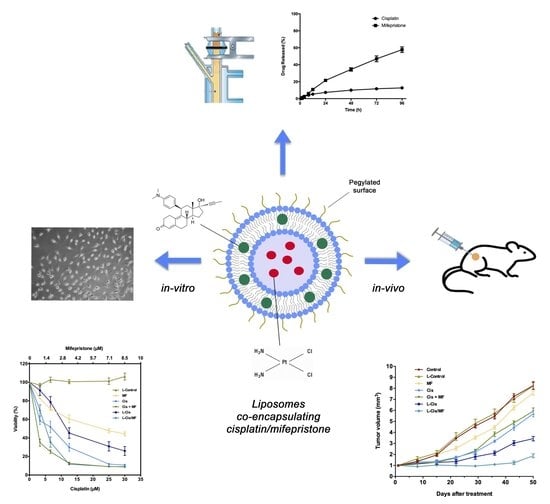
Liposomes Co-Encapsulating Cisplatin/Mifepristone Improve the Effect on Cervical Cancer: In Vitro and In Vivo Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Preparation of Cisplatin/Mifepristone-Loaded Liposomes
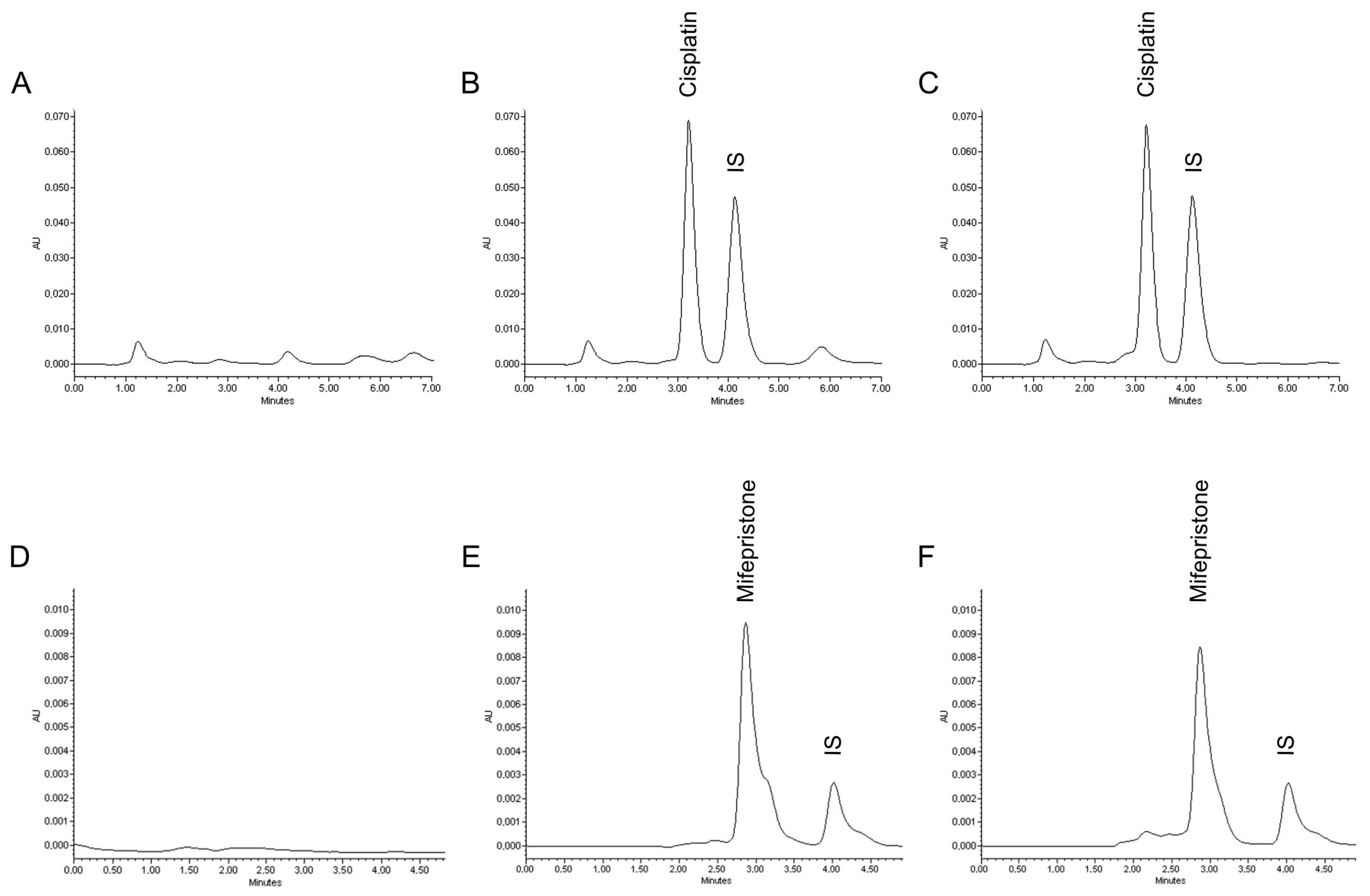
2.3. Characterization of Cisplatin/Mifepristone-Loaded Liposomes
2.4. Studies in Cervical Cancer Cell Line
2.5. Animals, Tumor Xenografts and Systemic Toxicity
2.6. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Cisplatin/Mifepristone-Loaded Liposomes
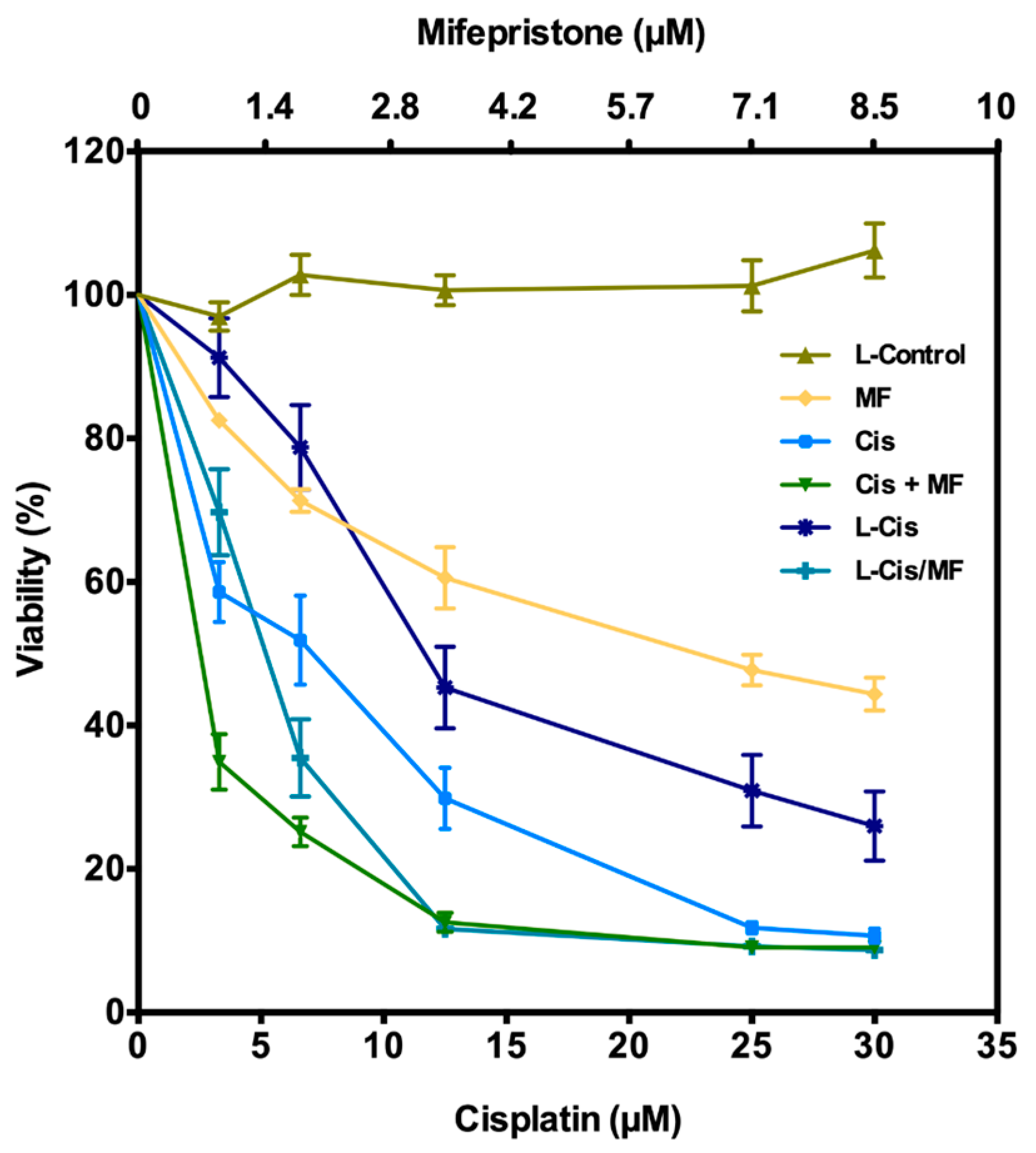
3.2. Post-Treatment Growth Inhibition of HeLa Cells
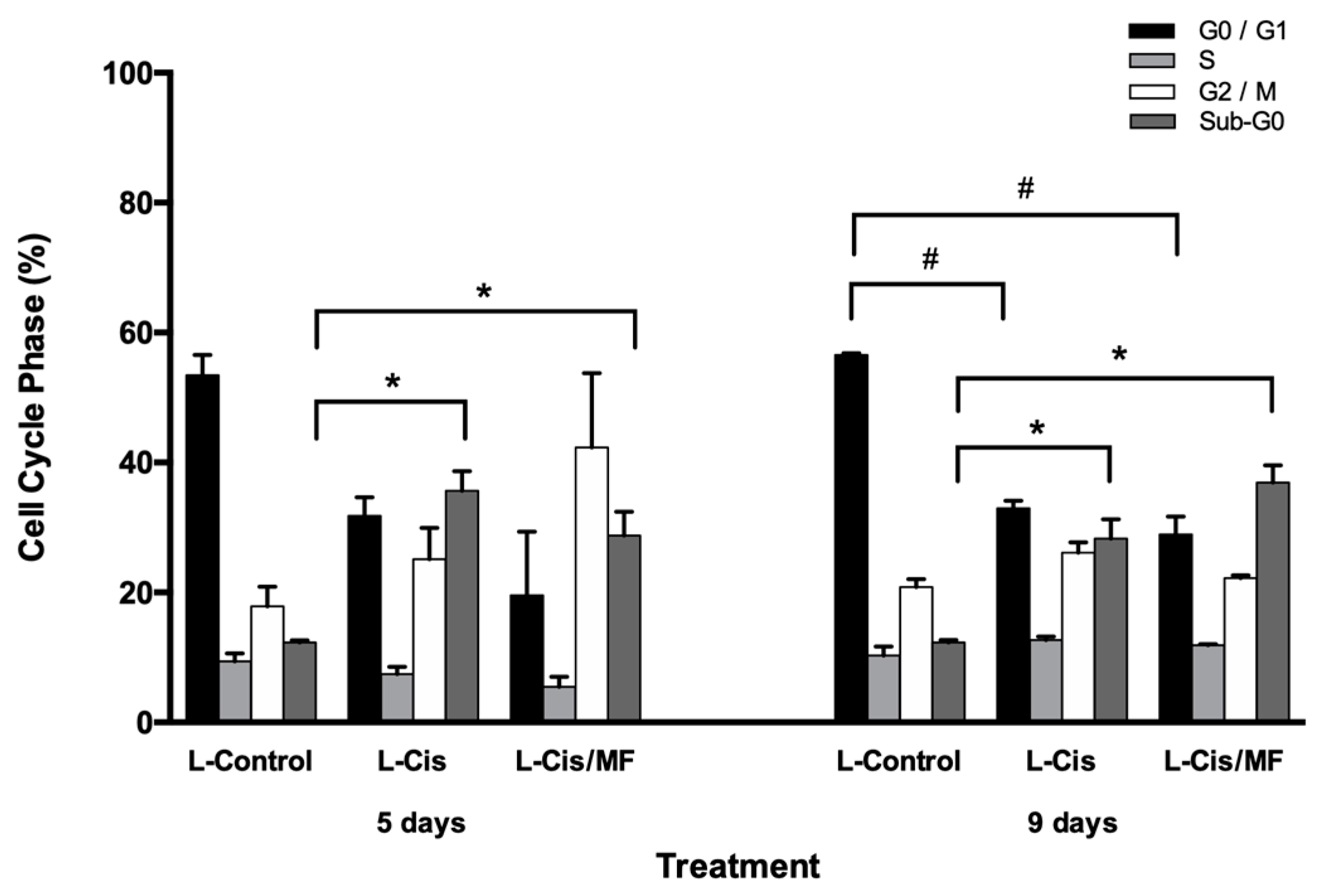
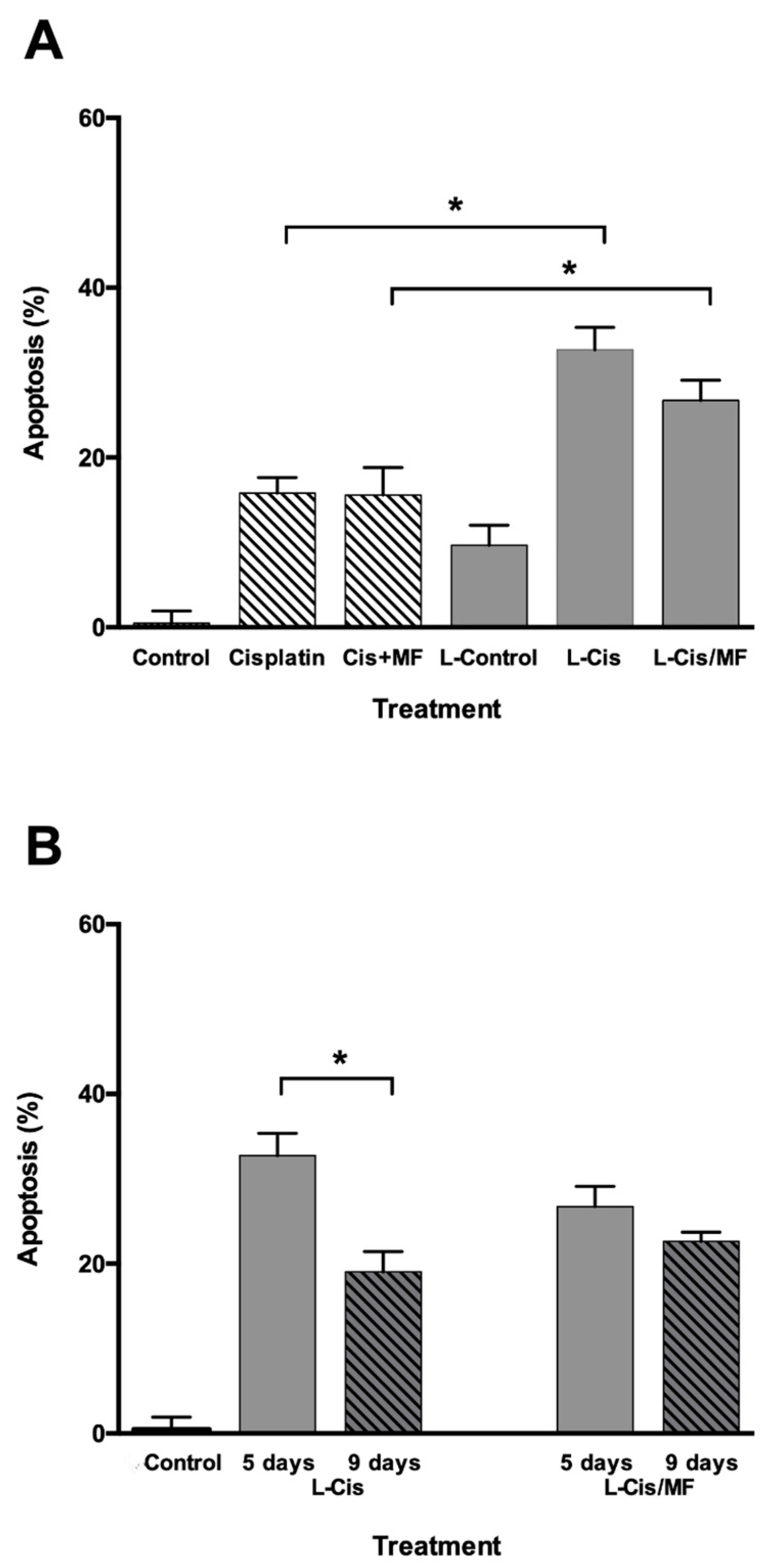
3.3. Cell Cycle and Apoptosis Analysis
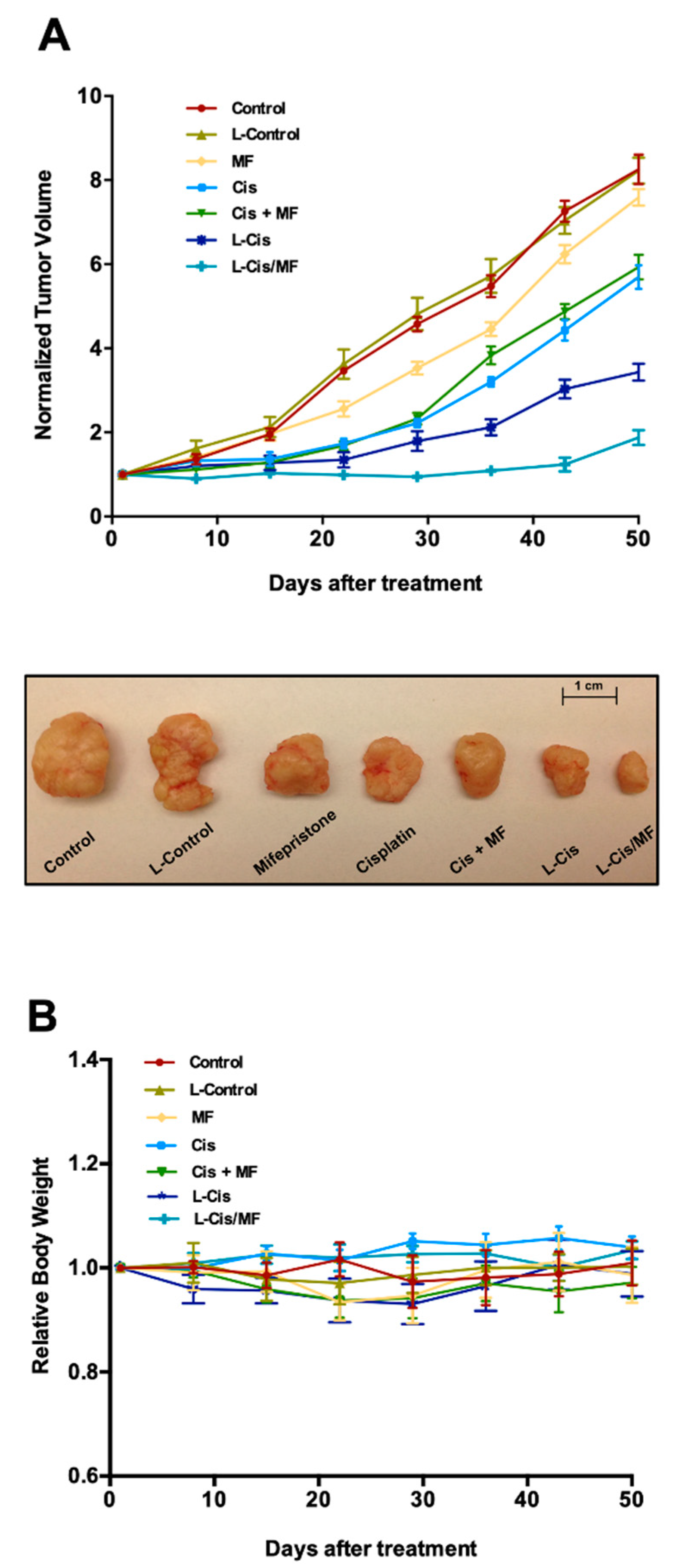
3.4. Tumor Growth Inhibition in Xenografts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arbyn, M.; Weiderpass, E.; Bruni, L.; De Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef]
- Shrestha, A.D.; Neupane, D.; Vedsted, P. Cervical cancer prevalence, incidence and mortality in low and middle income countries: A systematic review. Asian Pac. J. Cancer Prev. 2018, 19, 319–324. [Google Scholar] [PubMed]
- Sushmita, P.; Lalit, K.; Ravindra, M.P.; Ashish, U.; Soumyajit, R.; Vatsla, D.; Renu, M.; Subhash, C. Impact of treatment time on chemoradiotherapy in locally advanced cervical carcinoma. Asian Pac. J. Cancer Prev. 2015, 16, 5075–5079. [Google Scholar]
- Eifel, P.J.; Winter, K.; Morris, M.; Levenback, C.; Grigsby, P.W.; Cooper, J. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer. J. Clin. Oncol. 2004, 22, 872–880. [Google Scholar] [CrossRef]
- Rose, P.G.; Bundy, B.N.; Watkins, E.B.; Thigpen, J.T.; Deppe, G.; Maiman, M.A. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999, 340, 1144–1153. [Google Scholar] [CrossRef]
- Tsuda, N.; Watari, H.; Ushijima, K. Chemotherapy and molecular targeting therapy for recurrent cervical cancer. Chin. J. Cancer Res. 2016, 28, 241–253. [Google Scholar] [CrossRef]
- Benedetti, P.P.; Greggi, S.; Scambia, G. High-dose cisplatin and bleomycin neoadjuvant chemotherapy plus radical surgery in locally advanced cervical carcinoma: A preliminary report. Gynecol. Oncol. 1991, 41, 212–216. [Google Scholar]
- Chabner, B.A.; Longo, D.L. Cancer chemotherapy and biotherapy. In Principles and Practice, 5th ed.; Lipincott Williams &Wilkins (Wolters Kluwer): Philadelphia, PA, USA, 2011; pp. 96–105. [Google Scholar]
- Zhu, H.; Luo, H.; Zhang, W.; Shen, Z.; Hu, X.; Zhu, X. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des. Devel. Ther. 2016, 10, 1885–1895. [Google Scholar] [CrossRef]
- Lee, N.; Kim, S.I.; Lee, M.; Kim, H.S.; Kim, J.W.; Park, N.H.; Song, Y.S. Bevacizumab efficacy and recurrence pattern of persistent and metastatic cervical cancer. In Vivo 2019, 33, 863–868. [Google Scholar] [CrossRef]
- Llaguno-Munive, M.; Medina, L.A.; Jurado, R.; Romero-Pina, M.; Garcia-Lopez, P. Mifepristone improves chemo-radiation response in glioblastoma xenografts. Cancer Cell. Int. 2013, 13, 29. [Google Scholar] [CrossRef]
- Segovia-Mendoza, M.; Jurado, R.; Mir, R.; Medina, L.A.; Prado-Garcia, H.; Garcia-Lopez, P. Antihormonal agents as a strategy to improve the effect of chemo-radiation in cervical cancer: In vitro and in vivo study. BMC Cancer 2015, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Jurado, R.; Lopez-Flores, A.; Alvarez, A.; Garcia-Lopez, P. Cisplatin cytotoxicity is increased by mifepristone in cervical carcinoma: An in vitro and in vivo study. Oncol. Rep. 2009, 22, 1237–1245. [Google Scholar] [PubMed]
- Soutter, W.P.; Leake, R.A. Steroid hormone receptors in gynaecological cancer. In Recent Advances in Obstetrics and Gynaecology; Bonnard, J., Ed.; Churchill Livingstone: Edinburgh, UK, 1987; p. 175. [Google Scholar]
- Kapperman, H.E.; Goyeneche, A.A.; Telleria, C.M. Mifepristone inhibits non-small cell lung carcinoma cellular escape from DNA damaging cisplatin. Cancer Cell Int. 2018, 18, 185. [Google Scholar] [CrossRef] [PubMed]
- Gamarra-Luques, C.D.; Hapon, M.B.; Goyeneche, A.A.; Telleria, C.M. Resistance to cisplatin and paclitaxel does not affect the sensitivity of human ovarian cancer cells to antiprogestin-induced cytotoxicity. J. Ovarian Res. 2014, 7, 45. [Google Scholar] [CrossRef]
- Gamarra-Luques, C.D.; Goyeneche, A.A.; Hapon, M.B.; Telleria, C.M. Mifepristone prevents repopulation of ovarian cancer cells escaping cisplatin-paclitaxel therapy. BMC Cancer 2012, 12, 200. [Google Scholar] [CrossRef]
- Li, B.; Fan, J.; Liu, X.; Qi, R.; Bo, L.; Gu, J.; Qian, C.; Liu, X. Suppression of colorectal tumor growth by regulated survivin targeting. J. Mol. Med. 2006, 84, 1077–1086. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Qasim, M.; Kim, J.H. Nanoparticle-mediated combination therapy: Two-in-one approach for cancer. Int. J. Mol. Sci. 2018, 19, 3264. [Google Scholar] [CrossRef]
- Oku, N.; Namba, Y. Long-circulating liposomes. Crit. Rev. Ther. Drug Carrier Syst. 1994, 11, 231–270. [Google Scholar]
- Marqués-Gallego, P.; De Kroon, A.I. Ligation strategies for targeting liposomal nanocarriers. BioMed Res. Int. 2014, 2014, 129458. [Google Scholar] [CrossRef]
- Mozar, F.S.; Chowdhury, E.H. Impact of PEGylated nanoparticles on tumor targeted drug delivery. Curr. Pharm. Des. 2018, 24, 3283–3296. [Google Scholar] [CrossRef] [PubMed]
- Szoka, F.; Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA 1978, 75, 4194–4198. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- Toro-Córdova, A.; Ledezma-Gallegos, F.; Mondragon-Fuentes, L.; Jurado, R.; Medina, L.A.; Pérez-Rojas, J.M.; Garcia-Lopez, P. Determination of liposomal Cisplatin by high-performance liquid chromatography and its application in pharmacokinetic. J. Chromatogr. Sci. 2016, 54, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wang, S.; Wei, D.; Zhai, J. Development of a high-performance liquid chromatographic method for the determination of mifepristone in human plasma using norethisterone as an internal standard: Application to pharmacokinetic study. Contraception 2007, 76, 228–232. [Google Scholar] [CrossRef]
- Medina, L.A.; Herrera-Penilla, B.I.; Castro-Morales, M.A.; García-López, P.; Jurado, R.; Pérez-Cárdenas, E.; Chanona-Vilchis, J.; Brandan, M.E. Use of an orthovoltage X-ray treatment unit as a radiation research system in a small-animal cancer model. J. Exp. Clin. Cancer Res. 2008, 27, 57. [Google Scholar] [CrossRef]
- Starha, P.; Hošek, J.; Vančo, J.; Dvořák, Z.; Suchý, P.; Popa, I.; Pražanová, G.; Trávníček, Z. Pharmacological and molecular effects of platinum(II) complexes involving 7-azaindole derivatives. PLoS ONE 2014, 9, e90341. [Google Scholar] [CrossRef]
- Canta, A.; Chiorazzi, A.; Carozzi, V.; Meregalli, C.; Oggioni, N.; Sala, B.; Crippa, L.; Avezza, F.; Forestieri, D.; Rotella, G.; et al. In vivo comparative study of the cytotoxicity of a liposomal formulation of cisplatin (lipoplatin™). Cancer Chemother. Pharmacol. 2011, 68, 1001–1008. [Google Scholar] [CrossRef]
- Xu, B.; Zeng, M.; Zeng, J.; Feng, J.; Yu, L. Meta-analysis of clinical trials comparing the efficacy and safety of liposomal cisplatin versus conventional nonliposomal cisplatin in nonsmall cell lung cancer (NSCLC) and squamous cell carcinoma of the head and neck (SCCHN). Medicine 2018, 97, e13169. [Google Scholar] [CrossRef]
- Friedman, A.D.; Claypool, S.E.; Liu, R. The smart targeting of nanoparticles. Curr. Pharm. Des. 2013, 19, 6315–6329. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, L.; Saunders, D.N.; Ranson, M.; Thurecht, K.J.; Storm, G.; Vine, K.L. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. J. Control. Release 2018, 277, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Toro-Cordova, A.; Flores-Cruz, M.; Santoyo-Salazar, J.; Carrillo-Nava, E.; Jurado, R.; Figueroa-Rodriguez, P.A.; Lopez-Sanchez, P.; Medina, L.A.; Garcia-Lopez, P. Liposomes loaded with Cisplatin and magnetic nanoparticles: Physicochemical characterization, pharmacokinetics, and in-vitro efficacy. Molecules 2018, 23, 2272. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.W.; Cradock, J.C.; Vishnuvajjala, B.R.; Flora, K.P. Stability of cisplatin, iproplatin, carboplatin, and tetraplatin in commonly used intravenous solutions. Am. J. Hosp. Pharm. 1987, 44, 124–130. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Düzgüneş, N.; Nir, S. Mechanisms and kinetics of liposome-cell interactions. Adv. Drug Deliv. Rev. 1999, 40, 3–18. [Google Scholar] [CrossRef]
- Przybylo, M.; Glogocka, D.; Dobrucki, J.W.; Fraczkowska, K.; Podbielska, H.; Kopaczynska, M.; Borowik, T.; Langner, M. The cellular internalization of liposome encapsulated protoporphyrin IX by HeLa cells. Eur. J. Pharm. Sci. 2016, 85, 39–46. [Google Scholar] [CrossRef]
- Krasnici, S.; Werner, A.; Eichhorn, M.E.; Schmitt-Sody, M.; Pahernik, S.A.; Sauer, B.; Schulze, B.; Teifel, M.; Michaelis, U.; Naujoks, K.; et al. Effect of the surface charge of liposomes on their uptake by angiogenic tumor vessels. Int. J. Cancer 2003, 105, 561–567. [Google Scholar] [CrossRef]
- Clogston, J.D.; Patri, A.K. Zeta potential measurement. In Characterization of Nanoparticles Intended for Drug Delivery. Methods in Molecular Biology (Methods and Protocols), 1st ed.; McNeil, S., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 697, pp. 63–70. [Google Scholar]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef]
- Murugan, K.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; Du Toit, L.C.; Pillay, V. Parameters and characteristics governing cellular internalization and trans-barrier trafficking of nanostructures. Int. J. Nanomed. 2015, 10, 2191–2206. [Google Scholar]
- Chen, J.; Wang, J.; Shao, J.; Gao, Y.; Xu, J.; Yu, S.; Liu, Z.; Jia, L. The unique pharmacological characteristics of mifepristone (RU486): From terminating pregnancy to preventing cancer metastasis. Med. Res. Rev. 2014, 34, 979–1000. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Che-Ming, H.; Fu, V.; Zhang, L. Nanoparticle drug delivery enhances the cytotoxicity of hydrophobic–hydrophilic drug conjugates. J. Mater. Chem. 2012, 22, 994–999. [Google Scholar] [CrossRef]
- Carvalho Júnior, A.D.; Vieira, F.P.; de Melo, V.J.; Lopes, M.T.; Silveira, J.N.; Ramaldes, G.A.; Garnier-Suillerot, A.; Pereira-Maia, E.C.; de Oliveira, M.C. Preparation and cytotoxicity of cisplatin-containing liposomes. Braz. J. Med. Biol. Res. 2007, 40, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Minematsu, H.; Hiramatsu, Y.; Kitagawa, H.; Otani, T.; Iwashita, S.; Kudoh, T.; Chen, L.; Li, Y.; Okada, M.; et al. Novel and simple loading procedure of cisplatin into liposomes and targeting tumor endothelial cells. Int. J. Pharm. 2010, 391, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Xie, C.; Huang, K.; Zhu, C. The production and characteristics of solid lipid nanoparticles (SLNs). Biomaterials 2003, 24, 1781–1785. [Google Scholar] [CrossRef]
- Doijad, R.C.; Manvi, F.V.; Godhwani, D.M.; Joseph, R.; Deshmukh, N.V. Formulation and targeting efficiency of Cisplatin engineered solid lipid nanoparticles. Indian J. Pharm. Sci. 2008, 70, 203–207. [Google Scholar] [CrossRef]
- Aldawsari, H.M.; Singh, S. Rapid microwave-assisted Cisplatin-loaded solid lipid nanoparticles: Synthesis, characterization and anticancer study. Nanomaterials 2020, 10, 510. [Google Scholar] [CrossRef]
- Rew, Y.; Du, X.; Eksterowicz, J.; Zhou, H.; Jahchan, N.; Zhu, L.; Yan, X.; Kawai, H.; McGee, L.R.; Medina, J.C.; et al. Discovery of a potent and selective steroidal glucocorticoid receptor antagonist (ORIC-101). J. Med. Chem. 2018, 61, 7767–7784. [Google Scholar] [CrossRef]
- Sequeira, G.; Vanzulli, S.I.; Rojas, P.; Lamb, C.; Colombo, L.; May, M.; Molinolo, A.; Lanari, C. The effectiveness of nano chemotherapeutic particles combined with mifepristone depends on the PR isoform ratio in preclinical models of breast cancer. Oncotarget 2014, 5, 3246–3260. [Google Scholar] [CrossRef][Green Version]
- Sang, L.; Wang, X.; Zhao, X. Mifepristone inhibits the migration of cervical cancer cells by inhibiting exocrine secretion. Pharmacology 2018, 101, 322–329. [Google Scholar] [CrossRef]
- Dostál, Z.; Kosina, P.; Mlejnek, P.; Kikalová, K.; Modrianský, M. Mifepristone potentiates etoposide toxicity in Hep G2 cells by modulating drug transport. Toxicol. In Vitro 2019, 54, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sampson, A.; Peterson, B.G.; Tan, K.W.; Iram, S.H. Doxorubicin as a fluorescent reporter identifies novel MRP1 (ABCC1) inhibitors missed by calcein-based high content screening of anticancer agents. Biomed. Pharmacother. 2019, 118, 109289. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zou, S.; Sheng, B.; Zhao, M.; Sun, L.Z.; Zhu, X. Mifepristone inhibits IGF-1 signaling pathway in the treatment of uterine leiomyomas. Drug Des. Dev. Ther. 2019, 13, 3161–3170. [Google Scholar] [CrossRef] [PubMed]
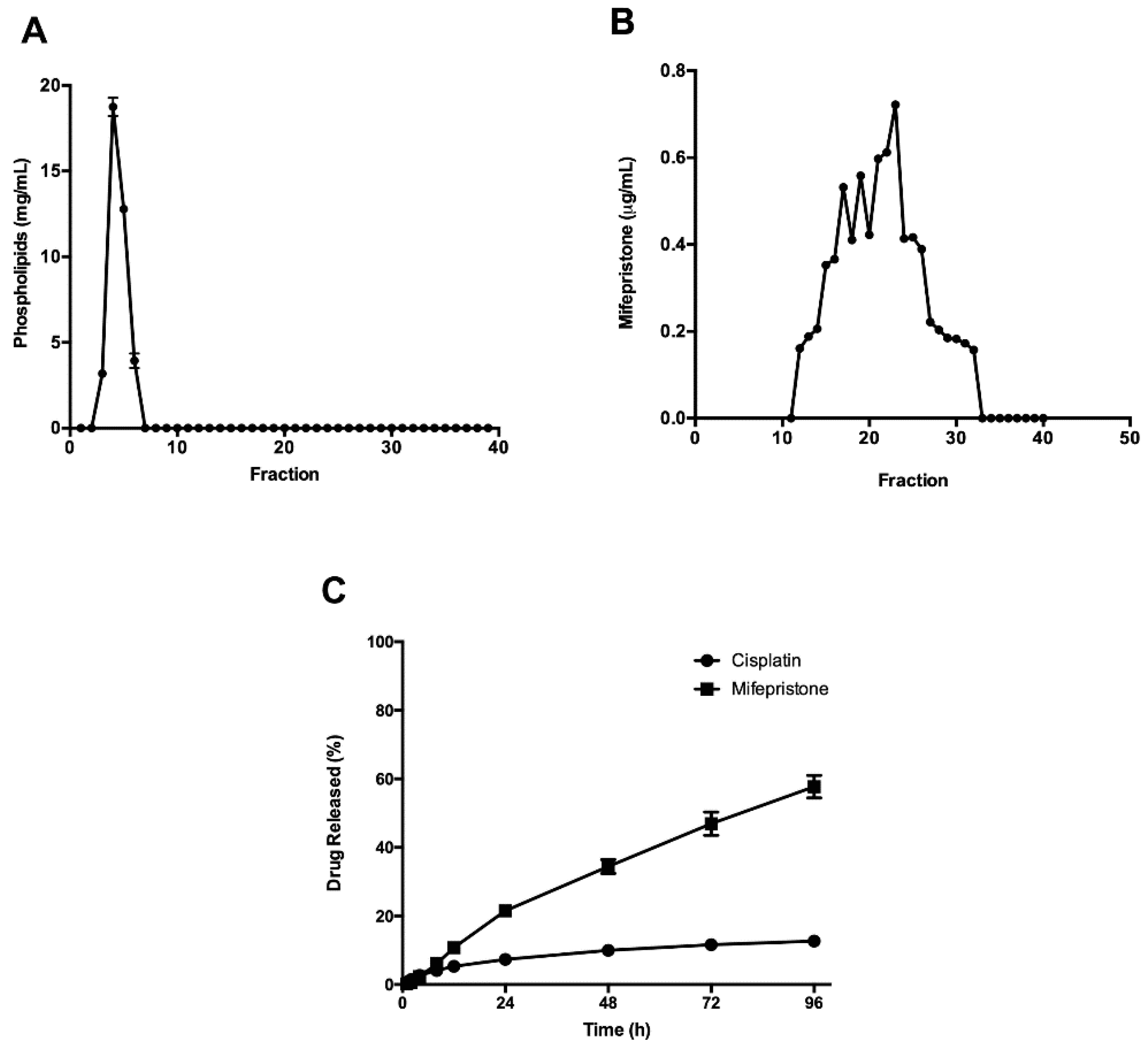
| Parameter | L-Cis | L-Cis/MF |
|---|---|---|
| Particle Size (nm) | 132 ± 6.1 | 109 ± 5.4 |
| Zeta Potential (mV) | −36.1 ± 1.1 | −38.7 ± 1.2 |
| Lipids (mg/mL) | 29.8 ± 3.4 | 37.6 ± 0.4 |
| Polydispersity Index (PDI) | 0.133 ± 0.03 | 0.11 ± 0.02 |
| Cisplatin (µg/mL) | 989.7 ± 82.9 | 813 ± 103 |
| Mifepristone (µg/mL) | - | 381.2 ± 19.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledezma-Gallegos, F.; Jurado, R.; Mir, R.; Medina, L.A.; Mondragon-Fuentes, L.; Garcia-Lopez, P. Liposomes Co-Encapsulating Cisplatin/Mifepristone Improve the Effect on Cervical Cancer: In Vitro and In Vivo Assessment. Pharmaceutics 2020, 12, 897. https://doi.org/10.3390/pharmaceutics12090897
Ledezma-Gallegos F, Jurado R, Mir R, Medina LA, Mondragon-Fuentes L, Garcia-Lopez P. Liposomes Co-Encapsulating Cisplatin/Mifepristone Improve the Effect on Cervical Cancer: In Vitro and In Vivo Assessment. Pharmaceutics. 2020; 12(9):897. https://doi.org/10.3390/pharmaceutics12090897
Chicago/Turabian StyleLedezma-Gallegos, Fabricio, Rafael Jurado, Roser Mir, Luis Alberto Medina, Laura Mondragon-Fuentes, and Patricia Garcia-Lopez. 2020. "Liposomes Co-Encapsulating Cisplatin/Mifepristone Improve the Effect on Cervical Cancer: In Vitro and In Vivo Assessment" Pharmaceutics 12, no. 9: 897. https://doi.org/10.3390/pharmaceutics12090897
APA StyleLedezma-Gallegos, F., Jurado, R., Mir, R., Medina, L. A., Mondragon-Fuentes, L., & Garcia-Lopez, P. (2020). Liposomes Co-Encapsulating Cisplatin/Mifepristone Improve the Effect on Cervical Cancer: In Vitro and In Vivo Assessment. Pharmaceutics, 12(9), 897. https://doi.org/10.3390/pharmaceutics12090897