Evaluation of Novel Doxorubicin-Loaded Magnetic Wax Nanocomposite Vehicles as Cancer Combinatorial Therapy Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterisation of Magnetic Wax Nanocomposite Vehicles (mWNVs)
2.2. Cell Culture
2.3. Proliferation Assay
2.4. Flow Cytometry Analysis
2.5. Multidrug Resistance Assay
2.6. Confocal Microscopy
2.7. D-MCTS Growth Inhibition Assays
2.8. Statistical Analysis
3. Results
3.1. Preparation and Characterization of DOX-Loaded Magnetic Wax Nanocomposite Vehicles (mWNV-DOX)
3.2. Vehicle Biocompatibility in 2D Cultured Cells
3.3. Chemotherapeutic Effect in 2D Culture Cells
3.4. Encapsulation Effect on a DOX-Resistant In Vitro Model
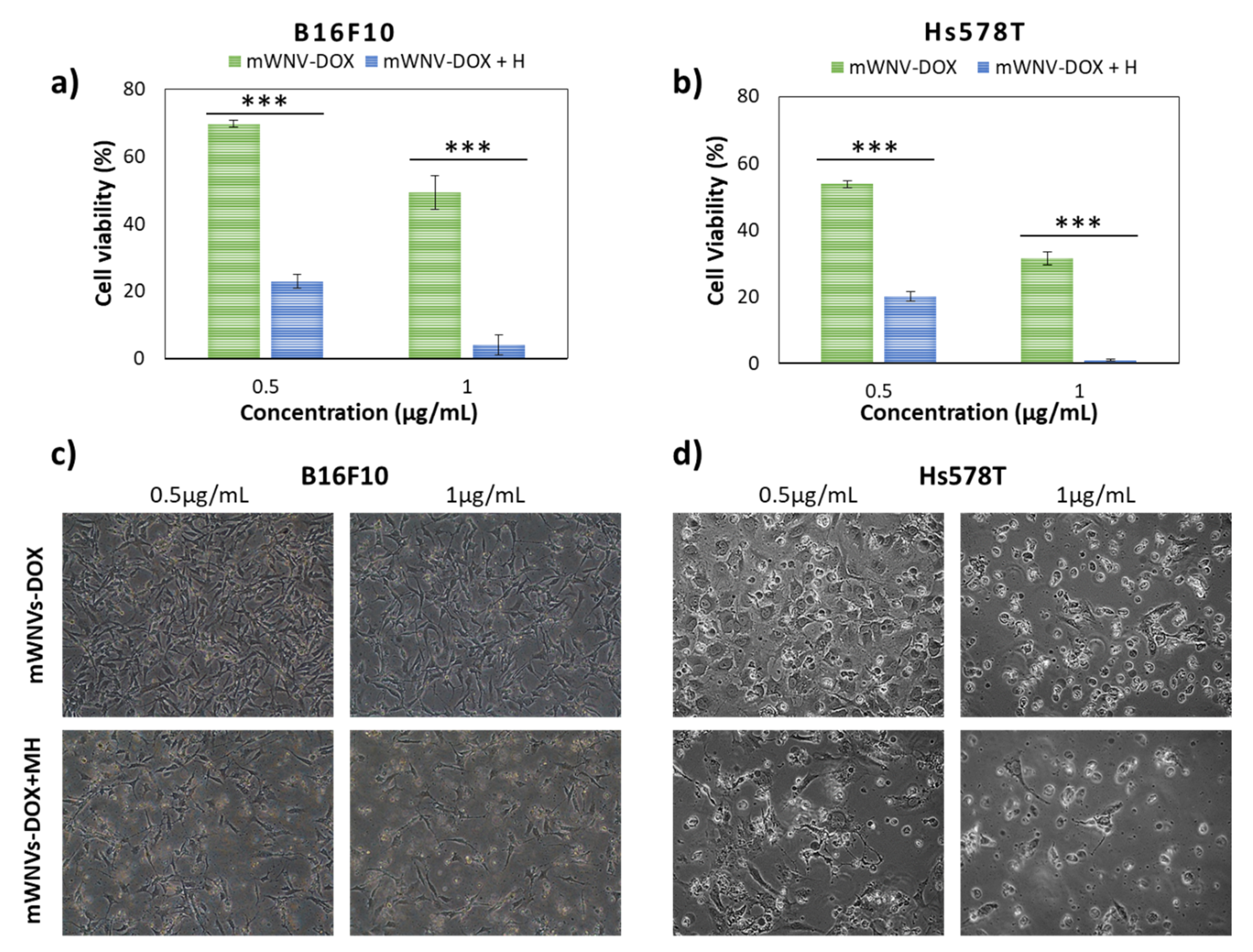
3.5. Combinatorial DOX-MH Treatment in 2D Cultured Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wright, J. Nanotechnology: Deliver on a promise. Sci. Am. 2014, 311, 112–113. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Batist, G.; Barton, J.; Chaikin, P.; Swenson, C.; Welles, L. Myocet (liposome-encapsulated doxorubicin citrate): A new approach in breast cancer therapy. Expert Opin. Pharmacother. 2002, 3, 1739–1751. [Google Scholar] [CrossRef] [PubMed]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99. [Google Scholar]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Zhong, H.; Chan, G.; Hu, Y.; Hu, H.; Ouyang, D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics 2018, 10, 263. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Mirbagheri, M.; Adibnia, V.; Hughes, B.R.; Waldman, S.D.; Banquy, X.; Hwang, D.K. Advanced cell culture platforms: A growing quest for emulating natural tissues. Mater. Horiz. 2019, 6, 45–71. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures – A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910. [Google Scholar]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Lazzari, G.; Couvreur, P.; Mura, S. Multicellular tumor spheroids: A relevant 3D model for the: In vitro preclinical investigation of polymer nanomedicines. Polym. Chem. 2017, 8, 4947–4969. [Google Scholar] [CrossRef]
- Lu, H.; Stenzel, M.H. Multicellular Tumor Spheroids (MCTS) as a 3D In Vitro Evaluation Tool of Nanoparticles. Small 2018, 14, 1702858. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining imaging and therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Dinu, C.Z.; Bale, S.S.; Zhu, G.; Dordick, J.S. Tubulin encapsulation of carbon nanotubes into functional hybrid assemblies. Small 2009, 5, 310–315. [Google Scholar] [CrossRef]
- Zhao, N.; Yan, L.; Zhao, X.; Chen, X.; Li, A.; Zheng, D.; Zhou, X.; Dai, X.; Xu, F.J. Versatile Types of Organic/Inorganic Nanohybrids: From Strategic Design to Biomedical Applications. Chem. Rev. 2018, 119, 1666–1762. [Google Scholar] [CrossRef]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef]
- Li, Z.; Ye, E.; David; Lakshminarayanan, R.; Loh, X.J. Recent Advances of Using Hybrid Nanocarriers in Remotely Controlled Therapeutic Delivery. Small 2016, 12, 4782–4806. [Google Scholar] [CrossRef]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic Drug Delivery: Where the Field Is Going. Front. Chem. 2018, 6, 619. [Google Scholar] [CrossRef]
- McBain, S.C.; Yiu, H.H.P.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar]
- Moura, C.L.; Gallo, J.; García-Hevia, L.; Pessoa, O.D.L.; Ricardo, N.M.P.S.; Bañobre-López, M. Magnetic Hybrid Wax Nanocomposites as Externally Controlled Theranostic Vehicles: High MRI Enhancement and Synergistic Magnetically Assisted Thermo/Chemo Therapy. Chem. Eur. J. 2020, 26, 4531–4538. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; Mcmahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.Y.; Yeap, S.K.; Ho, C.L.; Rahim, R.A.; Alitheen, N.B. Development of Multicellular Tumor Spheroid (MCTS) Culture from Breast Cancer Cell and a High Throughput Screening Method Using the MTT Assay. PLoS ONE 2012, 7, e44640. [Google Scholar] [CrossRef]
- De Almeida, R.R.; Gallo, J.; Da Silva, A.C.C.; Da Silva, A.K.O.; Pessoa, O.D.L.; Araújo, T.G.; Leal, L.K.A.M.; Fechine, P.B.A.; Bañobre-López, M.; Ricardo, N.M.P.S. Preliminary Evaluation of Novel Triglyceride-Based Nanocomposites for Biomedical Applications. J. Braz. Chem. Soc. 2017, 28, 1547–1556. [Google Scholar] [CrossRef]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef]
- Krishna, R.; Mayer, L.D. Liposomal doxorubicin circumvents PSC 833-free drug interactions, resulting in effective therapy of multidrug-resistant solid tumors. Cancer Res. 1997, 57, 5246–5253. [Google Scholar] [PubMed]
- Krishna, R.; St-Louis, M.; Mayer, L.D. Increased intracellular drug accumulation and complete chemosensitization achieved in multidrug-resistant solid tumors by co- administering valspodar (PSC 833) with sterically stabilized liposomal doxorubicin. Int. J. Cancer 2000, 85, 131–141. [Google Scholar] [CrossRef]
- Wang, J.C.; Liu, X.Y.; Lu, W.L.; Chang, A.; Zhang, Q.; Goh, B.C.; Lee, H.S. Pharmacokinetics of intravenously administered stealth liposomal doxorubicin modulated with verapamil in rats. Eur. J. Pharm. Biopharm. 2006, 62, 44–51. [Google Scholar] [CrossRef]
- Lai, G.M.; Chen, Y.N.; Mickley, L.A.; Fojo, A.T.; Bates, S.E. P-glycoprotein expression and schedule dependence of adriamycin cytotoxicity in human colon carcinoma cell lines. Int. J. Cancer 1991, 49, 696–703. [Google Scholar] [CrossRef]
- Nandwana, V.; De, M.; Chu, S.; Jaiswal, M.; Rotz, M.; Meade, T.J.; Dravid, V.P. Theranostic magnetic nanostructures (MNS) for Cancer. Cancer Treat. Res. 2015, 166, 51–83. [Google Scholar]
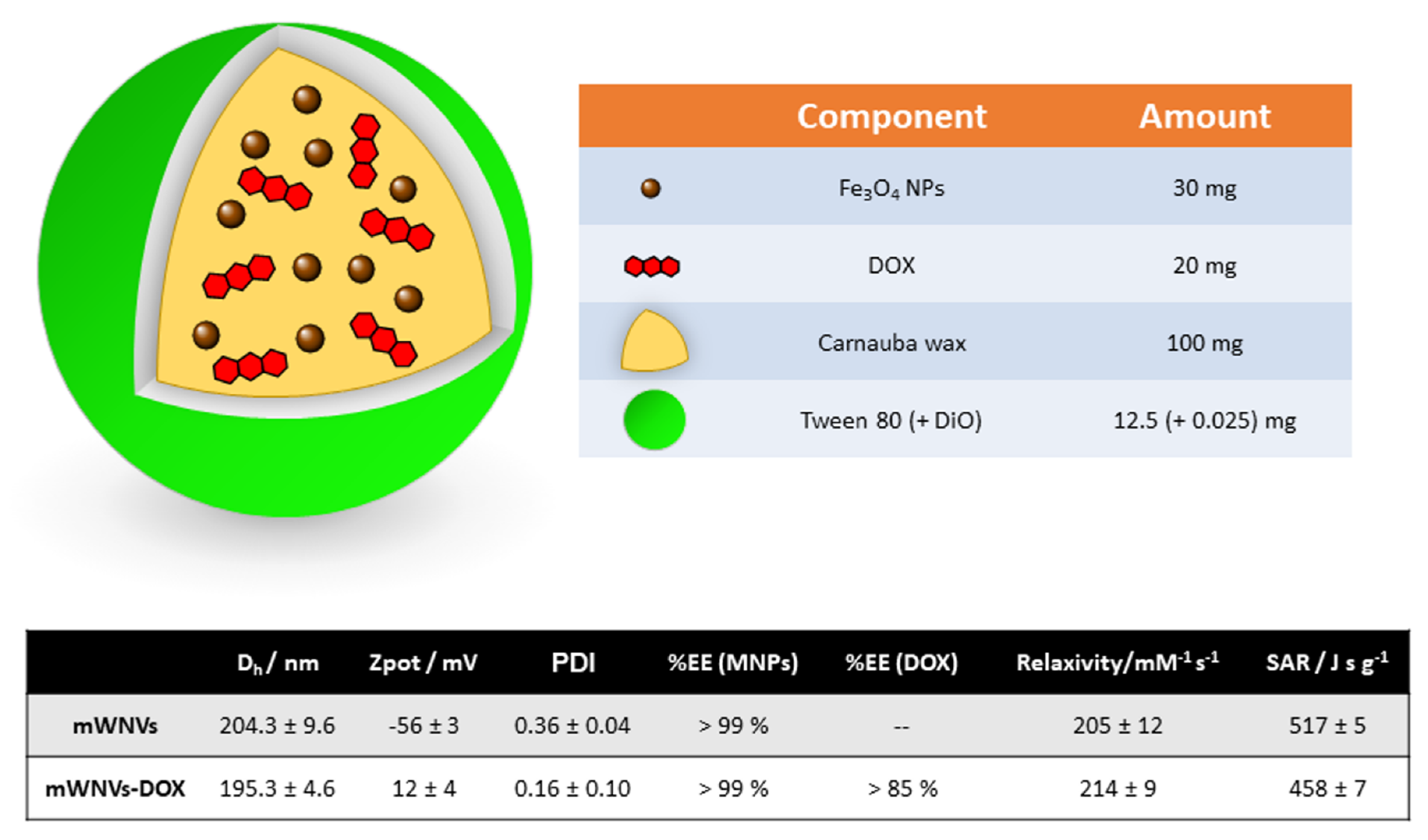
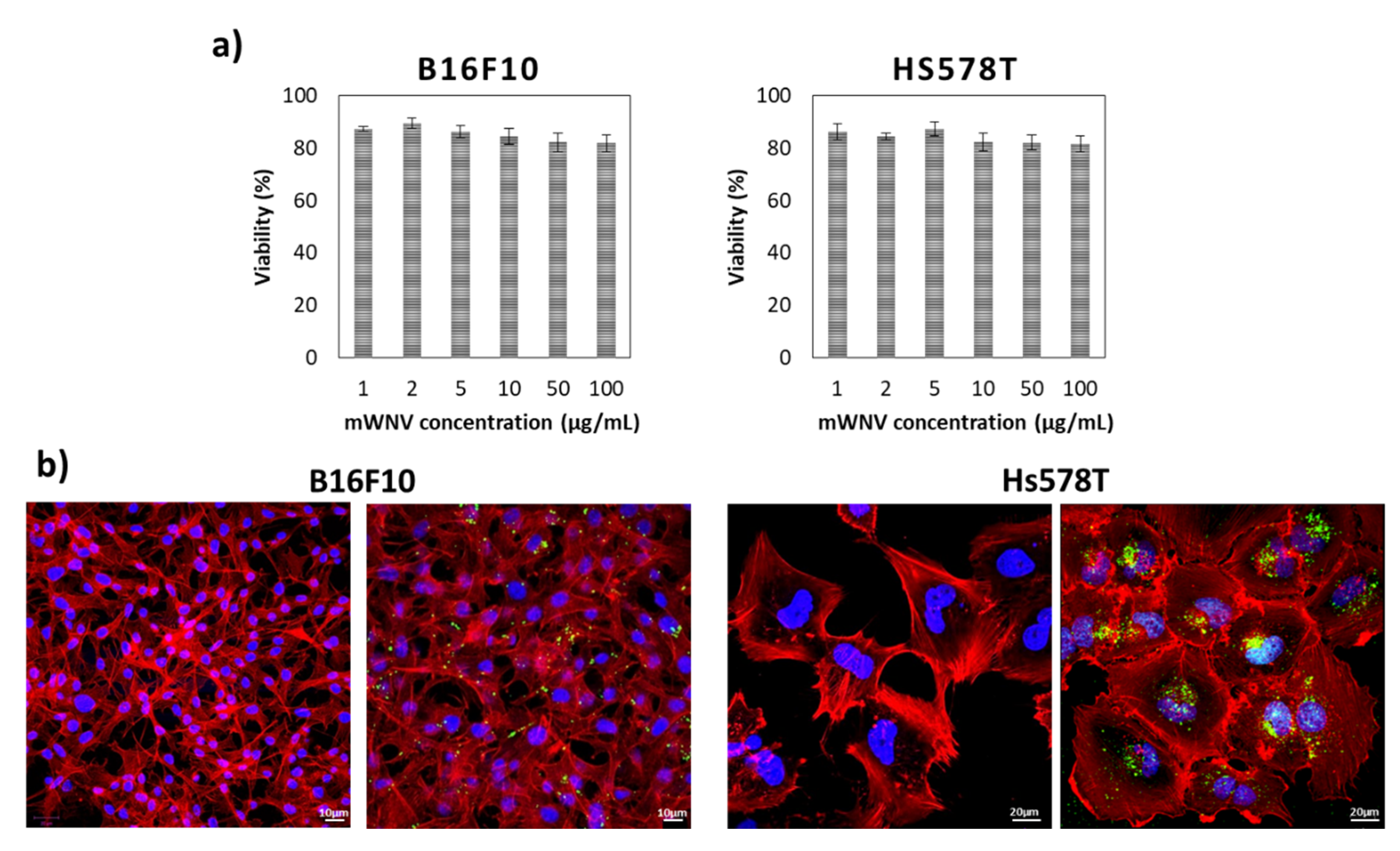
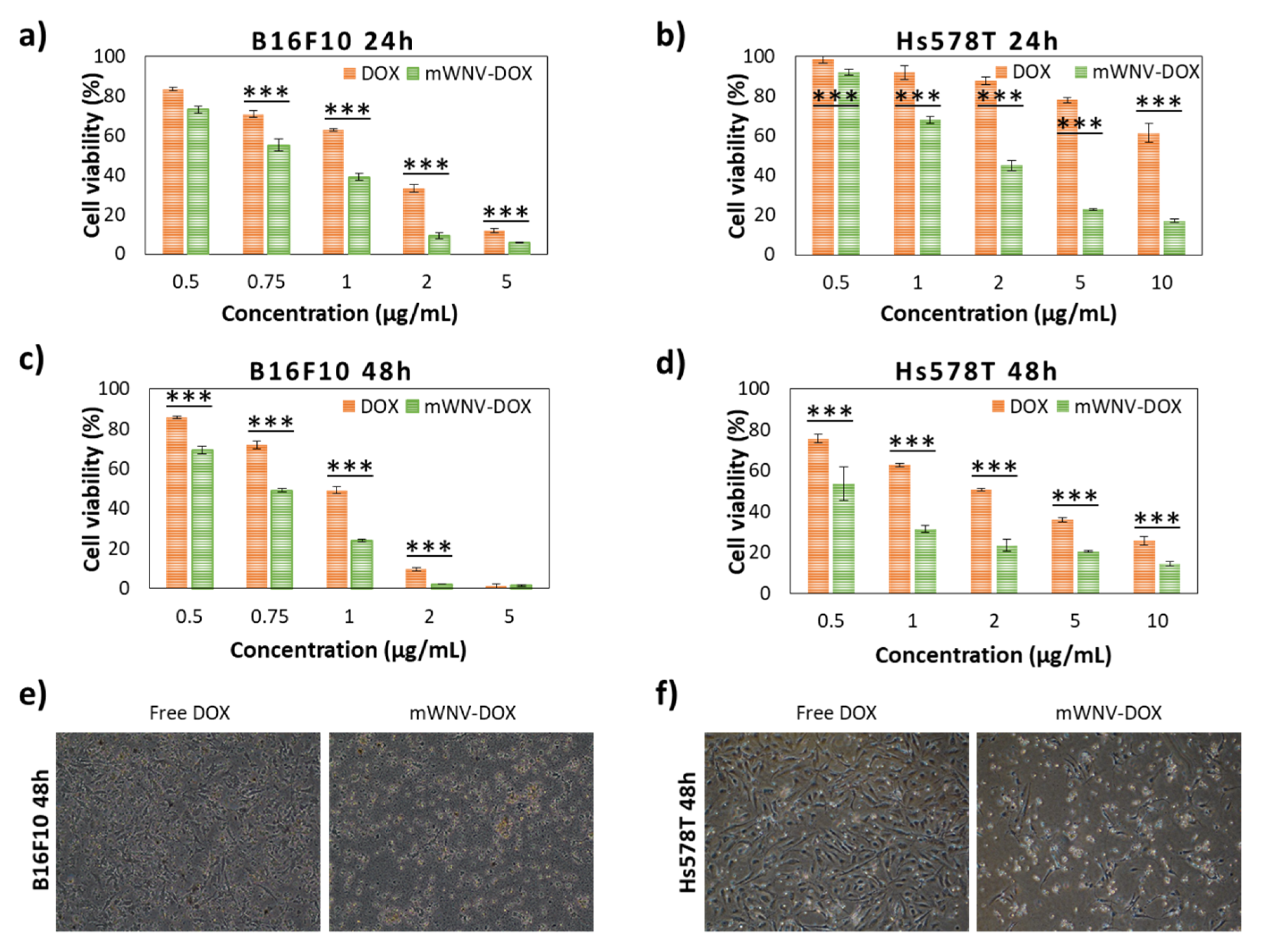
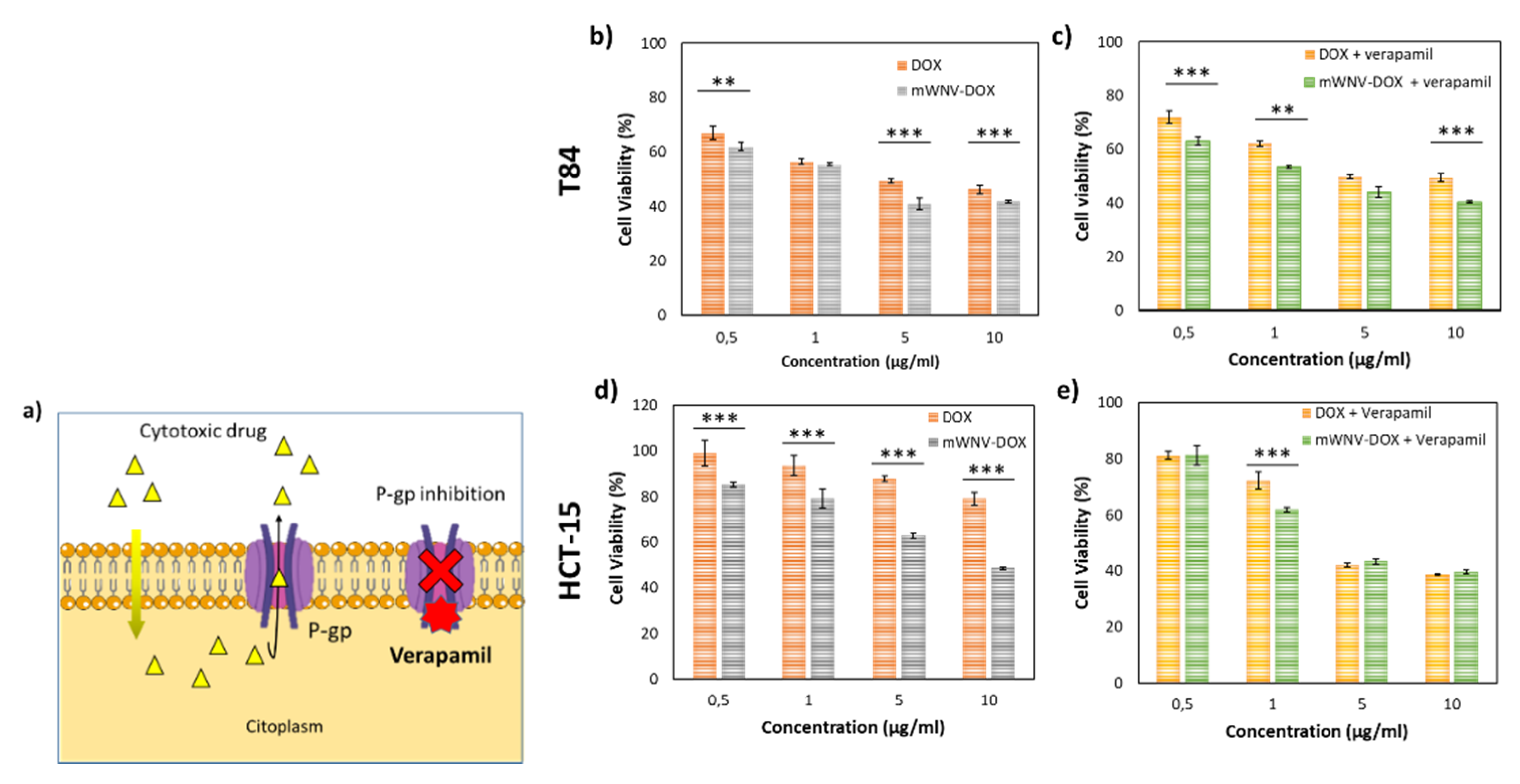


| Cell Culture | Time | Samples | Ic50 | Encapsulation Improvement | Therapeutic Index | |
|---|---|---|---|---|---|---|
| 2D | B16F10 | 24h | DOX | 1.4 µg/mL | 42.9% | 1.75 |
| mWNV-DOX | 0.8 µg/mL | |||||
| 48h | DOX | 1.0 µg/mL | 25% | 1.33 | ||
| mWNV-DOX | 0.75 µg/mL | |||||
| Hs578T | 24h | DOX | 13.4 µg/mL | 86.7% | 7.53 | |
| mWNV-DOX | 1.78 µg/mL | |||||
| 48h | DOX | 2.1 µg/mL | 72.4% | 3.62 | ||
| mWNV-DOX | 0.58 µg/mL | |||||
| T84 | 48h | DOX | 3.7 µg/mL | 45.9% | 1.85 | |
| mWNV-DOX | 2.0 µg/mL | |||||
| DOX + V | 4.9 µg/mL | 69.4% | 3.27 | |||
| mWNV-DOX + V | 1.5 µg/mL | |||||
| HCT15 | 48h | DOX | 56.4 µg/mL | 83.2% | 5.95 | |
| mWNV-DOX | 9.5 µg/mL | |||||
| DOX + V | 1.8 µg/mL | −8.2% | 0.92 | |||
| mWNV-DOX + V | 2.0 µg/mL |
| Cell Culture | Time | Samples | Normalized Final Volume | Encapsulation Improvement | |
|---|---|---|---|---|---|
| 3D | B16F10 | 7d | Vf(DOX/control) | 30.5% | 37.0% |
| Vf(mWNV-DOX+MH/control) | 19.2% | ||||
| Hs578T | 7d | Vf(DOX/control) | 83.8% | 31.0% | |
| Vf(mWNV-DOX+MH/control) | 57.8% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-López, J.; García-Hevia, L.; Melguizo, C.; Prados, J.; Bañobre-López, M.; Gallo, J. Evaluation of Novel Doxorubicin-Loaded Magnetic Wax Nanocomposite Vehicles as Cancer Combinatorial Therapy Agents. Pharmaceutics 2020, 12, 637. https://doi.org/10.3390/pharmaceutics12070637
Jiménez-López J, García-Hevia L, Melguizo C, Prados J, Bañobre-López M, Gallo J. Evaluation of Novel Doxorubicin-Loaded Magnetic Wax Nanocomposite Vehicles as Cancer Combinatorial Therapy Agents. Pharmaceutics. 2020; 12(7):637. https://doi.org/10.3390/pharmaceutics12070637
Chicago/Turabian StyleJiménez-López, Julia, Lorena García-Hevia, Consolación Melguizo, Jose Prados, Manuel Bañobre-López, and Juan Gallo. 2020. "Evaluation of Novel Doxorubicin-Loaded Magnetic Wax Nanocomposite Vehicles as Cancer Combinatorial Therapy Agents" Pharmaceutics 12, no. 7: 637. https://doi.org/10.3390/pharmaceutics12070637
APA StyleJiménez-López, J., García-Hevia, L., Melguizo, C., Prados, J., Bañobre-López, M., & Gallo, J. (2020). Evaluation of Novel Doxorubicin-Loaded Magnetic Wax Nanocomposite Vehicles as Cancer Combinatorial Therapy Agents. Pharmaceutics, 12(7), 637. https://doi.org/10.3390/pharmaceutics12070637






