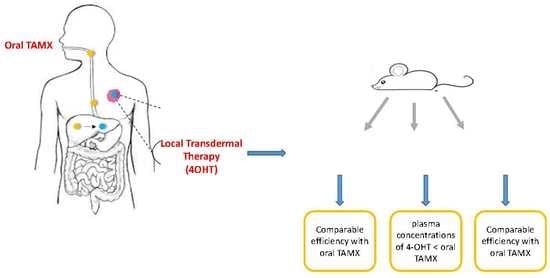
Efficacy of Emu Oil Transfersomes for Local Transdermal Delivery of 4-OH Tamoxifen in the Treatment of Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals (Mice)
2.2. Cell Line
2.3. Test Drugs
2.4. Chemicals
2.5. Transfersome Formulation
2.6. Skin Irritancy Studies
2.7. Efficacy of Topical Application of 4-Hydroxytamoxifen (4-OHT) Transfersomal Formulations
2.8. Quantification of Tamoxifen (TAMX) and Its Metabolite 4-OHT in Mice Plasma
2.9. Histopathology Studies
2.10. Statistical Analysis
3. Results and Discussion
3.1. Skin Irritancy Studies
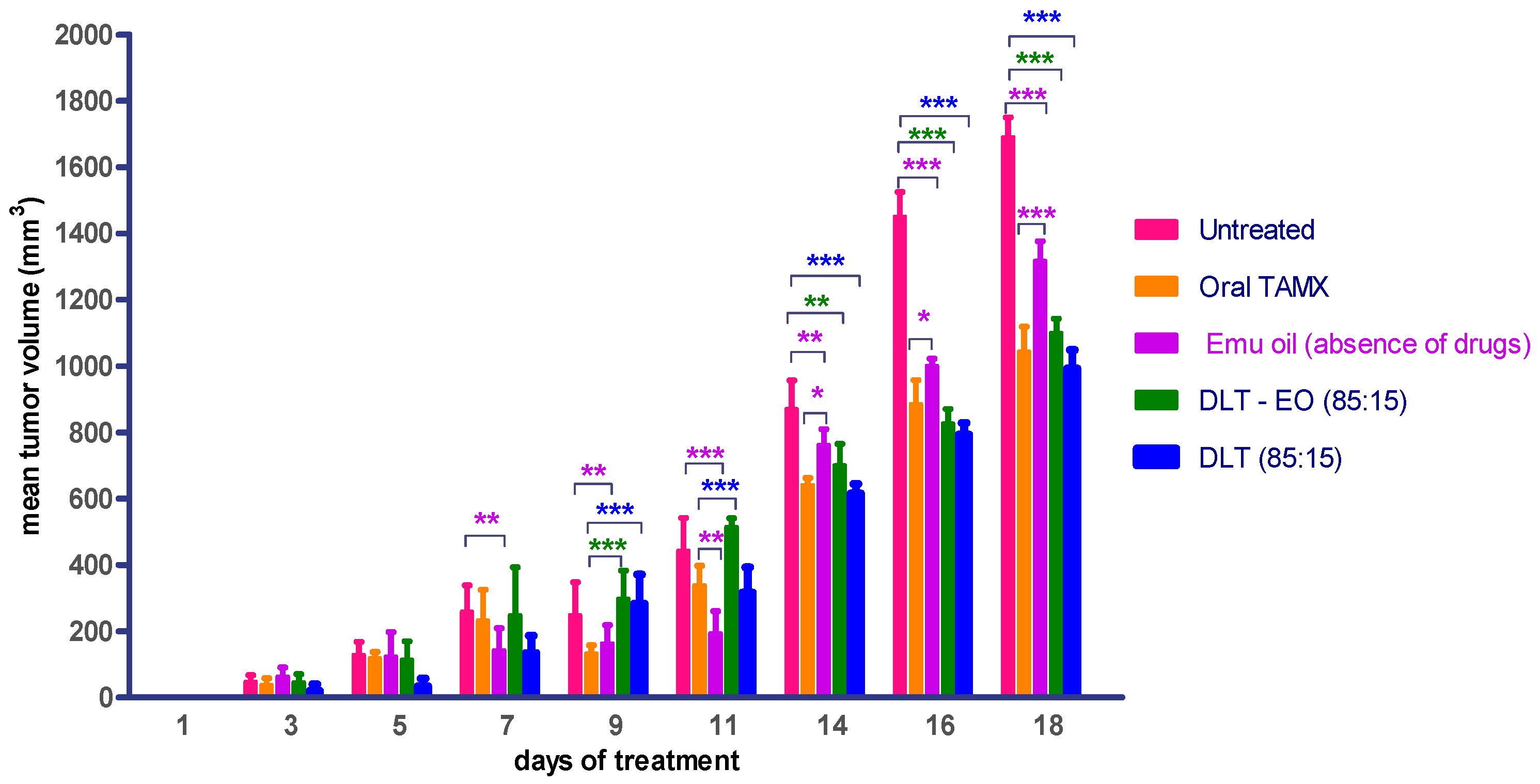
3.2. Treatment with Emu Oil and 4-OHT Transfersomal Formulations
Quantification of TAMX and 4-OHT in the BALB/c Mice Plasma
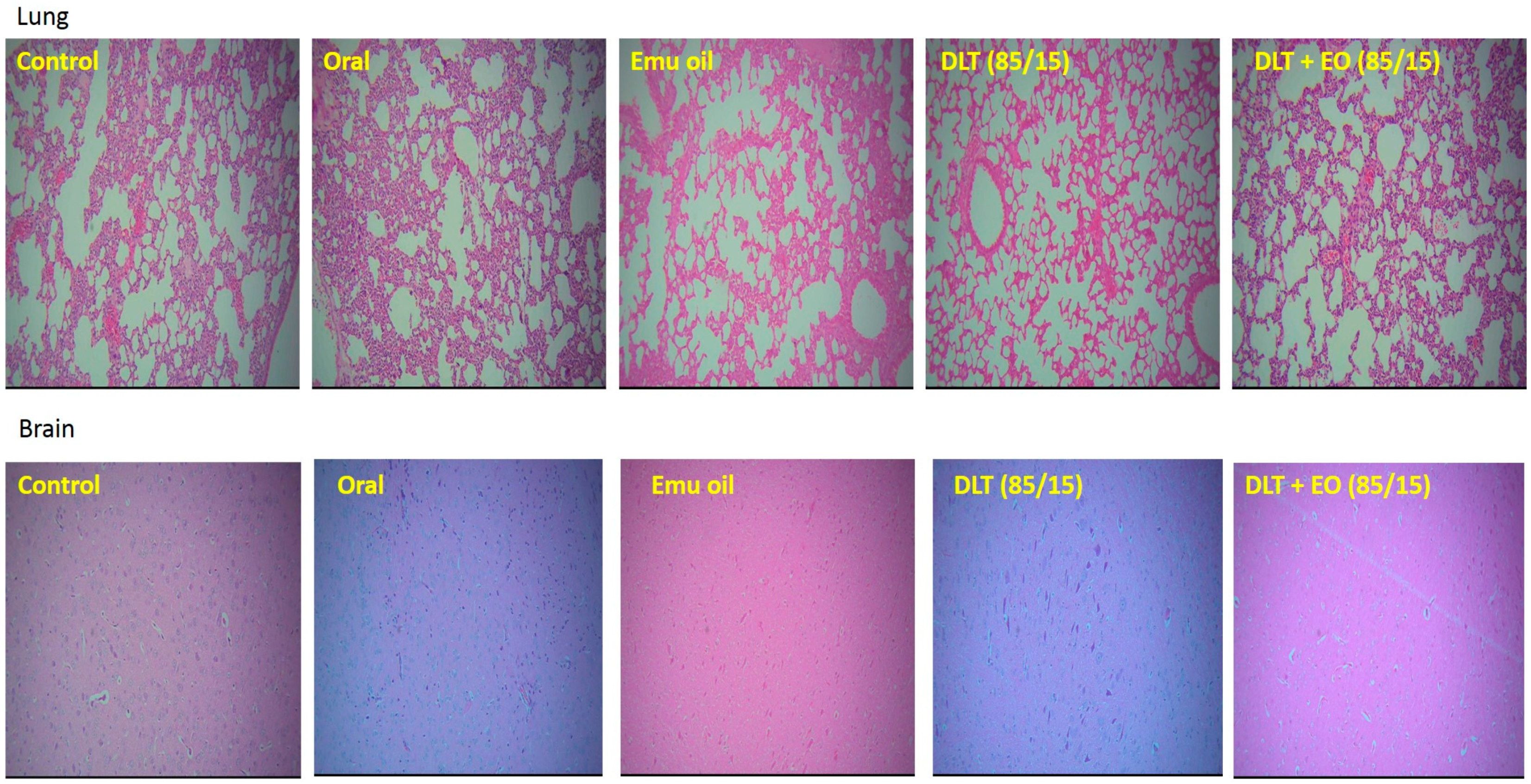
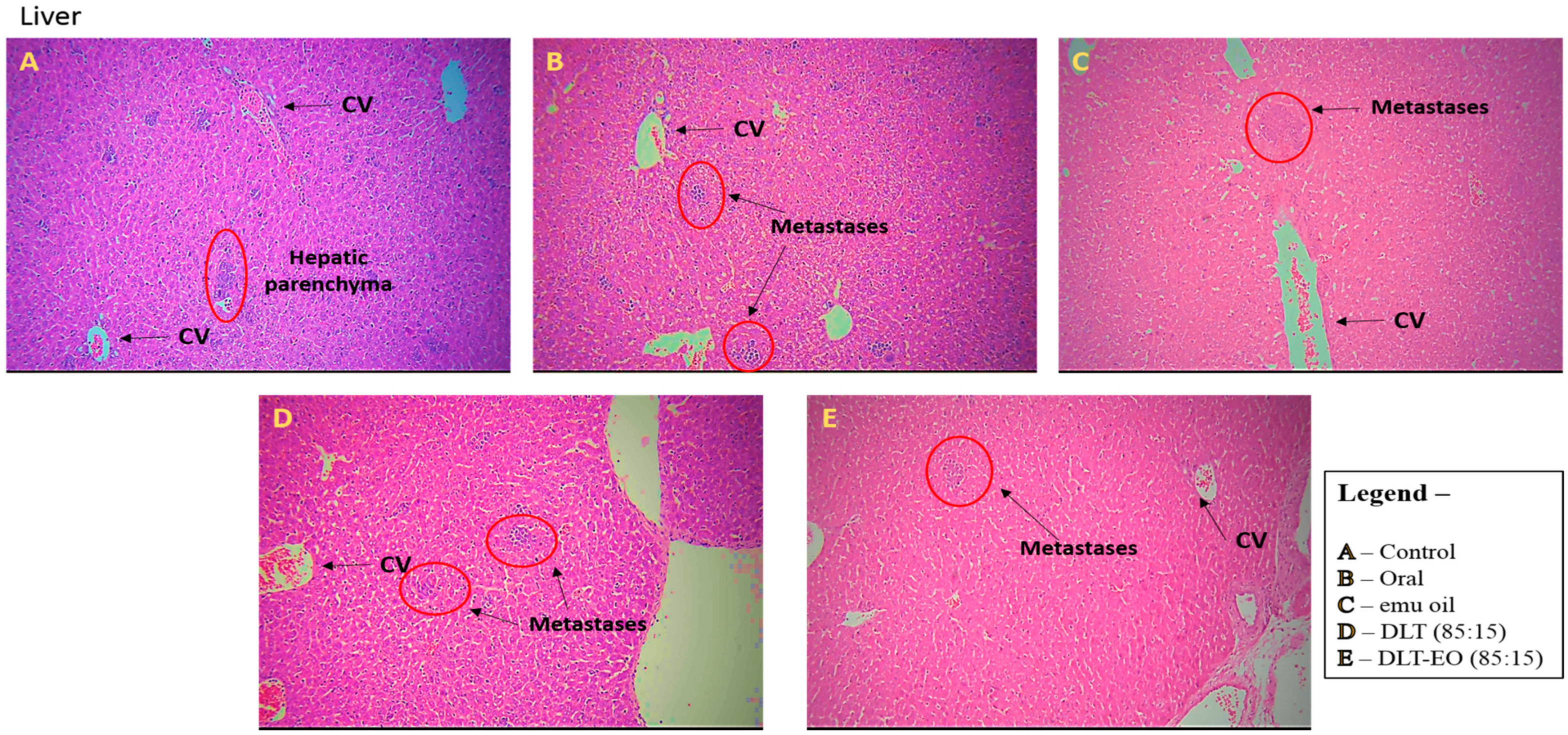
3.3. Histopathology Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GLOBACON. Breast Cancer: Estimated Incidence, Mortality and Prevalence Worldwide in 2013, in World Health Organization (WHO); IAF Research, Ed.; World Health Organization (WHO): Geneva, Switzerland, 2013. [Google Scholar]
- Van Seijen, M.; Lips, E.H.; Thompson, A.M.; Nik-Zainal, S.; Futreal, A.; Hwang, E.S.; Verschuur, E.; Lane, J.; Jonkers, J.; Rea, D.W.; et al. Ductal carcinoma in situ: To treat or not to treat, that is the question. Br. J. Cancer 2019, 121, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.M.; DeSantis, C.E.; Lin, C.C.; Kramer, J.L.; Jemal, A.; Kohler, B.; Brawley, O.W.; Gansler, T. Cancer statistics: Breast cancer in situ. CA Cancer J. Clin. 2015, 65, 481–495. [Google Scholar] [CrossRef]
- Elmore, J.G.; Fenton, J.J. Ductal carcinoma in situ (DCIS): Raising signposts on an Ill-Marked Treatment Path. JNCI J. Natl. Cancer Inst. 2012, 104, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Eng-Wong, J.; Costantino, J.P.; Swain, M.S. The Impact of Systemic Therapy Following Ductal Carcinoma In Situ. J. Natl. Cancer Inst. Monogr. 2010, 2010, 200–203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donker, M.; Litière, S.; Werutsky, G.; Julien, J.-P.; Fentiman, I.S.; Agresti, R.; Rouanet, P.; Lara, C.T.D.; Bartelink, H.; Duez, N.; et al. Breast-Conserving Treatment With or Without Radiotherapy in Ductal Carcinoma In Situ: 15-Year Recurrence Rates and Outcome After a Recurrence, from the EORTC 10853 Randomized Phase III Trial. J. Clin. Oncol. 2013, 31, 4054–4059. [Google Scholar] [CrossRef] [PubMed]
- Allred, D.C.; Anderson, S.J.; Paik, S.; Wickerham, D.L.; Nagtegaal, I.D.; Swain, S.M.; Mamounas, E.P.; Julian, T.B.; Geyer, C.E., Jr.; Costantino, J.P.; et al. Adjuvant Tamoxifen Reduces Subsequent Breast Cancer in Women With Estrogen Receptor–Positive Ductal Carcinoma in Situ: A Study Based on NSABP Protocol B-24. J. Clin. Oncol. 2012, 30, 1268–1273. [Google Scholar] [CrossRef]
- Cronin-Fenton, D.P.; Damkier, P.; Lash, T.L. Metabolism and Transport of Tamoxifen in Relation to Its Effectiveness: New Perspectives on an Ongoing Controversy; Future Oncology: London, UK, 2014; Volume 10, pp. 107–122. [Google Scholar]
- Fisher, B.; Costantino, J.P.; Wickerham, D.L.; Redmond, C.K.; Kavanah, M.; Cronin, W.M.; Vogel, V.; Robidoux, A.; Dimitrov, N.; Atkins, J.; et al. Tamoxifen for Prevention of Breast Cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998, 90, 1371–1388. [Google Scholar] [CrossRef]
- Morrow, M.; Jordan, V.C. Risk factors and the prevention of breast cancer with tamoxifen. Cancer Surv. 1993, 18, 211–229. [Google Scholar]
- Jordan, V.C.; Murphy, C.S. Endocrine Pharmacology of Antiestrogens as Antitumor Agents. Endocr. Rev. 1990, 11, 578–610. [Google Scholar] [CrossRef]
- Brigger, I.; Chaminade, P.; Marsaud, V.; Appel, M.; Besnard, M.; Gurny, R.; Renoir, M.; Couvreur, P.L. Tamoxifen encapsulation within polyethylene glycol-coated nanospheres. A new antiestrogen formulation. Int. J. Pharm. 2001, 214, 37–42. [Google Scholar] [CrossRef]
- Christiane Brems, J.B.; Parret, V.C.; Metzger, J.; Johnson, M.E. Alternative and Complementary Treatment Needs and Experiences of Women with Breast Cancer. J. Altern. Complement. Med. 2013, 19, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Nancy, K.; Janz, M.M.; Chung, L.K.; Lantz, P.M.; Hawley, S.T.; Morrow, M.; Schwartz, K.; Katz, S.J. Symptom Experience and Quality of Life of Women Following Breast Cancer Treatment. J. Womens Health 2007, 16, 1348–1361. [Google Scholar]
- Ackerman, A.B.; Kessler, G.; Gyorfi, T.; Tsou, H.C.; Gottlieb, G.J. Contrary view: The breast is not an organ per se, but a distinctive region of skin and subcutaneous tissue. Am. J. Dermatopathol. 2007, 29, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Sundralingam, U.; Khan, T.M.; Elendran, S.; Muniyandy, S.; Palanisamy, U.D. Patient-controlled transdermal 4-hydroxytamoxifen (4-OHT) vs. oral tamoxifen: A systematic review and meta analysis. Pak. J. Pharm. Sci. 2019, 32, 1121–1128. [Google Scholar]
- Gupta, A.; Aggarwal, G.; Singla, S.; Arora, R. Transfersomes: A Novel Vesicular Carrier for Enhanced Transdermal Delivery of Sertraline: Development, Characterization, and Performance Evaluation. Sci. Pharm. 2012, 80, 1061–1080. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G. Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim. Biophys. Acta (BBA) Biomembr. 1992, 1104, 226–232. [Google Scholar] [CrossRef]
- Rai, K.; Gupta, Y.; Jain, A.; Jain, S.K. Transfersomes: Self-optimizing carriers for bioactives. PDA J. Pharm. Sci. Technol. 2008, 62, 362–379. [Google Scholar]
- Duangjit, S.; Opanasopit, P.; Rojanarata, T.; Ngawhirunpat, T. Characterization and in vitro skin permeation of meloxicam-loaded liposomes versus transfersomes. J. Drug Deliv. 2011, 2011. [Google Scholar] [CrossRef]
- Hoffman, L.C.; Brand, M.M.; Cloete, S.W.P.; Muller, M. The fatty acid composition of muscles and fat depots of ostriches as influenced by genotype. S. Afr. J. Anim. Sci. 2012, 42, 256–265. [Google Scholar] [CrossRef][Green Version]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Raghu Nadhanan, R.; Abimosleh, S.M.; Su, Y.-W.; Scherer, M.A.; Howarth, G.S.; Xian, C.J. Dietary emu oil supplementation suppresses 5-fluorouracil chemotherapy-induced inflammation, osteoclast formation, and bone loss. Am. J. Physiol. Endocrinol. Metab. 2012, 302, E1440–E1449. [Google Scholar] [CrossRef] [PubMed]
- Donna, J.; Carrico, W.M.S. The Efficacy of Emu Oil vs. Placebo in Minimizing Vulvar Pain Levels in Women—A Randomized, Double Blinded, Placebo-Controlled Trial; ClinicalTrials.gov.: Michigan, MI, USA, 2011.
- Rollmann, D.C.; Novotny, P.J.; Petersen, I.A.; Garces, Y.I.; Bauer, H.J.; Yan, E.S.; Wahner-Roedler, D.; Vincent, A.; Sloan, J.A.; Laack, N.N.I. Double-Blind, Placebo-Controlled Pilot Study of Processed Ultra Emu Oil Versus Placebo in the Prevention of Radiation Dermatitis. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Attarzadeh, Y.; Asilian, A.; Shahmoradi, Z.; Adibi, N. Comparing the efficacy of emu oil with clotrimazole and hydrocortisone in the treatment of seborrheic dermatitis: A clinical trial. J. Res. Med. Sci. 2013, 18, 477. [Google Scholar] [PubMed]
- Sundralingam, U.; Muniyandy, S.; Radhakrishnan, A.K.; Palanisamy, U.D. Ratite oils for local transdermal delivery of 4-OH Tamoxifen: Development, Characterization and Ex-vivo Evaluation. J. Liposome Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Sousa, A.; Cabrita, A.S.; Reis, C.P.; Figueiredo, V.F. 12—A New Approach for cancer Treatment: From Specific Induction of Breast Cancer to Innovative Gold-Nanoparticle Mediated Thermal Therapies. In Nanomedicines for Breast Cancer Theranostics; Thorat, N.D., Bauer, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 269–298. [Google Scholar]
- Jain, S.; Jain, P.; Umamaheshwari, R.B.; Jain, N.K. Transfersomes—A Novel Vesicular Carrier for Enhanced Transdermal Delivery: Development, Characterization, and Performance Evaluation. Drug Dev. Ind. Pharm. 2003, 29, 1013–1026. [Google Scholar] [CrossRef]
- Cevc, G.; Gebauer, D. Hydration-driven transport of deformable lipid vesicles through fine pores and the skin barrier. Biophys. J. 2003, 84, 1010–1024. [Google Scholar] [CrossRef]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and Eosin Staining of Tissue and Cell Sections. Cold Spring Harb. Protoc. 2008, 2008, pdb.prot4986. [Google Scholar] [CrossRef]
- Hafid, S.R.A.; Chakravarthi, S.; Nesaretnam, K.; Radhakrishnan, A.K. Tocotrienol-Adjuvanted Dendritic Cells Inhibit Tumor Growth and Metastasis: A Murine Model of Breast Cancer. PLoS ONE 2013, 8, e74753. [Google Scholar]
- FDA. Guidance for Industry, Bioanalytical Method Validation; US Department of Health and Human Services, Ed.; FDA: Washington, DC, USA, 2001.
- Kuwajima, K.; Nakamura, T. Antitumor Complexes Formed by Oleic Acid and Molten Globule Intermediates of Proteins; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- MacLean, C.H.; Newberry, S.J.; Mojica, W.A.; Khanna, P.; Issa, A.M.; Suttorp, M.J.; Lim, Y.-W.; Traina, S.B.; Hilton, L.; Garland, R.; et al. Effects of Omega-3 Fatty Acids on Cancer RiskA Systematic Review. JAMA 2006, 295, 403–415. [Google Scholar] [CrossRef]
- Begin, M.E.; Ells, G.; Horrobin, D.F. Polyunsaturated Fatty Acid-Induced Cytotoxicity Against Tumor Cells and Its Relationship to Lipid Peroxidation. JNCI J. Natl. Cancer Inst. 1988, 80, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Van Loco, J.; Elskens, M.; Croux, C.; Beernaert, H. Linearity of Calibration Curves: Use and Misuse of the Correlation Coefficient; Katholieke Universiteit Leuven, Open Access Publications from Katholieke Universiteit Leuven: Leuven, Belgium, 2002; Volume 7, pp. 281–285. [Google Scholar]
- ICH. Impurities: Guideline for Residual Solvents Q3C(R5); In Step 4; ICH: Geneva, Switzerland, 2011. [Google Scholar]
- Asp, M.L.; Martindale, J.J.; Metzger, J.M. Direct, differential Effects of Tamoxifen, 4-Hydroxytamoxifen, and Raloxifene on Cardiac Myocyte Contractility and Calcium Handling. PLoS ONE 2013, 8, e78768. [Google Scholar] [CrossRef] [PubMed]
- Crewe, H.K.; Ellis, S.W.; Lennard, M.S.; Tucker, G.T. Variable contribution of cytochromes p450 2d6, 2c9 and 3a4 to the 4-hydroxylation of tamoxifen by human liver microsomes. Biochem. Pharmacol. 1997, 53, 171–178. [Google Scholar] [CrossRef]
- Robinson, S.P.; Langan-Fahey, S.M.; Johnson, D.A.; Jordan, V.C. Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab. Dispos. 1991, 19, 36–43. [Google Scholar]
- Kisanga, E.R.; Gjerde, J.; Schjøtt, J.; Mellgren, G.; Lien, E.A. Tamoxifen administration and metabolism in nude mice and nude rats. J. Steroid Biochem. Mol. Biol. 2003, 84, 361–367. [Google Scholar] [CrossRef]
- Lee, O.; Page, K.; Ivancic, D.; Helenowski, I.; Parini, V.; Sullivan, M.E.; Margenthaler, J.I.; Robert, T.; Jovanovic, B.; Dunn, B.K.; et al. A randomized phase II presurgical trial of transdermal 4-hydroxytamoxifen gel versus oral tamoxifen in women with ductal carcinoma in situ of the breast. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 3672–3682. [Google Scholar] [CrossRef]
- Rouanet, P.; Linares-Cruz, G.; Dravet, F.; Poujol, S.; Gourgou, S.; Simony-Lafontaine, J.; Kramar, A.; Girault, J.; Nestour, E.L.; Maudelonde, T. Neoadjuvant Percutaneous 4-Hydroxytamoxifen Decreases Breast Tumoral Cell Proliferation: A Prospective Controlled Randomized Study Comparing Three Doses of 4-Hydroxytamoxifen Gel to Oral Tamoxifen. J. Clin. Oncol. 2005, 23, 2980–2987. [Google Scholar] [CrossRef]
- Pujol, H.; Girault, J.; Rouanet, P.; Fournier, S.; Grenier, J.; Simony, J.; Fourtillan, J.B.; Pujol, J.L. Phase I Study of percutaneous 4-hydroxy-tamoxifen with analyses of 4-hydroxy-tamoxifen concentrations in breast cancer and normal breast tissue. Cancer Chemother. Pharmacol. 1995, 36, 493–498. [Google Scholar] [CrossRef]
- Reed, C.A.; Berndtson, A.K.; Nephew, K.P. Dose-dependent effects of 4-hydroxytamoxifen, the active metabolite of tamoxifen, on estrogen receptor-α expression in the rat uterus. Anti Cancer Drugs 2005, 16, 559–567. [Google Scholar] [CrossRef]
- Yang, G.; Nowsheen, S.; Aziz, K.; Georgakilas, A.G. Toxicity and adverse effects of Tamoxifen and other anti-estrogen drugs. Pharmacol. Ther. 2013, 139, 392–404. [Google Scholar] [CrossRef]
- DuPré, S.A.; Redelman, D.; Hunter, K.W., Jr. The mouse mammary carcinoma 4T1: Characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int. J. Exp. Pathol. 2007, 88, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.H.; Jackson, J.T.; Haynes, N.M.; Teng, M.W.; Moeller, M.; Hayakawa, Y.; Street, S.E.; Cameron, R.; Tanner, J.E.; Trapani, J.A.; et al. Gene-Engineered T Cells as a Superior Adjuvant Therapy for Metastatic Cancer. J. Immunol. 2004, 173, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- Altundag, K.; Bondy, M.L.; Mirza, N.Q.; Kau, S.W.; Broglio, K.; Hortobagyi, G.N.; Rivera, E. Clinicopathologic characteristics and prognostic factors in 420 metastatic breast cancer patients with central nervous system metastasis. Cancer 2007, 110, 2640–2647. [Google Scholar] [CrossRef]
- Tsavellas, G.; Patel, H.; Allen-Mersh, T.G. Detection and clinical significance of occult tumour cells in colorectal cancer. BJS Br. J. Surg. 2001, 88, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Beitsch, P.D.; Clifford, E. Detection of carcinoma cells in the blood of breast cancer patients. Am. J. Surg. 2000, 180, 446–449. [Google Scholar] [CrossRef]
- Gilbey, A.M.; Burnett, D.; Coleman, R.E.; Holen, I. The detection of circulating breast cancer cells in blood. J. Clin. Pathol. 2004, 57, 903–911. [Google Scholar] [CrossRef]
- Van Oort, I.M.; Witjes, J.A.; Kok, D.E.G.; Kiemeney, L.A.L.M.; Hulsbergen-vandeKaa, C.A. Maximum tumor diameter is not an independent prognostic factor in high-risk localized prostate cancer. World J. Urol. 2008, 26, 237–241. [Google Scholar] [CrossRef][Green Version]
- Epstein, J.I.; Carmichael, M.; Partin, A.W.; Walsh, P.C. Is Tumor Volume an Independent Predictor of Progression Following Radical Prostatectomy? A Multivariate Analysis of 185 Clinical Stage B Adenocarcinomas of the Prostate with 5 Years of Followup. J. Urol. 1993, 149, 1478–1481. [Google Scholar] [CrossRef]



| Statistical Parameters | TAMX a | 4-OHT a |
|---|---|---|
| Concentration range (ng mL−1) | 50–0.5 | 50–0.5 |
| Regression equation | y = 0.8144x + 7.116 | y = 1.6851x + 1.705 |
| Correlation equation (r) | 0.9935 | 0.9956 |
| Limit of detection (LOD) (ng mL−1) | 0.1 | 0.1 |
| Limit of quantification (LOQ) (ng mL−1) | 0.3 | 0.3 |
| Standard error | 5.74 | 2.20 |
| F | 3974.99 | 1120.60 |
| SS (residual) | 263.66 | 24.11 |
| MS (residual) | 32.96 | 4.82 |
| SS (regression) | 5404.55 | 1.31 × 105 |
| MS (regression) | 5404.55 | 1.31 × 105 |
| Lower 95% | 0.98167 | 3.960 |
| Upper 95% | 4.3916 | 13.696 |
| Compounds | Quality Control | Nominal Concentration (ng·mL−1) | Precision (% RSD) a | Accuracy (%) a | |
|---|---|---|---|---|---|
| Intra-Assay | Inter-Assay | ||||
| TAMX | QCL | 1 | 2.03 | 2.01 | 96.7 |
| QCM | 20 | 0.67 | 0.67 | 93.9 | |
| QCH | 200 | 2.37 | 2.35 | 107.1 | |
| 4-OHT | QCL | 1 | 1.71 | 1.69 | 95.5 |
| QCM | 20 | 0.50 | 0.50 | 95.7 | |
| QCH | 200 | 1.48 | 1.47 | 108.0 | |
| Types of Formulations | Dose Administered (µg) | Tamoxifen (ng/mL) | % of TAMX Compared to Dose Administered | 4-OHT (ng/mL) | % of 4-OHT Compared to Dose Administered |
|---|---|---|---|---|---|
| Control | ND | - | - | - | - |
| Topical Pure EO | - | - | - | - | - |
| Oral TAMX | 120 | 325.21 ± 3.78 | 0.27 | 634.42 ± 7.54 | 0.53 |
| DLT (85:15) + 4-OHT Transfersome | 100 | BQL | - | 32.45 ± 0.48 *** | 0.03 |
| DLT + EO (85:15) + 4-OHT Transfersome | 100 | BQL | - | 10.24 ± 0.07 *** | 0.01 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sundralingam, U.; Chakravarthi, S.; Radhakrishnan, A.K.; Muniyandy, S.; Palanisamy, U.D. Efficacy of Emu Oil Transfersomes for Local Transdermal Delivery of 4-OH Tamoxifen in the Treatment of Breast Cancer. Pharmaceutics 2020, 12, 807. https://doi.org/10.3390/pharmaceutics12090807
Sundralingam U, Chakravarthi S, Radhakrishnan AK, Muniyandy S, Palanisamy UD. Efficacy of Emu Oil Transfersomes for Local Transdermal Delivery of 4-OH Tamoxifen in the Treatment of Breast Cancer. Pharmaceutics. 2020; 12(9):807. https://doi.org/10.3390/pharmaceutics12090807
Chicago/Turabian StyleSundralingam, Usha, Srikumar Chakravarthi, Ammu Kutty Radhakrishnan, Saravanan Muniyandy, and Uma D. Palanisamy. 2020. "Efficacy of Emu Oil Transfersomes for Local Transdermal Delivery of 4-OH Tamoxifen in the Treatment of Breast Cancer" Pharmaceutics 12, no. 9: 807. https://doi.org/10.3390/pharmaceutics12090807
APA StyleSundralingam, U., Chakravarthi, S., Radhakrishnan, A. K., Muniyandy, S., & Palanisamy, U. D. (2020). Efficacy of Emu Oil Transfersomes for Local Transdermal Delivery of 4-OH Tamoxifen in the Treatment of Breast Cancer. Pharmaceutics, 12(9), 807. https://doi.org/10.3390/pharmaceutics12090807