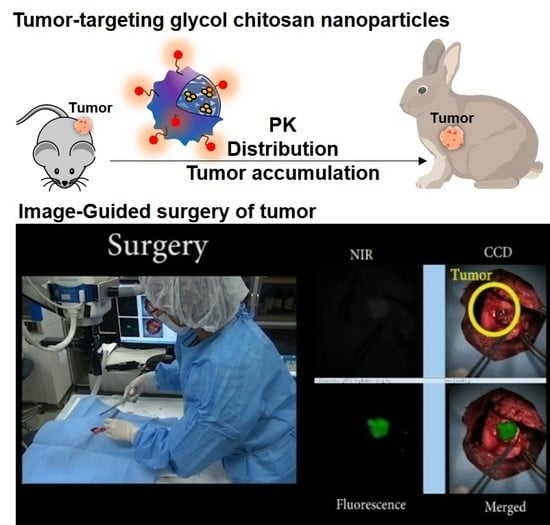
Tumor-Targeting Glycol Chitosan Nanoparticles for Image-Guided Surgery of Rabbit Orthotopic VX2 Lung Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
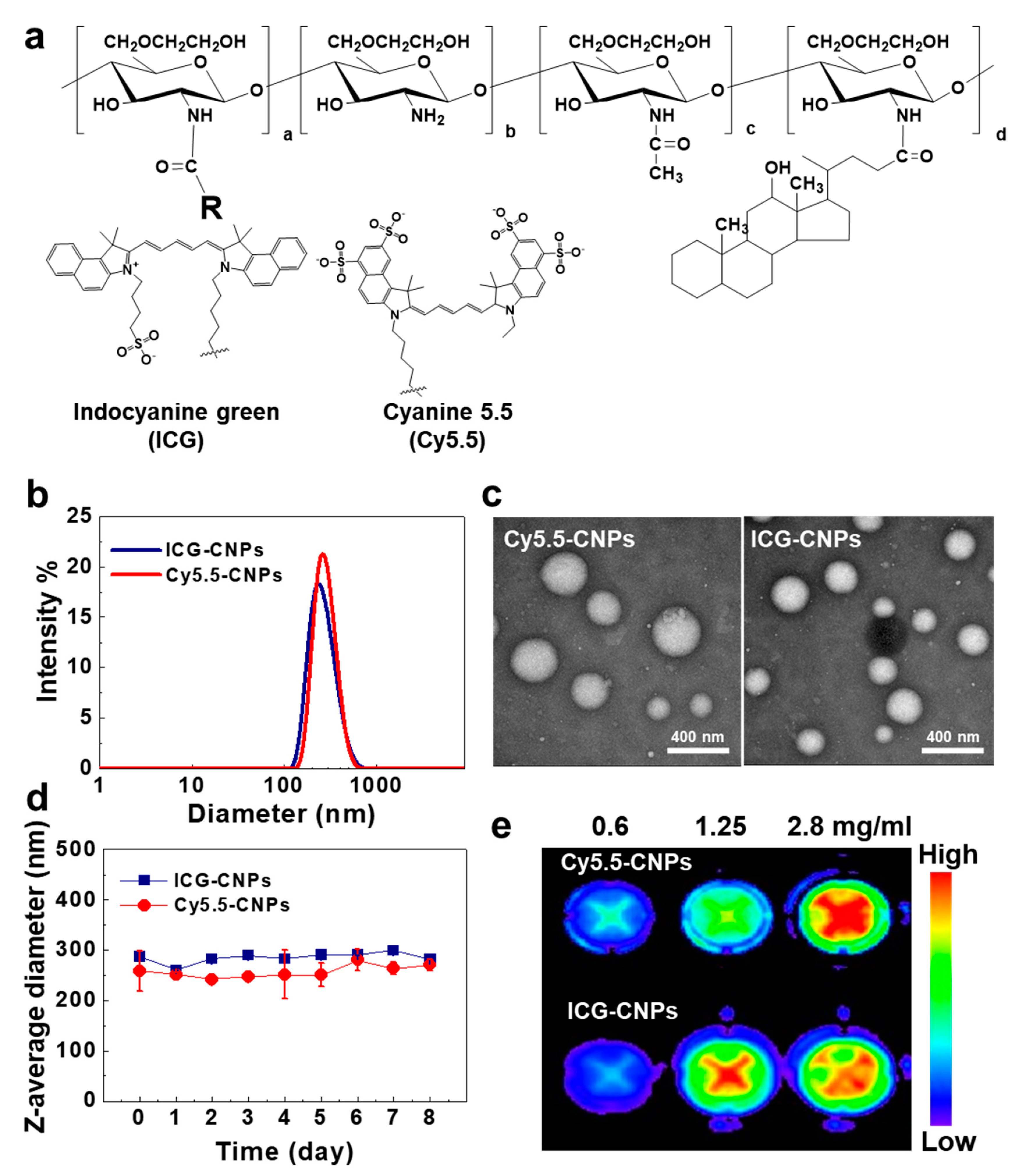
2.2. Preparation and Characterization of Glycol Chitosan Nanoparticles (CNPs)
2.3. Cellular Uptake and Cytotoxicity of CNPs
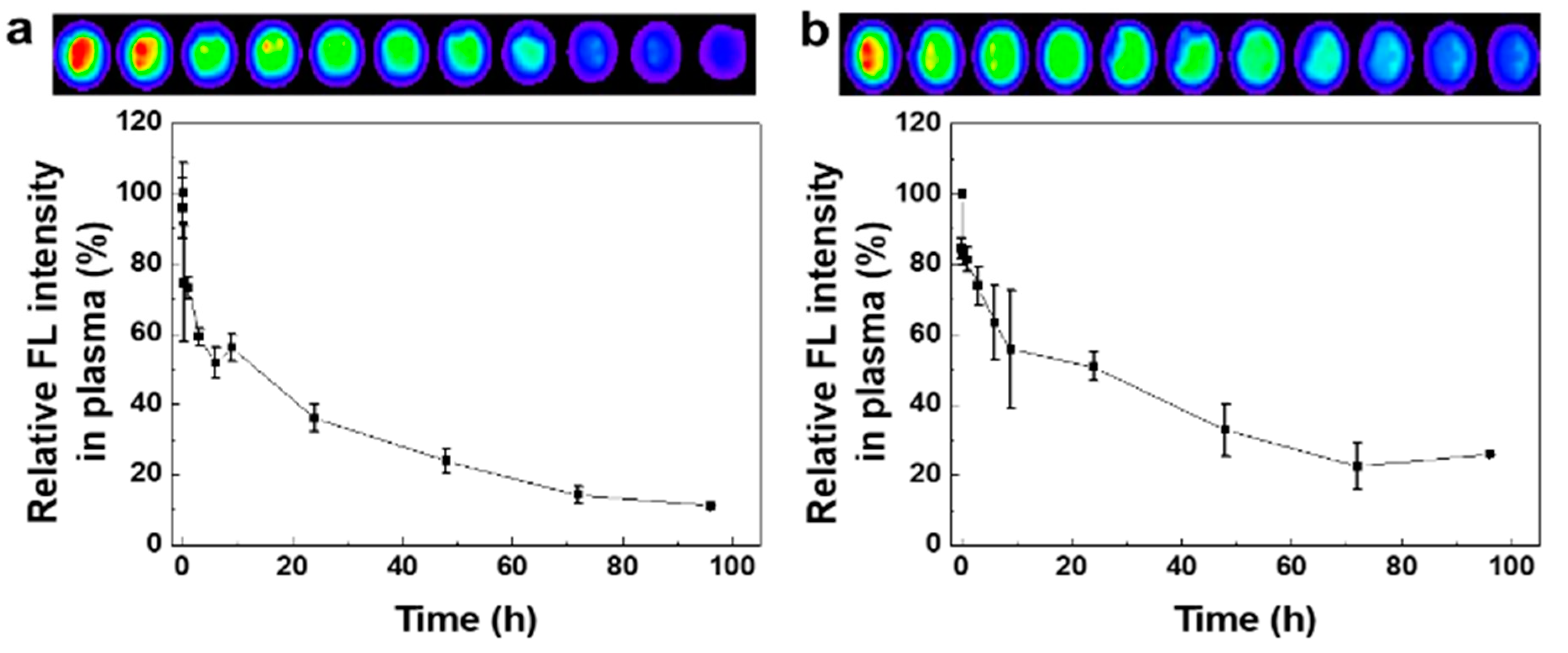
2.4. Pharmacokinetic Analysis of CNPs Using Mouse and Rabbit Blood
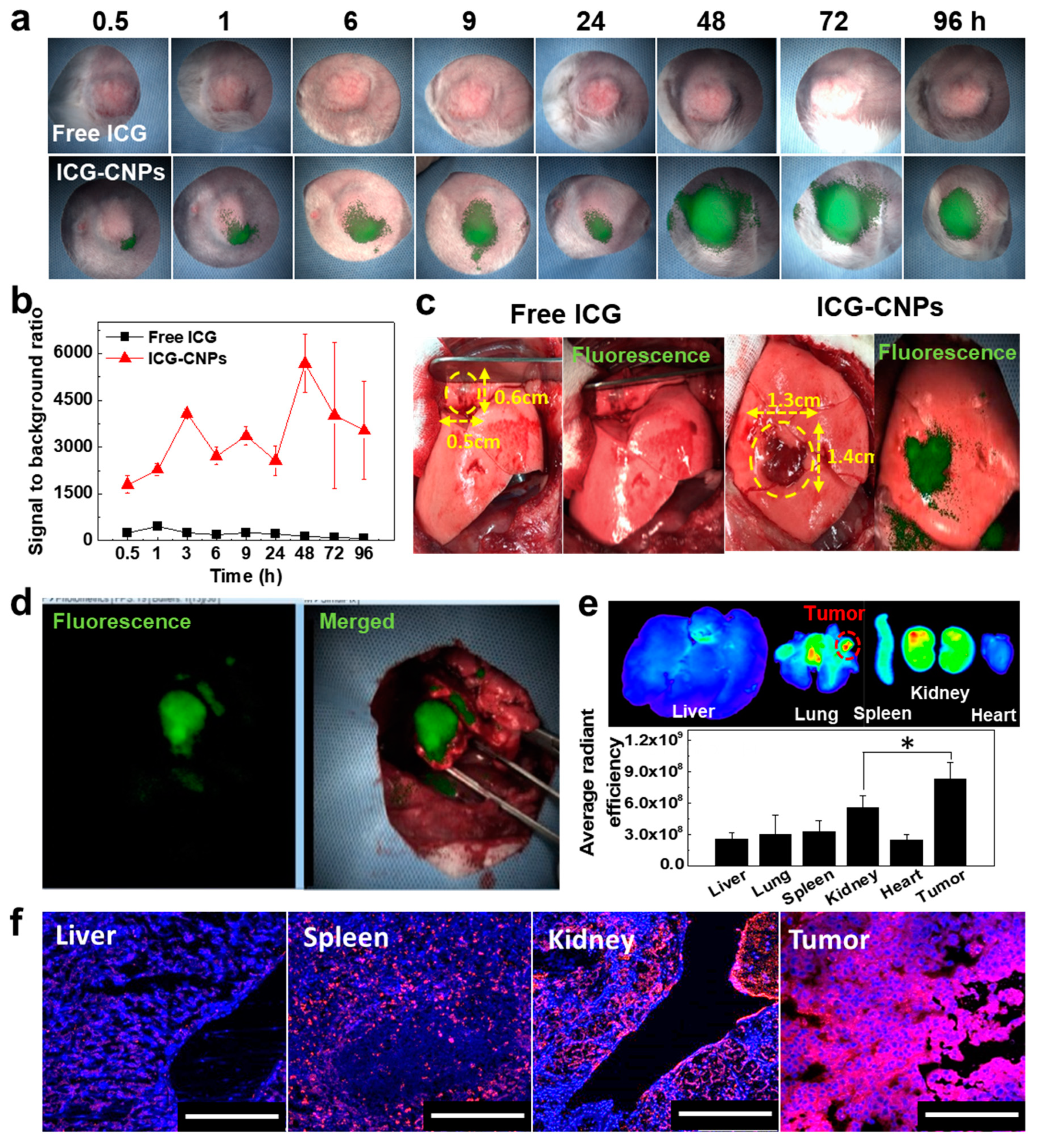
2.5. In Vivo Tumor Accumulation of CNPs in the Mouse and Rabbit VX2 Tumor Models
2.6. Image-Guided Surgery of Rabbit Orthotopic VX2 Lung Tumors after CNP Injection
2.7. Tissue Fluorescence Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Glycol Chitosan Nanoparticles (CNPs)
3.2. Cellular Uptake and Cytotoxicity of CNPs
3.3. Pharmacokinetic Analysis of CNPs Using the Mouse and Rabbit Blood
3.4. In Vivo Tumor Accumulation of CNPs in the Mouse VX2 Tumor Model
3.5. Image-Guided Surgery of Rabbit Orthotopic VX2 Lung Tumor after ICG-CNP Injection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, Y.; Kopecek, J. Biological rationale for the design of polymeric anti-cancer nanomedicines. J. Drug Target. 2013, 21, 1–26. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Jeon, S.; Yoon, H.Y.; Shim, M.K.; Ko, H.; Min, J.; Na, J.H.; Chang, H.; Han, H.; Kim, J.-H.; et al. Effects of tumor microenvironments on targeted delivery of glycol chitosan nanoparticles. J. Control. Release 2017, 267, 223–231. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor targeting via EPR: Strategies to enhance patient responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Kaliraj, K.; Poudel, K.; Jin, S.G.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Polypeptide Derivative of Metformin with the Combined Advantage of a Gene Carrier and Anticancer Activity. ACS. Biomater. Sci. Eng. 2019, 5, 5159–5168. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Gioux, S.; Choi, H.S.; Frangioni, J.V. Image-guided surgery using invisible near-infrared light: Fundamentals of clinical translation. Mol. Imaging 2010, 9, 237–255. [Google Scholar] [CrossRef]
- Weissleder, R.; Tung, C.H.; Mahmood, U.; Bogdanov, A., Jr. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999, 17, 375. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, J.H.; Park, K.; Lee, A.; Lee, S.-Y.; Youn, I.-C.; Ahn, C.-H.; Yoon, S.M.; Myung, S.-J.; Moon, D.H.; et al. Polymeric nanoparticle-based activatable near-infrared nanosensor for protease determination in vivo. Nano Lett. 2009, 9, 4412–4416. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Na, J.H.; Ko, H.K.; You, D.G.; Park, S.; Jun, E.; Yeom, H.J.; Seo, D.H.; Park, J.H.; Jeong, S.Y.; et al. Non-invasive optical imaging of cathepsin B with activatable fluorogenic nanoprobes in various metastatic models. Biomaterials 2014, 35, 2302–2311. [Google Scholar] [CrossRef]
- Crane, L.M.A.; Themelis, G.; Arts, H.J.G.; Buddingh, K.T.; Brouwers, A.H.; Ntziachristos, V.; van Dam, G.M.; Van Der Zee, A.G.J. Intraoperative near-infrared fluorescence imaging for sentinel lymph node detection in vulvar cancer: First clinical results. Gynecol. Oncol. 2011, 120, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, O.R.; Klop, W.M.C.; Buckle, T.; Vermeeren, L.; van den Brekel, M.W.M.; Balm, A.J.M.; Nieweg, O.E.; Olmos, R.A.V.; van Leeuwen, F.W.B. Feasibility of sentinel node biopsy in head and neck melanoma using a hybrid radioactive and fluorescent tracer. Ann. Surg. Oncol. 2012, 19, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Lee, Y.-S.; Quan, Y.H.; Choi, Y.; Jeong, J.M.; Kim, B.-M.; Kim, H.K. Thoracoscopic color and fluorescence imaging system for sentinel lymph node mapping in porcine lung using indocyanine green-neomannosyl human serum albumin: Intraoperative image-guided sentinel nodes navigation. Ann. Surg. Oncol. 2014, 21, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Vuijk, F.A.; Hilling, D.E.; Mieog, J.S.D.; Vahrmeijer, A.L. Fluorescent-guided surgery for sentinel lymph node detection in gastric cancer and carcinoembryonic antigen targeted fluorescent-guided surgery in colorectal and pancreatic cancer. Ann. Surg. Oncol. 2018, 118, 315–323. [Google Scholar] [CrossRef]
- Abran, M.; Stähli, B.E.; Merlet, N.; Mihalache-Avram, T.; Mecteau, M.; Rhéaume, E.; Busseuil, D.; Tardif, J.-C.; Lesage, F. Validating a bimodal intravascular ultrasound (IVUS) and near-infrared fluorescence (NIRF) catheter for atherosclerotic plaque detection in rabbits. Biomed. Opt. Express 2015, 6, 3989–3999. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kim, J.-H.; Choi, K.Y.; Yoon, H.Y.; Kim, K.; Kwon, I.C.; Choi, K.; Lee, B.-H.; Park, J.H.; Kim, I.-S. Hyaluronic acid nanoparticles for active targeting atherosclerosis. Biomaterials 2015, 53, 341–348. [Google Scholar] [CrossRef]
- Raymond, S.B.; Skoch, J.; Hills, I.D.; Nesterov, E.E.; Swager, T.M.; Bacskai, B.J. Smart optical probes for near-infrared fluorescence imaging of Alzheimer’s disease pathology. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 93–98. [Google Scholar] [CrossRef]
- Wunder, A.; Klohs, J.; Dirnagl, U. Non-invasive visualization of CNS inflammation with nuclear and optical imaging. Neuroscience 2009, 158, 1161–1173. [Google Scholar] [CrossRef]
- Tanaka, E.; Choi, H.S.; Fujii, H.; Bawendi, M.G.; Frangioni, J.V. Image-guided oncologic surgery using invisible light: Completed pre-clinical development for sentinel lymph node mapping. Ann. Surg. Oncol. 2006, 13, 1671–1681. [Google Scholar] [CrossRef]
- Hirche, C.; Engel, H.; Kolios, L.; Cognie, J.; Hünerbein, M.; Lehnhardt, M.; Kremer, T. An experimental study to evaluate the Fluobeam 800 imaging system for fluorescence-guided lymphatic imaging and sentinel node biopsy. Surg. Innov. 2013, 20, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Tobis, S.; Knopf, J.; Silvers, C.; Yao, J.; Rashid, H.; Wu, G.; Golijanin, D. Near infrared fluorescence imaging with robotic assisted laparoscopic partial nephrectomy: Initial clinical experience for renal cortical tumors. J. Urol. 2011, 186, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Al-Taher, M.; van den Bos, J.; Schols, R.M.; Bouvy, N.D.; Stassen, L.P. Fluorescence ureteral visualization in human laparoscopic colorectal surgery using methylene blue. J. Laparoendosc. Adv. Surg. Tech. 2016, 26, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Choi, Y.H. The feasibility of single-incision video-assisted thoracoscopic major pulmonary resection performed by surgeons experienced with a two-incision technique. Interact. Cardiovasc. Thorac. Surg. 2014, 20, 310–315. [Google Scholar] [CrossRef][Green Version]
- Vahrmeijer, A.L.; Hutteman, M.; van der Vorst, J.R.; van de Velde, C.J.H.; Frangioni, J.V. Image-guided cancer surgery using near-infrared fluorescence. Nat. Rev. Clin. Oncol. 2013, 10, 507. [Google Scholar] [CrossRef]
- Owens, E.A.; Lee, S.; Choi, J.; Henary, M.; Choi, H.S. NIR fluorescent small molecules for intraoperative imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 828–838. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, G.; Hong, G.H.; Choi, J.; Choi, H.S. Design considerations for targeted optical contrast agents. Quant. Imag. Med. Surg. 2012, 2, 266. [Google Scholar]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Choi, Y.; Lim, S.; Yoon, H.Y.; Kim, B.S.; Kwon, I.C.; Kim, K. Tumor-targeting glycol chitosan nanocarriers: Overcoming the challenges posed by chemotherapeutics. Expert Opin. Drug Deliv. 2019, 16, 835–846. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Son, S.; Kim, S.H.; Park, K.; Choi, K.; Kwon, I.C. Self-assembled glycol chitosan nanoparticles for disease-specific theranostics. J. Control. Release 2014, 193, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Son, S.; Lee, S.J.; You, D.G.; Yhee, J.Y.; Park, J.H.; Swierczewska, M.; Lee, S.; Kwon, I.C.; Kim, S.H. Glycol chitosan nanoparticles as specialized cancer therapeutic vehicles: Sequential delivery of doxorubicin and Bcl-2 siRNA. Sci. Rep. 2014, 4, 6878. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Shin, M.L.; Shim, M.K.; Lee, S.; Na, J.H.; Koo, H.; Lee, H.; Kim, J.-H.; Lee, K.Y.; Kim, K. Artificial chemical reporter targeting strategy using bioorthogonal click reaction for improving active-targeting efficiency of tumor. Mol. Pharm. 2017, 14, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Quan, Y.H.; Kim, M.; Kim, B.-M.; Kim, H.K. Intraoperative fluorescence image-guided pulmonary segmentectomy. J. Surg. Res. 2015, 199, 287–293. [Google Scholar] [CrossRef]
- Choi, B.H.; Young, H.S.; Quan, Y.H.; Rho, J.; Eo, J.S.; Han, K.N.; Choi, Y.H.; Hyun Koo, K. Real-time computed tomography fluoroscopy-guided solitary lung tumor model in a rabbit. PLoS ONE 2017, 12, e0179220. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Park, J.H.; Chung, H.; Kwon, I.C.; Jeong, S.Y.; Kim, I.-S. Physicochemical Characteristics of Self-Assembled Nanoparticles Based on Glycol Chitosan Bearing 5β-Cholanic Acid. Langmuir 2003, 19, 10188–10193. [Google Scholar] [CrossRef]
- Oshiro, H.; Fukumura, H.; Nagahama, K.; Sato, I.; Sugiura, K.; Iobe, H.; Okiyama, E.; Nagao, T.; Nagashima, Y.; Aoki, I.; et al. Establishment of successively transplantable rabbit VX2 cancer cells that express enhanced green fluorescent protein. Med. Mol. Morphol. 2015, 48, 13–23. [Google Scholar] [CrossRef]
- Yhee, J.Y.; Yoon, H.Y.; Kim, H.; Jeon, S.; Hergert, P.; Im, J.; Panyam, J.; Kim, K.; Nho, R.S. The effects of collagen-rich extracellular matrix on the intracellular delivery of glycol chitosan nanoparticles in human lung fibroblasts. Int. J. Nanomed. 2017, 12, 6089–6105. [Google Scholar] [CrossRef]
- Nam, H.Y.; Kwon, S.M.; Chung, H.; Lee, S.Y.; Kwon, S.H.; Jeon, H.; Kim, Y.; Park, J.H.; Kim, J.; Her, S.; et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J. Control. Release 2009, 135, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Bugno, J.; Lantvit, D.; Burdette, J.E.; Hong, S. Prolonged blood circulation and enhanced tumor accumulation of folate-targeted dendrimer-polymer hybrid nanoparticles. J. Control. Release 2014, 191, 115–122. [Google Scholar] [CrossRef]
- Harashima, H.; Komatsu, S.; Kojima, S.; Yanagi, C.; Morioka, Y.; Naito, M.; Kiwada, H. Species difference in the disposition of liposomes among mice, rats, and rabbits: Allometric relationship and species dependent hepatic uptake mechanism. Pharm. Res. 1996, 13, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Vinegoni, C.; Botnaru, I.; Aikawa, E.; Calfon, M.A.; Iwamoto, Y.; Folco, E.J.; Ntziachristos, V.; Weissleder, R.; Libby, P.; Jaffer, F.A. Indocyanine green enables near-infrared fluorescence imaging of lipid-rich, inflamed atherosclerotic plaques. Sci. Transl. Med. 2011, 3, 84ra45. [Google Scholar] [CrossRef] [PubMed]
- Sarko, D.; Georges, R. Kidney-specific drug delivery: Review of opportunities, achievements, and challenges. J. Anal. Pharm. Res. 2016, 2, 33–38. [Google Scholar] [CrossRef]
- Kim, C.S.; Mathew, A.P.; Uthaman, S.; Moon, M.J.; Bae, E.H.; Kim, S.W.; Park, I.-K. Glycol chitosan-based renal docking biopolymeric nanomicelles for site-specific delivery of the immunosuppressant. Carbohydr. Polym. 2020. [Google Scholar] [CrossRef]
- Lee, D.-E.; Na, J.H.; Lee, S.; Kang, C.M.; Kim, H.N.; Han, S.J.; Kim, H.; Choe, Y.S.; Jung, K.-H.; Lee, K.C. Facile method to radiolabel glycol chitosan nanoparticles with 64Cu via copper-free click chemistry for MicroPET imaging. Mol. Pharm. 2013, 10, 2190–2198. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
On, K.C.; Rho, J.; Yoon, H.Y.; Chang, H.; Yhee, J.Y.; Yoon, J.S.; Jeong, S.Y.; Kim, H.K.; Kim, K. Tumor-Targeting Glycol Chitosan Nanoparticles for Image-Guided Surgery of Rabbit Orthotopic VX2 Lung Cancer. Pharmaceutics 2020, 12, 621. https://doi.org/10.3390/pharmaceutics12070621
On KC, Rho J, Yoon HY, Chang H, Yhee JY, Yoon JS, Jeong SY, Kim HK, Kim K. Tumor-Targeting Glycol Chitosan Nanoparticles for Image-Guided Surgery of Rabbit Orthotopic VX2 Lung Cancer. Pharmaceutics. 2020; 12(7):621. https://doi.org/10.3390/pharmaceutics12070621
Chicago/Turabian StyleOn, Kyeong Cheol, Jiyun Rho, Hong Yeol Yoon, Hyeyoun Chang, Ji Young Yhee, Jun Sik Yoon, Seo Young Jeong, Hyun Koo Kim, and Kwangmeyung Kim. 2020. "Tumor-Targeting Glycol Chitosan Nanoparticles for Image-Guided Surgery of Rabbit Orthotopic VX2 Lung Cancer" Pharmaceutics 12, no. 7: 621. https://doi.org/10.3390/pharmaceutics12070621
APA StyleOn, K. C., Rho, J., Yoon, H. Y., Chang, H., Yhee, J. Y., Yoon, J. S., Jeong, S. Y., Kim, H. K., & Kim, K. (2020). Tumor-Targeting Glycol Chitosan Nanoparticles for Image-Guided Surgery of Rabbit Orthotopic VX2 Lung Cancer. Pharmaceutics, 12(7), 621. https://doi.org/10.3390/pharmaceutics12070621