Effects of Formulation Excipients on Skin Barrier Function in Creams Used in Pediatric Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
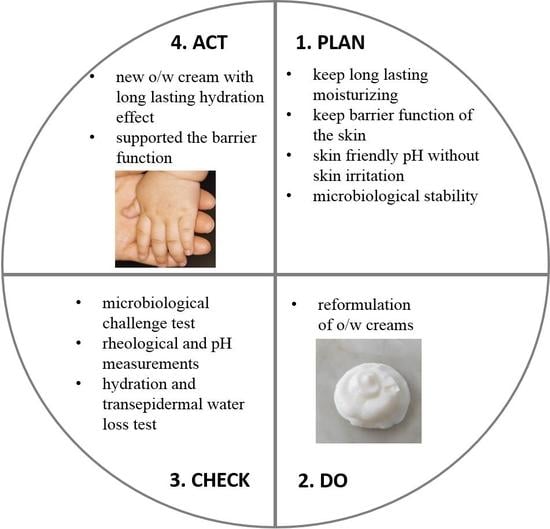
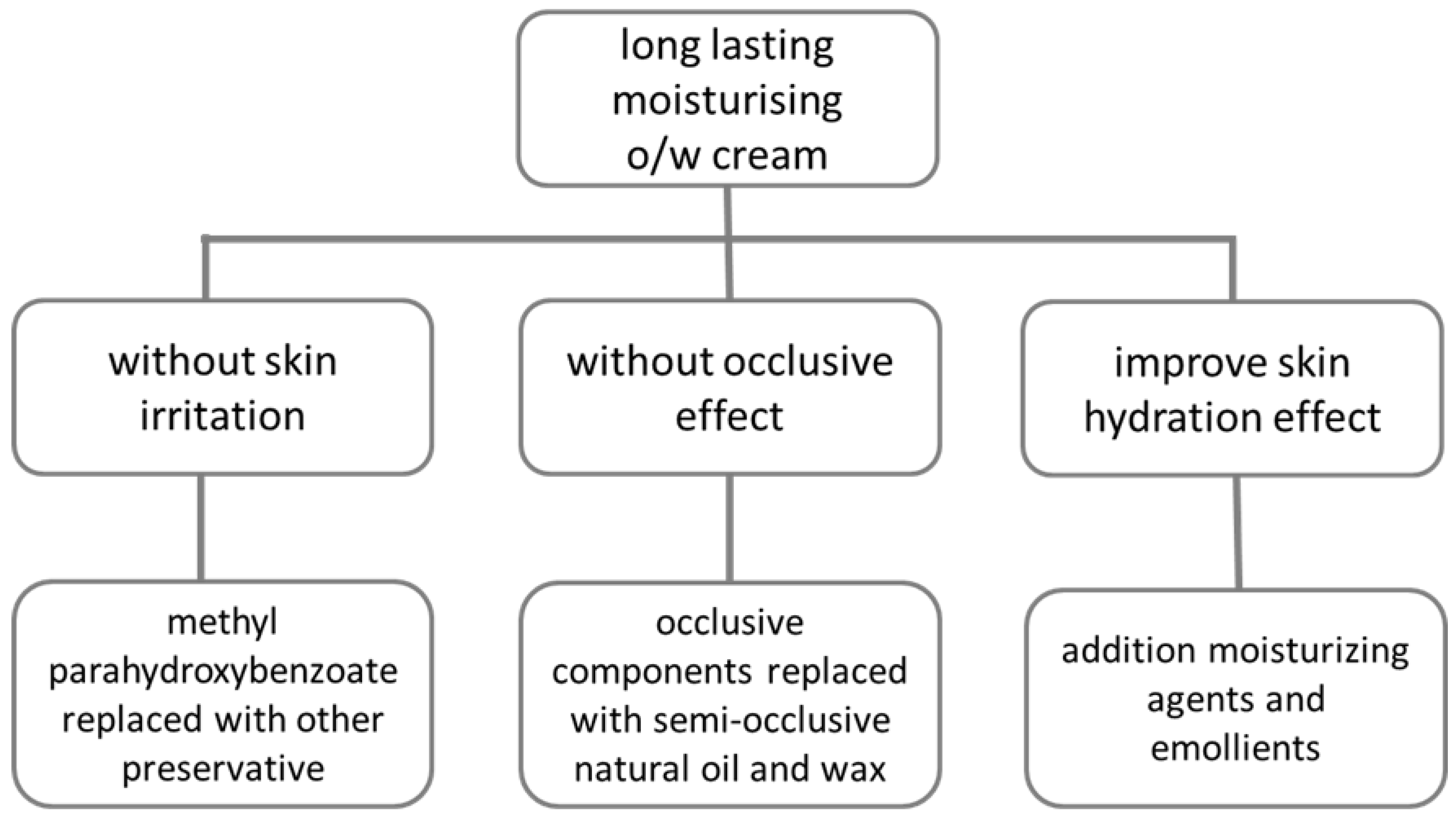
2.2. Plan of Reformulation
2.3. Preparation of the Samples
2.4. Methods
2.4.1. Microbiological Quality of Non-Sterile Pharmaceutical and Cosmetic Preparations for Dermal Use
- Pseudomonas aeruginosa;
- Staphylococcus aureus;
- Aspergillus brasiliensis; and
- Candida albicans.
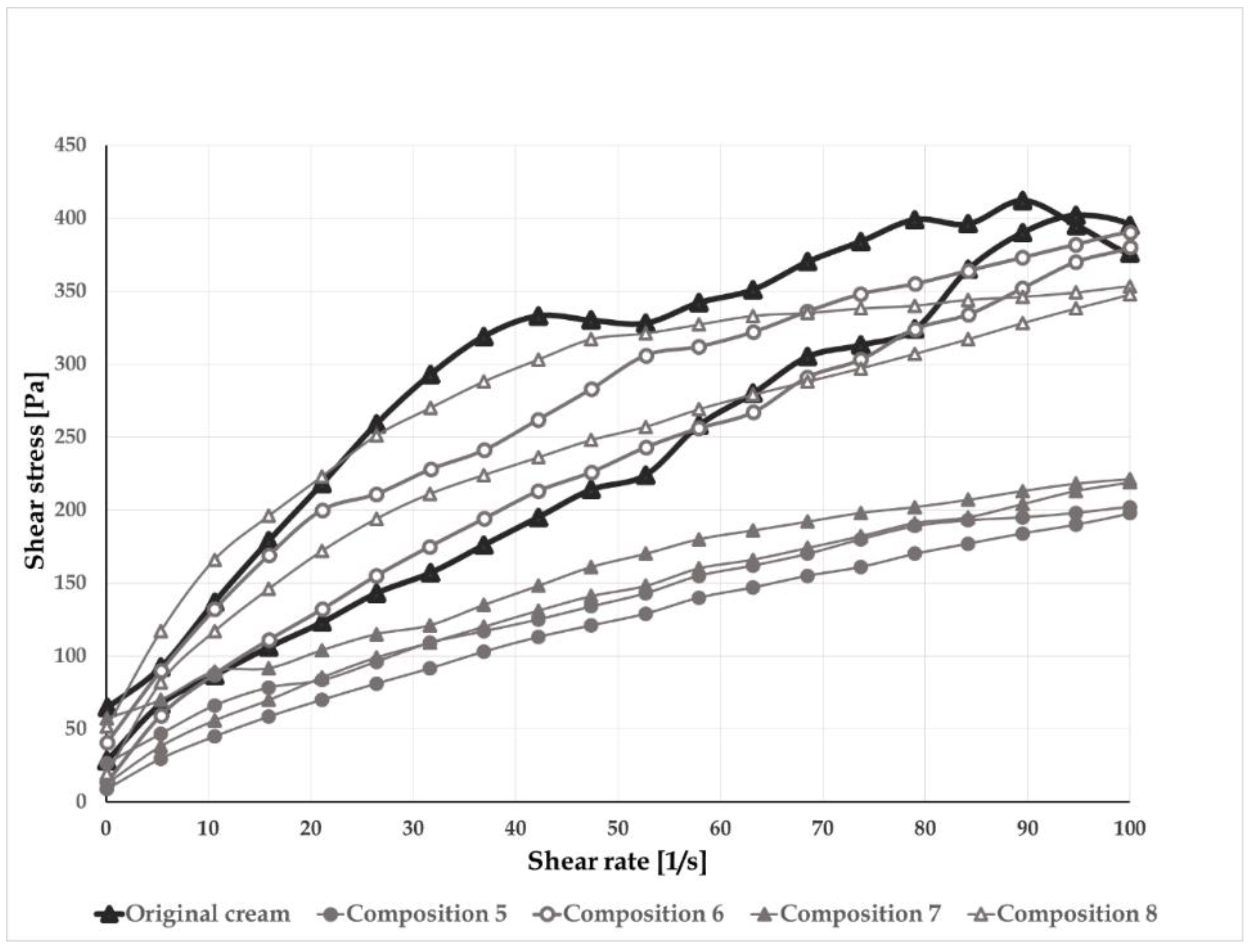
2.4.2. Rheological and pH Measurements
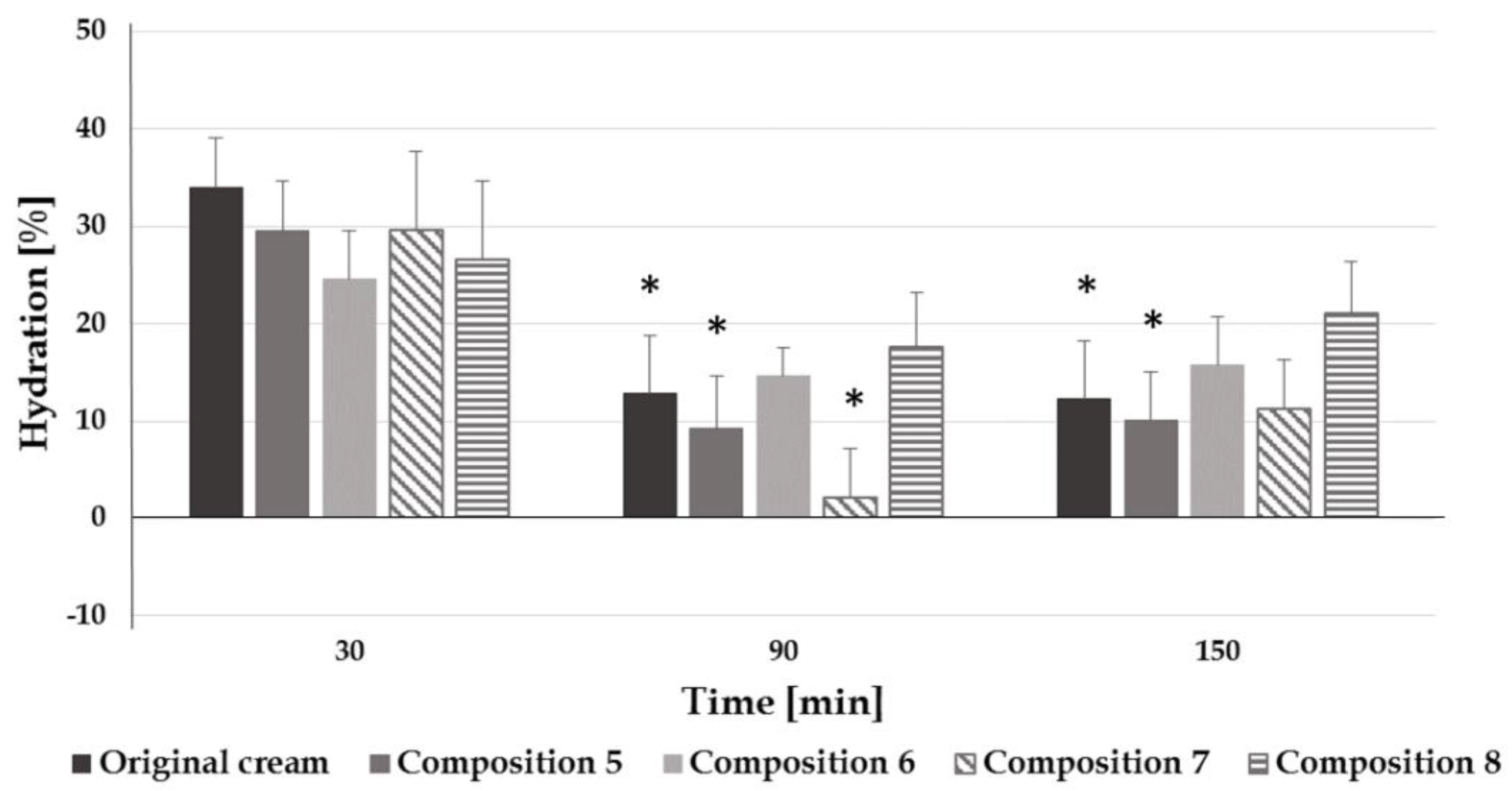
2.4.3. Hydration and Transepidermal Water Loss Tests
2.4.4. Statistical Analysis
3. Results and Discussion
3.1. Reformulation Phase 1
3.2. Reformulation Phase 2
3.3. Reformulation Phase 3
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ishiwatari, S.; Suzuki, T.; Hitomi, T.; Yoshino, T.; Matsukuma, S.; Tsuji, T. Effects of methyl paraben on skin keratinocytes. J. Appl. Toxicol. 2007, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moral-Sanchez, J.M.; Gonzalez-Alvarez, I.; Gonzalez-Alvarez, M.; Navarro-Ruiz, A.; Bermejo, M. Availability of Authorizations from EMA and FDA for Age-Appropriate Medicines Contained in the WHO Essential Medicines List for Children 2019. Pharmaceutics 2020, 12, 316. [Google Scholar] [CrossRef] [PubMed]
- Stamatas, G.N.; Nikolovski, J.; Luedtke, M.A.; Kollias, N.; Wiegand, B.C. Infant skin microstructure assessed in vivo differs from adult skin in organization and at the cellular level. Pediatr. Dermatol. 2010, 27, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Stamatas, G.N.; Nikolovski, J.; Mack, M.C.; Kollias, N. Infant skin physiology and development during the first years of life: A review of recent findings based on in vivo studies. Int. J. Cosmet. Sci. 2011, 33, 17–24. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Darlenski, R.; Lachmann, N.; Baudouin, C.; Msika, P.; Belilovsky, C.D.; Hachem, J. Infant epidermal skin physiology: Adaptation after birth. Br. J. Dermatol. 2012, 166, 483–490. [Google Scholar] [CrossRef]
- Lund, C.; Kuller, J.; Lane, A.; Wright Lott, J.; Raines, D.A. Neonatal skin care: The scientific basis for practice. Neonatal Netw. 1999, 18, 15–26. [Google Scholar] [CrossRef]
- Kelleher, M.M.; O’Carroll, M.; Gallagher, A.; Murray, D.M.; Dunn Galvin, A.; Irvine, A.D.; O’BHourihane, J. Newborn transepidermal water loss values: A reference dataset. Pediatr. Dermatol. 2013, 30, 712–716. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, 342, 59–209. [Google Scholar]
- Chandra, S.A.; Peterson, R.A.; Melich, D.; Merrill, C.M.; Bailey, D.; Mellon-Kusibab, K.; Adler, R. Dermal irritation of petrolatum in rabbits but not in mice, rats or minipigs. J. Appl. Toxicol. 2014, 34, 857–861. [Google Scholar] [CrossRef]
- Chew, A.-L.; Maibach, H.I. Classification of Irritant Contact Dermatitis. In Handbook of Cosmetic Science and Technology, 3rd ed.; Barel, A.O., Howard, M.P., Maibach, I., Eds.; Informa Healthcare: New York, NY, USA, 2009; p. 439. [Google Scholar]
- Pavlis, J.; Yosipovitch, G. Management of Itch in Atopic Dermatitis. Am. J. Clin. Dermatol. 2018, 19, 319–332. [Google Scholar] [CrossRef]
- Nistico, S.P.; Del Duca, E.; Tamburi, F.; Pignataro, E.; De Carvalho, N.; Farnetani, F.; Pellacani, G. Superiority of a vitamin B12-barrier cream compared with standard glycerol-petrolatum-based emollient cream in the treatment of atopic dermatitis: A randomized, left-to-right comparative trial. Dermatol. Ther. 2017, 30, e12523. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press An imprint of RPS Publishing 1 Lambeth High Street: London, UK, 2009; pp. 441–488. [Google Scholar]
- Commisson Regulation (EU) No 358/2014 of 9 April 2014 amending Annexes II and V to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Off. J. Eur. Union 2014, 107, 5–9.
- Annex to the European Commission Guideline on ‘Excipients in The Labelling and Package Leaflet of Medicinal Products for Human Use’ (SANTE-2017-11668) Excipients and Information for the Package Leaflet EMA/CHMP/302620/2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/annex-european-commission-guideline-excipients-labelling-package-leaflet-medicinal-products-human_en.pdf (accessed on 3 August 2020).
- Macy, E.; Schatz, M.; Zeiger, R.S. Immediate Hypersensitivity to Methylparaben Causing False-Positive Results of Local Anesthetic Skin Testing or Provocative Dose Testing. Perm. J. 2002, 6, 17–21. [Google Scholar]
- Thompson, J.E. (Ed.) A Practical Guide to Contemporary Pharmacy Practice, 3rd ed.; Ointment Bases Lippincott Williams & Wilkins: Baltimore, MD, USA, 2009. [Google Scholar]
- United States Pharmacopeia and National Formulary (USP 41-NF 36); United States Pharmacopeial Convention: Rockville, MD, USA, 2018.
- Hungarian Pharmacopoeia, 7th ed.; Medicina: Budapest, Hungary, 1986.
- European Pharmacopoeia. Efficacy of Antimicrobial Preservation, 7th ed.; Council of Europe: Strasbourg, France, 2011; pp. 505–506. [Google Scholar]
- Danby, S.G.; Alenezi, T.; Sultan, A.; Lavender, T.; Chittock, J.; Brown, K.; Cork, M.J. Effect of olive and sunflower seed oil on the adult skin barrier: Implications for neonatal skin care. Pediatr. Dermatol. 2013, 30, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S. (Ed.) Beeswax: Production, Properties Composition and Control. Beeswax Book; Bee Product Science: Muehlethurnen, Switzerland, 2009; Chapter 2. [Google Scholar]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.F.R.; Barreiro, M.F. Cosmetics Preservation: A Review on Present Strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [PubMed]
- Siegert, W. Comparison of microbial challenge testing methods for cosmetics. Househ. Pers. Care Today 2013, 8, 32–34. [Google Scholar]
- Gönüllü, Ü.; Yener, G.; Üner, M.; Incegül, T. Moisturizing potentials of ascorbyl palmitate and calcium ascorbate in various topical formulations. Int. J. Cosmet. Sci. 2004, 26, 31–36. [Google Scholar] [CrossRef]
- Yilmaz, E.; Borchert, H.H. Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity and erythema—An in vivo study. Int. J. Pharm. 2006, 307, 232–238. [Google Scholar] [CrossRef]
- Rosado, C.; Pinto, P.; Rodrigues, L.M. Comparative assessment of the performance of twogenerations of Tewameter TM210 and TM300. Int. J. Cosmet. Sci. 2005, 27, 237–241. [Google Scholar] [CrossRef]
- Gloor, M.; Senger, B.; Langenauer, M.; Fluhr, J.W. On the course of the irritant reaction after irritation with sodium lauryl sulphate. Skin Res. Technol. 2004, 10, 144–148. [Google Scholar] [CrossRef]
- Jensen, J.M.; Schütze, S.; Neumann, C.; Proksch, E.J. Impaired cutaneous permeability barrier function, skin hydration, and sphingomyelinase activity in keratin 10 deficient mice. J. Investig. Dermatol. 2000, 115, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Kónya, M.; Bohus, P.; Paglino, L.; Csóka, I.; Csányi, E.; Erős, I. Coherent emulsions containing alkylpolyglucoside esters as emulsifiers. Prog. Colloid Polym. Sci. 2004, 125, 161–166. [Google Scholar]
- Committee for Medicinal Products for Human Use (CHMP). Draft Guideline on Quality and Equivalence of Topical Products, 2018. European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/scientificguideline/draft-guideline-quality-equivalence-topical-products_en.pdf (accessed on 3 August 2020).





| Component | Concentration [w/w%] | Function |
|---|---|---|
| Phase A | ||
| Polysorbate 60 | 4 | nonionic o/w emulsifier |
| Liquid paraffin | 4 | oil phase and consistency softener |
| Cetostearyl alcohol | 12 | nonionic w/o emulsifier and consistency-increasing agent |
| White petrolatum | 20 | base of the cream |
| Phase B | ||
| Purified water | 58 | water phase of the cream |
| Phase C | ||
| Methyl parahydroxybenzoate | 0.2 | preservative |
| Ethanol 96% | 1.8 | solvent of preservative |
| Not Infected | Pseudomonas aeruginosa | Staphylococcus aureus | Aspergillus brasiliensis | Candida albicans | Mixed 25–25% Reinfected | |
|---|---|---|---|---|---|---|
| Control A | Initial time: 0 | Initial time: 2 × 105 | Initial time: 3 × 105 | Initial time: 1 × 105 | Initial time: 1 × 105 | Initial time: 1.75 × 105 |
| 1 day–6 weeks: 0 | 1 day–6 weeks: 1 × 105 | 1 day: 2 × 105 | 1 day: 1 × 105 | 1 day: 7 × 104 | 1 day: 1.2 × 105 | |
| 1–6 weeks: 0 | 1–6 weeks: 2 × 101 | 1–6 weeks: 6 × 104 | 1–6 weeks: 3 × 103 | |||
| Control B | Initial time: 0 | Initial time: 2 × 105 | Initial time: 3 × 105 | Initial time: 1 × 105 | Initial time: 1 × 105 | Initial time: 1.75 × 105 |
| 1 day–6 weeks: 0 | 1 day–6 weeks: 1 × 105 | 1 day: 2 × 105 | 1 day: 1 × 105 | 1 day: 7 × 104 | 1 day: 1.2 × 105 | |
| 1–6 weeks: 0 | 1–6 weeks: 2 × 101 | 1–6 weeks: 6 × 104 | 1–6 weeks: 3 × 103 |
| Not Infected | Pseudomonas aeruginosa | Staphylococcus aureus | Aspergillus brasiliensis | Candida albicans | Mixed 25–25% Reinfected | |
|---|---|---|---|---|---|---|
| Sample1 A | Initial time: 0 | Initial time: 2 × 105 | Initial time: 3 × 105 | Initial time: 1 × 105 | Initial time: 1 × 105 | Initial time: 2–3 × 105 |
| 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | |
| Sample1 B | Initial time: 0 | Initial time: 2 × 105 | Initial time: 3 × 105 | Initial time: 1 × 105 | Initial time: 1 × 105 | Initial time: 2–3 × 105 |
| 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | |
| Sample2 A | Initial time: 0 | Initial time: 2 × 105 | Initial time: 3 × 105 | Initial time: 1 × 105 | Initial time: 1 × 105 | Initial time: 1.75 × 105 |
| 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day: 4 × 104 | 1 day: 1 × 105 (Candida!) | |
| 1–6 weeks: 2 × 104 | 1–6 weeks: 1 × 103(Candida!) | |||||
| Sample2 B | Initial time: 0 | Initial time: 2 × 105 | Initial time: 3 × 105 | Initial time: 1 × 105 | Initial time: 1 × 105 | Initial time: 1.75 × 105 |
| 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day: 4 × 104 | 1 day: 1 × 105 (Candida!) | |
| 1–6 weeks: 2 × 104 | 1–6 weeks: 1 × 103(Candida!) | |||||
| Sample3 A | Initial time: 0 | Initial time: 2 × 105 | Initial time: 3 × 105 | Initial time: 1 × 105 | Initial time: 1 × 105 | Initial time: 2–3 × 105 |
| 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | |
| Sample3 B | Initial time: 0 | Initial time: 2 × 105 | Initial time: 3 × 105 | Initial time: 1 × 105 | Initial time: 1 × 105 | Initial time: 2–3 × 105 |
| 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | |
| Sample4 A | Initial time: 0 | Initial time: 2 × 105 | Initial time: 3 × 105 | Initial time: 1 × 105 | Initial time: 1 × 105 | Initial time: 2–3 × 105 |
| 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | |
| Sample4 B | Initial time: 0 | Initial time: 2 × 105 | Initial time: 3 × 105 | Initial time: 1 × 105 | Initial time: 1 × 105 | Initial time: 2–3 × 105 |
| 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 | 1 day–6 weeks: 0 |
| Components | Original Cream [w/w %] | Composition 1 [w/w %] | Composition 2 [w/w %] | Composition 3 [w/w %] | Composition 4 [w/w %] |
|---|---|---|---|---|---|
| Phase A | |||||
| Polysorbate 60 | 4 | 4 | 4 | 4 | 4 |
| Liquid paraffin | 4 | - | - | - | - |
| Cetostearyl alcohol | 12 | 12 | 12 | 12 | 12 |
| White petrolatum | 20 | - | - | - | - |
| Sunflower oil | - | 4 | 4 | 4 | 8 |
| White beeswax | - | 20 | 15 | 10 | 5 |
| Phase B | |||||
| Purified water | up to 100 | up to 100 | up to 100 | up to 100 | up to 100 |
| Phase C | |||||
| Methyl parahydroxy-benzoate | 0.2 | - | - | - | - |
| Ethanol 96% | 1.8 | - | - | - | - |
| Phenoxyethanol | - | 0.5 | 0.5 | 0.5 | 0.5 |
| Rheological Data | Original Cream | Composition 1 | Composition 2 | Composition 3 | Composition 4 |
|---|---|---|---|---|---|
| η100 (Pa *s) | 4.07 ± 0.31 | 3.38 ± 0.50 | 2.84 ± 0.27 | 3.26 ± 0.08 | 3.98 ± 0.15 |
| SR Pa s−s mL−1 | 39,639 ± 4631 | 21,028 ± 6683 | 21,973 ± 2593 | 26,504 ± 6561 | 18,341 ± 4618 |
| Original Cream | Composition 1 | Composition 2 | Composition 3 | Composition 4 |
|---|---|---|---|---|
| 4.52 | 4.81 | 4.76 | 4.89 | 4.88 |
| Components | Original Cream [w/w%] | Composition 5 [w/w%] | Composition 6 [w/w%] | Composition 7 [w/w%] | Composition 8 [w/w%] |
|---|---|---|---|---|---|
| Phase A | |||||
| Polysorbate 60 | 4 | 4 | 4 | 4 | 4 |
| Liquid paraffin | 4 | - | - | - | - |
| Cetostearyl alcohol | 12 | 6 | 12 | 6 | 12 |
| White petrolatum | 20 | - | - | - | - |
| Sunflower oil | - | 4 | 4 | 4 | 4 |
| White beeswax | - | 10 | 10 | 10 | 8 |
| Cocoa butter | - | 6 | - | 6 | 8 |
| Phase B | |||||
| Urea | - | - | 1 | 1 | - |
| Glycerol (85%) | - | - | - | - | 5 |
| Purified water | up to 100 | up to 100 | up to 100 | up to 100 | up to 100 |
| Phase C | |||||
| Methyl parahydroxy-benzoate | 0.2 | - | - | - | - |
| Ethanol 96% | 1.8 | - | - | - | - |
| Phenoxyethanol | - | 0.5 | 0.5 | 0.5 | 0.5 |
| Rheological Data | Original Cream | Composition 5 | Composition 6 | Composition 7 | Composition 8 |
|---|---|---|---|---|---|
| η100 (Pa*s) | 4.07 ± 0.31 | 1.94 ± 0.12 | 3.54 ± 0.38 | 2.11 ± 0.14 | 3.50 ± 0.05 |
| SR Pa s−s mL−1 | 39,639 ± 4631 | 11,696 ± 643 | 24,218 ± 2811 | 6051 ± 28 | 15,698 ± 1104 |
| Original Cream | Composition 5 | Composition 6 | Composition 7 | Composition 8 |
|---|---|---|---|---|
| 4.52 | 4.65 | 5.07 | 5.02 | 4.60 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, A.; Péter-Héderi, D.; Perei, K.; Budai-Szűcs, M.; Léber, A.; Gácsi, A.; Csányi, E.; Berkó, S. Effects of Formulation Excipients on Skin Barrier Function in Creams Used in Pediatric Care. Pharmaceutics 2020, 12, 729. https://doi.org/10.3390/pharmaceutics12080729
Kovács A, Péter-Héderi D, Perei K, Budai-Szűcs M, Léber A, Gácsi A, Csányi E, Berkó S. Effects of Formulation Excipients on Skin Barrier Function in Creams Used in Pediatric Care. Pharmaceutics. 2020; 12(8):729. https://doi.org/10.3390/pharmaceutics12080729
Chicago/Turabian StyleKovács, Anita, Dóra Péter-Héderi, Katalin Perei, Mária Budai-Szűcs, Attila Léber, Attila Gácsi, Erzsébet Csányi, and Szilvia Berkó. 2020. "Effects of Formulation Excipients on Skin Barrier Function in Creams Used in Pediatric Care" Pharmaceutics 12, no. 8: 729. https://doi.org/10.3390/pharmaceutics12080729
APA StyleKovács, A., Péter-Héderi, D., Perei, K., Budai-Szűcs, M., Léber, A., Gácsi, A., Csányi, E., & Berkó, S. (2020). Effects of Formulation Excipients on Skin Barrier Function in Creams Used in Pediatric Care. Pharmaceutics, 12(8), 729. https://doi.org/10.3390/pharmaceutics12080729