Development of a Surface Coating Technique with Predictive Value for Bead Coating in the Manufacturing of Amorphous Solid Dispersions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of MCC Tablets
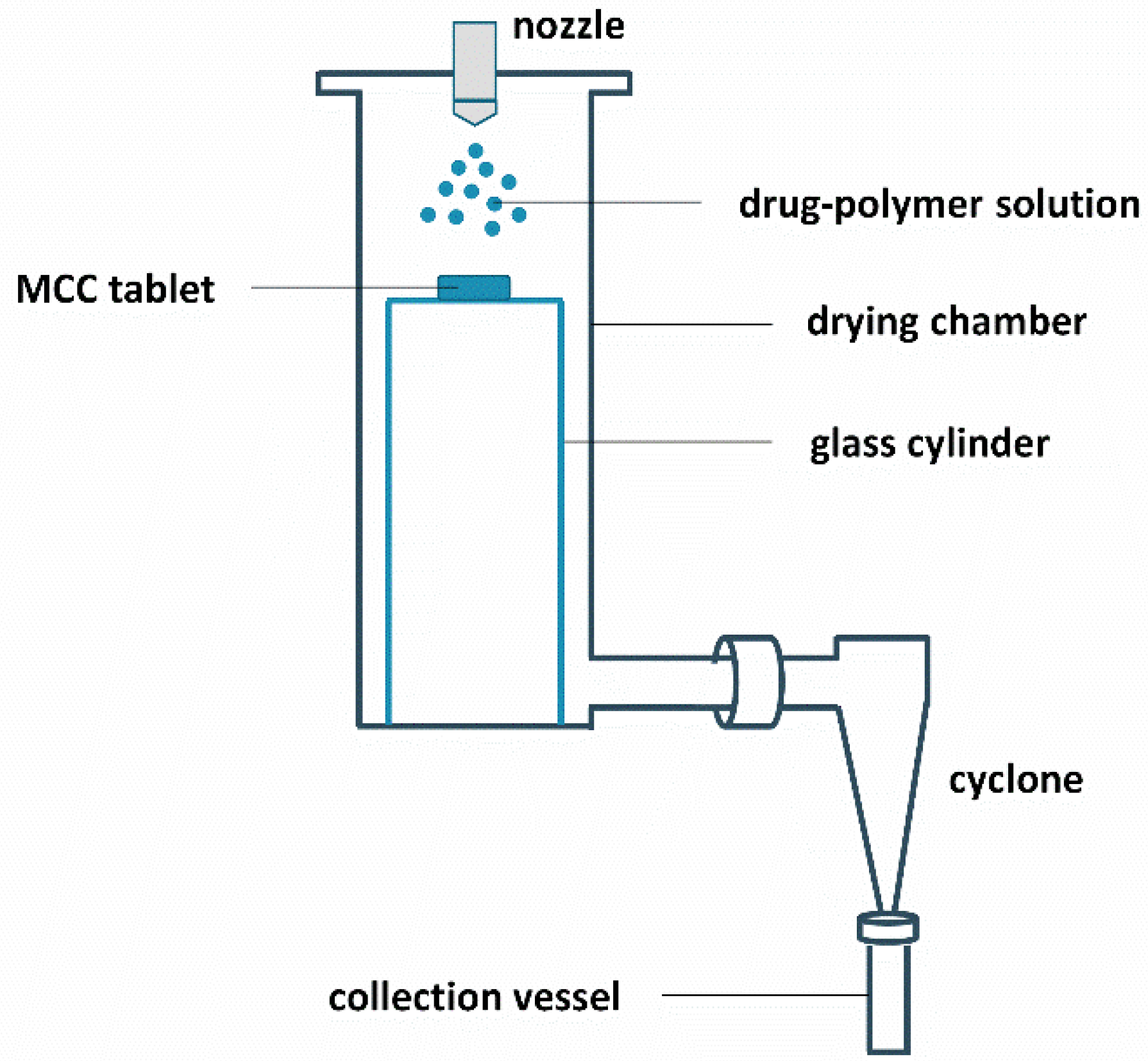
2.3. Surface Coating and Spray Drying
2.4. Film Casting
2.5. Bead Coating
2.6. Solid-State Characterization
2.6.1. Modulated Differential Scanning Calorimetry (mDSC)
2.6.2. X-ray Powder Diffraction (XRPD)
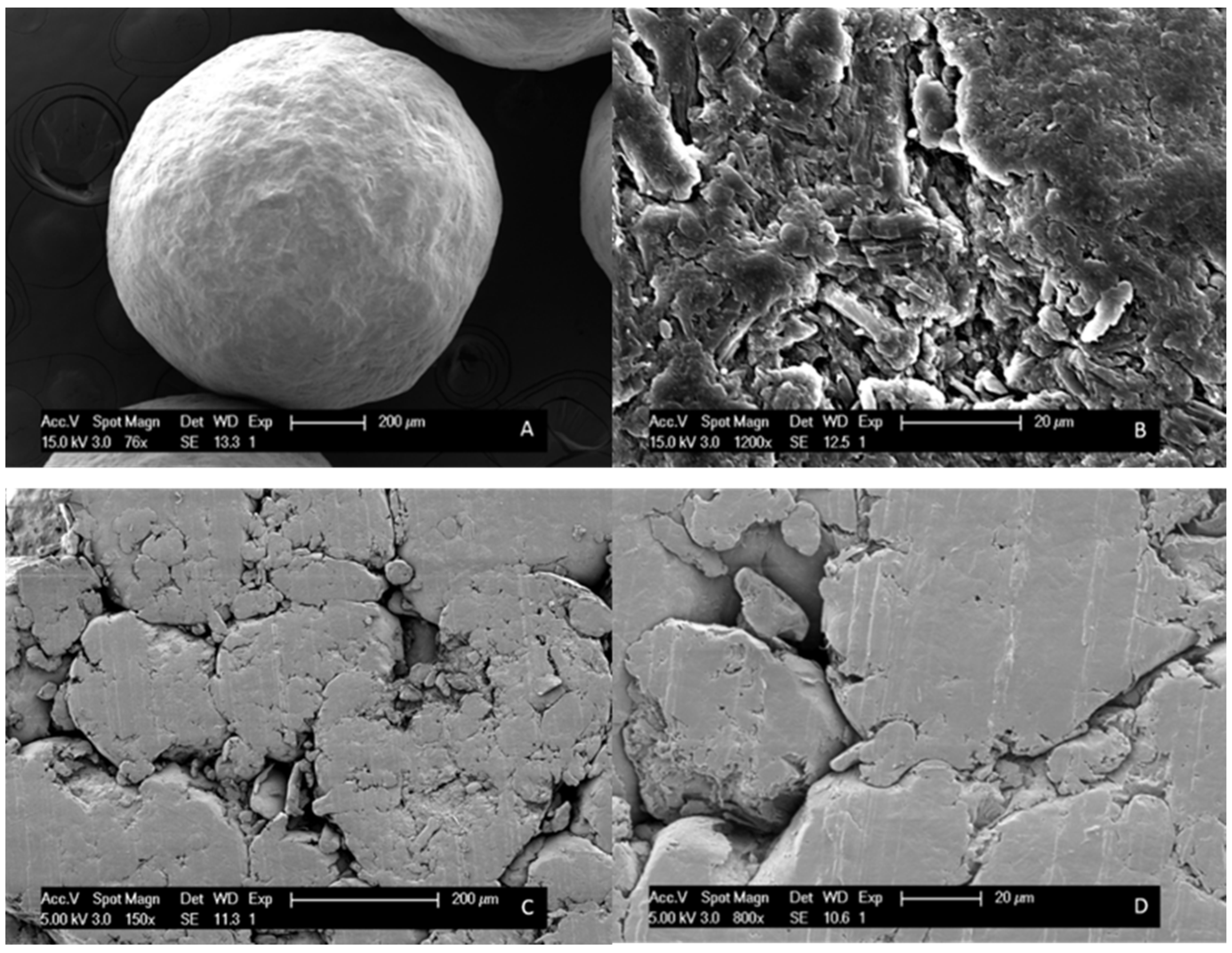
2.7. Scanning Electron Microscopy (SEM)
2.8. Thermogravimetric Analysis (TGA)
3. Results
3.1. Drug Selection
3.2. Determination of the Highest Possible Drug Loading: Film Casting and Bead Coating
3.2.1. Film Casting
3.2.2. Bead Coating
3.3. Development of a Surface Coating Technique
3.3.1. Compression Force
3.3.2. Spraying Procedure
3.3.3. Comparison to Bead Coating
3.3.4. Repeatability
3.4. Determination of the Highest Possible Drug Loading: Spray Drying
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ricarte, R.G.; Van Zee, N.J.; Li, Z.; Johnson, L.M.; Lodge, T.P.; Hillmyer, M.A. Recent Advances in Understanding the Micro-and Nanoscale Phenomena of Amorphous Solid Dispersions. Mol. Pharm. 2019, 16, 4089–4103. [Google Scholar] [CrossRef] [PubMed]
- Filo Vasconcelos, T.; Sarmento, B.; Costa, P. Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs. Drug Discov. Today 2007, 12, 1068–1075. [Google Scholar] [CrossRef]
- Jermain, S.V.; Brough, C.; Iii, R.O.W. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2017, 535, 379–392. [Google Scholar] [CrossRef]
- Luebbert, C.; Huxoll, F.; Sadowski, G. Amorphous-amorphous phase separation in API/polymer formulations. Molecules 2017, 22, 296. [Google Scholar] [CrossRef]
- Hrovat, K. Methods of amorphization and investigation of the amorphous state. Acta Pharm. 2013, 63, 305–334. [Google Scholar]
- Patel, B.B.; Patel, J.K.; Chakraborty, S.; Shukla, D. Revealing facts behind spray dried solid dispersion technology used for solubility enhancement. Saudi Pharm. J. 2015, 23, 352–365. [Google Scholar] [CrossRef] [Green Version]
- Vaka, S.; Bommana, M.; Desai, D.; Djordjevic, J.; Phuapradit, W.; Shah, N. Chapter 4: Excipients for amorphous solid dispersions. In Amorphous Solid Dispersions; Springer: New York, NY, USA, 2014; pp. 123–161. [Google Scholar]
- Jijun, F.; Lishuang, X.; Xiaoli, W.; Shu, Z.; Xiaoguang, T.; Xingna, Z.; Haibing, H.; Xing, T. Nimodipine (NM) tablets with high dissolution containing NM solid dispersions prepared by hot-melt extrusion. Drug Dev. Ind. Pharm. 2011, 37, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Worku, Z.A.; Paudel, A.; Van den Mooter, G. Can compression induce demixing in amorphous solid dispersions ? A case study of naproxen—PVP K25. Eur. J. Pharm. Biopharm. 2012, 81, 207–213. [Google Scholar]
- Worku, Z.A.; Paudel, A.; Rombaut, P.; Van den Mooter, G. Effect of Compression on Non-isothermal Crystallization Behaviour of Amorphous Indomethacin. Pharm. Res. 2012, 29, 2489–2498. [Google Scholar]
- Singh, A.; Bharati, A.; Frederiks, P.; Verkinderen, O.; Goderis, B.; Cardinaels, R.; Moldenaers, P.; Van Humbeeck, J.; Van den Mooter, G. Effect of Compression on the Molecular Arrangement of Itraconazole−Soluplus Solid Dispersions: Induction of Liquid Crystals or Exacerbation of Phase Separation? Mol. Pharm. 2016, 13, 1879–1893. [Google Scholar] [CrossRef]
- Prasad, M.B.; Vidyadhara, S.; Sasidhar, R.L.C.; Balakrishna, T.; Trilochani, P. Development and evaluation of diltiazem hydrochloride controlled-release pellets by fluid bed coating process. J. Adv. Pharm. Technol. Res. 2013, 4, 101–107. [Google Scholar]
- Dereymaker, A.; Scurr, D.J.; Steer, E.D.; Roberts, C.J.; Van den Mooter, G. Controlling the Release of Indomethacin from Glass Solutions Layered with a Rate Controlling Membrane Using Fluid-Bed Processing. Part 1: Surface and Cross-Sectional Chemical Analysis. Mol. Pharm. 2017, 14, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, G.; Klein, S. Dissolution testing of oral modified-release dosage forms. J. Pharm. Pharmacol. 2011, 64, 944–968. [Google Scholar] [CrossRef] [PubMed]
- Van Gyseghem, E.; Baert, L.; Van Remoortere, P.; Van ’t Klooster, G.; Rouan, M.-C.; Voorspoels, J.; De Kock, H.; Schueller, L.; Rosier, J.; Grooten, L.; et al. Co-administration of darunavir and a new pharmacokinetic booster: Formulation strategies and evaluation in dogs. Eur. J. Pharm. Sci. 2010, 41, 193–200. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, N.; Wu, B.; Lu, Y.; Guan, T.; Wu, W. Physical characterization of lansoprazole/PVP solid dispersion prepared by fluid-bed coating technique. Powder Technol. 2008, 182, 480–485. [Google Scholar] [CrossRef]
- Kolašinac, N.; Kachrimanis, K.; Djuriš, J.; Homšek, I.; Grujić, B.; Ibrić, S. Spray coating as a powerful technique in preparation of solid dispersions with enhanced desloratadine dissolution rate. Drug Dev. Ind. Pharm. 2013, 39, 1020–1027. [Google Scholar] [CrossRef]
- Sun, N.; Wei, X.; Wu, B.; Chen, J.; Lu, Y.; Wu, W. Enhanced dissolution of silymarin/polyvinylpyrrolidone solid dispersion pellets prepared by a one-step fluid-bed coating technique. Powder Technol. 2008, 182, 72–80. [Google Scholar] [CrossRef]
- Guns, S.; Dereymaker, A.; Kayaert, P.; Mathot, V.; Martens, J.A.; Van den Mooter, G. Comparison Between Hot-Melt Extrusion and Spray-Drying for Manufacturing Solid Dispersions of the Graft Copolymer of Ethylene Glycol and Vinylalcohol. Pharm. Res. 2011, 28, 673–682. [Google Scholar] [CrossRef]
- Van Eerdenbrugh, B.; Baird, J.A.; Taylor, L.S. Crystallization tendency of active pharmaceutical ingredients following rapid solvent evaporation—Classification and comparison with crystallization tendency from undercooled melts. J. Pharm. Sci. 2010, 99, 3826–3838. [Google Scholar] [CrossRef]
- Baird, J.A.; Taylor, L.S. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv. Drug Deliv. Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef]
- Peeters, O.M.; Blaton, N.M.; Ranter, C.J.D.E. The crystal structure of 1-(2,4-dichloro-beta-((2,4-dichloro-benzyl)oxy)phenethyl) imidazole hemihydrate. Bull. Soc. Chim. Belg. 1979, 88, 265–272. [Google Scholar] [CrossRef]
- Kersten, K.M.; Breen, M.E.; Mapp, A.K.; Matzger, A.J. Pharmaceutical solvate formation for the incorporation of the antimicrobial agent hydrogen peroxide. Chem. Commun. 2018, 54, 9286–9289. [Google Scholar] [CrossRef] [PubMed]
- Boel, E.; Smeets, A.; Vergaelen, M.; De La Rosa, V.R.; Hoogenboom, R.; Van den Mooter, G. Comparative study of the potential of poly(2-ethyl-2-oxazoline) as carrier in the formulation of amorphous solid dispersions of poorly soluble drugs. Eur. J. Pharm. Biopharm. 2019, 144, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]

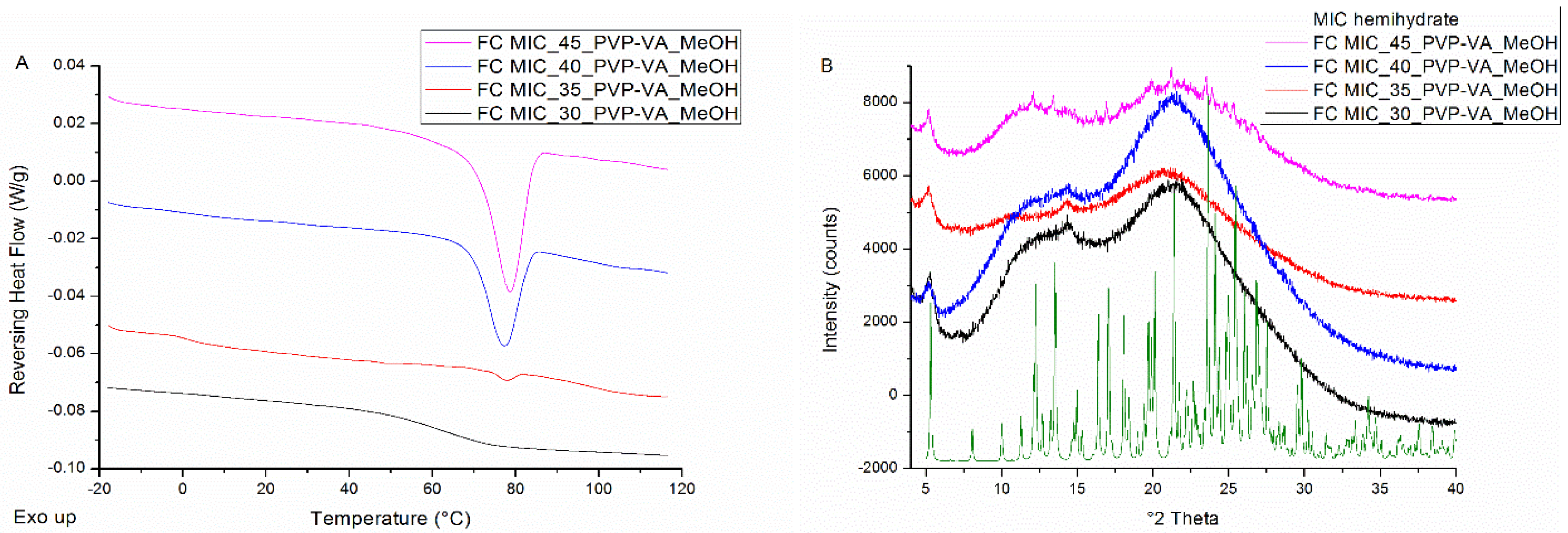
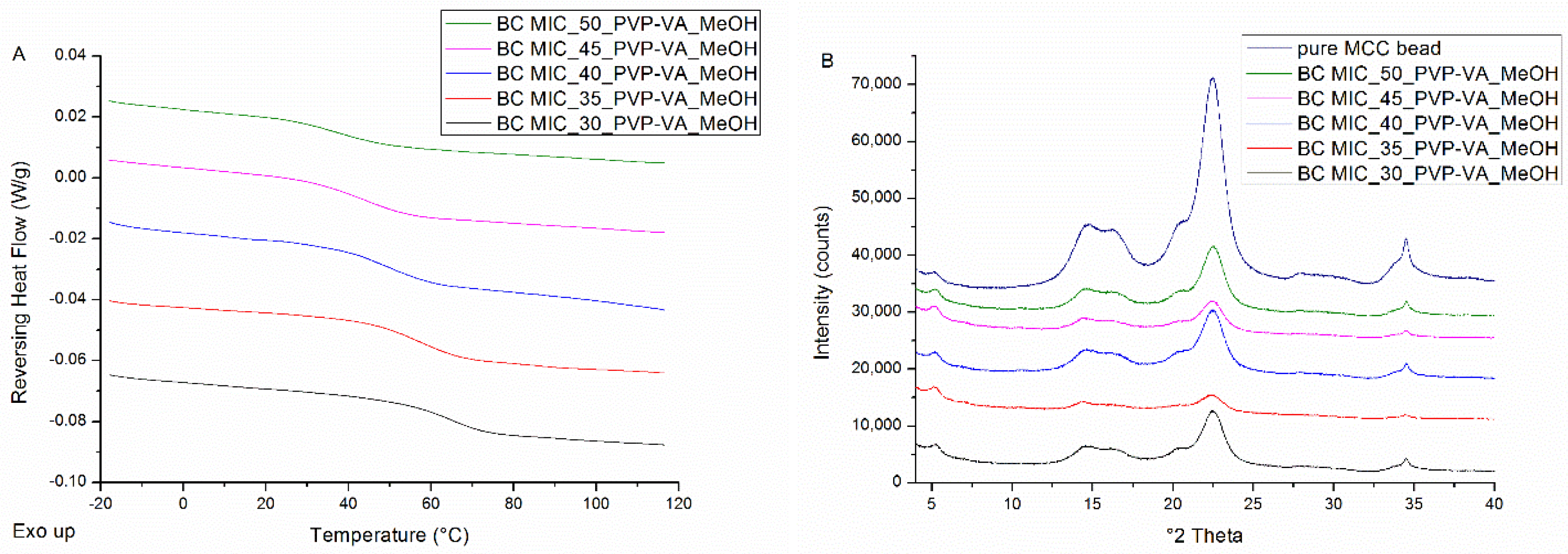
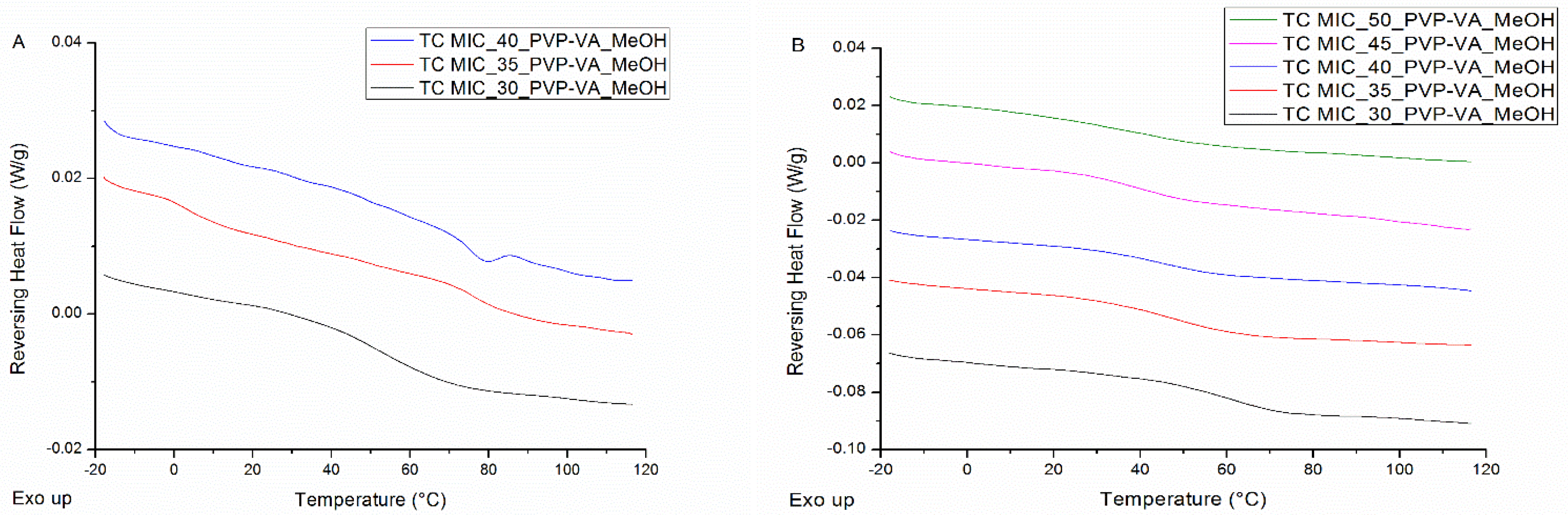
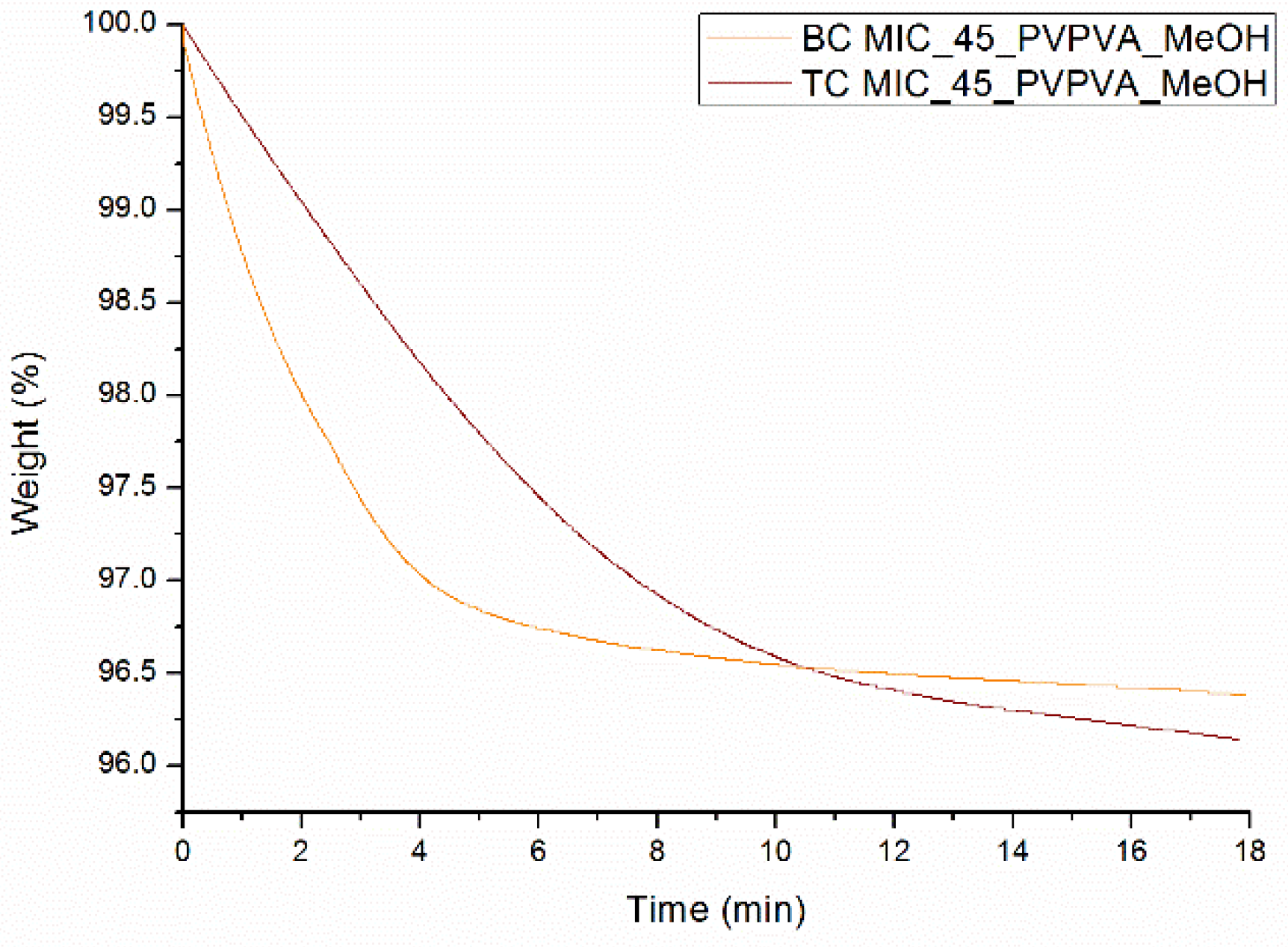

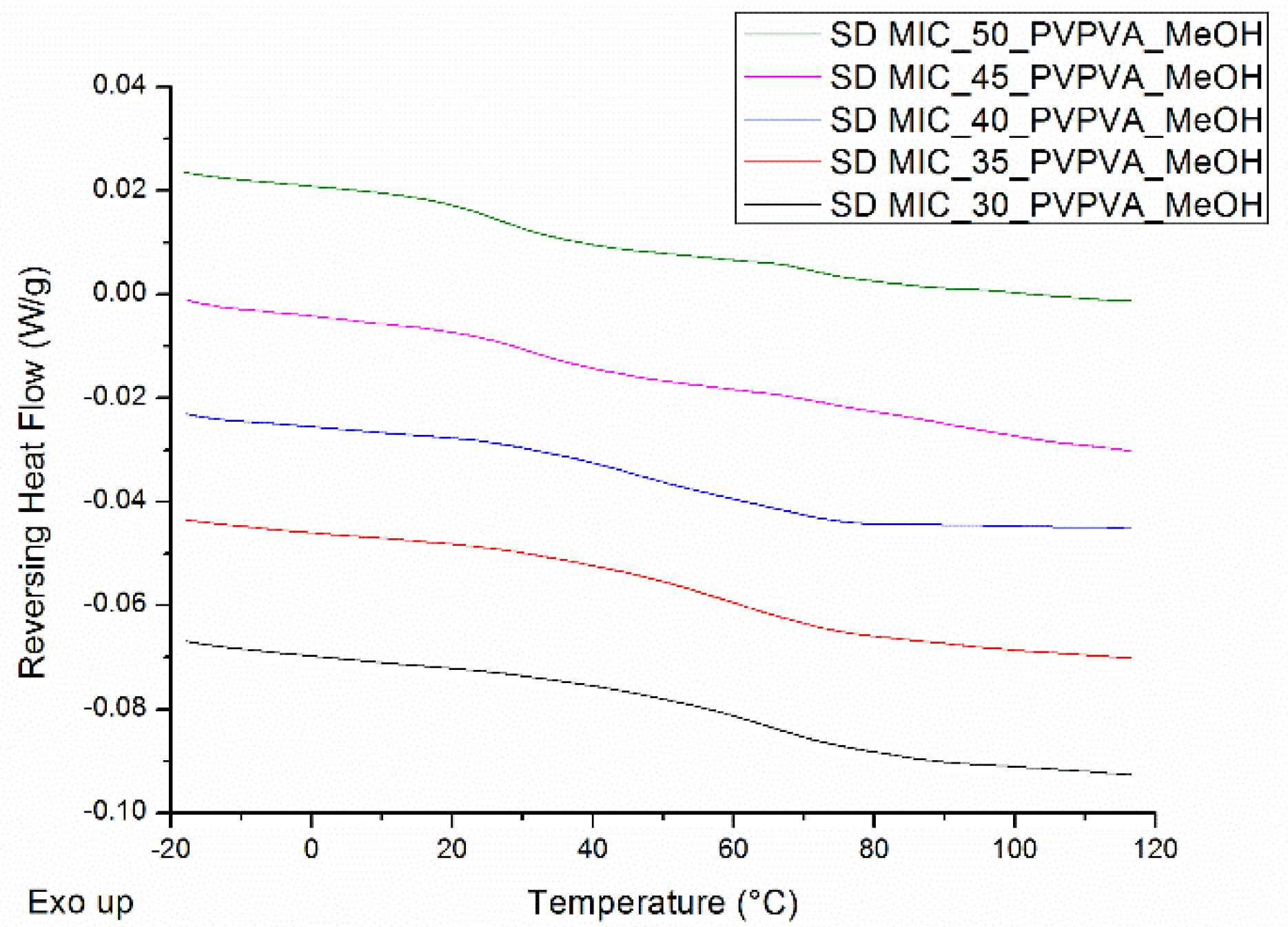
| Drug Weight Fraction (%) | Average Tg Value ± Sd (°C) | Average Tg Width ± Sd (°C) | Average Tm,onset ± Sd (°C) | Average Melting Enthalpy ± Sd (J/g) |
|---|---|---|---|---|
| 30 | 62.4 ± 0.8 | 23.7 ± 4.7 | / | / |
| 35 | 1.7 ± 0.9 (*) | 7.6 ± 1.6 | 75.7 ± 0.6 | 1.1 ± 0.2 |
| 40 | / | / | 75.1 ± 1.2 | 3.6 ± 2.0 |
| 45 | / | / | 69.7 ± 2.9 | 18.0 ± 4.6 |
| Drug Weight Fraction (%) | Average Tg Value ± Sd (°C) | Average Tg Width ± Sd (°C) |
|---|---|---|
| 30 | 65.3 ± 0.9 | 14.1 ± 0.6 |
| 35 | 57.2 ± 1.6 | 14.7 ± 1.0 |
| 40 | 49.6 ± 0.8 | 16.7 ± 3.3 |
| 45 | 43.8 ± 1.0 | 18.5 ± 1.1 |
| 50 | 36.4 ± 0.3 | 17.9 ± 1.4 |
| Drug Weight Fraction (%) | Average Tg Value ± Sd (°C) IM 1 | Average Tg Width ± Sd (°C) IM 1 | Average Tg Value ± Sd (°C) IM 2 | Average Tg Width ± Sd (°C) IM 2 |
|---|---|---|---|---|
| 30 | 60.1 ± 2.5 | 18.4 ± 3.8 | 60.0 ± 1.7 | 15.8 ± 1.6 |
| 35 | 50.5 ± 0.9 | 23.1 ± 3.3 | 47.8 ± 0.5 | 24.7 ± 2.8 |
| 40 | 45.0 ± 0.4 | 17.7 ± 1.0 | 45.5 ± 1.5 | 16.1 ± 2.7 |
| 45 | 37.0 ± 1.1 | 19.8 ± 0.7 | 35.9 ± 2.2 | 16.8 ± 8.0 |
| 50 | 31.8 ± 5.1 | 30.4 ± 7.6 | 34.1 ± 0.3 | 17.9 ± 0.8 |
| Sample | Average Tg Value ± Sd (°C) Batch 1 | Average Tg Value ± Sd (°C) Batch 2 | Average Tg Value ± Sd (°C) Batch 3 |
|---|---|---|---|
| TC_MIC_35_PVP-VA_MeOH_IM1 | 50.5 ± 0.9 | 54.1 ± 0.7 | 49.1 ± 6.1 |
| TC_MIC_35_PVP-VA_MeOH_IM2 | 47.8 ± 0.5 | 53.3 ± 1.2 | 55.3 ± 1.0 |
| TC_MIC_45_PVP-VA_MeOH_IM1 | 37.0 ± 1.1 | 33.1 ± 4.4 | 37.5 ± 3.1 |
| TC_MIC_45_PVP-VA_MeOH_IM2 | 35.9 ± 2.2 | 35.4 ± 1.6 | 38.4 ± 2.5 |
| Sample | Average Tg Value(s) ± Sd (°C) Batch 1 | Average Tg Value(s) ± Sd (°C) Batch 2 | Average Tg Value(s) ± Sd (°C) Batch 3 |
|---|---|---|---|
| SD_MIC_35_PVP-VA_MeOH_IM1 | 52.6 ± 4.7 | 51.6 ± 0.4 | 51.2 ± 3.2 |
| SD_MIC_35_PVP-VA_MeOH_IM2 | 52.5 ± 4.6 | 50.0 ± 1.3 | 49.6 ± 2.5 |
| SD_MIC_45_PVP-VA_MeOH_IM1 | 34.7 ± 3.3 71.4 ± 0.5 | 36.1 ± 0.6 71.8 ± 1.6 | 36.9 ± 1.9 69.9 ± 2.6 |
| SD_MIC_45_PVP-VA_MeOH_IM2 | 35.5 ± 3.7 71.7 ± 3.4 | 37.6 ± 0.9 (*) | 37.4 ± 0.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boel, E.; Panini, P.; Van den Mooter, G. Development of a Surface Coating Technique with Predictive Value for Bead Coating in the Manufacturing of Amorphous Solid Dispersions. Pharmaceutics 2020, 12, 878. https://doi.org/10.3390/pharmaceutics12090878
Boel E, Panini P, Van den Mooter G. Development of a Surface Coating Technique with Predictive Value for Bead Coating in the Manufacturing of Amorphous Solid Dispersions. Pharmaceutics. 2020; 12(9):878. https://doi.org/10.3390/pharmaceutics12090878
Chicago/Turabian StyleBoel, Eline, Piyush Panini, and Guy Van den Mooter. 2020. "Development of a Surface Coating Technique with Predictive Value for Bead Coating in the Manufacturing of Amorphous Solid Dispersions" Pharmaceutics 12, no. 9: 878. https://doi.org/10.3390/pharmaceutics12090878
APA StyleBoel, E., Panini, P., & Van den Mooter, G. (2020). Development of a Surface Coating Technique with Predictive Value for Bead Coating in the Manufacturing of Amorphous Solid Dispersions. Pharmaceutics, 12(9), 878. https://doi.org/10.3390/pharmaceutics12090878






