Hydrogel Delivery System Containing Calendulae flos Lyophilized Extract with Chitosan as a Supporting Strategy for Wound Healing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation and Analysis of Calendulae flos Lyophilized Extract
2.2.1. Extract Preparation
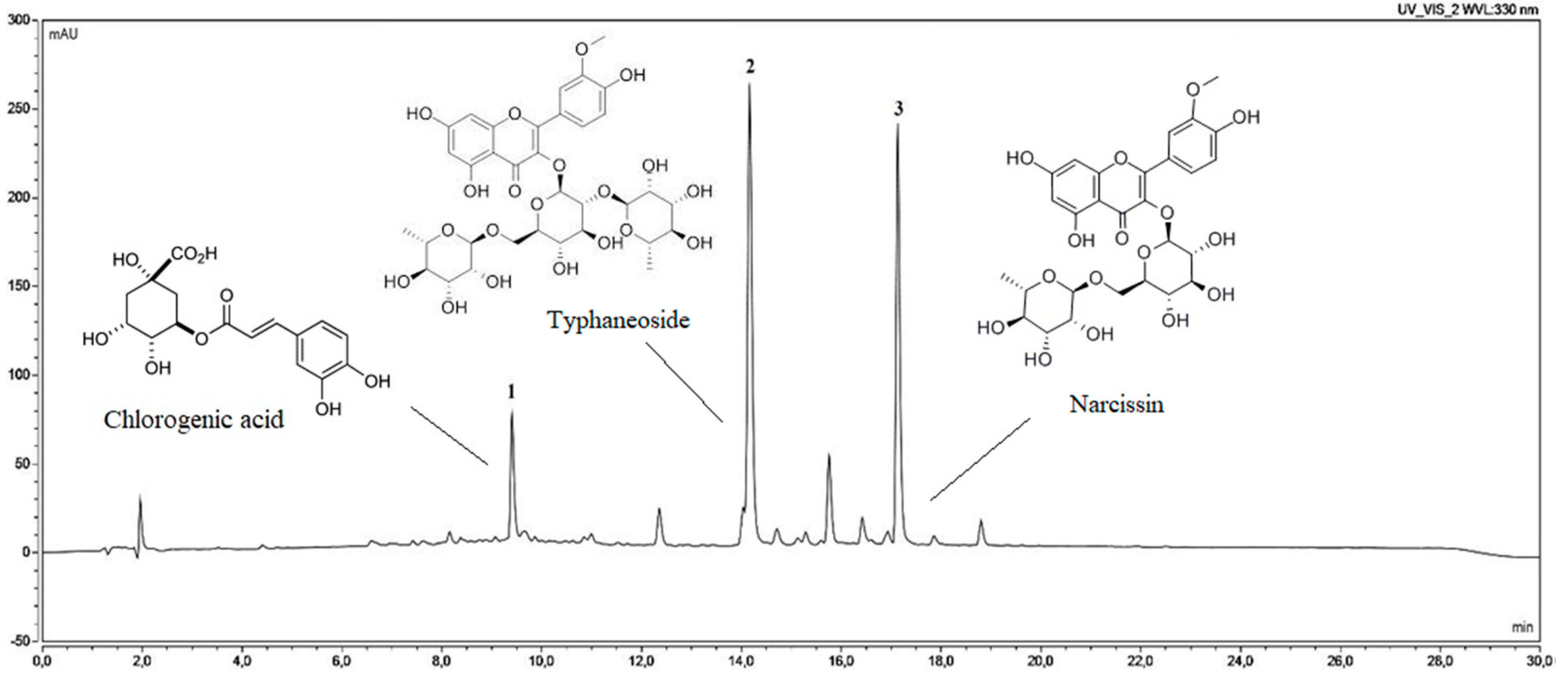
2.2.2. Determination of Chlorogenic Acid and Narcissin Content
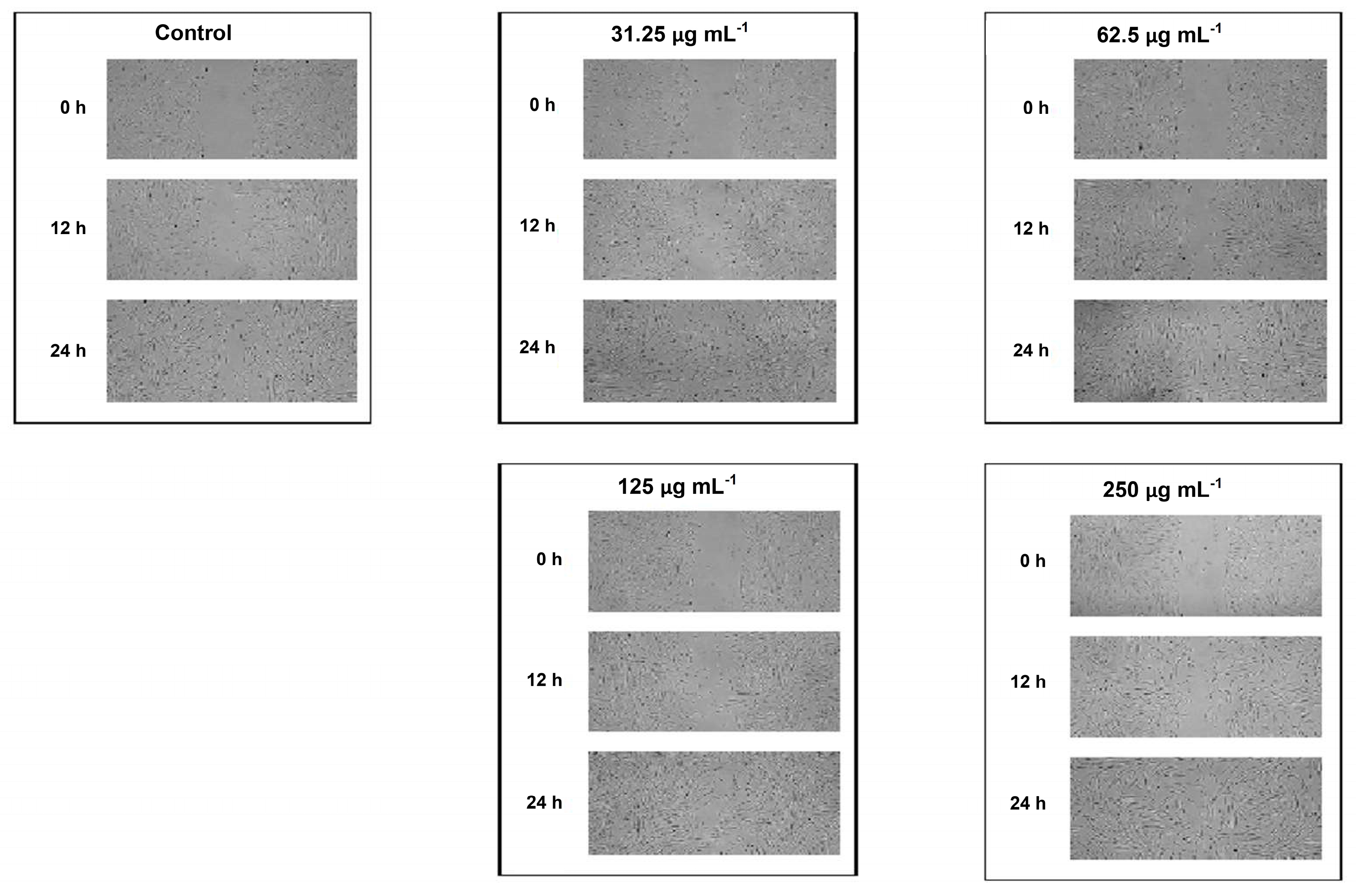
2.2.3. Scratch Wound Healing Assay
2.3. Preparation, Characterization, and Biological Activity of Chitosan Delivery System with Calendulae flos Lyophilized Extract
2.3.1. Chitosan Binary System
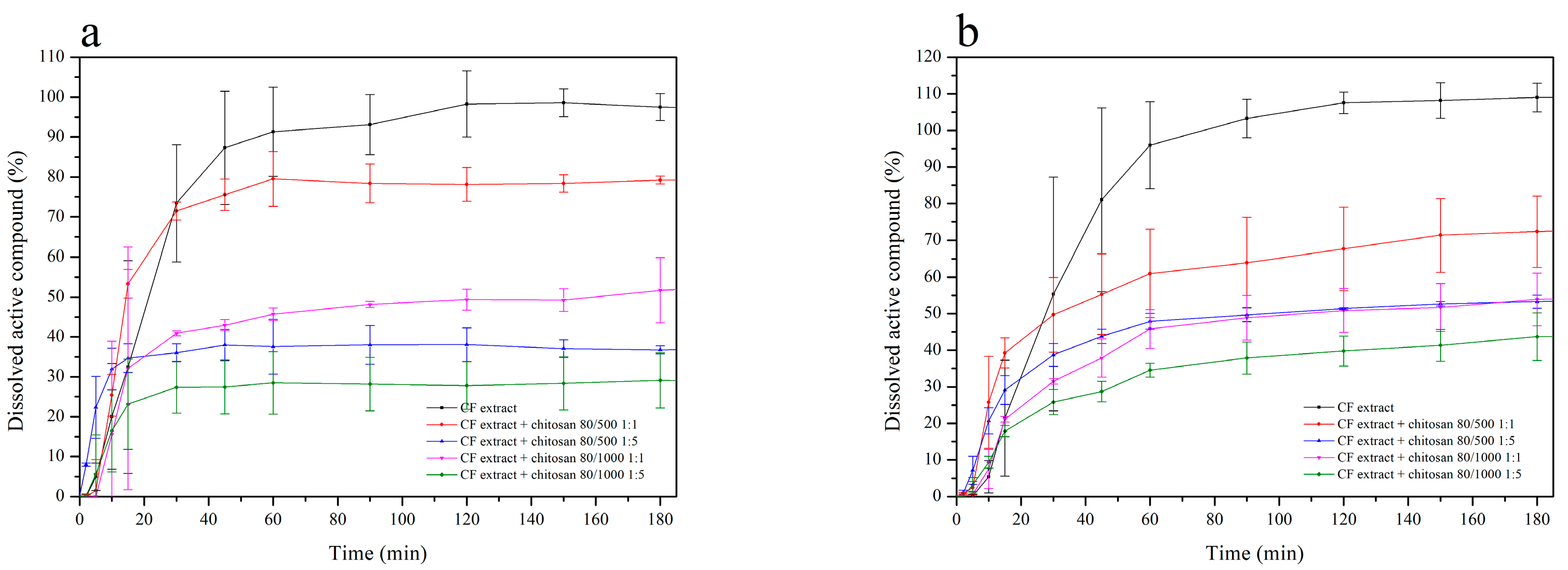
2.3.2. Dissolution Studies of Chitosan Delivery Systems
2.3.3. Permeability Studies of Chitosan Delivery Systems
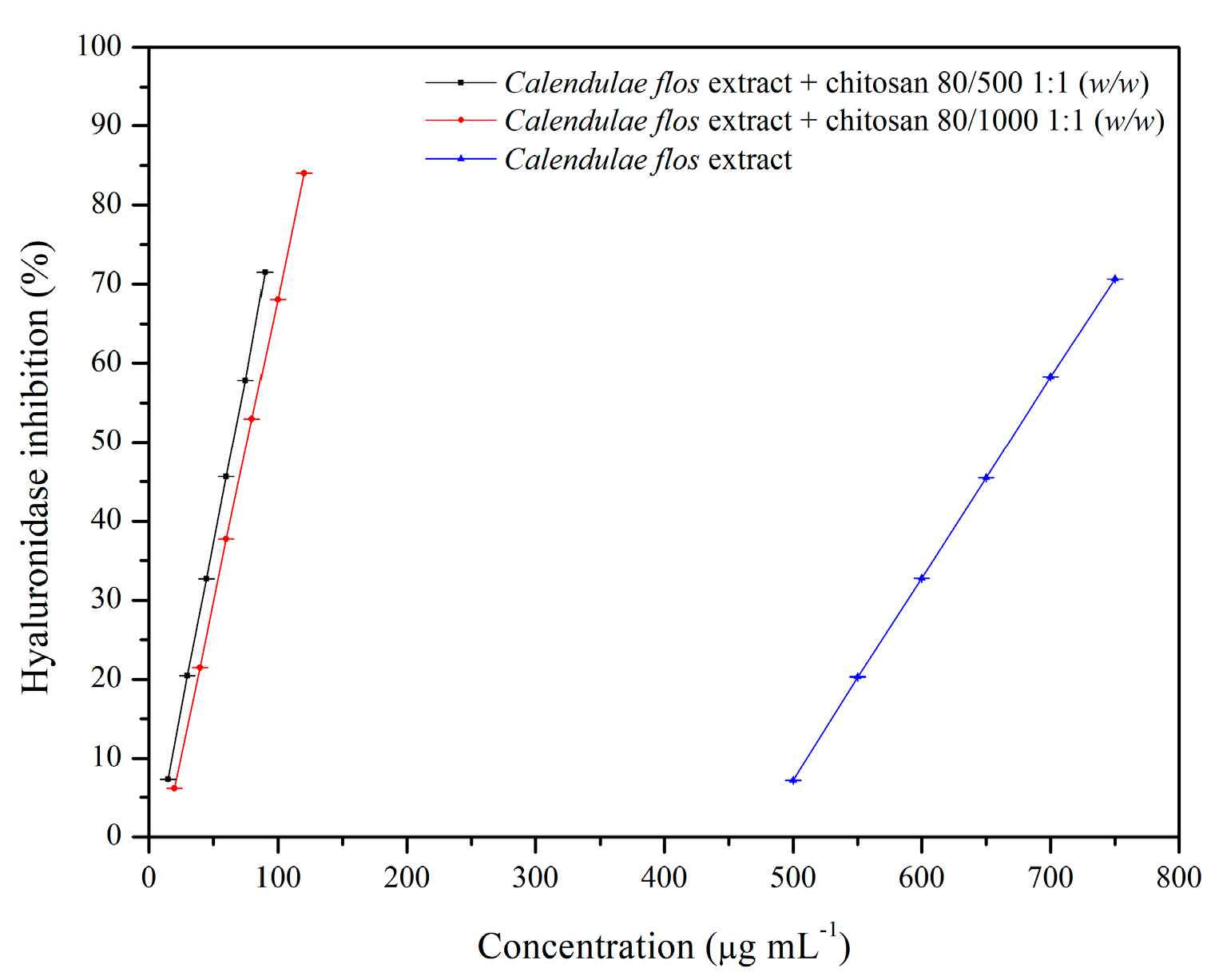
2.3.4. Anti-Hyaluronidase Activity
2.3.5. Antimicrobial Activity
2.4. Formulation of the Hydrogel Topical Pharmaceutical Dosage Containing Chitosan Delivery System with Calendulae flos Lyophilized Extract
2.4.1. Microscopic Structure of Hydrogels by Scanning Electron Microscopy (SEM)
2.4.2. Rheological Analysis
2.4.3. Drug Release Profiles
2.4.4. Antimicrobial Activity
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Teplicki, E.; Ma, Q.; Castillo, D.E.; Zarei, M.; Hustad, A.P.; Chen, J.; Li, J. The Effects of Aloe vera on Wound Healing in Cell Proliferation, Migration, and Viability. Wounds 2018, 30, 263–268. [Google Scholar]
- Espiritu, A.A.; Lao, S.N.; Guerrero, J.J. Burn wound healing potential of Bixa orellana Linn [Bixaceae] leaf extracts on albino mice. J. Med. Plants Stud. 2016, 4, 84–87. [Google Scholar]
- Alhashim, M.; Lombardo, J. Mechanism of Action of Topical Garlic on Wound Healing. Dermatol. Surg. 2018, 44, 630–634. [Google Scholar] [CrossRef]
- Medical Economics Company. PDR for Herbal Medicines, 2nd ed.; Medical Economics Company: Montvale, NJ, USA, 2000; pp. 497–499. [Google Scholar]
- European Medicines Agency (EMA). Assessment Report on Calendula Officinalis L., Flower. 2018. Available online: https://www.ema.europa.eu/en/medicines/herbal/calendulae-flos (accessed on 17 May 2020).
- Della Loggia, R.; Tubaro, A.; Sosa, S.; Becker, H.; Saar, S.; Isaac, O. The role of triterpenoids in the topical anti-inflammatory activity of Calendula officinalis flowers. Planta Medica 1994, 60, 516–520. [Google Scholar] [CrossRef]
- Zitterl-Eglseer, K.; Sosa, S.; Jurenitsch, J.; Schubert-Zsilavecz, M.; Della Loggia, R.; Tubaro, A.; Bertoldi, M.; Franz, C. Anti-oedematous activities of the main triterpendiol esters of marigold (Calendula officinalis L.). J. Ethnopharmacol. 1997, 57, 139–144. [Google Scholar] [CrossRef]
- Hamburger, M.; Adler, S.; Baumann, D.; Förg, A.; Weinreich, B. Preparative purification of the major anti-inflammatory triterpenoid esters from Marigold (Calendula officinalis). Fitoterapia 2003, 74, 328–338. [Google Scholar] [CrossRef]
- Nicolaus, C.; Junghanns, S.; Murillo, R.; Merfort, I. Triterpene alcohols from Calendula officinalis L. flowers and in vitro studies on their wound healing activity. Planta Med. 2014, 80, P2B63. [Google Scholar] [CrossRef]
- Patrick, K.F.; Kumar, S.; Edwardson, P.A.; Hutchinson, J.J. Induction of vascularisation by an aqueous extract of the flowers of Calendula officinalis L. the European marigold. Phytomedicine 1996, 3, 11–18. [Google Scholar] [CrossRef]
- Dinda, M.; Mazumdar, S.; Das, S.; Ganguly, D.; Dasgupta, U.B.; Dutta, A.; Jana, K.; Karmakar, P. The Water Fraction of Calendula officinalis Hydroethanol Extract Stimulates In Vitro and In Vivo Proliferation of Dermal Fibroblasts in Wound Healing. Phytother. Res. 2016, 30, 1696–1707. [Google Scholar] [CrossRef]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef]
- Preethi, K.C.; Kuttan, R. Wound healing activity of flower extract of Calendula officinalis. J. Basic Clin. Physiol. Pharmacol. 2009, 20, 73–79. [Google Scholar] [CrossRef]
- Parente, L.M.; Rde, S.L.J.; Tresvenzol, L.M.; Vinaud, M.C.; de Paula, J.R.; Paulo, N.M. Wound healing and anti-inflammatory effect in animal models of Calendula officinalis L. growing in Brazil. Evid. Based Complement. Alternat. Med. 2012, 2012, 375671. [Google Scholar] [CrossRef]
- Efstratiou, E.; Hussain, A.I.; Nigam, P.S.; Moore, J.E.; Ayub, M.A.; Rao, J.R. Antimicrobial activity of Calendula officinalis petal extracts against fungi, as well as Gram-negative and Gram-positive clinical pathogens. Complement. Ther. Clin. Pract. 2012, 18, 173–176. [Google Scholar] [CrossRef]
- El-Ganiny, A.M.; Shaker, G.H.; Aboelazm, A.A.; El-Dash, H.A. Prevention of Bacterial Biofilm Formation on Soft Contact Lenses Using Natural Compounds. J. Ophthalmic Inflamm. Infect. 2017, 7, 11. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.P.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the wound healing effect of Calendula extracts using the scratch assay with 3T3 fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef]
- Hormozi, M.; Gholami, M.; Babaniazi, A.; Gharravi, A.M. Calendula officinalis stimulate proliferation of mouse embryonic fibroblasts via expression of growth factors TGFβ1 and bFGF. Inflamm. Regen. 2019, 39, 7. [Google Scholar] [CrossRef]
- Jahdi, F.; Khabbaz, A.H.; Kashian, M.; Taghizadeh, M.; Haghani, H. The impact of calendula ointment on cesarean wound healing: A randomized controlled clinical trial. J. Fam. Med. Prim. Care 2018, 7, 893–897. [Google Scholar] [CrossRef]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A systematic review of Calendula officinalis extract for wound healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef]
- Mati-Baouche, N.; Elchinger, P.H.; de Baynast, H.; Pierre, G.; Delattre, C.; Michaud, P. Chitosan as an adhesive. Eur. Polym. J. 2014, 60, 198–212. [Google Scholar] [CrossRef]
- Grabovac, V.; Guggi, D.; Bernkop-Schnürch, A. Comparison of the Mucoadhesive Properties of Various Polymers. Adv. Drug Deliv. Rev. 2005, 57, 1713–1723. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros Ciência Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Menda, J.P.; Reddy, T.; Deepika, R.; Devi, P.M.; Sastry, T.P. Preparation and Characterization of Wound Healing Composites of Chitosan, Aloe Vera and Calendula officinalis a Comparative Study. AJPCT 2014, 2, 61–76. [Google Scholar]
- Assainar, S.K.; Nair, S.P. Action of Chitosan and its derivatives on clinical pathogens. Int. J. Curr. Microbiol. App. Sci 2014, 3, 748–759. [Google Scholar]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Yadav, T.C.; Srivastava, A.K.; Raghuwanshi, N.; Kumar, N.; Prasad, R.; Pruthi, V. Wound Healing Potential of Natural Polymer: Chitosan “A Wonder Molecule”. In Integrating Green Chemistry and Sustainable Engineering; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 527–579. [Google Scholar] [CrossRef]
- Okamoto, Y.; Watanabe, M.; Miyatake, K.; Morimoto, M.; Shigemasa, Y.; Minami, S. Effects of chitin/chitosan and Their oligomers/monomers on Migrations of Fibroblasts and Vascular Endothelium. Biomaterials 2002, 23, 1975–1979. [Google Scholar] [CrossRef]
- Masuoka, K.; Ishihara, M.; Asazuma, T.; Hattori, H.; Matsui, T.; Takase, B.; Kanatani, Y.; Fujita, M.; Saito, Y.; Yura, H.; et al. The interaction of chitosan with fibroblast growth factor-2 and its protection from inactivation. Biomaterials 2005, 26, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Revi, D.; Paul, W.; Anilkumar, T.V.; Sharma, C.P. Chitosan scaffold co-cultured with keratinocyte and fibroblast heals full thickness skin wounds in rabbit. J. Biomed. Mater. Res. A. 2014, 102, 3273–3281. [Google Scholar] [CrossRef]
- Ikemoto, S.; Mochizuki, M.; Yamada, M.; Takeda, A.; Uchinuma, E.; Yamashina, S.; Nomizu, M.; Kadoya, Y. Laminin peptide-conjugated chitosan membrane: Application for keratinocyte delivery in wounded skin. J. Biomed. Mater. Res. A 2006, 79, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shu, W.; Yu, Q.; Qu, W.; Wang, Y.; Li, R. Functional Biomaterials for Treatment of Chronic Wound. Front. Bioeng. Biotechnol. 2020, 8, 516. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.V.; Nanduri, L.S.Y. Interaction of chitin/chitosan with salivary and other epithelial cells—An overview. Int. J. Biol Macromol. 2017, 104, 1398–1406. [Google Scholar] [CrossRef]
- Paczkowska, M.; Chanaj-Kaczmarek, J.; Romaniuk-Drapała, A.; Rubiś, B.; Szymanowska, D.; Kobus-Cisowska, J.; Szymańska, E.; Winnicka, K.; Cielecka-Piontek, J. Mucoadhesive chitosan delivery system with Chelidonii herba lyophilized extract as a promising strategy for vaginitis treatment. J. Clin. Med. 2020, 9, 1208. [Google Scholar] [CrossRef]
- European Medicines Agency. European Pharmacopoeia (01/2011:1297) Monograph Calendulae flos; EDQM Council of Europe: Strasbourg, France, 2011. [Google Scholar]
- ICH. Validation of analytical procedures Q2(R2). In Proceedings of the International Conference of Harmonisation, Geneva, Switzerland, 14 November 2018. [Google Scholar]
- Moore, J.W.; Flanner, H.H. Mathematical comparison of curves with an emphasis on in vitro dissolution profiles. Pharm. Technol. 1996, 20, 64–74. [Google Scholar]
- Yee, S. In vitro permeability across Caco3 cells (colonic) can predict in vivo (small intestinal) absorption in man—Fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Dudek-Makuch, M.; Chanaj-Kaczmarek, J.; Czepulis, N.; Korybalska, K.; Rutkowski, R.; Łuczak, J.; Grabowska, K.; Bylka, W.; Witowski, J. Anti-inflammatory Activity and Phytochemical Profile of Galinsoga Parviflora Cav. Molecules 2018, 23, 2133. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szczepaniak, O.; Kmiecik, D.; Przeor, M.; Gramza-Michałowska, A.; Cielecka-Piontek, J.; Smuga-Kogut, M.; Szulc, P. Composition and in vitro effects of cultivars of Humulus lupulus L. Hops on cholinesterase activity and microbial growth. Nutrients 2019, 11, 1377. [Google Scholar] [CrossRef] [PubMed]
- Kobus-Cisowska, J.; Szymanowska, D.; Maciejewska, P.; Kmiecik, D.; Gramza-Michałowska, A.; Kulczyński, B.; Cielecka-Piontek, J. In vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron. J. Biotechnol. 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ko, C.Y.; Meyers, E.E.; Pedroja, B.S.; Pelaez, N.; Bernstein, A.M. Concentration-dependent effects of transforming growth factor β1 on corneal wound healing. Mol. Vis. 2011, 17, 2835–2846. [Google Scholar]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Akobirshoeva, A.; Zilfikarov, I.N.; Vennos, C. Isorhamnetin and Quercetin Derivatives as Anti-Acetylcholinesterase Principles of Marigold (Calendula officinalis) Flowers and Preparations. Int. J. Mol. Sci. 2017, 18, 1685. [Google Scholar] [CrossRef]
- Dhawan, S.; Singla, A.K.; Sinha, V.R. Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS Pharm. Sci. Tech. 2004, 5, e67. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Yadav, P.N. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018. [Google Scholar] [CrossRef]
- Nan, W.; Ding, L.; Chen, H.; Khan, F.; Yu, L.; Sui, X.; Shi, X. Topical Use of Quercetin-Loaded Chitosan Nanoparticles Against Ultraviolet B Radiation. Front. Pharmacol. 2018, 9, 826. [Google Scholar] [CrossRef]
- Tran, N.Q.; Joung, Y.K.; Lih, E.; Park, K.D. In situ forming and rutin-releasing chitosan hydrogels as injectable dressings for dermal wound healing. Biomacromolecules 2011, 12, 2872–2880. [Google Scholar] [CrossRef]
- Nguyen, V.C.; Nguyen, V.B.; Hsieh, M.F. Curcumin-Loaded Chitosan/Gelatin Composite Sponge for Wound Healing Application. Int. J. Polym. Sci. 2013. [Google Scholar] [CrossRef]
- Karri, V.V.S.R.; Kuppusamy, G.; Talluri, S.V.; Mannemala, S.S.; Kollipara, R.; Wadhwani, A.D.; Mulukutla, S.; Raju, K.R.S.; Malayandi, R. Curcumin loaded chitosan nanoparticles impregnated into collagen-alginate scaffolds for diabetic wound healing. Int. J. Biol. Macromol. 2016, 93, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Wani, T.U.; Raza, S.N.; Khan, N.A. Rosmarinic acid loaded chitosan nanoparticles for wound healing in rats. IJPSR 2019, 10, 1138–1147. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Rajabzadeh, A.; Zarei, L. Effect of Chitosan/Propolis Biodegradable Film on Full-Thickness Wound Healing in Rats. Iran. J. Vet. 2019, 14. [Google Scholar] [CrossRef]
- Mirzaei, E.; Sarkar, S.; Rezayat, S.M.; Faridi-Majidi, R. Herbal Extract Loaded Chitosan-Based Nanofibers as a Potential Wound-Dressing. JAMSAT 2016, 2, 141–150. [Google Scholar] [CrossRef]
- Melo, M.D.S.F.; Almeida, L.A.D.; Castro, K.C.D.; Campo, M.G.N. Chitosan membrane incorporated with Passiflora edulis Sims extract for potential application as wound dressing. Adv. Tissue Eng. Regen. Med. 2019, 5, 103–108. [Google Scholar] [CrossRef]
- Franco, P.B.; de Almeida, L.A.; Marques, R.F.C.; da Silva, M.A.; Campos, M.G.N. Chitosan Associated with the Extract of Unripe Banana Peel for Potential Wound Dressing Application. Int. J. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Paul, A.; Das, S.; Das, J.; Samadder, A.; Khuda-Bukhsh, A.R. Cytotoxicity and apoptotic signalling cascade induced by chelidonine-loaded PLGA nanoparticles in HepG2 cells in vitro and bioavailability of nano-chelidonine in mice in vivo. Toxicol. Lett. 2013, 222, 10–22. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Farrell, T.L.; Dew, T.P.; Poquet, L.; Hanson, P.; Williamson, G. Absorption and metabolism of chlorogenic acids in cultured gastric epithelial monolayers. Drug Metab. Dispos. 2011, 39, 2338–2346. [Google Scholar] [CrossRef]
- Scherbl, D.; Muentnich, S.; Richling, E. In vitro absorption studies of chlorogenic acids from coffee using the Ussing chamber model. Food Res. Int. 2014, 63, 456–463. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Kobayashi, S. Transepithelial transport of chlorogenic acid, caffeic acid, and their colonic metabolites in intestinal caco-2 cell monolayers. J. Agric. Food Chem. 2004, 52, 2518–2526. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Yang, X.; Wang, K.; Yang, X. The efflux of flavonoids morin, isorhamnetin-3-O-rutinoside and diosmetin-7-O-beta-D-xylopyranosyl-(1–6) -beta-D-glucopyranoside in the human intestinal cell line caco-2. Pharm Res. 2006, 23, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.P.; Landsman, A. Preclinical and Clinical Studies of Hyaluronic Acid in Wound Care: A Case Series and Literature Review. Wounds 2019, 31, 41–48. [Google Scholar] [PubMed]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a Crucial Regulator of Inflammation. Front. Immunol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Maity, N.; Nema, N.K.; Abedy, M.K.; Sarkar, B.K.; Mukherjee, P.K. Exploring Tagetes erecta Linn flower for the elastase, hyaluronidase and MMP-1 inhibitory activity. J. Ethnopharmacol. 2011, 137, 1300–1305. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, G.H. Evaluation of Antioxidant and Inhibitory Activities for Different Subclasses Flavonoids on Enzymes for Rheumatoid Arthritis. J. Food Sci. 2010, 75, 212–217. [Google Scholar] [CrossRef]
- Ao, C.; Higa, T.; Ming, H.; Ding, Y.; Tawata, S. Isolation and identification of antioxidant and hyaluronidase inhibitory compounds from Ficus microcarpa L. fil. Bark. J. Enzym. Inhib. Med. Chem. 2010, 25, 406–413. [Google Scholar] [CrossRef]
- Liu, M.; Yin, H.; Dong, J.; Xiao, L.; Liu, G.; Qian, Z.; Miao, J. Inhibition and Interaction with Hyaluronidase by Compounds from Hop (Humulus lupulus L.) Flowers. Asian J. Chem. 2013, 25, 10262–10266. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef]
- Coppo, E.; Marchese, A. Antibacterial activity of polyphenols. Curr. Pharm. Biotechnol. 2014, 15, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- Correa-Pacheco, Z.N.; Bautista-Baños, S.; Hernández-López, M.; Corona-Rangel, M.L. Evaluation of Physical Properties and Antibacterial Activity of Bioactive Compounds-Loaded Chitosan Nanoparticles. Mater. Sci. Forum 2018, 936, 3–7. [Google Scholar] [CrossRef]
- Abd El-Aziz, A.R.M.; Al-Othman, M.R.; Mahmoud, M.A.; Shehata, S.M.; Abdelazim, N.S. Chitosan nanoparticles as a carrier for Mentha longifolia extract: Synthesis, characterization and antifungal activity. Curr. Sci. 2018, 114, 2116–2122. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Rufato, K.B.; Galdino, J.P.; Ody, K.S.; Pereira, A.G.B.; Corradini, E.; Martins, A.F.; Paulino, A.T.; Fajardo, A.R.; Aouada, F.A.; La Porta, F.A.; et al. Hydrogels Based on Chitosan and Chitosan Derivatives for Biomedical Applications, Hydrogels—Smart Materials for Biomedical Applications. IntechOpen 2018, 1–40. [Google Scholar] [CrossRef]
- Kaur, G.; Grewal, J.; Jyoti, K.; Jain, U.K.; Chandra, R.; Madan, J. Oral controlled and sustained drug delivery systems: Concepts, advances, preclinical, and clinical status. Drug Target. Stimuli Sensitive Drug Deliv. Syst. 2018, 567–626. [Google Scholar] [CrossRef]
- Pandey, A.; Jagtap, J.V.; Polshettiwar, S.A.; Kuchekar, B.S. Formulation and Evaluation of Antibacterial and Antifungal Activity of Herbal Gel Containing Aloe vera, Azadirachta indica and Lycopersicon esculentum Seed Extract. Res. J. Pharm. Technol. 2011, 4, 552–554. [Google Scholar]
- Pounikar, Y.; Jain, P.; Khurana, N.; Omray, L.K.; Patil, S.; Gajbhiye, A. Formulation and characterization of Aloe vera cosmetic herbal hydrogel. Int. J. Pharm. Pharm. Sci. 2012, 4, 85–86. [Google Scholar]
- Aslani, A.; Zolfaghari, B.; Fereidani, Y. Design, formulation, and evaluation of a herbal gel contains melissa, sumac, licorice, rosemary, and geranium for treatment of recurrent labial herpes infections. Dent. Res. J. 2018, 15, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Chen, Y.-H. Thermo and pH-responsive methylcellulose and hydroxypropyl methylcellulose hydrogels containing K2SO4 for water retention and a controlled-release water-soluble fertilizer. Sci. Total Environ. 2019, 655, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Gehrke, S.H. Microporous, fast response cellulose ether hydrogel prepared by freeze-drying. Colloid Surf. B 2004, 38, 191–196. [Google Scholar] [CrossRef]
- Kumar, S.; Joonseok, K. Physiochemical, optical and biological activity of chitosan-chromone derivative for biomedical applications. Int. J. Mol. Sci. 2012, 13, 6102–6116. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Barati, A.; Salehi, E.; Tonelli, A.E.; Hamedi, H. Preparation and characterization of chitosan based hydrogels containing cyclodextrin inclusion compounds or nanoemulsions of thyme oil. Polym. Int. 2019, 68, 1891–1902. [Google Scholar] [CrossRef]
- McCarty, S.M.; Percival, S.L. Proteases and Delayed Wound Healing. Adv. Wound Care 2013, 2, 438–447. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Xu, W.; Tu, S.; Yan, L. Antibacterial hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef]

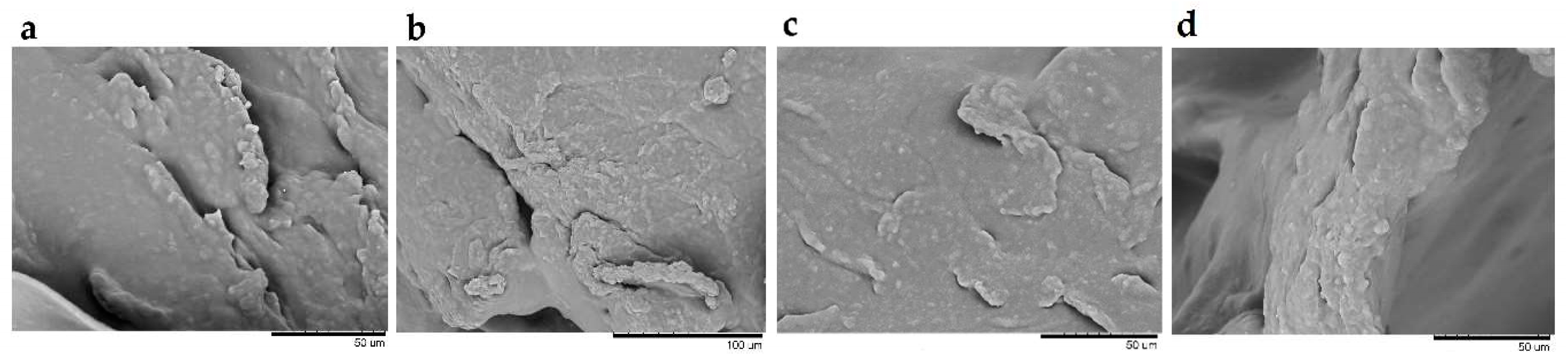
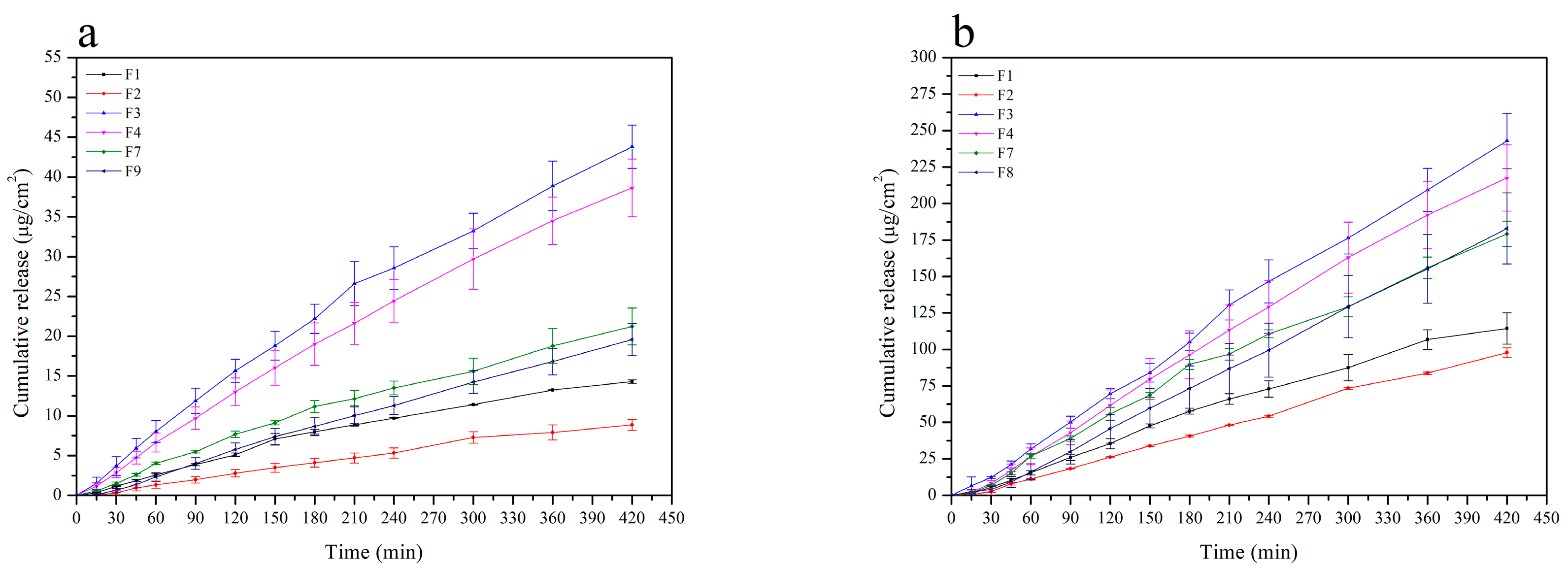
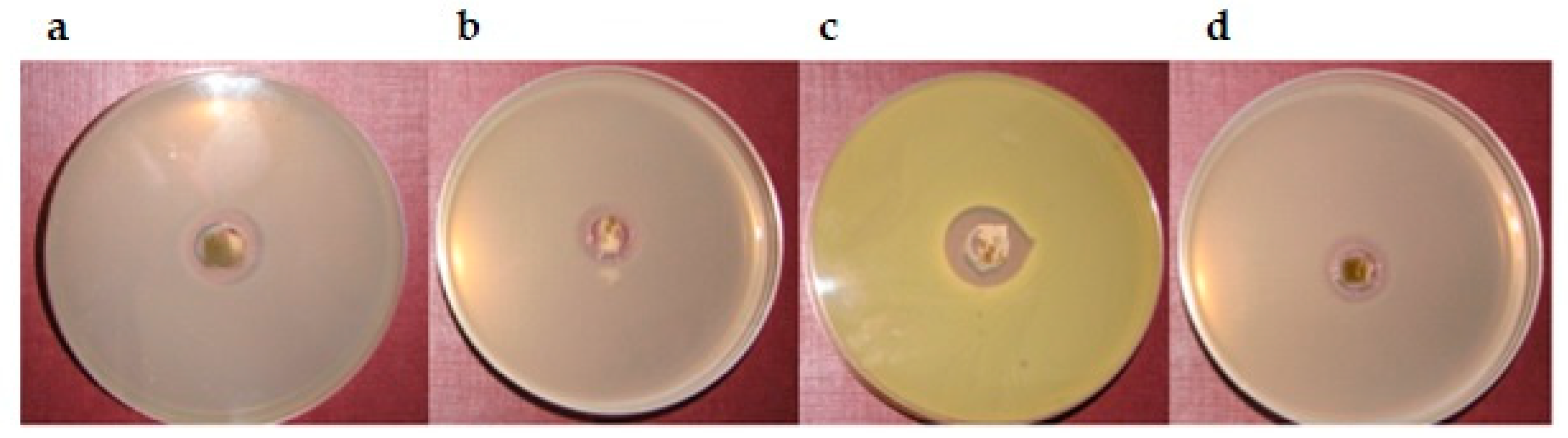
| Component | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Calendulae flos lyophilized extract | 0.3 g (3%) | 0.3 g (3%) | 1.0 g (10%) | 1.0 g (10%) | - | - | - | - | - |
| Calendulae flos lyophilized extract—chitosan 80/500 1:1 | - | - | - | - | 0.3 g (3%) | 0.3 g (3%) | 1.0 g (10%) | 1.0 g (10%) | - |
| Calendulae flos lyophilized extract—chitosan 80/1000 1:1 | - | - | - | - | - | - | - | - | 1.0 g (10%) |
| HPMC | 0.22 g (2%) | 0.32 g (3%) | 0.2 g (2%) | 0.3 g (3%) | 0.22 g (2%) | 0.32 g (3%) | 0.2 g (2%) | 0.3 g (3%) | 0.22 g (2%) |
| Water | up 10.0 g | up 10.0 g | up 10.0 g | up 10.0 g | up 10.0 g | up 10.0 g | up 10.0 g | up 10.0 g | up 10.0 g |
| Peak | Rt (min) | UV-VIS (λmax) | [M + H]+ | m/z Fragments | Formula | Content mg g−1 ± SD | Identification | Mode of Identification |
|---|---|---|---|---|---|---|---|---|
| 1 | 9.45 | 241, 324 | - | - | C16H18O9 | 4.22 ± 0.05 | chlorogenic acid | standard |
| 2 | 14.15 | 253, 355 | 771 | 625, 479, 317 | C34H42O20 | - | typhaneoside (isorhamnetin 3-O-rhamnosylorutinoside) | literature data |
| 3 | 17.12 | 254, 355 | 625 | 479, 317 | C28H32O16 | 64.63 ± 0.39 | narcissin (isorhamnetin 3-O-rutinoside) | standard |
| Substance | Papp ± SD (×10−6 cm s−1) | |
|---|---|---|
| Chlorogenic Acid | Narcissin | |
| Reference substances | 2.42 ± 1.18 | 2.35 ± 1.46 |
| Calendulae flos lyophilized extract | 2.90 ± 1.76 | 1.44 ± 0.68 |
| Calendulae flos lyophilized extract—chitosan 80/500 1:1 (w/w) | 2.97 ± 0.01 | 1.63 ± 0.01 |
| Calendulae flos lyophilized extract—chitosan 80/1000 1:1 (w/w) | 3.20 ± 0.41 | 1.60 ± 0.06 |
| Microorganism | Calendulae flos Lyophilized Extract | Lyophilized Extract + Chitosan 80/500 1:1 | Lyophilized Extract + Chitosan 80/500 1:5 | Lyophilized Extract + Chitosan 80/1000 1:1 | Lyophilized Extract + Chitosan 80/1000 1:5 | Chitosan 80/500 | Chitosan 80/1000 |
|---|---|---|---|---|---|---|---|
| MBC (mg mL−1) | |||||||
| S. aureus | 8 | 16 | 32 | 16 | 32 | 125 | 250 |
| S. epidermidis | 8 | 16 | 32 | 16 | 32 | 125 | 250 |
| E. faecalis | 32 | 64 | 125 | 16 | 125 | 125 | 250 |
| E. faecium | 32 | 64 | 125 | 32 | 125 | 125 | 250 |
| S. pyogenes | 64 | 64 | 125 | 32 | 125 | 125 | 250 |
| E. coli | 64 | 64 | 64 | 32 ↓ | 64 | 64 | 125 |
| P. aeruginosa | 4 | 8 | 64 | 16 | 32 | 125 | 125 |
| P. acnes | 16 | 8 ↓ | 8 ↓ | 4 ↓ | 125 | 125 | 125 |
| Candida albicans | 64 | 32 ↓ | 32 ↓ | 8 ↓ | 64 | 250 | 250 |
| Formulation | Thixotropy | Ostwald’s Model | ||
|---|---|---|---|---|
| Power-Law Index (n) | Consistency (K; Pa·sn) | Correlation Coefficient | ||
| F5 | 1.9 ± 0.5 | 0.8491 ± 0.0082 | 4.87 ± 0.16 | 0.9998 |
| F6 | 5.6 ± 2.0 | 0.7371± 0.0142 | 23.48 ± 0.57 | 0.9991 |
| F7 | 0.9 ± 0.4 | 0.7940 ± 0.0045 | 8.24 ± 0.18 | 0.9995 |
| F8 | 13.3 ± 4.0 | 0.6550 ± 0.0037 | 53.25 ± 0.25 | 0.998 |
| Formulation | Drug Flux (Jss; µg/cm2 h) | Correlation Coefficient (r) | Average Cumulative Amount per Area at 7 h (Q7h; µg/cm2) |
|---|---|---|---|
| Chlorogenic acid | |||
| F1 | 2.13 ± 0.01 | 0.9880 ± 0.0570 | 14.31 ± 0.22 |
| F2 | 1.70 ± 0.13 | 0.9950 ± 0.0017 | 11.21 ± 0.87 |
| F3 | 6.41 ± 0.36 | 0.9936 ± 0.0038 | 43.81 ± 2.71 |
| F4 | 5.82 ± 0.62 | 0.9965 ± 0.0019 | 38.63 ± 3.61 |
| F7 | 3.04 ± 0.37 | 0.9923 ± 0.0064 | 21.23 ± 2.31 |
| F9 | 2.92 ± 0.28 | 0.9985 ± 0.0013 | 19.57 ± 2.02 |
| Narcissin | |||
| F1 | 17.54 ± 1.43 | 0.9945 ± 0.0035 | 114.29 ± 10.69 |
| F2 | 13.80 ± 2.09 | 0.9960 ± 0.0016 | 97.74 ± 3.38 |
| F3 | 35.83 ± 2.48 | 0.9968 ± 0.0008 | 242.92 ± 18.93 |
| F4 | 32.84 ± 3.50 | 0.9964 ± 0.0046 | 217.59 ± 22.72 |
| F7 | 26.53 ± 1.57 | 0.9957 ± 0.0015 | 179.20 ± 8.69 |
| F9 | 27.46 ± 3.64 | 0.9988 ± 0.0013 | 182.95 ± 24.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanaj-Kaczmarek, J.; Paczkowska, M.; Osmałek, T.; Kaproń, B.; Plech, T.; Szymanowska, D.; Karaźniewicz-Łada, M.; Kobus-Cisowska, J.; Cielecka-Piontek, J. Hydrogel Delivery System Containing Calendulae flos Lyophilized Extract with Chitosan as a Supporting Strategy for Wound Healing Applications. Pharmaceutics 2020, 12, 634. https://doi.org/10.3390/pharmaceutics12070634
Chanaj-Kaczmarek J, Paczkowska M, Osmałek T, Kaproń B, Plech T, Szymanowska D, Karaźniewicz-Łada M, Kobus-Cisowska J, Cielecka-Piontek J. Hydrogel Delivery System Containing Calendulae flos Lyophilized Extract with Chitosan as a Supporting Strategy for Wound Healing Applications. Pharmaceutics. 2020; 12(7):634. https://doi.org/10.3390/pharmaceutics12070634
Chicago/Turabian StyleChanaj-Kaczmarek, Justyna, Magdalena Paczkowska, Tomasz Osmałek, Barbara Kaproń, Tomasz Plech, Daria Szymanowska, Marta Karaźniewicz-Łada, Joanna Kobus-Cisowska, and Judyta Cielecka-Piontek. 2020. "Hydrogel Delivery System Containing Calendulae flos Lyophilized Extract with Chitosan as a Supporting Strategy for Wound Healing Applications" Pharmaceutics 12, no. 7: 634. https://doi.org/10.3390/pharmaceutics12070634
APA StyleChanaj-Kaczmarek, J., Paczkowska, M., Osmałek, T., Kaproń, B., Plech, T., Szymanowska, D., Karaźniewicz-Łada, M., Kobus-Cisowska, J., & Cielecka-Piontek, J. (2020). Hydrogel Delivery System Containing Calendulae flos Lyophilized Extract with Chitosan as a Supporting Strategy for Wound Healing Applications. Pharmaceutics, 12(7), 634. https://doi.org/10.3390/pharmaceutics12070634










