The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation
Abstract
1. Introduction
2. Overview of the Techniques Used for Fabrication of Porous Scaffolds for Tissue Engineering Applications
2.1. Electrospinning
2.2. Additive Manufacturing
2.3. Particles Leaching
2.4. Foaming
2.5. Hydrogels
3. Materials Used for Scaffold Compositions
3.1. Polymers
3.2. Ceramics
3.3. Metals
4. Review of the Influence of the Scaffold Architecture on Cell Behavior
4.1. Effect of Porosity
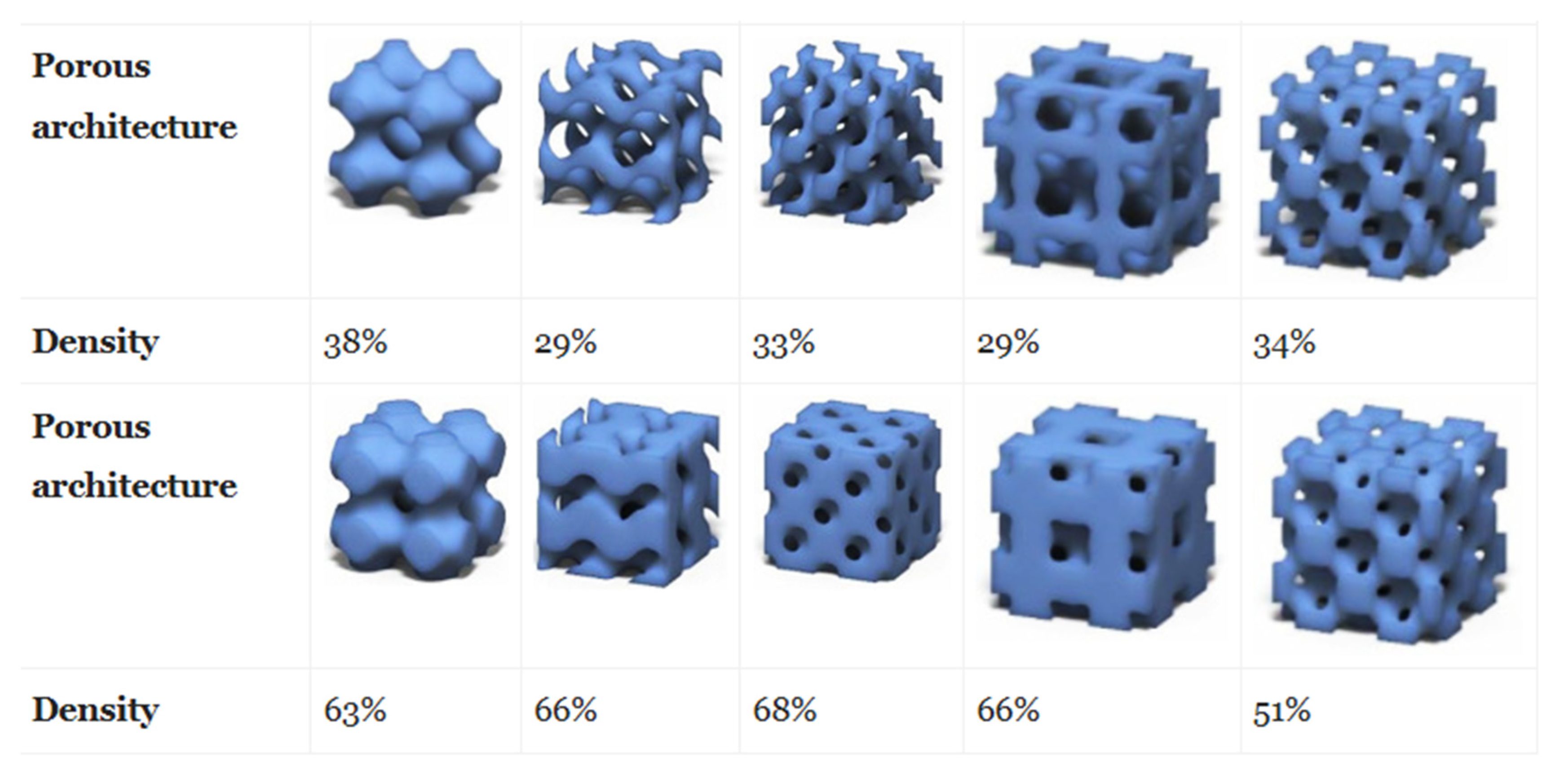
4.2. Effect of Pore Size and Shape
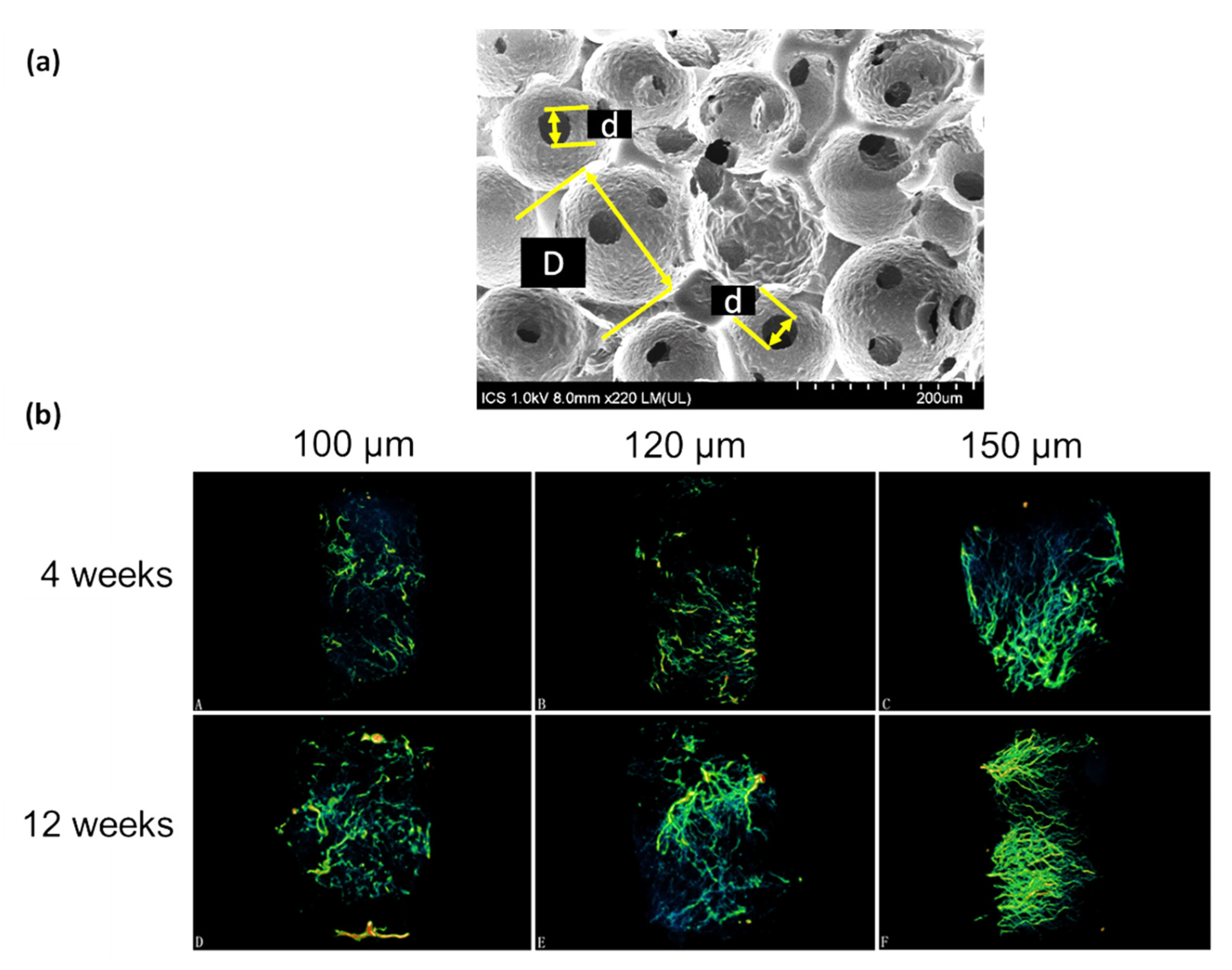
4.3. Effect of Pore Interconnectivity
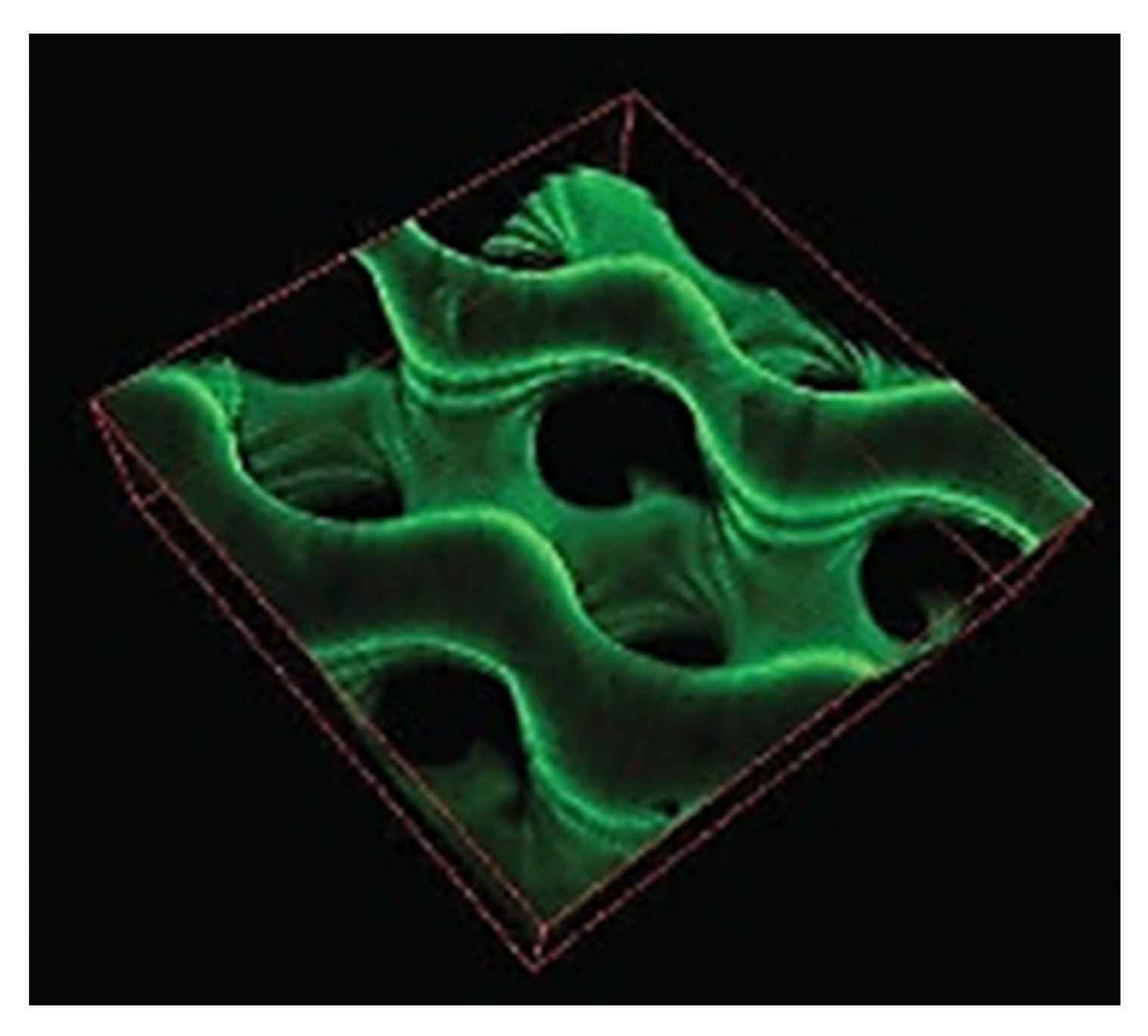
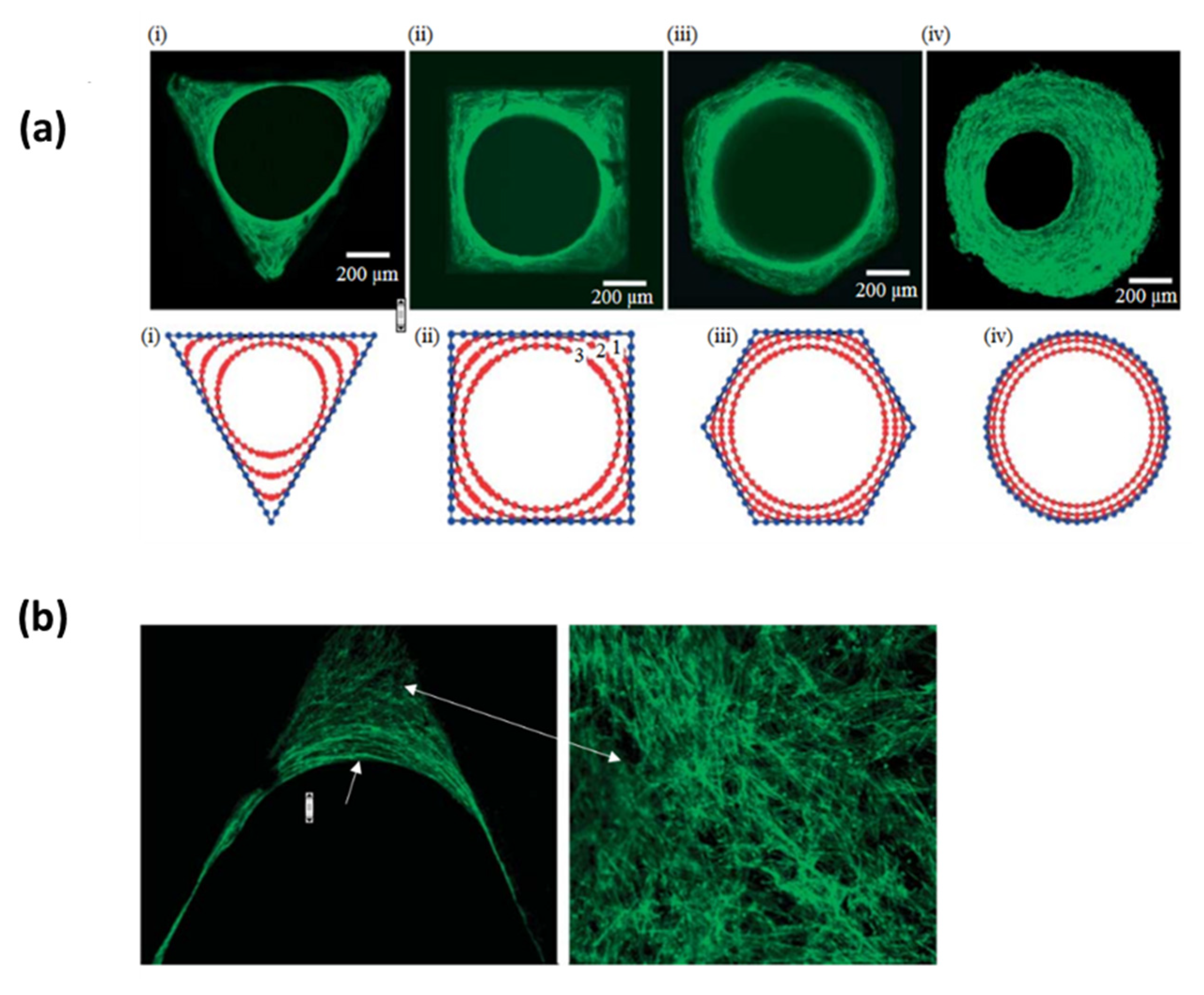
4.4. The Effect of the Curvature
4.5. Mechanical Properties
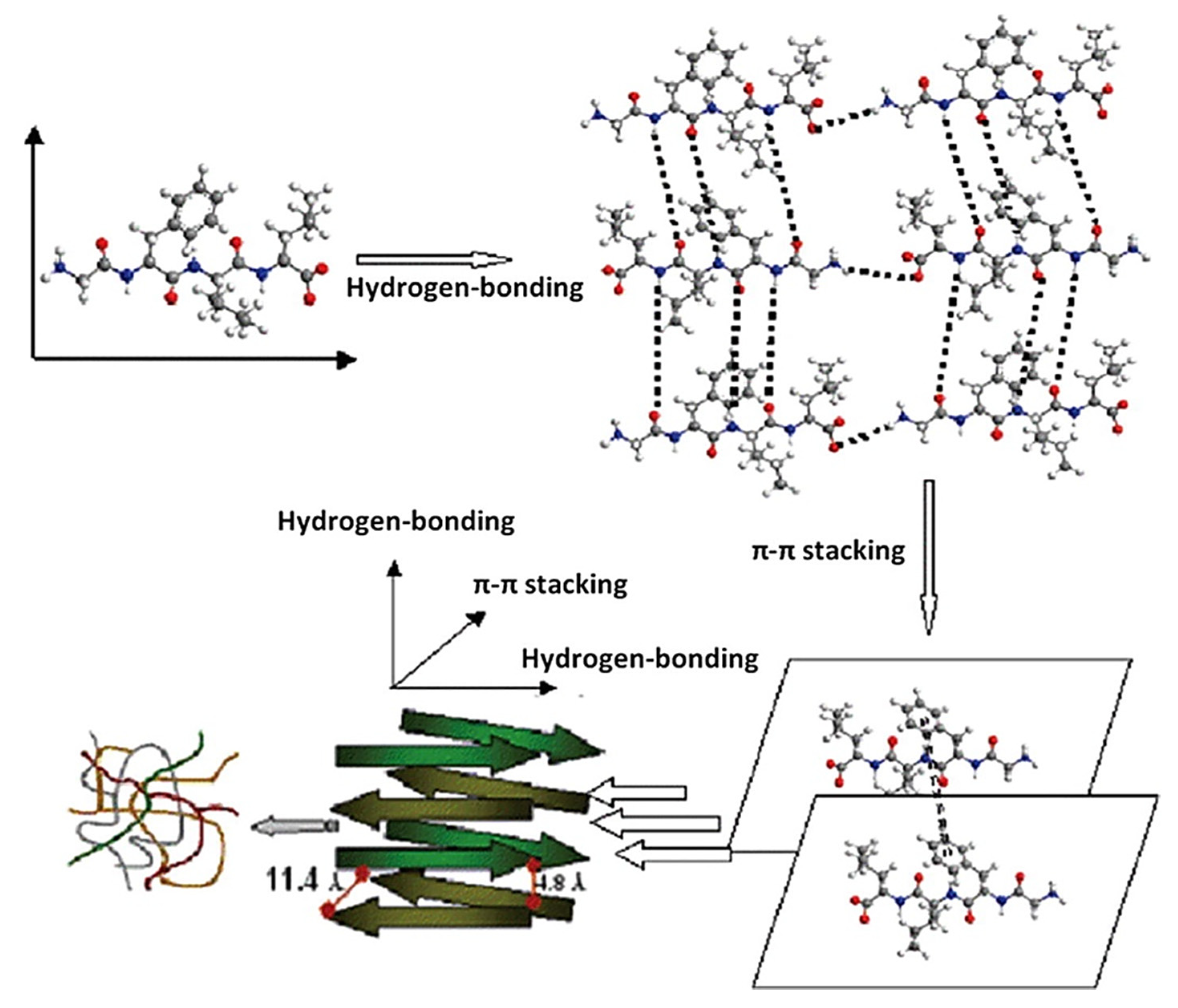
4.6. Bioactive Scaffolds
5. In Vivo Outcomes and Clinical Trials
6. General Conclusion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D. A pore way to heal and regenerate: 21st century thinking on biocompatibility. Regen. Biomater. 2016, 3, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Tissue Engineering Market Size, Share & Trends Report Tissue Engineering Market Size, Share & Trends Analysis Report By Application. Available online: https://www.grandviewresearch.com/industry-analysis/tissue-engineering-and-regeneration-industry (accessed on 28 February 2020).
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-dimensional cell culture: A breakthrough in vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Surface micropatterning to regulate cell functions. Biomaterials 1999, 20, 2333–2342. [Google Scholar] [CrossRef]
- Lucas, B.; Pérez, L.M.; Gálvez, B.G. Importance and regulation of adult stem cell migration. J. Cell. Mol. Med. 2018, 22, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef]
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent advancements in electrospinning design for tissue engineering applications: A review. J. Biomed. Mater. Res. Part A 2017, 105, 2892–2905. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Ndreu, A.; Nikkola, L.; Ylikauppila, H.; Ashammakhi, N.; Hasirci, V. Electrospun biodegradable nanofibrous mats for tissue engineering. Nanomedicine 2008, 3, 45–60. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Müller, K.; Quinn, J.F.; Johnston, A.P.R.; Becker, M.; Greiner, A.; Caruso, F. Polyelectrolyte functionalization of electrospun fibers. Chem. Mater. 2006, 18, 2397–2403. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly (ethylene-co-vinylacetate), poly (lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef]
- Yu, D.-G.; Shen, X.-X.; Branford-White, C.; White, K.; Zhu, L.-M.; Bligh, S.W.A. Oral fast-dissolving drug delivery membranes prepared from electrospun polyvinylpyrrolidone ultrafine fibers. Nanotechnology 2009, 20, 55104. [Google Scholar] [CrossRef]
- Powell, H.M.; Boyce, S.T. Engineered Human Skin Fabricated Using Electrospun Collagen–PCL Blends: Morphogenesis and Mechanical Properties. Tissue Eng. Part A 2009, 15, 2177–2187. [Google Scholar] [CrossRef]
- Shields, K.J.; Beckman, M.J.; Bowlin, G.L.; Wayne, J.S. Mechanical Properties and Cellular Proliferation of Electrospun Collagen Type II. Tissue Eng. 2004, 10, 1510–1517. [Google Scholar] [CrossRef]
- Halili, A.N.; Kürüm, B.; Karahan, S.; Hasirci, V. Collagen Based Multilayer Scaffolds for Meniscus Tissue Engineering: In Vivo Test Results. Biomater. Med. Appl. 2018, 9, 2. [Google Scholar]
- Stachewicz, U.; Szewczyk, P.K.; Kruk, A.; Barber, A.H.; Czyrska-Filemonowicz, A. Pore shape and size dependence on cell growth into electrospun fiber scaffolds for tissue engineering: 2D and 3D analyses using SEM and FIB-SEM tomography. Mater. Sci. Eng. C 2019, 95, 397–408. [Google Scholar] [CrossRef]
- Gizaw, M.; Faglie, A.; Pieper, M.; Poudel, S.; Chou, S.-F. The Role of Electrospun Fiber Scaffolds in Stem Cell Therapy for Skin Tissue Regeneration. Med One 2019, 4, e190002. [Google Scholar]
- Kuo, T.-Y.; Lin, C.-M.; Hung, S.-C.; Hsien, T.-Y.; Wang, D.-M.; Hsieh, H.-J. Incorporation and selective removal of space-forming nanofibers to enhance the permeability of cytocompatible nanofiber membranes for better cell growth. J. Taiwan Inst. Chem. Eng. 2018, 91, 146–154. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Yoo, J.; Mironov, V. 3D Printing and Biofabrication; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Lee, J.-Y.; An, J.; Chua, C.K. Fundamentals and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Liu, A.; Xue, G.; Sun, M.; Shao, H.; Ma, C.; Gao, Q.; Gou, Z.; Yan, S.; Liu, Y.; He, Y. 3D printing surgical implants at the clinic: A experimental study on anterior cruciate ligament reconstruction. Sci. Rep. 2016, 6, 21704. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.; Lee, H.; Kim, G.H. Combining a micro/nano-hierarchical scaffold with cell-printing of myoblasts induces cell alignment and differentiation favorable to skeletal muscle tissue regeneration. Biofabrication 2016, 8, 35021. [Google Scholar] [CrossRef]
- Castilho, M.; Dias, M.; Gbureck, U.; Groll, J.; Fernandes, P.; Pires, I.; Gouveia, B.; Rodrigues, J.; Vorndran, E. Fabrication of computationally designed scaffolds by low temperature 3D printing. Biofabrication 2013, 5, 35012. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Sun, A.X.; Lin, H.; Beck, A.M.; Kilroy, E.J.; Tuan, R.S. Projection stereolithographic fabrication of human adipose stem cell-incorporated biodegradable scaffolds for cartilage tissue engineering. Front. Bioeng. Biotechnol. 2015, 3, 115. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T.; Lima, D.; Lotz, M. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat. Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef]
- Bella, C.; Fosang, A.; Donati, D.M.; Wallace, G.G.; Choong, P.F.M. 3D bioprinting of cartilage for orthopedic surgeons: Reading between the lines. Front. Surg. 2015, 2, 39. [Google Scholar] [CrossRef]
- Mosadegh, B.; Xiong, G.; Dunham, S.; Min, J.K. Current progress in 3D printing for cardiovascular tissue engineering. Biomed. Mater. 2015, 10, 34002. [Google Scholar] [CrossRef]
- Shahriari, D.; Koffler, J.Y.; Tuszynski, M.H.; Campana, W.M.; Sakamoto, J.S. Hierarchically ordered porous and high-volume polycaprolactone microchannel scaffolds enhanced axon growth in transected spinal cords. Tissue Eng. Part A 2017, 23, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Lutzweiler, G.; Barthès, J.; Koenig, G.; Kerdjoudj, H.; Mayingi, J.; Boulmedais, F.; Schaaf, P.; Drenckhan, W.; Vrana, N.E. Modulation of Cellular Colonization of Porous Polyurethane scaffolds via the control of pore interconnection size and nanoscale surface modifications. ACS Appl. Mater. Interfaces 2019, 11, 19819–19829. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Tang, Y.F.; Qin, Y.S.; Luo, D.F. Polymer template fabrication of porous hydroxyapatite scaffolds with interconnected spherical pores. J. Eur. Ceram. Soc. 2011, 31, 225–229. [Google Scholar] [CrossRef]
- Linnes, M.P.; Ratner, B.D.; Giachelli, C.M. A fibrinogen-based precision microporous scaffold for tissue engineering. Biomaterials 2007, 28, 5298–5306. [Google Scholar] [CrossRef] [PubMed]
- Lutzweiler, G.; Farago, J.; Oliveira, E.; Jacomine, L.; Erverdi, O.; Vrana, N.E.; Testouri, A.; Schaaf, P.; Drenckhan, W. Validation of Milner’s visco-elastic theory of sintering for the generation of porous polymers with finely tuned morphology. Soft Matter 2020, 16, 1810–1824. [Google Scholar] [CrossRef]
- Descamps, M.; Duhoo, T.; Monchau, F.; Lu, J.; Hardouin, P.; Hornez, J.C.; Leriche, A. Manufacture of macroporous β-tricalcium phosphate bioceramics. J. Eur. Ceram. Soc. 2008, 28, 149–157. [Google Scholar] [CrossRef]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous Implants Modulate Healing and Induce Shifts in Local Macrophage Polarization in the Foreign Body Reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef]
- Somo, S.I.; Akar, B.; Bayrak, E.S.; Larson, J.C.; Appel, A.A.; Mehdizadeh, H.; Cinar, A.; Brey, E.M. Pore Interconnectivity Influences Growth Factor-Mediated Vascularization in Sphere-Templated Hydrogels. Tissue Eng. Part C Methods 2015, 21, 773–785. [Google Scholar] [CrossRef]
- Silbert, L.E. Jamming of frictional spheres and random loose packing. Soft Matter 2010, 6, 2918–2924. [Google Scholar] [CrossRef]
- Jacobs, L.J.M.; Kemmere, M.F.; Keurentjes, J.T.F. Sustainable polymer foaming using high pressure carbon dioxide: A review on fundamentals, processes and applications. Green Chem. 2008, 10, 731–738. [Google Scholar] [CrossRef]
- Costantini, M.; Barbetta, A. 6-Gas foaming technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds: Materials, Technologies, and Applications; Deng, Y., Kuiper, J.B.T., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 127–149. [Google Scholar]
- Deb, P.; Deoghare, A.B.; Borah, A.; Barua, E.; Lala, S. Das Scaffold Development Using Biomaterials: A Review. Mater. Today 2017, 5, 12909–12919. [Google Scholar]
- Dehli, F.; Rebers, L.; Stubenrauch, C.; Southan, A. Highly ordered gelatin methacryloyl hydrogel foams with tunable pore size. Biomacromolecules 2019, 20, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Drenckhan, W.; Saint-Jalmes, A. The science of foaming. Adv. Colloid Interface Sci. 2015, 222, 228–259. [Google Scholar] [CrossRef] [PubMed]
- Isabelle, C.; Sylvie, C.A.; Florence, E.; Francois, G.; Reinhard, H.; Olivier, P.; Florence, R.; Flatman, S.C. Foams: Structure and Dynamics; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Testouri, A.; Ranft, M.; Honorez, C.; Kaabeche, N.; Ferbitz, J.; Freidank, D.; Drenckhan, W. Generation of Crystalline Polyurethane Foams Using Millifluidic Lab-on-a-Chip Technologies. Adv. Eng. Mater. 2013, 15, 1086–1098. [Google Scholar] [CrossRef]
- Andrieux, S.; Drenckhan, W.; Stubenrauch, C. Highly ordered biobased scaffolds: From liquid to solid foams. Polymer 2017, 126, 425–431. [Google Scholar] [CrossRef]
- Andrieux, S.; Quell, A.; Stubenrauch, C.; Drenckhan, W. Liquid foam templating--A route to tailor-made polymer foams. Adv. Colloid Interface Sci. 2018, 256, 276–290. [Google Scholar] [CrossRef]
- Costantini, M.; Colosi, C.; Mozetic, P.; Jaroszewicz, J.; Tosato, A.; Rainer, A.; Trombetta, M.; Więszkowski, W.; Dentini, M.; Barbetta, A. Correlation between porous texture and cell seeding efficiency of gas foaming and microfluidic foaming scaffolds. Mater. Sci. Eng. C 2016, 62, 668–677. [Google Scholar] [CrossRef]
- Costantini, M.; Colosi, C.; Jaroszewicz, J.; Tosato, A.; Więszkowski, W.; Dentini, M.; Garstecki, P.; Barbetta, A. Microfluidic Foaming: A Powerful Tool for Tailoring the Morphological and Permeability Properties of Sponge-like Biopolymeric Scaffolds. ACS Appl. Mater. Interfaces 2015, 7, 23660–23671. [Google Scholar] [CrossRef]
- Rossmy, G.R.; Kollmeier, H.J.; Lidy, W.; Schator, H.; Wiemann, M. Cell-opening in one-shot flexible polyether based polyurethane foams. The Role of Silicone Surfactant and its Foundation in the Chemistry of Foam Formation. J. Cell. Plast. 1977, 13, 26–35. [Google Scholar] [CrossRef]
- Yasunaga, K.; Neff, R.A.; Zhang, X.D.; Macosko, C.W. Study of Cell Opening in Flexible Polyurethane Foam. J. Cell. Plast. 1996, 32, 427–448. [Google Scholar] [CrossRef]
- Li, H.; Liu, K.; Sang, Q.; Williams, G.R.; Wu, J.; Wang, H.; Wu, J.; Zhu, L.-M. A thermosensitive drug delivery system prepared by blend electrospinning. Colloids Surfaces B Biointerfaces 2017, 159, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Lutzweiler, G. Matériaux Poreux à Base de Polyuréthane Pour L’ingénierie Tissulaire; Université de Strasbourg: Rhine, France, 2019. [Google Scholar]
- Ng, W.S.; Lee, C.S.; Chuah, C.H.; Cheng, S.-F. Preparation and modification of water-blown porous biodegradable polyurethane foams with palm oil-based polyester polyol. Ind. Crops Prod. 2017, 97, 65–78. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-W.; Zhang, Y.; MacEwan, M.R.; Xia, Y. Neovascularization in biodegradable inverse opal scaffolds with uniform and precisely controlled pore sizes. Adv. Healthc. Mater. 2013, 2, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Chiessi, E.; Cavalieri, F.; Paradossi, G. Water and polymer dynamics in chemically cross-linked hydrogels of poly (vinyl alcohol): A molecular dynamics simulation study. J. Phys. Chem. B 2007, 111, 2820–2827. [Google Scholar] [CrossRef]
- Kang, W.; Bi, B.; Zhuo, R.; Jiang, X. Photocrosslinked methacrylated carboxymethyl chitin hydrogels with tunable degradation and mechanical behavior. Carbohydr. Polym. 2017, 160, 18–25. [Google Scholar] [CrossRef]
- Kumar, P.; Ciftci, S.; Barthes, J.; Knopf-Marques, H.; Muller, C.B.; Debry, C.; Vrana, N.E.; Ghaemmaghami, A.M. A composite Gelatin/hyaluronic acid hydrogel as an ECM mimic for developing mesenchymal stem cell-derived epithelial tissue patches. J. Tissue Eng. Regen. Med. 2020, 14, 45–57. [Google Scholar] [CrossRef]
- Satish, L.; Millan, S.; Das, S.; Jena, S.; Sahoo, H. Thermal aggregation of bovine serum albumin in conventional buffers: An insight into molecular level interactions. J. Solut. Chem. 2017, 46, 831–848. [Google Scholar] [CrossRef]
- Andersen, T.; Auk-Emblem, P.; Dornish, M. 3D cell culture in alginate hydrogels. Microarrays 2015, 4, 133–161. [Google Scholar] [CrossRef]
- Yang, K.-S.; Guo, X.; Meng, W.; Hyun, J.-Y.; Kang, I.-K.; Kim, Y. Behavior of hepatocytes inoculated in gelatin-immobilized polyurethane foam. Macromol. Res. 2003, 11, 488–494. [Google Scholar] [CrossRef]
- Schaschkow, A.; Sigrist, S.; Mura, C.; Barthes, J.; Vrana, N.E.; Czuba, E.; Lemaire, F.; Neidl, R.; Dissaux, C.; Lejay, A.; et al. Glycaemic control in diabetic rats treated with islet transplantation using plasma combined with hydroxypropylmethyl cellulose hydrogel. Acta Biomater. 2020, 102, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Almany, L.; Seliktar, D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 2005, 26, 2467–2477. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, D.; Liu, B.; Nian, G.; Li, X.; Yin, J.; Qu, S.; Yang, W. 3D Printing of Multifunctional Hydrogels. Adv. Funct. Mater. 2019, 29, 1900971. [Google Scholar] [CrossRef]
- Lee, C.R.; Grad, S.; Gorna, K.; Gogolewski, S.; Goessl, A.; Alini, M. Fibrin--polyurethane composites for articular cartilage tissue engineering: A preliminary analysis. Tissue Eng. 2005, 11, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Criado-Gonzalez, M.; Fores, J.R.; Wagner, D.; Schröder, A.P.; Carvalho, A.; Schmutz, M.; Harth, E.; Schaaf, P.; Jierry, L.; Boulmedais, F. Enzyme-assisted self-assembly within a hydrogel induced by peptide diffusion. Chem. Commun. 2019, 55, 1156–1159. [Google Scholar] [CrossRef]
- Frederix, P.W.J.M.; Scott, G.G.; Abul-Haija, Y.M.; Kalafatovic, D.; Pappas, C.G.; Javid, N.; Hunt, N.T.; Ulijn, R.V.; Tuttle, T. Exploring the sequence space for (tri-) peptide self-assembly to design and discover new hydrogels. Nat. Chem. 2015, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O. Viscoelastic hydrogels for 3D cell culture. Biomater. Sci. 2017, 5, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Yu, X.; Wu, H.; Li, J.; Lv, H.; Yang, X. Nonswellable and Tough Supramolecular Hydrogel Based on Strong Micelle Cross-Linkings. Biomacromolecules 2019, 20, 3399–3407. [Google Scholar] [CrossRef]
- Alakpa, E.V.; Jayawarna, V.; Lampel, A.; Burgess, K.V.; West, C.C.; Bakker, S.C.J.; Roy, S.; Javid, N.; Fleming, S.; Lamprou, D.A.; et al. Tunable supramolecular hydrogels for selection of lineage-guiding metabolites in stem cell cultures. Chem 2016, 1, 298–319. [Google Scholar] [CrossRef]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef]
- Szot, C.S.; Buchanan, C.F.; Freeman, J.W.; Rylander, M.N. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials 2011, 32, 7905–7912. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Rubin, J.; Deng, Y.; Huang, C.; Demirci, U.; Karp, J.M.; Khademhosseini, A. A cell-laden microfluidic hydrogel. Lab Chip 2007, 7, 756–762. [Google Scholar] [CrossRef]
- Bryant, S.J.; Cuy, J.L.; Hauch, K.D.; Ratner, B.D. Photo-patterning of porous hydrogels for tissue engineering. Biomaterials 2007, 28, 2978–2986. [Google Scholar] [CrossRef] [PubMed]
- Kostina, N.Y.; Blanquer, S.; Pop-Georgievski, O.; Rahimi, K.; Dittrich, B.; Höcherl, A.; Michálek, J.; Grijpma, D.W.; Rodriguez-Emmenegger, C. Zwitterionic Functionalizable Scaffolds with Gyroid Pore Architecture for Tissue Engineering. Macromol. Biosci. 2019, 19, 1800403. [Google Scholar] [CrossRef]
- Ahearne, M.; Yang, Y.; Liu, K.K. Mechanical characterisation of hydrogels for tissue engineering applications. Top. Tissue Eng. 2008, 4, 1–16. [Google Scholar]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2019, 1–11. [Google Scholar] [CrossRef]
- Foster, G.A.; Headen, D.M.; González, C.G.; Salmerón-Sánchez, M.; Shirwan, H.; Garc, A.J. Protease-degradable microgels for protein delivery for vascularization. Biomaterials 2017, 113, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pich, A.; Hellweg, T.; Hoare, T.; Lyon, L.A.; Crassous, J.J.; Suzuki, D.; Gumerov, R.A.; Schneider, S.; Potemkin, I.I.; et al. Nanogels and microgels: From model colloids to applications, recent developments, and future trends. Langmuir 2019, 35, 6231–6255. [Google Scholar] [CrossRef]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Negahi Shirazi, A.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Castilla-Cortázar, I.; Más-Estellés, J.; Meseguer-Dueñas, J.M.; Escobar Ivirico, J.L.; Marí, B.; Vidaurre, A. Hydrolytic and enzymatic degradation of a poly(ε-caprolactone) network. Polym. Degrad. Stab. 2012, 97, 1241–1248. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Hamilton, G.; Seifalian, A.M. Current status of prosthetic bypass grafts: A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74B, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Winnacker, M. Polyamides and their functionalization: Recent concepts for their applications as biomaterials. Biomater. Sci. 2017, 5, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Lutzweiler, G.; Barthes, J.; Vrana, N.E.; Rawiso, M.; Louis, B.; Mayingi, J.; Carre, A.; Drenckhan, W.; Schaaf, P. Adjustment of Cell Adhesion on Polyurethane Structures via Control of the Hard/Soft Segment Ratio. Macromol. Mater. Eng. 2020, 305, 2000093. [Google Scholar] [CrossRef]
- Adipurnama, I.; Yang, M.-C.; Ciach, T.; Butruk-Raszeja, B. Surface modification and endothelialization of polyurethane for vascular tissue engineering applications: A review. Biomater. Sci. 2017, 5, 22–37. [Google Scholar] [CrossRef]
- Ma, Z.; Mao, Z.; Gao, C. Surface modification and property analysis of biomedical polymers used for tissue engineering. Colloids Surfaces B Biointerfaces 2007, 60, 137–157. [Google Scholar] [CrossRef]
- Williams, R. Surface Modification of Biomaterials: Methods Analysis and Applications; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Mason, T.O.; Shimanovich, U. Fibrous Protein Self-Assembly in Biomimetic Materials. Adv. Mater. 2018, 30, 1706462. [Google Scholar] [CrossRef]
- Neves, S.C.; Pereira, R.F.; Araújo, M.; Barrias, C.C. 4-Bioengineered peptide-functionalized hydrogels for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C., Eds.; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Trujillo, S.; Gonzalez-Garcia, C.; Rico, P.; Reid, A.; Windmill, J.; Dalby, M.J.; Salmeron-Sanchez, M. Engineered full-length Fibronectin-based hydrogels sequester and present growth factors to promote regenerative responses in vitro and in vivo. bioRxiv 2019. [Google Scholar] [CrossRef]
- Dinoro, J.; Maher, M.; Talebian, S.; Jafarkhani, M.; Mehrali, M.; Orive, G.; Foroughi, J.; Lord, M.S.; Dolatshahi-Pirouz, A. Sulfated polysaccharide-based scaffolds for orthopaedic tissue engineering. Biomaterials 2019, 214, 119214. [Google Scholar] [CrossRef]
- Malafaya, P.B.; Silva, G.A.; Reis, R.L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Gerecht, S.; Burdick, J.A.; Ferreira, L.S.; Townsend, S.A.; Langer, R.; Vunjak-Novakovic, G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 11298–11303. [Google Scholar] [CrossRef] [PubMed]
- Elisseeff, J.; Ferran, A.; Hwang, S.; Varghese, S.; Zhang, Z. The Role of Biomaterials in Stem Cell Differentiation: Applications in the Musculoskeletal System. Stem Cells Dev. 2006, 15, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Sabir, M.I.; Xu, X.; Li, L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009, 44, 5713–5724. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry—Scope and Perspectives Molecules, Supermolecules, and Molecular Devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2015, 15, 13. [Google Scholar] [CrossRef]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implications for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 2002, 99, 9996–10001. [Google Scholar] [CrossRef]
- Ellis-Behnke, R.G.; Liang, Y.-X.; You, S.-W.; Tay, D.K.C.; Zhang, S.; So, K.-F.; Schneider, G.E. Nano neuro knitting: Peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc. Natl. Acad. Sci. USA 2006, 103, 5054–5059. [Google Scholar] [CrossRef]
- Naskar, J.; Palui, G.; Banerjee, A. Tetrapeptide-Based Hydrogels: For Encapsulation and Slow Release of an Anticancer Drug at Physiological pH. J. Phys. Chem. B 2009, 113, 11787–11792. [Google Scholar] [CrossRef]
- Kutz, M. Others Standard Handbook of Biomedical Engineering and Design; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Thomas, S.; Balakrishnan, P.; Sreekala, M.S. Fundamental Biomaterials: Ceramics; Woodhead Publishing: Sawston, UK, 2018. [Google Scholar]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Ducheyne, P.; Mauck, R.L.; Smith, D.H. Biomaterials in the repair of sports injuries. Nat. Mater. 2012, 11, 652. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- De Bruijn, J.D.; Bovell, Y.P.; Davies, J.E.; van Blitterswijk, C.A. Osteoclastic resorption of calcium phosphates is potentiated in postosteogenic culture conditions. J. Biomed. Mater. Res. 1994, 28, 105–112. [Google Scholar] [CrossRef]
- Doi, Y.; Shibutani, T.; Moriwaki, Y.; Kajimoto, T.; Iwayama, Y. Sintered carbonate apatites as bioresorbable bone substitutes. J. Biomed. Mater. Res. 1998, 39, 603–610. [Google Scholar] [CrossRef]
- Paramsothy, M.; Ramakrishna, S. Biodegradable Materials for Clinical Applications: A Review. Rev. Adv. Sci. Eng. 2015, 4, 221–238. [Google Scholar] [CrossRef]
- Gotman, I. Characteristics of metals used in implants. J. Endourol. 1997, 11, 383–389. [Google Scholar] [CrossRef]
- Welsch, G.; Boyer, R.; Collings, E.W. Materials Properties Handbook: Titanium Alloys; ASM International: Almere, The Netherlands, 1993. [Google Scholar]
- Duerig, T.; Pelton, A.; Stöckel, D. An overview of nitinol medical applications. Mater. Sci. Eng. A 1999, 273, 149–160. [Google Scholar] [CrossRef]
- Kunčická, L.; Kocich, R.; Lowe, T.C. Advances in metals and alloys for joint replacement. Prog. Mater. Sci. 2017, 88, 232–280. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, C.; Fan, H.; Fan, Y.; Jiang, Q.; Song, P.; Fan, H.; Chen, Y.; Zhang, X. Bio-Functional Design, Application and Trends in Metallic Biomaterials. Int. J. Mol. Sci. 2018, 19, 24. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Brandt, M. Laser Additive Manufacturing: Materials, Design, Technologies, and Applications; Woodhead Publishing: Sawston, UK, 2016. [Google Scholar]
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal fabrication by additive manufacturing using laser and electron beam melting technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Kolken, H.M.A.; Janbaz, S.; Leeflang, S.M.A.; Lietaert, K.; Weinans, H.H.; Zadpoor, A.A. Rationally designed meta-implants: A combination of auxetic and conventional meta-biomaterials. Mater. Horizons 2018, 5, 28–35. [Google Scholar] [CrossRef]
- Ali, M.N.; Busfield, J.J.C.; Rehman, I.U. Auxetic oesophageal stents: Structure and mechanical properties. J. Mater. Sci. Mater. Med. 2014, 25, 527–553. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Haiying, Y.; Howard, W.M.; Paul, H.W.; Shang-you, Y. Effect of Porosity and Pore Size on Microstructures and Mechanical Properties of Poly-e-Caprolactone-Hydroxyapatite Composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 86, 541–547. [Google Scholar]
- Sobral, J.M.; Caridade, S.G.; Sousa, R.A.; Mano, J.F.; Reis, R.L. Three-dimensional plotted scaffolds with controlled pore size gradients: Effect of scaffold geometry on mechanical performance and cell seeding efficiency. Acta Biomater. 2011, 7, 1009–1018. [Google Scholar] [CrossRef]
- Shimko, D.A.; Shimko, V.F.; Sander, E.A.; Dickson, K.F.; Nauman, E.A. Effect of porosity on the fluid flow characteristics and mechanical properties of tantalum scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 73, 315–324. [Google Scholar] [CrossRef]
- Danilevicius, P.; Georgiadi, L.; Pateman, C.J.; Claeyssens, F.; Chatzinikolaidou, M.; Farsari, M. The effect of porosity on cell ingrowth into accurately defined, laser-made, polylactide-based 3D scaffolds. Appl. Surf. Sci. 2015, 336, 2–10. [Google Scholar] [CrossRef]
- Torres-Sanchez, C.; Al Mushref, F.R.A.; Norrito, M.; Yendall, K.; Liu, Y.; Conway, P.P. The effect of pore size and porosity on mechanical properties and biological response of porous titanium scaffolds. Mater. Sci. Eng. C 2017, 77, 219–228. [Google Scholar] [CrossRef]
- Wang, Y.F.; Barrera, C.M.; Dauer, E.A.; Gu, W.; Andreopoulos, F.; Huang, C.Y.C. Systematic characterization of porosity and mass transport and mechanical properties of porous polyurethane scaffolds. J. Mech. Behav. Biomed. Mater. 2017, 65, 657–664. [Google Scholar] [CrossRef]
- Ikeda, R.; Fujioka, H.; Nagura, I.; Kokubu, T.; Toyokawa, N.; Inui, A.; Makino, T.; Kaneko, H.; Doita, M.; Kurosaka, M. The effect of porosity and mechanical property of a synthetic polymer scaffold on repair of osteochondral defects. Int. Orthop. 2009, 33, 821–828. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Q.; Jiang, Y.; Zhang, Y.; Ye, Z.; Tan, W.; Lang, M. Effect of porosity on long-term degradation of poly ($\varepsilon$-caprolactone) scaffolds and their cellular response. Polym. Degrad. Stab. 2013, 98, 209–218. [Google Scholar] [CrossRef]
- Murphy, W.L.; Kohn, D.H.; Mooney, D.J. Growth of continuous bonelike mineral within porous poly (lactide-co-glycolide) scaffolds in vitro. J. Biomed. Mater. Res. 2000, 50, 50–58. [Google Scholar] [CrossRef]
- Montazerian, H.; Davoodi, E.; Asadi-Eydivand, M.; Kadkhodapour, J.; Solati-Hashjin, M. Porous scaffold internal architecture design based on minimal surfaces: A compromise between permeability and elastic properties. Mater. Des. 2017, 126, 98–114. [Google Scholar] [CrossRef]
- Bobbert, F.S.L.; Lietaert, K.; Eftekhari, A.A.; Pouran, B.; Ahmadi, S.M.; Weinans, H.; Zadpoor, A.A. Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties. Acta Biomater. 2017, 53, 572–584. [Google Scholar] [CrossRef]
- Matsiko, A.; Gleeson, J.P.; O’Brien, F.J. Scaffold Mean Pore Size Influences Mesenchymal Stem Cell Chondrogenic Differentiation and Matrix Deposition. Tissue Eng. Part A 2014, 21, 486–497. [Google Scholar] [CrossRef]
- Yang, J.; Shi, G.; Bei, J.; Wang, S.; Cao, Y.; Shang, Q.; Yang, G.; Wang, W. Fabrication and surface modification of macroporous poly (L-lactic acid) and poly (L-lactic-co-glycolic acid)(70/30) cell scaffolds for human skin fibroblast cell culture. J. Biomed. Mater. Res. 2002, 62, 438–446. [Google Scholar] [CrossRef]
- Wang, H.; Pieper, J.; Péters, F.; van Blitterswijk, C.A.; Lamme, E.N. Synthetic scaffold morphology controls human dermal connective tissue formation. J. Biomed. Mater. Res. Part A 2005, 74A, 523–532. [Google Scholar] [CrossRef]
- Di Luca, A.; Ostrowska, B.; Lorenzo-Moldero, I.; Lepedda, A.; Swieszkowski, W.; Van Blitterswijk, C.; Moroni, L. Gradients in pore size enhance the osteogenic differentiation of human mesenchymal stromal cells in three-dimensional scaffolds. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, T.H.; Im, G.I.; Lee, J.H. Investigation of pore size effect on chondrogenic differentiation of adipose stem cells using a pore size gradient scaffold. Biomacromolecules 2010, 11, 1948–1955. [Google Scholar] [CrossRef]
- Bružauskait, I.; Bironait, D.; Bagdonas, E.; Bernotien, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.R.; Mortisen, D.J.; Sussman, E.M.; Dupras, S.K.; Fugate, J.A.; Cuy, J.L.; Hauch, K.D.; Laflamme, M.A.; Murry, C.E.; Ratner, B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar] [CrossRef] [PubMed]
- Rüdrich, U.; Lasgorceix, M.; Champion, E.; Pascaud-Mathieu, P.; Damia, C.; Chartier, T.; Brie, J.; Magnaudeix, A. Pre-osteoblast cell colonization of porous silicon substituted hydroxyapatite bioceramics: Influence of microporosity and macropore design. Mater. Sci. Eng. C 2019, 97, 510–528. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Arns, C.H.; Hutmacher, D.W.; Milthorpe, B.K.; Sheppard, A.P.; Knackstedt, M.A. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 2009, 30, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Karande, T.S.; Ong, J.L.; Agrawal, C.M. Diffusion in musculoskeletal tissue engineering scaffolds: Design issues related to porosity, permeability, architecture, and nutrient mixing. Ann. Biomed. Eng. 2004, 32, 1728–1743. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, W.; Liu, D.; Zhang, H.; Gao, P.; Geng, L.; Yuan, Y.; Lu, J.; Wang, Z. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci. Rep. 2015, 5, 9409. [Google Scholar] [CrossRef]
- Choi, S.-W.; Zhang, Y.; Xia, Y. Three-dimensional scaffolds for tissue engineering: The importance of uniformity in pore size and structure. Langmuir 2010, 26, 19001–19006. [Google Scholar] [CrossRef]
- Marshall, A.J.; Ratner, B.D. Quantitative characterization of sphere-templated porous biomaterials. AIChE J. 2005, 51, 1221–1232. [Google Scholar] [CrossRef]
- Murphy, W.L.; Dennis, R.G.; Kileny, J.L.; Mooney, D.J. Salt fusion: An approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002, 8, 43–52. [Google Scholar] [CrossRef]
- Kemppainen, J.M.; Hollister, S.J. Differential effects of designed scaffold permeability on chondrogenesis by chondrocytes and bone marrow stromal cells. Biomaterials 2010, 31, 279–287. [Google Scholar] [CrossRef]
- Rumpler, M.; Woesz, A.; Dunlop, J.W.C.; van Dongen, J.T.; Fratzl, P. The effect of geometry on three-dimensional tissue growth. J. R. Soc. Interface 2008, 5, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Bidan, C.M.; Kommareddy, K.P.; Rumpler, M.; Kollmannsberger, P.; Fratzl, P.; Dunlop, J.W.C. Geometry as a Factor for Tissue Growth: Towards Shape Optimization of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2013, 2, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Magnaudeix, A.; Usseglio, J.; Lasgorceix, M.; Lalloue, F.; Damia, C.; Brie, J.; Pascaud-Mathieu, P.; Champion, E. Quantitative analysis of vascular colonisation and angio-conduction in porous silicon-substituted hydroxyapatite with various pore shapes in a chick chorioallantoic membrane (CAM) model. Acta Biomater. 2016, 38, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Guyot, Y.; Papantoniou, I.; Chai, Y.C.; Van Bael, S.; Schrooten, J.; Geris, L. A computational model for cell/ECM growth on 3D surfaces using the level set method: A bone tissue engineering case study. Biomech. Model. Mechanobiol. 2014, 13, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Pieuchot, L.; Marteau, J.; Guignandon, A.; Dos Santos, T.; Brigaud, I.; Chauvy, P.-F.; Cloatre, T.; Ponche, A.; Petithory, T.; Rougerie, P.; et al. Curvotaxis directs cell migration through cell-scale curvature landscapes. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.; Kurniawan, N.A.; Korus, G.; Bouten, C.V.C.; Petersen, A. Mesoscale substrate curvature overrules nanoscale contact guidance to direct bone marrow stromal cell migration. J. R. Soc. Interface 2018, 15, 20180162. [Google Scholar] [CrossRef]
- Buenzli, P.R.; Lanaro, M.; Wong, C.S.; McLaughlin, M.P.; Allenby, M.C.; Woodruff, M.A.; Simpson, M.J. Cell proliferation and migration explain pore bridging dynamics in 3D printed scaffolds of different pore size. bioRxiv 2020. [Google Scholar] [CrossRef]
- He, X.; Jiang, Y. Substrate curvature regulates cell migration. Phys. Biol. 2017, 14, 35006. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802. [Google Scholar] [CrossRef]
- Pek, Y.S.; Wan, A.C.A.; Ying, J.Y. The effect of matrix stiffness on mesenchymal stem cell differentiation in a 3D thixotropic gel. Biomaterials 2010, 31, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Lozoya, O.A.; Wauthier, E.; Turner, R.A.; Barbier, C.; Prestwich, G.D.; Guilak, F.; Superfine, R.; Lubkin, S.R.; Reid, L.M. Regulation of hepatic stem/progenitor phenotype by microenvironment stiffness in hydrogel models of the human liver stem cell niche. Biomaterials 2011, 32, 7389–7402. [Google Scholar] [CrossRef] [PubMed]
- Levy-Mishali, M.; Zoldan, J.; Levenberg, S. Effect of scaffold stiffness on myoblast differentiation. Tissue Eng. Part A 2009, 15, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Ryan, E.J.; Kearney, C.J.; Kelly, D.J.; O’Brien, F.J. Macrophage polarization in response to collagen scaffold stiffness is dependent on cross-linking agent used to modulate the stiffness. ACS Biomater. Sci. Eng. 2018, 5, 544–552. [Google Scholar] [CrossRef]
- Petersen, A.; Joly, P.; Bergmann, C.; Korus, G.; Duda, G.N. The Impact of Substrate Stiffness and Mechanical Loading on Fibroblast-Induced Scaffold Remodeling. Tissue Eng. Part A 2012, 18, 1804–1817. [Google Scholar] [CrossRef]
- Baker, B.M.; Shah, R.P.; Huang, A.H.; Mauck, R.L. Dynamic Tensile Loading Improves the Functional Properties of Mesenchymal Stem Cell-Laden Nanofiber-Based Fibrocartilage. Tissue Eng. Part A 2011, 17, 1445–1455. [Google Scholar] [CrossRef]
- Wernike, E.; Li, Z.; Alini, M.; Grad, S. Effect of reduced oxygen tension and long-term mechanical stimulation on chondrocyte-polymer constructs. Cell Tissue Res. 2008, 331, 473–483. [Google Scholar] [CrossRef]
- Roether, J.; Bertels, S.; Oelschlaeger, C.; Bastmeyer, M.; Willenbacher, N. Microstructure, local viscoelasticity and cell culture suitability of 3D hybrid HA/collagen scaffolds. PLoS ONE 2018, 13, e0207397. [Google Scholar] [CrossRef]
- Grier, W.K.; Iyoha, E.M.; Harley, B.A.C. The influence of pore size and stiffness on tenocyte bioactivity and transcriptomic stability in collagen-GAG scaffolds. J. Mech. Behav. Biomed. Mater. 2017, 65, 295–305. [Google Scholar] [CrossRef]
- Harley, B.A.; Freyman, T.M.; Wong, M.Q.; Gibson, L.J. A new technique for calculating individual dermal fibroblast contractile forces generated within collagen-GAG scaffolds. Biophys. J. 2007, 93, 2911–2922. [Google Scholar] [CrossRef]
- Freyman, T.M.; Yannas, I.V.; Yokoo, R.; Gibson, L.J. Fibroblast contraction of a collagen--GAG matrix. Biomaterials 2001, 22, 2883–2891. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2015, 15, 326. [Google Scholar] [CrossRef]
- McKinnon, D.D.; Domaille, D.W.; Cha, J.N.; Anseth, K.S. Biophysically Defined and Cytocompatible Covalently Adaptable Networks as Viscoelastic 3D Cell Culture Systems. Adv. Mater. 2014, 26, 865–872. [Google Scholar] [CrossRef]
- Ranga, A.; Gobaa, S.; Okawa, Y.; Mosiewicz, K.; Negro, A.; Lutolf, M.P. 3D niche microarrays for systems-level analyses of cell fate. Nat. Commun. 2014, 5, 4324. [Google Scholar] [CrossRef]
- Wang, C.; Hou, W.; Guo, X.; Li, J.; Hu, T.; Qiu, M.; Liu, S.; Mo, X.; Liu, X. Two-phase electrospinning to incorporate growth factors loaded chitosan nanoparticles into electrospun fibrous scaffolds for bioactivity retention and cartilage regeneration. Mater. Sci. Eng. C 2017, 79, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Carrow, J.K.; Di Luca, A.; Dolatshahi-Pirouz, A.; Moroni, L.; Gaharwar, A.K. 3D-printed bioactive scaffolds from nanosilicates and PEOT/PBT for bone tissue engineering. Regen. Biomater. 2018, 6, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kang, Y.; Bao, J.; Zhang, Y.; Yang, Y.; Zhou, X. Modeling vascularized bone regeneration within a porous biodegradable CaP scaffold loaded with growth factors. Biomaterials 2013, 34, 4971–4981. [Google Scholar] [CrossRef] [PubMed]
- Elsdale, T.; Bard, J. Collagen substrata for studies on cell behavior. J. Cell Biol. 1972, 54, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.; Turner, L.-A.; Saeed, A.; Burgess, K.; Blackburn, G.; Reynolds, P.; Wells, J.; Mountford, J.; Gadegaard, N.; Salmeron-Sanchez, M.; et al. Nanotopography reveals metabolites that maintain the immunosuppressive phenotype of mesenchymal stem cells. bioRxiv 2019. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.; Park, S.; Kim, S.; Lee, D.; Kim, W.; Kim, J. Nanopatterned scaffolds for neural tissue engineering and regenerative medicine. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Springer: Berlin/Heidelberg, Germany, 2018; pp. 421–443. [Google Scholar]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. In vitro dissolution of melt-derived 45S5 and sol-gel derived 58S bioactive glasses. J. Biomed. Mater. Res. 2002, 61, 301–311. [Google Scholar] [CrossRef]
- Jones, J.R. New trends in bioactive scaffolds: The importance of nanostructure. J. Eur. Ceram. Soc. 2009, 29, 1275–1281. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. J. Biol. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef]
- Benedetto, A.; Accetta, G.; Fujita, Y.; Charras, G. Spatiotemporal control of gene expression using microfluidics. Lab Chip 2014, 14, 1336–1347. [Google Scholar] [CrossRef]
- Perrier-Groult, E.; Ronzière, M.-C.; Bareille, R.; Pinzano, A.; Mallein-Gerin, F.; Freyria, A.-M. Analysis of collagen expression during chondrogenic induction of human bone marrow mesenchymal stem cells. Biotechnol. Lett. 2011, 33, 2091–2101. [Google Scholar] [CrossRef]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.K.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef]
- Shah, N.J.; Macdonald, M.L.; Beben, Y.M.; Padera, R.F.; Samuel, R.E.; Hammond, P.T. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials 2011, 32, 6183–6193. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef]
- Koons, G.L.; Mikos, A.G. Progress in three-dimensional printing with growth factors. J. Control. release 2019, 295, 50–59. [Google Scholar] [CrossRef]
- D’Souza, S.E.; Ginsberg, M.H.; Plow, E.F. Arginyl-glycyl-aspartic acid (RGD): A cell adhesion motif. Trends Biochem. Sci. 1991, 16, 246–250. [Google Scholar] [CrossRef]
- Rodriguez-Cabello, J.C.; De Torre, I.G.; Ibañez-Fonseca, A.; Alonso, M. Bioactive scaffolds based on elastin-like materials for wound healing. Adv. Drug Deliv. Rev. 2018, 129, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-H.; Jeon, S.H.; Park, J.-Y.; Chung, J.-H.; Choung, Y.-H.; Choung, H.-W.; Kim, E.-S.; Choung, P.-H. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng. Part A 2011, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Naves, A.F.; Motay, M.; Mérindol, R.; Davi, C.P.; Felix, O.; Catalani, L.H.; Decher, G. Layer-by-Layer assembled growth factor reservoirs for steering the response of 3T3-cells. Colloids Surfaces B Biointerfaces 2016, 139, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Harper, M.M.; Connolly, M.L.; Goldie, L.; Irvine, E.J.; Shaw, J.E.; Jayawarna, V.; Richardson, S.M.; Dalby, M.J.; Lightbody, D.; Ulijn, R. V Biogelx: Cell Culture on Self-Assembling Peptide Gels BT-Peptide Self-Assembly: Methods and Protocols. In Peptide Self-Assembly: Methods and Protocols; Nilsson, B.L., Doran, T.M., Eds.; Springer: New York, NY, USA, 2018; pp. 283–303. [Google Scholar]
- Dankers, P.Y.W.; Harmsen, M.C.; Brouwer, L.A.; Van Luyn, M.J.A.; Meijer, E.W. A modular and supramolecular approach to bioactive scaffolds for tissue engineering. Nat. Mater. 2005, 4, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.A.; Motwani, M.S.; Faull, P.A.; Seymour, A.J.; Yu, T.T.L.; Enayati, M.; Taheem, D.K.; Salzlechner, C.; Haghighi, T.; Kania, E.M.; et al. Bi-directional cell-pericellular matrix interactions direct stem cell fate. Nat. Commun. 2018, 9, 4049. [Google Scholar] [CrossRef]
- Loebel, C.; Mauck, R.L.; Burdick, J.A. Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat. Mater. 2019, 18, 883–891. [Google Scholar] [CrossRef]
- Saunders, L.; Ma, P.X. Self-Healing Supramolecular Hydrogels for Tissue Engineering Applications. Macromol. Biosci. 2019, 19, 1800313. [Google Scholar] [CrossRef]
- Azevedo, S.; Costa, A.M.S.; Andersen, A.; Choi, I.S.; Birkedal, H.; Mano, J.F. Bioinspired Ultratough Hydrogel with Fast Recovery, Self-Healing, Injectability and Cytocompatibility. Adv. Mater. 2017, 29, 1700759. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Wang, X.; Huang, L.; Chen, Y.; Bao, C. Glucose-sensitive delivery of metronidazole by using a photo-crosslinked chitosan hydrogel film to inhibit Porphyromonas gingivalis proliferation. Int. J. Biol. Macromol. 2019, 122, 19–28. [Google Scholar] [CrossRef]
- Bacelar, A.H.; Cengiz, I.F.; Silva-Correia, J.; Sousa, R.A.; Oliveira, J.M.; Reis, R.L. “Smart” hydrogels in tissue engineering and regenerative medicine applications. In Handbook of Intelligent Scaffolds for Tissue Engineering and Regenerative Medicine; Jenny Stanford: Singapore, 2017; pp. 333–367. [Google Scholar]
- Tienen, T.G.; Heijkants, R.G.J.C.; de Groot, J.H.; Pennings, A.J.; Schouten, A.J.; Veth, R.P.H.; Buma, P. Replacement of the knee meniscus by a porous polymer implant: A study in dogs. Am. J. Sports Med. 2006, 34, 64–71. [Google Scholar] [CrossRef] [PubMed]
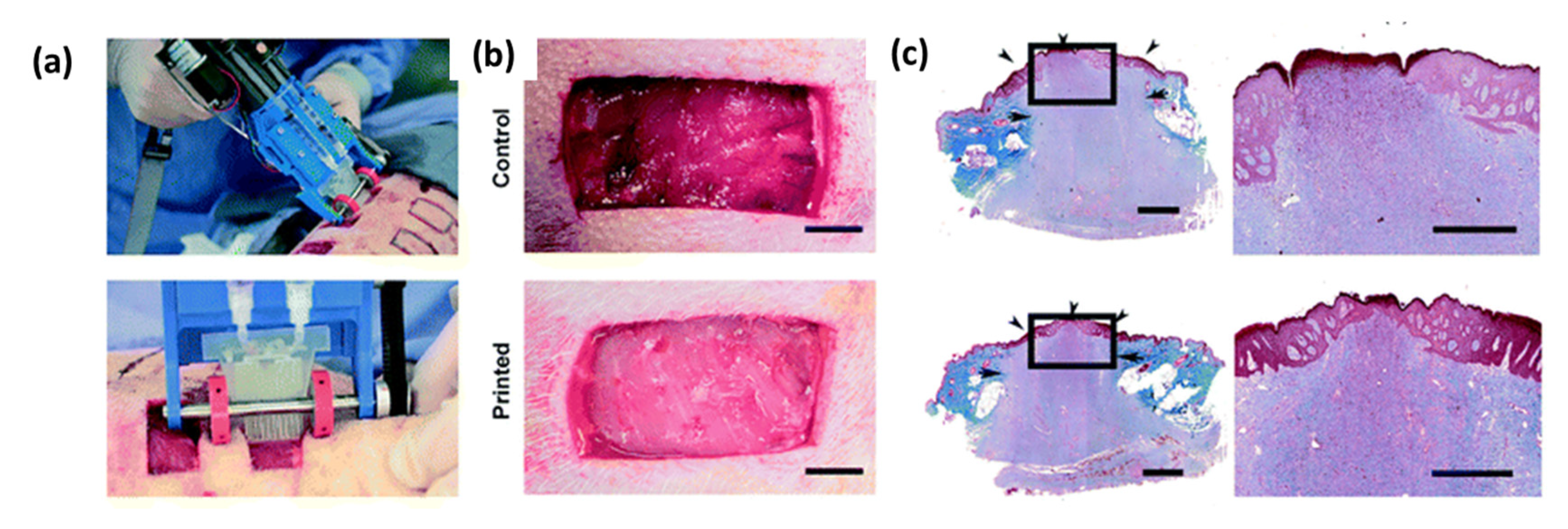
- Kim, H.Y.; Jung, S.Y.; Lee, S.J.; Lee, H.J.; Truong, M.-D.; Kim, H.S. Fabrication and characterization of 3D-printed elastic auricular scaffolds: A pilot study. Laryngoscope 2019, 129, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ma, H.; Zhang, L.; Bryers, J.D. Precision-porous templated scaffolds of varying pore size drive dendritic cell activation. Biotechnol. Bioeng. 2018, 115, 1086–1095. [Google Scholar] [CrossRef]
- Feng, B.; Jinkang, Z.; Zhen, W.; Jianxi, L.; Jiang, C.; Jian, L.; Guolin, M.; Xin, D. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecture in vivo. Biomed. Mater. 2011, 6, 15007. [Google Scholar] [CrossRef] [PubMed]
- Rieger, E.; Dupret-Bories, A.; Salou, L.; Metz-Boutigue, M.-H.; Layrolle, P.; Debry, C.; Lavalle, P.; Engin Vrana, N. Controlled implant/soft tissue interaction by nanoscale surface modifications of 3D porous titanium implants. Nanoscale 2015, 7, 9908–9918. [Google Scholar] [CrossRef]
- Vrana, N.E.; Dupret-Bories, A.; Schultz, P.; Debry, C.; Vautier, D.; Lavalle, P. Titanium Microbead-Based Porous Implants: Bead Size Controls Cell Response and Host Integration. Adv. Healthc. Mater. 2013, 3, 79–87. [Google Scholar] [CrossRef]
- Lu, T.; Feng, S.; He, F.; Ye, J. Enhanced osteogenesis of honeycomb β-tricalcium phosphate scaffold by construction of interconnected pore structure: An in vivo study. J. Biomed. Mater. Res. Part A 2020, 108, 645–653. [Google Scholar] [CrossRef]
- Webber, M.J.; Khan, O.F.; Sydlik, S.A.; Tang, B.C.; Langer, R. A Perspective on the Clinical Translation of Scaffolds for Tissue Engineering. Ann. Biomed. Eng. 2015, 43, 641–656. [Google Scholar] [CrossRef]
- Kon, E.; Roffi, A.; Filardo, G.; Tesei, G.; Marcacci, M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 767–775. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Pan, W.; Yang, F.; Jiang, W.; Wu, X.; Kong, X.; Dai, K.; Hao, Y. In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci. Rep. 2016, 6, 34072. [Google Scholar] [CrossRef]
- Moradi, L.; Vasei, M.; Dehghan, M.M.; Majidi, M.; Farzad Mohajeri, S.; Bonakdar, S. Regeneration of meniscus tissue using adipose mesenchymal stem cells-chondrocytes co-culture on a hybrid scaffold: In vivo study. Biomaterials 2017, 126, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, N.; Cheng, R.; Leng, L.; Sotoudehfar, M.; Ba, P.Q.; Bakhtyar, N.; Amini-Nik, S.; Jeschke, M.G.; Günther, A. Handheld skin printer: In situ formation of planar biomaterials and tissues. Lab Chip 2018, 18, 1440–1451. [Google Scholar] [CrossRef]
- Fadia, N.B.; Bliley, J.M.; DiBernardo, G.A.; Crammond, D.J.; Schilling, B.K.; Sivak, W.N.; Spiess, A.M.; Washington, K.M.; Waldner, M.; Liao, H.-T.; et al. Long-gap peripheral nerve repair through sustained release of a neurotrophic factor in nonhuman primates. Sci. Transl. Med. 2020, 12, eaav7753. [Google Scholar] [CrossRef]
- Okesola, B.O.; Ni, S.; Derkus, B.; Galeano, C.C.; Hasan, A.; Wu, Y.; Ramis, J.; Buttery, L.; Dawson, J.I.; D’Este, M.; et al. Growth-Factor Free Multicomponent Nanocomposite Hydrogels That Stimulate Bone Formation. Adv. Funct. Mater. 2020, 30, 1906205. [Google Scholar] [CrossRef]
- Madry, H.; Alini, M.; Stoddart, M.J.; Evans, C.; Miclau, T.; Steiner, S. Barriers and strategies for the clinical translation of advanced orthopaedic tissue engineering protocols. Eur. Cell Mater. 2014, 27, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Naughton, G.; Mansbridge, J.; Gentzkow, G. A Metabolically Active Human Dermal Replacement for the Treatment of Diabetic Foot Ulcers. Artif. Organs 1997, 21, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Sabolinski, M. A bilayered living skin construct (APLIGRAF®) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999, 7, 201–207. [Google Scholar] [CrossRef]
- Machens, H.-G.; Berger, A.C.; Mailaender, P. Bioartificial skin. Cells Tissues Organs 2000, 167, 88–94. [Google Scholar] [CrossRef]
- Debry, C.; Vrana, N.E.; Dupret-Bories, A. Implantation of an Artificial Larynx after Total Laryngectomy. N. Engl. J. Med. 2017, 376, 97–98. [Google Scholar] [CrossRef]
- Leroy, A.; Beaufils, P.; Faivre, B.; Steltzlen, C.; Boisrenoult, P.; Pujol, N. Actifit® polyurethane meniscal scaffold: MRI and functional outcomes after a minimum follow-up of 5 years. Orthop. Traumatol. Surg. Res. 2017, 103, 609–614. [Google Scholar] [CrossRef]
- Xiao, Z.; Tang, F.; Tang, J.; Yang, H.; Zhao, Y.; Chen, B.; Han, S.; Wang, N.; Li, X.; Cheng, S.; et al. One-year clinical study of NeuroRegen scaffold implantation following scar resection in complete chronic spinal cord injury patients. Sci. China Life Sci. 2016, 59, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Theodore, N.; Hlubek, R.; Danielson, J.; Neff, K.; Vaickus, L.; Ulich, T.R.; Ropper, A.E. First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: A clinical pilot study for safety and feasibility. Neurosurgery 2016, 79, E305–E312. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.D.; Lavik, E.B.; Qu, X.; Park, K.I.; Ourednik, J.; Zurakowski, D.; Langer, R.; Snyder, E.Y. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- El Shazley, N.; Hamdy, A.; El-Eneen, H.A.; El Backly, R.M.; Saad, M.M.; Essam, W.; Moussa, H.; El Tantawi, M.; Jain, H.; Marei, M.K. Bioglass in alveolar bone regeneration in orthodontic patients: Randomized controlled clinical trial. JDR Clin. Transl. Res. 2016, 1, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Brunton, P.A.; Davies, R.P.W.; Burke, J.L.; Smith, A.; Aggeli, A.; Brookes, S.J.; Kirkham, J. Treatment of early caries lesions using biomimetic self-assembling peptides--a clinical safety trial. Br. Dent. J. 2013, 215, E6. [Google Scholar] [CrossRef]
- Saha, S.; Yang, X.B.; Wijayathunga, N.; Harris, S.; Feichtinger, G.A.; Davies, R.P.W.; Kirkham, J. A biomimetic self-assembling peptide promotes bone regeneration in vivo: A rat cranial defect study. Bone 2019, 127, 602–611. [Google Scholar] [CrossRef]
- Alvarez, M.M.; Liu, J.C.; Trujillo-de Santiago, G.; Cha, B.H.; Vishwakarma, A.; Ghaemmaghami, A.M.; Khademhosseini, A. Delivery strategies to control inflammatory response: Modulating M1-M2 polarization in tissue engineering applications. J. Control. Release 2015, 240, 349–363. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Marshall, A.J. Biomaterials with tightly controlled poresize that promote vascular in-growth. Polym. Prepr. 2004, 45, 100–101. [Google Scholar]
- Sadtler, K.; Estrellas, K.; Allen, B.W.; Wolf, M.T.; Fan, H.; Tam, A.J.; Patel, C.H.; Luber, B.S.; Wang, H.; Wagner, K.R.; et al. Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 2016, 352, 366–370. [Google Scholar] [CrossRef]
- Hook, A.L.; Anderson, D.G.; Langer, R.; Williams, P.; Davies, M.C.; Alexander, M.R. High throughput methods applied in biomaterial development and discovery. Biomaterials 2010, 31, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Barthes, J.; Cazzola, M.; Muller, C.; Dollinger, C.; Debry, C.; Ferraris, S.; Spriano, S.; Vrana, N.E. Controlling porous titanium/soft tissue interactions with an innovative surface chemical treatment: Responses of macrophages and fibroblasts. Mater. Sci. Eng. C 2020, 112, 110845. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Vrana, N.E.; Ghaemmaghami, A.M.; Zorlutuna, P. Adverse Reactions to Biomaterials: State of the Art in Biomaterial Risk Assessment, Immunomodulation and in vitro Models for Biomaterial Testing. Front. Bioeng. Biotechnol. 2019, 7, 15. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutzweiler, G.; Ndreu Halili, A.; Engin Vrana, N. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics 2020, 12, 602. https://doi.org/10.3390/pharmaceutics12070602
Lutzweiler G, Ndreu Halili A, Engin Vrana N. The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics. 2020; 12(7):602. https://doi.org/10.3390/pharmaceutics12070602
Chicago/Turabian StyleLutzweiler, Gaëtan, Albana Ndreu Halili, and Nihal Engin Vrana. 2020. "The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation" Pharmaceutics 12, no. 7: 602. https://doi.org/10.3390/pharmaceutics12070602
APA StyleLutzweiler, G., Ndreu Halili, A., & Engin Vrana, N. (2020). The Overview of Porous, Bioactive Scaffolds as Instructive Biomaterials for Tissue Regeneration and Their Clinical Translation. Pharmaceutics, 12(7), 602. https://doi.org/10.3390/pharmaceutics12070602




