Ex-Vivo Trans-Corneal and Trans-Scleral Diffusion Studies with Ocular Formulations of Glutathione as an Antioxidant Treatment for Ocular Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
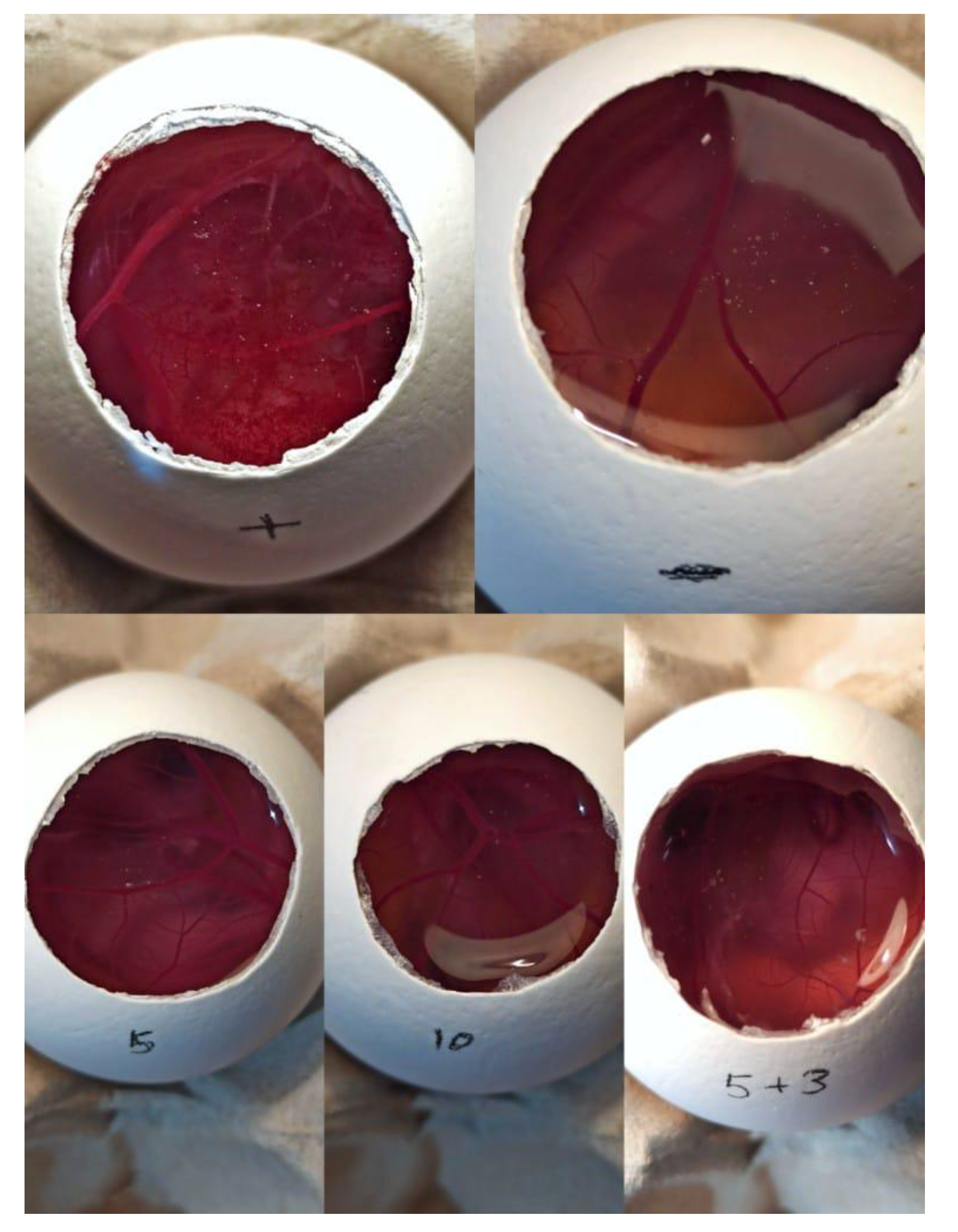
2.2. Ocular Tolerance Test (HET-CAM Test) Fertile
2.3. Stability Studies of GSH
2.4. Ocular Insert with GSH
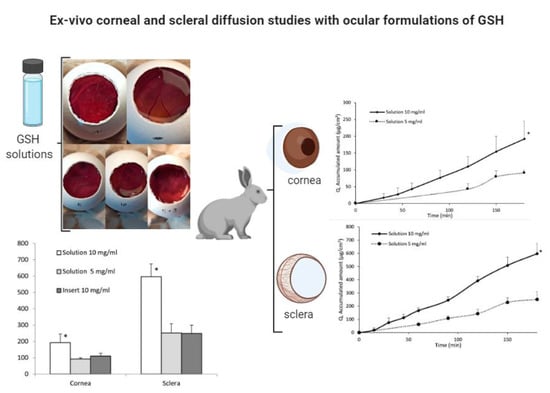
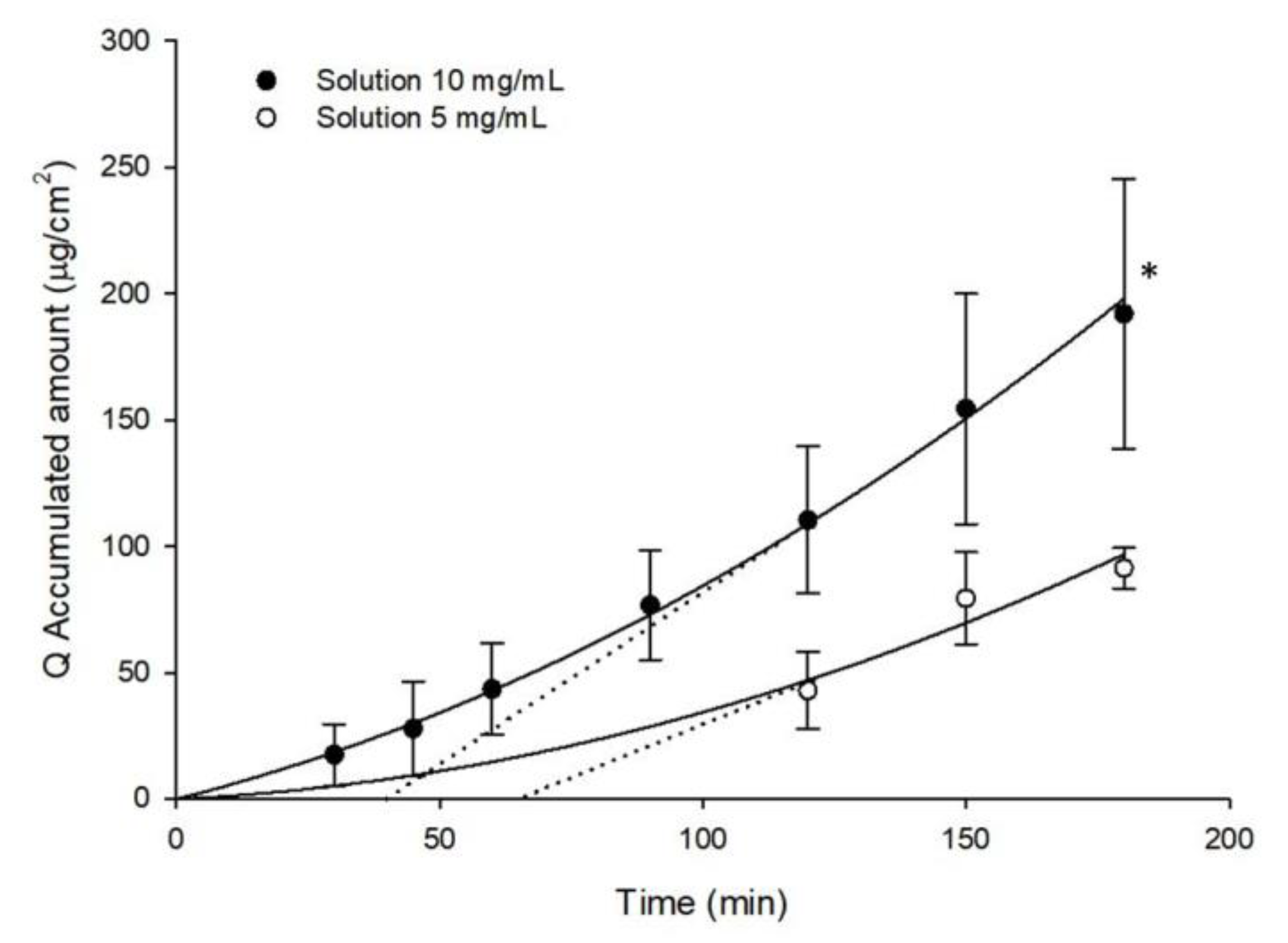
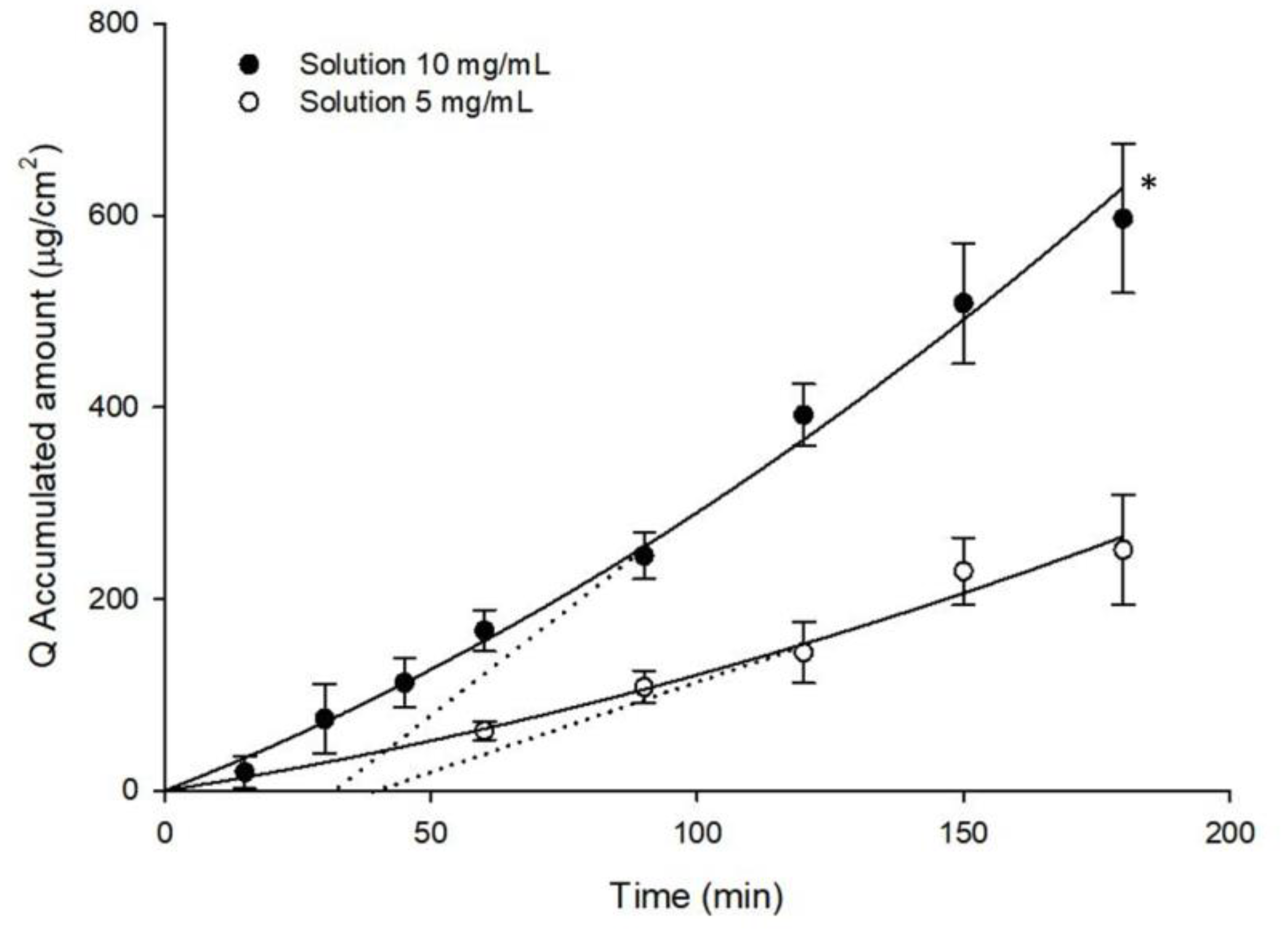
2.5. Ex-Vivo Ocular Diffusion Studies
2.6. Determination of GSH
2.7. Data Analysis
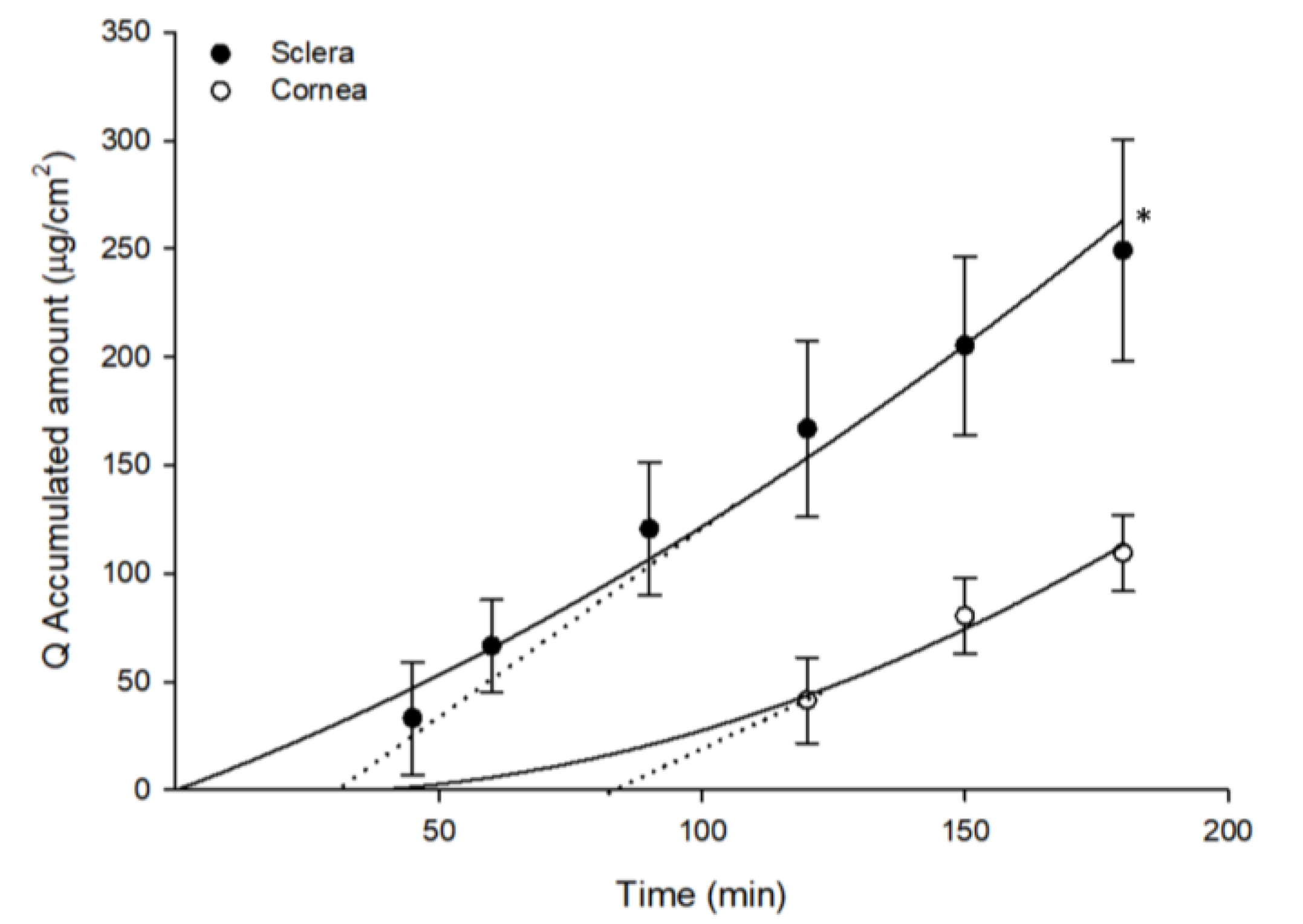
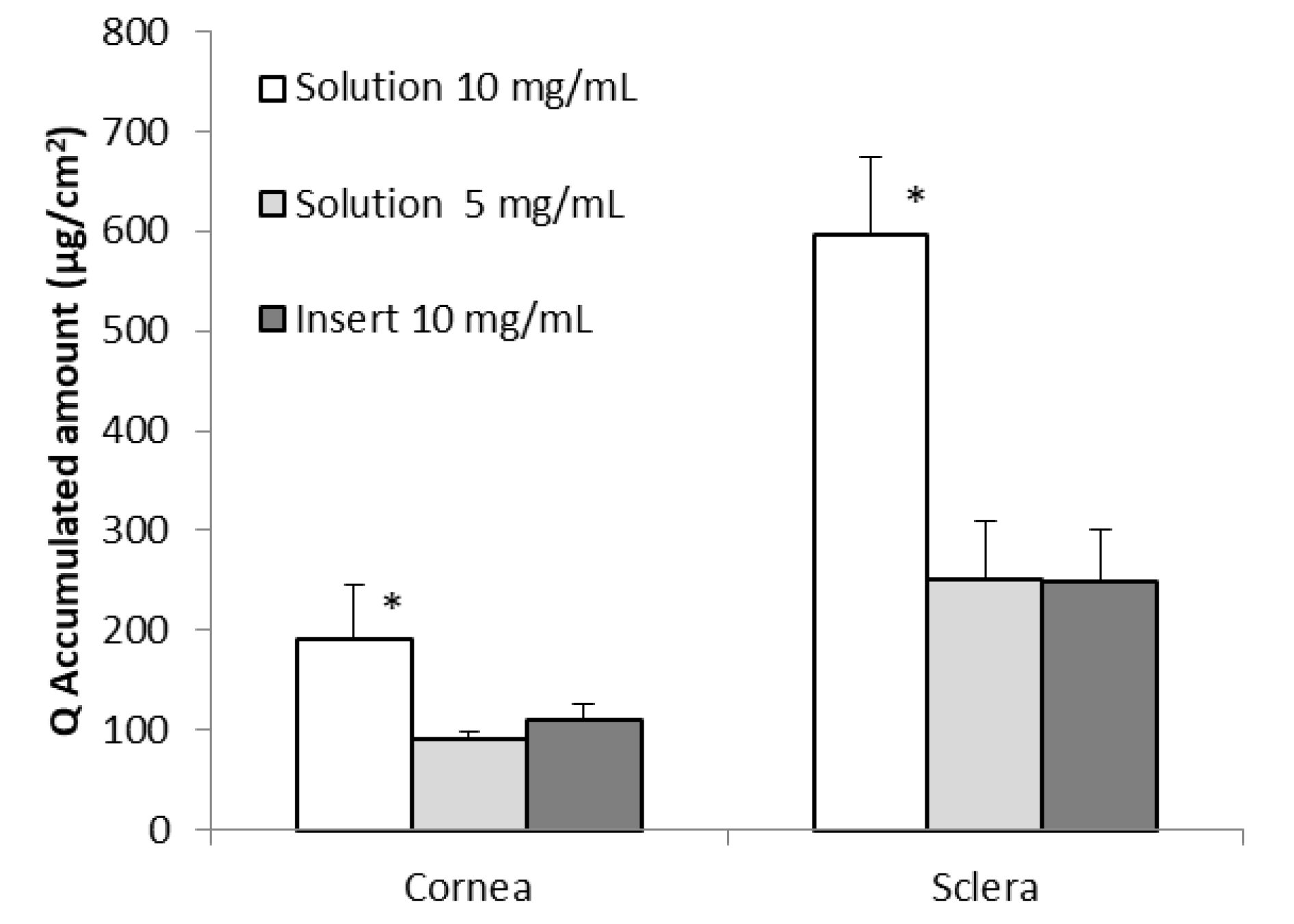
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sies, H. Oxidative stress: From basic research to clinical application. Am. J. Med. 1991, 91, S31–S38. [Google Scholar] [CrossRef]
- Vizzarri, F.; Palazzo, M.; Bartollino, S.; Casamassima, D.; Parolini, B.; Troiano, P.; Caruso, C.; Costagliola, C. Effects of an Antioxidant Protective Topical Formulation on Eye Exposed to Ultraviolet-Irradiation: A Study in Rabbit Animal Model. Physiol. Res. 2018, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant Defenses in the ocular surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef]
- Umapathy, A.; Donaldson, P.; Lim, J. Antioxidant Delivery Pathways in the Anterior Eye. Biomed Res. Int. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Riley, M.V.; Meyer, R.F.; Yates, E.M. Glutathione in the aqueous humor of human and other species. Investig. Ophthalmol. Vis. Sci. 1980, 19, 94–96. [Google Scholar]
- Brubaker, R.F.; Bourne, W.M.; Bachman, L.A.; McLaren, J.W. Ascorbic acid content of human corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1681–1683. [Google Scholar]
- Koc, M.; Bostanci, B.; Demirel, O.O.; Genc, F.; Tekin, K.; Koban, Y.; Dincel, A.S.; Sen, M.; Yilmazbas, P. The Effect of Ascorbic Acid (Vitamin C) on Transepithelial Corneal Cross-Linking in Rabbits. J. Ocul. Pharmacol. Ther. 2017, 33, 525–529. [Google Scholar] [CrossRef]
- Braakhuis, A.; Raman, R.; Vaghefi, E. The Association between Dietary Intake of Antioxidants and Ocular Disease. Diseases 2017, 5. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Kaplowitz, N.; Aw, T.Y.; Ookhtens, M. The regulation of hepatic glutathione. Annu. Rev. Pharmacol. Toxicol. 1985, 25, 715–744. [Google Scholar] [CrossRef]
- Meister, A.; Tate, S.S. Glutathione and Related γ-Glutamyl Compounds: Biosynthesis and Utilization. Annu. Rev. Biochem. 1976, 45, 559–604. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N.; Krance, S.M.; Marchan, R.; Hammond, C.L. Plasma membrane glutathione transporters and their roles in cell physiology and pathophysiology. Mol. Asp. Med. 2009, 30, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, A.; Wiedemann, P. Müller glial cells in retinal disease. Ophthalmologica 2012, 227, 1–19. [Google Scholar] [CrossRef]
- Sánchez-Vallejo, V.; Benlloch-Navarro, S.; Trachsel-Moncho, L.; López-Pedrajas, R.; Almansa, I.; Romero, F.J.; Miranda, M. Alterations in glutamate cysteine ligase content in the retina of two retinitis pigmentosa animal models. Free Radic. Biol. Med. 2016, 96, 245–254. [Google Scholar] [CrossRef]
- Fan, X.; Monnier, V.M.; Whitson, J. Lens glutathione homeostasis: Discrepancies and gaps in knowledge standing in the way of novel therapeutic approaches. Exp. Eye Res. 2017, 156, 103–111. [Google Scholar] [CrossRef]
- Lomaestro, B.M.; Malone, M. Glutathione in health and disease: Pharmacotherapeutic issues. Ann. Pharmacother. 1995, 29, 1263–1273. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Thakur, A.; Kaur, J.; Zulkifli, M. Glutathione transporters. Biochim. Biophys. Acta 2013, 1830, 3154–3164. [Google Scholar] [CrossRef]
- Izumi, H.; Sato, K.; Kojima, K.; Saito, T.; Saido, T.C.; Fukunaga, K. Oral glutathione administration inhibits the oxidative stress and the inflammatory responses in AppNL-G-F/NL-G-F knock-in mice. Neuropharmacology 2020, 168, 108026. [Google Scholar] [CrossRef]
- Sinha, R.; Sinha, I.; Calcagnotto, A.; Trushin, N.; Haley, J.S.; Schell, T.D.; Richie, J.P. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur. J. Clin. Nutr. 2018, 72, 105–111. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Shankland, E.G.; Wilbur, T.K.; Padowski, J.M. Phase IIb Study of Intranasal Glutathione in Parkinson’s Disease. J. Parkinsons Dis. 2017, 7, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.M.; Johnson, L.E.; Ahuja, S.; Ekström, P.A.R.; Romero, J.; van Veen, T. Significant photoreceptor rescue by treatment with a combination of antioxidants in an animal model for retinal degeneration. Neuroscience 2007, 145, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Arnal, E.; Ahuja, S.; Alvarez-Nölting, R.; López-Pedrajas, R.; Ekström, P.; Bosch-Morell, F.; van Veen, T.; Romero, F.J. Antioxidants rescue photoreceptors in rd1 mice: Relationship with thiol metabolism. Free Radic. Biol. Med. 2010, 48, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.G.; Dias, K.; Pereira, T.A.; Bernardi, D.S.; Lopez, R.F. V Topical delivery of ocular therapeutics: Carrier systems and physical methods. J. Pharm. Pharmacol. 2014, 66, 507–530. [Google Scholar] [CrossRef]
- Vandervoort, J.; Ludwig, A. Ocular drug delivery: Nanomedicine applications. Nanomedicine 2007, 2, 11–21. [Google Scholar] [CrossRef]
- Mundada, A.S.; Shrikhande, B.K. Design and Evaluation of Soluble Ocular Drug Insert for Controlled Release of Ciprofloxacin Hydrochloride. Drug Dev. Ind. Pharm. 2006, 32, 443–448. [Google Scholar] [CrossRef]
- Mundada, A.S.; Shrikhande, B.K. Formulation and Evaluation of Ciprofloxacin Hydrochloride Soluble Ocular Drug Insert. Curr. Eye Res. 2008, 33, 469–475. [Google Scholar] [CrossRef]
- Rathore, K.; Nema, R. Review on ocular inserts. Int. J. Pharm. Tech. Res. 2009, 1, 164–169. [Google Scholar]
- Rathore, K.S.; Nema, R.K. An Insight into Ophthalmic Drug Delivery System. Int. J. Pharm. Sci. Drug Res. 2009, 1, 1–5. [Google Scholar] [CrossRef]
- Kalweit, S.; Besoke, R.; Gerner, I.; Spielmann, H. A national validation project of alternative methods to the Draize rabbit eye test. Toxicol. In Vitro 1990, 4, 702–706. [Google Scholar] [CrossRef]
- Rodríguez, I.C.; Cerezo, A.; Salem, I.I. Sistemas de liberación bioadhesivos. ARS Pharm. 2000, 1, 115–128. [Google Scholar]
- Ramkanth, S.; Chetty, C. Design and evaluation of diclofenac sodium ocusert. Int. J. PharmTech Res. 2009, 1, 1219–1223. [Google Scholar]
- Pawar, P.K.; Katara, R.; Majumdar, D.K. Design and evaluation of moxifloxacin hydrochloride ocular inserts. Acta Pharm. 2012, 62, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Chandran, S.; Roy, A.; Saha, R.N. Effect of pH and formulation variables on in vitro transcorneal permeability of flurbiprofen: A technical note. AAPS PharmSciTech 2008, 9, 1031–1037. [Google Scholar] [CrossRef][Green Version]
- Srirangam, R.; Majumdar, S. Passive asymmetric transport of hesperetin across isolated rabbit cornea. Int. J. Pharm. 2010, 394, 60–67. [Google Scholar] [CrossRef]
- Sebastián-Morelló, M.; Calatayud-Pascual, M.A.; Rodilla, V.; Balaguer-Fernández, C.; López-Castellano, A. Ex vivo rabbit cornea diffusion studies with a soluble insert of moxifloxacin. Drug Deliv. Transl. Res. 2018, 8, 132–139. [Google Scholar] [CrossRef]
- Kalevar, V. Donor corneae for preservation. A modified dissection technique. Br. J. Ophthalmol. 1968, 52, 695. [Google Scholar] [CrossRef][Green Version]
- Fu, R.C.-C.; Lidgate, D.M. In Vitro Rabbit Corneal Permeability Study of Ketorolac, Tromethamine, a non-Steroidal Anti-Inflammatory Agent. Drug Dev. Ind. Pharm. 1986, 12, 2403–2430. [Google Scholar] [CrossRef]
- Majumdar, S.; Hingorani, T.; Srirangam, R.; Gadepalli, R.S.; Rimoldi, J.M.; Repka, M.A. Transcorneal permeation of L- and D-aspartate ester prodrugs of acyclovir: Delineation of passive diffusion versus transporter involvement. Pharm. Res. 2009, 26, 1261–1269. [Google Scholar] [CrossRef]
- Okamoto, N.; Ito, Y.; Nagai, N.; Murao, T.; Takiguchi, Y.; Kurimoto, T.; Mimura, O. Preparation of Ophthalmic Formulations Containing Cilostazol as an Anti-glaucoma Agent and Improvement in Its Permeability through the Rabbit Cornea. J. Oleo Sci. 2010, 59, 423–430. [Google Scholar] [CrossRef]
- Reed, D.; Babson, J.; Beatty, P.; Brodie, A.E.; Ellis, W.W.; Potter, D.W. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal. Biochem. 1980, 106, 55–62. [Google Scholar] [CrossRef]
- Alambiaga-Caravaca, A.M.; Calatayud-Pascual, M.A.; Rodilla, V.; Concheiro, A.; López-Castellano, A.; Alvarez-Lorenzo, C. Micelles of Progesterone for Topical Eye Administration: Interspecies and Intertissues Differences in Ex Vivo Ocular Permeability. Pharmaceutics 2020, 12, 702. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Veiga, B.; Sigurdsson, H.H.; Loftsson, T.; Alvarez-Lorenzo, C. Cyclodextrin—Amphiphilic Copolymer Supramolecular Assemblies for the Ocular Delivery of Natamycin. Nanomaterials 2019, 9. [Google Scholar] [CrossRef]
- Shang, F.; Lu, M.; Dudek, E.; Reddan, J.; Taylor, A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free Radic. Biol. Med. 2003, 34, 521–530. [Google Scholar] [CrossRef]
- Smuda, M.; Glomb, M.A. Maillard Degradation Pathways of Vitamin C. Angew. Chem. Int. Ed. 2013, 52, 4887–4891. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Winkler, B.S.; Orselli, S.M.; Rex, T.S. The redox couple between glutathione and ascorbic acid: A chemical and physiological perspective. Free Radic. Biol. Med. 1994, 17, 333–349. [Google Scholar] [CrossRef]
- Van Eyk, A.D.; Seifart, H.I.; Meyer, D.; van der Bijl, P. In Vitro Transcorneal Diffusion of the Antimicrobial Macrolides Azithromycin and Clarithromycin and the Impact on Microbial Keratitis. Cornea 2009, 28, 441–446. [Google Scholar] [CrossRef]
- Van Der Bijl, P.; Engelbrecht, A.H.; Van Eyk, A.D.; Meyer, D. Comparative permeability of human and rabbit corneas to cyclosporin and tritiated water. J. Ocul. Pharmacol. Ther. 2002, 18, 419–427. [Google Scholar] [CrossRef]
- Tortora, G.J.; Derrickson, B. (Eds.) Principios de Anatomía y Fisiología, 13th ed.; Panamerican: Madrid, Spain, 2013. [Google Scholar]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Zhang, W.; Prausnitz, M.R.; Edwards, A. Model of transient drug diffusion across cornea. J. Control. Release 2004, 99, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lutz, R.J.; Wang, N.S.; Robinson, M.R. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007, 39, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Rotman, M.; Welling, M.M.; Bunschoten, A.; de Backer, M.E.; Rip, J.; Nabuurs, R.J.A.; Gaillard, P.J.; van Buchem, M.A.; van der Maarel, S.M.; van der Weerd, L. Enhanced glutathione PEGylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for Alzheimer’s disease. J. Control. Release 2015, 203, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Rip, J.; Chen, L.; Hartman, R.; van den Heuvel, A.; Reijerkerk, A.; van Kregten, J.; van der Boom, B.; Appeldoorn, C.; de Boer, M.; Maussang, D.; et al. Glutathione PEGylated liposomes: Pharmacokinetics and delivery of cargo across the blood-brain barrier in rats. J. Drug Target. 2014, 22, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Paquet-Durand, F. Degenerating photoreceptors in Retinitis pigmentosa models release cGMP. A way of self protection? In Proceedings of the Research: A Vision for Hpe ARVO 2016, Seattle, WA, USA, 1–5 May 2016. [Google Scholar]
- Kumari, A.; Sharma, P.K.; Garg, V.K.; Garg, G. Ocular inserts—Advancement in therapy of eye diseases. J. Adv. Pharm. Technol. Res. 2010, 1, 291–296. [Google Scholar] [CrossRef]
- Dekina, S.S.; Romanovskaya, I.I.; Ovsepyan, A.M.; Balashova, M.V. Sterilization of Ocular Medical Inserts with Immobilized Proteins. Pharm. Chem. J. 2015, 49, 275–279. [Google Scholar] [CrossRef]
- Naguib, S.S.; Hathout, R.M.; Mansour, S. Optimizing novel penetration enhancing hybridized vesicles for augmenting the in-vivo effect of an anti-glaucoma drug. Drug Deliv. 2017, 24, 99–108. [Google Scholar] [CrossRef]
- Miranda, M.; Benlloch-Navarro, S.; Sánchez-Vallejo, V.; Trachsel-Moncho, L.; Soria, J.M.; Almansa, I.; Araiz, J. Effect of glutathione ethyl ester, lipoic acid and progesterone on photoreceptor survival in the retina of rd10 mice. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1733. [Google Scholar]
- Sánchez-Vallejo, V.; Benlloch-Navarro, S.; López-Pedrajas, R.; Romero, F.J.; Miranda, M. Neuroprotective actions of progesterone in an in vivo model of retinitis pigmentosa. Pharmacol. Res. 2015, 99, 276–288. [Google Scholar] [CrossRef]
- Cantó, A.; Olivar, T.; Romero, F.J.; Miranda, M. Nitrosative Stress in Retinal Pathologies: Review. Antioxidants 2019, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Alvarez-Nölting, R.; Araiz, J.; Romero Gómez, F.J. Antioxidant therapy in retinitis pigmentosa. Nov. Asp. Neuroprot. 2010, 661, 1–15. [Google Scholar]
- Gomez, F.J.R.; Miranda, M.; Arnal, E.; Ahuja, S.; Garcia-Delpech, S.; Ekström, P.; van Veen, T. Antioxidants Reduce Cell Death in a Model of Retinitis Pigmentosa: Relationship With Glutathione Metabolism. Investig. Ophthalmol. Vis. Sci. 2009, 50, 700. [Google Scholar]
- Sancho-Pelluz, J.; Arango-Gonzalez, B.; Kustermann, S.; Romero, F.J.; van Veen, T.; Zrenner, E.; Ekström, P.; Paquet-Durand, F. Photoreceptor Cell Death Mechanisms in Inherited Retinal Degeneration. Mol. Neurobiol. 2008, 38, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Baudouin-Cornu, P.; Lagniel, G.; Kumar, C.; Huang, M.-E.; Labarre, J. Glutathione Degradation Is a Key Determinant of Glutathione Homeostasis. J. Biol. Chem. 2012, 287, 4552–4561. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Gil, H.-W.; Yang, J.-O.; Lee, E.-Y.; Kim, H.-K.; Kim, S.-H.; Chung, Y.-H.; Hwang, S.-K.; Lee, Z.-W. Pharmacokinetics of Glutathione and Its Metabolites in Normal Subjects. J. Korean Med. Sci. 2005, 20, 721. [Google Scholar] [CrossRef][Green Version]
- Del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Ranta, V.-P.; Urtti, A. Transscleral drug delivery to the posterior eye: Prospects of pharmacokinetic modeling. Adv. Drug Deliv. Rev. 2006, 58, 1164–1181. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Gunda, S.; Hariharan, S.; Mitra, A.K. Corneal Absorption and Anterior Chamber Pharmacokinetics of Dipeptide Monoester Prodrugs of Ganciclovir (GCV): In Vivo Comparative Evaluation of These Prodrugs with Val-GCV and GCV in Rabbits. J. Ocul. Pharmacol. Ther. 2006, 22, 465–476. [Google Scholar] [CrossRef]
| Component | Amount |
|---|---|
| Polyvinylpyrrolidone K30 | 600 mg |
| Hydroxypropyl methylcellulose 4500 | 400 mg |
| Polyethylene glycol | 0.5 mL |
| Water | qs for a final volume of 10 mL |
| Glycerol | 25 mg |
| Glutathione | 100 mg |
| Solutions | Day | 25 °C Light | 25 °C Darkness | 4 °C Darkness |
|---|---|---|---|---|
| GSH | 2 | 97.56 ± 3.27 | 97.30 ± 3.95 | 99.44 ± 2.35 |
| 7 | 88.88 ± 1.21 | 89.37 ± 2.26 | 99.19 ± 7.78 | |
| 14 | 88.87 ± 1.21 | 87.44 ± 0.45 | 98.78 ± 7.29 | |
| 30 | 71.14 ± 0.35 | 72.92 ± 2.71 | 92.27 ± 5.78 | |
| GSH + Vit C | 2 | 86.81 ± 5.09 | 95.86 ± 2.54 | 96.93 ± 8.28 |
| 7 | 77.17 ± 10.16 | 86.99 ± 0.39 | 94.72 ± 6.25 | |
| 14 | 73.87 ± 4.81 | 78.40 ± 3.00 | 91.47 ± 4.57 | |
| 30 | 58.71 ± 0.25 | 64.64 ± 3.20 | 89.97 ± 1.21 |
| Conditions | Kp × 103 (cm/h) | ||
|---|---|---|---|
| Cornea | Sclera | ||
| Solutions | 5 mg/mL | 6.08 ± 0.83 | 17.63 ± 3.62 * |
| 10 mg/mL | 6.54 ± 1.79 | 20.74 ± 2.08 * | |
| Insert | 10 mg/mL | 3.44 ± 0.64 | 8.76 ± 1.58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebastián-Morelló, M.; Alambiaga-Caravaca, A.M.; Calatayud-Pascual, M.A.; Rodilla, V.; Balaguer-Fernández, C.; Miranda, M.; López-Castellano, A. Ex-Vivo Trans-Corneal and Trans-Scleral Diffusion Studies with Ocular Formulations of Glutathione as an Antioxidant Treatment for Ocular Diseases. Pharmaceutics 2020, 12, 861. https://doi.org/10.3390/pharmaceutics12090861
Sebastián-Morelló M, Alambiaga-Caravaca AM, Calatayud-Pascual MA, Rodilla V, Balaguer-Fernández C, Miranda M, López-Castellano A. Ex-Vivo Trans-Corneal and Trans-Scleral Diffusion Studies with Ocular Formulations of Glutathione as an Antioxidant Treatment for Ocular Diseases. Pharmaceutics. 2020; 12(9):861. https://doi.org/10.3390/pharmaceutics12090861
Chicago/Turabian StyleSebastián-Morelló, María, Adrián M. Alambiaga-Caravaca, María Aracely Calatayud-Pascual, Vicent Rodilla, Cristina Balaguer-Fernández, María Miranda, and Alicia López-Castellano. 2020. "Ex-Vivo Trans-Corneal and Trans-Scleral Diffusion Studies with Ocular Formulations of Glutathione as an Antioxidant Treatment for Ocular Diseases" Pharmaceutics 12, no. 9: 861. https://doi.org/10.3390/pharmaceutics12090861
APA StyleSebastián-Morelló, M., Alambiaga-Caravaca, A. M., Calatayud-Pascual, M. A., Rodilla, V., Balaguer-Fernández, C., Miranda, M., & López-Castellano, A. (2020). Ex-Vivo Trans-Corneal and Trans-Scleral Diffusion Studies with Ocular Formulations of Glutathione as an Antioxidant Treatment for Ocular Diseases. Pharmaceutics, 12(9), 861. https://doi.org/10.3390/pharmaceutics12090861