Porous Nanomaterials Targeting Autophagy in Bone Regeneration
Abstract
:1. Introduction
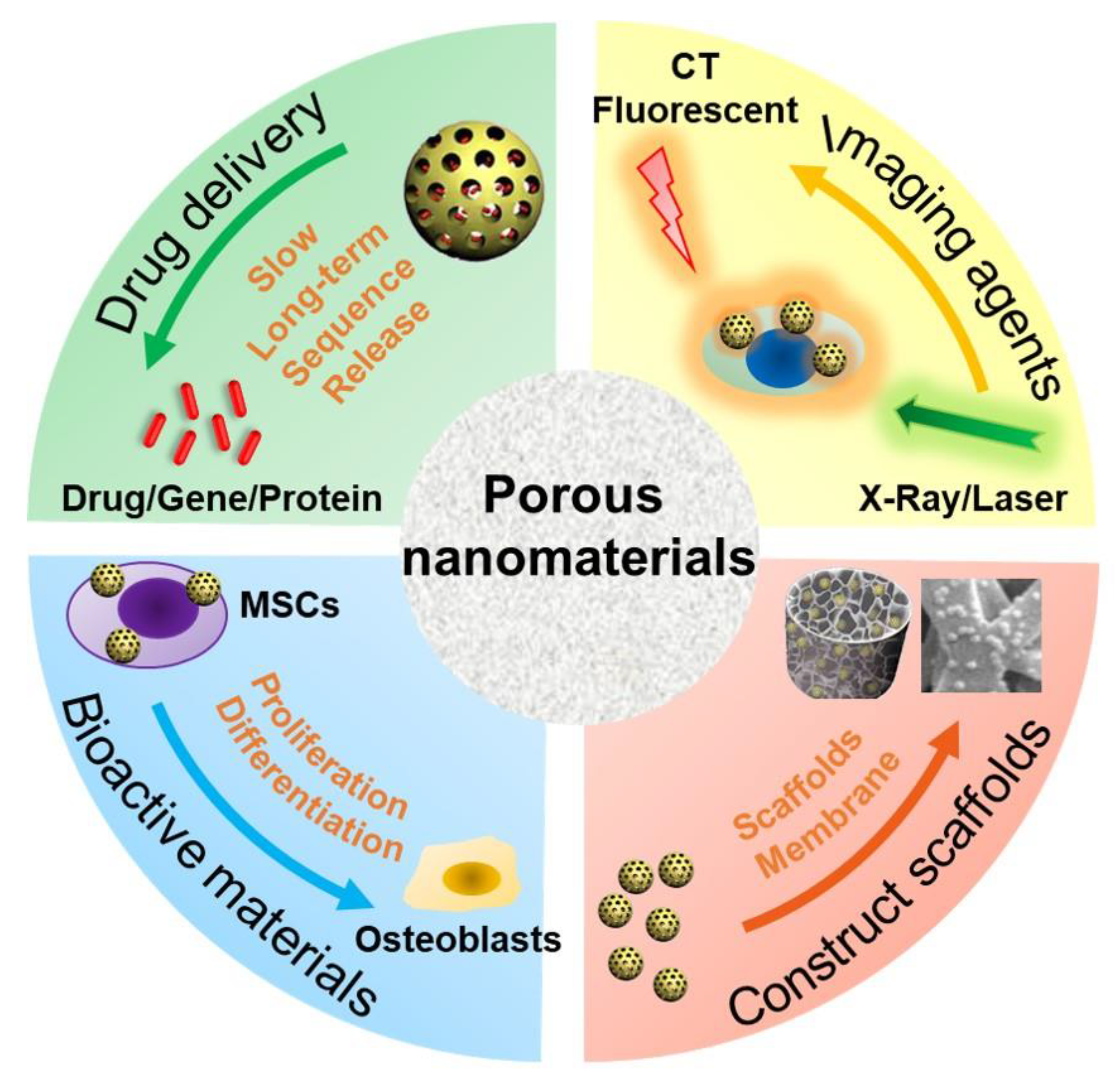
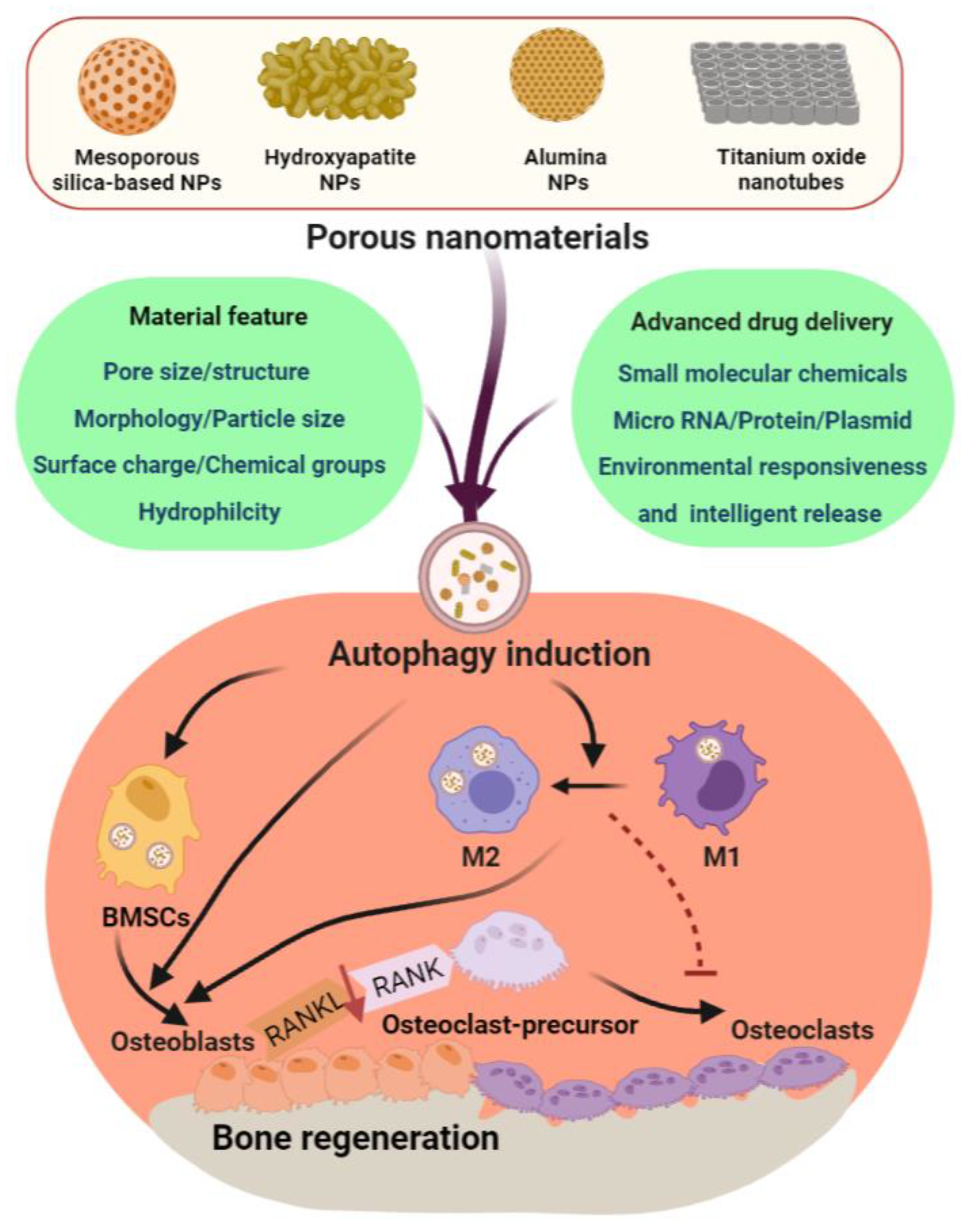
2. PNMs for Bone Regeneration
3. Autophagy Modulation and Bone Reconstruction
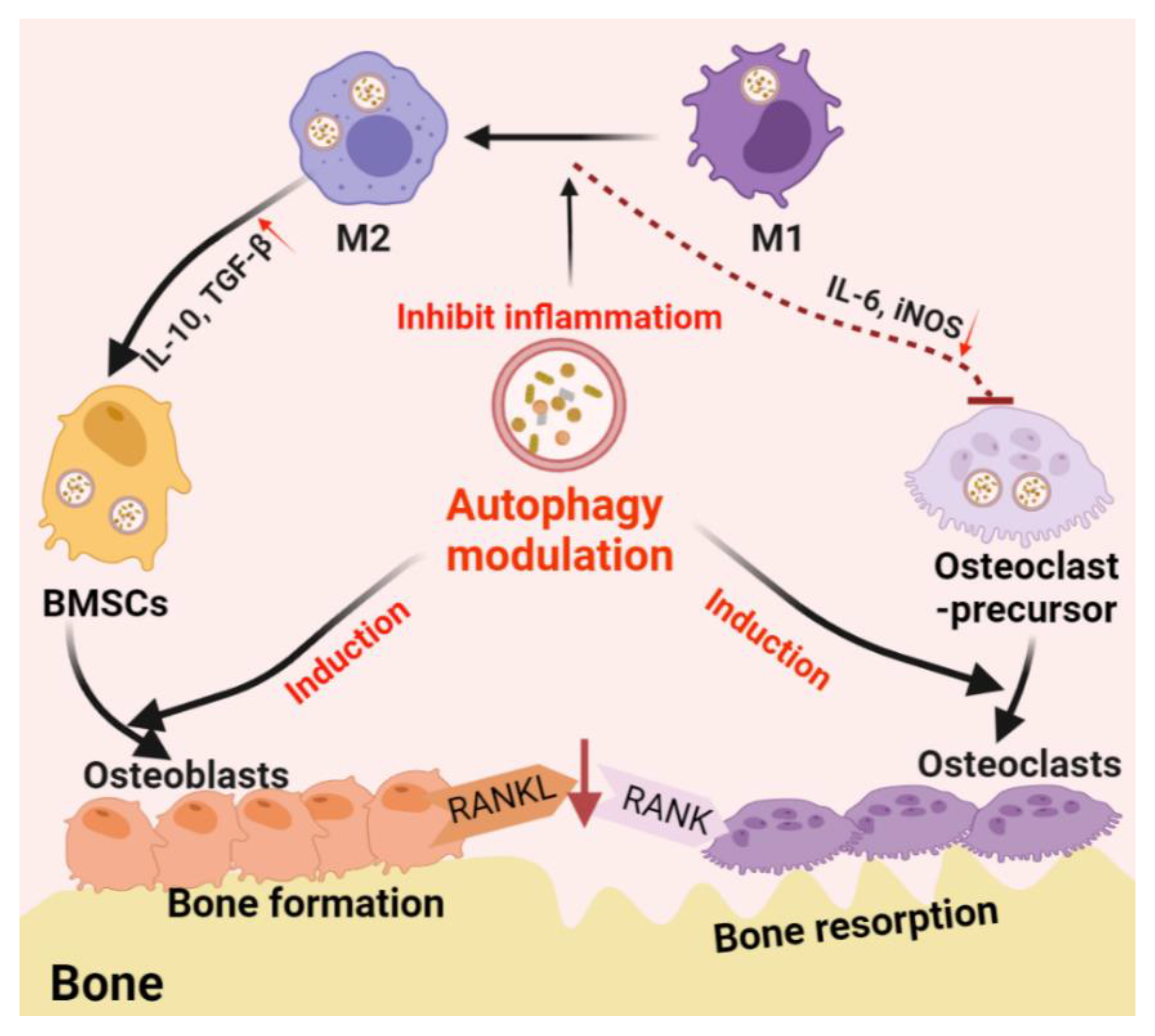
3.1. Autophagy in the Differentiation/Function of Osteoclasts and Osteoblasts
3.2. Autophagy-Associated Immunomodulation in Bone Remodeling
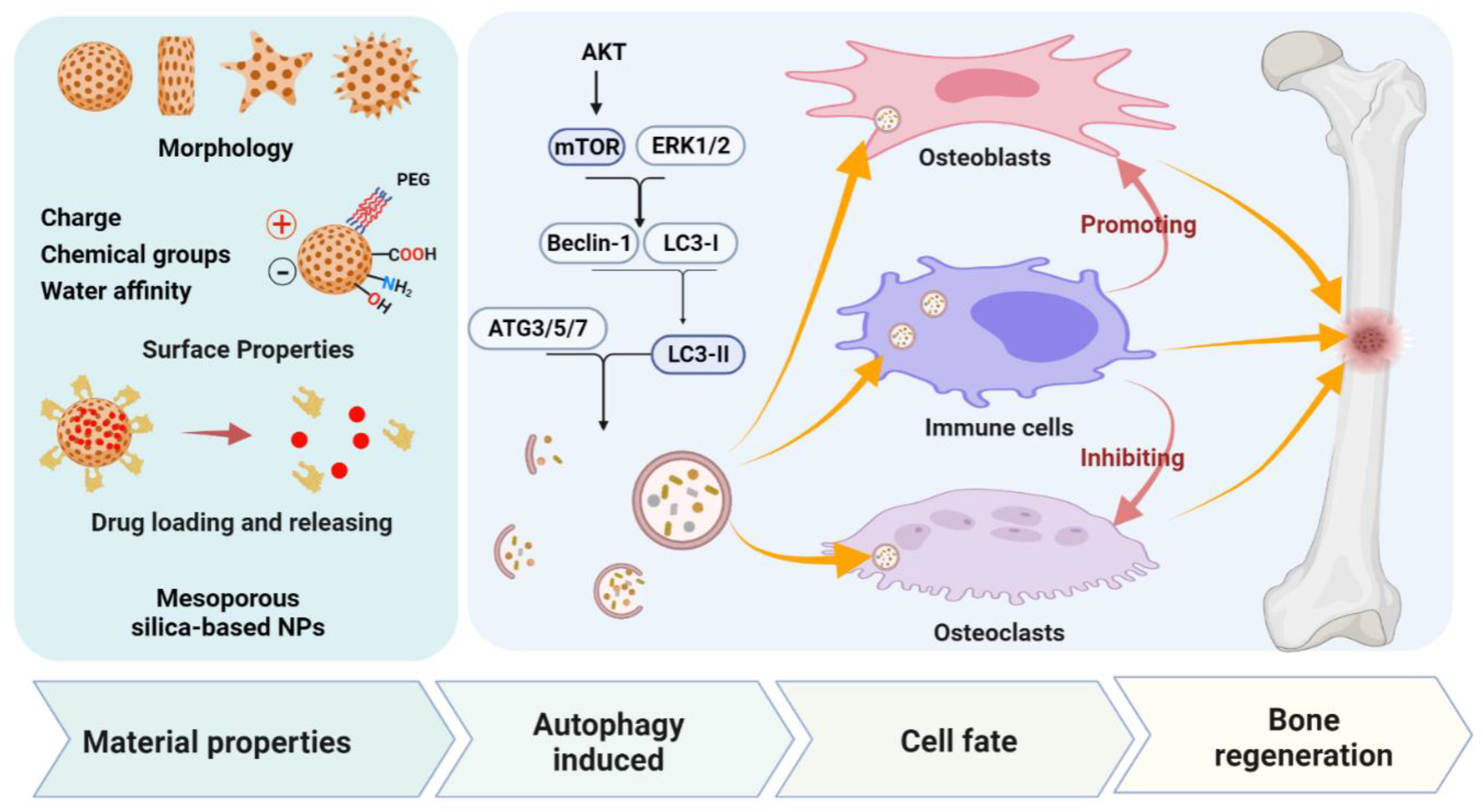
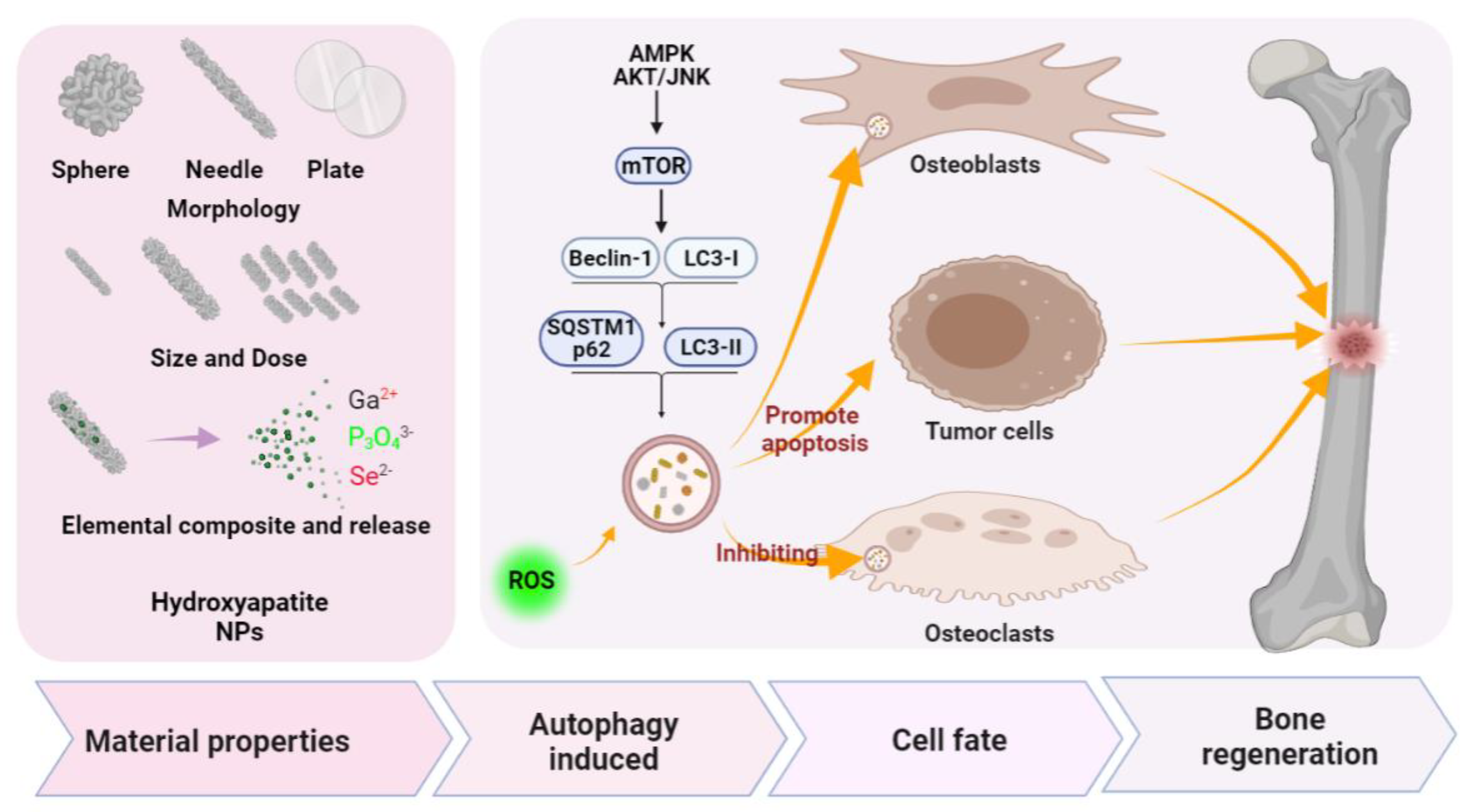
4. PNMs Regulate Autophagy in Bone Regeneration
4.1. Mesoporous Silica-Based Nanomaterials (MSNs)
4.2. Porous Nano-Hydroxyapatite (nHAP)
4.3. Titanium Dioxide Nanotubes (TiO2 NTs) and Alumina Nanoparticles (Al2O3)
5. Challenges and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviation
| PNMs | porous nanomaterials |
| MSNs | mesoporous silica nanoparticles |
| HAPs | hydroxyapatite nanoparticles |
| MBGNs | mesoporous bioactive glass |
| MCeO2 | mesoporous ceria |
| MRI | magnetic resonance |
| CT | computed tomography |
| PI | photoacoustic imaging |
| FI | fluorescence imaging |
| TiO2 NTs | Titanium dioxide nanotubes |
| ALP | alkaline phosphatase |
| Osx | osterix |
| COL-I | collagen-I |
| ERK | extracellular signal-regulated kinase |
| LC3 I | microtubule-associated protein 1A/1B-light chain 3 |
| LC3 II | LC3-phosphatidylethanolamine conjugate |
| ROS | reactive oxygen species |
| IL-6 | interleukin 6 |
| iNOS | inducible nitric oxide synthase |
| IL-10 | interleukin 10 |
| TGF-β | transforming growth factor-beta |
| RANKL | the receptor activator of nuclear factor-κB ligand |
| LPS | lipopolysaccharide |
| BMDMs | bone marrow-derived macrophages |
| AKT | v-Akt murine thymoma viral oncogene |
| mTOR | the mammalian target of rapamycin |
| PEG | polyethylene glycol |
| CTAB | cetyl trimethyl ammonium bromide |
| hPDLSCs | human periodontal ligament stem cells |
| B-SeHANs | selenium-doped hydroxyapatite nanoparticles |
| Ga2+ | calcium ion |
| P3O43− | Phosphate ion |
| Se2− | Selenium ion |
| Al2O3 | alumina nanoparticle |
| BMP2 | bone morphogenetic protein 2 |
| GLUT1 | glucose transport protein type 1 |
| BMSCs | bone marrow stem cells |
References
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C. Nanomaterial-based bone regeneration. Nanoscale 2017, 9, 4862–4874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Webster, T.J. Nanotechnolology and nanomaterials: Promises for improved tissue regeneration. Nano Today 2009, 4, 66–80. [Google Scholar] [CrossRef]
- Jayaraman, P.; Gandhimathi, C.; Venugopal, J.R.; Becker, D.L.; Ramakrishna, S.; Srinivasan, D.K. Controlled release of drugs in electrosprayed nanoparticles for bone tissue engineering. Adv. Drug Deliv. Rev. 2015, 94, 77–95. [Google Scholar] [CrossRef]
- Lead, J.R.; Batley, G.E.; Alvarez, P.; Croteau, M.-N.; Handy, R.D.; McLaughlin, M.J.; Judy, J.D.; Schirmer, K. Nanomaterials in the environment: Behavior, fate, bioavailability, and effects-An updated review. Environ. Toxicol. Chem. 2018, 37, 2029–2063. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, Y.; Zhang, C.; Lai, X.; Zhang, Y.; Wu, J.; Hu, C.; Shao, L. Nanomaterial-mediated autophagy: Coexisting hazard and health benefits in biomedicine. Part. Fibre Toxicol. 2020, 17, 1–26. [Google Scholar] [CrossRef]
- Liao, Y.; Yu, H.; Lv, J.; Cai, Y.; Liu, F.; He, Z.; He, S. Targeting autophagy is a promising therapeutic strategy to overcome chemoresistance and reduce metastasis in osteosarcoma. Int. J. Oncol. 2019, 55, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Hu, Y.; Hou, Y.; Li, M.; Chen, M.; Mu, C.; Tao, B.; Zhu, W.; Luo, Z.; Cai, K. Differentiation regulation of mesenchymal stem cells via autophagy induced by structurally-different silica based nanobiomaterials. J. Mater. Chem. B 2019, 7, 2657–2666. [Google Scholar] [CrossRef]
- Ali, A.; Suhail, M.; Mathew, S.; Shah, M.A.; Harakeh, S.M.; Ahmad, S.; Kazmi, Z.; Alhamdan, M.A.R.; Chaudhary, A.; Damanhouri, G.A.; et al. Nanomaterial Induced Immune Responses and Cytotoxicity. J. Nanosci. Nanotechnol. 2016, 16, 40–57. [Google Scholar] [CrossRef]
- Li, D.; Wang, C.; Li, Z.; Wang, H.; He, J.; Zhu, J.; Zhang, Y.; Shen, C.; Xiao, F.; Gao, Y.; et al. Nano-sized Al2O3 particle-induced autophagy reduces osteolysis in aseptic loosening of total hip arthroplasty by negative feedback regulation of RANKL expression in fibroblasts. Cell Death Dis. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Liu, N.; Liu, K.; Zhou, G.; Gan, J.; Shi, T.; Zhenheng, W.; Wang, L.; Tongguo, S.; Bao, N.; et al. Autophagy mediated CoCrMo particle-induced peri-implant osteolysis by promoting osteoblast apoptosis. Autophagy 2015, 11, 2358–2369. [Google Scholar] [CrossRef]
- Liu, N.; Meng, J.; Wang, Z.; Zhou, G.; Shi, T.; Zhao, J. Autophagy mediated TiAl 6 V 4 particle-induced peri-implant osteolysis by promoting expression of TNF-α. Biochem. Biophys. Res. Commun. 2016, 473, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xiao, Y. The Autophagy in Osteoimmonology: Self-Eating, Maintenance, and Beyond. Front. Endocrinol. 2019, 10, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyakumar, P.; Saravanakumar, S.S.; Kulathuraan, K.; Ramadas, V.; Natarajan, B. Functionalization Of Biomolecules With Nanostructured Porous Silicon For Biomedical Application. Surf. Rev. Lett. 2015, 22, 1550022. [Google Scholar] [CrossRef]
- Lou, X.-Y.; Li, Y.-P.; Yang, Y.-W. Gated Materials: Installing Macrocyclic Arenes-Based Supramolecular Nanovalves on Porous Nanomaterials for Controlled Cargo Release. Biotechnol. J. 2019, 14, e1800354. [Google Scholar] [CrossRef] [Green Version]
- Davoodi, E.; Zhianmanesh, M.; Montazerian, H.; Milani, A.S.; Hoorfar, M. Nano-porous anodic alumina: Fundamentals and applications in tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 1–16. [Google Scholar] [CrossRef]
- Hassanpour, P.; Panahi, Y.; Ebrahimi-Kalan, A.; Akbarzadeh, A.; Davaran, S.; Nasibova, A.N.; Khalilov, R.; Kavetskyy, T. Biomedical applications of aluminium oxide nanoparticles. Micro Nano Lett. 2018, 13, 1227–1231. [Google Scholar] [CrossRef]
- Mohandas, G.; Oskolkov, N.; McMahon, M.T.; Walczak, P.; Janowski, M. Porous tantalum and tantalum oxide nanoparticles for regenerative medicine. Acta Neurobiol. Exp. 2014, 74, 188–196. [Google Scholar]
- Luo, J.; Ding, X.; Song, W.; Bai, J.-Y.; Liu, J.; Li, Z.; Meng, F.-H.; Chen, F.-H.; Zhang, Y.-M. Inducing Macrophages M2 Polarization by Dexamethasone Laden Mesoporous Silica Nanoparticles from Titanium Implant Surface for Enhanced Osteogenesis. Acta Met. Sin. 2019, 32, 1253–1260. [Google Scholar] [CrossRef] [Green Version]
- Mann, A.P.; Tanaka, T.; Somasunderam, A.; Liu, X.; Gorenstein, D.G.; Ferrari, M. E-Selectin-Targeted Porous Silicon Particle for Nanoparticle Delivery to the Bone Marrow. Adv. Mater. 2011, 23, H278–H282. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, G.; Xing, X.; Liu, J.; Cheng, Y.; Ye, T.; Wang, Q.; Xiao, X.; Li, Z.; Deng, H. Near-Infrared Light-Triggered Porous AuPd Alloy Nanoparticles To Produce Mild Localized Heat To Accelerate Bone Regeneration. J. Phys. Chem. Lett. 2019, 10, 4185–4191. [Google Scholar] [CrossRef]
- Baeza, A.; Ruiz-Molina, D.; Vallet-Regí, M. Recent advances in porous nanoparticles for drug delivery in antitumoral applications: Inorganic nanoparticles and nanoscale metal-organic frameworks. Expert Opin. Drug Deliv. 2016, 14, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Castro, T.A.; Ibarra-Alonso, M.C.; Oliva, J.; Martínez-Luévanos, A. Porous aerogel and core/shell nanoparticles for controlled drug delivery: A review. Mater. Sci. Eng. C 2019, 96, 915–940. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, C.; Wang, X.; Zhang, W.; Wang, Y.; Vahabi, V. Porphyrin-like porous nanomaterials as drug delivery systems for ibuprofen drug. Mol. Phys. 2019, 118, e1678776. [Google Scholar] [CrossRef]
- Ha, S.-W.; Weitzmann, M.N.; Beck, G.R., Jr. Bioactive silica nanoparticles promote osteoblast differentiation through stimulation of autophagy and direct association with LC3 and p62. ACS Nano 2014, 8, 5898–5910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Guo, Y.; Yu, M.; Wang, M.; Ma, P.X.; Lei, B. Monodispersed Bioactive Glass Nanoclusters with Ultralarge Pores and Intrinsic Exceptionally High miRNA Loading for Efficiently Enhancing Bone Regeneration. Adv. Health Mater. 2017, 6, 1700630. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhu, Y.; Ni, B.; Xie, N.; Lu, X.; Shi, J.; Zeng, Y.; Guo, X. Mesoporous Silica Nanoparticles/Hydroxyapatite Composite Coated Implants to Locally Inhibit Osteoclastic Activity. ACS Appl. Mater. Interfaces 2014, 6, 5456–5466. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, M.; Kumar, P.S.; Das, B.; Dhara, S.; Chattopadhyay, S. Structurally Tuned Antimicrobial Mesoporous Hydroxyapatite Nanorods by Cyclic Oligosaccharides Regulation to Release a Drug for Osteomyelitis. Cryst. Growth Des. 2016, 17, 433–445. [Google Scholar] [CrossRef]
- Sistanipour, E.; Meshkini, A.; Oveisi, H. Catechin-conjugated mesoporous hydroxyapatite nanoparticle: A novel nano-antioxidant with enhanced osteogenic property. Colloids Surf. B Biointerfaces 2018, 169, 329–339. [Google Scholar] [CrossRef]
- Qiu, Y.; Xu, X.; Guo, W.; Zhao, Y.; Su, J.; Chen, J. Mesoporous Hydroxyapatite Nanoparticles Mediate the Release and Bioactivity of BMP-2 for Enhanced Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 2323–2335. [Google Scholar] [CrossRef]
- Walpole, A.R.; Briggs, E.P.; Karlsson, M.; Pålsgård, E.; Wilshaw, P.R. Nano-porous Alumina Coatings for Improved Bone Implant Interfaces. Mater. Werkst. 2003, 34, 1064–1068. [Google Scholar] [CrossRef]
- Briggs, E.P.; Walpole, A.R.; Wilshaw, P.R.; Karlsson, M.; Pålsgård, E. Formation of highly adherent nano-porous alumina on Ti-based substrates: A novel bone implant coating. J. Mater. Sci. Mater. Med. 2004, 15, 1021–1029. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Lee, J.H.; Lee, E.-J.; Kim, H.-W. Collagen hydrogels incorporated with surface-aminated mesoporous nanobioactive glass: Improvement of physicochemical stability and mechanical properties is effective for hard tissue engineering. Acta Biomater. 2013, 9, 9508–9521. [Google Scholar] [CrossRef]
- Westhauser, F.; Wilkesmann, S.; Nawaz, Q.; Hohenbild, F.; Rehder, F.; Saur, M.; Fellenberg, J.; Moghaddam, A.; Ali, M.S.; Peukert, W.; et al. Effect of manganese, zinc, and copper on the biological and osteogenic properties of mesoporous bioactive glass nanoparticles. J. Biomed. Mater. Res. Part A 2021, 109, 1457–1467. [Google Scholar] [CrossRef]
- Patel, K.D.; Buitrago, J.O.; Parthiban, S.P.; Lee, J.-H.; Singh, R.K.; Knowles, J.C.; Kim, H.-W. Combined Effects of Nanoroughness and Ions Produced by Electrodeposition of Mesoporous Bioglass Nanoparticle for Bone Regeneration. ACS Appl. Bio Mater. 2019, 2, 5190–5203. [Google Scholar] [CrossRef]
- Kim, J.-J.; Singh, R.K.; Patel, K.D.; Kim, H.-W. Delivery of Small Genetic Molecules through Hollow Porous Nanoparticles Silences Target Gene and in Turn Stimulates Osteoblastic Differentiation. Part. Part. Syst. Charact. 2016, 33, 878–886. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Lu, B.; Zhu, D.-Y.; Yin, J.-H.; Xu, H.; Zhang, C.-Q.; Ke, Q.-F.; Gao, Y.-S.; Guo, Y.-P. Incorporation of cerium oxide in hollow mesoporous bioglass scaffolds for enhanced bone regeneration by activating the ERK signaling pathway. Biofabrication 2019, 11, 25012. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.-T.; Wang, L.; Yu, B. Superparamagnetic iron oxide promotes osteogenic differentiation of rat adipose-derived stem cells. Int. J. Clin. Exp. Med. 2015, 8, 698–705. [Google Scholar]
- Yun, W.S.; Aryal, S.; Ahn, Y.J.; Seo, Y.J.; Key, J. Engineered iron oxide nanoparticles to improve regenerative effects of mesenchymal stem cells. Biomed. Eng. Lett. 2020, 10, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Everett, D.H. Manual of Symbols and Terminology for Physicochemical Quantities and Units, Appendix II: Definitions, Terminology and Symbols in Colloid and Surface Chemistry. Pure Appl. Chem. 1972, 31, 577–638. [Google Scholar] [CrossRef]
- Zhou, X.; Weng, W.; Chen, B.; Feng, W.; Wang, W.; Nie, W.; Chen, L.; Mo, X.-M.; Su, J.; He, C. Mesoporous silica nanoparticles/gelatin porous composite scaffolds with localized and sustained release of vancomycin for treatment of infected bone defects. J. Mater. Chem. B 2018, 6, 740–752. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Wang, Z.; Liu, B.; Zhu, S.; Zhu, L.; Peng, B. Licorice isoliquiritigenin-encapsulated mesoporous silica nanoparticles for osteoclast inhibition and bone loss prevention. Theranostics 2019, 9, 5183–5199. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Liu, Q.; Yang, L.; Wang, K.; Sun, T.; Ji, Y.; Liu, L.; Yu, W.; Qu, Y.; Wang, J.; et al. Sustained Delivery of BMP-2-Related Peptide from the True Bone Ceramics/Hollow Mesoporous Silica Nanoparticles Scaffold for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Zarif, F.; Tabassum, S.; Jamal, A.; Gul, U.; Gilani, M.A.; Sharif, F.; Zahid, S.; Asif, A.; Chaudhry, A.A.; Rehman, I.U. Surface-grafted remedial hydroxyapatite nanoparticles to avoid operational infections. Mon. Chem. Chem. Mon. 2019, 150, 605–615. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Liu, C.-H.; Liang, Y.-H.; Lin, F.-H.; Wu, K.C.-W. Hollow mesoporous hydroxyapatite nanoparticles (hmHANPs) with enhanced drug loading and pH-responsive release properties for intracellular drug delivery. J. Mater. Chem. B 2013, 1, 2447–2450. [Google Scholar] [CrossRef]
- Subhapradha, N.; Abudhahir, M.; Aathira, A.; Srinivasan, N.; Moorthi, A. Polymer coated mesoporous ceramic for drug delivery in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 65–73. [Google Scholar] [CrossRef]
- Lei, L.; Liu, Z.; Yuan, P.; Jin, R.; Wang, X.; Jiang, T.; Chen, X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chem. B 2019, 7, 2722–2735. [Google Scholar] [CrossRef]
- Liang, H.; Jin, C.; Ma, L.; Feng, X.; Deng, X.; Wu, S.; Liu, X.; Yang, C. Accelerated Bone Regeneration by Gold-Nanoparticle-Loaded Mesoporous Silica through Stimulating Immunomodulation. ACS Appl. Mater. Interfaces 2019, 11, 41758–41769. [Google Scholar] [CrossRef]
- Mizuno, H.; Tobita, M.; Orbay, H.; Uysal, A.C.; Lu, F. Adipose-Derived Stem Cells as a Novel Tool for Future Regenerative Medicine. In Stem Cells and Cancer Stem Cells; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; Volume 12, pp. 165–174. [Google Scholar]
- Kiessling, F.; Mertens, M.E.; Grimm, J.; Lammers, T. Nanoparticles for Imaging: Top or Flop? Radiology 2014, 273, 10–28. [Google Scholar] [CrossRef] [Green Version]
- Vieira, S.; Vial, S.; Reis, R.L.; Oliveira, J.M. Nanoparticles for bone tissue engineering. Biotechnol. Prog. 2017, 33, 590–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempen, P.; Greasley, S.; Parker, K.A.; Campbell, J.C.; Chang, H.-Y.; Jones, J.; Sinclair, R.; Gambhir, S.S.; Jokerst, J.V. Theranostic Mesoporous Silica Nanoparticles Biodegrade after Pro-Survival Drug Delivery and Ultrasound/Magnetic Resonance Imaging of Stem Cells. Theranostics 2015, 5, 631–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, D.; Chen, D.; Li, K.; Qu, Y.; Sun, K.; Tao, K.; Dai, K.; Ai, S. Gold Nanoparticles as a Potential Cellular Probe for Tracking of Stem Cells in Bone Regeneration Using Dual-Energy Computed Tomography. ACS Appl. Mater. Interfaces 2016, 8, 32241–32249. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Khademi, C.; Gambhir, S.S. Intracellular Aggregation of Multimodal Silica Nanoparticles for Ultrasound-Guided Stem Cell Implantation. Sci. Transl. Med. 2013, 5, 177ra35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokerst, J.; Thangaraj, M.; Kempen, P.; Sinclair, R.; Gambhir, S.S. Photoacoustic Imaging of Mesenchymal Stem Cells in Living Mice via Silica-Coated Gold Nanorods. ACS Nano 2012, 6, 5920–5930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, C.; Ow, H. Commercial Nanoparticles for Stem Cell Labeling and Tracking. Theranostics 2013, 3, 544–560. [Google Scholar] [CrossRef]
- Van Schooneveld, M.M.; Cormode, D.P.; Koole, R.; Van Wijngaarden, J.T.; Calcagno, C.; Skajaa, T.; Hilhorst, J.; Hart, D.C.T.; Fayad, Z.A.; Mulder, W.J.M.; et al. A fluorescent, paramagnetic and PEGylated gold/silica nanoparticle for MRI, CT and fluorescence imaging. Contrast Media Mol. Imaging 2010, 5, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Ma, M.; Wang, J.; Wang, F.; Chern, S.-X.; Zhao, E.R.; Jhunjhunwala, A.; Darmadi, S.; Chen, H.; Jokerst, J.V. Exosome-like silica nanoparticles: A novel ultrasound contrast agent for stem cell imaging. Nanoscale 2017, 9, 402–411. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Shen, Y.; Shao, Y.; He, H.; Tan, Y.; Tian, X.; Xie, F. Gadolinium3+-doped mesoporous silica nanoparticles as a potential magnetic resonance tracer for monitoring the migration of stem cells in vivo. Int. J. Nanomed. 2013, 8, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Xia, Z.; Czernuszka, J. Design and Development of Three-Dimensional Scaffolds for Tissue Engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Das, K.; Bose, S.; Bandyopadhyay, A. TiO2 nanotubes on Ti: Influence of nanoscale morphology on bone cell-materials interaction. J. Biomed. Mater. Res. Part A 2008, 90, 225–237. [Google Scholar] [CrossRef]
- Han, C.-M.; Kim, H.-E.; Koh, Y.-H. Creation of hierarchical micro/nano-porous TiO2 surface layer onto Ti implants for improved biocompatibility. Surf. Coat. Technol. 2014, 251, 226–231. [Google Scholar] [CrossRef]
- Kim, M.-J.; Lim, H.-J.; Lee, B.G.; Kim, J.-H.; Choi, J.; Kang, H.-G. Establishment of Validation Methods to Test the Biocompatibility of Titanium Dioxide. Bull. Korean Chem. Soc. 2013, 34, 1857–1863. [Google Scholar] [CrossRef] [Green Version]
- Awad, N.K.; Edwards, S.L.; Morsi, Y. A review of TiO2 NTs on Ti metal: Electrochemical synthesis, functionalization and potential use as bone implants. Mater. Sci. Eng. C 2017, 76, 1401–1412. [Google Scholar] [CrossRef]
- von Wilmowsky, C.; Bauer, S.; Lutz, R.; Meisel, M.; Neukam, F.W.; Toyoshima, T.; Schmuki, P.; Nkenke, E.; Schlegel, K.A. In vivo evaluation of anodic TiO2 nanotubes: An experimental study in the pig. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 165–171. [Google Scholar] [CrossRef]
- Bjursten, L.M.; Rasmusson, L.; Oh, S.; Smith, G.C.; Brammer, K.S.; Jin, S. Titanium dioxide nanotubes enhance bone bonding in vivo. J. Biomed. Mater. Res. Part A 2010, 92, 1218–1224. [Google Scholar]
- Wang, N.; Li, H.; Lü, W.; Li, J.; Wang, J.; Zhang, Z.; Liu, Y. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 2011, 32, 6900–6911. [Google Scholar] [CrossRef]
- Li, J.; Li, J.J.; Zhang, J.; Wang, X.; Kawazoe, N.; Chen, G. Gold nanoparticle size and shape influence on osteogenesis of mesenchymal stem cells. Nanoscale 2016, 8, 7992–8007. [Google Scholar] [CrossRef]
- Kong, C.H.; Steffi, C.; Shi, Z.; Wang, W. Development of mesoporous bioactive glass nanoparticles and its use in bone tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2878–2887. [Google Scholar] [CrossRef]
- Molino, G.; Palmieri, M.C.; Montalbano, G.; Fiorilli, S.L.; Vitale-Brovarone, C. Biomimetic and mesoporous nano-hydroxyapatite for bone tissue application: A short review. Biomed. Mater. 2019, 15, 22001. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Torre, E.; Bari, A.; Taccardi, N.; Cassinelli, C.; Morra, M.; Fiorilli, S.; Vitale-Brovarone, C.; Iviglia, G.; Boccaccini, A.R. Antioxidant mesoporous Ce-doped bioactive glass nanoparticles with anti-inflammatory and pro-osteogenic activities. Mater. Today Bio 2020, 5, 100041. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Baghbaderani, M.T.; Hernández, V.V.; Naruphontjirakul, P.; Li, S.; McFarlane, T.; Hachim, D.; Stevens, M.M.; Porter, A.E.; Jones, J.R. Nanoceria provides antioxidant and osteogenic properties to mesoporous silica nanoparticles for osteoporosis treatment. Acta Biomater. 2020, 122, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shi, M.; Jones, J.; Chen, Z.; Chang, J.; Wu, C.; Xiao, Y. Strategies to direct vascularisation using mesoporous bioactive glass-based biomaterials for bone regeneration. Int. Mater. Rev. 2017, 62, 392–414. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Zhu, X.-R.; Du, J.-H. Autophagy: A potential target for the treatment of intraocular neovascularization. Int. J. Ophthalmol. 2018, 11, 695–698. [Google Scholar] [CrossRef]
- Liu, L.; Xia, R.; Liu, X. MiR-125b-5p Promotes Autophagy in Systemic Lupus Erythematosus Cells by Up Regulating the Expression of Genes Related to UV Resistance. Acta Microsc. 2020, 29, 2856–2865. [Google Scholar]
- Kabeya, Y.; Mizushima, N.; Yamamoto, A.; Oshitani-Okamoto, S.; Ohsumi, Y.; Yoshimori, T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell Sci. 2004, 117, 2805–2812. [Google Scholar] [CrossRef] [Green Version]
- Chun, Y.; Kim, J. Autophagy: An Essential Degradation Program for Cellular Homeostasis and Life. Cells 2018, 7, 278. [Google Scholar] [CrossRef] [Green Version]
- Avivar-Valderas, A.; Salas, E.; Bobrovnikova-Marjon, E.; Diehl, J.A.; Nagi, C.; Debnath, J.; Aguirre-Ghiso, J.A. PERK Integrates Autophagy and Oxidative Stress Responses to Promote Survival during Extracellular Matrix Detachment. Mol. Cell. Biol. 2011, 31, 3616–3629. [Google Scholar] [CrossRef] [Green Version]
- Rambold, A.S.; Cohen, S.; Lippincott-Schwartz, J. Fatty Acid Trafficking in Starved Cells: Regulation by Lipid Droplet Lipolysis, Autophagy, and Mitochondrial Fusion Dynamics. Dev. Cell 2015, 32, 678–692. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, N.; Levine, B. Autophagy in mammalian development and differentiation. Nat. Cell Biol. 2010, 12, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Rodriguez-Enriquez, S.; Lemasters, J.J. Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys. 2007, 462, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Deng, Z.; Ma, Y.; Jin, J.; Qi, F.; Li, S.; Liu, C.; Lyu, F.-J.; Zheng, Q. The Role of Autophagy and Mitophagy in Bone Metabolic Disorders. Int. J. Biol. Sci. 2020, 16, 2675–2691. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakayamada, S.; Okada, Y. Osteoblasts and Osteoclasts in Bone Remodeling and Inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-F.; Chen, G.; Ren, J.-G.; Zhang, W.; Wu, Z.-X.; Liu, B.; Zhao, Y. The Adaptor Protein p62 Is Involved in RANKL-induced Autophagy and Osteoclastogenesis. J. Histochem. Cytochem. 2014, 62, 879–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSelm, C.; Miller, B.; Zou, W.; Beatty, W.L.; van Meel, E.; Takahata, Y.; Klumperman, J.; Tooze, S.; Teitelbaum, S.L.; Virgin, H.W. Autophagy Proteins Regulate the Secretory Component of Osteoclastic Bone Resorption. Dev. Cell 2011, 21, 966–974. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Su, J.; Sun, W.; Cai, L.; Deng, Z. AMP-activated protein kinase stimulates osteoblast differentiation and mineralization through autophagy induction. Int. J. Mol. Med. 2018, 41, 2535–2544. [Google Scholar] [CrossRef]
- Nollet, M.; Santucci-Darmanin, S.; Breuil, V.; Al-Sahlanee, R.; Cros, C.; Topi, M.; Momier, D.; Samson, M.; Pagnotta, S.; Cailleteau, L.; et al. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy 2014, 10, 1965–1977. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Zhang, R.; Pajarinen, J.; Lin, T.-H.; Goodman, S.B.; Yao, Z. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res. Ther. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Böse, T.; Unger, R.E.; Jansen, J.A.; Kirkpatrick, C.J.; Beucken, J.J.J.P.V.D. Macrophage type modulates osteogenic differentiation of adipose tissue MSCs. Cell Tissue Res. 2017, 369, 273–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Klein, T.; Murray, R.Z.; Crawford, R.; Chang, J.; Wu, C.; Xiao, Y. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today 2016, 19, 304–321. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, Y.; Jia, L.; Pan, L.; Li, H.; Du, J. Atg5 deficiency-mediated mitophagy aggravates cardiac inflammation and injury in response to angiotensin II. Free Radic. Biol. Med. 2014, 69, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Symington, J.W.; Wang, C.; Twentyman, J.; Owusu-Boaitey, N.; Schwendener, R.A.; Núñez, G.; Schilling, J.D.; Mysorekar, I.U. ATG16L1 deficiency in macrophages drives clearance of uropathogenic E. coli in an IL-1β-dependent manner. Mucosal Immunol. 2015, 8, 1388–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Zhao, E.; Ilyas, G.; Lalazar, G.; Lin, Y.; Haseeb, M.; Tanaka, K.E.; Czaja, M.J. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 2015, 11, 271–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Mitochondria and the Autophagy-Inflammation-Cell Death Axis in Organismal Aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Bachhuka, A.; Han, S.; Wei, F.; Lu, S.; Visalakshan, R.M.; Vasilev, K.; Xiao, Y. Tuning Chemistry and Topography of Nanoengineered Surfaces to Manipulate Immune Response for Bone Regeneration Applications. ACS Nano 2017, 11, 4494–4506. [Google Scholar] [CrossRef]
- Shen, G.; Ren, H.; Shang, Q.; Qiu, T.; Yu, X.; Zhang, Z.; Huang, J.; Zhao, W.; Zhang, Y.; Liang, D.; et al. Autophagy as a target for glucocorticoid-induced osteoporosis therapy. Cell. Mol. Life Sci. 2018, 75, 2683–2693. [Google Scholar] [CrossRef]
- Vomero, M.; Barbati, C.; Colasanti, T.; Perricone, C.; Novelli, L.; Ceccarelli, F.; Spinelli, F.R.; Di Franco, M.; Conti, F.; Valesini, G.; et al. Autophagy and Rheumatoid Arthritis: Current Knowledges and Future Perspectives. Front. Immunol. 2018, 9, 1577. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.Y.; Wang, W.-C.; Lin, P.Y.; Huang, C.P.; Chen, C.Y.; Chen, Y.K. The roles of autophagy and hypoxia in human inflammatory periapical lesions. Int. Endod. J. 2018, 51, e125–e145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, F.; Yang, Z.; Xu, L.; Sun, Y. Targeted tumor therapy by autophagy of nanoparticles. Futur. Oncol. 2020, 16, 793–803. [Google Scholar] [CrossRef]
- Cao, B.; Dai, X.; Wang, W. Knockdown of TRPV4 suppresses osteoclast differentiation and osteoporosis by inhibiting autophagy through Ca2+–calcineurin–NFATc1 pathway. J. Cell. Physiol. 2019, 234, 6831–6841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, J.; Cheng, L.; Lin, K. Enhancement of osteoporotic bone regeneration by strontium-substituted 45S5 bioglass via time-dependent modulation of autophagy and the Akt/mTOR signaling pathway. J. Mater. Chem. B 2021, 9, 3489–3501. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, M.; Wang, D.; Bu, W.; Wang, Z.; Shen, Y.; Zhang, K.; Zhou, D.; Yang, B.; Sun, H. Bone formation promoted by bone morphogenetic protein-2 plasmid-loaded porous silica nanoparticles with the involvement of autophagy. Nanoscale 2019, 11, 21953–21963. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Han, S.; Shi, M.; Liu, G.; Chen, Z.; Chang, J.; Wu, C.; Xiao, Y. Immunomodulatory effects of mesoporous silica nanoparticles on osteogenesis: From nanoimmunotoxicity to nanoimmunotherapy. Appl. Mater. Today 2018, 10, 184–193. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, X.; Zhou, M.; Kuang, Y.; Xiang, M.; Li, J.; Song, J. Effect of metformin on human periodontal ligament stem cells cultured with polydopamine-templated hydroxyapatite. Eur. J. Oral Sci. 2019, 127, 210–221. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, Y.; Zhou, P.; Wei, K.; Wang, H.; Wang, J.; Fang, H.; Zhang, S. Hierarchically constructed selenium-doped bone-mimetic nanoparticles promote ROS-mediated autophagy and apoptosis for bone tumor inhibition. Biomaterials 2020, 257, 120253. [Google Scholar] [CrossRef]
- Yang, G.; Liu, H.; Hu, X.; Chen, Z.; Friis, T.E.; Wang, J.; Xiao, Y.; Zhang, S. Bio-inspired hybrid nanoparticles promote vascularized bone regeneration in a morphology-dependent manner. Nanoscale 2017, 9, 5794–5805. [Google Scholar] [CrossRef]
- Wang, R.; Hu, H.; Guo, J.; Wang, Q.; Cao, J.; Wang, H.; Li, G.; Mao, J.; Zou, X.; Chen, D.; et al. Nano-Hydroxyapatite Modulates Osteoblast Differentiation Through Autophagy Induction via mTOR Signaling Pathway. J. Biomed. Nanotechnol. 2019, 15, 405–415. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, X.; Xu, L.; Hu, X.; Deng, F.; Yang, Z.; Jiang, L.; Fu, T.; Zhou, P.; Song, J. Nanosized alumina particle and proteasome inhibitor bortezomib prevented inflammation and osteolysis induced by titanium particle via autophagy and NF-κB. Signal. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, M.; Ren, R.; Gao, C.; Cheng, H.; Li, Y.; Gao, B.; Wei, Y.; Fu, J.; Sun, J.; et al. Improved Immunoregulation of Ultra-Low-Dose Silver Nanoparticle-Loaded TiO2 Nanotubes via M2 Macrophage Polarization by Regulating GLUT1 and Autophagy. Int. J. Nanomed. 2020, 15, 2011–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, F.; Li, M.; Crawford, R.; Zhou, Y.; Xiao, Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019, 86, 480–492. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nat. Cell Biol. 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Slowing, I.; Vivero-Escoto, J.L.; Wu, K.C.-W.; Lin, V.S.-Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Gisbert-Garzarán, M.; Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for the Treatment of Complex Bone Diseases: Bone Cancer, Bone Infection and Osteoporosis. Pharmaceutics 2020, 12, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenholm, J.M.; Zhang, J.; Linden, M.; Sahlgren, C. Mesoporous silica nanoparticles in tissue engineering—A perspective. Nanomedicine 2016, 11, 391–402. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; He, C. Mesoporous silica nanoparticles for tissue-engineering applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2019, 11, e1573. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Chenab, K.K.; Taheri-Ledari, R.; Mosafer, J.; Hashemi, S.M.; Mokhtarzadeh, A.; Maleki, A.; Hamblin, M.R. Recent advances in the application of mesoporous silica-based nanomaterials for bone tissue engineering. Mater. Sci. Eng. C 2020, 107, 110267. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; He, N.; Zhao, Y.; Liu, T.; Deng, Y. Autophagy Modulated by Inorganic Nanomaterials. Theranostics 2020, 10, 3206–3222. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, Y.; Yü, Y.; Li, Y.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Silica nanoparticles enhance autophagic activity, disturb endothelial cell homeostasis and impair angiogenesis. Part. Fibre Toxicol. 2014, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Yang, M.; Jing, L.; Wang, J.; Yu, Y.; Li, Y.; Duan, J.; Zhou, X.; Li, Y. Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int. J. Nanomed. 2016, 11, 5257–5276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Lee, J.; Kwak, M.; Cho, Y.-L.; Hwang, B.; Cho, M.J.; Lee, N.G.; Park, J.; Lee, S.-H.; Park, J.-G.; et al. Two distinct cellular pathways leading to endothelial cell cytotoxicity by silica nanoparticle size. J. Nanobiotechnol. 2019, 17, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, Y.; Lu, K.; Yang, M.; Li, Y.; Zhou, X.; Sun, Z. Silica nanoparticles induce autophagy dysfunction via lysosomal impairment and inhibition of autophagosome degradation in hepatocytes. Int. J. Nanomed. 2017, 12, 809–825. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Cheng, F.; Xue, C.; Wang, H.; Chen, C.; Du, Q.; Ge, D.; Sun, B. Surface modification of Stöber silica nanoparticles with controlled moiety densities determines their cytotoxicity profiles in macrophages. Langmuir 2019, 35, 14688–14695. [Google Scholar] [CrossRef]
- Marquardt, C.; Fritsch-Decker, S.; Al-Rawi, M.; Diabaté, S.; Weiss, C. Autophagy induced by silica nanoparticles protects RAW264.7 macrophages from cell death. Toxicology 2017, 379, 40–47. [Google Scholar] [CrossRef]
- El-Fattah, H.A.; Helmy, Y.; El-Kholy, B.; Marie, M. In vivo animal histomorphometric study for evaluating biocompatibility and osteointegration of nano-hydroxyapatite as biomaterials in tissue engineering. J. Egypt. Natl. Cancer Inst. 2010, 22, 241–250. [Google Scholar]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Hu, X.; Zhang, C.; Chen, S.; Li, Z.; Yang, X.; Liu, H.; Jia, G.; Liu, D.; Ge, K.; et al. Hybrid Mesoporous Silica-Based Drug Carrier Nanostructures with Improved Degradability by Hydroxyapatite. ACS Nano 2015, 9, 9614–9625. [Google Scholar] [CrossRef]
- Nga, N.K.; Chau, N.T.T.; Viet, P.H. Facile synthesis of hydroxyapatite nanoparticles mimicking biological apatite from eggshells for bone-tissue engineering. Colloids Surf. B Biointerfaces 2018, 172, 769–778. [Google Scholar] [CrossRef]
- Frasnelli, M.; Cristofaro, F.; Sglavo, V.M.; Dirè, S.; Callone, E.; Ceccato, R.; Bruni, G.; Cornaglia, A.I.; Visai, L. Synthesis and characterization of strontium-substituted hydroxyapatite nanoparticles for bone regeneration. Mater. Sci. Eng. C 2017, 71, 653–662. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Pazarçeviren, A.E.; Tezcaner, A.; Evis, Z. Mesoporous strontium doped nano sized sulphate hydroxyapatite as a novel biomaterial for bone tissue applications. RSC Adv. 2016, 6, 68058–68071. [Google Scholar] [CrossRef]
- Walmsley, G.G.; McArdle, A.; Tevlin, R.; Momeni, A.; Atashroo, D.; Hu, M.S.; Feroze, A.H.; Wong, V.W.; Lorenz, P.H.; Longaker, M.T.; et al. Nanotechnology in bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1253–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Jin, M.; Zheng, Y.-D.; Guan, Y.-P.; Lu, X.; Luo, J.-L. Nanotubular surface modification of metallic implants via electrochemical anodization technique. Int. J. Nanomed. 2014, 9, 4421–4435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, K.-S.; Choi, E.-J.; Bae, J.-M.; Park, Y.-B.; Oh, S. Visible Light-Enhanced Antibacterial and Osteogenic Functionality of Au and Pt Nanoparticles Deposited on TiO2 Nanotubes. Materials 2020, 13, 3721. [Google Scholar] [CrossRef] [PubMed]
- Gunputh, U.F.; Le, H.; Handy, R.D.; Tredwin, C. Anodised TiO2 nanotubes as a scaffold for antibacterial silver nanoparticles on titanium implants. Mater. Sci. Eng. C 2018, 91, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, G.; Webster, T.J. Reduced bacterial growth and increased osteoblast proliferation on titanium with a nanophase TiO2 surface treatment. Int. J. Nanomed. 2017, 12, 363–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, S.; Wang, F.; Bi, Y.; Wang, P.; Zhang, R.; Bohatko-Naismith, J.; Zhang, X.; Wang, H. Autophagy regulates the Wnt/GSK3β/β-catenin/cyclin D1 pathway in mesenchymal stem cells (MSCs) exposed to titanium dioxide nanoparticles (TiO2NPs). Toxicol. Rep. 2020, 7, 1216–1222. [Google Scholar] [CrossRef]
- Prashanth, P.; Raveendra, R.; Krishna, R.H.; Ananda, S.; Bhagya, N.; Nagabhushana, B.; Lingaraju, K.; Naika, H.R. Synthesis, characterizations, antibacterial and photoluminescence studies of solution combustion-derived α-Al2O3 nanoparticles. J. Asian Ceram. Soc. 2015, 3, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Abbas, Y.; Zuhra, Z.; Butler, I.S. Synthesis of γ-alumina (Al2O3) nanoparticles and their potential for use as an adsorbent in the removal of methylene blue dye from industrial wastewater. Nanoscale Adv. 2019, 1, 213–218. [Google Scholar] [CrossRef] [Green Version]
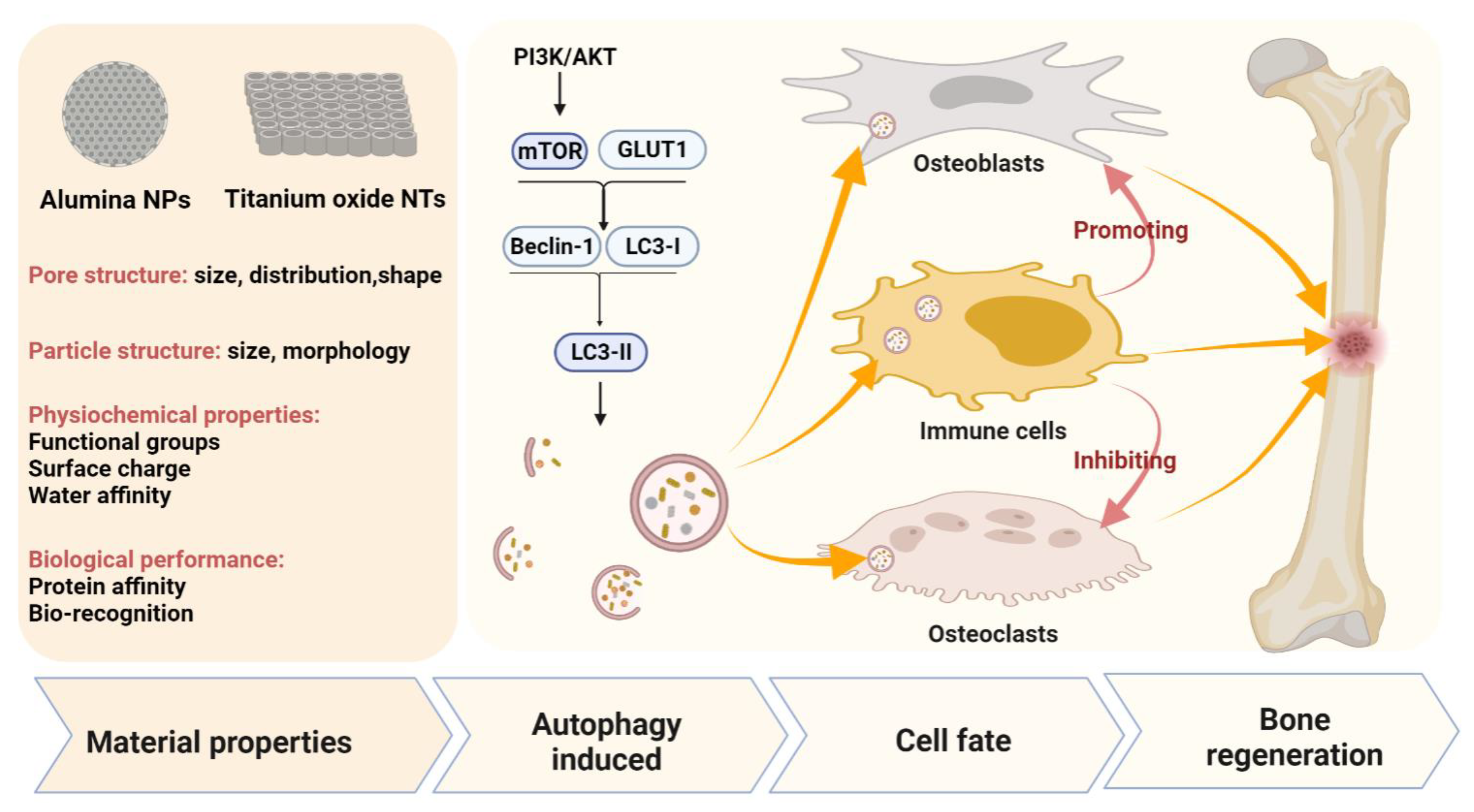
| Item | Nanoparticles | Compound Carried/ Combination Drugs | Target Cells | Autophagy Markers (Down/Up) | Autophagy Mechanism | Osteogenesis Marker (Down/up) | Biological Effect | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | Silica nanoparticles | Cobalt ferrite magnetic metal core | MC3T3-E1 cells | LC3II/LC3I ↑ P62 ↑ | ERK1/2-LC3 and P62 | ALP ↑ Alizarin red ↑ OSC ↑ | Autophagy and promoted osteoblast differentiation and mineralization | [25] |
| 2 | Mesoporous silica nanoparticles | No combination | RAW 264.7 cells | LC3II/LC3I ↑ | Not reported | Alizarin Red S ↑ | Autophagy and inhibited inflammation and promoted osteogenesis | [108] |
| 3 | Silica nanoparticles | Load BMP-2 plasmid | MC3T3-E1 | LC3II ↑ | Not reported | Alizarin red S ↑ | Stimulated autophagy, osteogenic differentiation, and bone regeneration | [107] |
| 4 | Silica-based nano-biomaterials | No combination | MSCs | LC3-II ↑ p-ERK/ERK ↑ p-AKT/mAKT ↓ P-mTOR/mTOR ↓ | ERK1/2 and AKT/mTOR | ALP, mineralization level, COLI, OPG, OCN, OPN, and RUNX2 ↑ | Enhanced the differentiation potential by enhancing autophagy | [8] |
| 5 | 45S5 bioglass | Sr doped | OVX-BMSCs | AKT/mTOR | ALP, alizarin red S staining ↑ | Improved autophagy, promoted osteogenic differentiation of OVX-BMSCs and bone regeneration in osteoporotic bone defects | [106] | |
| 6 | Nano-hydroxyapatite | No combination | MC3T3-E1 cells | LC3II/LC3I ↑ | mTOR | ALP, BMP2, BSP, COL-I, OSC, and Runx2 ↑ | Autophagy and modulated osteoblast differentiation | [112] |
| 7 | Hydroxyapatite NPs | Integrating nanoparticles within gelatin | rMSCs | LC3A/LC3B ↑ P62 ↑ | Not reported | OCN, OPN ↑ | Autophagy activation and promoted vascularized and bone regeneration | [111] |
| 8 | Selenium-doped hydroxyapatite nanoparticles (B-SeHANs) | No combination | Human MNNG/HOS osteosarcoma cells | LC3B II ↑ Beclin-1 ↑ SQSTM1/P62 ↓ | AKT/mTOR and JNK | MMP-9 ↑ bone destruction ↓ | Promoted autophagy and apoptosis to inhibit tumor growth while profoundly reducing bone destruction | [110] |
| 9 | Polydopamine-templated hydroxyapatite (tHA) | Combined metformin | hPDLSCs | LC3B II ↑ Beclin-1 ↑ | AMPK/mTOR | OPN, Runx2, ALP activity, and Alizarin red ↑ | THA combined with metformin regulated autophagy, improved the activity of hPDLSCs, and promoted osteogenic differentiation | [109] |
| 10 | Nanosized alumina particle | Proteasome inhibitor, bortezomib (BTZ) | MG-63 cells | LC3 ↑ | Not reported | Apoptotic cell ↓ | Activated autophagy and inhibited apoptosis | [113] |
| 11 | Nanosized Al2O3 particle | No combination | Human fibroblasts | LC3II ↑ Beclin-1↑ | BECN-1 | RANKL ↓ | Autophagy inhibited the expression of RANkL and inhibited osteolysis | [10] |
| 12 | Titanium oxide nanotubes | BMP2-stimulated macrophage-derived exosomes | hBMSCs | LC3II/LC3I ↑ ATG5 ↑ | Not reported | ALP, BMP2, BMP7, Runx2, OCN, and Col-I, OPN ↑ | Activated autophagy during osteogenic differentiation | [115] |
| 13 | TiO2 nanotubes | Silver nanoparticle loaded | RAW 264.7 and MC3T3-E1 | LC3II/LC3I and Beclin-1 ↑ | PI3K/AKT and GLUT1 | ALP, RUNX2, OCN, and OPG ↑ | Activated autophagy and promoted osteogenesis by regulating bone immunity | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Xiao, L.; Xiao, Y. Porous Nanomaterials Targeting Autophagy in Bone Regeneration. Pharmaceutics 2021, 13, 1572. https://doi.org/10.3390/pharmaceutics13101572
Zhang Q, Xiao L, Xiao Y. Porous Nanomaterials Targeting Autophagy in Bone Regeneration. Pharmaceutics. 2021; 13(10):1572. https://doi.org/10.3390/pharmaceutics13101572
Chicago/Turabian StyleZhang, Qing, Lan Xiao, and Yin Xiao. 2021. "Porous Nanomaterials Targeting Autophagy in Bone Regeneration" Pharmaceutics 13, no. 10: 1572. https://doi.org/10.3390/pharmaceutics13101572
APA StyleZhang, Q., Xiao, L., & Xiao, Y. (2021). Porous Nanomaterials Targeting Autophagy in Bone Regeneration. Pharmaceutics, 13(10), 1572. https://doi.org/10.3390/pharmaceutics13101572








