Rheology by Design: A Regulatory Tutorial for Analytical Method Validation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of HC Cream Formulations
2.2.2. Rheological Characterization
Rotational Measurements
Oscillatory Measurements
2.2.3. Rheological Method Validation
Risk Assessment
Equipment Qualification
Precision
Discriminatory Power
Robustness
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Rheological Method Validation
3.1.1. HC Cream Rheological Characterization
3.1.2. Equipment Qualification
3.1.3. Precision
3.1.4. Discriminatory Power
3.1.5. Robustness
3.1.6. Updated Risk Assessment
3.1.7. Standardizing the Procedure
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| CAA | Critical analytical attribute |
| CMA | Critical material attribute |
| CPP | Critical process parameter |
| CQA | Critical quality attribute |
| CR | Controlled-rate |
| CS | Controlled-stress |
| DoE | Design of experiment |
| F5 | Hydrocortisone cream formulation manufactured with 5% w/w of glyceryl monostearate amount |
| F10 | Hydrocortisone cream formulation manufactured with 10% w/w of glyceryl monostearate amount |
| F20 | Hydrocortisone cream formulation manufactured with 20% w/w of glyceryl monostearate amount |
| F10NC | Negative control of hydrocortisone cream formulation manufactured with 10% w/w of glyceryl monostearate amount |
| G″ | Loss modulus |
| G′ | Storage modulus |
| HC | Hydrocortisone |
| LVR | Linear viscoelastic region |
| o/w | Oil-in-water |
| PAT | Process analytical technology |
| QbD | Quality by Design |
| REM | Risk estimation matrix |
| RSD | Relative standard deviation |
| SR | Thixotropic relative area |
| Tan δ | Loss tangent |
| τf | Flow point |
| τ0.OSC | Oscillatory yield point |
| τ0.ROT | Rotational yield point |
| ƞ∞ | Infinite-shear viscosity |
| ƞL | Lower-shear thinning viscosity |
| ƞU | Upper-shear thinning viscosity |
| ƞ0 | Zero-shear viscosity |
References
- Krishnaiah, Y.S.R.; Xu, X.; Rahman, Z.; Yang, Y.; Katragadda, U.; Lionberger, R.; Peters, J.R.; Uhl, K.; Khan, M.A. Development of performance matrix for generic product equivalence of acyclovir topical creams. Int. J. Pharm. 2014, 475, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Isaac, V.L.B.; Chiari-Andréo, B.G.; Marto, J.M.; Moraes, J.D.D.; Leone, B.A.; Corrêa, M.A.; Ribeiro, H.M. Rheology as a Tool to Predict the Release of Alpha-Lipoic Acid from Emulsions Used for the Prevention of Skin Aging. Biomed. Res. Int. 2015, 2015, 818656. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.L.; Gough, T.; Isreb, M.; Dhumal, R.; Jones, J.W.; Nicholson, S.; Paradkar, A.; Gough, T.; Isreb, M.; Dhumal, R.; et al. In-process rheometry as a PAT tool for hot melt extrusion. Drug Dev. Ind. Pharm. 2018, 44, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Van Heugten, A.J.P.; Braal, C.L.; Versluijs-Helder, M.; Vromans, H. The influence of cetomacrogol ointment processing on structure: A definitive screening design. Eur. J. Pharm. Sci. 2017, 99, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ethier, A.; Bansal, P.; Baxter, J.; Langley, N.; Richardson, N.; Patel, A.M. The Role of Excipients in the Microstructure of Topical Semisolid Drug Products. In The Role of Microstructure in Topical Drug Product Development; Langley, N., Michniak-Kohn, B., Osborne, D.W., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 155–193. ISBN 978-3-030-17355-5. [Google Scholar]
- Mangas-Sanjuán, V.; Pleguezuelos-Villa, M.; Merino-Sanjuán, M.; Hernández, M.J.; Nácher, A.; García-Arieta, A.; Peris, D.; Hidalgo, I.; Soler, L.; Sallan, M.; et al. Assessment of the Inter-Batch Variability of Microstructure Parameters in Topical Semisolids and Impact on the Demonstration of Equivalence. Pharmaceutics 2019, 11, 503. [Google Scholar] [CrossRef]
- Ghica, M.V.; Hîrj, M. Flow and Thixotropic Parameters for Rheological characetrization of hydrogels. Molecules 2016, 21, 786. [Google Scholar] [CrossRef]
- Lauterbach, A.; Müller-Goymann, C.C. Comparison of rheological properties, follicular penetration, drug release, and permeation behavior of a novel topical drug delivery system and a conventional cream. Eur. J. Pharm. Biopharm. 2014, 88, 614–624. [Google Scholar] [CrossRef]
- Marto, J.; Baltazar, D.; Duarte, A.; Fernandes, A.; Gouveia, L.; Militão, M.; Salgado, A.; Simões, S.; Oliveira, E.; Ribeiro, H.M. Topical gels of etofenamate: In vitro and in vivo evaluation. Pharm. Dev. Technol. 2015, 20, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Namjoshi, S.; Dabbaghi, M.; Roberts, M.S.; Grice, J.E. Quality by Design: Development of the Quality Target Product Profile (QTPP) for Semisolid Topical Products. Pharmaceutics 2020, 12, 287. [Google Scholar] [CrossRef]
- Simões, A.; Veiga, F.; Vitorino, C. Developing Cream Formulations: Renewed Interest in an Old Problem. J. Pharm. Sci. 2019, 108, 3240–3251. [Google Scholar] [CrossRef]
- Binder, L.; Mazál, J.; Petz, R.; Klang, V.; Valenta, C. The role of viscosity on skin penetration from cellulose ether-based hydrogels. Ski. Res. Technol. 2019, 25, 725–734. [Google Scholar] [CrossRef]
- Sivaraman, A.; Ganti, S.S.; Nguyen, H.X.; Birk, G.; Wieber, A.; Lubda, D.; Banga, A.K. Development and evaluation of a polyvinyl alcohol based topical gel. J. Drug Deliv. Sci. Technol. 2017, 39, 210–216. [Google Scholar] [CrossRef]
- Soriano-Ruiz, J.L.; Calpena-Capmany, A.C.; Cañadas-Enrich, C.; Febrer, N.B.; Suñer-Carbó, J.; Souto, E.B.; Clares-Naveros, B. Biopharmaceutical profile of a clotrimazole nanoemulsion: Evaluation on skin and mucosae as anticandidal agent. Int. J. Pharm. 2019, 554, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Al Baraghthi, T.; Alkilani, A.Z.; Abu-Huwaij, R. Correlation Between Rheological Properties and In Vitro Drug Release from Penetration Enhancer-Loaded Carbopol® Gels. J. Pharm. Innov. 2016, 11, 339–351. [Google Scholar] [CrossRef]
- Simões, A.; Veiga, F.; Figueiras, A.; Vitorino, C. A practical framework for implementing Quality by design to the development of topical drug products: Nanosystem-based dosage forms. Int. J. Pharm. 2018, 548, 385–399. [Google Scholar] [CrossRef]
- ICH Expert Working Group. ICH Pharmaceutical Development Q8. ICH Harmon. Tripart. Guidel. 2009, 8, 1–28. [Google Scholar]
- Henriques, J.; Sousa, J.; Veiga, F.; Cardoso, C.; Vitorino, C. Process analytical technologies and injectable drug products: Is there a future? Int. J. Pharm. 2019, 554, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Qwist, P.K.; Sander, C.; Okkels, F.; Jessen, V.; Baldursdottir, S.; Rantanen, J. On-line rheological characterization of semi-solid formulations. Eur. J. Pharm. Sci. 2019, 128, 36–42. [Google Scholar] [CrossRef]
- Van Heugten, A.J.P.; Vromans, H. Scale up of Semisolid Dosage Forms Manufacturing Based on Process Understanding: From Lab to Industrial Scale. AAPS PharmSciTech 2018, 19, 2330–2334. [Google Scholar] [CrossRef] [PubMed]
- EMA. Draft Guideline on Quality and Equivalence of Topical Products. 2018. Available online: https://www.ema.europa.eu/en/quality-equivalence-topical-products (accessed on 27 August 2020).
- Miranda, M.; Cardoso, C.; Vitorino, C. Quality and Equivalence of Topical Products: A Critical Appraisal. Eur. J. Pharm. Sci. 2019, 148, 105082. [Google Scholar] [CrossRef]
- Pleguezuelos-Villa, M.; Merino-Sanjuán, M.; Hernández, M.J.; Nácher, A.; Peris, D.; Hidalgo, I.; Soler, L.; Sallan, M.; Merino, V. Relationship between rheological properties, in vitro release and in vivo equivalency of topical formulations of diclofenac. Int. J. Pharm. 2019, 572, 118755. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.-K.; Raw, A.; Lionberger, R.; Yu, L. Generic Development of Topical Dermatologic Products: Formulation Development, Process Development, and Testing of Topical Dermatologic Products. AAPS J. 2013, 15, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Benson, H.A.E.; Watkinson, A.C. Topical and Transdermal Drug Delivery: Principles and Practice; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 1118140494. [Google Scholar]
- Rath, S.; Kanfer, I. A validated IVRT method to assess topical creams containing metronidazole using a novel approach. Pharmaceutics 2020, 12, 119. [Google Scholar] [CrossRef]
- ICH. Validation of Analytical Procedures: Text and Methodology Q2 (R1); ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Simões, A.; Veiga, F.; Vitorino, C. Progressing Towards the Sustainable Development of Cream Formulations. Pharmaceutics 2020, 12, 647. [Google Scholar] [CrossRef]
- EMA. Guideline on Bioanalytical Method Validation. 2009. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation (accessed on 27 August 2019).
- Simões, A.; Veiga, F.; Vitorino, C.; Figueiras, A. A Tutorial for Developing a Topical Cream Formulation Based on the Quality by Design Approach. J. Pharm. Sci. 2018, 107, 2653–2662. [Google Scholar] [CrossRef] [PubMed]
- U.S. FDA. Draft Guidance on Acyclovir; U.S. FDA: Silver Spring, ML, USA, 2016.
- EMA. Guideline on quality of oral modified release products. Eur. Med. Agency 2014, 44, 1–16. [Google Scholar] [CrossRef]
- Miranda, M.; Pais, A.A.C.C.; Cardoso, C.; Vitorino, C. aQbD as a platform for IVRT method development—A regulatory oriented approach. Int. J. Pharm. 2019, 572, 118695. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook; Vincentz Network GmbH & Co. KG: Hanover, Germany, 2010; Volume 59, ISBN 9783866306509. [Google Scholar]
- Ili, T.; Daniels, R. Critical quality attributes, in vitro release and correlated in vitro skin permeation—In vivo tape stripping collective data for demonstrating therapeutic (non) equivalence of topical semisolids: A case study of “ready-to-use” vehicles. Int. J. Pharm. 2017, 528, 253–267. [Google Scholar] [CrossRef]
- Tiffner, K.I.; Kanfer, I.; Augustin, T.; Raml, R.; Raney, S.G.; Sinner, F. A comprehensive approach to qualify and validate the essential parameters of an in vitro release test (IVRT) method for acyclovir cream, 5%. Int. J. Pharm. 2018, 535, 217–227. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, J.Y.; Lee, E.J.; Park, S.K. Rheological properties and microstructures of Carbopol gel network system. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Ribeiro, H.M.; Morais, J.A.; Eccleston, G.M. Structure and rheology of semisolid o/w creams containing cetyl alcohol/non-ionic surfactant mixed emulsifier and different polymers. Int. J. Cosmet. Sci. 2004, 26, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Clares-Naveros, B.; Suñer-Carbó, J.; Calpena-Campmany, A.; Rodriguez-Lagunas, M.; Halbaut-Bellowa, L.; Zamarbide-Losada, J.; Boix-Montañés, A.; Barbolini, E. Biopharmaceutical Development of a Bifonazole Multiple Emulsion for Enhanced Epidermal Delivery. Pharmaceutics 2019, 11, 66. [Google Scholar] [CrossRef]
- Mahdi, E.S.; Noor, A.M.; Sakeena, M.H.; Abdullah, G.Z.; Abdulkarim, M.F.; Sattar, M.A. Formulation and in vitro release evaluation of newly synthesized palm kernel oil esters-based nanoemulsion delivery system for 30 % ethanolic dried extract derived from local Phyllanthus urinaria for skin antiaging. Int. J. Nanomed. 2011, 6, 2499–2512. [Google Scholar] [CrossRef]
- De Souza Mendes, P.R. Modeling the thixotropic behavior of structured fluids. J. Nonnewton. Fluid Mech. 2009, 164, 66–75. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J. A mathematical model of rheological behavior of novel bio-based isocyanate-terminated polyurethane prepolymers. Ind. Crops Prod. 2014, 60, 123–129. [Google Scholar] [CrossRef]
- Mewis, J.; Wagner, N.J. Thixotropy. Adv. Colloid Interface Sci. 2009, 147, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.F. Rheology of Emulsions. In Rheology of Dispersions; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 121–147. ISBN 9783527631568. [Google Scholar]
- Pisal, P.B.; Patil, S.S.; Pokharkar, V.B. Rheological investigation and its correlation with permeability coefficient of drug loaded carbopol gel: Influence of absorption enhancers. Drug Dev. Ind. Pharm. 2013, 39, 593–599. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Liu, J.; Fang, L. Correlation between rheological properties, in vitro release, and percutaneous permeation of tetrahydropalmatine. AAPS PharmSciTech 2011, 12, 1002–1010. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Li, F.; Li, H.; Wong, B.S.; Low, C.Y.; Liu, X.Y.; Kang, L. Drug permeation through skin is inversely correlated with carrier gel rigidity. Mol. Pharm. 2015, 12, 444–452. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Puri, A.; Banga, A.K. Methods to simulate rubbing of topical formulation for in vitro skin permeation studies. Int. J. Pharm. 2017, 519, 22–33. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Wang, X.; Tang, H.; Wu, N.; Wu, F.; Yu, D.; Elfalleh, W. Effects of (+)-catechin on a rice bran protein oil-in-water emulsion: Droplet size, zeta-potential, emulsifying properties, and rheological behavior. Food Hydrocoll. 2020, 98, 105306. [Google Scholar] [CrossRef]
- Batheja, P.; Sheihet, L.; Kohn, J.; Singer, A.J.; Michniak-Kohn, B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J. Control. Release 2011, 149, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Bruschi, M.L.; De Freitas, O.; Gremião, M.P.D.; Lara, E.H.G.; Andrews, G.P. Rheological, mechanical and mucoadhesive properties of thermoresponsive, bioadhesive binary mixtures composed of poloxamer 407 and carbopol 974P designed as platforms for implantable drug delivery systems for use in the oral cavity. Int. J. Pharm. 2009, 372, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Brown, A.F.; Woolfson, A.D. Rheological characterization of bioadhesive, antimicrobial, semisolids designed for the treatment of periodontal diseases: Transient and dynamic viscoelastic and continuous shear analysis. J. Pharm. Sci. 2001, 90, 1978–1990. [Google Scholar] [CrossRef]
- Goodrum, J.W.; Geller, D.P.; Adams, T.T. Rheological characterization of yellow grease and poultry fat. J. Am. Oil Chem. Soc. 2002, 79, 961–964. [Google Scholar] [CrossRef]
- Wong, P.C.H.; Heng, P.W.S.; Chan, L.W. Viscosity-temperature relationship of lipid-based excipients amenable for spray congealing: Derivation of a rheological parameter with good correlation to particle size. Eur. J. Lipid Sci. Technol. 2016, 118, 1062–1073. [Google Scholar] [CrossRef]
- ICH. ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology. Int. Conf. Harmon. 2005, 1994, 17. [Google Scholar]
- Rawat, A.; Gupta, S.S.; Kalluri, H.; Lowenborg, M.; Bhatia, K.; Warner, K. Rheological Characterization in the Development of Topical Drug Products. In The Role of Microstructure in Topical Drug Product Development; AAPS Advances in the Pharmaceutical Sciences Series; Springer: Berlin/Heidelberg, Germany, 2019; Volume 36, pp. 3–45. [Google Scholar]
- Carriço, C.; Pinto, P.; Graça, A.; Gonçalves, L.M.; Ribeiro, H.M.; Marto, J. Design and characterization of a new quercus suber-based pickering emulsion for topical application. Pharmaceutics 2019, 11, 131. [Google Scholar] [CrossRef]
- Chanamai, R.; McClements, D.J. Dependence of creaming and rheology of monodisperse oil-in-water emulsions on droplet size and concentration. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 172, 79–86. [Google Scholar] [CrossRef]
- Vianna-Filho, R.P.; Petkowicz, C.L.O.; Silveira, J.L.M. Rheological characterization of O/W emulsions incorporated with neutral and charged polysaccharides. Carbohydr. Polym. 2013, 93, 266–272. [Google Scholar] [CrossRef]
- Tadros, T.F. Emulsion Formation, Stability, and Rheology. Emulsion Formation and Stability; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, Germany, 2013; pp. 1–75. [Google Scholar]
- Tricks, B.R.T. Rheology Essentials of Cosmetic and Food Emulsions; Springer: Berlin/Heidelberg, Germany, 2006; ISBN 3-540-25553-2. [Google Scholar]
- Djalili-Moghaddam, M.; Ebrahimzadeh, R.; Toll, S. Study of geometry effects in torsional rheometry of fibre suspensions. Rheol. Acta 2004, 44, 29–37. [Google Scholar] [CrossRef]
- Fernanda, P. Methods Used in the Study of the Physical Properties of Fats. In Structure-Function Analysis of Edible Fats; Elsevier: Amsterdam, The Netherlands, 2018; pp. 313–385. ISBN 9780128140420. [Google Scholar]
- Chhabra, R.P.; Richardson, J.F. Non-Newtonian Flow and Applied Rheology; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780750685320. [Google Scholar]
- Van Vliet, T. Measuring Apparatus. Rheology and Fracture Mechanics of Foods; CRC Press: Boca Raton, FL, USA, 2013; ISBN 9781439827031. [Google Scholar]
- Aho, J.; Hvidt, S.; Baldursdottir, S. Rheology in Pharmaceutical Sciences. In Analytical Techniques in the Pharmaceutical Sciences; Springer: New York, NY, USA, 2016; pp. 719–750. [Google Scholar]





| Test | Viscosity Curve | Thixotropic Behaviour | Amplitude Sweep | Frequency Sweep | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAA | η0 | η∞ | ηU | ηL | τ0.ROT | SR | LVR plateau | τ0.OSC | τf | G′ | G″ | Tan δ |
| CMV | ||||||||||||
| Geometry | M | M | M | M | M | M | M | M | M | M | M | L |
| Temperature | M | M | M | M | M | M | M | M | M | M | M | L |
| Sample application | M | M | M | M | M | M | M | M | M | M | M | L |
| Gap and trimming | L | L | L | L | L | L | L | L | L | L | L | L |
| Data acquisition mode | L | L | L | L | L | L | L | L | L | L | L | L |
| Integration time | M | M | M | M | M | M | M | M | M | M | M | L |
| Sample amount | M | M | M | M | M | M | M | M | M | M | M | L |
| Analyst | L | L | L | L | L | L | L | L | L | L | L | L |
| Shear stress ramp | H | H | H | H | H | H | H | H | ||||
| Step duration | M | M | M | M | M | M | M | M | M | M | M | L |
| Shear rate ramp | H | |||||||||||
| Shear load time | H | |||||||||||
| Shear recovery time | H | |||||||||||
| Frequency value | M | M | M | |||||||||
| Shear stress within LVR plateau | H | H | L | |||||||||
| Frequency ramp | M | M | L | |||||||||
| Temperature | CAA | Acceptance Criteria | Results | Status |
|---|---|---|---|---|
| Standard 25 °C | ƞ (Pa.s) | 4.984 Precision (RSD) < 15% Accuracy (Bias) < 15% | Mean = 5.27 ± 0.14 RSD = 2.67% Bias = 5.74% | C |
| Standard 32 °C | 4.360 Precision (RSD) < 15% Accuracy (Bias) < 15% | Mean = 4.8 ± 0.2 RSD = 4.17% Bias = 10.09% | C |
| CAA | Results | |||||
|---|---|---|---|---|---|---|
| Acceptance Criteria | Intraday Variability | Interday Variability | Status | |||
| Mean ± SD | RSD (%) | Mean ± SD | RSD (%) | |||
| ƞ0 (Pa.s) | [CAA ± 15%] | 26,293 ± 6538 | 24.87 | 26,338 ± 7474 | 28.38 | NC |
| ƞ∞ (Pa.s) | 17.3 ± 1.5 | 8.85 | 17.3 ± 1.6 | 9.28 | C | |
| ƞU (Pa.s) | 23,277 ± 7231 | 31.06 | 23,277 ± 8168 | 35.09 | NC | |
| ƞL (Pa.s) | 40.8 ± 5.8 | 14.14 | 40.8 ± 6.1 | 14.83 | C | |
| τ0.ROT (Pa) | 27.2 ± 1.7 | 6.36 | 27.2 ± 1.8 | 6.71 | C | |
| SR (Pa/s) | 25,041 ± 2548 | 10.17 | 24,576 ± 3238 | 13.17 | C | |
| LVR plateau (Pa) | 6649 ± 454 | 6.83 | 6659 ± 492 | 7.38 | C | |
| τ0.OSC (Pa) | 34.6 ± 4.5 | 13.00 | 34.7 ± 4.6 | 13.38 | C | |
| τf (Pa) | 76.6 ± 5.3 | 6.88 | 76.9 ± 6.4 | 8.30 | C | |
| G′ (Pa) | 6867 ± 484 | 7.05 | 6853 ±634 | 9.25 | C | |
| G″ (Pa) | 1942 ± 148 | 7.63 | 1941 ± 184 | 9.49 | C | |
| Tan δ | 0.28 ± 0.02 | 5.57 | 0.28 ± 0.02 | 6.87 | C | |
| Sensitivity | Specificity | Selectivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CAA | Results | Acceptance Criteria | Status | Results | Acceptance Criteria | Status | Acceptance Criteria | Status | |||
| F5 | F10 | F20 | F10NC | ||||||||
| Mean ± SD | R2 | ||||||||||
| ƞ0 (Pa.s) | 8600 ± 2409 | 26,338 ± 7474 | 62,870 ± 6630 | 19,785 ± 6121 | CAA [F5] < CAA [F10] < CAA [F20] | C | 1.000 | R2 > 0.9 | C | CAA [F5] ≠ CAA [F10] ≠ CAA [F20] ≠ CAA [F10NC] | C |
| η∞ (Pa.s) | 3.57 ± 0.56 | 17.3 ± 1.6 | 17.3 ± 0.4 | 6.96 ± 0.55 | C | 0.972 | C | C | |||
| ƞU (Pa.s) | 6422 ± 553 | 23,278 ± 8168 | 69,250 ± 5260 | 12,815 ± 5969 | C | 0.995 | C | C | |||
| ƞL (Pa.s) | 18.4 ± 2.3 | 40.8 ± 6.1 | 56.2 ± 3.4 | 32.2 ± 4.9 | C | 0.914 | C | C | |||
| τ0.ROT (Pa) | 10.8 ± 0.1 | 27.2 ± 1.8 | 55.2 ± 7.4 | 10.7 ± 0.05 | C | 0.998 | C | C | |||
| SR (Pa/s) | 5006 ± 325 | 24,576 ± 3228 | 136,625 ± 9419 | 9062 ± 1195 | C | 0.962 | C | C | |||
| LVR plateau (Pa) | 1636 ± 06 | 665 ± 491 | 33,721 ± 2446 | 4081 ± 900 | C | 0.965 | C | C | |||
| τ0.OSC (Pa) | 16 ± 2.2 | 34.7 ± 4.6 | 67.9 ± 5.4 | 10.9 ± 0.2 | C | 0.999 | C | C | |||
| τf (Pa) | 44.4 ± 1.9 | 76.9 ± 6.4 | 124 ± 10 | 26.4 ± 3.5 | C | 0.993 | C | C | |||
| G′ (Pa) | 1649 ± 97 | 6853 ± 634 | 35,787 ± 634 | 3419 ± 487 | C | 0.964 | C | C | |||
| G″ (Pa) | 509 ± 35 | 1941 ± 184 | 15,739 ± 184 | 1169 ± 202 | C | 0.940 | C | C | |||
| Tan δ | 0.23 ± 0.13 | 0.28 ± 0.02 | 0.44 ± 0.02 | 0.28 ± 0.12 | C | 0.991 | C | C | |||
| CAA | F10 vs. F5 | F10 vs. F20 | F10 vs. F10.NC |
|---|---|---|---|
| ƞ0 (Pa.s) | Normal distribution? Yes. CI: [−27,445–8031] p-value: < 0.0001 | Normal distribution? Yes. CI: [−34,029–10,854] p-value: < 0.0001 | Normal distribution? Yes. CI: [−5034–18,141] p-value: 0.4403 |
| ƞ∞ (Pa.s) | Normal distribution? Yes. CI: [−15.49–12.03] p-value: < 0.0001 | Normal distribution? Yes. CI: [−17.23–12.52] p-value: < 0.0001 | Normal distribution? Yes. CI: [8.311–12.44] p-value: < 0.0001 |
| ƞU (Pa.s) | Normal distribution? Yes. CI: [−26,774–6936] p-value: 0.0003 | Normal distribution? Yes. CI: [−61,072–30,873] p-value: < 0.0001 | Normal distribution? Yes. CI: [−492–21,417] p-value: 0.0659 |
| ƞL (Pa.s) | Normal distribution? Yes. CI: [−29.36–15.52] p-value: < 0.0001 | Normal distribution? Yes. CI: [−26.67–4.147] p-value: 0.0040 | Normal distribution? Yes. CI: [0.3611–16.81] p-value: 0.0380 |
| τ0.ROT (Pa) | Normal distribution? Yes. CI: [−21.04–11.90] p-value: < 0.0001 | Normal distribution? Yes. CI: [−31.92–23.91] p-value: < 0.0001 | Normal distribution? Yes. CI: [12.01–21.15] p-value: < 0.0001 |
| SR (Pa/s) | Normal distribution? Yes. CI: [−25,597–13,543] p-value: < 0.0001 | Normal distribution? Yes. CI: [−118,645–105,452] p-value: < 0.0001 | Normal distribution? Yes. CI: [9487–21,541] p-value: < 0.0001 |
| LVR plateau (Pa) | Normal distribution? Yes. CI: [−6224–3821] p-value: < 0.0001 | Normal distribution? Yes. CI: [−28,200–25,925] p-value: < 0.0001 | Normal distribution? Yes. CI: [1376–3779] p-value: < 0.0001 |
| τ0.OSC (Pa) | Normal distribution? Yes. CI: [−23.67–13.6] p-value: < 0.0001 | Normal distribution? Yes. CI: [−37.97–28.41] p-value: < 0.0001 | Normal distribution? Yes. CI: [18.41–29.11] p-value:< 0.0001 |
| τf (Pa) | Normal distribution? No. CI: [−40.5–24.55] p-value: < 0.0001 | Normal distribution? No. CI: [−54.46–39.88] p-value: < 0.0001 | Normal distribution? No. CI: [42.52–58.49] p-value: < 0.0001 |
| G′ (Pa) | Normal distribution? Yes. CI: [−6958–3451] p-value: < 0.0001 | Normal distribution? Yes. CI: [−30,402–27,466] p-value: < 0.0001 | Normal distribution? Yes. CI: [1966–4902] p-value: < 0.0001 |
| G″ (Pa) | Normal distribution? Yes. CI: [−2120–745.8] p-value: < 0.0001 | Normal distribution? Yes. CI: [−14,373–13,223] p-value: < 0.0001 | Normal distribution? Yes. CI: [197.6–1348] p-value: < 0.0044 |
| Tan δ | Normal distribution? Yes. CI: [−0.1384–0.03657] p-value: 0.4165 | Normal distribution? Yes. CI: [−0.2315–0.08499] p-value: < 0.0001 | Normal distribution? Yes. CI: [−0.06649–0.08001] p-value: 0.9947 |
| CAA | TEMPERATURE | GEOMETRY | APPLICATION | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acceptance Criteria | Condition | Mean ± SD | RSD (%) | Status | Acceptance Criteria | Condition | Mean ± SD | RSD (%) | Status | Acceptance Criteria | Condition | Mean ± SD | RSD (%) | Status | |
| ƞ0 (Pa.s) | [CAA ± 15%] | 32 ± 2 °C | 25,953 ± 6810 | 26.24 | NC | [CAA ± 15%] | C35-P35 vs. P35-P35 | 22,981 ± 10,560 | 45.95 | NC | [CAA ± 15%] | Syringe vs. Spatula | 26,363 ± 7070 | 26.82 | NC |
| ƞ∞ (Pa.s) | 17.2 ± 1.6 | 9.17 | C | 15.7 ± 4.4 | 27.93 | NC | 17.7 ± 2.0 | 11.38 | C | ||||||
| ƞU (Pa.s) | 22,194 ± 7370 | 33.21 | NC | 20,702 ± 10,313 | 49.82 | NC | 22,203 ± 8126 | 36.60 | NC | ||||||
| ƞL (Pa.s) | 41 ± 6 | 14.06 | C | 37 ± 11 | 30.17 | NC | 44 ± 10 | 22.46 | NC | ||||||
| τ0.ROT (Pa) | 27.0 ± 2.1 | 7.74 | C | 28.4 ± 5.8 | 20.49 | NC | 27.3 ± 1.7 | 6.36 | C | ||||||
| SR (Pa/s) | 27,602 ± 6397 | 23.18 | NC | 30,014 ± 5245 | 60.53 | NC | 27,349 ± 8034 | 29.38 | NC | ||||||
| LVR plateau (Pa) | 6539 ± 453 | 8.61 | C | P35-P35 vs. P20-P20 | 6879 ± 996 | 14.48 | C | 6704 ± 503 | 7.51 | C | |||||
| τ0.OSC (Pa) | 39 ± 9 | 25.43 | NC | 36 ± 7 | 19.92 | NC | 38 ± 9 | 23.31 | NC | ||||||
| τf (Pa) | 87 ± 35 | 26.69 | NC | 81 ± 17 | 20.96 | NC | 85 ± 20 | 24.03 | NC | ||||||
| G′ (Pa) | 6783 ± 623 | 9.18 | C | 7430 ± 2251 | 30.30 | NC | 6961 ± 709 | 10.18 | C | ||||||
| G″ (Pa) | 1932 ± 179 | 9.28 | C | 2143 ± 712 | 33.21 | NC | 1997 ± 263 | 13.17 | C | ||||||
| Tan δ | 0.286 ± 0.018 | 6.44 | C | 0.288 ± 0.023 | 7.97 | C | 0.287 ± 0.021 | 7.39 | C | ||||||
| Test | Viscosity Curve | Thixotropic Behaviour | Amplitude Sweep | Frequency Sweep | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAA | η0 | η∞ | ηU | ηL | τ0.ROT | SR | LVR Plateau | τ0.OSC | τf | G′ | G″ | Tan δ |
| CMV | ||||||||||||
| Temperature | H | L | H | L | L | H | L | H | H | L | L | L |
| Geometry | H | H | H | H | H | H | L | H | H | H | H | L |
| Sample application | H | L | H | H | L | H | L | H | H | L | L | L |
| Gap and trimming | L | L | L | L | L | L | L | L | L | L | L | L |
| Data acquisition mode | M | M | M | M | M | L | L | L | L | L | L | L |
| Integration time | M | M | M | M | M | M | M | M | M | M | M | L |
| Sample amount | M | M | M | M | M | M | M | M | M | M | M | L |
| Analyst | M | M | M | M | M | M | M | M | M | M | M | L |
| Shear stress ramp | H | H | H | H | H | H | H | H | ||||
| Step duration | M | M | M | M | M | M | M | M | M | M | M | L |
| Shear rate ramp | H | |||||||||||
| Shear load time | H | |||||||||||
| Shear recovery time | H | |||||||||||
| Frequency value | M | M | M | |||||||||
| Shear stress within LVR plateau | H | H | L | |||||||||
| Frequency ramp | M | M | L | |||||||||
| Test | Pre-Setting | CMV | Highly Relevant Caas | Interpretation | Typical Graphical Representation |
|---|---|---|---|---|---|
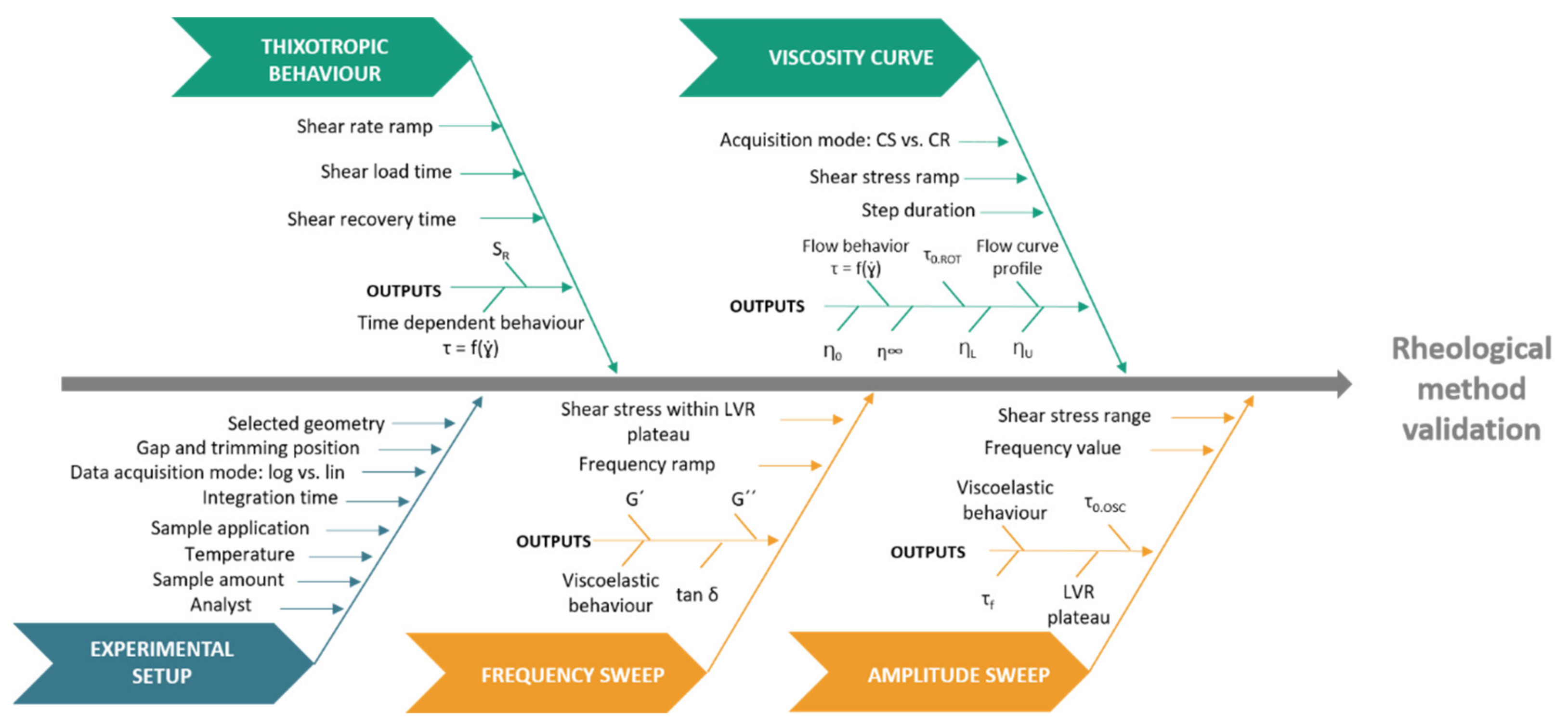
| Rotational: viscosity curve behaviour | Acquisition mode Shear stress ramp Step duration | Temperature: medium risk variable Geometry: high risk variable Sample application: medium risk variable | η0 ηU ηL η∞ | Higher η0, ηU, ηL, η∞ and τ0.ROT suggest more structured systems | 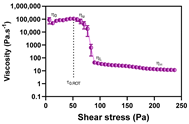 |
| Rotational: shear stress/deformation diagram | τ0.ROT | 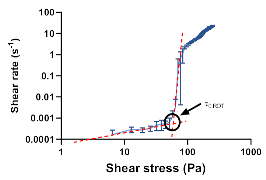 | |||
| Rotational: thixotropic behaviour | Step duration Shear rate ramp Shear load time Shear recovery time | SR | Larger SR is indicative of more structured and consistent (high viscous and less flowable samples) systems |  | |
| Oscillatory: amplitude sweep. | Frequency value | LVR plateau τf | Larger LVR and superior τf are indicative of more structured systems. |  | |
| Oscillatory: frequency sweep | Shear stress within LVR plateau Frequency ramp | G′ G″ Tan δ | G′ > G″, prevalence of elastic properties Tan δ < 1, Viscoelastic with prevalence of elastic properties, meaning gel/solid—like structures |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simões, A.; Miranda, M.; Cardoso, C.; Veiga, F.; Vitorino, C. Rheology by Design: A Regulatory Tutorial for Analytical Method Validation. Pharmaceutics 2020, 12, 820. https://doi.org/10.3390/pharmaceutics12090820
Simões A, Miranda M, Cardoso C, Veiga F, Vitorino C. Rheology by Design: A Regulatory Tutorial for Analytical Method Validation. Pharmaceutics. 2020; 12(9):820. https://doi.org/10.3390/pharmaceutics12090820
Chicago/Turabian StyleSimões, Ana, Margarida Miranda, Catarina Cardoso, Francisco Veiga, and Carla Vitorino. 2020. "Rheology by Design: A Regulatory Tutorial for Analytical Method Validation" Pharmaceutics 12, no. 9: 820. https://doi.org/10.3390/pharmaceutics12090820
APA StyleSimões, A., Miranda, M., Cardoso, C., Veiga, F., & Vitorino, C. (2020). Rheology by Design: A Regulatory Tutorial for Analytical Method Validation. Pharmaceutics, 12(9), 820. https://doi.org/10.3390/pharmaceutics12090820








