Mathematical Modelling, Simulation and Optimisation of Microneedles for Transdermal Drug Delivery: Trends and Progress
Abstract
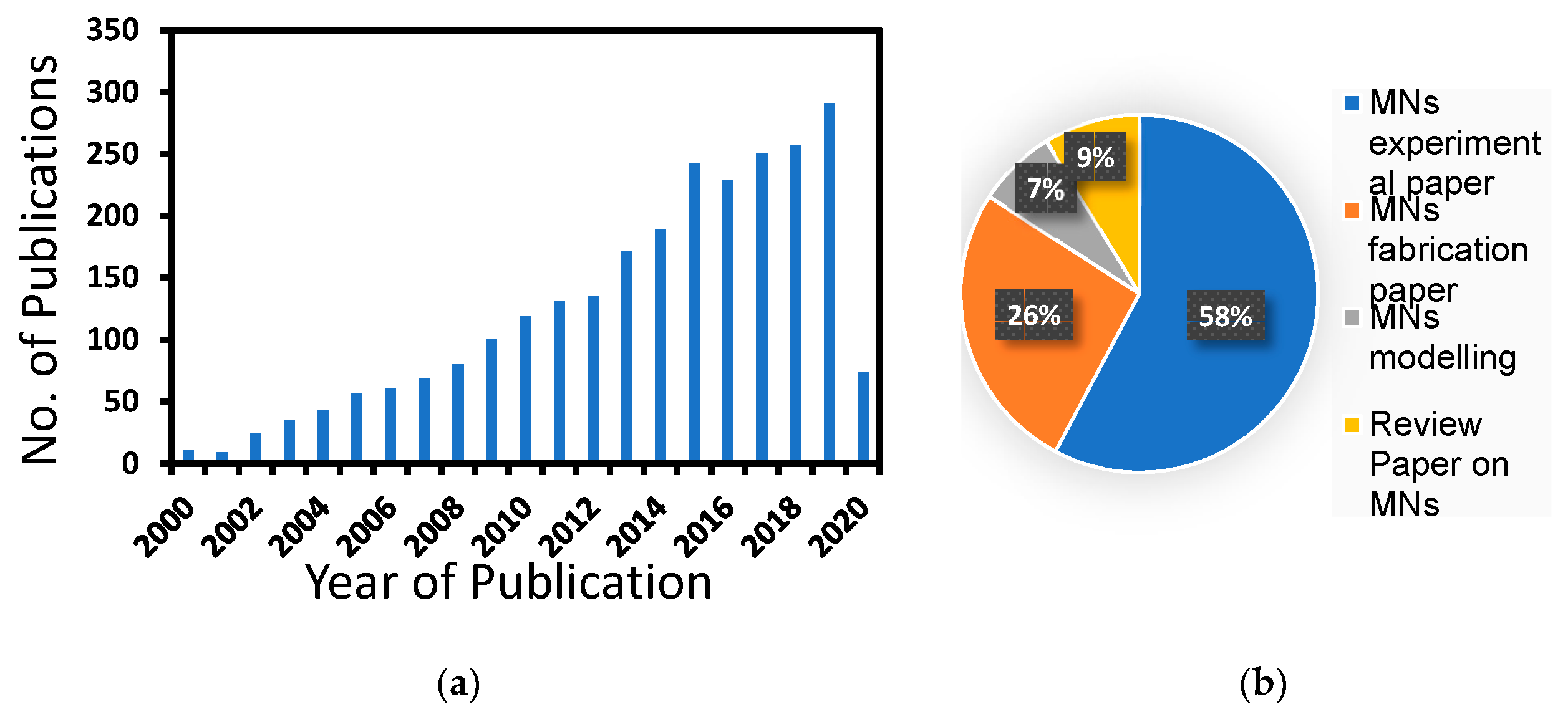
1. Introduction
2. MN Features
2.1. Morphological Variation of MNs
2.2. Materials of MN
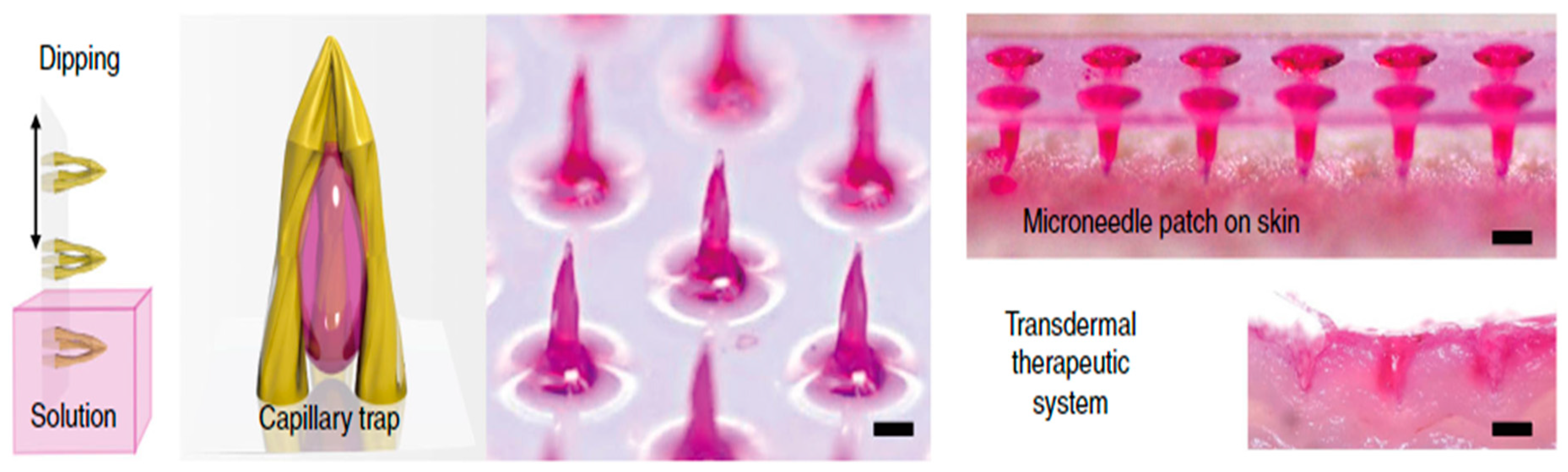
2.3. Various Design of MNs
3. MN Modelling Approaches
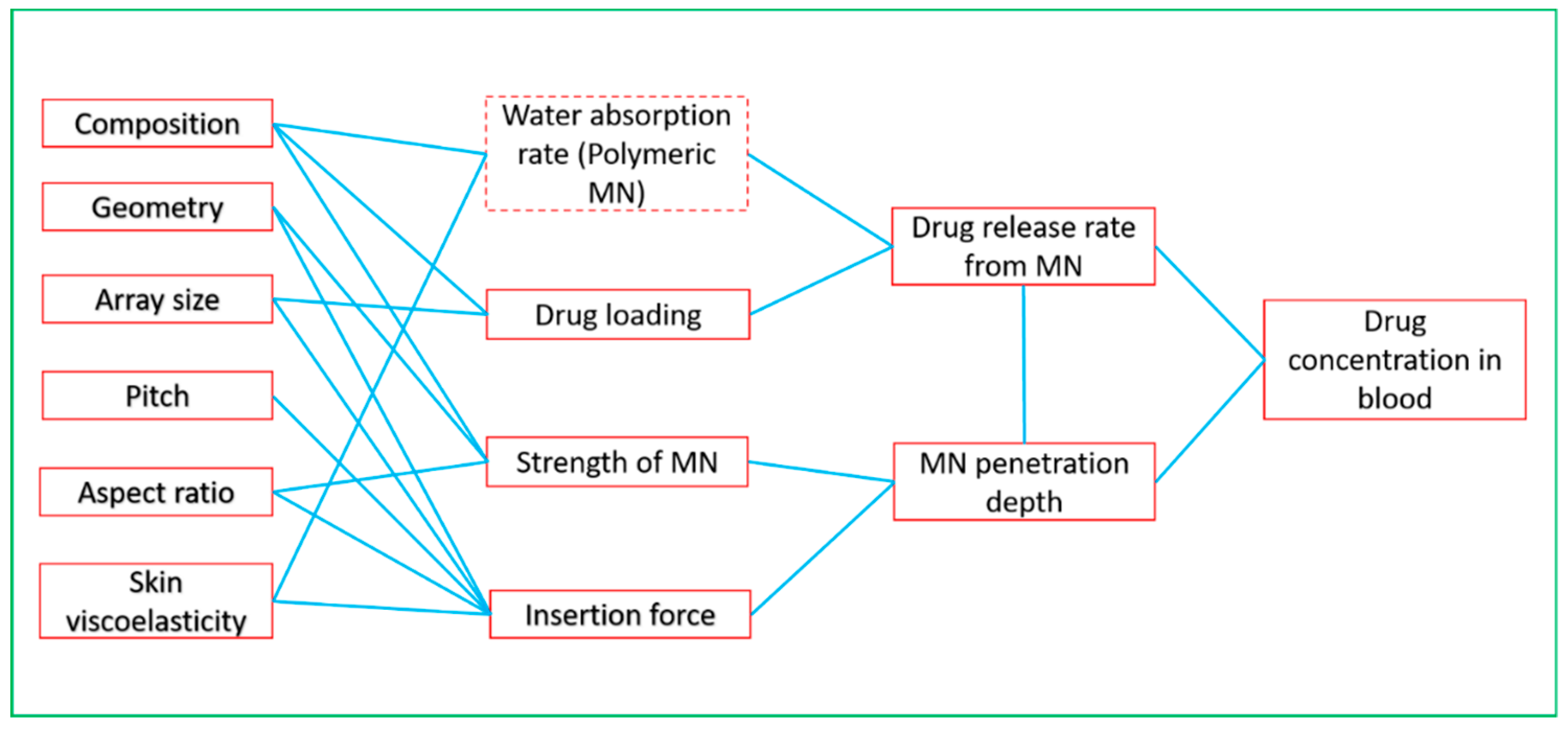
3.1. Parameters for MN Modelling
3.2. MN Modelling Tools
4. Case Studies on MN Simulation and Optimisation
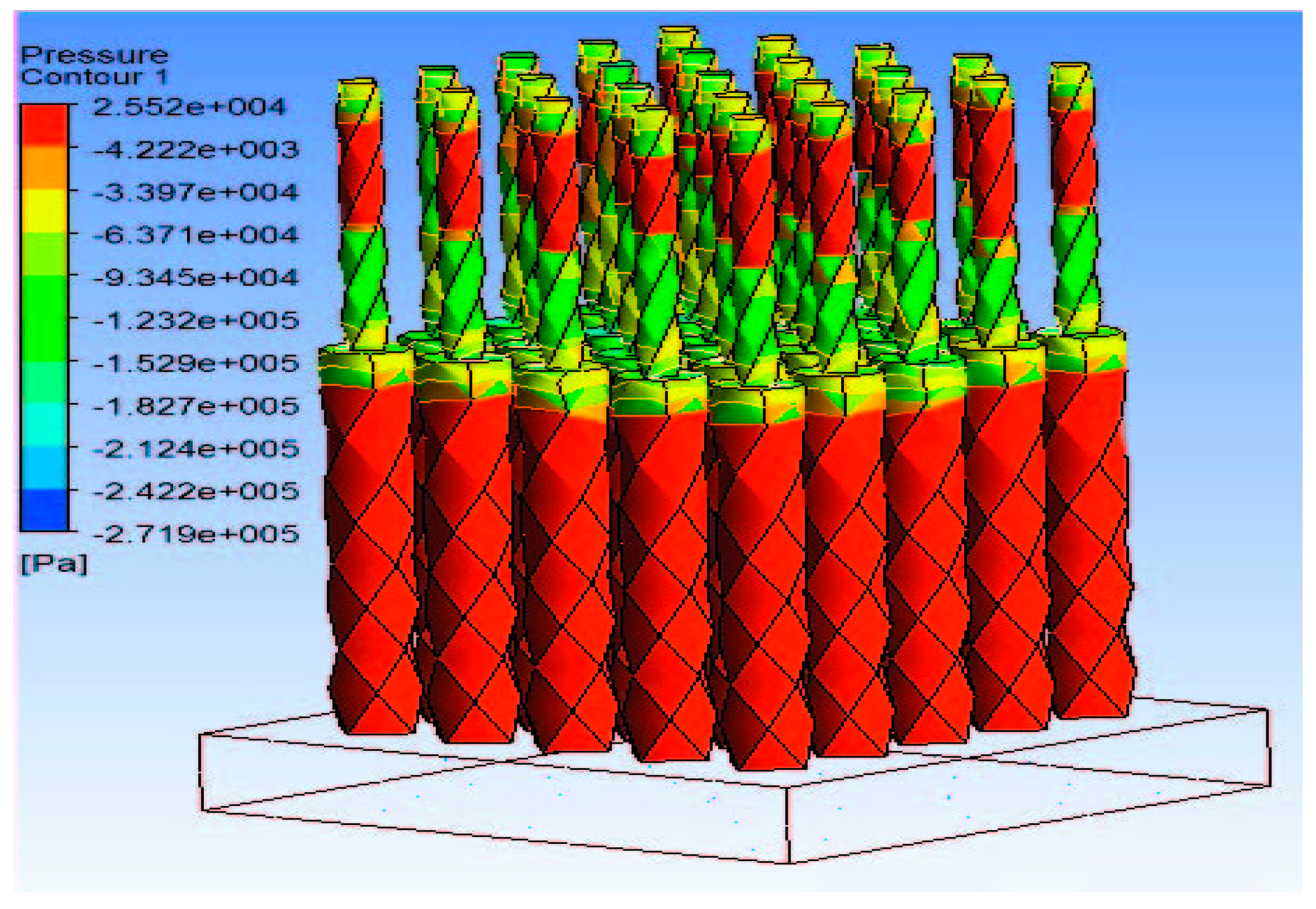
4.1. Simulation of MN Insertion Force and Effect of Skin Properties
4.2. Simulation of MN Enhanced TDD
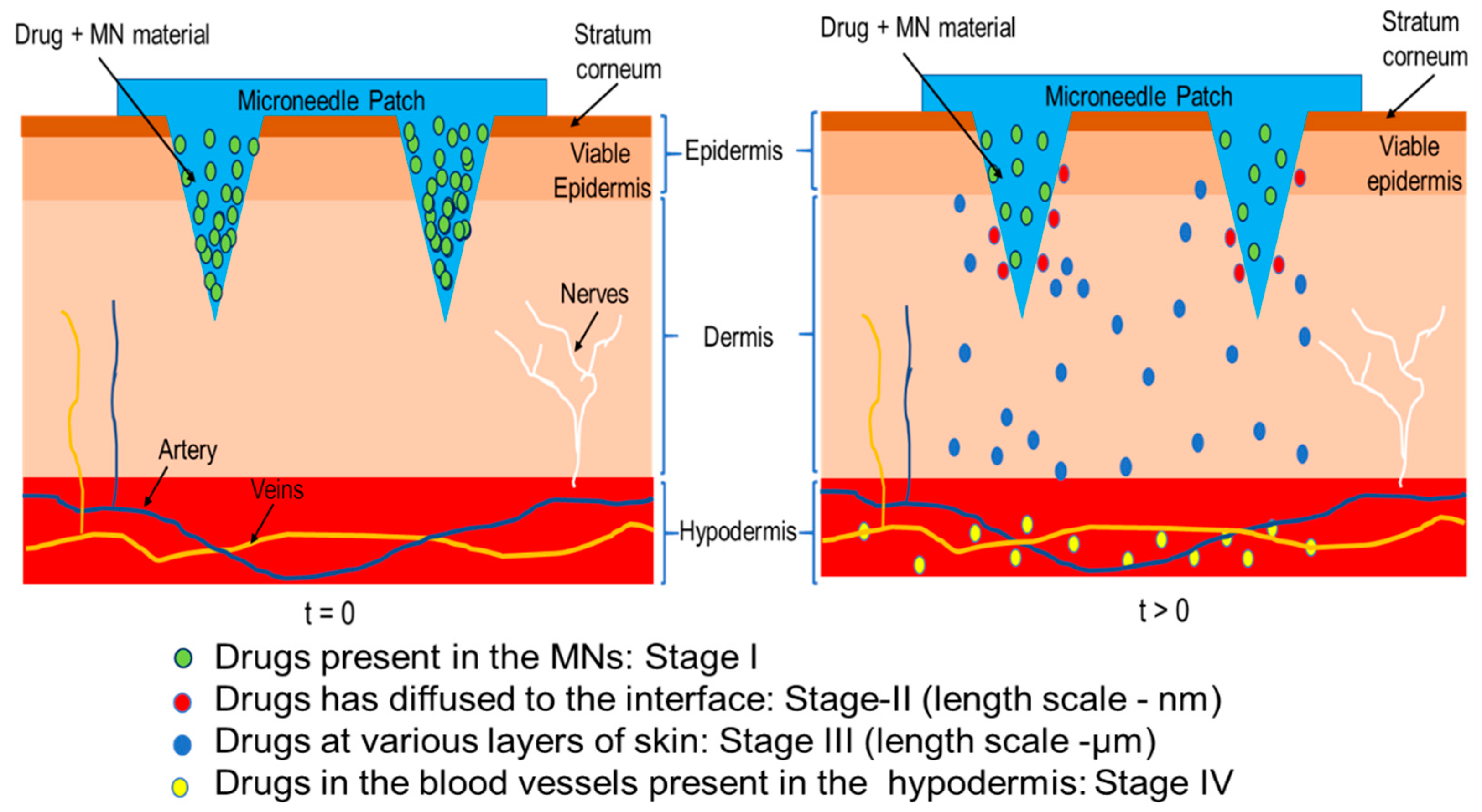
4.3. Governing Equations for Drug Transport in MN Treated Skin
- 1.
- The diffusion coefficient can be deduced from the partition coefficient considering skin as a multilayer structure. The values of the diffusion coefficient are different for each layer of the skin, which can be calculated separately using Equation (10) [120].Here, Ki/j is the partition coefficient between different diffusion compartments (donor compartment, SC, VE and DE), h is the thickness of the skin layer and c0 is the initial drug concentration in the skin layer.
- 2.
- To further explore the properties of different layers, the skin is considered as a porous material in which tortuous channels are defined to exist as pathways for the transportation of drug molecules. Equation (9) provides diffusion coefficients for every layer in the skin. While the drug molecules are inside those channels, the hindrance factor H(λ) also needs to be included where λ is the ratio of molecule radius over skin pore radius. Therefore, the size of the molecules decides the significance of the hindrance factor. Equation (11) shows the hindrance factor in cases where λ is less than 0.4 [121].After the hindrance factor is calculated, it is imported into Fick’s law along with the porosity and tortuosity of the skin which have been shown in Equations (12) and (13) [121,122].where D∞ is the diffusion coefficient of the drug solution at infinite dilution, τ is the tortuosity of the channels and ε is the average porosity of the skin.
- 3.
- The third method aims to acquire the diffusion coefficient via experiment where the time lag (the time duration when the diffusion reaches its steady state) needs to be measured. Although this method considers the skin as homogeneous material, the diffusion coefficient is still more accurate than that obtained using Equation (6). The theoretical relation between the diffusion coefficient and the time lag is shown in Equation (14) [123]:where tlag is the time lag and l is the thickness of the skin. There are also other studies which consider factors such as back diffusion of the drug molecules in skin [124], however, they are not elaborated in this review.
4.4. Simulations of TDD in MNs Treated Skin
4.4.1. Simulation of Solid MNs
4.4.2. Simulation of Hollow MNs
4.4.3. Simulation of Dissolving MNs
5. Conclusions
Funding
Conflicts of Interest
References
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles: A Novel Approach to Transdermal Drug Delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Das, D.B. Microneedles for drug delivery: Trends and progress. Drug Deliv. 2014, 23, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.-S.; Kong, Y.; Wang, Y.; Luo, Y.; Fan, X.; Xie, X.; Yang, B.-R.; Wu, M.X. Microneedles for transdermal diagnostics: Recent advances and new horizons. Biomaterials 2019, 232, 119740. [Google Scholar] [CrossRef] [PubMed]
- Duarah, S.; Sharma, M.; Wen, J. Recent advances in microneedle-based drug delivery: Special emphasis on its use in paediatric population. Eur. J. Pharm. Biopharm. 2019, 136, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, J.; Kahkoska, A.R.; Wang, J.; Buse, J.B.; Gu, Z. Advances in transdermal insulin delivery. Adv. Drug Deliv. Rev. 2019, 139, 51–70. [Google Scholar] [CrossRef]
- Dangol, M.; Kim, S.; Li, C.G.; Lahiji, S.F.; Jang, M.; Ma, Y.; Huh, I.; Jung, H. Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J. Control. Release 2017, 265, 41–47. [Google Scholar] [CrossRef]
- Ameri, M.; Kadkhodayan, M.; Nguyen, J.; Bravo, J.A.; Su, R.; Chan, K.; Samiee, A.; Daddona, P.E. Human Growth Hormone Delivery with a Microneedle Transdermal System: Preclinical Formulation, Stability, Delivery and PK of Therapeutically Relevant Doses. Pharmaceutics 2014, 6, 220–234. [Google Scholar] [CrossRef]
- Nayak, A.; Das, D.B.; Vladisavljević, G.T. Microneedle-Assisted Permeation of Lidocaine Carboxymethylcellulose with Gelatine Co-polymer Hydrogel. Pharm. Res. 2013, 31, 1170–1184. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, H.-S.; Hwang, Y.-H.; Kim, J.-J.; Kang, K.-Y.; Kim, S.J.; Kim, H.K.; Kim, J.D.; Jeong, D.H.; Paik, M.-J.; et al. Enhanced anti-tumor immunotherapy by dissolving microneedle patch loaded ovalbumin. PLoS ONE 2019, 14, e0220382. [Google Scholar] [CrossRef]
- Caudill, C.L.; Perry, J.L.; Tian, S.; Luft, J.C.; DeSimone, J.M. Spatially controlled coating of continuous liquid interface production microneedles for transdermal protein delivery. J. Control. Release 2018, 284, 122–132. [Google Scholar] [CrossRef]
- Courtenay, A.J.; McCrudden, M.T.C.; McAvoy, K.J.; McCarthy, H.O.; Donnelly, R.F. Microneedle-Mediated Transdermal Delivery of Bevacizumab. Mol. Pharm. 2018, 15, 3545–3556. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wang, H.; Xie, Y.; Fu, Y.; Li, Y.; Liu, P.; Du, H.; Zhu, J.; Dong, L.; Hussain, M.; et al. Transdermal delivery of rapamycin with poor water-solubility by dissolving polymeric microneedles for anti-angiogenesis. J. Mater. Chem. B 2020, 8, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, B.; Zhou, Y.; Chen, Z.; Jiang, L.; Yuan, W.; Liang, L. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS ONE 2017, 12, e0172043. [Google Scholar] [CrossRef] [PubMed]
- Naito, S.; Ito, Y.; Kiyohara, T.; Kataoka, M.; Ochiai, M.; Takada, K. Antigen-loaded dissolving microneedle array as a novel tool for percutaneous vaccination. Vaccine 2012, 30, 1191–1197. [Google Scholar] [CrossRef]
- Yao, G.; Quan, G.; Lin, S.; Peng, T.; Wang, Q.; Ran, H.; Chen, H.; Zhang, Q.; Wang, L.; Pan, X.; et al. Novel dissolving microneedles for enhanced transdermal delivery of levonorgestrel: In vitro and in vivo characterization. Int. J. Pharm. 2017, 534, 378–386. [Google Scholar] [CrossRef]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Park, J.-H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef]
- Pierre, M.R.; Rossetti, F. Microneedle-Based Drug Delivery Systems for Transdermal Route. Curr. Drug Targets 2014, 15, 281–291. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Indermun, S.; Luttge, R.; Choonara, Y.E.; Kumar, P.; Du Toit, L.C.; Modi, G.; Pillay, V. Current advances in the fabrication of microneedles for transdermal delivery. J. Control. Release 2014, 185, 130–138. [Google Scholar] [CrossRef]
- Rajeswari, R.; Malliga, P. Design of MEMS Based Microneedle for Drug Delivery System. Procedia Eng. 2014, 97, 2001–2010. [Google Scholar] [CrossRef]
- Nejad, H.R.; Sadeqi, A.; Kiaee, G.; Sonkusale, S.R. Low-cost and cleanroom-free fabrication of microneedles. Microsyst. Nanoeng. 2018, 4, 17073. [Google Scholar] [CrossRef]
- Aoyagi, S.; Izumi, H.; Isono, Y.; Fukuda, M.; Ogawa, H. Laser fabrication of high aspect ratio thin holes on biodegradable polymer and its application to a microneedle. Sens. Actuators A Phys. 2007, 139, 293–302. [Google Scholar] [CrossRef]
- Uddin, J.; Scoutaris, N.; Economidou, S.N.; Giraud, C.; Chowdhry, B.Z.; Donnelly, R.F.; Douroumis, D. 3D printed microneedles for anticancer therapy of skin tumours. Mater. Sci. Eng. C 2019, 107, 110248. [Google Scholar] [CrossRef] [PubMed]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, J.; Windmill, J.F.C.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C 2019, 102, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Perennes, F.; Marmiroli, B.; Matteucci, M.; Tormen, M.; Vaccari, L.; Di Fabrizio, E. Sharp beveled tip hollow microneedle arrays fabricated by LIGA and 3D soft lithography with polyvinyl alcohol. J. Micromech. Microeng. 2006, 16, 473–479. [Google Scholar] [CrossRef]
- Koutsonanos, D.G.; Martin, M.D.P.; Zarnitsyn, V.G.; Sullivan, S.P.; Compans, R.W.; Prausnitz, M.R.; Skountzou, I. Transdermal Influenza Immunization with Vaccine-Coated Microneedle Arrays. PLoS ONE 2009, 4, e4773. [Google Scholar] [CrossRef]
- Vinayakumar, K.B.; Kulkarni, P.G.; Nayak, M.M.; Dinesh, N.S.; Hegde, G.M.; Ramachandra, S.G.; Rajanna, K. A hollow stainless steel microneedle array to deliver insulin to a diabetic rat. J. Micromech. Microeng. 2016, 26, 65013. [Google Scholar] [CrossRef]
- Davis, S.; Martanto, W.; Allen, M.; Prausnitz, M. Hollow Metal Microneedles for Insulin Delivery to Diabetic Rats. IEEE Trans. Biomed. Eng. 2005, 52, 909–915. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Mooney, K.; Caffarel-Salvador, E.; Torrisi, B.M.; Eltayib, E.; McElnay, J.C. Microneedle-Mediated Minimally Invasive Patient Monitoring. Ther. Drug Monit. 2013, 36, 10–17. [Google Scholar] [CrossRef]
- Sharma, S.; Hatware, K.; Bhadane, P.; Sindhikar, S.; Mishra, D.K. Recent advances in microneedle composites for biomedical applications: Advanced drug delivery technologies. Mater. Sci. Eng. C 2019, 103, 109717. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, K.; Wang, Y.; Singh, T.R.R.; Donnelly, R.F. Microneedles for enhanced transdermal and intraocular drug delivery. Curr. Opin. Pharmacol. 2017, 36, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Martanto, W.; Moore, J.S.; Kashlan, O.; Kamath, R.; Wang, P.M.; O’Neal, J.M.; Prausnitz, M.R. Microinfusion Using Hollow Microneedles. Pharm. Res. 2006, 23, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Johnston, C.R. Human blood rheology in MEMS-based microneedles. Smart Mater. Nano-Micro-Smart Syst. 2005, 5651, 185–195. [Google Scholar]
- Miller, P.R.; Taylor, R.M.; Tran, B.Q.; Boyd, G.; Glaros, T.; Chavez, V.H.; Krishnakumar, R.; Sinha, A.; Poorey, K.; Williams, K.P.; et al. Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Commun. Boil. 2018, 1, 173. [Google Scholar] [CrossRef]
- Ranamukhaarachchi, S.A.; Padeste, C.; Dübner, M.; Hafeli, U.O.; Stoeber, B.; Cadarso, V.J. Integrated hollow microneedle-optofluidic biosensor for therapeutic drug monitoring in sub-nanoliter volumes. Sci. Rep. 2016, 6, 29075. [Google Scholar] [CrossRef]
- Kiang, T.K.L.; Ranamukhaarachchi, S.A.; Ensom, M.H.H. Revolutionizing Therapeutic Drug Monitoring with the Use of Interstitial Fluid and Microneedles Technology. Pharmaceutics 2017, 9, 43. [Google Scholar] [CrossRef]
- Römgens, A.M.; Bader, D.L.; Bouwstra, J.A.; Oomens, C.W.J. Predicting the optimal geometry of microneedles and their array for dermal vaccination using a computational model. Comput. Methods Biomech. Biomed. Eng. 2016, 19, 1599–1609. [Google Scholar] [CrossRef]
- Li, W.Z.; Huo, M.R.; Zhou, J.P.; Zhou, Y.Q.; Hao, B.H.; Liu, T.; Zhang, Y. Super-short solid silicon microneedles for transdermal drug delivery applications. Int. J. Pharm. 2010, 389, 122–129. [Google Scholar]
- Nguyen, J.; Ita, K.B.; Morra, M.J.; Popova, I.E. The influence of solid microneedles on the transdermal delivery of selected antiepileptic drugs. Pharmaceutics 2016, 8, 33. [Google Scholar] [CrossRef]
- Hoang, M.T.; Ita, K.B.; Bair, D.A. Solid microneedles for transdermal delivery of amantadine hydrochloride and pramipexole dihydrochloride. Pharmaceutics 2015, 7, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Cormier, M.; Johnson, B.; Ameri, M.; Nyam, K.; Libiran, L.; Zhang, D.D.; Daddona, P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J. Control. Release 2004, 97, 503–511. [Google Scholar] [CrossRef]
- Baek, S.-H.; Shin, J.-H.; Kim, Y.-C. Drug-coated microneedles for rapid and painless local anesthesia. Biomed. Microdevices 2017, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Modulation of transdermal drug delivery with coated microneedles. J. Drug Deliv. Sci. Technol. 2018, 45, 203–212. [Google Scholar] [CrossRef]
- Vrdoljak, A.; McGrath, M.G.; Carey, J.B.; Draper, S.J.; Hill, A.V.; O’Mahony, C.; Crean, A.M.; Moore, A.C. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J. Control. Release 2012, 159, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, J.-H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef]
- Dillon, C.; Hughes, H.; O’Reilly, N.J.; McLoughlin, P. Formulation and characterisation of dissolving microneedles for the transdermal delivery of therapeutic peptides. Int. J. Pharm. 2017, 526, 125–136. [Google Scholar] [CrossRef]
- Migdadi, E.M.; Courtenay, A.J.; Tekko, I.; McCrudden, M.T.; Kearney, M.-C.; McAlister, E.; McCarthy, H.O.; Donnelly, R.F. Hydrogel-forming microneedles enhance transdermal delivery of metformin hydrochloride. J. Control. Release 2018, 285, 142–151. [Google Scholar] [CrossRef]
- Yang, S.; Wu, F.; Liu, J.; Fan, G.; Welsh, W.; Zhu, H.; Jin, T. Phase-Transition Microneedle Patches for Efficient and Accurate Transdermal Delivery of Insulin. Adv. Funct. Mater. 2015, 25, 4633–4641. [Google Scholar] [CrossRef]
- Yin, Z.; Kuang, D.; Wang, S.; Zheng, Z.; Yadavalli, V.K.; Lu, S. Swellable silk fibroin microneedles for transdermal drug delivery. Int. J. Boil. Macromol. 2018, 106, 48–56. [Google Scholar] [CrossRef]
- Yuan, W.; Hong, X.; Wu, Z.; Chen, L.; Liu, Z.; Wu, F.; Wei, L.L. Dissolving and biodegradable microneedle technologies for transdermal sustained delivery of drug and vaccine. Drug Des. Dev. Ther. 2013, 7, 945–952. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.; Alkilani, A.Z.; McCrudden, C.M.; McAlister, E.; McCarthy, H.O.; Woolfson, A.D.; Donnelly, R.F. Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. J. Control. Release 2014, 180, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wu, C.-W. Microneedle, bio-microneedle and bio-inspired microneedle: A review. J. Control. Release 2017, 251, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Pires, L.; Vinayakumar, K.; Turos, M.; Miguel, V.; Gaspar, J. A Perspective on Microneedle-Based Drug Delivery and Diagnostics in Paediatrics. J. Pers. Med. 2019, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.W.; Tayyaba, S.; Afzulpurkar, N. Micro Electromechanical Systems (MEMS) Based Microfluidic Devices for Biomedical Applications. Int. J. Mol. Sci. 2011, 12, 3648–3704. [Google Scholar] [CrossRef]
- Amin, F.; Ahmed, S. Design, modeling and simulation of MEMS-based silicon Microneedles. J. Phys. Conf. Ser. 2013, 439, 012049. [Google Scholar] [CrossRef]
- Narayanan, S.P.; Raghavan, S. Solid silicon microneedles for drug delivery applications. Int. J. Adv. Manuf. Technol. 2016, 93, 407–422. [Google Scholar] [CrossRef]
- Chabri, F.; Bouris, K.; Jones, T.; Barrow, D.; Hann, A.; Allender, C.; Brain, K.; Birchall, J.C. Microfabricated silicon microneedles for nonviral cutaneous gene delivery. Br. J. Dermatol. 2004, 150, 869–877. [Google Scholar] [CrossRef]
- Roxhed, N.; Griss, P.; Stemme, G. Membrane-sealed hollow microneedles and related administration schemes for transdermal drug delivery. Biomed. Microdevices 2008, 10, 271–279. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Yang, R.; Laffitte, Y.; Schmill, U.; Hu, W.; Kaddoura, M.; Blondeel, E.J.M.; Cui, B. Fabrication of sharp silicon hollow microneedles by deep-reactive ion etching towards minimally invasive diagnostics. Microsyst. Nanoeng. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- McGrath, M.G.; Vrdoljak, A.; O’Mahony, C.; Oliveira, J.C.; Moore, A.; Crean, A.M. Determination of parameters for successful spray coating of silicon microneedle arrays. Int. J. Pharm. 2011, 415, 140–149. [Google Scholar] [CrossRef]
- Bal, S.M.; Caussin, J.; Pavel, S.; Bouwstra, J.A. In vivo assessment of safety of microneedle arrays in human skin. Eur. J. Pharm. Sci. 2008, 35, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Li, Z.; Yu, Z. Microneedles for transdermal delivery of insulin. J. Drug Deliv. Sci. Technol. 2015, 28, 11–17. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.Z.; Wang, Q.L.; Jin, X.; Guo, X.D. Fabrication of coated polymer microneedles for transdermal drug delivery. J. Control. Release 2017, 265, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.P.; Koutsonanos, D.G.; Martin, M.D.P.; Lee, J.W.; Zarnitsyn, V.; Choi, S.-O.; Murthy, N.; Compans, R.W.; Skountzou, I.; Prausnitz, M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010, 16, 915–920. [Google Scholar] [CrossRef]
- Mansoor, I.; Hafeli, U.O.; Stoeber, B. Hollow Out-of-Plane Polymer Microneedles Made by Solvent Casting for Transdermal Drug Delivery. J. Microelectromech. Syst. 2011, 21, 44–52. [Google Scholar] [CrossRef]
- Lhernould, M.S. Optimizing hollow microneedles arrays aimed at transdermal drug delivery. Microsyst. Technol. 2013, 19, 1–8. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, J.; Wen, D.; Kahkoska, A.R.; Gu, Z. Polymeric microneedles for transdermal protein delivery. Adv. Drug Deliv. Rev. 2018, 127, 106–118. [Google Scholar] [CrossRef]
- You, X.; Chang, J.-H.; Ju, B.-K.; Pak, J. Rapidly dissolving fibroin microneedles for transdermal drug delivery. Mater. Sci. Eng. C 2011, 31, 1632–1636. [Google Scholar] [CrossRef]
- Kolli, C.S.; Banga, A.K. Characterization of Solid Maltose Microneedles and their Use for Transdermal Delivery. Pharm. Res. 2008, 25, 104–113. [Google Scholar] [CrossRef]
- Olatunji, O.; Igwe, C.C.; Ahmed, A.S.; Alhassan, D.O.A.; Asieba, G.O.; Das Diganta, B. Microneedles from fish scale biopolymer. J. Appl. Polym. Sci. 2014, 131, 40377. [Google Scholar] [CrossRef]
- Chen, C.-H.; Shyu, V.B.-H.; Chen, C.-T. Dissolving Microneedle Patches for Transdermal Insulin Delivery in Diabetic Mice: Potential for Clinical Applications. Materials 2018, 11, 1625. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, Y.; Ye, Y.; Disanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef]
- Wei, J.; Haridass, I.N.; Crichton, M.; Mohammed, Y.; Meliga, S.C.; Sanchez, W.Y.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S.; Kendall, M.A. Space- and time-resolved investigation on diffusion kinetics of human skin following macromolecule delivery by microneedle arrays. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coating Formulations for Microneedles. Pharm. Res. 2007, 24, 1369–1380. [Google Scholar] [CrossRef] [PubMed]
- Brazzle, J.; Papautsky, I.; Frazier, A. Micromachined needle arrays for drug delivery or fluid extraction. IEEE Eng. Med. Biol. Mag. 1999, 18, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Das, D.B.; Rielly, C.D. Microneedle assisted micro-particle delivery by gene guns: Mathematical model formulation and experimental verification. Chem. Eng. Sci. 2015, 125, 176–190. [Google Scholar] [CrossRef]
- Sabitha, B.; Muniraj, N.J.R. Structural analysis of hollow titanium microneedle for amniotic fluidic extraction. In Proceedings of the 2013 International Conference on Optical Imaging Sensor and Security (ICOSS), Coimbatore, India, 2–3 July 2013; pp. 1–5. [Google Scholar]
- Tayyaba, S.; Ashraf, M.W.; Nisar, A.; Afzulpurkar, N.; Ashraf, M.N. Simulation of dual radii polymeric microneedle array for blood extraction. In Proceedings of the 2010 6th International Conference on Emerging Technologies (ICET), Islamabad, Pakistan, 18–19 October 2010; pp. 110–113. [Google Scholar]
- Khumpuang, S.; Sugiyama, S. Fabrication and simulation of novel crown-shaped microneedle array. In Biomedical Applications of Micro-and Nanoengineering II; International Society for Optics and Photonics: Bellingham, WA, USA, 2005; Volume 5651, pp. 288–297. [Google Scholar]
- Lim, J.; Tahk, D.; Yu, J.; Min, D.-H.; Jeon, N.L. Design rules for a tunable merged-tip microneedle. Microsyst. Nanoeng. 2018, 4, 1–10. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, Y.; Lee, W.; Ren, L.; Liu, B.; Liang, L.; Wang, Z.; Jiang, L. Additive Manufacturing of Honeybee-Inspired Microneedle for Easy Skin Insertion and Difficult Removal. ACS Appl. Mater. Interfaces 2018, 10, 29338–29346. [Google Scholar] [CrossRef]
- Ghosh, T.K. Dermal Drug Delivery: From Innovation to Production; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Sangshetti, J.N.; Deshpande, M.; Zaheer, Z.; Shinde, D.B.; Arote, R. Quality by design approach: Regulatory need. Arab. J. Chem. 2017, 10, S3412–S3425. [Google Scholar] [CrossRef]
- Jiang, S.; Li, P.; Yu, Y.; Liu, J.; Yang, Z. Experimental study of needle–tissue interaction forces: Effect of needle geometries, insertion methods and tissue characteristics. J. Biomech. 2014, 47, 3344–3353. [Google Scholar] [CrossRef]
- Held, J.; Gaspar, J.; Ruther, P.; Hagner, M.; Cismak, A.; Heilmann, A.; Paul, O. Design of experiment characterization of microneedle fabrication processes based on dry silicon etching. J. Micromech. Microeng. 2010, 20, 25024. [Google Scholar] [CrossRef]
- Al-Qallaf, B.; Das, D.B.; Mori, D.; Cui, Z. Modelling transdermal delivery of high molecular weight drugs from microneedle systems. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2007, 365, 2951–2967. [Google Scholar] [CrossRef] [PubMed]
- Loizidou, E.Z.; Williams, N.A.; Barrow, D.A.; Eaton, M.; McCrory, J.P.; Evans, S.; Allender, C. Structural characterisation and transdermal delivery studies on sugar microneedles: Experimental and finite element modelling analyses. Eur. J. Pharm. Biopharm. 2015, 89, 224–231. [Google Scholar] [CrossRef]
- Gomaa, Y.A.; Garland, M.J.; McInnes, F.J.; Donnelly, R.F.; El-Khordagui, L.K.; Wilson, C.G. Flux of ionic dyes across microneedle-treated skin: Effect of molecular characteristics. Int. J. Pharm. 2012, 438, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Groves, R.B. Quantifying the Mechanical Properties of Skin In Vivo and Ex Vivo to Optimise Microneedle Device Design. Ph.D. Thesis, Cardiff University, Wales, UK, 2012. [Google Scholar]
- Boonma, A.; Narayan, R.J.; Lee, Y.-S. Analytical Modeling and Evaluation of Microneedles Apparatus with Deformable Soft Tissues for Biomedical Applications. Comput. Des. Appl. 2013, 10, 139–157. [Google Scholar] [CrossRef]
- Rajoli, R.K.R.; Flexner, C.; Chiong, J.; Owen, A.; Donnelly, R.F.; Larrañeta, E.; Siccardi, M. Modelling the intradermal delivery of microneedle array patches for long-acting antiretrovirals using PBPK. Eur. J. Pharm. Biopharm. 2019, 144, 101–109. [Google Scholar] [CrossRef]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Ronnander, P.; Simon, L.; Spilgies, H.; Koch, A. Modelling the in-vitro dissolution and release of sumatriptan succinate from polyvinylpyrrolidone-based microneedles. Eur. J. Pharm. Sci. 2018, 125, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Stoeber, B.; Liepmann, D. Arrays of hollow out-of-plane microneedles for drug delivery. J. Microelectromech. Syst. 2005, 14, 472–479. [Google Scholar] [CrossRef]
- Oh, J.-H.; Park, H.-H.; Do, K.-Y.; Han, M.; Hyun, D.-H.; Kim, C.; Lee, S.S.; Hwang, S.-J.; Shin, S.-C. Influence of the delivery systems using a microneedle array on the permeation of a hydrophilic molecule, calcein. Eur. J. Pharm. Biopharm. 2008, 69, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Warner, K.S.; Zhang, J.; Sharma, S.; Gale, B.K.; Sharma, S. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int. J. Pharm. 2010, 391, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, Y.A.; Morrow, D.I.; Garland, M.J.; Donnelly, R.F.; El-Khordagui, L.K.; Meidan, V.M. Effects of microneedle length, density, insertion time and multiple applications on human skin barrier function: Assessments by transepidermal water loss. Toxicol. In Vitro 2010, 24, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.; Kuntsche, J.; Fahr, A. Skin penetration enhancement by a microneedle device (Dermaroller®) in vitro: Dependency on needle size and applied formulation. Eur. J. Pharm. Sci. 2009, 36, 511–523. [Google Scholar] [CrossRef]
- Verbaan, F.; Bal, S.M.; Berg, D.-J.V.D.; Groenink, W.; Verpoorten, H.; Lüttge, R.; Bouwstra, J. Assembled microneedle arrays enhance the transport of compounds varying over a large range of molecular weight across human dermatomed skin. J. Control. Release 2007, 117, 238–245. [Google Scholar] [CrossRef]
- Uppuluri, C.T.; Shaik, A.S.; Han, T.; Nayak, A.; Nair, K.J.; Whiteside, B.R.; Nalluri, B.N.; Das, D.B. Effect of Microneedle Type on Transdermal Permeation of Rizatriptan. AAPS Pharm. Sci. Tech. 2017, 18, 1495–1506. [Google Scholar] [CrossRef][Green Version]
- Chen, S.; Wu, D.; Liu, Y.; Huang, Y.; Xu, H.; Gao, W.; Zhang, J.; Sun, J.; Zhuang, J. Optimal scaling analysis of polymeric microneedle length and its effect on transdermal insulin delivery. J. Drug Deliv. Sci. Technol. 2020, 56, 101547. [Google Scholar] [CrossRef]
- Verbaan, F.; Bal, S.M.; Berg, D.-J.V.D.; Dijksman, J.; Van Hecke, M.; Verpoorten, H.; Berg, A.V.D.; Lüttge, R.; Bouwstra, J. Improved piercing of microneedle arrays in dermatomed human skin by an impact insertion method. J. Control. Release 2008, 128, 80–88. [Google Scholar] [CrossRef]
- Watanabe, T.; Hagino, K.; Sato, T. Evaluation of the effect of polymeric microneedle arrays of varying geometries in combination with a high-velocity applicator on skin permeability and irritation. Biomed. Microdevices 2014, 16, 591–597. [Google Scholar] [CrossRef]
- Aggarwal, P.; Johnston, C.R. Geometrical effects in mechanical characterizing of microneedle for biomedical applications. Sens. Actuators B Chem. 2004, 102, 226–234. [Google Scholar] [CrossRef]
- Al-Qallaf, B.; Das, D.B. Optimizing Microneedle Arrays to Increase Skin Permeability for Transdermal Drug Delivery. Ann. N. Y. Acad. Sci. 2009, 1161, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.; Al-Qallaf, B.; Das, D.B. Transdermal drug delivery by coated microneedles: Geometry effects on effective skin thickness and drug permeability. Chem. Eng. Res. Des. 2008, 86, 1196–1206. [Google Scholar] [CrossRef]
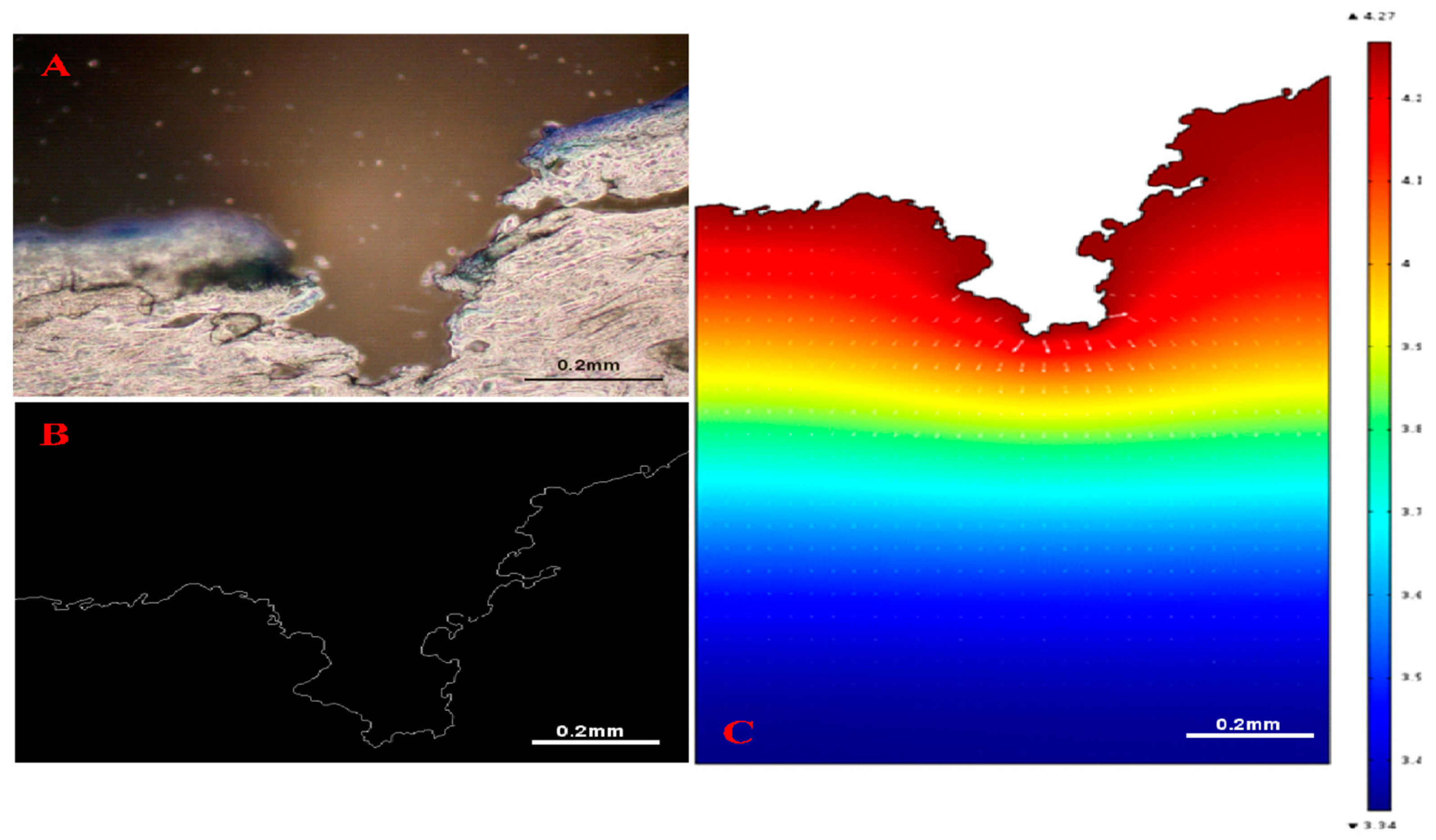
- Han, T.; Das, D.B. A New Paradigm for Numerical Simulation of Microneedle-Based Drug Delivery Aided by Histology of Microneedle-Pierced Skin. J. Pharm. Sci. 2015, 104, 1993–2007. [Google Scholar] [CrossRef] [PubMed]
- Bodhale, D.W.; Nisar, A.; Afzulpurkar, N.V. Structural and microfluidic analysis of hollow side-open polymeric microneedles for transdermal drug delivery applications. Microfluid. Nanofluid. 2009, 8, 373–392. [Google Scholar] [CrossRef]
- Haldkar, R.K.; Sheorey, T.; Gupta, V.K. Modeling and flow analysis of piezoelectric based micropump with various shapes of microneedle. J. Mech. Sci. Technol. 2017, 31, 2933–2941. [Google Scholar] [CrossRef]
- Al-Qallaf, B.; Das, D.B. Optimization of square microneedle arrays for increasing drug permeability in skin. Chem. Eng. Sci. 2008, 63, 2523–2535. [Google Scholar] [CrossRef]
- Olatunji, O.; Das, D.B.; Garland, M.J.; Belaid, L.; Donnelly, R.F. Influence of Array Interspacing on the Force Required for Successful Microneedle Skin Penetration: Theoretical and Practical Approaches. J. Pharm. Sci. 2013, 102, 1209–1221. [Google Scholar] [CrossRef]
- Ahn, B. Optimal Microneedle Design for Drug Delivery Based on Insertion Force Experiments with Variable Geometry. Int. J. Control. Autom. Syst. 2019, 18, 143–149. [Google Scholar] [CrossRef]
- Aoyagi, S.; Izumi, H.; Fukuda, M. Biodegradable polymer needle with various tip angles and consideration on insertion mechanism of mosquito’s proboscis. Sens. Actuators A Phys. 2008, 143, 20–28. [Google Scholar] [CrossRef]
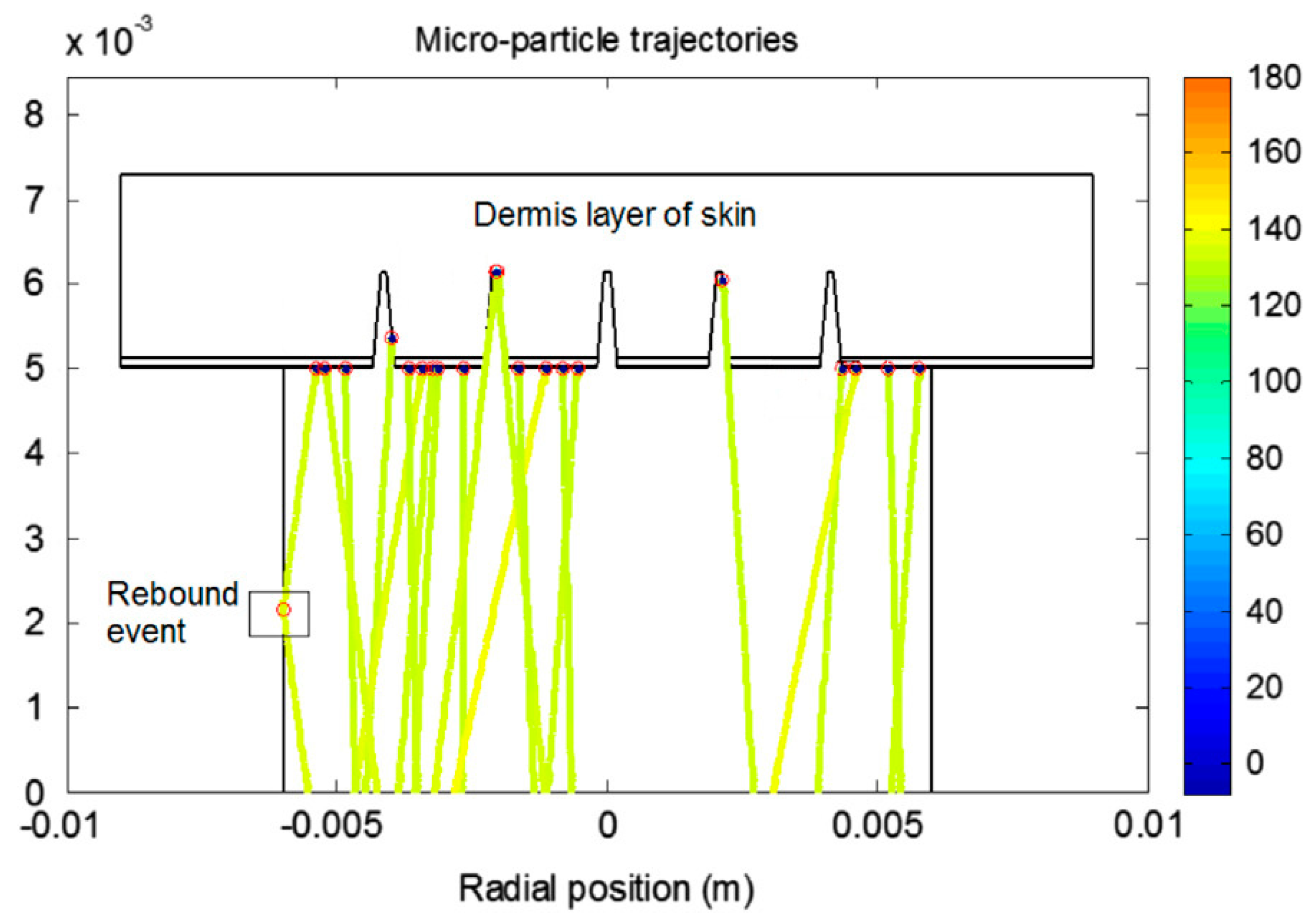
- Kong, X.Q.; Zhou, P.; Wu, C.-W. Numerical simulation of microneedles’ insertion into skin. Comput. Methods Biomech. Biomed. Eng. 2011, 14, 827–835. [Google Scholar] [CrossRef]
- Kim, B.; Seong, K.-Y.; You, I.; Selvaraj, V.; Yim, S.-G.; O’Cearbhaill, E.; Jeong, U.; Yang, S.Y. Touch-actuated transdermal delivery patch for quantitative skin permeation control. Sens. Actuators B Chem. 2018, 256, 18–26. [Google Scholar] [CrossRef]
- Al-Qallaf, B.; Mori, D.; Olatunji, L.; Das, D.B.; Cui, Z. Transdermal Drug Delivery by Microneedles: Does Skin Metabolism Matter? Int. J. Chem. React. Eng. 2009, 7, 69. [Google Scholar] [CrossRef]
- Milewski, M.; Paudel, K.S.; Brogden, N.K.; Ghosh, P.; Banks, S.L.; Hammell, D.C.; Stinchcomb, A.L. Microneedle-Assisted Percutaneous Delivery of Naltrexone Hydrochloride in Yucatan Minipig: In Vitro–In Vivo Correlation. Mol. Pharm. 2013, 10, 3745–3757. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McAllister, D.V.; Wang, P.M.; Davis, S.P.; Park, J.-H.; Canatella, P.J.; Allen, M.G.; Prausnitz, M.R. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: Fabrication methods and transport studies. Proc. Natl. Acad. Sci. USA 2003, 100, 13755–13760. [Google Scholar] [CrossRef]
- Hansen, S.; Henning, A.; Naegel, A.; Heisig, M.; Wittum, G.; Neumann, D.; Kostka, K.-H.; Zbytovska, J.; Lehr, C.-M.; Schaefer, U.F. In-silico model of skin penetration based on experimentally determined input parameters. Part I: Experimental determination of partition and diffusion coefficients. Eur. J. Pharm. Biopharm. 2008, 68, 352–367. [Google Scholar] [CrossRef]
- Olatunji, O.; Das, D.B.; Nassehi, V. Modelling Transdermal Drug Delivery Using Microneedles: Effect of Geometry on Drug Transport Behaviour. J. Pharm. Sci. 2012, 101, 164–175. [Google Scholar] [CrossRef]
- Kushner, J.; Blankschtein, D.; Langer, R. Evaluation of the porosity, the tortuosity, and the hindrance factor for the transdermal delivery of hydrophilic permeants in the context of the aqueous pore pathway hypothesis using dual-radiolabeled permeability experiments. J. Pharm. Sci. 2007, 96, 3263–3282. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1958. [Google Scholar]
- Morofuji, R.; Hikima, T.; Tojo, K. Effect of Diffusive Direction across the Skin on the Penetration Profile of Chemicals in Vitro. Boil. Pharm. Bull. 2013, 36, 1760–1765. [Google Scholar] [CrossRef]
- Rzhevskiy, A.S.; Telaprolu, K.C.; Mohammed, Y.; Grice, J.E.; Roberts, M.S.; Anissimov, Y. Using a simple equation to predict the microporation-enhanced transdermal drug flux. Eur. J. Pharm. Biopharm. 2018, 127, 12–18. [Google Scholar] [CrossRef]
- Lyashko, S.I.; Klyushin, D.A.; Onotskyi, V.V.; Lyashko, N.I. Optimal Control of Drug Delivery from Microneedle Systems. In Cybernetics and Systems Analysis; Springer: Berlin/Heidelberg, Germany, 2018; Volume 54, pp. 357–365. [Google Scholar]
- Shibata, T.; Nakanishi, A.; Sakai, T.; Kato, N.; Kawashima, T.; Mineta, T.; Makino, E. Fabrication and Mechanical Characterization of Microneedle Array for Cell Surgery. In Proceedings of the TRANSDUCERS 2007—2007 International Solid-State Sensors, Actuators and Microsystems Conference, Institute of Electrical and Electronics Engineers (IEEE), Lyon, France, 10–14 June 2007; pp. 719–722. [Google Scholar]
- Lv, Y.-G.; Liu, J.; Gao, Y.-H.; Xu, B. Modeling of transdermal drug delivery with a microneedle array. J. Micromech. Microeng. 2006, 16, 2492–2501. [Google Scholar] [CrossRef]
- Liu, T.-T.; Chen, K.; Pan, M. Experimental and modelling characterisation of adjustable hollow Micro-needle delivery systems. Med. Eng. Phys. 2017, 49, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Pan, M.; Feng, Z.-G. Modeling of Drug Delivery by A Pump Driven Micro-Needle Array System. Open Biomed. Eng. J. 2016, 10, 19–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, K.S.; Ita, K.; Simon, L. Modelling of dissolving microneedles for transdermal drug delivery: Theoretical and experimental aspects. Eur. J. Pharm. Sci. 2015, 68, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Ronnander, P.; Simon, L.; Koch, A. Experimental and mathematical study of the transdermal delivery of sumatriptan succinate from polyvinylpyrrolidone-based microneedles. Eur. J. Pharm. Biopharm. 2020, 146, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Zoudani, E.; Soltani, M. A new computational method of modeling and evaluation of dissolving microneedle for drug delivery applications: Extension to theoretical modeling of a novel design of microneedle (array in array) for efficient drug delivery. Eur. J. Pharm. Sci. 2020, 150, 105339. [Google Scholar] [CrossRef]
- Watanabe, S.; Iimori, M.; Chan, D.V.; Hara, E.; Kitao, H.; Maehara, Y. MDC1 methylation mediated by lysine methyltransferases EHMT1 and EHMT2 regulates active ATM accumulation flanking DNA damage sites. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Chavoshi, S.; Rabiee, M.; Rafizadeh, M.; Rabiee, N.; Shamsabadi, A.S.; Bagherzadeh, M.; Salarian, R.; Tahriri, M.; Tayebi, L. Mathematical modeling of drug release from biodegradable polymeric microneedles. Bio-Des. Manuf. 2019, 2, 96–107. [Google Scholar] [CrossRef]
- Siegel, S.J.; Kahn, J.B.; Metzger, K.; Winey, K.I.; Werner, K.; Dan, N. Effect of drug type on the degradation rate of PLGA matrices. Eur. J. Pharm. Biopharm. 2006, 64, 287–293. [Google Scholar] [CrossRef]
- Amodwala, S.; Kumar, P.; Thakkar, H. Statistically optimized fast dissolving microneedle transdermal patch of meloxicam: A patient friendly approach to manage arthritis. Eur. J. Pharm. Sci. 2017, 104, 114–123. [Google Scholar] [CrossRef]
- Chen, S.; Chen, J.; Li, N. Finite element analysis of microneedle insertion into skin. Micro Nano Lett. 2012, 7, 1206–1209. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, P.; Dalton, C.; Jullien, G. Modeling of drug delivery into tissues with a microneedle array using mixture theory. Biomech. Model. Mechanobiol. 2009, 9, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.H.; Liu, J.L.; Zhu, D.D.; Hao, Y.Y.; Guo, X.D. Multiscale simulations of drug distributions in polymer dissolvable microneedles. Colloids Surf. B Biointerfaces 2020, 189, 110844. [Google Scholar] [CrossRef] [PubMed]

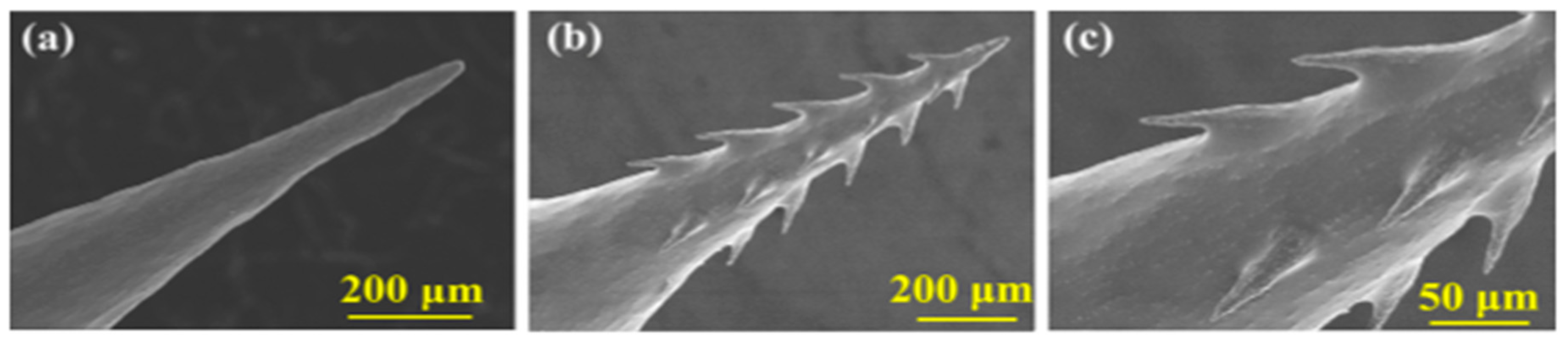


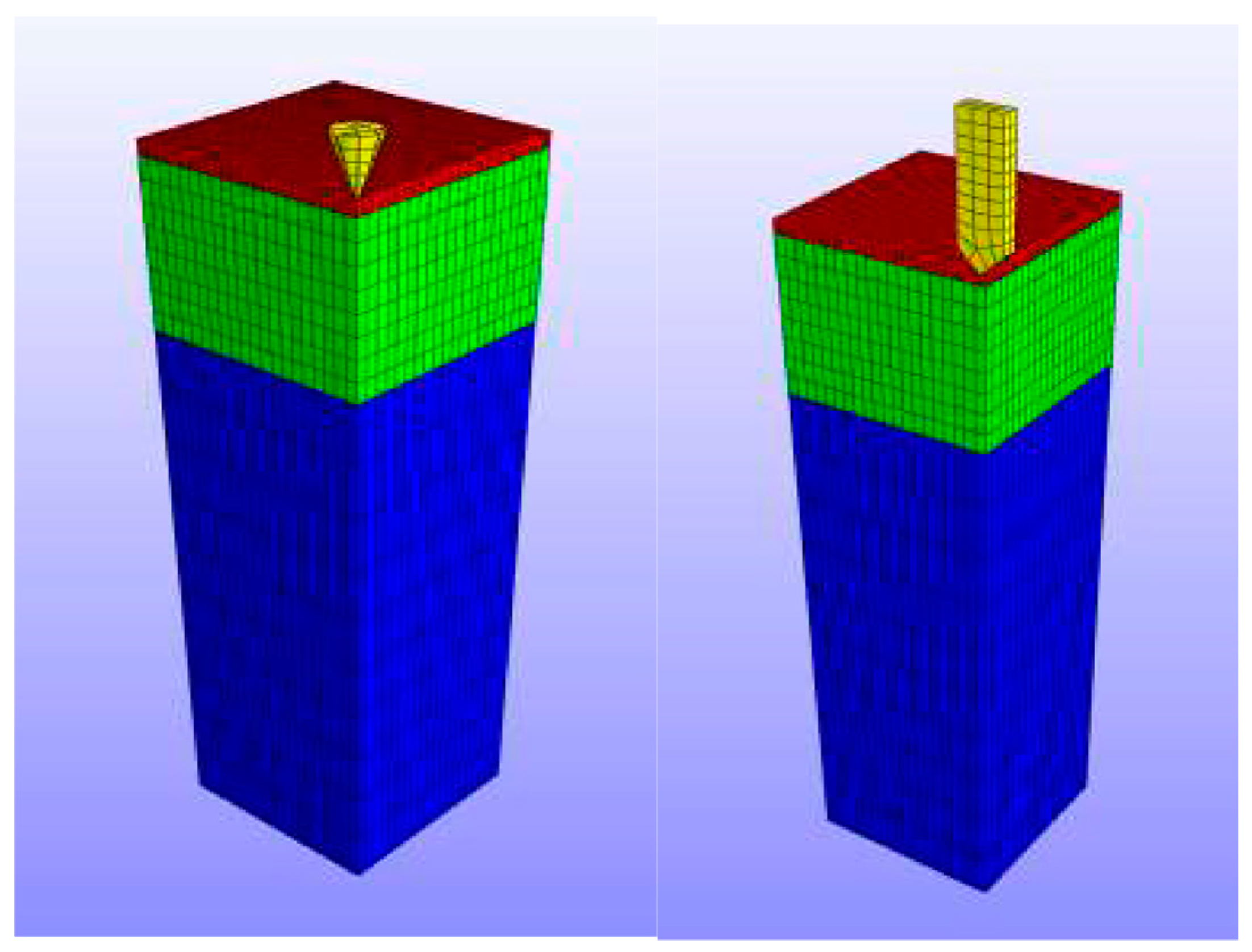
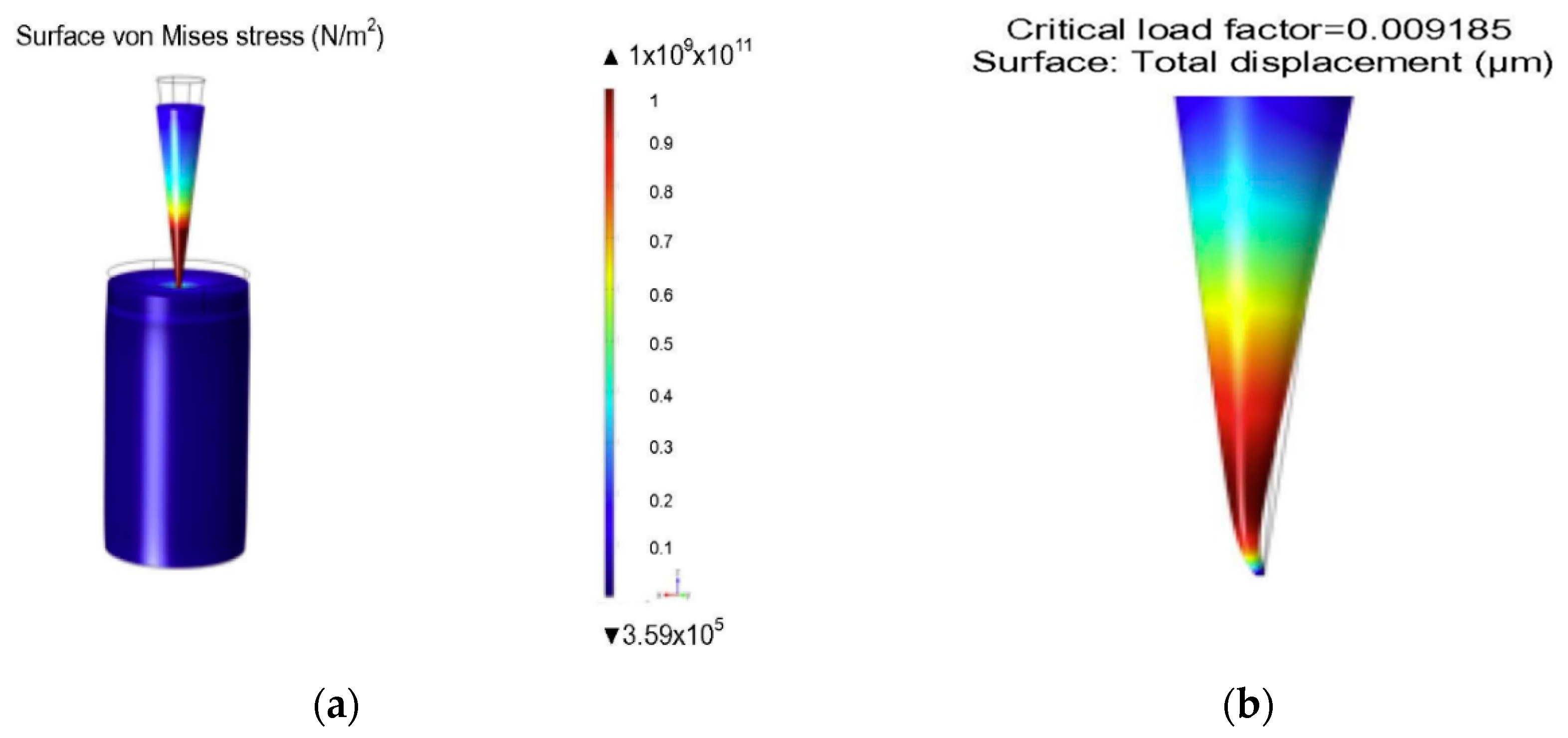


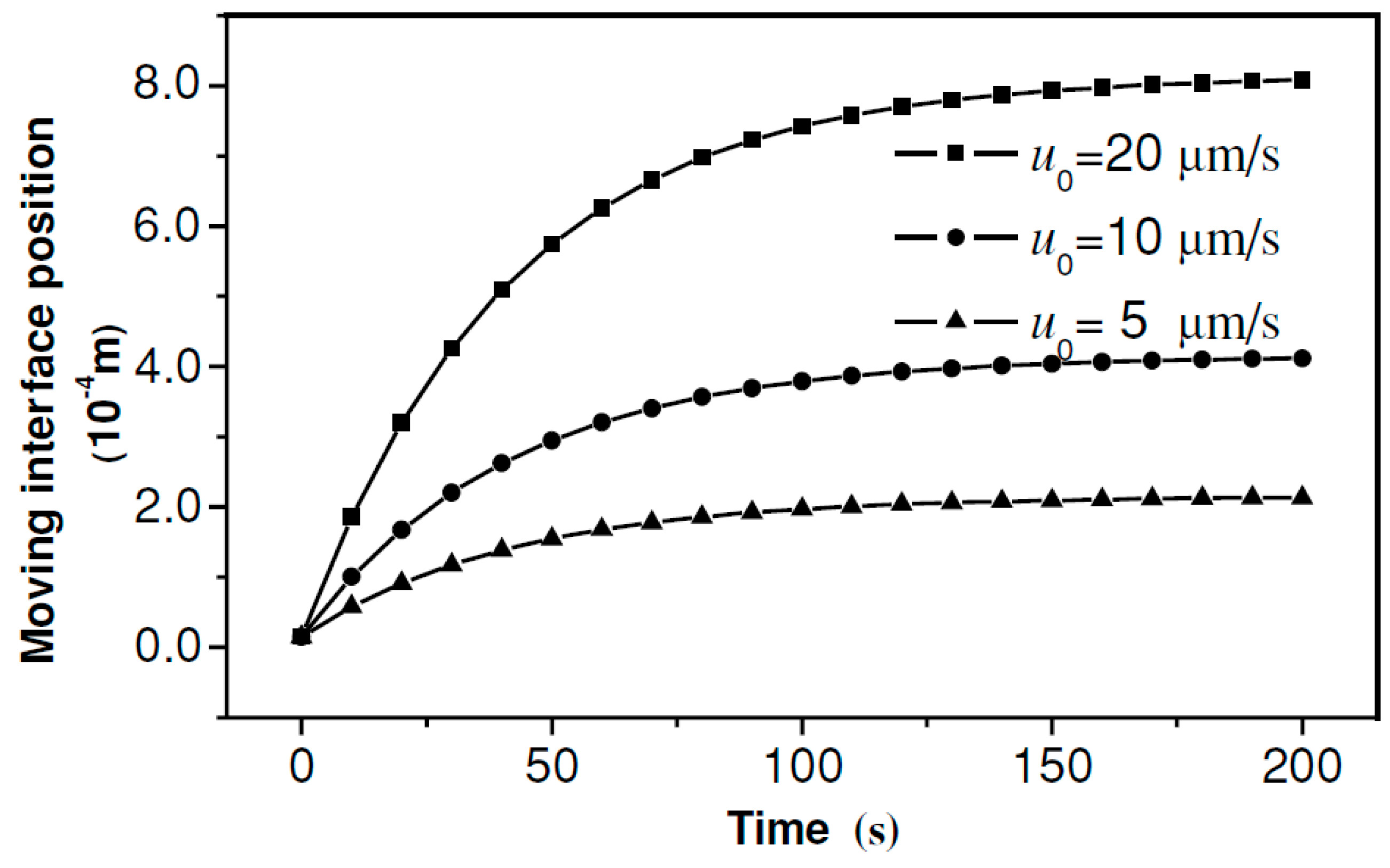
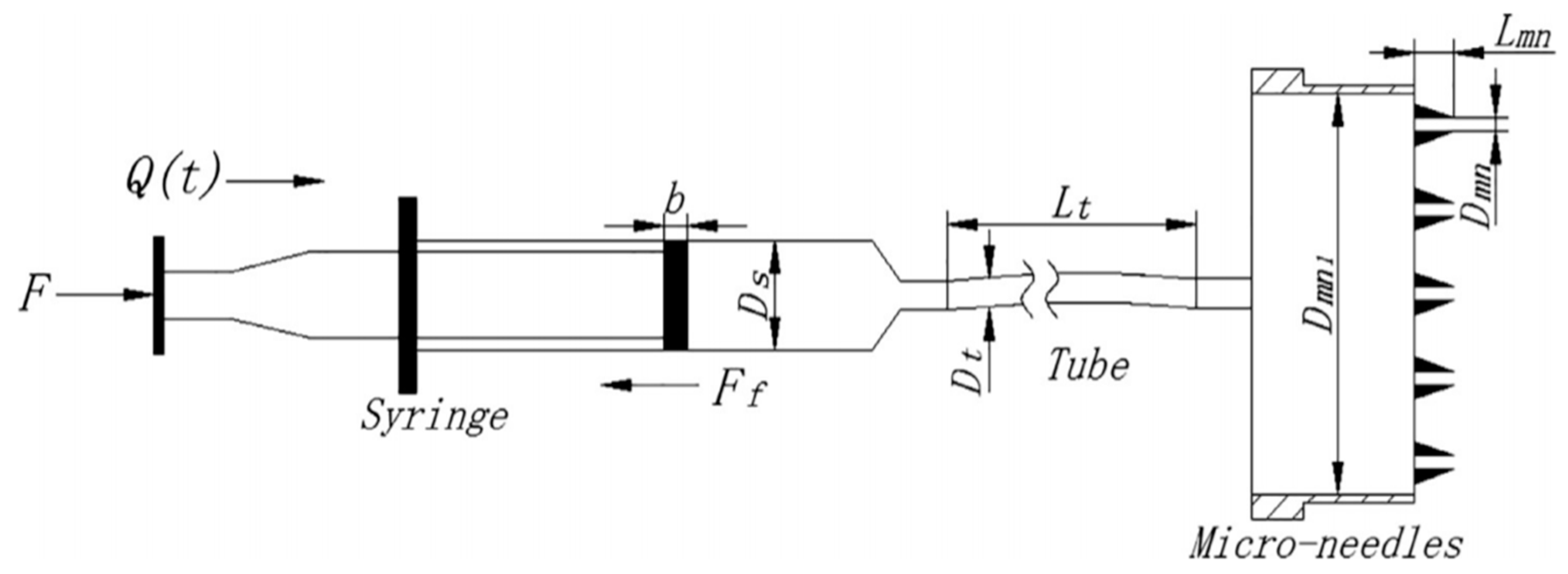
| MN Distribution in a Patch | Permeability (cm/s) |
|---|---|
| Square | K = 1.6185 × D − 0.0008 |
| Diamond | K = 0.8125 × D − 0.0029 |
| Triangular | K = 0.936 × D − 0.0007 |
| Rectangular | K = 1.622 × D − 0.0002 |
| Constants | Definition | Values | Variables | Definition | Unit |
|---|---|---|---|---|---|
| Dmn | Inner diameter of MN | Q(t) | Volumetric flow rate of liquid drug | m3/s | |
| Dmn1 | Inner diameter of micro-needle cavity | F | Force that the pump push block applies on syringe | [N] | |
| Ds | Inner diameter of syringe | ζ | Friction coefficient at syringe outlet | - | |
| Dt | Inner diameter of soft tube | ζ 1 | Friction coefficient at connection from soft tube to micro-needle cavity | - | |
| Lmn | Length of MN | ζ 2 | Friction coefficient at connection from MN cavity to needle | - | |
| Ρ | Density of liquid drug | 1000 Kg/m3 | Ff | Friction force between seal ring of piston and syringe wall | [N] |
| Μ | Dynamic viscosity of liquid drug | λ | Friction factor | - | |
| N | Number of MNs | 5 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, P.R.; Han, T.; Olatunji, O.; Pattanayek, S.K.; Das, D.B. Mathematical Modelling, Simulation and Optimisation of Microneedles for Transdermal Drug Delivery: Trends and Progress. Pharmaceutics 2020, 12, 693. https://doi.org/10.3390/pharmaceutics12080693
Yadav PR, Han T, Olatunji O, Pattanayek SK, Das DB. Mathematical Modelling, Simulation and Optimisation of Microneedles for Transdermal Drug Delivery: Trends and Progress. Pharmaceutics. 2020; 12(8):693. https://doi.org/10.3390/pharmaceutics12080693
Chicago/Turabian StyleYadav, Prateek Ranjan, Tao Han, Ololade Olatunji, Sudip K. Pattanayek, and Diganta Bhusan Das. 2020. "Mathematical Modelling, Simulation and Optimisation of Microneedles for Transdermal Drug Delivery: Trends and Progress" Pharmaceutics 12, no. 8: 693. https://doi.org/10.3390/pharmaceutics12080693
APA StyleYadav, P. R., Han, T., Olatunji, O., Pattanayek, S. K., & Das, D. B. (2020). Mathematical Modelling, Simulation and Optimisation of Microneedles for Transdermal Drug Delivery: Trends and Progress. Pharmaceutics, 12(8), 693. https://doi.org/10.3390/pharmaceutics12080693







