Formulation of Sodium Valproate Nanospanlastics as a Promising Approach for Drug Repurposing in the Treatment of Androgenic Alopecia
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of SV-Loaded Spanlastic Nanovesicles (SNVs)
2.2.2. In Vitro Characterization of SV-Loaded SNVs
Determination of Entrapment Efficiency of SV-Loaded SNVs
In Vitro Release Study of SV-Loaded SNVs
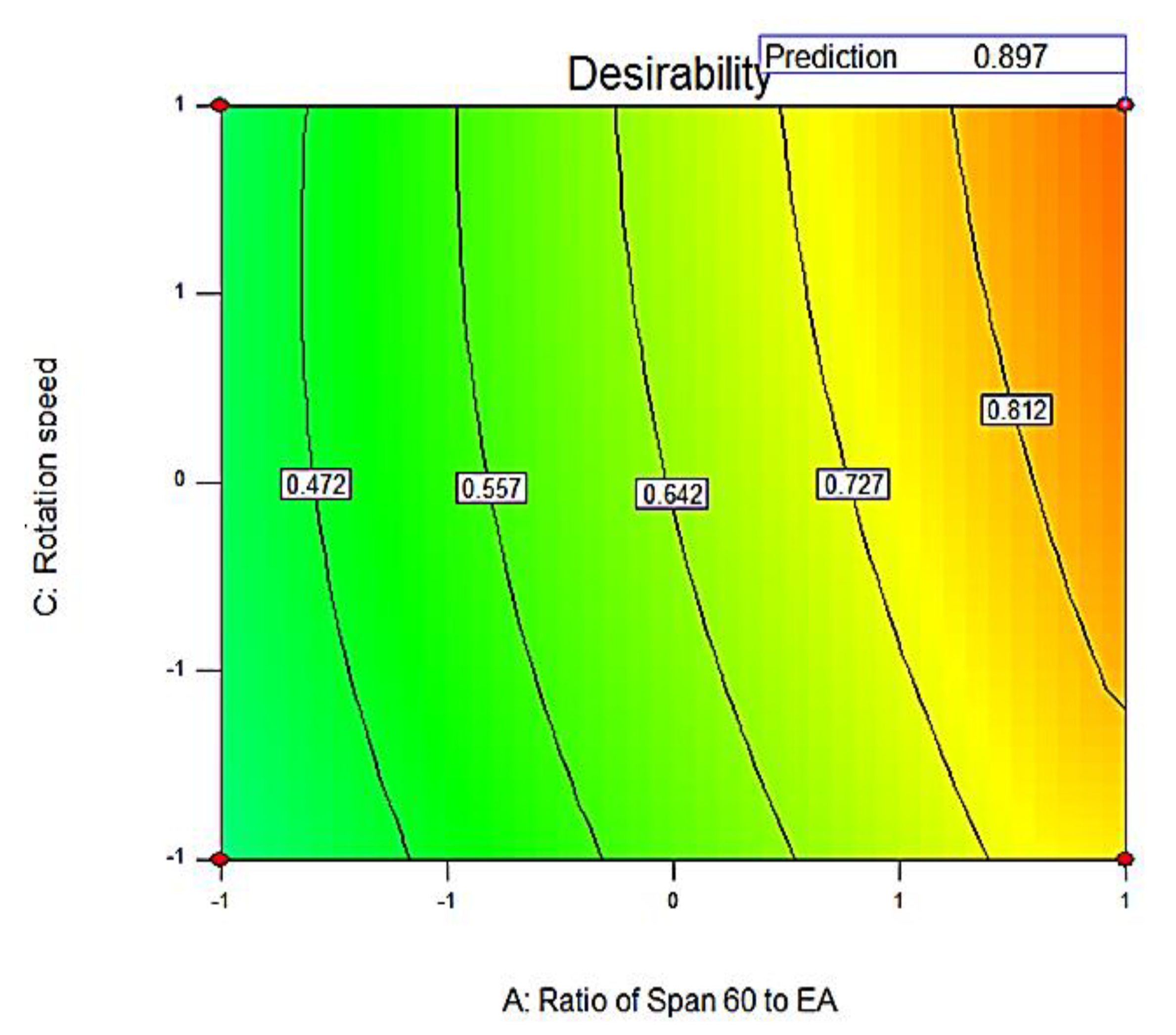
Statistical Optimization of SV-Loaded SNVs
2.2.3. Characterization of the Optimized SV-Loaded SNVs
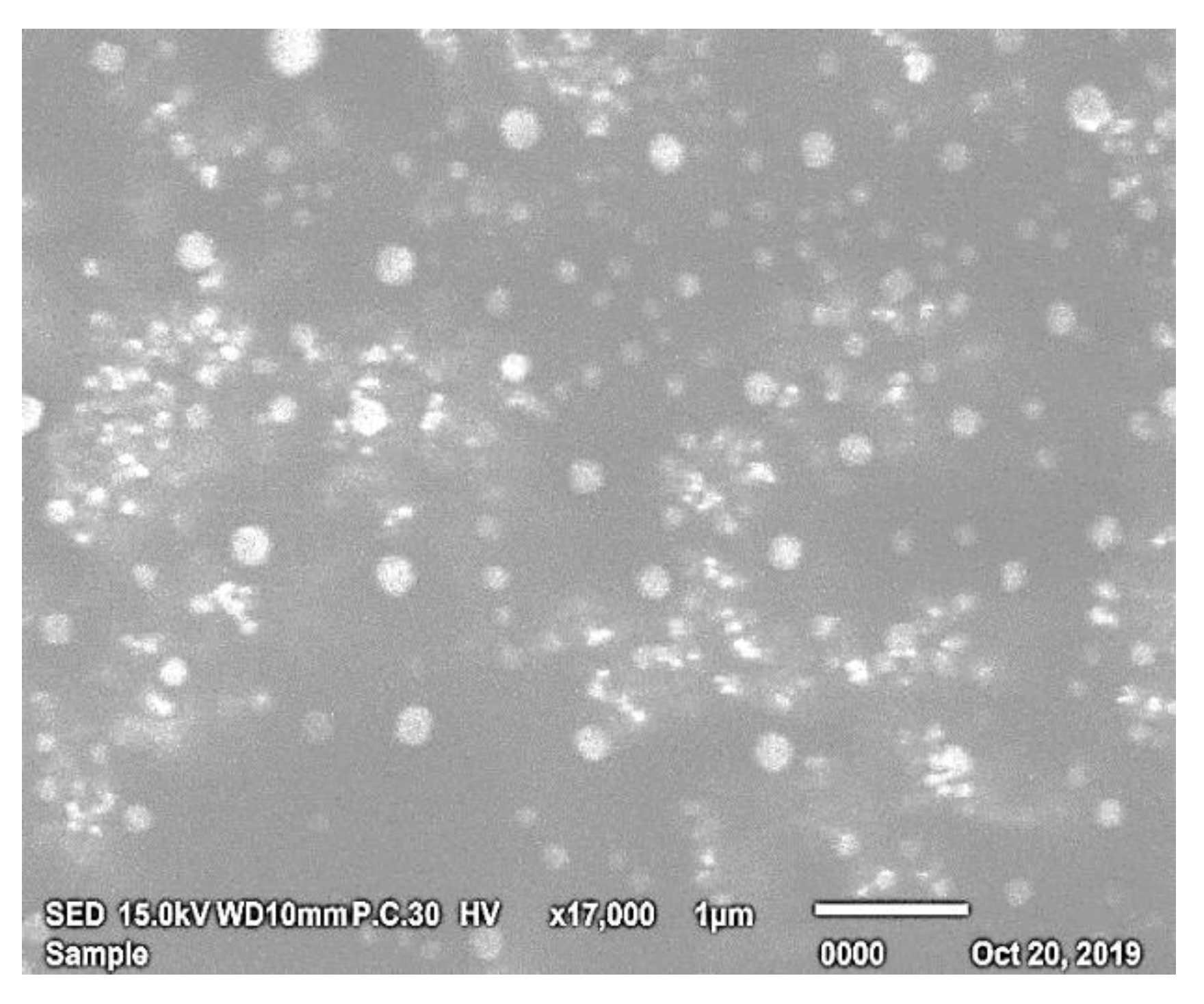
Morphological Characterization by Scanning Electron Microscopy (SEM)
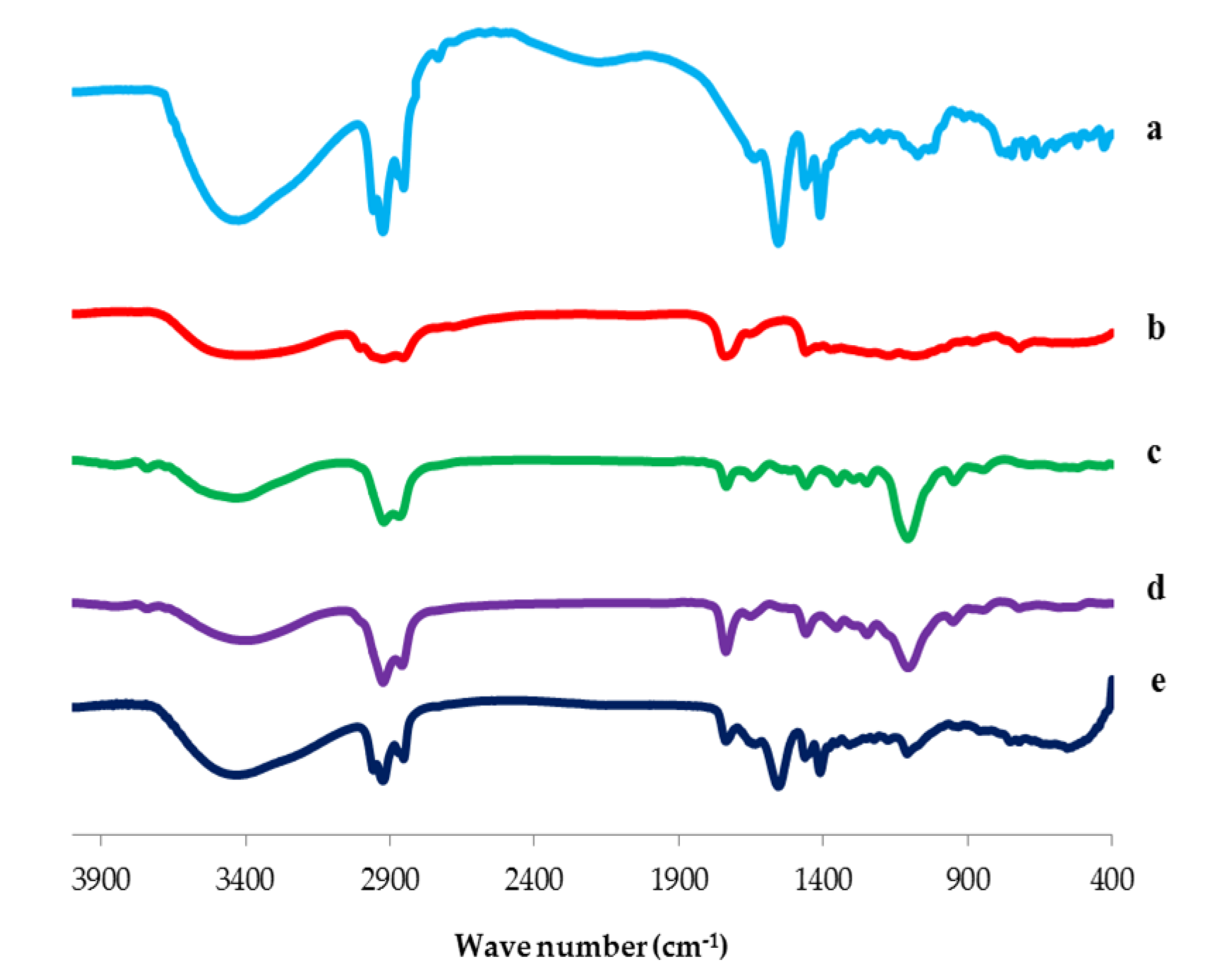
Fourier Transform Infrared Spectroscopy (FTIR)
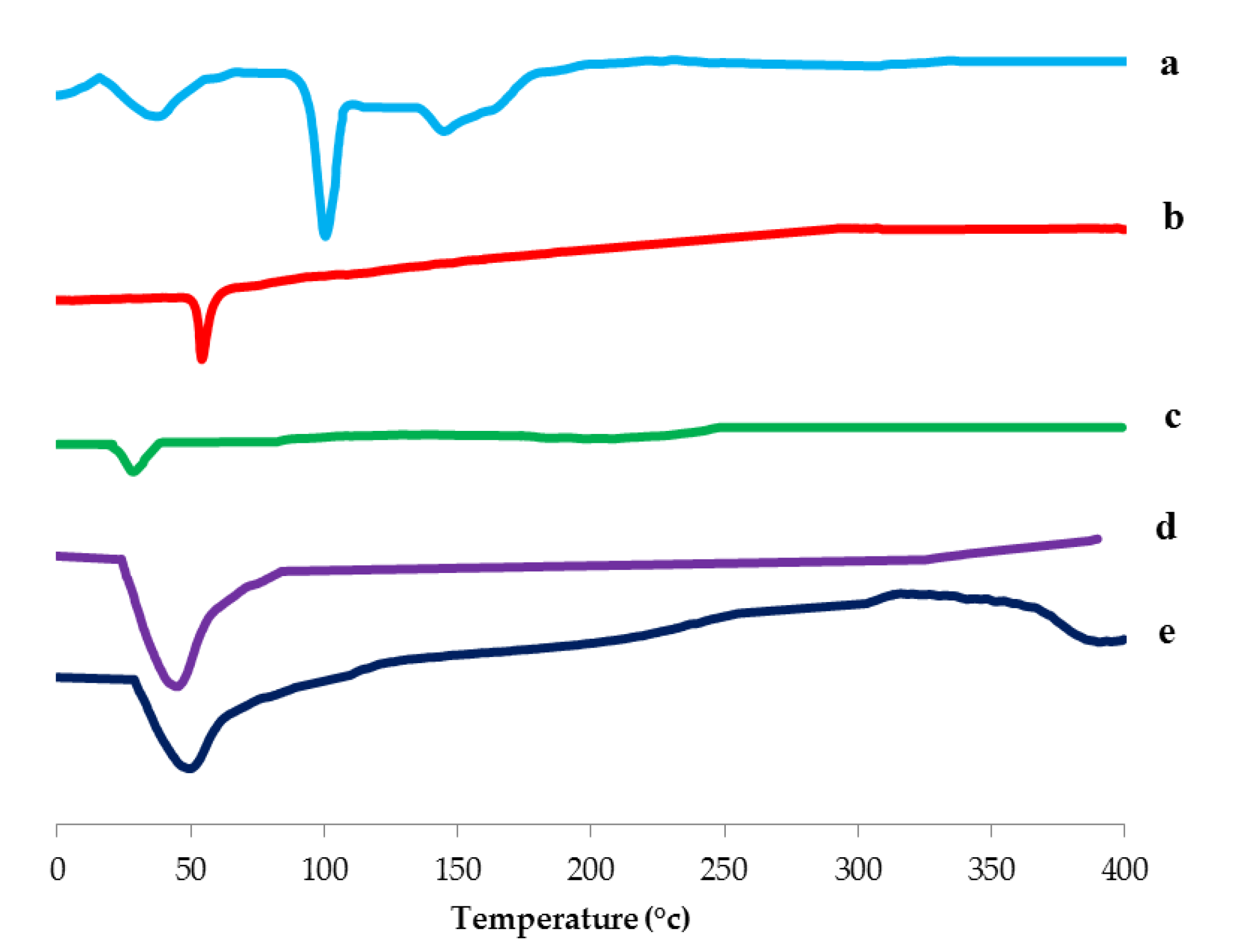
Differential Scanning Calorimetry (DSC) Study
Determination of Particle Size and Zeta Potential of SV-Loaded SNVs
Measurement of Vesicle Elasticity
Comparative Ex Vivo Permeation between the Optimized SV-Loaded SNVs and Free Drug
2.2.4. Comparative Study between the Optimized SV-Loaded SVs and Marketed Minoxidil Lotion by Dermoscopic Evaluation for the Treatment of Androgenic Alopecia
Ethical Consideration
Patient Selection
- (a)
- Inclusion Criteria
- (b)
- Exclusion Criteria
Method
2.2.5. HPLC Assay of SV
2.2.6. Statistical Analysis
3. Results
3.1. The Preliminary Screening Study
3.2. Analysis of Factorial Design
3.2.1. The Effect of Formulation Variables on EE% of SV-Loaded SNVs
3.2.2. The Effect of Formulation Variables on Q12h from SV-Loaded SNVs
3.2.3. Optimization of SV-Loaded Nanospanlastics
3.3. Characterization of the Optimized SV-Loaded SNVs
3.3.1. Morphological Characterization by SEM
3.3.2. Fourier Transform Infrared (FTIR) Spectroscopy
3.3.3. Differential Scanning Calorimetry (DSC) Study
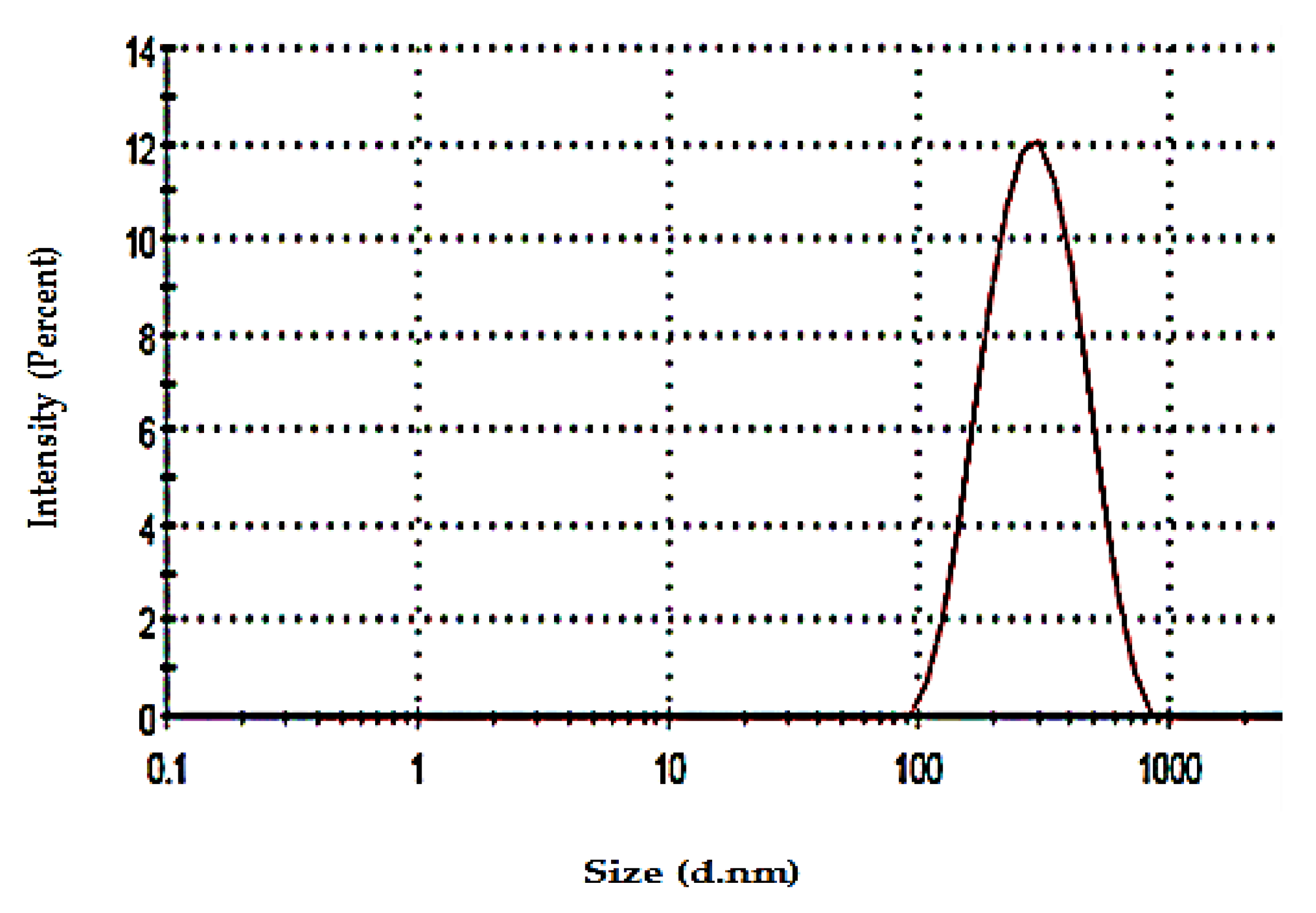
3.3.4. Determination of Particle Size and Zeta Potential
3.3.5. Measurement of Vesicle Elasticity
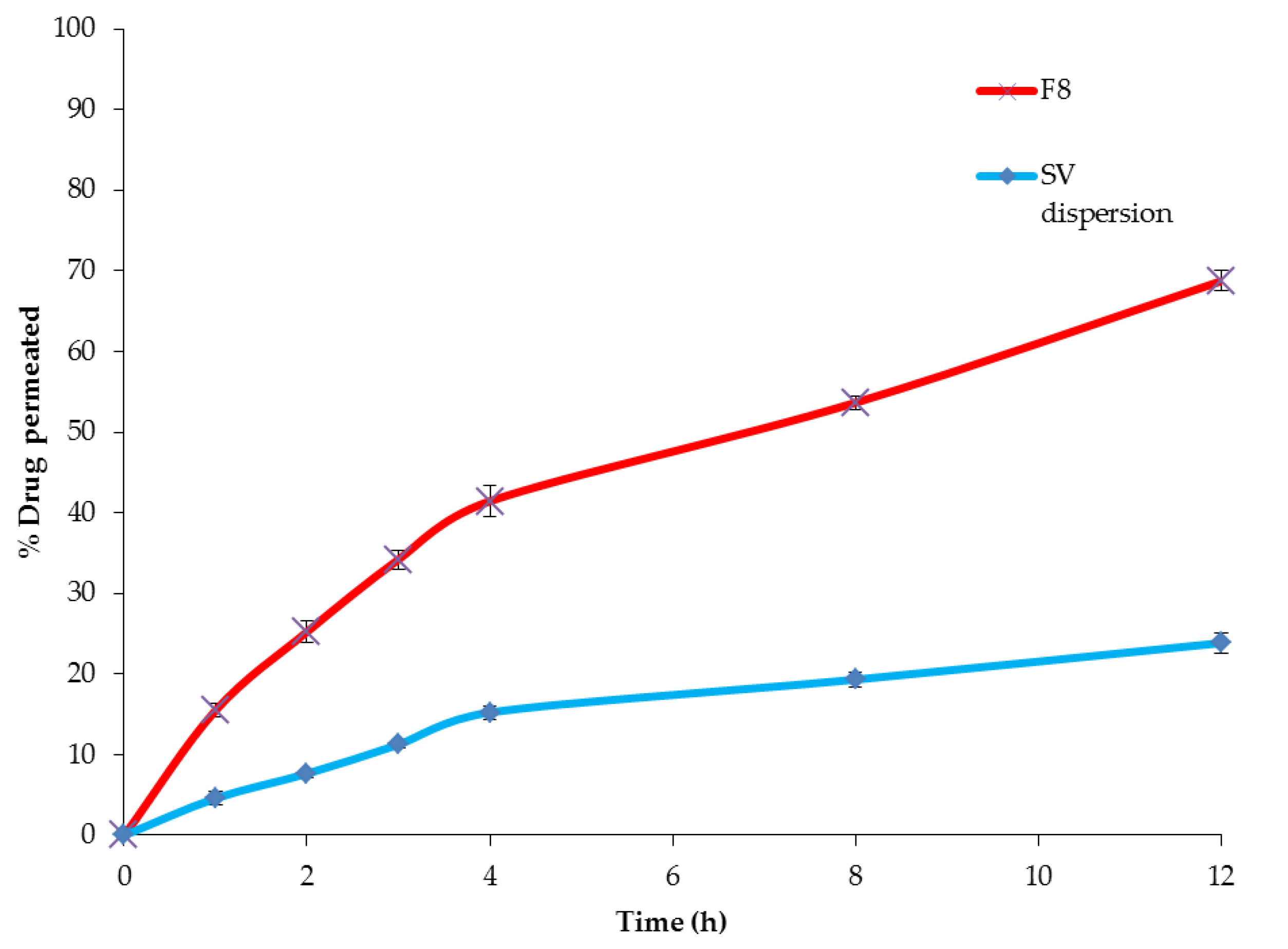
3.3.6. Comparative ex vivo Permeation between the Optimized SV-Loaded SNVs and Free Drug
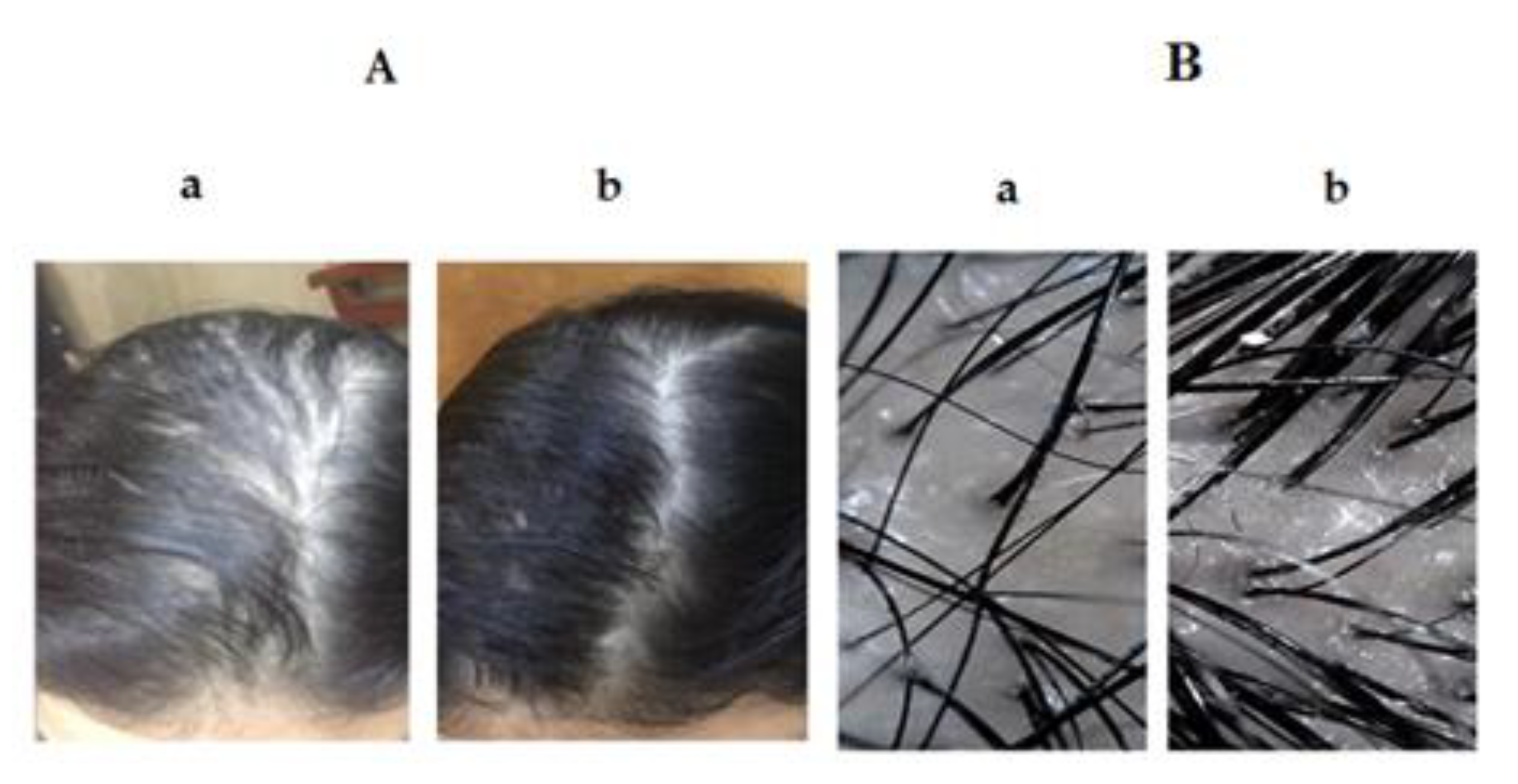
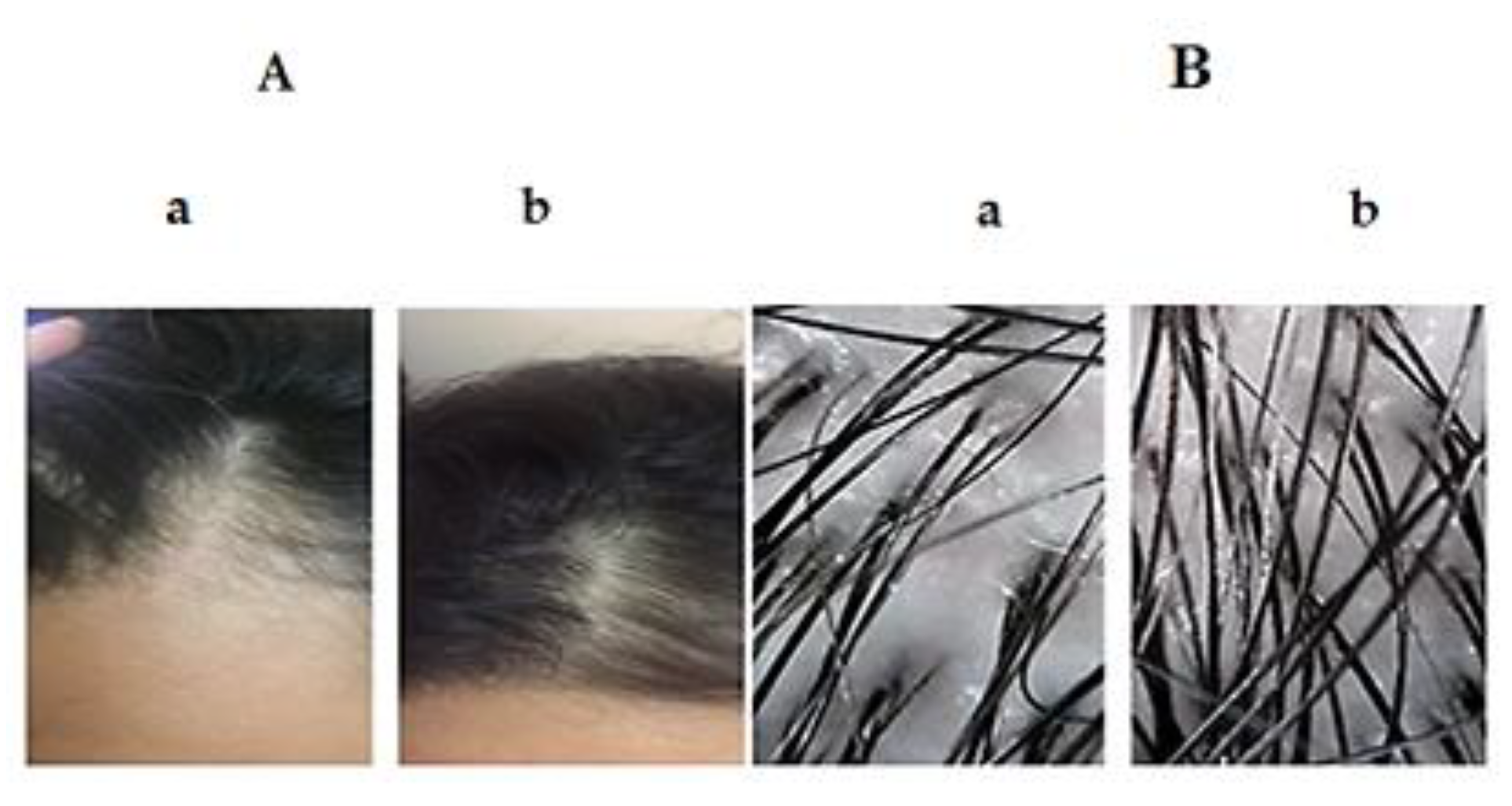
3.4. Comparative Study between the Optimized SV-Loaded SVs and Marketed Minoxidil Lotion by Dermoscopic Evaluation for Treatment of AGA
3.4.1. Distribution of Cases in the Study Groups
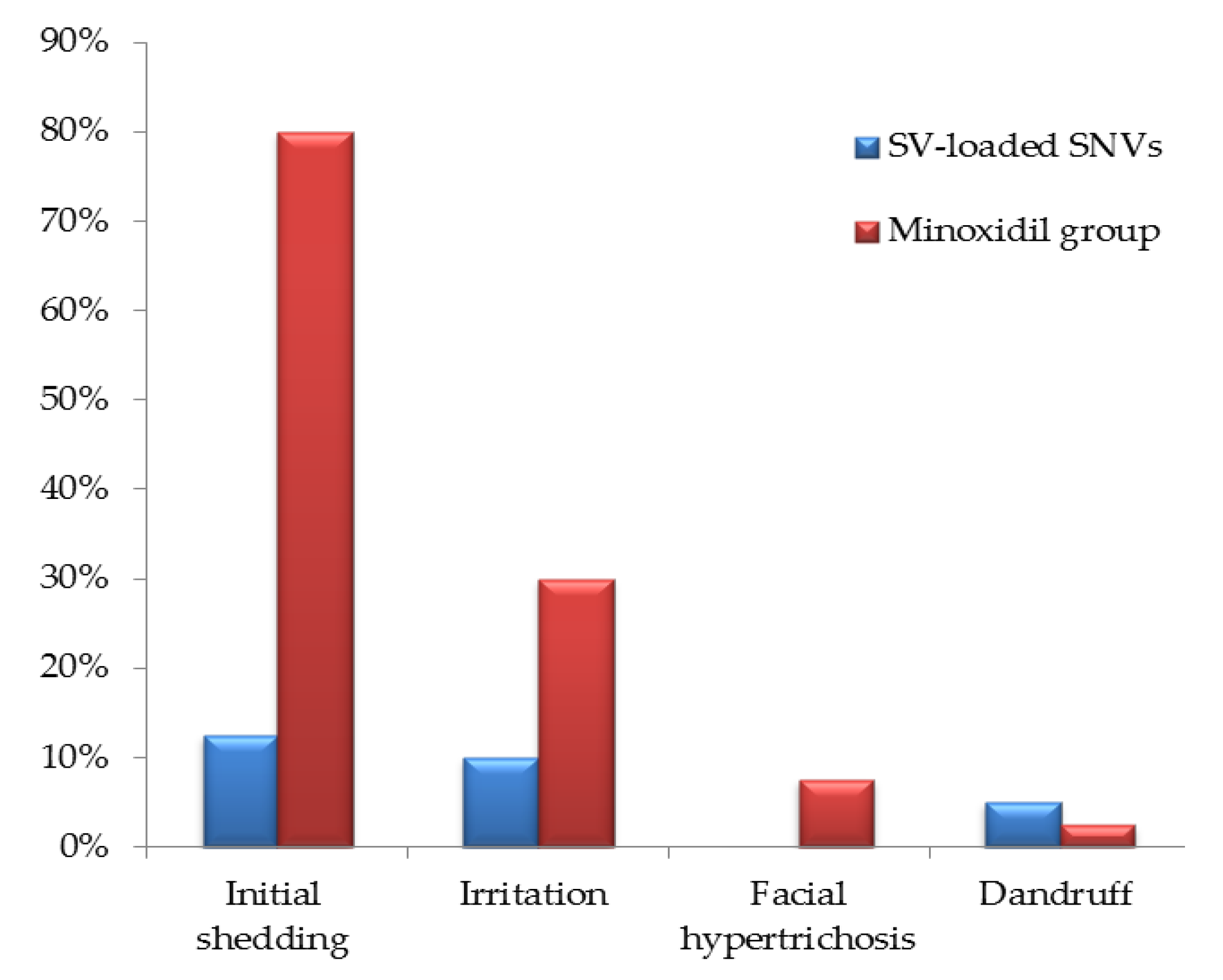
3.4.2. Result Analysis Using Dermoscopic Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zarkesh, K.; Khazaeli, P.; Pardakhty, A.; Rezaifar, M. Preparation and Physicochemical Characterization of Topical Niosomal Formulation of Minoxidil and Tretinoin; School of Pharmacy, Kerman University of Medical Sciences: Kerman, Iran, 2016. [Google Scholar]
- Agarwal, S.; Godse, K.; Mahajan, A.; Patil, S.; Nadkarni, N. Application of the basic and specific classification on patterned hair loss in Indians. Int. J. Trichol. 2013, 5, 126. [Google Scholar] [CrossRef]
- Gajjar, P.C.; Mehta, H.H.; Barvaliya, M.; Sonagra, B. Comparative study between mesotherapy and topical 5% minoxidil by dermoscopic evaluation for androgenic alopecia in male: A randomized controlled trial. Int. J. Trichol. 2019, 11, 58. [Google Scholar] [CrossRef]
- Levy, L.L.; Emer, J.J. Female pattern alopecia: Current perspectives. Int. J. Women’s Health 2013, 5, 541. [Google Scholar]
- Egbuna, C.; Kumar, S.; Ifemeje, J.C.; Ezzat, S.M.; Kaliyaperumal, S. Phytochemicals as Lead Compounds for New Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Chandrashekar, B.; Nandhini, T.; Vasanth, V.; Sriram, R.; Navale, S. Topical minoxidil fortified with finasteride: An account of maintenance of hair density after replacing oral finasteride. Indian Dermatol. Online J. 2015, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- Kakunje, A.; Sindhu, P.; Prabhu, A.; Karkal, R.; Kumar, P.; Gupta, N.; Pookoth, R. Topical Valproate Solution for Hair Growth. Online J. Health Allied Sci. 2018, 17, 14. [Google Scholar]
- Despland, P. Tolerance to and unwanted effects of valproate sodium. Praxis 1994, 83, 1132–1139. [Google Scholar] [PubMed]
- Hamidi, M.; Azadi, A.; Mohamadi-Samani, S.; Rafiei, P.; Ashrafi, H. Valproate-Loaded hydrogel nanoparticles: Preparation and characterization. J. Appl. Polym. Sci. 2012, 124, 4686–4693. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.-S.; Min, D.S.; Kim, H.-Y.; Choi, K.-Y. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef]
- Jo, S.J.; Shin, H.; Park, Y.W.; Paik, S.H.; Park, W.S.; Jeong, Y.S.; Shin, H.J.; Kwon, O. Topical valproic acid increases the hair count in male patients with androgenetic alopecia: A randomized, comparative, clinical feasibility study using phototrichogram analysis. J. Dermatol. 2014, 41, 285–291. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, H.D.; Kim, B.J.; Kim, M.N.; Han, D.H. A case of androgenetic alopecia treated with valproic acid. Int. J. Dermatol. 2014, 53, e214–e215. [Google Scholar] [CrossRef]
- Ramezani, V.; Honarvar, M.; Seyedabadi, M.; Karimollah, A.; Ranjbar, A.M.; Hashemi, M. Formulation and optimization of transfersome containing minoxidil and caffeine. J. Drug Deliv. Sci. Technol. 2018, 44, 129–135. [Google Scholar] [CrossRef]
- Farghaly, D.A.; Aboelwafa, A.A.; Hamza, M.Y.; Mohamed, M.I. Topical delivery of fenoprofen calcium via elastic nano-vesicular spanlastics: Optimization using experimental design and in vivo evaluation. AAPS PharmSciTech 2017, 18, 2898–2909. [Google Scholar] [CrossRef] [PubMed]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Enhanced non invasive trans-tympanic delivery of ciprofloxacin through encapsulation into nano-spanlastic vesicles: Fabrication, in-vitro characterization, and comparative ex-vivo permeation studies. Int. J. Pharm. 2017, 522, 157–164. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Database of Select Committee on GRAS Substances (SCOGS) Reviews; FDA Database: Silver Spring, MD, USA, 2012.
- US Food and Drug Administration. Inactive Ingredient Search for Approved Drug Products; FDA Database: Silver Spring, MD, USA, 2017.
- Salim, M.; Minamikawa, H.; Sugimura, A.; Hashim, R. Amphiphilic designer nano-carriers for controlled release: From drug delivery to diagnostics. MedChemComm 2014, 5, 1602–1618. [Google Scholar] [CrossRef]
- Abdelbary, G.A.; Amin, M.M.; Zakaria, M.Y. Ocular ketoconazole-loaded proniosomal gels: Formulation, ex vivo corneal permeation and in vivo studies. Drug Deliv. 2017, 24, 309–319. [Google Scholar] [CrossRef]
- Fahmy, A.M.; El-Setouhy, D.A.; Ibrahim, A.B.; Habib, B.A.; Tayel, S.A.; Bayoumi, N.A. Penetration enhancer-containing spanlastics (PECSs) for transdermal delivery of haloperidol: In vitro characterization, ex vivo permeation and in vivo biodistribution studies. Drug Deliv. 2018, 25, 12–22. [Google Scholar] [CrossRef]
- El Gamal, S.S.; Naggar, V.F.; Allam, A.N. Optimization of acyclovir oral tablets based on gastroretention technology: Factorial design analysis and physicochemical characterization studies. Drug Dev. Ind. Pharm. 2011, 37, 855–867. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- Larsson, J. Methods for Measurement of Solubility and Dissolution Rate of Sparingly Soluble Drugs. Master’s Thesis, Lund University, Lund, Sweden, 2009. [Google Scholar]
- El-Menshawe, S.F.; Ali, A.A.; Halawa, A.A.; El-Din, A.S.S. A novel transdermal nanoethosomal gel of betahistine dihydrochloride for weight gain control: In-vitro and in-vivo characterization. Drug Des. Dev. Ther. 2017, 11, 3377. [Google Scholar] [CrossRef][Green Version]
- Ciurba, A.; Todoran, N.; Tăurean, A.; Antonoaea, P.; Hancu, G.; Moisei, A.; Sipos, E. Kinetic analysis of in vitro drug release from valproic acid and sodium valproate suppositories. Farmacia 2014, 62, 1143–1156. [Google Scholar]
- Chaudhari, S.P.; Chatur, V.M. Development of valproic acid niosomal in situ nasal gel formulation for epilepsy. Indian J. Pharm. Educ. Res. 2013, 16, 31–41. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.K.; Handa, V.; Kathuria, H. Oleic acid nanovesicles of minoxidil for enhanced follicular delivery. Medicines 2018, 5, 103. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.R. Body Temperature Regulation; University of Cincinnati Raymond Walters College: Blue Ash, OH, USA, 1996. [Google Scholar]
- Abd-Elal, R.M.; Shamma, R.N.; Rashed, H.M.; Bendas, E.R. Trans-nasal zolmitriptan novasomes: In-vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug Deliv. 2016, 23, 3374–3386. [Google Scholar] [CrossRef]
- Mazyed, E.A.; Abdelaziz, A.E. Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study. Pharmaceutics 2020, 12, 465. [Google Scholar] [CrossRef]
- Bansal, S.; Aggarwal, G.; Chandel, P.; Harikumar, S. Design and development of cefdinir niosomes for oral delivery. J. Pharm. Bioallied Sci. 2013, 5, 318. [Google Scholar]
- Mazyed, E.A.; Zakaria, S. Enhancement of dissolution characteristics of clopidogrel bisulphate by proniosomes. Int. J. Appl. Pharm. 2019, 11, 77–85. [Google Scholar] [CrossRef]
- Gupta, A.; Prajapati, S.K.; Balamurugan, M.; Singh, M.; Bhatia, D. Design and development of a proniosomal transdermal drug delivery system for captopril. Trop. J. Pharm. Res. 2007, 6, 687–693. [Google Scholar] [CrossRef]
- Al-mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Nano-transfersomal ciprofloxacin loaded vesicles for non-invasive trans-tympanic ototopical delivery: In-vitro optimization, ex-vivo permeation studies, and in-vivo assessment. Int. J. Pharm. 2014, 472, 304–314. [Google Scholar] [CrossRef]
- Mali, N.; Darandale, S.; Vavia, P. Niosomes as a vesicular carrier for topical administration of minoxidil: Formulation and in vitro assessment. Drug Deliv. Transl. Res. 2013, 3, 587–592. [Google Scholar] [CrossRef]
- Hollands, C. The Animals (scientific procedures) Act 1986. Lancet 1986, 2, 32. [Google Scholar] [CrossRef]
- Directive, E. 63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.A.; Rizq, W.Y. Finasteride nano-transferosomal gel formula for management of androgenetic alopecia: Ex vivo investigational approach. Drug Des. Dev. Ther. 2018, 12, 2259. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Ahmad, F.J.; Singh, R.M.; Singh, G.N. 3-Acetyl-11-keto-β-boswellic acid loaded-polymeric nanomicelles for topical anti-inflammatory and anti-arthritic activity. J. Pharm. Pharmacol. 2010, 62, 273–278. [Google Scholar] [CrossRef]
- Lemaire, F.J. A European Directive for Clinical Research; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373. [Google Scholar]
- Hamilton, J.B. Patterned loss of hair in man: Types and incidence. Ann. N. Y. Acad. Sci. 1951, 53, 708–728. [Google Scholar] [CrossRef]
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br. J. Dermatol. 1977, 97, 247–254. [Google Scholar] [CrossRef]
- Kim, J.; Shin, W. How to do random allocation (randomization). Clin. Orthop. Surg. 2014, 6, 103–109. [Google Scholar] [CrossRef]
- Phaechamud, T.; Mueannoom, W.; Tuntarawongsa, S.; Chitrattha, S. Preparation of coated valproic acid and sodium valproate sustained-release matrix tablets. Indian J. Pharm. Sci. 2010, 72, 173. [Google Scholar] [CrossRef]
- ICH, I. Q2 (R1): Validation of analytical procedures: Text and methodology. In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, 8–13 November 2005. [Google Scholar]
- Li, D.; Martini, N.; Wu, Z.; Wen, J. Development of an isocratic HPLC method for catechin quantification and its application to formulation studies. Fitoterapia 2012, 83, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.; El-gendy, N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Patel, D.; Raval, J. Formulation and evaluation of propranolol hydrochloride-loaded carbopol-934p/ethyl cellulose mucoadhesive microspheres. IJPR 2010, 9, 221. [Google Scholar]
- Araujo, J.; Gonzalez-Mira, E.; Egea, M.; Garcia, M.; Souto, E. Optimization and physicochemical characterization of a triamcinolone acetonide-loaded NLC for ocular antiangiogenic applications. Int. J. Pharm. 2010, 393, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Turk, C.T.S.; Oz, U.C.; Serim, T.M.; Hascicek, C. Formulation and optimization of nonionic surfactants emulsified nimesulide-loaded PLGA-based nanoparticles by design of experiments. AAPS PharmSciTech 2014, 15, 161–176. [Google Scholar] [CrossRef]
- Kao, L.S.; Green, C.E. Analysis of variance: Is there a difference in means and what does it mean? J. Surg. Res. 2008, 144, 158–170. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; Elnaggar, Y.S.; Hamdy, D.A.; Abdallah, O.Y. Novel cremochylomicrons for improved oral bioavailability of the antineoplastic phytomedicine berberine chloride: Optimization and pharmacokinetics. Int. J. Pharm. 2018, 535, 316–324. [Google Scholar] [CrossRef]
- Petchsomrit, A.; Sermkaew, N.; Wiwattanapatapee, R. Effect of alginate and surfactant on physical properties of oil entrapped alginate bead formulation of curcumin. Int. J. Med. Health Biomed Bioeng Pharm. Eng. 2013, 7, 864–868. [Google Scholar]
- Setthacheewakul, S.; Mahattanadul, S.; Phadoongsombut, N.; Pichayakorn, W.; Wiwattanapatapee, R. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur. J. Pharm. Biopharm. 2010, 76, 475–485. [Google Scholar] [CrossRef]
- Modi, A.; Tayade, P. A comparative solubility enhancement profile of valdecoxib with different solubilization approaches. Indian J. Pharm. Sci. 2007, 69, 274. [Google Scholar]
- Moorthi, C.; Krishnan, K.; Manavalan, R.; Kathiresan, K. Preparation and characterization of curcumin–piperine dual drug loaded nanoparticles. Asian Pac. J. Trop. Biomed. 2012, 2, 841–848. [Google Scholar] [CrossRef]
- Basalious, E.B.; Shawky, N.; Badr-Eldin, S.M. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: Development and optimization. Int. J. Pharm. 2010, 391, 203–211. [Google Scholar] [CrossRef] [PubMed]
- John, B. Application of desirability function for optimizing the performance characteristics of carbonitrided bushes. Int. J. Ind. Eng. Comput. 2013, 4, 305–314. [Google Scholar] [CrossRef]
- Das, M.K.; Palei, N.N. Sorbitan ester niosomes for topical delivery of rofecoxib. Indian J. Exp. Biol. 2011, 49, 438–445. [Google Scholar] [PubMed]
- Alsarra, I.A.; Al-Omar, M.; Belal, F. Valproic acid and sodium valproate: Comprehensive profile. Profiles Drug Subst. Excip. Relat. Methodol. 2005, 32, 209–240. [Google Scholar] [PubMed]
- Petruševski, G.; Naumov, P.; Jovanovski, G.; Bogoeva-Gaceva, G.; Ng, S.W. Solid-state forms of sodium valproate, active component of the anticonvulsant drug epilim. ChemMedChem Chem. Enabling Drug Discov. 2008, 3, 1377–1386. [Google Scholar]
- El Maghraby, G.M.; Ghanem, S.F. Preparation and evaluation of rapidly dissolving tablets of raloxifene hydrochloride by ternary system formation. Int. J. Pharm. Pharm. Sci. 2016, 8, 127–136. [Google Scholar]
- El-Sayed, M.M.; Hussein, A.K.; Sarhan, H.A.; Mansour, H.F. Flurbiprofen-loaded niosomes-in-gel system improves the ocular bioavailability of flurbiprofen in the aqueous humor. Drug Dev. Ind. Pharm. 2017, 43, 902–910. [Google Scholar] [CrossRef]
- Srikrishna, T.; Sai Lalith Kumar, M.V.; Vasanth Kumar, P.M.; Sujitha, Y.N.; Shobaran, D. Formulation and evaluation of Sodium Valproate Sustained Released Tablets. J. Glob. Trends Pharm. Sci. 2015, 6, 2446–2452. [Google Scholar]
- Rahman, S.A.; Abdelmalak, N.S.; Badawi, A.; Elbayoumy, T.; Sabry, N.; Ramly, A.E. Formulation of tretinoin-loaded topical proniosomes for treatment of acne: In-vitro characterization, skin irritation test and comparative clinical study. Drug Deliv. 2015, 22, 731–739. [Google Scholar] [CrossRef]
- Yeo, L.; Olusanya, T.; Chaw, C.; Elkordy, A. Brief effect of a small hydrophobic drug (cinnarizine) on the physicochemical characterisation of Niosomes produced by thin-film hydration and microfluidic methods. Pharmaceutics 2018, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Kaur, I.P. A novel nanovesicular carrier system to deliver drug topically. Pharm. Dev. Technol. 2013, 18, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Sengodan, T.; Sunil, B.; Vaishali, R.; Chandra, R.J.; Nagar, S.; Nagar, O. Formulation and evaluation of maltodextrin based proniosomes loaded with indomethacin. Int. J. PharmTech Res. 2009, 1, 517–523. [Google Scholar]
- Basha, M.; Abd El-Alim, S.H.; Shamma, R.N.; Awad, G.E. Design and optimization of surfactant-based nanovesicles for ocular delivery of Clotrimazole. J. Liposome Res. 2013, 23, 203–210. [Google Scholar] [CrossRef]
- Caracciolo, G.; Pozzi, D.; Caminiti, R.; Marianecci, C.; Moglioni, S.; Carafa, M.; Amenitsch, H. Effect of hydration on the structure of solid-supported Niosomal membranes investigated by in situ energy dispersive X-ray diffraction. Chem. Phys. Lett. 2008, 462, 307–312. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Teeranachaideekul, V.; Supaperm, T. Effect of charged and non-ionic membrane additives on physicochemical properties and stability of niosomes. AAPS PharmSciTech 2008, 9, 851. [Google Scholar] [CrossRef]
- Essa, E.A. Effect of formulation and processing variables on the particle size of sorbitan monopalmitate niosomes. Asian J. Pharm. (AJP) Free Full Text Artic. Asian J. Pharm. 2014, 4, 227–233. [Google Scholar] [CrossRef]
- Pawar, H.A.; Attarde, V.B.; Parag Subhash, G. Optimization of Bifonazole-Loaded Nisomal Formulation Using Plackett-Burman Design and 23 Factorial Design. Open Pharm. Sci. J. 2016, 3, 31–48. [Google Scholar] [CrossRef][Green Version]
- Uchechi, O.; Ogbonna, J.D.; Attama, A.A. Nanoparticles for dermal and transdermal drug delivery. Appl. Nanotechnol. Drug Deliv. 2014, 4, 193–227. [Google Scholar]
- Garg, V.; Singh, H.; Bhatia, A.; Raza, K.; Singh, S.K.; Singh, B.; Beg, S. Systematic development of transethosomal gel system of piroxicam: Formulation optimization, in vitro evaluation, and ex vivo assessment. AAPS PharmSciTech 2017, 18, 58–71. [Google Scholar] [CrossRef]
- Manosroi, A.; Jantrawut, P.; Manosroi, J. Anti-inflammatory activity of gel containing novel elastic niosomes entrapped with diclofenac diethylammonium. Int. J. Pharm. 2008, 360, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Leonyza, A.; Surini, S. Optimization of sodium deoxycholate-based transfersomes for percutaneous delivery of peptides and proteins. Int. J. Appl. Pharm. 2019, 11, 329–332. [Google Scholar] [CrossRef]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to evaluate skin penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef]
- Flaten, G.E.; Palac, Z.; Engesland, A.; Filipović-Grčić, J.; Vanić, Ž.; Škalko-Basnet, N. In vitro skin models as a tool in optimization of drug formulation. Eur. J. Pharm. Sci. 2015, 75, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Todo, H. Transdermal permeation of drugs in various animal species. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef]
- Tomomi, H.; Masami, I.; Kenji, S.; Yasunori, M. Prediction of skin permeability of drugs. II. Development of composite membrane as a skin alternative. Int. J. Pharm. 1992, 79, 21–28. [Google Scholar] [CrossRef]
- Watanabe, T.; Hasegawa, T.; Takahashi, H.; Ishibashi, T.; Takayama, K.; Sugibayashi, K. Utility of three-dimensional cultured human skin model as a tool to evaluate skin permeation of drugs. Altern. Anim. Test. Exp. 2001, 8, 1–14. [Google Scholar]
- Takeuchi, H.; Mano, Y.; Terasaka, S.; Sakurai, T.; Furuya, A.; Urano, H.; Sugibayashi, K. Usefulness of rat skin as a substitute for human skin in the in vitro skin permeation study. Exp. Anim. 2011, 60, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. Adv. Appl. 2016, 8, 163. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef]
- Salama, H.A.; Mahmoud, A.A.; Kamel, A.O.; Abdel Hady, M.; Awad, G.A. Brain delivery of olanzapine by intranasal administration of transfersomal vesicles. J. Liposome Res. 2012, 22, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Implementing Central Composite Design for Developing Transdermal Diacerein-Loaded Niosomes: Ex vivo Permeation and In vivo Deposition. Curr. Drug Deliv. 2018, 15, 1330–1342. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Whiting, D.; Iorizzo, M.; Pazzaglia, M.; Misciali, C.; Vincenzi, C.; Micali, G. The role of scalp dermoscopy in the diagnosis of alopecia areata incognita. J. Am. Acad. Dermatol. 2008, 59, 64–67. [Google Scholar] [CrossRef]
- Price, V.H.; Menefee, E.; Strauss, P.C. Changes in hair weight and hair count in men with androgenetic alopecia, after application of 5% and 2% topical minoxidil, placebo, or no treatment. J. Am. Acad. Dermatol. 1999, 41, 717–721. [Google Scholar] [CrossRef]
- Gugle, A.S.; Jadhav, V.M.; Kote, R.P.; Deshmukh, M.D.; Dalvi, A.V. Comparative Study of Efficacy of Topical Minoxidil 5% and Combination of Topical Minoxidil 5%, Topical Azelaic Acid 1.5% and Topical Tretinoin 0.01% on the Basis of Dermoscopic Analysis in Androgenetic Alopecia. MVP J. Med Sci. 2015, 2, 90–99. [Google Scholar] [CrossRef]
- Rathnayake, D.; Sinclair, R. Male androgenetic alopecia. Expert Opin. Pharmacother. 2010, 11, 1295–1304. [Google Scholar] [CrossRef]
- Whiting, D.A. Possible mechanisms of miniaturization during androgenetic alopecia or pattern hair loss. J. Am. Acad. Dermatol. 2001, 45, S81–S86. [Google Scholar] [CrossRef]
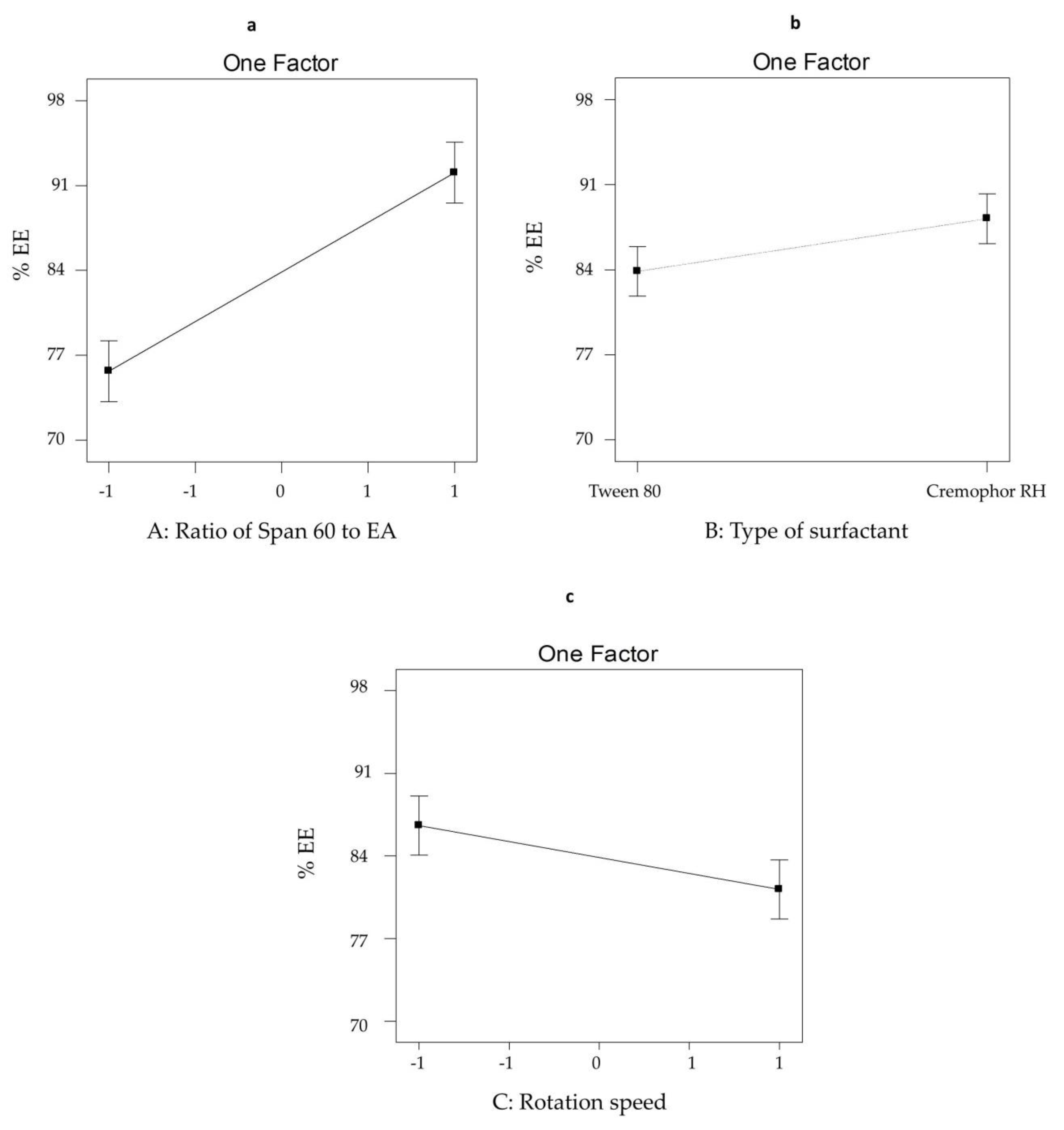
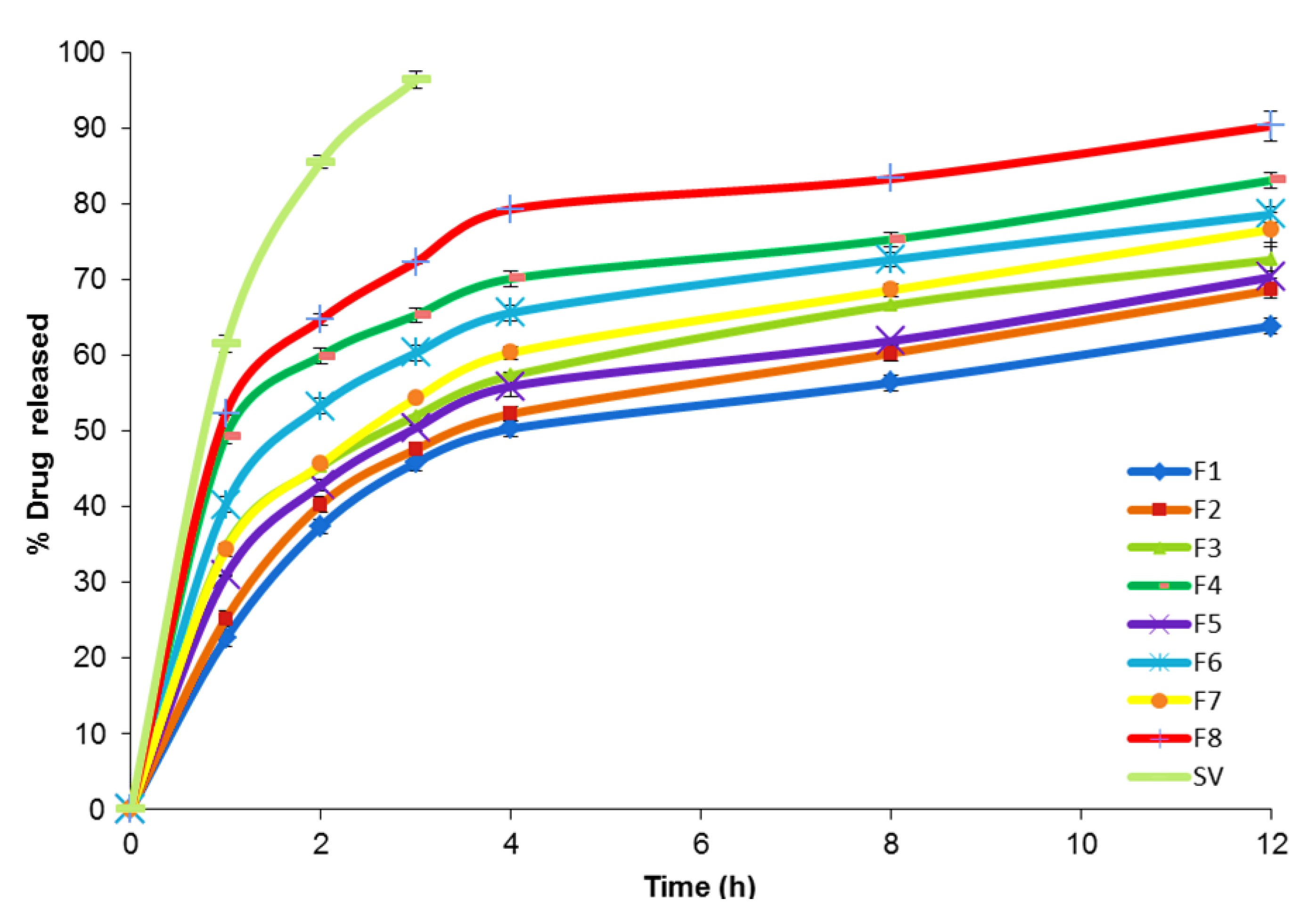
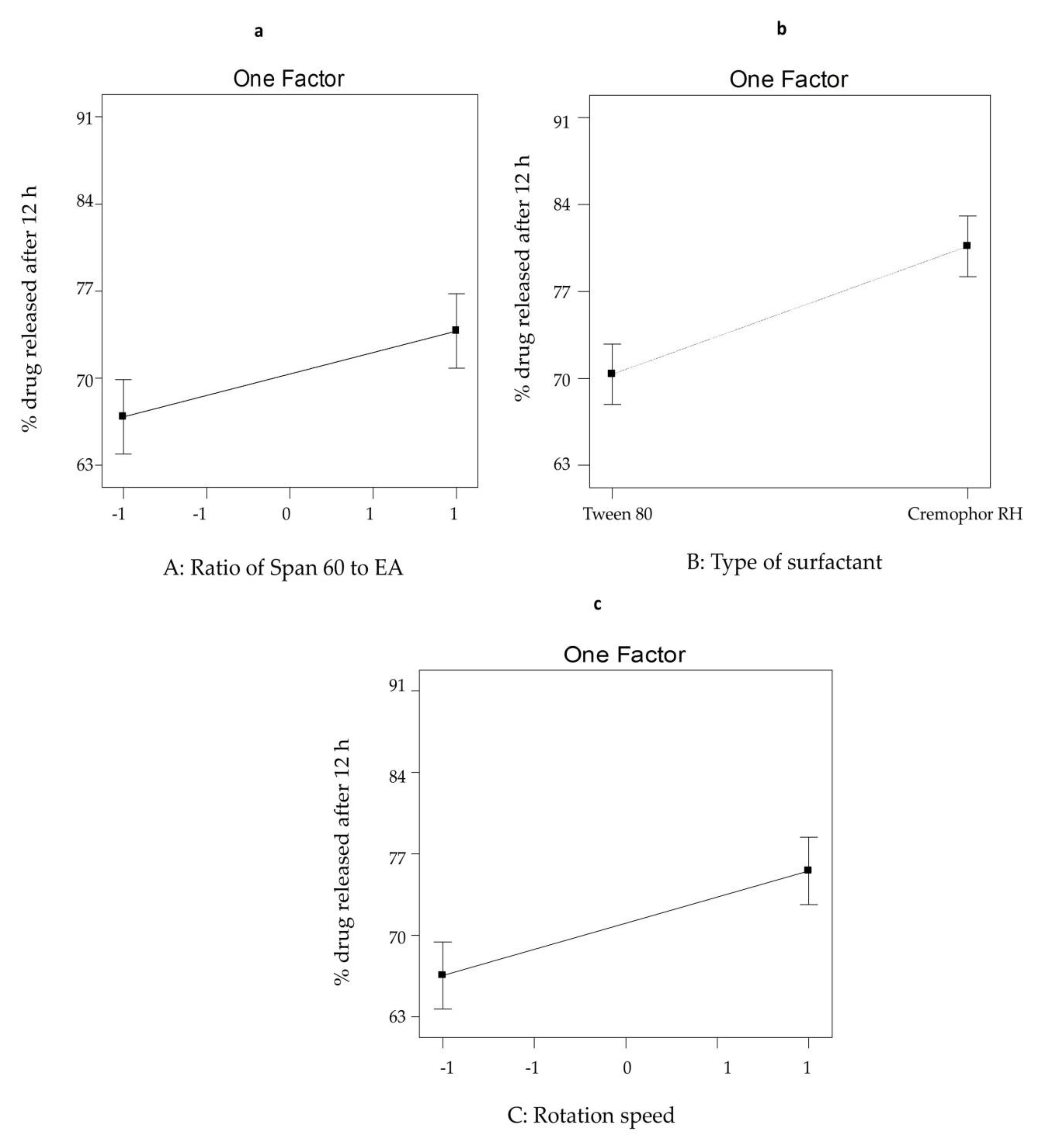
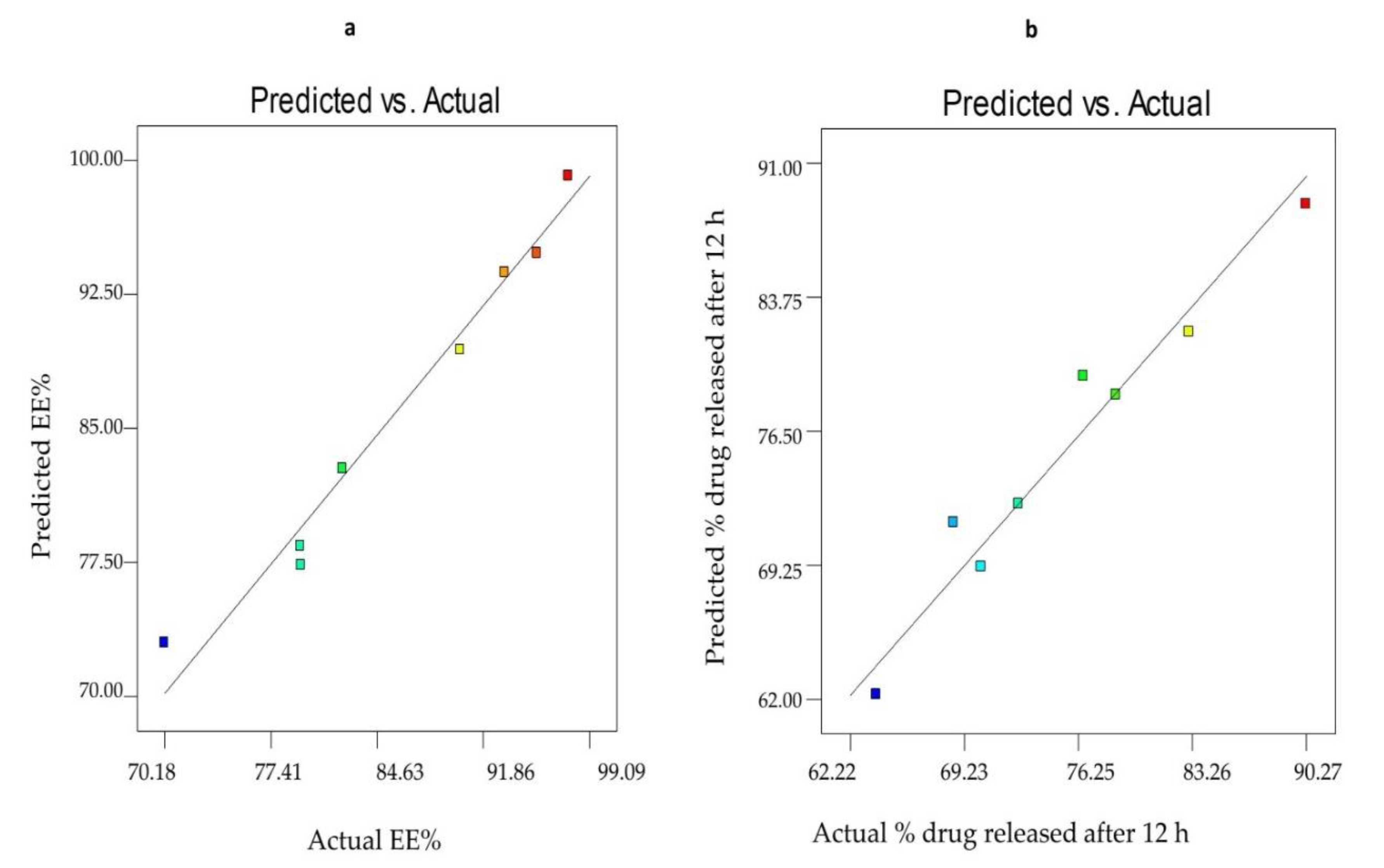

| Formula Code | Variables | ||||
|---|---|---|---|---|---|
| Independent | Dependent | ||||
| X1 | X2 | X3 | Y1 * | Y2 * | |
| F1 | −1 | −1 | −1 | 79.44 ± 1.15 | 63.84 ± 1.75 |
| F2 | −1 | −1 | 1 | 70.18 ± 0.62 | 68.59 ± 1.82 |
| F3 | −1 | 1 | −1 | 82.30 ± 0.98 | 72.59 ± 1.12 |
| F4 | −1 | 1 | 1 | 79.48 ± 1.33 | 83.09 ± 1.79 |
| F5 | 1 | −1 | −1 | 95.53 ± 1.46 | 70.29 ± 2.14 |
| F6 | 1 | −1 | 1 | 90.32 ± 2.18 | 78.59 ± 1.53 |
| F7 | 1 | 1 | −1 | 97.68 ± 1.93 | 76.58 ± 1.29 |
| F8 # | 1 | 1 | 1 | 93.34 ± 0.92 | 90.27 ± 1.98 |
| Independent Variables | Low (−1) | High (+1) | |||
| X1: Ratio of Span 60 to EA | 60:40 | 80:20 | |||
| X2: Type of EA | Tween 80 | Cremophor RH | |||
| X3: Rotation speed (rpm) | 500 | 1000 | |||
| Responses | R2 | Adjusted R2 | Predicted R2 | Adequate Precision |
|---|---|---|---|---|
| EE% (Y1) | 0.9731 | 0.9529 | 0.8924 | 17.669 |
| Q12h (Y2) | 0.9512 | 0.9146 | 0.8047 | 15.095 |
| Dependent Variable | Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|---|
| EE% (Y1) | Model | 631.81 | 3 | 210.65 | 48.23 | 0.0013 |
| X1 | 535.79 | 1 | 535.79 | 122.71 | 0.0004 | |
| X2 | 37.54 | 1 | 37.54 | 8.60 | 0.0427 | |
| X3 | 58.45 | 1 | 58.48 | 13.39 | 0.0216 | |
| Q12h (Y2) | Model | 481.10 | 3 | 160.37 | 25.98 | 0.0044 |
| X1 | 95.36 | 1 | 95.36 | 15.45 | 0.0171 | |
| X2 | 212.39 | 1 | 212.39 | 34.41 | 0.0042 | |
| X3 | 173.35 | 1 | 173.35 | 28.08 | 0.0061 |
| Formula | * Jss (µg cm−2 h−1) | * KP (cm h−1) | ER |
|---|---|---|---|
| SV dispersion | 2.77 ± 1.16 | 0.0069 ± 0.65 | ------- |
| F8 | 8.33 ± 1.28 | 0.0208 ± 1.15 | 3.00 |
| Demographic Data | SV-Loaded SNVs (n = 40) | Minoxidil (n = 40) | p-Value |
|---|---|---|---|
| Age/Years | |||
| Mean ± SD Min-Max | 32.32 ± 4.47 (24–41) | 34.27 ± 5.64 (25–44) | 0.125 |
| Sex | |||
| Male | 14 (35.0%) | 18 (45.0%) | 0.161 |
| Female | 26 (65.0%) | 22 (55.0%) | |
| Occupations | |||
| Worker | 25 (62.5%) | 22 (55%) | 0.226 |
| not working/housewife | 15 (37.5%) | 18 (45%) | |
| Disease Criteria | SV-Loaded SNVs (n = 40) | Minoxidil (n = 40) | p-Value |
|---|---|---|---|
| Disease Duration/Years | |||
| Mean ± SD Min-Max | 7.33 ± 2.05 (2–17) | 6.25 ± 2.64 (1–16) | 0.118 |
| Family History of Psoriasis | |||
| Positive | 25 (62.5%) | 23 (57.5%) | 0.361 |
| Negative | 15 (37.5%) | 17 (42.5%) | |
| Previous Treatment | |||
| Positive | 29 (72.5%) | 26 (65%) | 0.212 |
| Negative | 11 (27.5%) | 14 (35%) | |
| Classification of the Disease | SV-Loaded SNVs (n = 40) | Minoxidil (n = 40) | p-Value |
|---|---|---|---|
| Female Cases (Ludwig Classification) | (n = 26) | (n = 22) | |
| Ludwig I | 9 (34.6%) | 7 (31.8%) | 0.361 |
| Ludwig II | 10 (38.5%) | 9 (40.9%) | |
| Ludwig III | 7 (26.9%) | 6 (27.3) | |
| Male Cases (Hamilton Classification) | (n = 14) | (n = 18) | |
| Hamilton II | 5 (35.7%) | 7(38.9%) | 0.212 |
| Hamilton III | 7 (50%) | 8 (44.4%) | |
| Hamilton IV | 2 (14.3%) | 3 (16.7%) |
| Patients Satisfaction | SV-Loaded SNVs (n = 40) | Minoxidil (n = 40) | p-Value |
|---|---|---|---|
| Poor | 6 (15%) | 7 (17.5%) | 0.149 |
| Fair | 14 (35%) | 13 (32.5%) | |
| Good | 12 (30%) | 15 (37.5%) | |
| Excellent | 8 (20%) | 5 (12.5%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badria, F.A.; Fayed, H.A.; Ibraheem, A.K.; State, A.F.; Mazyed, E.A. Formulation of Sodium Valproate Nanospanlastics as a Promising Approach for Drug Repurposing in the Treatment of Androgenic Alopecia. Pharmaceutics 2020, 12, 866. https://doi.org/10.3390/pharmaceutics12090866
Badria FA, Fayed HA, Ibraheem AK, State AF, Mazyed EA. Formulation of Sodium Valproate Nanospanlastics as a Promising Approach for Drug Repurposing in the Treatment of Androgenic Alopecia. Pharmaceutics. 2020; 12(9):866. https://doi.org/10.3390/pharmaceutics12090866
Chicago/Turabian StyleBadria, Farid. A., Hassan A. Fayed, Amira K. Ibraheem, Ahmed F. State, and Eman A. Mazyed. 2020. "Formulation of Sodium Valproate Nanospanlastics as a Promising Approach for Drug Repurposing in the Treatment of Androgenic Alopecia" Pharmaceutics 12, no. 9: 866. https://doi.org/10.3390/pharmaceutics12090866
APA StyleBadria, F. A., Fayed, H. A., Ibraheem, A. K., State, A. F., & Mazyed, E. A. (2020). Formulation of Sodium Valproate Nanospanlastics as a Promising Approach for Drug Repurposing in the Treatment of Androgenic Alopecia. Pharmaceutics, 12(9), 866. https://doi.org/10.3390/pharmaceutics12090866





