Development of a Surface Coating Technique with Predictive Value for Bead Coating in the Manufacturing of Amorphous Solid Dispersions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of MCC Tablets
2.3. Surface Coating and Spray Drying
2.4. Film Casting
2.5. Bead Coating
2.6. Solid-State Characterization
2.6.1. Modulated Differential Scanning Calorimetry (mDSC)
2.6.2. X-ray Powder Diffraction (XRPD)
2.7. Scanning Electron Microscopy (SEM)
2.8. Thermogravimetric Analysis (TGA)
3. Results
3.1. Drug Selection
3.2. Determination of the Highest Possible Drug Loading: Film Casting and Bead Coating
3.2.1. Film Casting
3.2.2. Bead Coating
3.3. Development of a Surface Coating Technique
3.3.1. Compression Force
3.3.2. Spraying Procedure
3.3.3. Comparison to Bead Coating
3.3.4. Repeatability
3.4. Determination of the Highest Possible Drug Loading: Spray Drying
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ricarte, R.G.; Van Zee, N.J.; Li, Z.; Johnson, L.M.; Lodge, T.P.; Hillmyer, M.A. Recent Advances in Understanding the Micro-and Nanoscale Phenomena of Amorphous Solid Dispersions. Mol. Pharm. 2019, 16, 4089–4103. [Google Scholar] [CrossRef] [PubMed]
- Filo Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- Jermain, S.V.; Brough, C.; Iii, R.O.W. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2017, 535, 379–392. [Google Scholar] [CrossRef]
- Luebbert, C.; Huxoll, F.; Sadowski, G. Amorphous-amorphous phase separation in API/polymer formulations. Molecules 2017, 22, 296. [Google Scholar] [CrossRef]
- Hrovat, K. Methods of amorphization and investigation of the amorphous state. Acta Pharm. 2013, 63, 305–334. [Google Scholar]
- Patel, B.B.; Patel, J.K.; Chakraborty, S.; Shukla, D. Revealing facts behind spray dried solid dispersion technology used for solubility enhancement. Saudi Pharm. J. 2015, 23, 352–365. [Google Scholar] [CrossRef]
- Vaka, S.; Bommana, M.; Desai, D.; Djordjevic, J.; Phuapradit, W.; Shah, N. Chapter 4: Excipients for amorphous solid dispersions. In Amorphous Solid Dispersions; Springer: New York, NY, USA, 2014; pp. 123–161. [Google Scholar]
- Jijun, F.; Lishuang, X.; Xiaoli, W.; Shu, Z.; Xiaoguang, T.; Xingna, Z.; Haibing, H.; Xing, T. Nimodipine (NM) tablets with high dissolution containing NM solid dispersions prepared by hot-melt extrusion. Drug Dev. Ind. Pharm. 2011, 37, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Worku, Z.A.; Paudel, A.; Van den Mooter, G. Can compression induce demixing in amorphous solid dispersions ? A case study of naproxen—PVP K25. Eur. J. Pharm. Biopharm. 2012, 81, 207–213. [Google Scholar]
- Worku, Z.A.; Paudel, A.; Rombaut, P.; Van den Mooter, G. Effect of Compression on Non-isothermal Crystallization Behaviour of Amorphous Indomethacin. Pharm. Res. 2012, 29, 2489–2498. [Google Scholar]
- Singh, A.; Bharati, A.; Frederiks, P.; Verkinderen, O.; Goderis, B.; Cardinaels, R.; Moldenaers, P.; Van Humbeeck, J.; Van den Mooter, G. Effect of Compression on the Molecular Arrangement of Itraconazole−Soluplus Solid Dispersions: Induction of Liquid Crystals or Exacerbation of Phase Separation? Mol. Pharm. 2016, 13, 1879–1893. [Google Scholar] [CrossRef]
- Prasad, M.B.; Vidyadhara, S.; Sasidhar, R.L.C.; Balakrishna, T.; Trilochani, P. Development and evaluation of diltiazem hydrochloride controlled-release pellets by fluid bed coating process. J. Adv. Pharm. Technol. Res. 2013, 4, 101–107. [Google Scholar]
- Dereymaker, A.; Scurr, D.J.; Steer, E.D.; Roberts, C.J.; Van den Mooter, G. Controlling the Release of Indomethacin from Glass Solutions Layered with a Rate Controlling Membrane Using Fluid-Bed Processing. Part 1: Surface and Cross-Sectional Chemical Analysis. Mol. Pharm. 2017, 14, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, G.; Klein, S. Dissolution testing of oral modified-release dosage forms. J. Pharm. Pharmacol. 2011, 64, 944–968. [Google Scholar] [CrossRef] [PubMed]
- Van Gyseghem, E.; Baert, L.; Van Remoortere, P.; Van ’t Klooster, G.; Rouan, M.-C.; Voorspoels, J.; De Kock, H.; Schueller, L.; Rosier, J.; Grooten, L.; et al. Co-administration of darunavir and a new pharmacokinetic booster: Formulation strategies and evaluation in dogs. Eur. J. Pharm. Sci. 2010, 41, 193–200. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, N.; Wu, B.; Lu, Y.; Guan, T.; Wu, W. Physical characterization of lansoprazole/PVP solid dispersion prepared by fluid-bed coating technique. Powder Technol. 2008, 182, 480–485. [Google Scholar] [CrossRef]
- Kolašinac, N.; Kachrimanis, K.; Djuriš, J.; Homšek, I.; Grujić, B.; Ibrić, S. Spray coating as a powerful technique in preparation of solid dispersions with enhanced desloratadine dissolution rate. Drug Dev. Ind. Pharm. 2013, 39, 1020–1027. [Google Scholar] [CrossRef]
- Sun, N.; Wei, X.; Wu, B.; Chen, J.; Lu, Y.; Wu, W. Enhanced dissolution of silymarin/polyvinylpyrrolidone solid dispersion pellets prepared by a one-step fluid-bed coating technique. Powder Technol. 2008, 182, 72–80. [Google Scholar] [CrossRef]
- Guns, S.; Dereymaker, A.; Kayaert, P.; Mathot, V.; Martens, J.A.; Van den Mooter, G. Comparison Between Hot-Melt Extrusion and Spray-Drying for Manufacturing Solid Dispersions of the Graft Copolymer of Ethylene Glycol and Vinylalcohol. Pharm. Res. 2011, 28, 673–682. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Baird, J.A.; Taylor, L.S. Crystallization tendency of active pharmaceutical ingredients following rapid solvent evaporation—Classification and comparison with crystallization tendency from undercooled melts. J. Pharm. Sci. 2010, 99, 3826–3838. [Google Scholar] [CrossRef]
- Baird, J.A.; Taylor, L.S. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv. Drug Deliv. Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef]
- Peeters, O.M.; Blaton, N.M.; Ranter, C.J.D.E. The crystal structure of 1-(2,4-dichloro-beta-((2,4-dichloro-benzyl)oxy)phenethyl) imidazole hemihydrate. Bull. Soc. Chim. Belg. 1979, 88, 265–272. [Google Scholar] [CrossRef]
- Kersten, K.M.; Breen, M.E.; Mapp, A.K.; Matzger, A.J. Pharmaceutical solvate formation for the incorporation of the antimicrobial agent hydrogen peroxide. Chem. Commun. 2018, 54, 9286–9289. [Google Scholar] [CrossRef] [PubMed]
- Boel, E.; Smeets, A.; Vergaelen, M.; De La Rosa, V.R.; Hoogenboom, R.; Van den Mooter, G. Comparative study of the potential of poly(2-ethyl-2-oxazoline) as carrier in the formulation of amorphous solid dispersions of poorly soluble drugs. Eur. J. Pharm. Biopharm. 2019, 144, 79–90. [Google Scholar] [CrossRef] [PubMed]

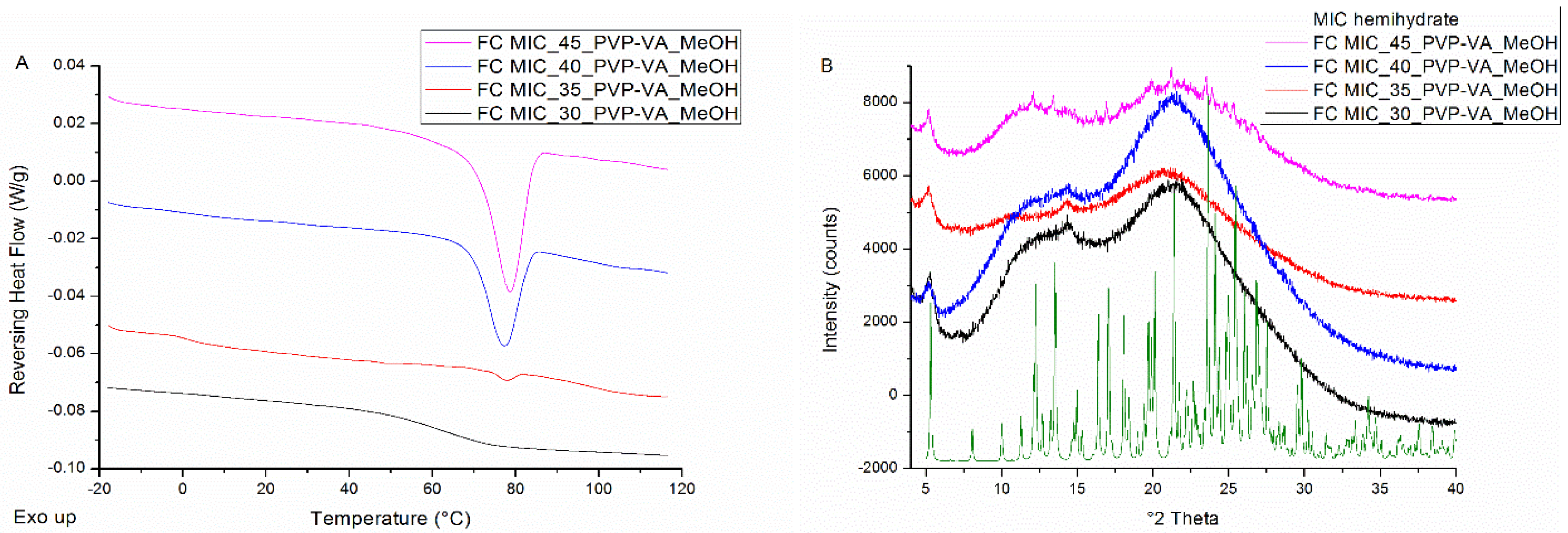
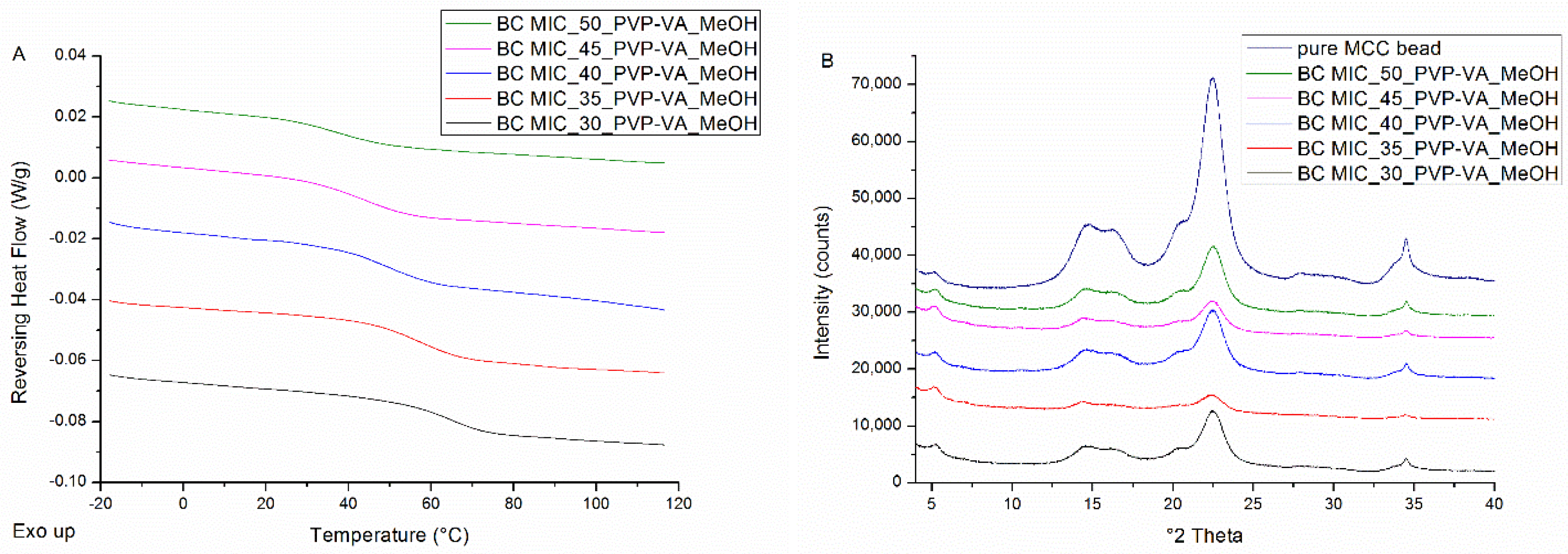
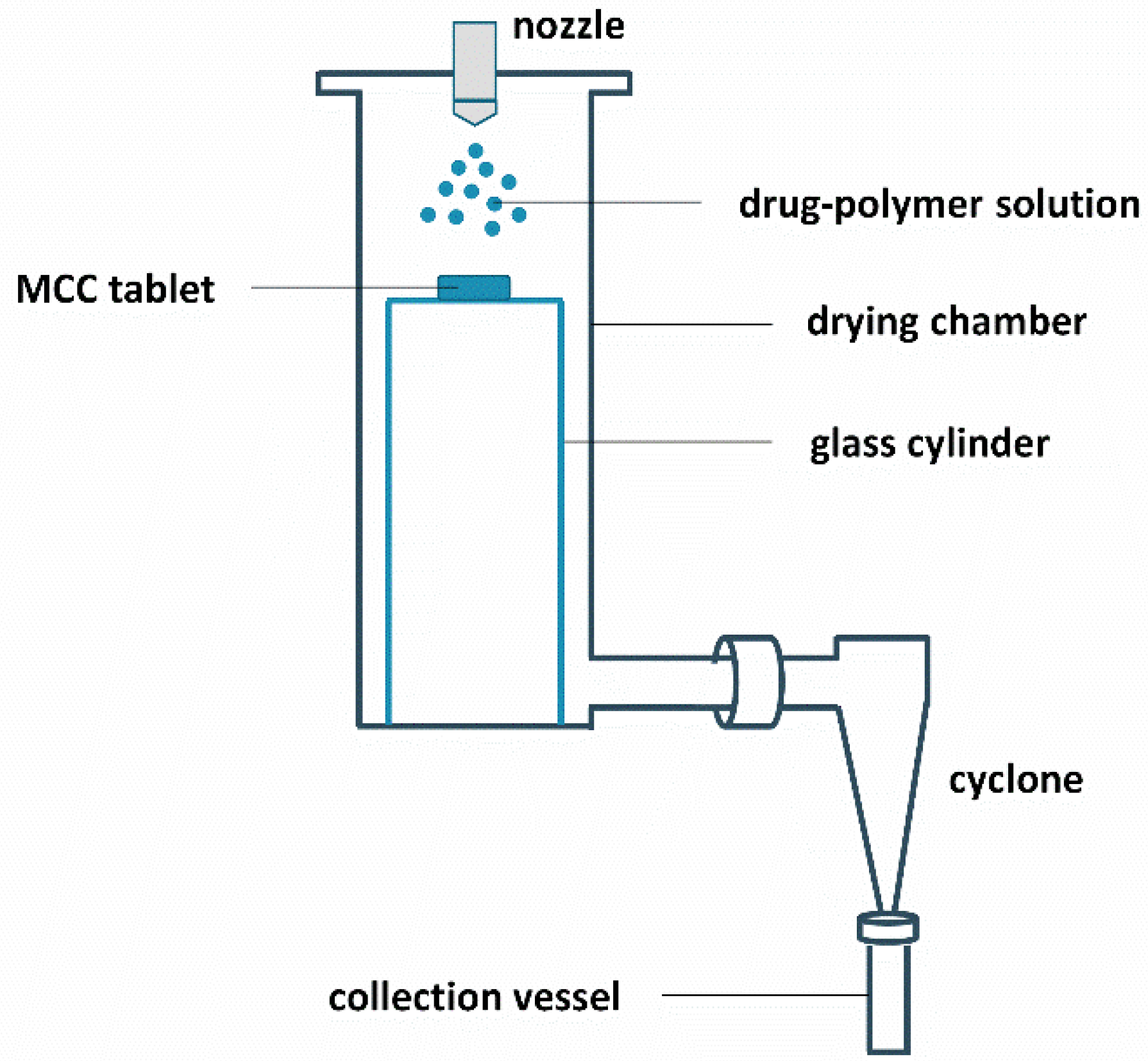
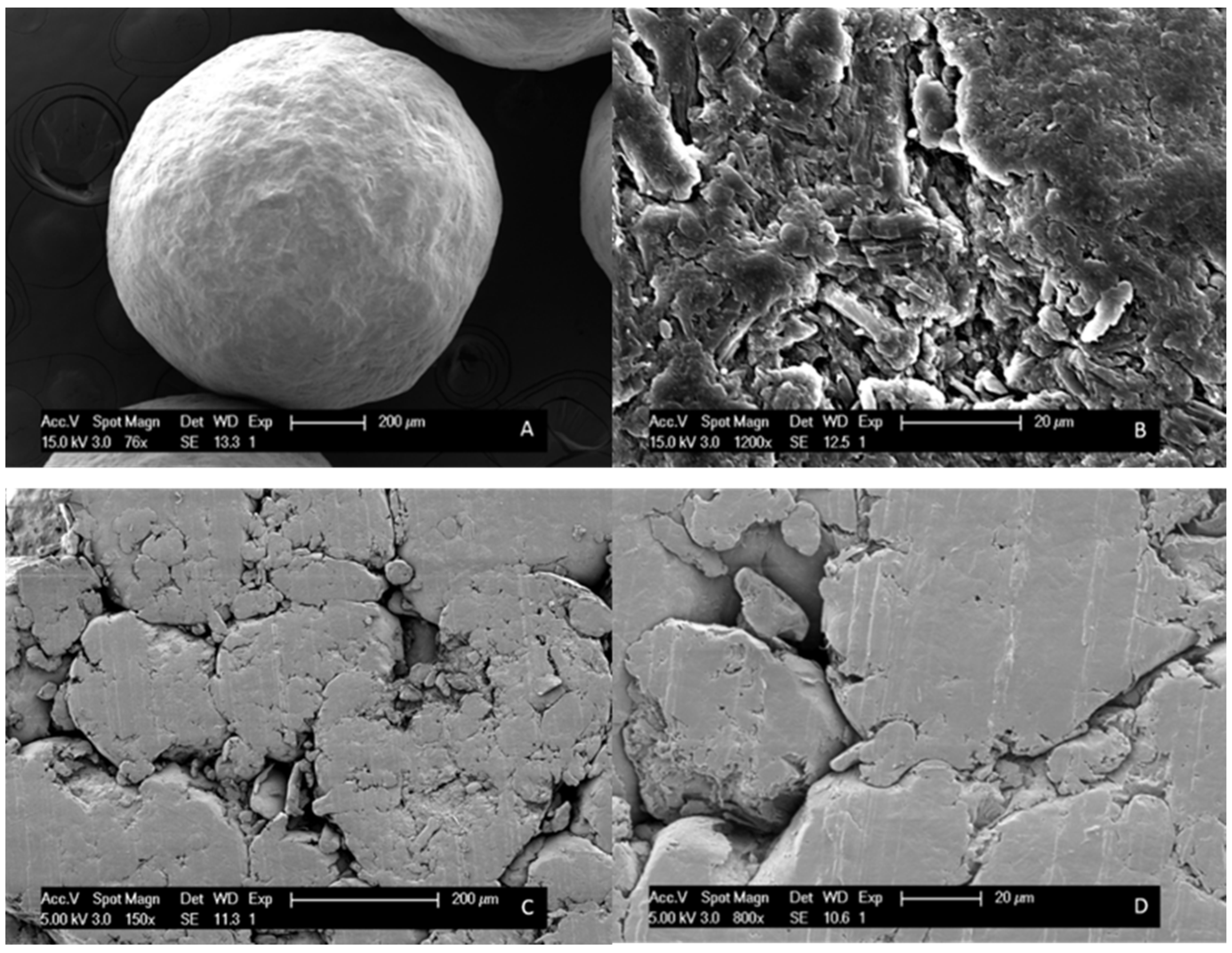
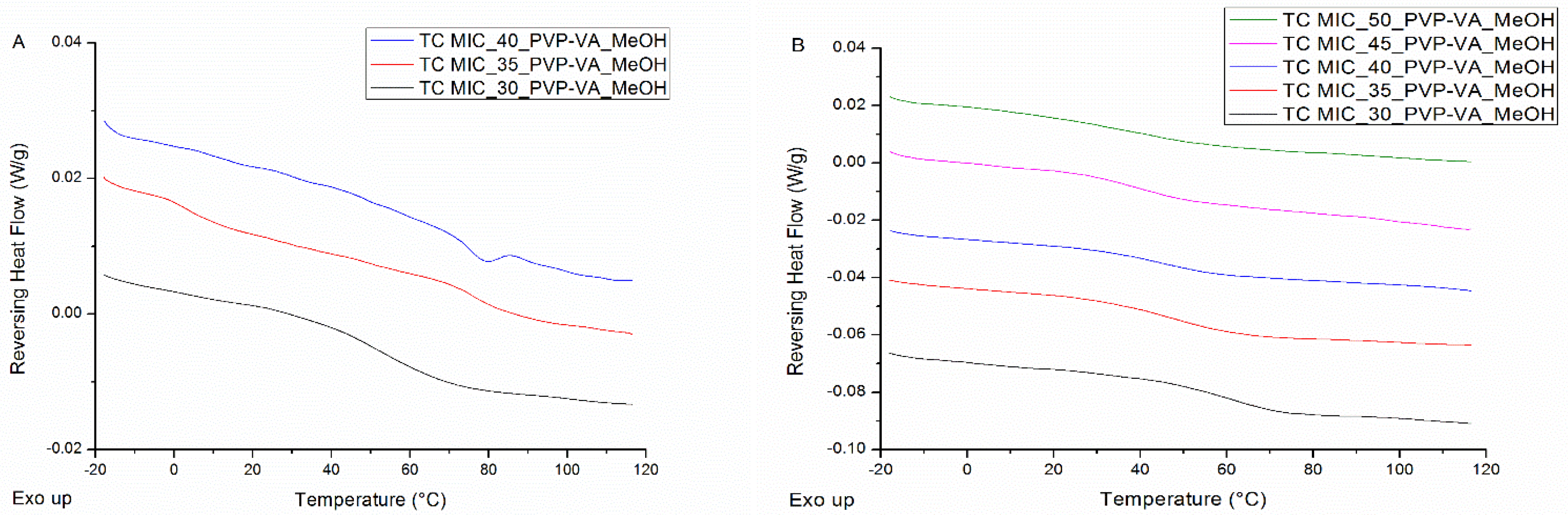
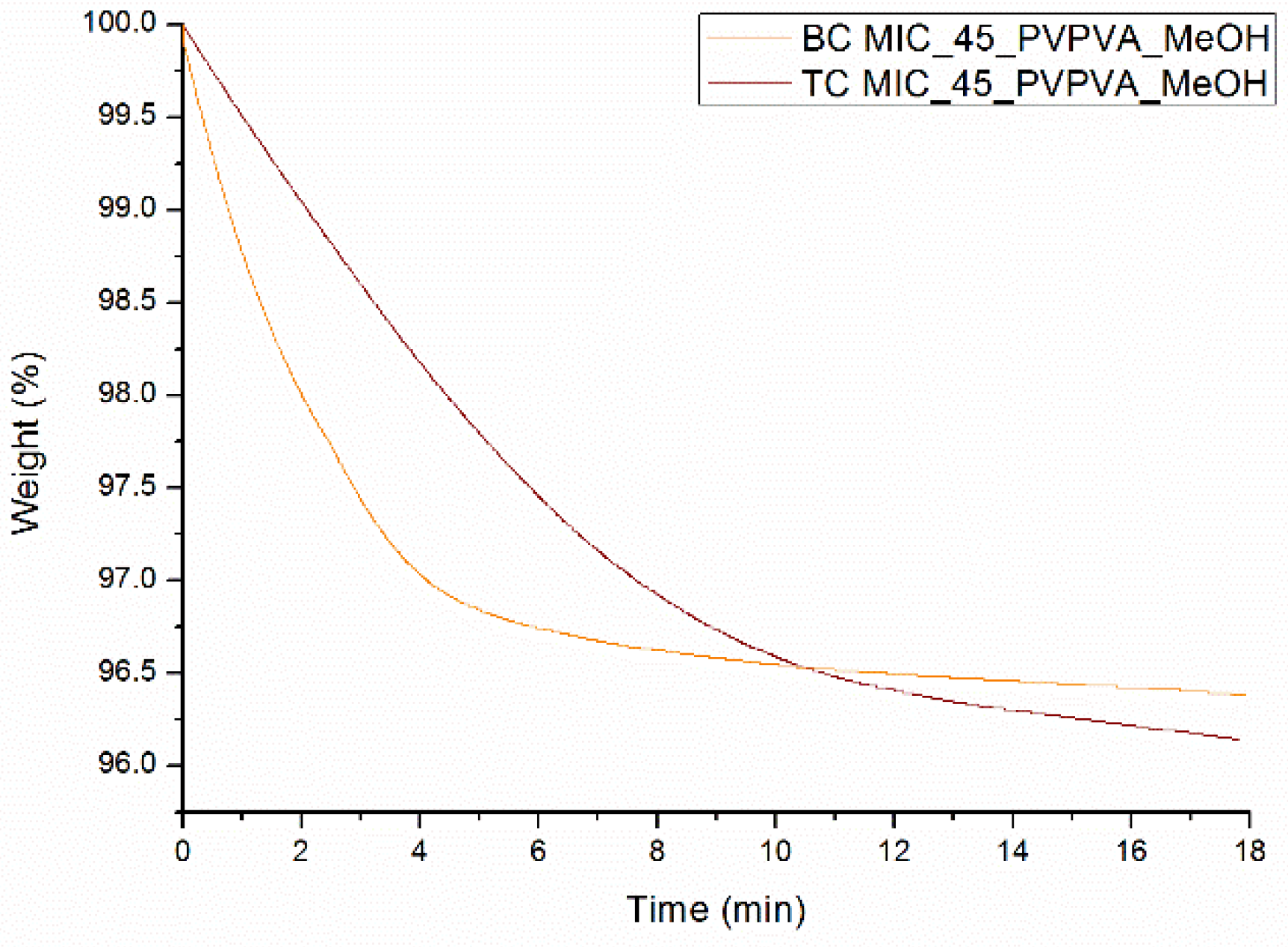

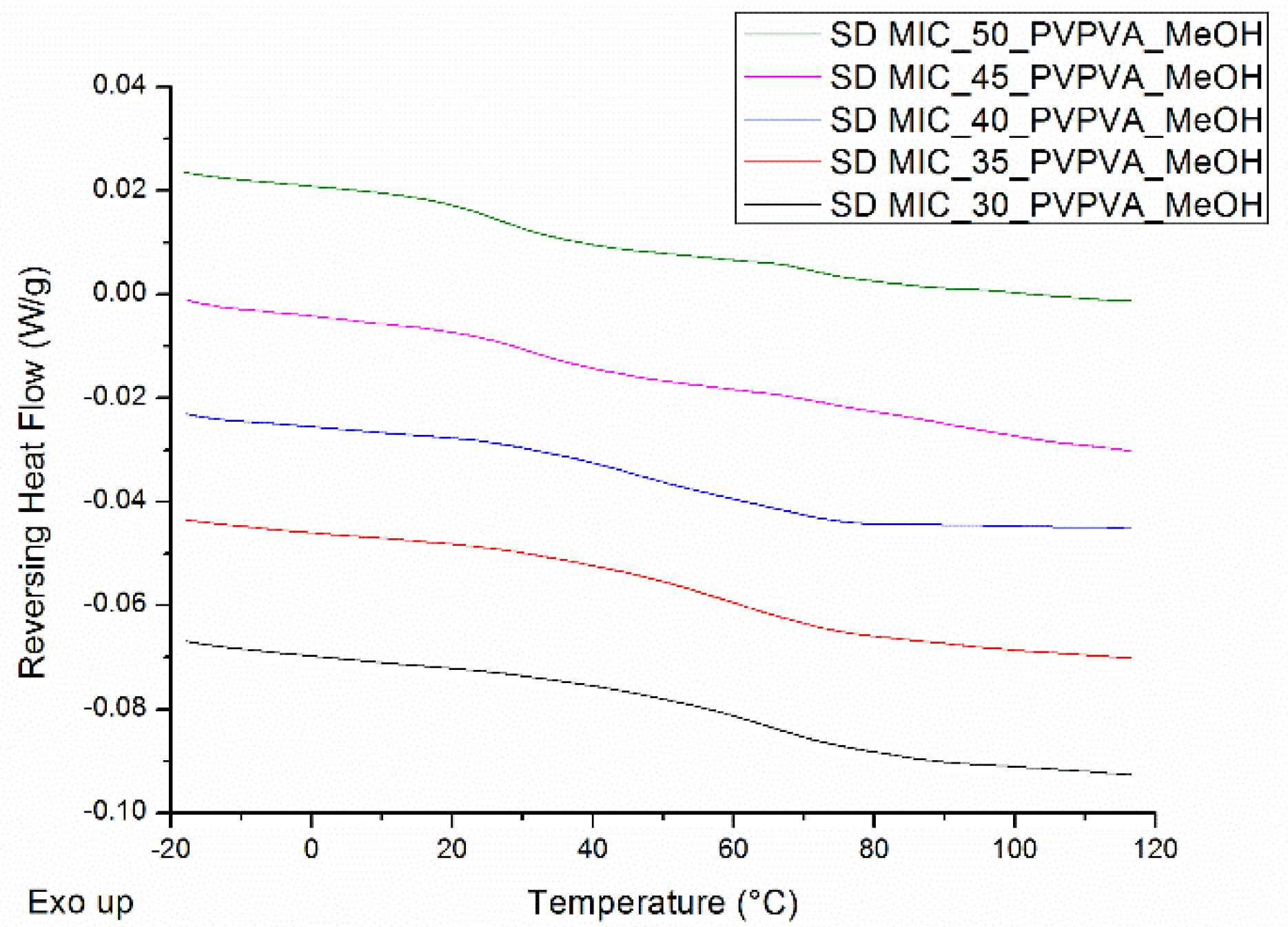
| Drug Weight Fraction (%) | Average Tg Value ± Sd (°C) | Average Tg Width ± Sd (°C) | Average Tm,onset ± Sd (°C) | Average Melting Enthalpy ± Sd (J/g) |
|---|---|---|---|---|
| 30 | 62.4 ± 0.8 | 23.7 ± 4.7 | / | / |
| 35 | 1.7 ± 0.9 (*) | 7.6 ± 1.6 | 75.7 ± 0.6 | 1.1 ± 0.2 |
| 40 | / | / | 75.1 ± 1.2 | 3.6 ± 2.0 |
| 45 | / | / | 69.7 ± 2.9 | 18.0 ± 4.6 |
| Drug Weight Fraction (%) | Average Tg Value ± Sd (°C) | Average Tg Width ± Sd (°C) |
|---|---|---|
| 30 | 65.3 ± 0.9 | 14.1 ± 0.6 |
| 35 | 57.2 ± 1.6 | 14.7 ± 1.0 |
| 40 | 49.6 ± 0.8 | 16.7 ± 3.3 |
| 45 | 43.8 ± 1.0 | 18.5 ± 1.1 |
| 50 | 36.4 ± 0.3 | 17.9 ± 1.4 |
| Drug Weight Fraction (%) | Average Tg Value ± Sd (°C) IM 1 | Average Tg Width ± Sd (°C) IM 1 | Average Tg Value ± Sd (°C) IM 2 | Average Tg Width ± Sd (°C) IM 2 |
|---|---|---|---|---|
| 30 | 60.1 ± 2.5 | 18.4 ± 3.8 | 60.0 ± 1.7 | 15.8 ± 1.6 |
| 35 | 50.5 ± 0.9 | 23.1 ± 3.3 | 47.8 ± 0.5 | 24.7 ± 2.8 |
| 40 | 45.0 ± 0.4 | 17.7 ± 1.0 | 45.5 ± 1.5 | 16.1 ± 2.7 |
| 45 | 37.0 ± 1.1 | 19.8 ± 0.7 | 35.9 ± 2.2 | 16.8 ± 8.0 |
| 50 | 31.8 ± 5.1 | 30.4 ± 7.6 | 34.1 ± 0.3 | 17.9 ± 0.8 |
| Sample | Average Tg Value ± Sd (°C) Batch 1 | Average Tg Value ± Sd (°C) Batch 2 | Average Tg Value ± Sd (°C) Batch 3 |
|---|---|---|---|
| TC_MIC_35_PVP-VA_MeOH_IM1 | 50.5 ± 0.9 | 54.1 ± 0.7 | 49.1 ± 6.1 |
| TC_MIC_35_PVP-VA_MeOH_IM2 | 47.8 ± 0.5 | 53.3 ± 1.2 | 55.3 ± 1.0 |
| TC_MIC_45_PVP-VA_MeOH_IM1 | 37.0 ± 1.1 | 33.1 ± 4.4 | 37.5 ± 3.1 |
| TC_MIC_45_PVP-VA_MeOH_IM2 | 35.9 ± 2.2 | 35.4 ± 1.6 | 38.4 ± 2.5 |
| Sample | Average Tg Value(s) ± Sd (°C) Batch 1 | Average Tg Value(s) ± Sd (°C) Batch 2 | Average Tg Value(s) ± Sd (°C) Batch 3 |
|---|---|---|---|
| SD_MIC_35_PVP-VA_MeOH_IM1 | 52.6 ± 4.7 | 51.6 ± 0.4 | 51.2 ± 3.2 |
| SD_MIC_35_PVP-VA_MeOH_IM2 | 52.5 ± 4.6 | 50.0 ± 1.3 | 49.6 ± 2.5 |
| SD_MIC_45_PVP-VA_MeOH_IM1 | 34.7 ± 3.3 71.4 ± 0.5 | 36.1 ± 0.6 71.8 ± 1.6 | 36.9 ± 1.9 69.9 ± 2.6 |
| SD_MIC_45_PVP-VA_MeOH_IM2 | 35.5 ± 3.7 71.7 ± 3.4 | 37.6 ± 0.9 (*) | 37.4 ± 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boel, E.; Panini, P.; Van den Mooter, G. Development of a Surface Coating Technique with Predictive Value for Bead Coating in the Manufacturing of Amorphous Solid Dispersions. Pharmaceutics 2020, 12, 878. https://doi.org/10.3390/pharmaceutics12090878
Boel E, Panini P, Van den Mooter G. Development of a Surface Coating Technique with Predictive Value for Bead Coating in the Manufacturing of Amorphous Solid Dispersions. Pharmaceutics. 2020; 12(9):878. https://doi.org/10.3390/pharmaceutics12090878
Chicago/Turabian StyleBoel, Eline, Piyush Panini, and Guy Van den Mooter. 2020. "Development of a Surface Coating Technique with Predictive Value for Bead Coating in the Manufacturing of Amorphous Solid Dispersions" Pharmaceutics 12, no. 9: 878. https://doi.org/10.3390/pharmaceutics12090878
APA StyleBoel, E., Panini, P., & Van den Mooter, G. (2020). Development of a Surface Coating Technique with Predictive Value for Bead Coating in the Manufacturing of Amorphous Solid Dispersions. Pharmaceutics, 12(9), 878. https://doi.org/10.3390/pharmaceutics12090878






