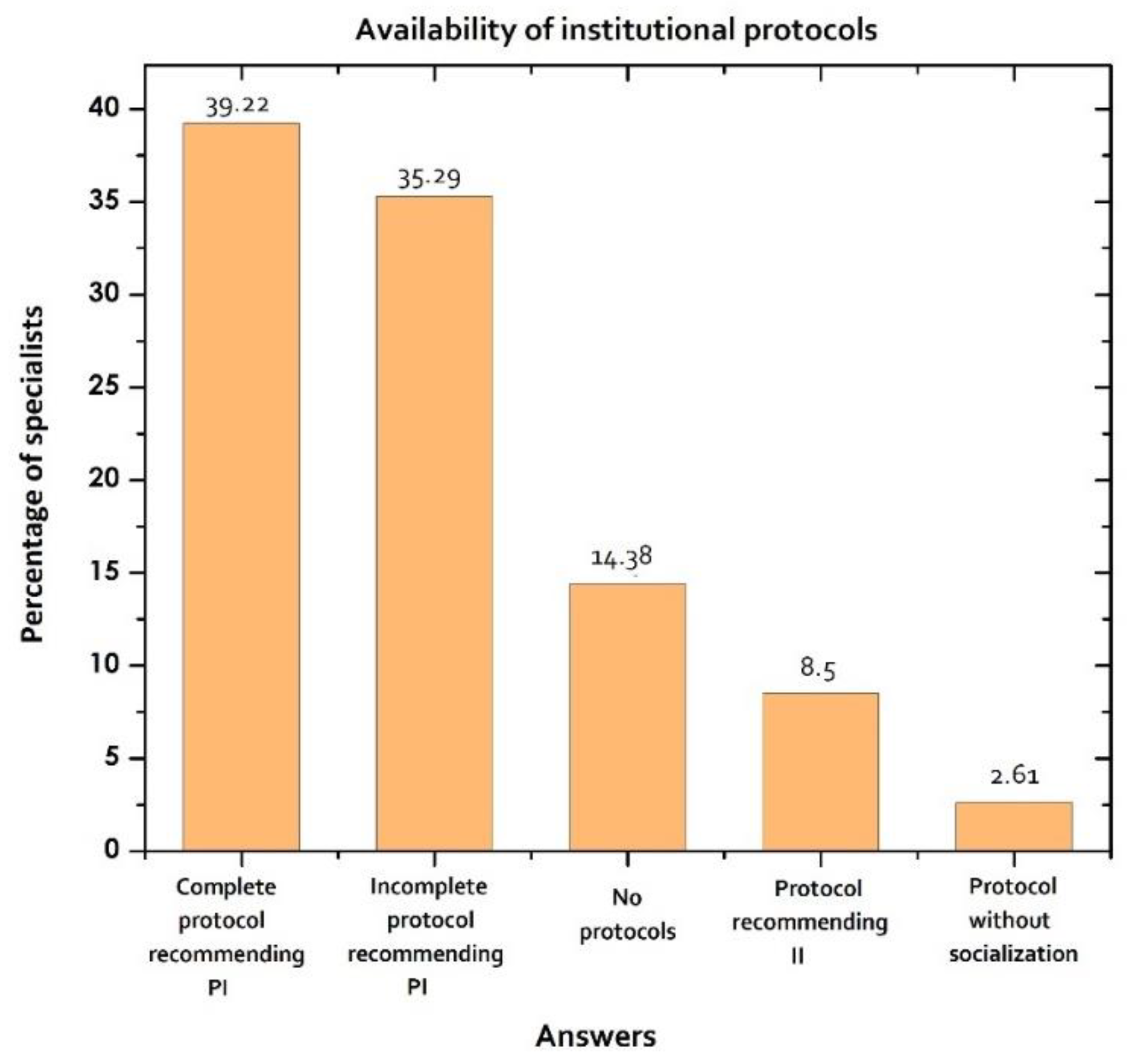
| Institutional protocol | ||||
| Yes | 85.62 | 131/153 | ||
| Complete protocol recommending PI | 39.22 | 60/153 | ||
| Incomplete protocol recommending PI | 35.29 | 54/153 | ||
| Protocol recommending II | 8.5 | 13/153 | ||
| Protocol without socialization | 2.61 | 4/153 | ||
| No | 14.38 | 22/153 | ||
| No protocols | 14.38 | 22/153 | ||
| Unnecessary | 0 | 0/153 | ||
| TDM availability | ||||
| TDM not available | 11.11 | 17/153 | ||
| <6 h | 21.57 | 33/153 | ||
| 6−12 h | 28.10 | 43/153 | ||
| 12−24 h | 9.15 | 14/153 | ||
| 24−48 h | 13.73 | 21/153 | ||
| >48 h | 16.34 | 25/153 | ||
| Pharmaceutical support | ||||
| Yes | 22.22 | 34/153 | ||
| No | 77.78 | 119/153 | ||
| Use of MIC | ||||
| Yes | 75.82 | 116/153 | ||
| No | 24.18 | 37/153 | ||
| Use of software | ||||
| Yes | 26.80 | 41/153 | ||
| No | 73.20 | 112/153 | ||
| Prolonged infusions | ||||
| Yes | 71.90 | 110/153 | ||
| No | 28.10 | 43/153 | ||
| Approach of clinical cases | 4.9 | (3.5−6.4) | ||
| Bivariate Analysis | ||
|---|---|---|
| Median (IQR) | p-value | |
| Sex | ||
| Male | 5.0 (4.4, 6.4) | 0.501 |
| Female | 4.9 (4.2, 6.4) | |
| University hospital | ||
| Yes | 4.9 (3.4, 6.4) | 0.946 |
| No | 4.8 (4.3, 6.2) | |
| Membership of the infection committee | ||
| Yes | 4.7 (3.3, 6.6) | 0.650 |
| No | 4.9 (4.2, 6.3) | |
| Antimicrobial education | ||
| Formal education | 4.9 (4.2, 6.4) | 0.469 |
| Informal education | 4.8 (3.5, 6.4) | |
| None | 4.8 (3.3, 5.9) | |
| Institutional protocol | ||
| Complete protocol recommending PI | 6 (4.4, 7.4) | 0.032 |
| Incomplete protocol recommending PI | 4.7 (4.3, 6.1) | |
| Protocol recommending II | 4.5 (3.3, 6.1) | |
| Protocol without socialization | 4.8 (3.9, 5.2) | |
| No protocols | 4.5 (3.0, 7.7) | |
| TDM availability | ||
| TDM not available | 4 (3.2, 4.9) | 0.026 |
| Vancomycin | 5.1 (4.3, 6.5) | |
| β-lactams and vancomycin | 4.6 (3.3, 5.7) | |
| Time to serum results | ||
| TDM not available | 4.5 (3.1, 5) | 0.076 |
| <6 h | 5 (3.4, 7.2) | |
| 6−12 h | 4.6 (3.7, 6.2) | |
| 12−24 h | 5 (4.7, 6.2) | |
| 24−48 h | 5.7 (4.6, 6.4) | |
| > 48 h | 4.9 (4.5, 6.4) | |
| Pharmaceutical support | ||
| Yes | 5.9 (3.4, 7.3) | 0.424 |
| No | 4.8 (4.3, 6.2) | |
| Use of MIC | ||
| Yes | 4.9 (3.4, 6.4) | 0.871 |
| No | 4.7 (4.3, 6.2) | |
| Use of software | ||
| Yes | 4.7 (3.3, 6.2) | 0.273 |
| No | 4.9 (4.3, 6.4) | |
| Bivariate Analysis | |||
|---|---|---|---|
| PI n = 110 | II n = 43 | p-value | |
| Age, median years (IQR) | |||
| 41.2 (34.1, 50.6) | 45.4 (34.2, 55.6) | 0.447 | |
| Sex, n (%) | |||
| Male | 76 (69) | 28 (65.1) | 0.778 |
| Female | 34 (30.9) | 15 (34.9) | |
| University hospital, n (%) | |||
| Yes | 62 (56.4) | 20 (46.5) | 0.358 |
| No | 48 (43.6) | 23 (53.5) | |
| Membership of the infection committee, n (%) | |||
| Yes | 35 (31.8) | 12 (27.9) | 0.782 |
| No | 75 (68.2) | 31 (72.1) | |
| Experience time, median years (IQR) | |||
| 10 (4, 16.7) | 8 (3, 17.5) | 0.205 | |
| Monthly dedication in ICU, median hours (IQR) | |||
| 240 (160, 280) | 200 (160, 240) | 0.159 | |
| Antimicrobial education, n (%) | |||
| Formal education | 30 (27.3) | 17 (39.5) | 0.32 |
| Informal education | 58 (52.7) | 18 (41.9) | |
| None | 22 (20) | 8 (18.6) | |
| Infectologist weekly visits, median days (IQR) | |||
| 3 (1, 5) | 3 (1, 7) | 0.675 | |
| Institutional protocol, n (%) | |||
| Complete protocol recommending PI | 50 (45.5) | 10 (23.3) | 0.093 |
| Incomplete protocol recommending PI | 36 (32.7) | 18 (41.9) | |
| Protocol recommending II | 9 (8.2) | 4 (9.3) | |
| Protocol without socialization | 3 (2.7) | 1 (2.3) | |
| No protocols | 12 (10.9) | 10 (23.2) | |
| TDM availability, n (%) | |||
| TDM not available | 23 (20.9) | 15 (34.9) | 0.102 |
| Vancomycin | 81 (73.6) | 24 (55.8) | |
| β-lactams and vancomycin | 6 (5.5) | 4 (9.3) | |
| Time to serum results, n (%) | |||
| TDM not available | 20 (18.2) | 13 (30.2) | 0.538 |
| <6 h | 31 (28.2) | 12(27.9) | |
| 6−12 h | 10 (9.1) | 4 (9.3) | |
| 12−24 h | 15 (13.6) | 6 (13.9) | |
| 24−48 h | 21 (19.1) | 4 (9.3) | |
| >48 h | 13 (11.8) | 4 (9.3) | |
| Pharmaceutical support, n (%) | |||
| Yes | 27 (24.5) | 7 (16.3) | 0.373 |
| No | 83 (75.4) | 36 (83.7) | |
| Use of MIC, n (%) | |||
| Yes | 83 (75.5) | 33 (76.7) | 1 |
| No | 27 (24.5) | 10 (23.3) | |
| Use of software, n (%) | |||
| Yes | 28 (25.5) | 13 (30.2) | 0.691 |
| No | 82 (74.5) | 30 (69.8) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, Y.V.; Blanco, J.; Díaz-Quijano, D.M.; Lechtig-Wasserman, S.; Liebisch-Rey, H.; Díaz-Pinilla, N.; Vergara-Ramirez, P.; Bustos, R.-H. Administration and Therapeutic Drug Monitoring of β-lactams and Vancomycin in Critical Care Units in Colombia: The ANTIBIOCOL Study. Pharmaceutics 2021, 13, 1577. https://doi.org/10.3390/pharmaceutics13101577
Fuentes YV, Blanco J, Díaz-Quijano DM, Lechtig-Wasserman S, Liebisch-Rey H, Díaz-Pinilla N, Vergara-Ramirez P, Bustos R-H. Administration and Therapeutic Drug Monitoring of β-lactams and Vancomycin in Critical Care Units in Colombia: The ANTIBIOCOL Study. Pharmaceutics. 2021; 13(10):1577. https://doi.org/10.3390/pharmaceutics13101577
Chicago/Turabian StyleFuentes, Yuli V., Jhosep Blanco, Diana Marcela Díaz-Quijano, Sharon Lechtig-Wasserman, Hans Liebisch-Rey, Nicolas Díaz-Pinilla, Peter Vergara-Ramirez, and Rosa-Helena Bustos. 2021. "Administration and Therapeutic Drug Monitoring of β-lactams and Vancomycin in Critical Care Units in Colombia: The ANTIBIOCOL Study" Pharmaceutics 13, no. 10: 1577. https://doi.org/10.3390/pharmaceutics13101577
APA StyleFuentes, Y. V., Blanco, J., Díaz-Quijano, D. M., Lechtig-Wasserman, S., Liebisch-Rey, H., Díaz-Pinilla, N., Vergara-Ramirez, P., & Bustos, R.-H. (2021). Administration and Therapeutic Drug Monitoring of β-lactams and Vancomycin in Critical Care Units in Colombia: The ANTIBIOCOL Study. Pharmaceutics, 13(10), 1577. https://doi.org/10.3390/pharmaceutics13101577









