Monoolein Assisted Oil-Based Transdermal Delivery of Powder Vaccine
Abstract
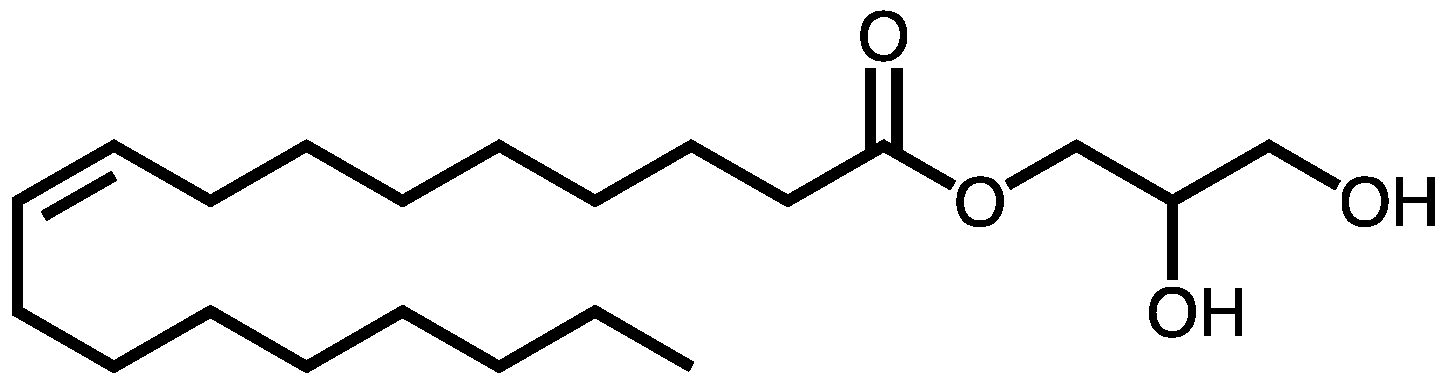
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Physical Properties of Biomacromolecules
2.4. Preparation of Liquid-Based Patch Apparatus Carrying OVA Powder
2.5. Ex-Vivo Skin Permeation Tests
2.6. OVA Stability in Oil Dispersion
2.7. Analyses of SC Structure
2.8. In Vivo Skin Permeation Tests
2.9. Antibody Responses in Mice
3. Results
3.1. Ex Vivo Permeation Test Using Yucatan Micropig (YMP) Skin
3.1.1. Effect of MO concentration
3.1.2. Factors Influencing Passive Delivery of Macromolecules
3.2. OVA Stability in Oil Vehicle
3.3. Structural Change in the Stratum Corneum
3.4. In Vivo Skin Permeation Test on Mice
3.5. Immune Response in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lagassé, H.A.D.; Alexaki, A.; Simhadri, V.L.; Katagiri, N.H.; Jankowski, W.; Sauna, Z.E.; Kimchi-Sarfaty, C. Recent advances in (therapeutic protein) drug development. F1000Research 2017, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30, e1705328. [Google Scholar] [CrossRef]
- Songkro, S. An overview of skin penetration enhancers: Penetration enhancing activity, skin irritation potential and mechanism of action. Songklanakarin J. Sci. Technol. 2009, 31, 299–321. [Google Scholar]
- Narangifard, A.; den Hollander, L.; Wennberg, C.L.; Lundborg, M.; Lindahl, E.; Iwai, I.; Han, H.; Masich, S.; Daneholt, B.; Norlén, L. Human skin barrier formation takes place via a cubic to lamellar lipid phase transition as analyzed by cryo-electron microscopy and EM-simulation. Exp. Cell Res. 2018, 366, 139–151. [Google Scholar] [CrossRef]
- Barry, B.W. Lipid–protein–partitioning theory of skin penetration enhancement. J. Control. Release 1991, 15, 237–248. [Google Scholar] [CrossRef]
- Yehia, R.; Hathout, R.M.; Attia, D.A.; Elmazar, M.M.; Mortada, N.D. Anti-tumor efficacy of an integrated methyl dihydrojasmonate transdermal microemulsion system targeting breast cancer cells: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2017, 155, 512–521. [Google Scholar] [CrossRef]
- ElMasry, S.R.; Hathout, R.M.; Abdel-Halim, M.; Mansour, S. In Vitro transdermal delivery of sesamol using oleic acid chemically-modified gelatin nanoparticles as a potential breast cancer medication. J. Drug Deliv. Sci. Technol. 2018, 48, 30–39. [Google Scholar] [CrossRef]
- Lopes, L.B.; Collett, J.H.; Bentley, M.V.L.B. Topical delivery of cyclosporin A: An in vitro study using monoolein as a penetration enhancer. Eur. J. Pharm. Biopharm. 2005, 60, 25–30. [Google Scholar] [CrossRef]
- Herai, H.; Gratieri, T.; Thomazine, J.A.; Bentley, M.V.L.B.; Lopez, R.F.V. Doxorubicin skin penetration from monoolein-containing propylene glycol formulations. Int. J. Pharm. 2007, 329, 88–93. [Google Scholar] [CrossRef]
- Quintão, W.d.S.C.; Matos, B.N.; Reis, T.A.; Barreto, L.C.d.S.; Gratieri, T.; Gelfuso, G.M. Influence of monoolein on progesterone transdermal delivery. Braz. J. Pharm. Sci. 2015, 51, 923–929. [Google Scholar]
- Lopes, L.B.; Lopes, J.L.C.; Oliveira, D.C.R.; Thomazini, J.A.; Garcia, M.T.J.; Fantini, M.C.A.; Collett, J.H.; Bentley, M.V.L.B. Liquid crystalline phases of monoolein and water for topical delivery of cyclosporin A: Characterization and study of in vitro and in vivo delivery. Eur. J. Pharm. Biopharm. 2006, 63, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Avrahami, M.; Aserin, A.; Garti, N. H(II) mesophase and peptide cell-penetrating enhancers for improved transdermal delivery of sodium diclofenac. Colloids Surf. B Biointerfaces 2010, 77, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Anbarasan, B.; Fatima Grace, X.; Shanmuganathan, S. An overview of cubosomes—Smart drug delivery system. Sri Ramachandra J. Med. 2015, 8, 1–4. [Google Scholar]
- Kozaka, S.; Tahara, Y.; Wakabayashi, R.; Nakata, T.; Ueda, T.; Kamiya, N.; Goto, M. Transcutaneous cancer vaccine using a reverse micellar antigen carrier. Mol. Pharm. 2020, 17, 645–655. [Google Scholar] [CrossRef]
- Harada, S.; Tkahashi, Y.; Nakagawa, H.; Yamashita, F.; Hashida, M. Effect of ehicle properties on skin penetration of emedastine. Biol. Pharm. Bull. 2000, 23, 1224–1228. [Google Scholar] [CrossRef]
- Ogiso, T.; Paku, T.; Iwaki, M.; Tanino, T. Percuraneous penetration of fluorescein isothiocyanate-dextrans and the mechanism for enhancement effect of enhancers on the intercellular penetration. Chem. Pharm. Bull. 1995, 18, 1566–1571. [Google Scholar] [CrossRef]
- Tamura, G.; Ichinose, M.; Fukuchi, Y.; Miyamoto, T. Transdermal tulobuterol patch, a long-acting β2-agonist. Allergol. Int. 2012, 61, 219–229. [Google Scholar] [CrossRef]
- Banerjee, A.; Ibsen, K.; Iwao, Y.; Zakrewsky, M.; Mitragotri, S. Transdermal protein delivery using choline and geranate (CAGE) deep eutectic solvent. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Yu, Y.; Kiran Kumar, M.N.; Wu, M.X. Delivery of allergen powder for safe and effective epicutaneous immunotherapy. J. Allergy Clin. Immunol. 2020, 145, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Shreffler, W.G.; Yang, W.H.; Sussman, G.L.; Brown-Whitehorn, T.F.; Nadeau, K.C.; Cheema, A.S.; Leonard, S.A.; Pongracic, J.A.; Sauvage-Delebarre, C.; et al. Effect of varying doses of epicutaneous immunotherapy vs. placebo on reaction to peanut protein exposure among patients with peanut sensitivity: A randomized clinical trial. J. Am. Med. Assoc. 2017, 318, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, M.; Imamura, K.; Hirakawa, Y.; Tahara, Y.; Kamiya, N.; Goto, M. Sucrose laurate-enhanced transcutaneous immunization with a solid-in-oil nanodispersion. Medchemcomm 2014, 5, 20–24. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yang, J.T. A new approach to the calculation of secondary structures of globular proteins by optical rotatory dispersion and circular dichroism. Biochem. Biophys. Res. Commun. 1971, 44, 1285–1291. [Google Scholar] [CrossRef]
- Cornwell, P.A.; Barry, B.W.; Bouwstra, J.A.; Gooris, G.S. Modes of action of terpene penetration enhancers in human skin; differential scanning calorimetry, small-angle X-ray diffraction and enhancer uptake studies. Int. J. Pharm. 1996, 127, 9–26. [Google Scholar] [CrossRef]
- Glombitza, B.; Müller-Goymann, C.C. Influence of different ceramides on the structure of in vitro model lipid systems of the stratum corneum lipid matrix. Chem. Phys. Lipids 2002, 117, 29–44. [Google Scholar] [CrossRef]
- Kitaoka, M.; Imamura, K.; Hirakawa, Y.; Tahara, Y.; Kamiya, N.; Goto, M. Needle-free immunization using a solid-in-oil nanodispersion enhanced by a skin-permeable oligoarginine peptide. Int. J. Pharm. 2013, 458, 334–339. [Google Scholar] [CrossRef]
- Matsuo, K.; Yonehara, R.; Gekko, K. Secondary-structure analysis of proteins by vacuum-ultraviolet circular dichroism spectroscopy. J. Biochem. 2004, 135, 405–411. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, A practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2004; pp. 1–23. [Google Scholar]
- Mendelsohn, R.; Moore, D.J. Infrared determination of conformational order and phase behavior in ceramides and stratum corneum models. Methods Enzymol. 2000, 312, 228–247. [Google Scholar]
- Kulkarni, C.V.; Wachter, W.; Iglesias-Salto, G.; Engelskirchen, S.; Ahualli, S. Monoolein: A magic lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef]
- Liebert, M.A. Final report on the safety assessment of trichloroethane. J. Am. Coll. Toxicol. 1986, 5, 391–412. [Google Scholar]
- Steluti, R.; De Rosa, F.S.; Collett, J.; Tedesco, A.C.; Bentley, M.V.L.B. Topical glycerol monooleate/propylene glycol formulations enhance 5-aminolevulinic acid in vitro skin delivery and in vivo protophorphyrin IX accumulation in hairless mouse skin. Eur. J. Pharm. Biopharm. 2005, 60, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, N.; Shobha Rani, R. Physicochemical and pharmacokinetic parameters in drug selection and loading for transdermal drug delivery. Indian J. Pharm. Sci. 2008, 70, 94–96. [Google Scholar] [CrossRef] [PubMed]
- Shingade, G.M.; Aamer, Q.; Sabale, P.M.; Grampurohit, N.D.; Gadhave, M.V.; Jadhav, S.L.; Gaikwad, D.D. Review on: Recent trend on transdermal drug delivery system. J. Drug Deliv. Ther. 2012, 2, 66–74. [Google Scholar] [CrossRef]
- Tokumoto, S.; Higo, N.; Todo, H.; Sugibayashi, K. Effect of combination of low-frequency sonophoresis or electroporation with iontophoresis on the mannitol flux or electroosmosis through excised skin. Biol. Pharm. Bull. 2016, 39, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef]
- Yang, J.T.; Wu, C.S.C.; Martinez, H.M. Calculation of protein conformation from circular dichroism. Methods Enzymol. 1986, 130, 208–269. [Google Scholar]
- Bouwstra, J.A.; Gooris, G.S.; van der Spek, J.A.; Bras, W. Structural investigations of human stratum corneum by small-angle X-ray scattering. J. Investig. Dermatol. 1991, 97, 1005–1012. [Google Scholar] [CrossRef]
- Ongpipattanakul, B.; Burnette, R.R.; Potts, R.O.; Francoeur, M.L. Evidence that oleic acid exists in a separate phase within strarum corneum lipids. Pharm. Res. 1991, 8, 350–354. [Google Scholar] [CrossRef]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Geneidi, A.S.; Guy, R.H. Uptake of microemulsion components into the stratum corneum and their molecular effects on skin barrier function. Mol. Pharm. 2010, 7, 1266–1273. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitaoka, M.; Oka, A.; Goto, M. Monoolein Assisted Oil-Based Transdermal Delivery of Powder Vaccine. Pharmaceutics 2020, 12, 814. https://doi.org/10.3390/pharmaceutics12090814
Kitaoka M, Oka A, Goto M. Monoolein Assisted Oil-Based Transdermal Delivery of Powder Vaccine. Pharmaceutics. 2020; 12(9):814. https://doi.org/10.3390/pharmaceutics12090814
Chicago/Turabian StyleKitaoka, Momoko, Atsushi Oka, and Masahiro Goto. 2020. "Monoolein Assisted Oil-Based Transdermal Delivery of Powder Vaccine" Pharmaceutics 12, no. 9: 814. https://doi.org/10.3390/pharmaceutics12090814
APA StyleKitaoka, M., Oka, A., & Goto, M. (2020). Monoolein Assisted Oil-Based Transdermal Delivery of Powder Vaccine. Pharmaceutics, 12(9), 814. https://doi.org/10.3390/pharmaceutics12090814