A Novel Vitamin E TPGS-Based Formulation Enhances Chlorhexidine Bioavailability in Corneal Layers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Line and Culture Conditions
2.3. Ex Vivo Determination of Corneal Drug Penetration in Franz Diffusion Cells
2.4. HPLC Analysis
2.5. In Vitro Toxicity by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
2.6. In Vivo Ocular Tolerance in Rabbits
2.7. Microorganisms
2.8. Determination of Minimal Inhibitory Concentrations (MICs), Minimum Bactericidal Concentrations (MBCs), and Minimum Fungicidal Concentration (MFC)
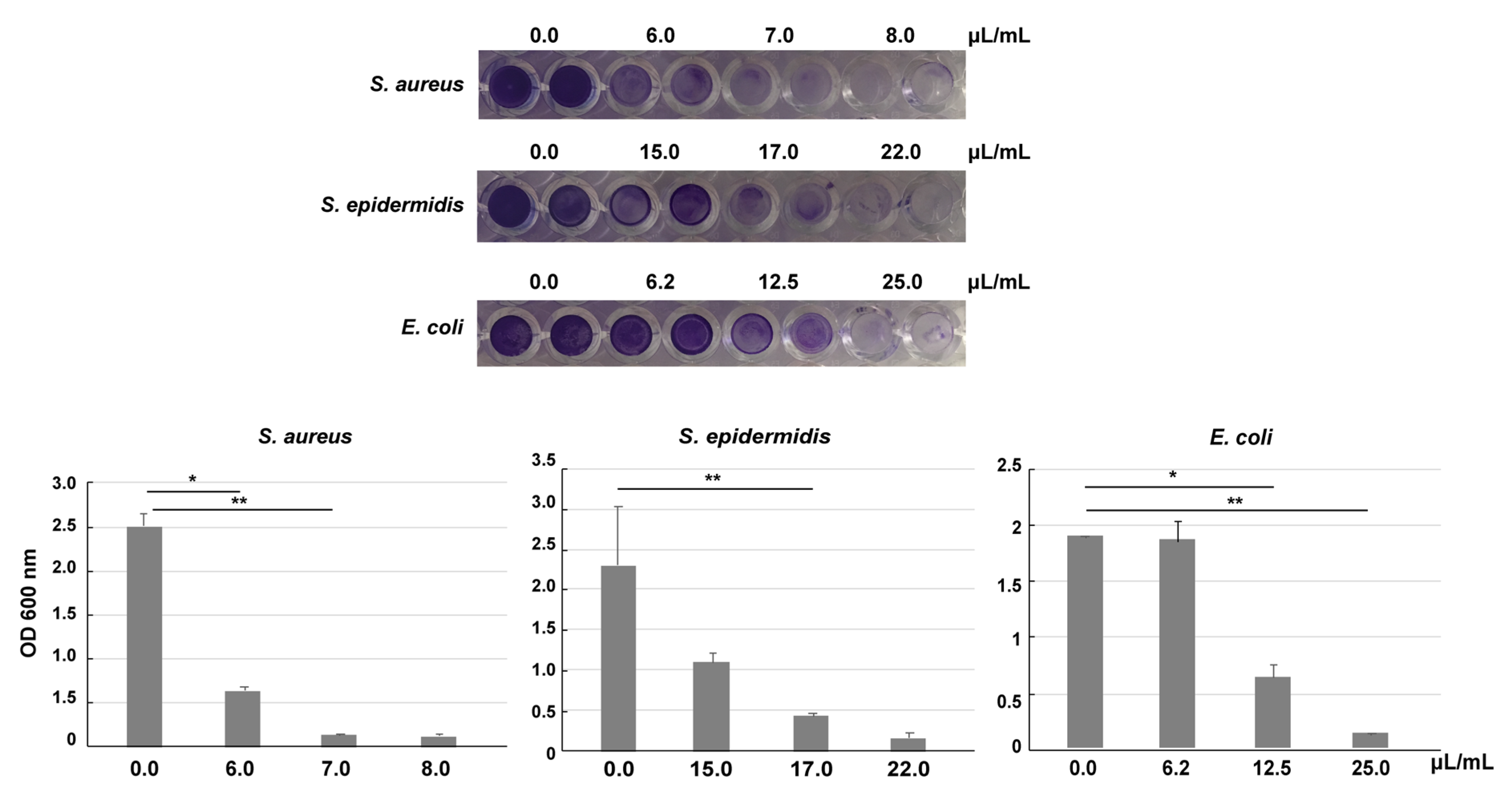
2.9. Biofilm Inhibition
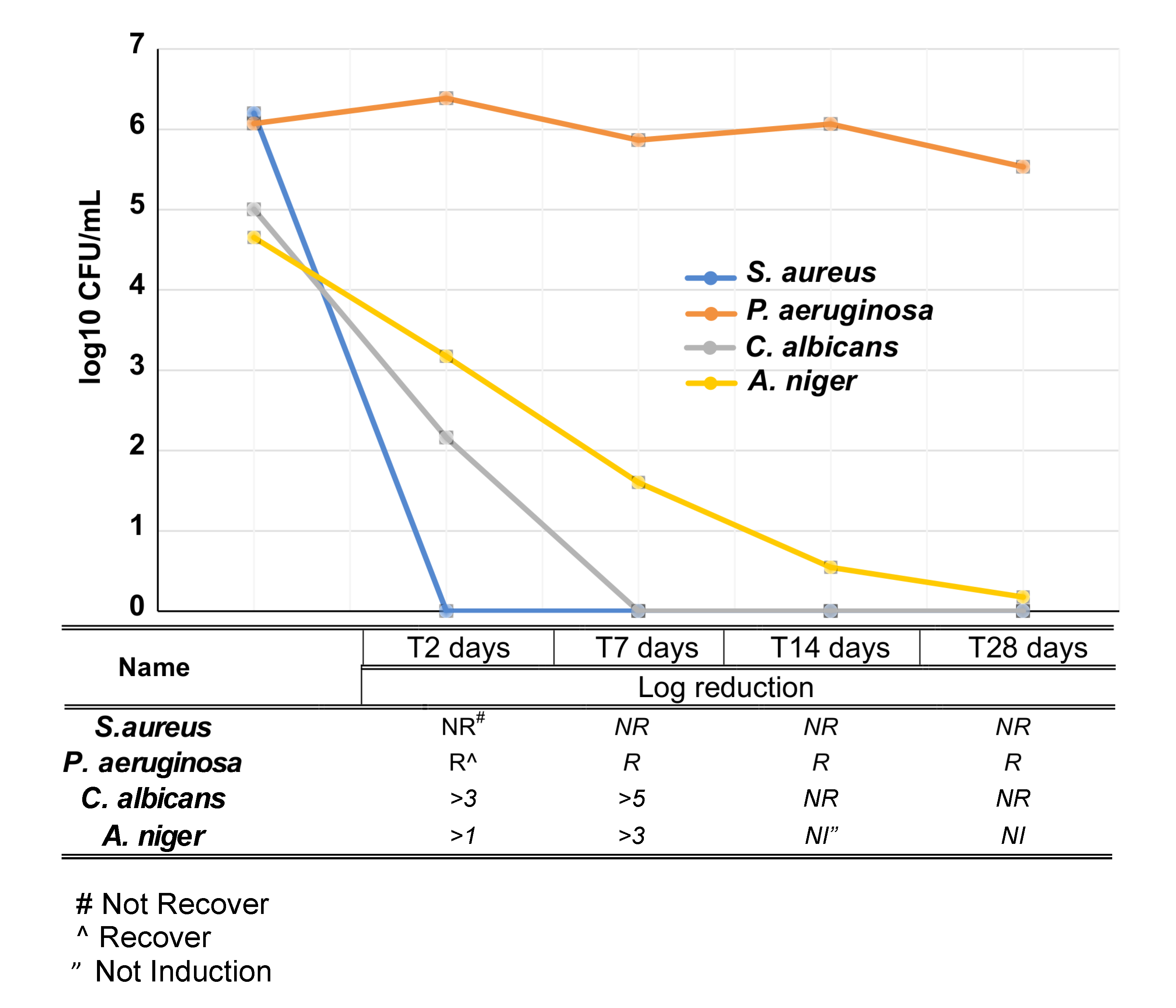
2.10. Challenge Test
3. Results
3.1. VE-TPGS Increases Corneal Accumulation of CHX
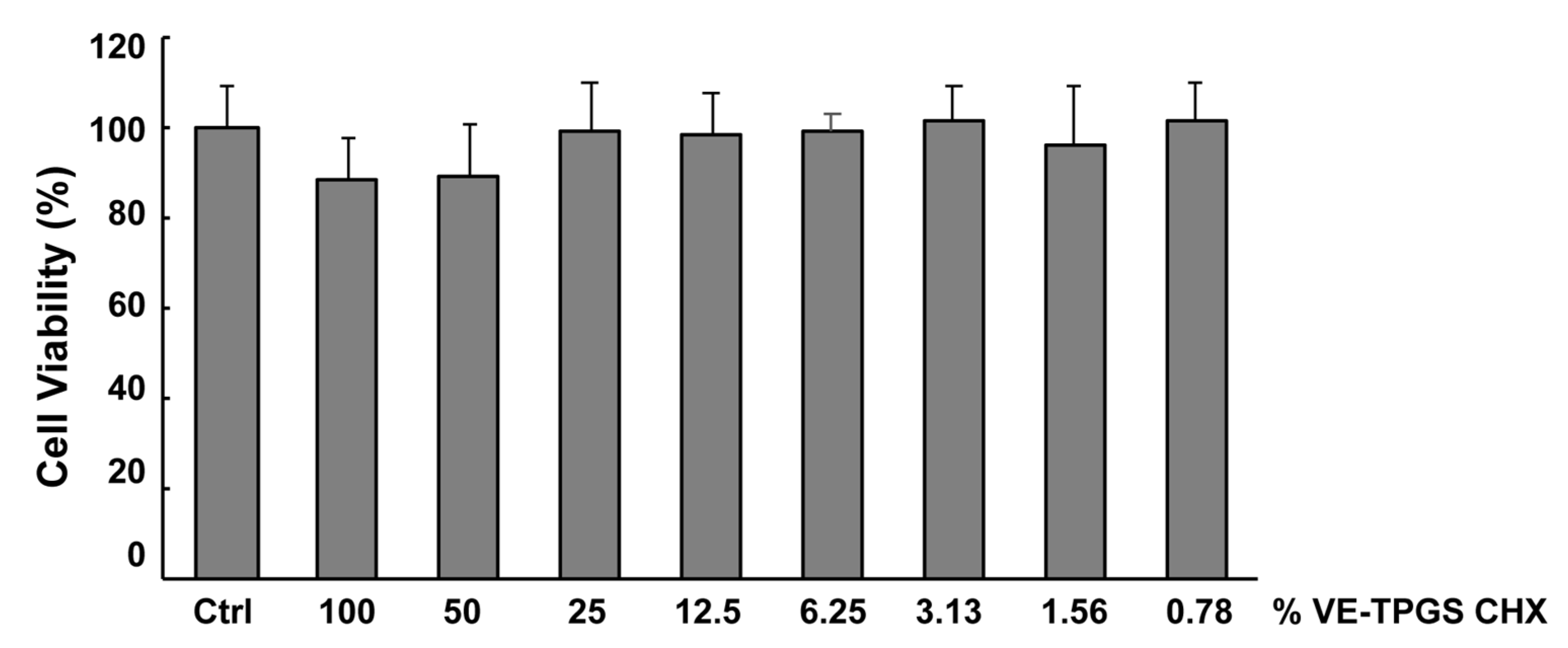
3.2. In Vitro Toxicity of VE-TPGS CHX Formulation
3.3. In Vivo Ocular Tolerance Study in Rabbits
3.4. Antimicrobial Activity of VE-TPGS Chlorhexidine Gluconate (CHX) Formulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Pathogenesis of microbial keratitis. Microb. Pathog. 2017, 104, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Ng, T.P.; Fong, K.S.; Tan, D.T. Risk factors and clinical outcomes between fungal and bacterial keratitis: A comparative study. CLAO J. 1997, 23, 275–281. [Google Scholar] [PubMed]
- Mark, E. Contact-lens-related microbial keratitis: Case report and review. J. Optom. 2011, 4, 122–127. [Google Scholar]
- Al-Mujaini, A.; Al-Kharusi, N.; Thakral, A.; Wali, U.K. Bacterial keratitis: Perspective on epidemiology, clinico-pathogenesis, diagnosis and treatment. Sultan Qaboos Univ. Med. J. 2009, 9, 184–195. [Google Scholar]
- Mills, R. Microbial keratitis: what’s the preferred initial therapy? View 1: Corneal scraping and combination antibiotic therapy is indicated. Br. J. Ophthalmol. 2003, 87, 1167–1169. [Google Scholar] [CrossRef]
- Jastaneiah, S.; Al-Rajhi, A.; Abbottb, D. Ocular mycosis at a referral center in Saudi Arabia: A 20-year study. Saudi J. Ophthalmol. 2011, 25, 231–238. [Google Scholar] [CrossRef]
- Ferrer, C.; Alio, J.L. Evaluation of molecular diagnosis in fungal keratitis. Ten years of experience. J. Ophthalmic Inflamm. Infect. 2011, 1, 15–22. [Google Scholar] [CrossRef]
- Tarrand, J.J.; Lichterfeld, M.; Warraich, I.; Luna, M.; Han, X.Y.; May, G.S.; Kontoyiannis, D.P. Diagnosis of Invasive Septate Mold Infections: A Correlation of Microbiological Culture and Histologic or Cytologic Examination. Am. J. Clin. Pathol. 2003, 119, 854–858. [Google Scholar] [CrossRef]
- Sharma, S.; Kunimoto, D.Y.; Gopinathan, U.; Athmanathan, S.; Garg, P.; Rao, G.N. Evaluation of corneal scraping smear examination methods in the diagnosis of bacterial and fungal keratitis: A survey of eight years of laboratory experience. Cornea 2002, 21, 643–647. [Google Scholar] [CrossRef]
- Tuli, S.; Gray, M. Surgical management of corneal infections. Curr. Opin. Ophthalmol. 2016, 27, 340–347. [Google Scholar] [CrossRef]
- Martins, S.A.; Combs, J.C.; Noguera, G.; Camacho, W.; Wittmann, P.; Walther, R.; Cano, M.; Dick, J.; Behrens, A. Antimicrobial efficacy of riboflavin/UVA combination (365 nm) in vitro for bacterial and fungal isolates: A potential new treatment for infectious keratitis. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3402–3408. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Krishna, S.N.; Kumar, S. Photo-activated riboflavin therapy of refractory corneal ulcers. Cornea 2012, 31, 1210–1213. [Google Scholar] [CrossRef]
- Sauer, A.; Letscher-Bru, V.; Speeg-Schatz, C.; Touboul, D.; Colin, J.; Candolfi, E.; Bourcier, T. In vitro efficacy of antifungal treatment using riboflavin/UV-A (365nm) combination and amphotericin B. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3950–3953. [Google Scholar] [CrossRef] [PubMed]
- Charukamnoetkanok, P.; Pineda, R. Controversies in management of bacterial keratitis. Int. Ophthalmol. Clin. 2005, 45, 199–210. [Google Scholar] [CrossRef]
- McBain, A.J.; Bartolo, R.G.; Catrenich, C.E.; Charbonneau, D.; Ledder, R.G.; Gilbert, P. Effects of a chlorhexidine gluconate-containing mouthwash on the vitality and antimicrobial susceptibility of in vitro oral bacterial ecosystems. Appl. Environ. Microbiol. 2003, 69, 4770–4776. [Google Scholar] [CrossRef] [PubMed]
- Tattawasart, U.; Hann, A.C.; Maillard, J.Y.; Furr, J.R.; Russell, A.D. Cytological changes in chlorhexidine-resistant isolates of Pseudomonas stutzeri. J. Antimicrob. Chemother. 2000, 45, 145–152. [Google Scholar] [CrossRef]
- Martin, M.J.; Rahman, M.R.; Johnson, G.J.; Srinivasan, M.; Clayton, Y.M. Mycotic keratitis: Susceptibility to antiseptic agents. Int. Ophthalmol. 1995, 19, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Kuyyakanond, T.; Quesnel, L.B. The mechanism of action of chlorhexidine. FEMS Microbiol. Lett. 1992, 79, 211–215. [Google Scholar] [CrossRef]
- Barrett-Bee, K.; Newboult, L.; Edwards, S. The membrane destabilising action of the antibacterial agent chlorhexidine. FEMS Microbiol. Lett. 1994, 119, 249–253. [Google Scholar] [CrossRef][Green Version]
- Audus, K.L.; Tavakoli-Saberi, M.R.; Zheng, H.; Boyce, E.N. Chlorhexidine effects on membrane lipid domains of human buccal epithelial cells. J. Dent. Res. 1992, 71, 1298–1303. [Google Scholar] [CrossRef]
- Paulson, D.S. Efficacy evaluation of a 4% chlorhexidine gluconate as a full-body shower wash. Am. J. Infect. Control 1993, 21, 205–209. [Google Scholar] [CrossRef]
- James, X.L.; Werner, J.; Kirsch, T.; Joseph, D.Z.; Virk, S.M. Cytotoxicity evaluation of chlorhexidine gluconate on human fibroblasts, myoblasts, and osteoblasts. J. Bone Jt. Infect. 2018, 3, 165–172. [Google Scholar]
- Giannelli, M.; Chellini, F.; Margheri, M.; Tonelli, P.; Tani, A. Effect of chlorhexidine digluconate on different cell types: A molecular and ultrastructural investigation. Toxicol. In Vitro 2008, 22, 308–317. [Google Scholar] [CrossRef]
- Hirsch, T.; Koerber, A.; Jacobsen, F.; Dissemond, J.; Steinau, H.U.; Gatermann, S.; Al-Benna, S.; Kesting, M.; Seipp, H.M.; Steinstraesser, L. Evaluation of toxic side effects of clinically used skin antiseptics in vitro. J. Surg. Res. 2010, 164, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, P.; Cogen, R.; Taubman, S. Cytopathologic effects of chlorhexidine on human cells. J. Periodontol. 1977, 48, 212–215. [Google Scholar] [CrossRef]
- Carrijo-Carvalho, L.C.; Sant’ana, V.P.; Foronda, A.S.; de Freitas, D.; de Souza Carvalho, F.R. Therapeutic agents and biocides for ocular infections by free-living amoebae of Acanthamoeba genus. Surv. Ophthalmol. 2017, 62, 203–218. [Google Scholar] [CrossRef]
- O’Day, D.M.; Head, W.S.; Robinson, R.D.; Clanton, J.A. Corneal penetration of topical amphotericin B and natamycin. Curr. Eye Res. 1986, 5, 877–882. [Google Scholar] [CrossRef]
- Vontobel, S.F.; Abad-Villar, E.M.; Kaufmann, C.; Zinkernagel, A.S.; Hauser, P.C.; Thiel, A.M. Corneal Penetration of Polyhexamethylene Biguanide and Chlorhexidine Digluconate. J. Clin. Exp. Ophthalmol. 2015, 6, 430. [Google Scholar] [CrossRef]
- Ghate, D.; Edelhauser, H.F. Ocular drug delivery. Expert Opin. Drug Deliv. 2006, 3, 275–287. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Vinarov, Z.; Katev, V.; Radeva, D.; Tcholakova, S.; Denkov, N.D. Micellar solubilization of poorly water-soluble drugs: Effect of surfactant and solubilizate molecular structure. Drug Dev. Ind. Pharm. 2018, 44, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Andal, A.; Bisht, R.; Rupenthal, I.D.; Mitra, A.K. Polymeric micelles for ocular drug delivery: From structural frameworks to recent preclinical studies. J. Control. Release 2017, 248, 96–116. [Google Scholar] [CrossRef] [PubMed]
- Etsuo, N. Evidence for beneficial effects of vitamin E. Korean J. Intern. Med. 2015, 30, 571–579. [Google Scholar]
- Costagliola, C.; Libondi, T.; Menzione, M.; Rinaldi, E.; Auricchio, G. Vitamin E and red blood cell glutathione. Metabolism 1985, 34, 712–714. [Google Scholar] [CrossRef]
- Sato, K.; Niki, E.; Shimasaki, H. Free radical-mediated chain oxidation of low density lipoprotein and its synergistic inhibition by vitamin E and vitamin C. Arch. Biochem. Biophys. 1990, 279, 402–405. [Google Scholar] [CrossRef]
- Bowry, V.W.; Ingold, K.U.; Stocker, R. Vitamin E in human low-density lipoprotein: When and how this antioxidant becomes a pro-oxidant. Biochem, J. 1992, 288 Pt 2, 341–344. [Google Scholar] [CrossRef]
- Niki, E.; Noguchi, N. Dynamics of antioxidant action of vitamin E. Acc. Chem. Res. 2004, 37, 45–51. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Ostacolo, C.; Caruso, C.; Tronino, D.; Troisi, S.; Laneri, S.; Pacente, L.; Del Prete, A.; Sacchi, A. Enhancement of corneal permeation of riboflavin-5′-phosphate through vitamin E TPGS: A promising approach in corneal trans-epithelial cross linking treatment. Int. J. Pharm. 2013, 440, 148–153. [Google Scholar] [CrossRef]
- Viñas, P.; Balsalobre, N.; López-Erroz, C.; Hernández-Córdoba, M. Liquid chromatographic analysis of riboflavin vitamers in foods using fluorescence detection. J. Agric. Food Chem. 2004, 52, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Horobin, R.W.; Colombo, L.L.; Blázquez-Castro, A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018, 120, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 83, 377–390. [Google Scholar]
- Wikler, M.A. Clinical and Laboratory Standard Institute. In Performance Standards for Antimicrobial Susceptibility Testing; 18th Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; Approved Standard; CLSI Document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Deluca, P.P.; Kostenbauder, H.B. Interaction of preservatives with macromolecules IV. Binding of quaternary ammonium compounds by nonionic agents. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1960, 49, 430–437. [Google Scholar] [CrossRef]
- Maurer, N.; Wong, K.F.; Hope, M.J.; Cullis, P.R. Anomalous solubility behavior of the antibiotic ciprofloxacin encapsulated in liposomes: A 1H-NMR study. Biochim. Biophys. Acta 1998, 1374, 9–20. [Google Scholar] [CrossRef][Green Version]
- Rossi, A.B.; Bacquey, A.; Nocera, T.; Thouvenin, M.D. Efficacy and Tolerability of a Medical Device Repairing Emollient Cream Associated with a Topical Corticosteroid in Adults with Atopic Dermatitis: An Open-label, Intra-individual Randomized Controlled Study. Dermatol. Ther. 2019, 9, 389. [Google Scholar] [CrossRef]
- Pinter, A.; Thouvenin, M.D.; Bacquey, A.; Rossi, A.B.; Nocera, T. Tolerability and Efficacy of a Medical Device Repairing Emollient Cream in Children and Adults with Mild to Moderate Atopic Dermatitis. Dermatol. Ther. 2019, 9, 309–319. [Google Scholar] [CrossRef]
- Barbour, M.E.; Maddocks, S.E.; Wood, N.J.; Collins, A.M. Synthesis, characterization, and efficacy of antimicrobial CHX hexametaphosphate nanoparticles for applications in biomedical materials and consumer products. Int. J. Nanomed. 2013, 8, 3507–3519. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Dintaman, J.M.; Silverman, J.A. Inhibition of P-glycoprotein by D-a-tocopheryl polyethylene glycol 1000 succinate (TPGS). Pharm. Res. 1999, 16, 1550–1556. [Google Scholar] [CrossRef]
- Fato, R.; Bergamini, C.; Leoni, S.; Pinna, A.; Carta, F.; Cardascia, N.; Ferrari, T.M.; Sborgia, C.; Lenaz, G. Coenzyme Q10 vitreous levels after administration of coenzyme Q10 eyedrops in patients undergoing vitrectomy. Acta Ophthalmol. 2010, 88, e150–e151. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.; Shafor, C.; Gause, S.; Hsu, K.H.; Powell, K.C.; Chauhan, A. Therapeutic contact lenses: A patent review. Expert Opin. Ther. Pat. 2015, 25, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Ayala, M.J.; Mulet, M.E.; Artola, A.; Ruiz, J.M.; Bellot, J. Antioxidant therapy in the treatment of experimental acute corneal inflammation. Ophthalmic Res. 1995, 27, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Costagliola, C.; Iuliano, G.; Menzione, M.; Rinaldi, E.; Vito, P.; Auricchio, G. Effect of vitamin E on glutathione content in red blood cells, aqueous humor and lens of humans and other species. Exp. Eye Res. 1986, 43, 905–914. [Google Scholar] [CrossRef]
- Stone, S. Vitamin E (wheat germ oil) in the treatment of interstitial keratitis. Arch. Ophthalmol. 1943, 30, 467–475. [Google Scholar] [CrossRef]
- Costagliola, C.; Di Giovanni, A.; Rinaldi, M.; Scibelli, G.; Fioretti, F. Photorefractive keratectomy and cataract. Surv. Ophthalmol. 1997, 42 (Suppl. S1), S133–S140. [Google Scholar] [CrossRef]
- Satterfield, D.; Taube, D.; Kenney, M.C. Effect of vitamin E on the production of collagen, DNA and fibronectin in keratocytes in vitro. Ophthalmic Res. 1988, 20, 227–231. [Google Scholar] [CrossRef]
- Pinna, A.; Usai, D.; Sechi, L.A.; Molicotti, P.; Zanetti, S.; Carta, A. Detection of virulence factors in Pseudomonas aeruginosa strains isolated from contact lens-associated corneal ulcers. Cornea 2008, 27, 320–326. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Starr, M.B.; Masket, S. Bacterial endophthalmitis prophylaxis for cataract surgery: An evidence-based update. Ophthalmology 2002, 109, 13–24. [Google Scholar] [CrossRef]
- Sanfilippo, C.M.; Morrissey, I.; Janes, R.; Morris, T.W. Surveillance of the activity of aminoglycosides and fluoroquinolones against ophthalmic pathogens from Europe in 2010–2011. Curr. Eye Res. 2015, 41, 581–589. [Google Scholar] [CrossRef]
- Hammond, S.A.; Morgan, J.R.; Russell, A.D. Comparative susceptibility of hospital isolates of Gram-negative bacteria to antiseptics and disinfectants. J. Hosp. Inject. 1987, 9, 255–264. [Google Scholar] [CrossRef]
- Dance, D.A.B.; Pearson, A.D.; Seal, D.V.; Lowes, J.A. A hospital outbreak caused by a chlorhexidine and antibiotic resistant Proteus mirabilis. J. Hosp. Inject. 1987, 10, 10–16. [Google Scholar] [CrossRef]
- Pachori, P.; Gothalwal, R.; Gandhi, P. Emergence of antibiotic resistance Pseudomonas aeruginosa in intensive care unit; a critical review. Genes Dis. 2019, 6, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Maillard, J.Y.; Lambert, R.J.; Russell, A.D. Development of resistance to chlorhexidine diacetate in Pseudomonas aeruginosa and the effect of a “residual” concentration. J. Hosp. Infect. 2000, 46, 297–303. [Google Scholar] [CrossRef]
- Tattawasart, U.; Maillard, J.Y.; Furr, J.R.; Russell, A.D. Development of resistance to chlorhexidine diacetate and cetylpyridinium chloride in Pseudomonas stutzeri and changes in antibiotic susceptibility. J. Hosp. Infect. 1999, 42, 219–229. [Google Scholar] [CrossRef]
| Formulation Code | CHX% | VE TPGS% |
|---|---|---|
| A | 0.02 | - |
| B1 | 0.02 | 0.010 |
| B2 | 0.02 | 0.050 |
| B3 | 0.02 | 0.100 |
| B4 | 0.02 | 0.250 |
| B5 | 0.02 | 0.500 |
| B6 | 0.02 | 1.000 |
| Formulation Code | CHX Accumulation (nmol/mg) |
|---|---|
| A | 0.032 ± 0.017 |
| B1 | 0.056 ± 0.033 |
| B2 | 0.104 ± 0.011 |
| B3 | 0.213 ± 0.027 |
| B4 | 0.297 ± 0.031 |
| B5 | 0.345 ± 0.041 |
| B6 | 0.376 ± 0.022 |
| Bacteria | MIC | MBC | MBC/MIC | Biofilm Inhibition |
|---|---|---|---|---|
| S. aureus | 9.0 | 35.0 | 3.9 | 7.5 |
| S. epidermis | 27.5 | 75.0 | 2.7 | 17.5 |
| E. coli | 30.0 | 40.0 | 1.3 | 25.0 |
| P. aeruginosa | >100 | >100 | - | >100 |
| Fungus | MIC | MFC | MFC/MIC | |
| C. albicans | 50.0 | 75.0 | 1.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, C.; Porta, A.; Tosco, A.; Eletto, D.; Pacente, L.; Bartollino, S.; Costagliola, C. A Novel Vitamin E TPGS-Based Formulation Enhances Chlorhexidine Bioavailability in Corneal Layers. Pharmaceutics 2020, 12, 642. https://doi.org/10.3390/pharmaceutics12070642
Caruso C, Porta A, Tosco A, Eletto D, Pacente L, Bartollino S, Costagliola C. A Novel Vitamin E TPGS-Based Formulation Enhances Chlorhexidine Bioavailability in Corneal Layers. Pharmaceutics. 2020; 12(7):642. https://doi.org/10.3390/pharmaceutics12070642
Chicago/Turabian StyleCaruso, Ciro, Amalia Porta, Alessandra Tosco, Daniela Eletto, Luigi Pacente, Silvia Bartollino, and Ciro Costagliola. 2020. "A Novel Vitamin E TPGS-Based Formulation Enhances Chlorhexidine Bioavailability in Corneal Layers" Pharmaceutics 12, no. 7: 642. https://doi.org/10.3390/pharmaceutics12070642
APA StyleCaruso, C., Porta, A., Tosco, A., Eletto, D., Pacente, L., Bartollino, S., & Costagliola, C. (2020). A Novel Vitamin E TPGS-Based Formulation Enhances Chlorhexidine Bioavailability in Corneal Layers. Pharmaceutics, 12(7), 642. https://doi.org/10.3390/pharmaceutics12070642








