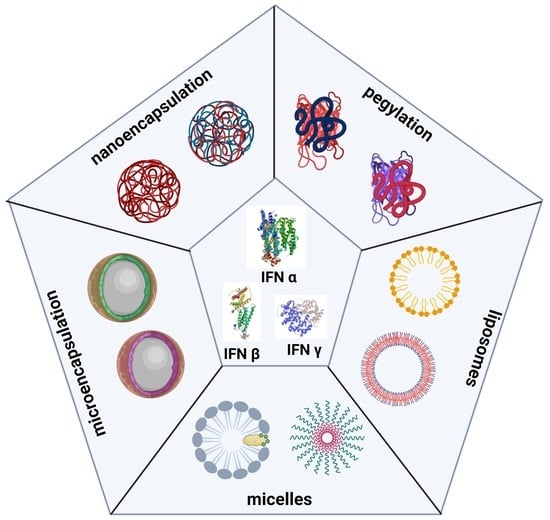
Forms and Methods for Interferon’s Encapsulation
Abstract
1. Introduction
2. IFN Delivery Systems
3. PEGylation of IFNs
4. Liposomes
5. Polymeric Micelles
6. Recent Encapsulation Forms of IFNs
6.1. Microencapsulation
6.1.1. IFN-α
6.1.2. IFN-β
6.1.3. IFN-γ
6.2. Nanoencapsulation
6.2.1. IFN-α
6.2.2. IFN-β
6.2.3. IFN-γ
| IFN Type | Encapsulating Matrix | Route of Administration | Encapsulation Method | Physical Properties | Formulation Objective | Advantages/Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| IFN-α | Microspheres of LEAVE | In vitro | Double emulsion/solvent evaporation | Size = 186 µm | Stabilization of IFN-α on PELA particles with sustained release and retention of antiviral activity for up to 11 days in in vitro studies. | A: stabilization of IFN in the matrix D: initial burst release | [119] |
| PLGA microspheres | In vitro | Double emulsion/solvent evaporation | Size = 1.8 µm | Sustained in vitro release of methoxy-PEG-IFN-α for up to 3 weeks, although they exhibited high release peaks. | A: solubility maintained D: initial burst release | [120] | |
| PLGA/ poloxamer | In vitro | Oil-in-oil solvent extraction | Size = 40 µm | Evaluation of microparticles and nanoparticles as an in vitro controlled release system. The MPs released IFN for up to 96 days. | A: integrity and activity of the molecule D: initial burst release | [54] | |
| Multivesicular liposomes | In vitro | Double emulsion/solvent evaporation | Size = ~20 µm | Development of a system for controlled and sustained release of PEG-IFN-α for up to 6 days in vitro. | A: high stability and encapsulation efficiency D: initial burst release | [101] | |
| Uni- and multivesicular liposomes | Intramuscular | Film hydration-dilution | Size = 101 nm | Prolonged retention of IFN-α-2b for up to 24 h at the application site after intramuscular administration in Kungming mice. | A: high retention at the application site D: loss of activity | [102] | |
| Lysine-coated gold nanoparticles | In vitro | Chloroauric acid and borohydride reduction | Size without IFN = 10 nm | in vitro transport of IFN-α on gold nanoparticles coupled to lysine found on the particle surface. | A: stable conjugation in water D: modification of the carboxyl groups of the molecule | [145] | |
| Poly(ether-ester) microspheres (Poly-Active) | Subcutaneous | Double emulsion/solvent evaporation | Size = ~30 µm | Phase IIB clinical study of Locteron®, a 14-day dose–response sustained-release formulation, well tolerated by patients at a dose of 80 µg. | A: significant decrease in adverse events D: scarce report of its physicochemical characterization | [121] | |
| PLGA microspheres | In vitro | Double emulsion/solvent evaporation | Size = 28.1 µm | Encapsulation of IFN-α in PLGA microparticles in vitro. No changes were detected in the physicochemical and biological characteristics of the molecule released by diffusion for 24 h at 37 °C. | A: uniform size distribution D: IFN instability | [127] | |
| PLGA microspheres | Intramuscular | Double emulsion/solvent evaporation | Size = 81.23 µm | Increased residence time of IFN-α in serum up to 18 days, and sustained release with activity up to 12 days in studies in rhesus monkeys. | A: increase in circulation time in vivo D: loss of biological activity | [128] | |
| Alginate microspheres chitosan | Intramuscular | Coacervation | Size = 2.18 µm | Evaluation of pharmacokinetics in ICR mice, revealing a 4-fold increase in the half-life of IFN-α, with no increased peak concentration, and reduced bioavailability | A: increase in maximum serum concentration D: low encapsulation efficiency | [129] | |
| PLA and PLGA microspheres | In vitro | Double emulsion/solvent evaporation with magnetite nanoparticles inclusion | Average size = 2.5 µm Size distribution = 0.5–3.5 µm | Particle loading with magnetite for site-specific delivery. In vitro antiviral assays in Vero cells against vesicular stomatitis virus indicated a slight reduction in the antiviral activity of the particles. | A: particle direction using magnetic field D: low encapsulation efficiency | [130] | |
| PLGA microspheres | In vitro | Double emulsion/solvent evaporation | Size distribution = 40.54–115.62 µm | Sustained release maintains the molecule’s biological activity for up to 7 days in in vitro studies in Wish cells against vesicular stomatitis virus. | A: high encapsulation efficiency D: in vivo performance was not evaluated. | [131] | |
| IFN-α | PLGA-PEGT/PBT microspheres | Subcutaneous | Double emulsion/solvent evaporation | Size = 28.94 µm | Extended cumulative release for up to 23 days in vitro, conforming to zero-order kinetics. Plasma levels were stable for 13 days in Sprague–Dawley rats, starting with a rapid release on day 1. | A: high encapsulation efficiency D: initial burst release | [132] |
| PLGA nanoparticles with adsorbed HBV antigens | Intravenous | Double emulsion | Size = 174 nm PZ = +30 mV | System aimed at treating hepatitis B. Studies in BALB/c mice indicated that nanoparticles transport IFN to hepatocytes, with good systemic circulation. | A: site-specific transport D: low encapsulation efficiency | [148] | |
| Liposomes | Intramuscular | Film hydration | Size = 82–172 nm PDI < 0.35 | Increased half-life, peak time, and bioavailability of encapsulated IFN-α-2b in Wistar rats. | A: accumulation in the liver D: non-uniform size | [103] | |
| Gold nanoparticles plus hyaluronic acid (HA) | Intravenous | Chloroauric acid reduction with citrate and reductive amination of HA | Size = 52.23 nm PDI = 0.089 | Selective transport to the liver for HCV treatment. Biological activity of IFN-α is similar to PEG-Intron in vitro (Daudi), in vivo (BALB/c mice). | A: serum stability D: slow initial release | [146] | |
| Protamine sulfate-impregnated gelatin microspheres | In vitro | Emulsion polymerization with glutaraldehyde as a crosslinker | Size = 28.94 µm | Protamine sulfate impregnation to increase the release time of IFN-α to 336 h and prolong the cytotoxic effect in vitro in ovarian cancer Skov3 cells | A: almost complete release D: no correlation with cytotoxicity | [133] | |
| Chondroitin sulfate and PVP | Intradermal | Two-solution system in polydimethylsiloxane molds | Arrangements of 12 × 12 microneedles. Dimensions: 680 × 380 μm | Transport of IFN-α in microneedles. In vivo studies (SD rats), the needles have good stability for two months and do not cause skin damage. | A: no injections required D: limited stability over time | [134] | |
| PLGA and PEG-PLGA nanoparticles | In vitro | Double emulsion/solvent evaporation | Size = 104–129 nm | Evaluation of sustained release of IFN-α under in vitro conditions: phosphate-buffered saline and blood plasma. | A: sustained and stable release D: in vivo pharmacokinetics not evaluated. | [149] | |
| Chitosan nanoparticles | Evaluation of the oral route | Ionotropic gelation | Size = 36 nm PZ = +30 mV | Nanoparticles for oral administration, with in vitro antiviral activity (MDBK) comparable to commercial IFN-α. IFN levels in plasma 1h after in vivo inoculation (in CF-1 mice). | A: high encapsulation efficiency D: non-specific release in the stomach | [150] | |
| PEGylated Liposomes | Franz Cell Diffusion System | Film hydration | Size = 181 nm PZ = −13 mV | Formulation for treatment of human papillomavirus. No in vitro release. Ex vivo studies in goat vaginal tissue with high penetration of the molecule into the tissue. | A: crosses mucosa D: in vitro and ex vivo release was not correlated | [105] | |
| POEGMA-PHPMA copolymer micelles | Intravenous | Self-assembly of copolymer blocks | Size = 64.9 nm | Formation of micelles by self-assembled copolymer blocks that encapsulated IFN-α, with increased half-life up to 83.8 h, and antitumor activity in mice with ovarian tumors | A: effective tumor suppression D: decrease in biological activity | [57] | |
| Chitosan nanoparticles | Oral | Ionotropic gelation | Size = 36 nm PDI = 0.47 Potential Z = +30 mV | Evaluation of oral administration of nanoparticles. In vitro (Caco-2:HT29-MTX (9:1)) and in vivo (BALB/c mice) studies confirmed improved pharmacokinetics and bioavailability. | A: crosses intestinal epithelium D: no analysis in disease models | [151] | |
| Core-shell nanoparticles; core: HSA-IFN-α, shell: PSS-CS-PSS | Subcutaneous | Core: aqueous precipitation; shell: layer-by-layer assembly | Size = 100 nm PZ = −50 mV | Sustained-release after ten days in Pannon rabbits, with biological activity similar to lyophilized HSA-IFN-α. | A: bioactivity maintained D: PSS is not biocompatible | [152] | |
| Elastin-like copolypeptide micelles | Intravenous | Self-assembly of two copolypeptide building blocks | Size = 48 nm | Formation of micelles by blocks of two self-assembled polypeptides that encapsulated IFN-α, with an increase in its half-life up to 54.7 h, and antitumor activity in mice with ovarian tumors. | A: efficient accumulation in tumors D: encapsulation efficiency is not reported. | [58] | |
| IFN-β | Poly(methacrylic acid-ethylene glycol) microparticles | Direct intestinal | UV polymerization using TEGDMA as crosslinker | Size < 53 µm | Encapsulation for intestinal delivery of IFN-ß. In vitro and in vivo results in Sprague–Dawley rats showed sustained release and improved pharmacokinetics. | A: pH-sensitive behavior D: incomplete release | [135] |
| TMC-PEGDMA-MAA microparticles | Oral | Suspension polymerization by free radicals | Size = 1–3.5 µm at intestinal pH (6.8) | pH-sensitive oral transport system for the treatment of multiple sclerosis. Most of the IFN-ß was released in vitro at intestinal pH. Release profile in New Zealand White rabbits exceeded 24 h. | A: pH-sensitive D: in vitro and in vivo release was not correlated | [136] | |
| PLGA and PEG-PLGA nanoparticles | Subcutaneous | Double emulsion/solvent evaporation | Size = 145 nm and 163 nm PZ = 17.7 and 18.8 mV | Treatment of Multiple Sclerosis. No toxicity in vitro, in vivo studies in Wistar rats showed mild toxic effects such as pale kidney and pyelectasis. | A: high encapsulation efficiency D: mild toxicity | [153] | |
| Chitosan nanoparticles/cyclodextrin | Intranasal | Gelation | Size = 206 nm PZ = 20 mV PDI = 0.13 | Nasal administration of the formulation for treating multiple sclerosis, with greater effectiveness, than free IFN-β in C57BL/6 mice with sclerosis. | A: reduction in encephalomyelitis D: no CD4+ lymphocyte downregulation | [154] | |
| IFN-γ | PLGA microspheres | In vitro | Double emulsion/solvent evaporation | Size = 30–50 µm | Stabilization of IFN-γ in microparticles, maintaining the native conformation and biological activity of the protein. | A: bioactivity maintained D: encapsulation destabilizes the protein | [137] |
| PLA microspheres | Oral | Double emulsion/solvent evaporation | Size = 1.27 µm | Sustained release in vitro for 400 h and increased absorption when administered orally in Wistar rats. | A: increase in porosity D: delayed release | [138] | |
| Liposomes | Inhalation | Freezing, thawing | Size = 170–180 nm | It demonstrated that encapsulation of IFN-γ and liposomal muramyl tripeptide with chitosan activated alveolar macrophages and increased survival in the treated group. In vivo study in a murine model. | A: increase in the activation of alveolar macrophages. D: loss of biological activity | [97] | |
| BSA nanoparticles | Intraperitoneal | Coacervation and chemical crosslinking | Size = ~340 nm PZ = −19.6 mV | Evaluation of macrophage activation for Brucella abortus. It increased the bactericidal effect of IFN-γ-activated macrophages in vitro and in vivo (BALB/c mice). | A: increased biological activity D: extended-release only for 20 h | [155] | |
| Liposomes with cyclic peptides | Intravenous | Film hydration | Size = 83.5 nm PDI = 0.067 | Selective liposome transport to hepatic stellate cells increased half-life and antifibrotic activity of IFN-γ with fewer adverse effects in Sprague–Dawley rats. | A: selective transport to hepatic cells D: low encapsulation efficiency | [104] | |
| PLGA core–shell nanoparticles containing IFN-γ and doxorubicin. | Intravenous | Nanoprecipitation | Size = ~100 nm | Melanoma immunotherapy. Female C57BL/6 murine model, free IFN at 8 h, encapsulated cleared after 48 h inoculated in mice. There was no toxicity in vital organs. | A: temperature-sensitive behavior D: conditional encapsulation efficiency | [156] | |
| PEGylated Liposomes | Intravenous | Thin-film hydration and extrusion | Size = 135 nm PDI = 0.05 | Preparation of IFN-γ-containing liposomes for colon cancer treatment. Sustained release in vitro for 144 h with an abrupt onset and increased cytokine-activated antitumor immune response in BALB/c mice with C-26 tumor cells. | A: significant induction of the antitumor response D: low encapsulation efficiency | [106] |
7. Discussion
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Negishi, H.; Taniguchi, T.; Yanai, H. The Interferon (IFN) Class of Cytokines and the IFN Regulatory Factor (IRF) Transcription Factor Family. Cold Spring Harb. Perspect. Biol. 2018, 10, a028423. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhao, P.; Ma, B.; Guo, G.; Sun, Y.; Xing, M. Cloning, expression and antiviral bioactivity of Red-crowned Crane interferon-α. Gene 2014, 544, 49–55. [Google Scholar] [CrossRef]
- Wang, B.X.; Fish, E.N. Global virus outbreaks: Interferons as 1st responders. Semin. Immunol. 2019, 43, 101300. [Google Scholar] [CrossRef]
- Li, S.-F.; Zhao, F.-R.; Shao, J.-J.; Xie, Y.-L.; Chang, H.-Y.; Zhang, Y.-G. Interferon-omega: Current status in clinical applications. Int. Immunopharmacol. 2017, 52, 253–260. [Google Scholar] [CrossRef]
- Blank, T.; Prinz, M. Type I interferon pathway in CNS homeostasis and neurological disorders. Glia 2017, 65, 1397–1406. [Google Scholar] [CrossRef]
- Schreiber, G. The molecular basis for differential type I interferon signaling. J. Biol. Chem. 2017, 292, 7285–7294. [Google Scholar] [CrossRef]
- Schoggins, J.W. Interferon-Stimulated Genes: What Do They All Do? Annu. Rev. Virol. 2019, 6, 567–584. [Google Scholar] [CrossRef]
- Lazear, H.M.; Schoggins, J.W.; Diamond, M.S. Shared and Distinct Functions of Type I and Type III Interferons. Immunity 2019, 50, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.X.; Fish, E.N. The yin and yang of viruses and interferons. Trends Immunol. 2012, 33, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Dickow, J.; Francois, S.; Kaiserling, R.-L.; Malyshkina, A.; Drexler, I.; Westendorf, A.M.; Lang, K.S.; Santiago, M.L.; Dittmer, U.; Sutter, K. Diverse Immunomodulatory Effects of Individual IFN-α Subtypes on Virus-Specific CD8+ T Cell Responses. Front. Immunol. 2019, 10, 2255. [Google Scholar] [CrossRef] [PubMed]
- Benedicenti, O.; Wang, T.; Morel, E.; Secombes, C.J.; Soleto, I.; Díaz-Rosales, P.; Tafalla, C. Type I Interferon Regulates the Survival and Functionality of B Cells in Rainbow Trout. Front. Immunol. 2020, 11, 1494. [Google Scholar] [CrossRef] [PubMed]
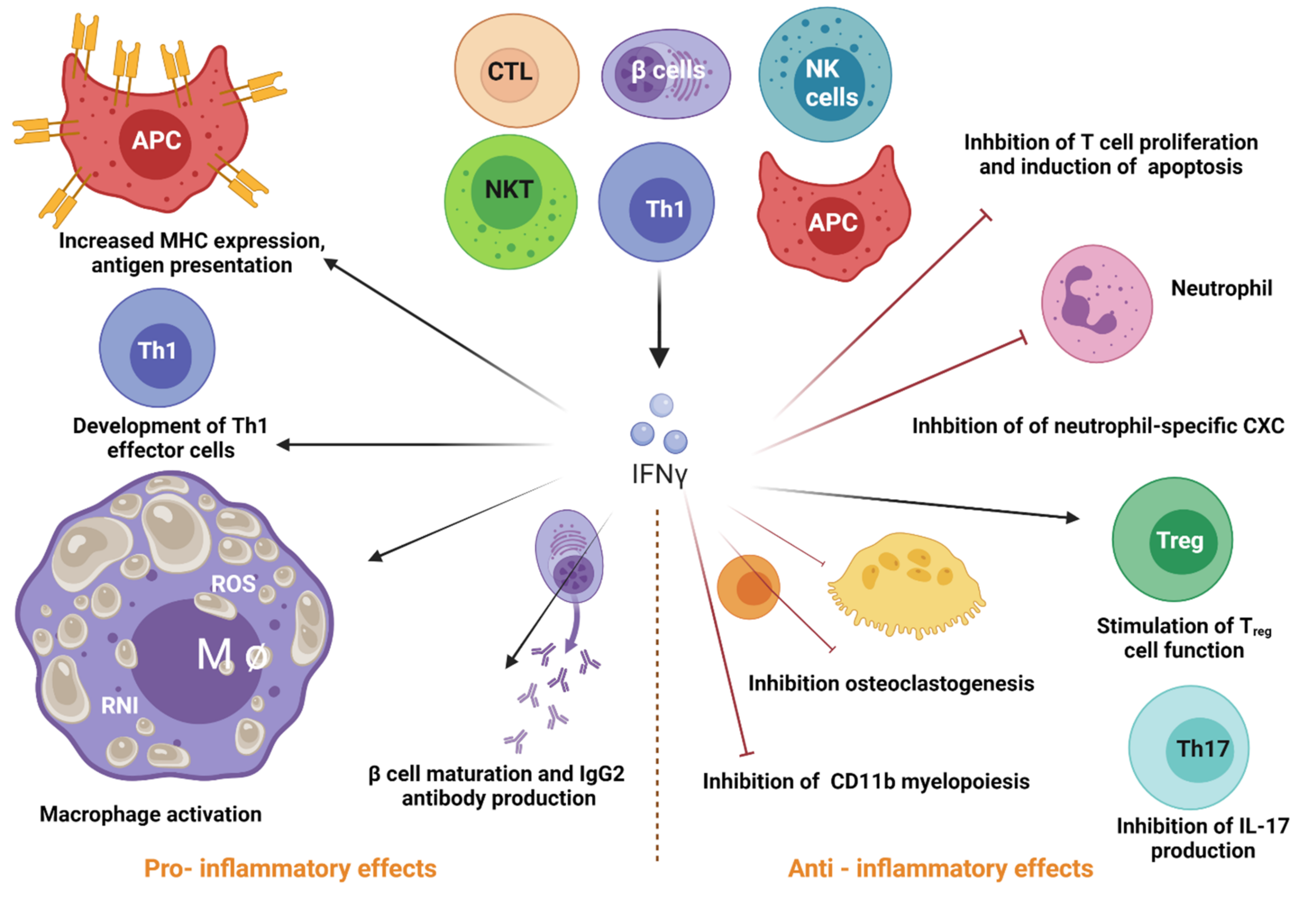
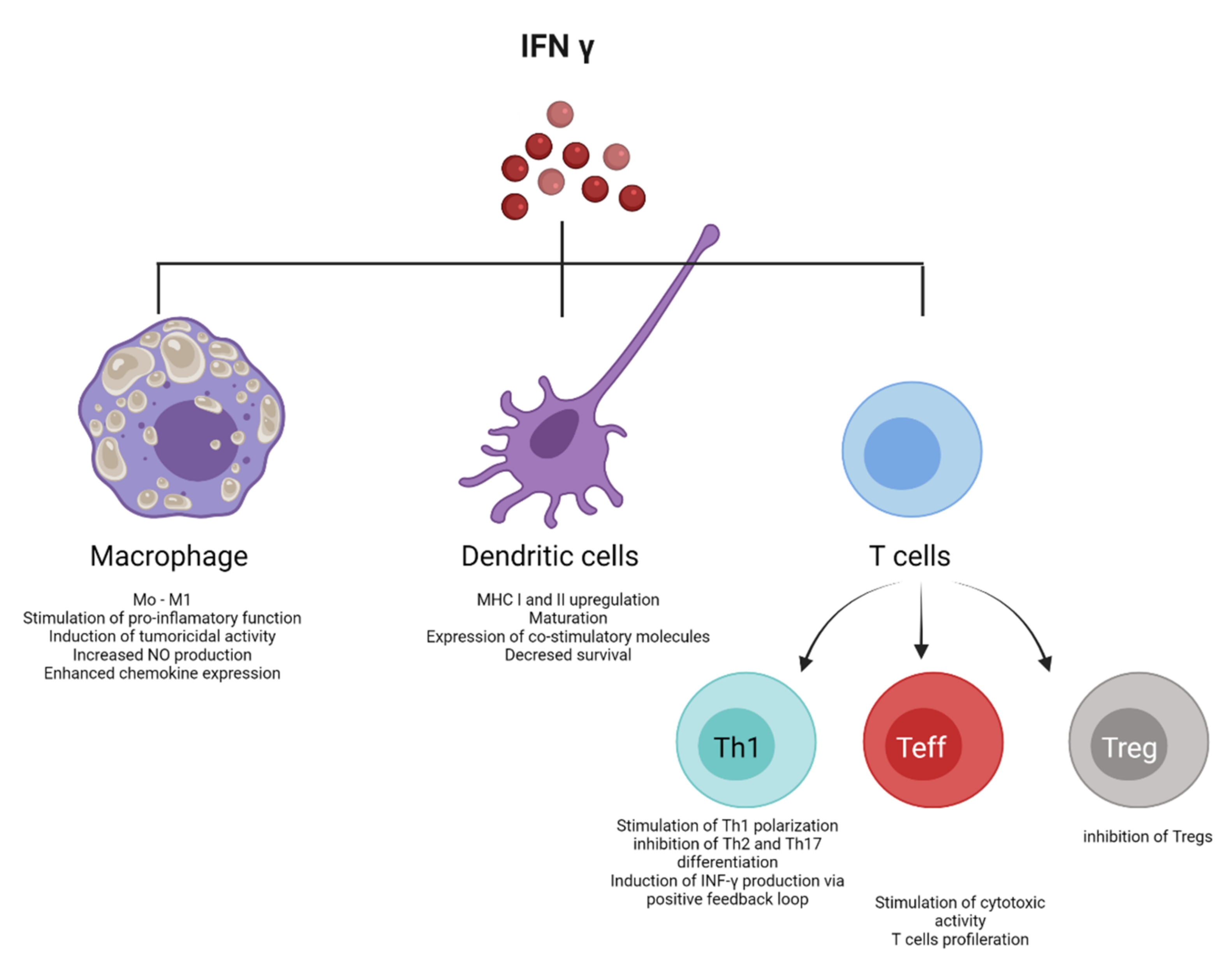
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon gamma and Its Important Roles in Promoting and Inhibiting Spontaneous and Therapeutic Cancer Immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Goldszmid, R.S.; Caspar, P.; Rivollier, A.; White, S.; Dzutsev, A.; Hieny, S.; Kelsall, B.; Trinchieri, G.; Sher, A. NK Cell-Derived Interferon-γ Orchestrates Cellular Dynamics and the Differentiation of Monocytes into Dendritic Cells at the Site of Infection. Immunity 2012, 36, 1047–1059. [Google Scholar] [CrossRef]
- Malone, R.M.; Cox, B.C.; Frogget, B.C.; Kaufman, M.I.; Tibbitts, A.; Tunnell, T.W.; Evans, S.C.; Herrmann, H.W.; Kim, Y.H.; Mack, J.M. Overview of the gamma reaction history diagnostic for the National Ignition Facility (NIF). In Proceedings of the International Optical Design Conference, Jackson Hole, WY, USA, 13–17 June 2010; p. ITuC3. [Google Scholar]
- Kaskow, B.J.; Baecher-Allan, C. Effector T Cells in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a029025. [Google Scholar] [CrossRef]
- Green, D.S.; Young, H.A.; Valencia, J.C. Current prospects of type II interferon γ signaling and autoimmunity. J. Biol. Chem. 2017, 292, 13925–13933. [Google Scholar] [CrossRef] [PubMed]
- Stifter, S.A.; Bhattacharyya, N.; Pillay, R.; Flórido, M.; Triccas, J.A.; Britton, W.J.; Feng, C.G. Functional Interplay between Type I and II InterferonsIs Essential to Limit Influenza A Virus-Induced Tissue Inflammation. PLoS Pathog. 2016, 12, e1005378. [Google Scholar] [CrossRef]
- Fenimore, J.; Young, H.A. Regulation of IFN-γ expression. In Regulation of Cytokine Gene Expression in Immunity and Diseases; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–19. [Google Scholar] [CrossRef]
- Borst, K.; Flindt, S.; Blank, P.; Larsen, P.-K.; Chhatbar, C.; Skerra, J.; Spanier, J.; Hirche, C.; König, M.; Alanentalo, T. Selective reconstitution of IFN-γ gene function in Ncr1+ NK cells is sufficient to control systemic vaccinia virus infection. PLOS Pathog. 2020, 16, e1008279. [Google Scholar] [CrossRef]
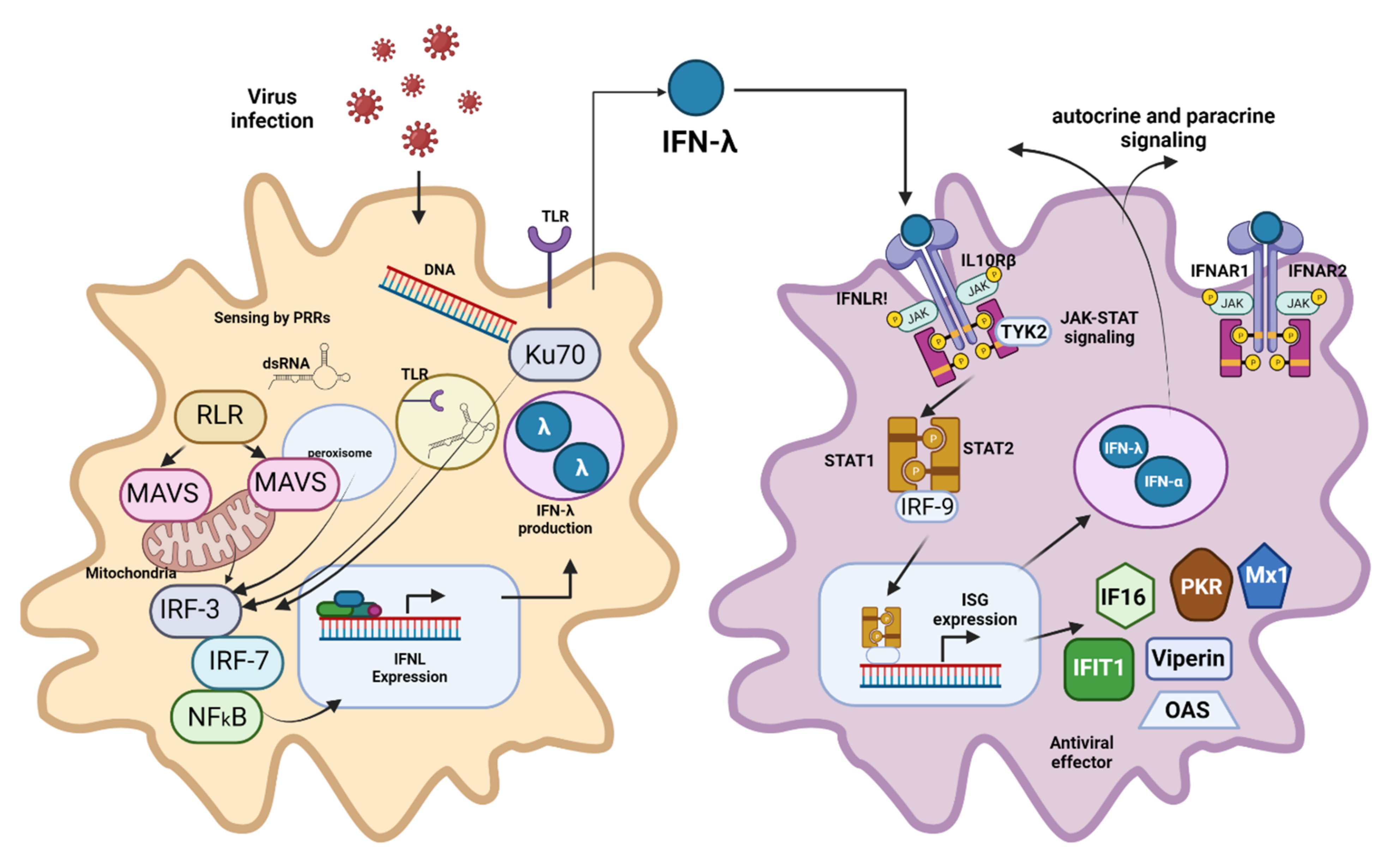
- Kotenko, S.V.; Gallagher, G.; Baurin, V.V.; Lewis-Antes, A.; Shen, M.; Shah, N.K.; Langer, J.A.; Sheikh, F.; Dickensheets, H.; Donnelly, R.P. IFN-λs mediate antiviral protection through a distinct class II cytokine receptor complex. Nat. Immunol. 2003, 4, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, P.; Kindsvogel, W.; Xu, W.; Henderson, K.; Schlutsmeyer, S.; Whitmore, T.E.; Kuestner, R.; Garrigues, U.; Birks, C.; Roraback, J. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat. Immunol. 2003, 4, 63–68. [Google Scholar] [CrossRef]
- Park, A.; Iwasaki, A. Type I and Type III Interferons—Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe 2020, 27, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Wang, L.; Song, L. The primitive interferon-like system and its antiviral function in molluscs. Dev. Comp. Immunol. 2021, 118, 103997. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, N.; Zdebik, A.; Bogusławska, J. Interferon Alpha 2a and 2b in Ophthalmology: A Review. J. Interf. Cytokine Res. 2019, 39, 259–272. [Google Scholar] [CrossRef]
- Lebbe, C.; Garbe, C.; Stratigos, A.J.; Harwood, C.; Peris, K.; del Marmol, V.; Malvehy, J.; Zalaudek, I.; Hoeller, C.; Dummer, R. Diagnosis and treatment of Kaposi’s sarcoma: European consensus-based interdisciplinary guideline (EDF/EADO/EORTC). Eur. J. Cancer 2019, 114, 117–127. [Google Scholar] [CrossRef]
- Lampertico, P.A.K.; Berg, T.; Buti, M.; Janssen, H.L.A.; Papatheodoridis, G.; Zoulim, F.; Tacke, F. Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Maughan, A.; Ogbuagu, O. Pegylated interferon alpha 2a for the treatment of hepatitis C virus infection. Expert Opin. Drug Metab. Toxicol. 2018, 14, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.N.; Westfechtel, L.; Dressler, C.; Nast, A. Anogenital warts and other HPV-associated anogenital lesions in the HIV-positive patient: A systematic review and meta-analysis of the efficacy and safety of interventions assessed in controlled clinical trials. Sex. Transm. Infect. 2017, 93, 543–550. [Google Scholar] [CrossRef]
- Chen, Y.C.; Figliozzi, R.W.; Hsia, S.V. Pilot Analyses of Interferon Subtype Expression Profiles in Patients with Herpes Zoster or Postherpetic Neuralgia. Viral Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.A.; Ravandi, F. How I manage patients with hairy cell leukaemia. Br. J. Haematol. 2017, 177, 543–556. [Google Scholar] [CrossRef]
- Jakimovski, D.; Kolb, C.; Ramanathan, M.; Zivadinov, R.; Weinstock-Guttman, B. Interferon β for Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a032003. [Google Scholar] [CrossRef] [PubMed]
- Abdolvahab, M.H.; Mofrad, M.; Schellekens, H. Interferon Beta: From Molecular Level to Therapeutic Effects. Int. Rev. Cell Mol. Biol. 2016, 326, 343–372. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Goncalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Baldridge, M.T. Interferon-Lambda: A Potent Regulator of Intestinal Viral Infections. Front. Immunol. 2017, 8, 749. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodriguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, A.; Harris, J.M. Improvements in protein PEGylation: Pegylated interferons for treatment of hepatitis C. J. Control. Release 2001, 72, 217–224. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Z.; Tian, H.; Chen, X. Production and clinical development of nanoparticles for gene delivery. Mol. Ther. Methods Clin. Dev. 2016, 3, 16023. [Google Scholar] [CrossRef]
- Cocco, E.; Marrosu, M.G. Profile of PEGylated interferon beta in the treatment of relapsing-remitting multiple sclerosis. Ther. Clin. Risk Manag. 2015, 11, 759–766. [Google Scholar] [CrossRef][Green Version]
- Beilharz, M.W.; Cummins, M.J.; Bennett, A.L.; Cummins, J.M. Oromucosal Administration of Interferon to Humans. Pharmaceuticals 2010, 3, 323–344. [Google Scholar] [CrossRef]
- Conlon, K.C.; Miljković, M.D.; Waldmann, T.A. Cytokines in the Treatment of Cancer. J. Interf. Cytokine Res. 2019, 39, 6–21. [Google Scholar] [CrossRef]
- Fagundes, R.N.; Ferreira, L.; Pace, F.H.L. Health-related quality of life and fatigue in patients with chronic hepatitis C with therapy with direct-acting antivirals agents interferon-free. PLoS ONE 2020, 15, e0237005. [Google Scholar] [CrossRef]
- Platis, D.; Foster, G.R. Interferon Proteins: Structure, Production and Purification. In The Interferons; Wiley: Hoboken, NJ, USA, 2006; pp. 73–83. [Google Scholar]
- Castro, L.S.; Lobo, G.S.; Pereira, P.; Freire, M.G.; Neves, M.C.; Pedro, A.Q. Interferon-Based Biopharmaceuticals: Overview on the Production, Purification, and Formulation. Vaccines 2021, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, L.; Martín, L.; Mangues, R.; Ferrer-Miralles, N.; Vázquez, E.; Villaverde, A. Recombinant pharmaceuticals from microbial cells: A 2015 update. Microb. Cell Fact. 2016, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.R. The Role of Structure in the Biology of Interferon Signaling. Front. Immunol. 2020, 11, 606489. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Lee, M.W.; Wolf, A.J.; Limon, J.J.; Becker, C.A.; Ding, M.; Murali, R.; Lee, E.Y.; Liu, G.Y.; Wong, G.C.L.; et al. Direct Antimicrobial Activity of IFN-β. J. Immunol. 2017, 198, 4036–4045. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.-J.; Xu, S.; Wang, H.-M.; Ling, Y.; Dong, J.; Xia, R.-D.; Sun, X.-H. Nanoparticles: Oral Delivery for Protein and Peptide Drugs. AAPS PharmSciTech 2019, 20, 1–11. [Google Scholar] [CrossRef]
- Lembo, D.; Cavalli, R. Nanoparticulate Delivery Systems for Antiviral Drugs. Antivir. Chem. Chemother. 2010, 21, 53–70. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Pandey, A. Solid lipid nanoparticles: A multidimensional drug delivery system. In Nanoscience in Medicine Vol. 1; Springer: Berlin/Heidelberg, Germany, 2020; pp. 249–295. [Google Scholar]
- Ye, C.; Chi, H. A review of recent progress in drug and protein encapsulation: Approaches, applications and challenges. Mater. Sci. Eng. C 2018, 83, 233–246. [Google Scholar] [CrossRef]
- Sánchez, A.; Tobío, M.; González, L.; Fabra, A.; Alonso, M.J. Biodegradable micro- and nanoparticles as long-term delivery vehicles for interferon-alpha. Eur. J. Pharm. Sci. 2003, 18, 221–229. [Google Scholar] [CrossRef]
- Dai, J.; Long, W.; Liang, Z.; Wen, L.; Yang, F.; Chen, G. A novel vehicle for local protein delivery to the inner ear: Injectable and biodegradable thermosensitive hydrogel loaded with PLGA nanoparticles. Drug Dev. Ind. Pharm. 2018, 44, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Majumder, N.; Das, N.G.; Das, S.K. Polymeric micelles for anticancer drug delivery. Ther. Deliv. 2020, 11, 613–635. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, M.; Sun, J.; Hu, J.; Wang, Z.; Guo, J.; Gao, W. Polymerization Induced Self-Assembly of a Site-Specific Interferon α-Block Copolymer Conjugate into Micelles with Remarkably Enhanced Pharmacology. J. Am. Chem. Soc. 2018, 140, 10435–10438. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, J.; Liu, X.; Sun, J.; Gao, W. Temperature-triggered micellization of interferon alpha-diblock copolypeptide conjugate with enhanced stability and pharmacology. J. Control. Release 2020, 328, 444–453. [Google Scholar] [CrossRef]
- Zazo, H.; Colino, C.I.; Lanao, J.M. Current applications of nanoparticles in infectious diseases. J. Control. Release 2016, 224, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Kang, P.M. Systemic Review of Biodegradable Nanomaterials in Nanomedicine. Nanomaterials 2020, 10, 656. [Google Scholar] [CrossRef]
- Lembo, D.; Donalisio, M.; Civra, A.; Argenziano, M.; Cavalli, R. Nanomedicine formulations for the delivery of antiviral drugs: A promising solution for the treatment of viral infections. Expert Opin. Drug Deliv. 2018, 15, 93–114. [Google Scholar] [CrossRef]
- Thitinan, S.; McConville, J.T. Interferon alpha delivery systems for the treatment of hepatitis C. Int. J. Pharm. 2009, 369, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Turecek, P.L.; Bossard, M.J.; Schoetens, F.; Ivens, I.A. PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J. Pharm. Sci. 2016, 105, 460–475. [Google Scholar] [CrossRef]
- Delgado, C.; Francis, G.E.; Fisher, D. The uses and properties of PEG-linked proteins. Crit. Rev. Ther. Drug Carr. Syst. 1992, 9, 249–304. [Google Scholar]
- Nucci, M.L.; Shorr, R.; Abuchowski, A. The therapeutic value of poly(ethylene glycol)-modified proteins. Adv. Drug Deliv. Rev. 1991, 6, 133–151. [Google Scholar] [CrossRef]
- Foster, G.R. Pegylated Interferons for the Treatment of Chronic Hepatitis C. Drugs 2010, 70, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Mero, A. The Impact of PEGylation on Biological Therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Ramon, J.; Saez, V.; Báez, R.; Aldana, R.; Hardy, E. PEGylated Interferon-α2b: A Branched 40K Polyethylene Glycol Derivative. Pharm. Res. 2005, 22, 1375–1387. [Google Scholar] [CrossRef]
- Basu, A.; Yang, K.; Wang, M.; Liu, S.; Chintala, R.; Palm, T.; Zhao, H.; Peng, P.; Wu, D.; Zhang, Z.; et al. Structure−Function Engineering of Interferon-β-1b for Improving Stability, Solubility, Potency, Immunogenicity, and Pharmacokinetic Properties by Site-Selective Mono-PEGylation. Bioconj. Chem. 2006, 17, 618–630. [Google Scholar] [CrossRef]
- Fee, C.J. Size comparison between proteins PEGylated with branched and linear poly(ethylene glycol) molecules. Biotechnol. Bioeng. 2007, 98, 725–731. [Google Scholar] [CrossRef]
- Asselah, T.; Lada, O.; Moucari, R.; Martinot, M.; Boyer, N.; Marcellin, P. Interferon Therapy for Chronic Hepatitis B. Clin. Liver Dis. 2007, 11, 839–849. [Google Scholar] [CrossRef]
- Trinh, V.A.; Zobniw, C.; Hwu, W.-J. The efficacy and safety of adjuvant interferon-alfa therapy in the evolving treatment landscape for resected high-risk melanoma. Expert Opin. Drug Saf. 2017, 16, 933–940. [Google Scholar] [CrossRef]
- Jansen, P.L.; De Bruijne, J. Controlled-release interferon alpha 2b, a new member of the interferon family for the treatment of chronic hepatitis C. Expert Opin. Investig. Drugs 2012, 21, 111–118. [Google Scholar] [CrossRef]
- Woo, A.S.J.; Kwok, R.; Ahmed, T. Alpha-interferon treatment in hepatitis B. Ann. Transl. Med. 2017, 5, 159. [Google Scholar] [CrossRef]
- Fam, C.M.; Eisenberg, S.P.; Carlson, S.J.; Chlipala, E.A.; Cox, G.N.; Rosendahl, M.S. PEGylation Improves the Pharmacokinetic Properties and Ability of Interferon Gamma to Inhibit Growth of a Human Tumor Xenograft in Athymic Mice. J. Interferon Cytokine Res. 2014, 34, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Boulestin, A.; Kamar, N.; Sandres-Saune, K.; Alric, L.; Vinel, J.-P.; Rostaing, L.; Izopet, J. Pegylation of IFN-α and Antiviral Activity. J. Interf. Cytokine Res. 2006, 26, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Meller, S.; Gerber, P.; Kislat, A.; Hevezi, P.; Göbel, T.; Wiesner, U.; Kellermann, S.; Bünemann, E.; Zlotnik, A.; Häussinger, D. Allergic sensitization to pegylated interferon-α results in drug eruptions. Allergy 2015, 70, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hu, J.; Gao, W. Tuning the molecular size of site-specific interferon-polymer conjugate for optimized antitumor efficacy. Sci. China Mater. 2017, 60, 563–570. [Google Scholar] [CrossRef]
- Bewersdorf, J.P.; Giri, S.; Wang, R.; Podoltsev, N.; Williams, R.T.; Rampal, R.K.; Tallman, M.S.; Zeidan, A.M.; Stahl, M. Interferon Therapy in Myelofibrosis: Systematic Review and Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2020, 20, e712–e723. [Google Scholar] [CrossRef]
- Schlapschy, M.; Binder, U.; Börger, C.; Theobald, I.; Wachinger, K.; Kisling, S.; Haller, D.; Skerra, A. PASylation: A biological alternative to PEGylation for extending the plasma half-life of pharmaceutically active proteins. Protein Eng. Des. Sel. 2013, 26, 489–501. [Google Scholar] [CrossRef]
- Binder, U.; Skerra, A. PASylation®: A versatile technology to extend drug delivery. Curr. Opin. Colloid Interface Sci. 2017, 31, 10–17. [Google Scholar] [CrossRef]
- Shamloo, A.; Rostami, P.; Mahmoudi, A. PASylation Enhances the Stability, Potency, and Plasma Half-Life of Interferon α-2a: A Molecular Dynamics Simulation. Biotechnol. J. 2020, 15, 1900385. [Google Scholar] [CrossRef]
- Zvonova, E.A.; Ershov, A.V.; Ershova, O.A.; Sudomoina, M.A.; Degterev, M.B.; Poroshin, G.N.; Eremeev, A.V.; Karpov, A.P.; Vishnevsky, A.Y.; Goldenkova-Pavlova, I.V.; et al. PASylation technology improves recombinant interferon-β1b solubility, stability, and biological activity. Appl. Microbiol. Biotechnol. 2017, 101, 1975–1987. [Google Scholar] [CrossRef]
- Xia, Y.; Schlapschy, M.; Morath, V.; Roeder, N.; Vogt, E.I.; Stadler, D.; Cheng, X.; Dittmer, U.; Sutter, K.; Heikenwalder, M.; et al. PASylated interferon α efficiently suppresses hepatitis B virus and induces anti-HBs seroconversion in HBV-transgenic mice. Antivir. Res. 2019, 161, 134–143. [Google Scholar] [CrossRef]
- Abbina, S.; Parambath, A. PEGylation and its alternatives: A summary. In Engineering of Biomaterials for Drug Delivery Systems; Parambath, A., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 363–376. [Google Scholar]
- Vahed, S.Z.; Salehi, R.; Davaran, S.; Sharifi, S. Liposome-based drug co-delivery systems in cancer cells. Mater. Sci. Eng. C 2017, 71, 1327–1341. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Zakeri-Milani, P.; Baradaran, B. Liposome and immune system interplay: Challenges and potentials. J. Control. Release 2019, 305, 194–209. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Gurari-Rotman, D.; Lelkes, P.I. Encapsulation of human fibroblast interferon activity in liposomes. Biochem. Biophys. Res. Commun. 1982, 107, 136–143. [Google Scholar] [CrossRef]
- Eppstein, D.A.; Marsh, Y.V.; van der Pas, M.; Felgner, P.L.; Schreiber, A.B. Biological activity of liposome-encapsulated murine interferon gamma is mediated by a cell membrane receptor. Proc. Natl. Acad. Sci. USA 1985, 82, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Sone, S.; Tandon, P.; Utsugi, T.; Ogawara, M.; Shimizu, E.; Nii, A.; Ogura, T. Synergism of recombinant human interferon gamma with liposome-encapsulated muramyl tripeptide in activation of the tumoricidal properties of human monocytes. Int. J. Cancer 1986, 38, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A.; Nayar, R. Encapsulation is not involved in the activities of recombinant gamma interferon associated with multilamellar phospholipid liposomes on murine bone marrow-derived macrophages. Lymphokine Res. 1989, 8, 415–425. [Google Scholar]
- Coradini, D.; Pellizzaro, C.; Biffi, A.; Lombardi, L.; Pirronello, E.; Riva, L.; Di Fronzo, G. Effect of liposome-encapsulated alpha- or beta-interferon on breast cancer cell lines. Anticancer Res. 1998, 18, 177–182. [Google Scholar]
- Killion, J.J.; Fan, D.; Bucana, C.D.; Frangos, D.N.; Price, J.E.; Fidler, I.J. Augmentation of Antiproliferative Activity of Interferon Alfa Against Human Bladder Tumor Cell Lines by Encapsulation of Interferon Alfa Within Liposomes. J. Natl. Cancer Inst. 1989, 81, 1387–1392. [Google Scholar] [CrossRef]
- Mellors, J.W.; Debs, R.J.; Ryan, J.L. Incorporation of recombinant gamma interferon into liposomes enhances its ability to induce peritoneal macrophage antitoxoplasma activity. Infect. Immun. 1989, 57, 132–137. [Google Scholar] [CrossRef]
- Goldbach, P.; Dumont, S.; Kessler, R.; Poindron, P.; Stamm, A. In situ activation of mouse alveolar macrophages by aerosolized liposomal IFN-gamma and muramyl tripeptide. Am. J. Physiol. Cell. Mol. Physiol. 1996, 270, L429–L434. [Google Scholar] [CrossRef] [PubMed]
- Rutenfranz, I.; Bauer, A.; Kirchner, H. Interferon gamma encapsulated into liposomes enhances the activity of monocytes and natural killer cells and has antiproliferative effects on tumor cells in vitro. Blut 1990, 61, 30–37. [Google Scholar] [CrossRef]
- Rutenfranz, I.; Bauer, A.; Kirchner, H. Pharmacokinetic Study of Liposome-Encapsulated Human Interferon-γ after Intravenous and Intramuscular Injection in Mice. J. Interf. Res. 1990, 10, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Saravolac, E.; Kournikakis, B.; Gorton, L.; Wong, J. Effect of liposome-encapsulation on immunomodulating and antiviral activities of interferon-γ. Antivir. Res. 1996, 29, 199–207. [Google Scholar] [CrossRef]
- Vyas, S.; Rawat, M.; Rawat, A.; Mahor, S.; Gupta, P. Pegylated Protein Encapsulated Multivesicular Liposomes: A Novel Approach for Sustained Release of Interferon α. Drug Dev. Ind. Pharm. 2006, 32, 699–707. [Google Scholar] [CrossRef]
- Yang, L.; Yang, W.; Bi, D.; Zeng, Q. A novel method to prepare highly encapsulated interferon-alpha-2b containing liposomes for intramuscular sustained release. Eur. J. Pharm. Biopharm. 2006, 64, 9–15. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.; Cheng, G.; Wei, H.-Y.; Zeng, Q. Encapsulation, pharmacokinetics and tissue distribution of interferon α-2b liposomes after intramuscular injection to rats. Arch. Pharmacal Res. 2011, 34, 941–948. [Google Scholar] [CrossRef]
- Li, F.; Li, Q.H.; Wang, J.Y.; Zhan, C.Y.; Xie, C.; Lu, W.Y. Effects of interferon-gamma liposomes targeted to platelet-derived growth factor receptor–beta on hepatic fibrosis in rats. J. Control. Release 2012, 159, 261–270. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Basnet, P.; Acharya, G.; Škalko-Basnet, N. PEGylated liposomes for topical vaginal therapy improve delivery of interferon alpha. Eur. J. Pharm. Biopharm. 2017, 113, 132–139. [Google Scholar] [CrossRef]
- Shamshiri, M.K.; Jaafari, M.R.; Badiee, A. Preparation of liposomes containing IFN-gamma and their potentials in cancer immunotherapy: In vitro and in vivo studies in a colon cancer mouse model. Life Sci. 2021, 264, 118605. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.C.I.M.; Butt, A.M.; Amjad, M.W.; Kesharwani, P. Chapter 5—Polymeric Micelles for Drug Targeting and Delivery. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Mishra, V., Kesharwani, P., Mohd Amin, M.C.I., Iyer, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 167–202. [Google Scholar]
- Bastakoti, B.P.; Liu, Z. Chapter 10—Multifunctional polymeric micelles as therapeutic nanostructures: Targeting, imaging, and triggered release. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 261–283. [Google Scholar]
- Liu, J.; Ai, X.; Zhang, H.; Zhuo, W.; Mi, P. Polymeric Micelles with Endosome Escape and Redox-Responsive Functions for Enhanced Intracellular Drug Delivery. J. Biomed. Nanotechnol. 2019, 15, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Movassaghian, S.; Merkel, O.M.; Torchilin, V.P. Applications of polymer micelles for imaging and drug delivery. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.K.; Meireles, M.A.A. Encapsulation of Food Compounds Using Supercritical Technologies: Applications of Supercritical Carbon Dioxide as an Antisolvent. Food Public Health 2014, 4, 247–258. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Paulo, F.; Santos, L. Design of experiments for microencapsulation applications: A review. Mater. Sci. Eng. C 2017, 77, 1327–1340. [Google Scholar] [CrossRef]
- Andrade, C. Sustained-Release, Extended-Release, and Other Time-Release Formulations in Neuropsychiatry. J. Clin. Psychiatry 2015, 76, e995–e999. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, N.V.N.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ogura, Y.; Tabata, Y.; Kimura, H.; Wiedemann, P.; Honda, Y. Drug delivery systems for vitreoretinal diseases. Prog. Retin. Eye Res. 2004, 23, 253–281. [Google Scholar] [CrossRef]
- Saez, V.; Ramon, J.; Peniche, C.; Hardy, E. Microencapsulation of Alpha Interferons in Biodegradable Microspheres. J. Interf. Cytokine Res. 2012, 32, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Deng, X.; He, S.; Li, X.; Jia, W.; Wei, D.; Zhang, Z.; Ma, J. Study on biodegradable microspheres containing recombinant interferon-α-2a. J. Pharm. Pharmacol. 2002, 54, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Diwan, M.; Park, T.G. Stabilization of recombinant interferon-α by pegylation for encapsulation in PLGA microspheres. Int. J. Pharm. 2003, 252, 111–122. [Google Scholar] [CrossRef]
- De Leede, L.G.; Humphries, J.E.; Bechet, A.C.; Van Hoogdalem, E.J.; Verrijk, R.; Spencer, D.G. Novel Controlled-Release Lemna-Derived IFN-α2b (Locteron): Pharmacokinetics, Pharmacodynamics, and Tolerability in a Phase I Clinical Trial. J. Interf. Cytokine Res. 2008, 28, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, J.S.; Parveen, S.; Saif, R.U. New Horizons and Perspectives in the Management of Chronic Hepatitis C. JMS Ski. 2010, 13, 41–47. [Google Scholar] [CrossRef]
- Di Bisceglie, A.; Ghalib, R.; Hamzeh, F.; Rustgi, V. Early virologic response after peginterferon alpha-2a plus ribavirin or peginterferon alpha-2b plus ribavirin treatment in patients with chronic hepatitis C. J. Viral Hepat. 2007, 14, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.J.; Teh, A.Y.; Twyman, R.M.; Ma, J.K. Target Product Selection—Where Can Molecular Pharming Make the Difference? Curr. Pharm. Des. 2013, 19, 5478–5485. [Google Scholar] [CrossRef]
- Pawlotsky, J.M. New Hepatitis C Virus (HCV) Drugs and the Hope for a Cure: Concepts in Anti-HCV Drug Development. Semin. Liver Dis. 2014, 34, 22–29. [Google Scholar] [CrossRef][Green Version]
- Vermehren, J.; Sarrazin, C. New hepatitis C therapies in clinical development. Eur. J. Med. Res. 2011, 16, 303. [Google Scholar] [CrossRef] [PubMed]
- Saez, V.; Ramón, J.; Aldana, R.; Pérez, D.; Hardy, E. Microencapsulation of recombinant interferon α-2b into poly (D, L-lactide-co-glycolide) microspheres. Biotecnol. Apl. 2008, 25, 31–41. [Google Scholar]
- Zhang, Y.-M.; Yang, F.; Yang, Y.-Q.; Song, F.-L.; Xu, A.-L. Recombinant interferon-alpha2b poly(lactic-co-glycolic acid) microspheres: Pharmacokinetics-pharmacodynamics study in rhesus monkeys following intramuscular administration. Acta Pharmacol. Sin. 2008, 29, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.H.; Yu, H.Y.; Gao, J.Q.; Sun, X.Y.; Liang, W.Q. Hydrophilic biodegradable microspheres of interferon-alpha and its pharmacokinetics in mice. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 85, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, J.; Sun, L.; Dai, Y.; Liu, L.; Li, X.; Wang, J.; Weng, J.; Jia, W.; Zhang, Z. Preparation and characterization of interferon-loaded magnetic biodegradable microspheres. J. Biomed. Mater. Res. Part B Appl. Biomater. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2008, 87B, 189–196. [Google Scholar] [CrossRef]
- Yang, F.; Song, F.-L.; Pan, Y.-F.; Wang, Z.-Y.; Yang, Y.-Q.; Zhao, Y.-M.; Liang, S.-Z.; Zhang, Y.-M. Preparation and characteristics of interferon-alpha poly(lactic-co-glycolic acid) microspheres. J. Microencapsul. 2010, 27, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, L.; Liu, Y.; Zhang, H.; Li, X.; Luo, F.; Mei, X. Development of interferon alpha-2b microspheres with constant release. Int. J. Pharm. 2011, 410, 48–53. [Google Scholar] [CrossRef]
- Gulia, M.; Rai, S.; Jain, U.K.; Katare, O.P.; Katyal, A.; Madan, J. Sustained-release protamine sulphate-impregnated microspheres may reduce the frequent administration of recombinant interferon alpha-2b in ovarian cancer: In-vitro characterization. AntiCancer Drugs 2014, 25, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, Y.; Zhang, S.; Gao, Y. Dissolving microneedle-based intradermal delivery of interferon-α-2b. Drug Dev. Ind. Pharm. 2016, 42, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Kamei, N.; Morishita, M.; Chiba, H.; Kavimandan, N.J.; Peppas, N.A.; Takayama, K. Complexation hydrogels for intestinal delivery of interferon β and calcitonin. J. Control. Release 2009, 134, 98–102. [Google Scholar] [CrossRef]
- Kondiah, P.P.; Tomar, L.K.; Tyagi, C.; Choonara, Y.E.; Modi, G.; du Toit, L.C.; Kumar, P.; Pillay, V. A novel pH-sensitive interferon-β (INF-β) oral delivery system for application in multiple sclerosis. Int. J. Pharm. 2013, 456, 459–472. [Google Scholar] [CrossRef]
- Cleland, J.L.; Jones, A.J. Stable Formulations of Recombinant Human Growth Hormone and Interferon-gamma for Microencapsulation in Biodegradable Mircospheres. Pharm. Res. 1996, 13, 1464–1475. [Google Scholar] [CrossRef]
- Conway, B.R.; Alpar, H. Single and Coencapsulation of lnterferon-γ in Biodegradable PLA Microspheres for Optimization of Multicomponent Vaccine Delivery Vehicles. Drug Deliv. 1997, 4, 75–80. [Google Scholar] [CrossRef]
- Eyles, J.; Alpar, H.; Conway, B.R.; Keswick, M. Oral Delivery and Fate of Poly(lactic acid) Microsphere-encapsulated Interferon in Rats. J. Pharm. Pharmacol. 1997, 49, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cleland, J.L. Factors Affecting the in Vitro Release of Recombinant Human Interferon-γ (rhIFN-γ) from PLGA Microspheres. J. Pharm. Sci. 1997, 86, 908–914. [Google Scholar] [CrossRef]
- Liang, J.; Li, F.; Fang, Y.; Yang, W.; An, X.; Zhao, L.; Xin, Z.; Cao, L.; Hu, Q. Synthesis, characterization and cytotoxicity studies of chitosan-coated tea polyphenols nanoparticles. Colloids Surf. B Biointerfaces 2011, 82, 297–301. [Google Scholar] [CrossRef]
- Saez, V.; Ramon, J.A.; Caballero, L.; Aldana, R.; Cruz, E.; Peniche, C.; Paez, R. Extraction of PLGA-Microencapsulated Proteins Using a Two-Immiscible Liquid Phases System Containing Surfactants. Pharm. Res. 2013, 30, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, E.; Furusyo, N.; Kajiwara, E.; Takahashi, K.; Nomura, H.; Tanabe, Y.; Satoh, T.; Maruyama, T.; Nakamuta, M.; Kotoh, K.; et al. Evaluation of the adverse effect of premature discontinuation of pegylated interferon α-2b and ribavirin treatment for chronic hepatitis C virus infection: Results from Kyushu University Liver Disease Study. J. Gastroenterol. Hepatol. 2012, 27, 1233–1240. [Google Scholar] [CrossRef]
- Jain, A.K.; Thareja, S. In vitro and in vivo characterization of pharmaceutical nanocarriers used for drug delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 524–539. [Google Scholar] [CrossRef]
- Aghdam, A.G.; Vossoughi, M.; Almzadeh, I.; Zeinali, M. Bioconjugation of Interferon-alpha Molecules to Lysine-Capped Gold Nanoparticles for Further Drug Delivery Applications. J. Dispers. Sci. Technol. 2008, 29, 1062–1065. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Yang, J.-A.; Jung, H.S.; Beack, S.; Choi, J.E.; Hur, W.; Koo, H.; Kim, K.; Yoon, S.K.; Hahn, S.K. Hyaluronic Acid–Gold Nanoparticle/Interferon α Complex for Targeted Treatment of Hepatitis C Virus Infection. ACS Nano 2012, 6, 9522–9531. [Google Scholar] [CrossRef]
- Zaman, R.; Islam, R.A.; Ibnat, N.; Othman, I.; Zaini, A.; Lee, C.Y.; Chowdhury, E.H. Current strategies in extending half-lives of therapeutic proteins. J. Control. Release 2019, 301, 176–189. [Google Scholar] [CrossRef]
- Giri, N.; Tomar, P.; Karwasara, V.S.; Pandey, R.S.; Dixit, V. Targeted novel surface-modified nanoparticles for interferon delivery for the treatment of hepatitis B. Acta Biochim. Biophys. Sin. 2011, 43, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Feczkó, T.; Fodor-Kardos, A.; Sivakumaran, M.; Haque Shubhra, Q.T. In vitro IFN-α release from IFN-α- and pegylated IFN-α-loaded poly(lactic-co-glycolic acid) and pegylated poly(lactic-co-glycolic acid) nanoparticles. Nanomedicine 2016, 11, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Cánepa, C.; Imperiale, J.C.; Berini, C.A.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Development of a Drug Delivery System Based on Chitosan Nanoparticles for Oral Administration of Interferon-α. Biomacromolecules 2017, 18, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, J.C.; Schlachet, I.; Lewicki, M.; Sosnik, A.; Biglione, M.M. Oral Pharmacokinetics of a Chitosan-Based Nano- Drug Delivery System of Interferon Alpha. Polymers 2019, 11, 1862. [Google Scholar] [CrossRef]
- Kristó, K.; Szekeres, M.; Makai, Z.; Márki, Á.; Kelemen, A.; Bali, L.; Pallai, Z.; Dékány, I.; Csóka, I. Preparation and investigation of core-shell nanoparticles containing human interferon-α. Int. J. Pharm. 2020, 573, 118825. [Google Scholar] [CrossRef] [PubMed]
- Fodor-Kardos, A.; Kiss, Á.F.; Monostory, K.; Feczkó, T. Sustained in vitro interferon-beta release and in vivo toxicity of PLGA and PEG-PLGA nanoparticles. RSC Adv. 2020, 10, 15893–15900. [Google Scholar] [CrossRef]
- González, L.F.; Acuña, E.; Arellano, G.; Morales, P.; Sotomayor, P.; Oyarzun-Ampuero, F.; Naves, R. Intranasal delivery of interferon-β-loaded nanoparticles induces control of neuroinflammation in a preclinical model of multiple sclerosis: A promising simple, effective, non-invasive, and low-cost therapy. J. Control. Release 2021, 331, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Segura, S.; Gamazo, C.; Irache, J.M.; Espuelas, S. Gamma Interferon Loaded onto Albumin Nanoparticles: In Vitro and In Vivo Activities against Brucella abortus. Antimicrob. Agents Chemother. 2007, 51, 1310–1314. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yin, Y.; Hu, Q.; Xu, C.; Qiao, Q.; Qin, X.; Song, Q.; Peng, Y.; Zhao, Y.; Zhang, Z. Co-delivery of Doxorubicin and Interferon-γ by Thermosensitive Nanoparticles for Cancer Immunochemotherapy. Mol. Pharm. 2018, 15, 4161–4172. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Saifullah, M.; Shishir, M.R.I.; Ferdowsi, R.; Tanver Rahman, M.R.; Van Vuong, Q. Micro and nano encapsulation, retention and controlled release of flavor and aroma compounds: A critical review. Trends Food Sci. Technol. 2019, 86, 230–251. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; De Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Smatti, M.K.; Cyprian, F.S.; Nasrallah, G.K.; Al Thani, A.A.; Almishal, R.O.; Yassine, H.M. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses 2019, 11, 762. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Sousa Lobo, J.M. Cytokines and growth factors. Adv. Biochem. Eng. Biotechnol. 2019, 171, 87–113. [Google Scholar] [CrossRef]
- ClinicalTrials.gov-Database. Search of: Interferon|Active, not Recruiting Studies—List Results. Available online: https://clinicaltrials.gov/ct2/results?term=interferon&Search=Apply&recrs=d&age_v=&gndr=&type=&rslt= (accessed on 23 June 2021).
- Zheng, B.; He, M.L.; Wong, K.L.; Ching, T.L.; Poon, L.L.M.; Peng, Y.; Guan, Y.; Lin, M.C.M.; Kung, H.F. Potent Inhibition of SARS-Associated Coronavirus (SCoV) Infection and Replication by Type I Interferons (IFN-α/β) but Not by Type II Interferon (IFN-γ). J. Interf. Cytokine Res. 2004, 24, 388–390. [Google Scholar] [CrossRef]
- De Wilde, A.H.; Raj, V.S.; Oudshoorn, D.; Bestebroer, T.M.; Van Nieuwkoop, S.; Limpens, R.W.A.L.; Posthuma, C.C.; Van Der Meer, Y.; Barcena, M.; Haagmans, B.L.; et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J. Gen. Virol. 2013, 94, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Moezzi, S.M.I.; Mosaddeghi, P.; Nadimi Parashkouhi, S.; Fazel Hoseini, S.M.; Badakhshan, F.; Negahdaripour, M. Interferon-inducer antivirals: Potential candidates to combat COVID-19. Int. Immunopharmacol. 2021, 91, 107245. [Google Scholar] [CrossRef]
- Lavigne, G.M.; Russell, H.; Sherry, B.; Ke, R. Autocrine and paracrine interferon signalling as ‘ring vaccination’ and ‘contact tracing’ strategies to suppress virus infection in a host. Proc. R. Soc. B 2021, 288, 20203002. [Google Scholar] [CrossRef]
- Jones, C.T.; Catanese, M.T.; Law, L.M.J.; Khetani, S.R.; Syder, A.J.; Ploss, A.; Oh, T.S.; Schoggins, J.W.; MacDonald, M.R.; Bhatia, S.N.; et al. Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat. Biotechnol. 2010, 28, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef] [PubMed]
- Bekisz, J.; Baron, S.; Balinsky, C.; Morrow, A.; Zoon, K.C. Antiproliferative Properties of Type I and Type II Interferon. Pharmaceuticals 2010, 3, 994–1015. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V. Evaluation of Routes of Administration of Interferon in Cancer: A Review and a Proposal. Cancer Drug Deliv. 1984, 1, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wang, Q.; Wang, H. New era of drug innovation in China. Acta Pharm. Sin. B 2019, 9, 1084. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Greish, K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 2010, 624, 25–37. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved Antiviral Drugs over the Past 50 Years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Scherzad, A.; Hagen, R.; Hackenberg, S. Current Understanding of Nasal Epithelial Cell Mis-Differentiation. J. Inflamm. Res. 2019, 12, 309–317. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Lei, T.; Qin, L.; Zhou, Y.; Hu, C.; Liu, Q.; Gao, H. The construction of in vitro nasal cavity-mimic M-cell model, design of M cell-targeting nanoparticles and evaluation of mucosal vaccination by nasal administration. Acta Pharm. Sin. B 2020, 10, 1094–1105. [Google Scholar] [CrossRef]
- Bustamante-Marin, X.M.; Ostrowski, L.E. Cilia and Mucociliary Clearance. Cold Spring Harb. Perspect. Biol. 2017, 9, a028241. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T. Axoneme Structure from Motile Cilia. Cold Spring Harb. Perspect. Biol. 2017, 9, a028076. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.C.; Karp, D.A.; Kahwaji, C.I. Physiology, Nasal; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Schmidt, M.E.; Varga, S.M. The CD8 T Cell Response to Respiratory Virus Infections. Front. Immunol. 2018, 9, 678. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A. Exploiting Mucosal Immunity for Antiviral Vaccines. Annu. Rev. Immunol. 2016, 34, 575–608. [Google Scholar] [CrossRef]
- Iwasaki, A.; Foxman, E.F.; Molony, R.D. Early local immune defences in the respiratory tract. Nat. Rev. Immunol. 2017, 17, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, L.; McCloskey, A.P.; Higgins, G.; Ramsey, J.M.; Cryan, S.A.; MacLoughlin, R. Effective nebulization of interferon-γ using a novel vibrating mesh. Respir. Res. 2019, 20, 66. [Google Scholar] [CrossRef]
- Pandey, V.; Gadeval, A.; Asati, S.; Jain, P.; Jain, N.; Roy, R.K.; Tekade, M.; Soni, V.; Tekade, R.K. Formulation strategies for nose-to-brain delivery of therapeutic molecules. Drug Deliv. Syst. 2019, 291–332. [Google Scholar] [CrossRef]
- Tanwar, H.; Sachdeva, R. Transdermal drug delivery system: A review. Int. J. Pharm. Sci. Res. 2016, 7, 2274–2290. [Google Scholar] [CrossRef]
- Shen, K.-L.; Yang, Y.-H. Diagnosis and treatment of 2019 novel coronavirus infection in children: A pressing issue. World J. Pediatr. 2020, 16, 219–221. [Google Scholar] [CrossRef]
- Scott, G. The Use of Interferons in Respiratory Viral Infections. In Viral and Other Infections of the Human Respiratory Tract; Springer: Berlin/Heidelberg, Germany, 1996; pp. 383–396. [Google Scholar]
- Kumaki, Y.; Day, C.W.; Bailey, K.W.; Wandersee, M.K.; Wong, M.-H.; Madsen, J.R.; Madsen, J.S.; Nelson, N.M.; Hoopes, J.D.; Woolcott, J.D. Induction of Interferon-γ-Inducible Protein 10 by SARS-CoV Infection, Interferon Alfacon 1 and Interferon Inducer in Human Bronchial Epithelial Calu-3 Cells and BALB/c Mice. Antivir. Chem. Chemother. 2010, 20, 169–177. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, T.; Li, C.; Chen, X.; Li, L.; Qin, X.; Li, H.; Luo, J. An experimental trial of recombinant human interferon alpha nasal drops to prevent coronavirus disease 2019 in medical staff in an epidemic area. MedRxiv 2020. [Google Scholar] [CrossRef]
- Wang, H.Q.; Ma, L.L.; Jiang, J.D.; Pang, R.; Chen, Y.J.; Li, Y.H. Recombinant human interferon alpha 2b broad-spectrum anti-respiratory viruses pharmacodynamics study in vitro. Yao Xue Xue Bao 2014, 49, 1547–1553. [Google Scholar]
- Jaffe, H.A.; Buhl, R.; Mastrangeli, A.; Holroyd, K.J.; Saltini, C.; Czerski, D.; Jaffe, H.; Kramer, S.; Sherwin, S.; Crystal, R. Organ specific cytokine therapy. Local activation of mononuclear phagocytes by delivery of an aerosol of recombinant interferon-gamma to the human lung. J. Clin. Investig. 1991, 88, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Condos, R.; Hull, F.P.; Schluger, N.W.; Rom, W.N.; Smaldone, G.C. Regional Deposition of Aerosolized Interferon-γ in Pulmonary Tuberculosis. Chest 2004, 125, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.U.; Shehzad, A.; Ahmed, M.B.; Lee, Y.S. Intranasal Delivery of Nanoformulations: A Potential Way of Treatment for Neurological Disorders. Molecules 2020, 25, 1929. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, T.I.; Villacis-Aguirre, C.A.; Santiago Vispo, N.; Santiago Padilla, L.; Pedroso Santana, S.; Parra, N.C.; Alonso, J.R.T. Forms and Methods for Interferon’s Encapsulation. Pharmaceutics 2021, 13, 1533. https://doi.org/10.3390/pharmaceutics13101533
Ramos TI, Villacis-Aguirre CA, Santiago Vispo N, Santiago Padilla L, Pedroso Santana S, Parra NC, Alonso JRT. Forms and Methods for Interferon’s Encapsulation. Pharmaceutics. 2021; 13(10):1533. https://doi.org/10.3390/pharmaceutics13101533
Chicago/Turabian StyleRamos, Thelvia I., Carlos A. Villacis-Aguirre, Nelson Santiago Vispo, Leandro Santiago Padilla, Seidy Pedroso Santana, Natalie C. Parra, and Jorge Roberto Toledo Alonso. 2021. "Forms and Methods for Interferon’s Encapsulation" Pharmaceutics 13, no. 10: 1533. https://doi.org/10.3390/pharmaceutics13101533
APA StyleRamos, T. I., Villacis-Aguirre, C. A., Santiago Vispo, N., Santiago Padilla, L., Pedroso Santana, S., Parra, N. C., & Alonso, J. R. T. (2021). Forms and Methods for Interferon’s Encapsulation. Pharmaceutics, 13(10), 1533. https://doi.org/10.3390/pharmaceutics13101533