A Precise Prediction Method for the Properties of API-Containing Tablets Based on Data from Placebo Tablets
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tablet Formulation
2.3. Evaluation of Tablet Properties
2.4. Construction and Comparison of the Response Surfaces for the Tablet Properties of Placebo- and API-Containing Tablets
2.5. Prediction of the Response Surfaces Using Regression Analyses and Data from Placebo Tablets
2.6. Evaluation of the Prediction Accuracy
2.7. Computer Programs
3. Results and Discussion
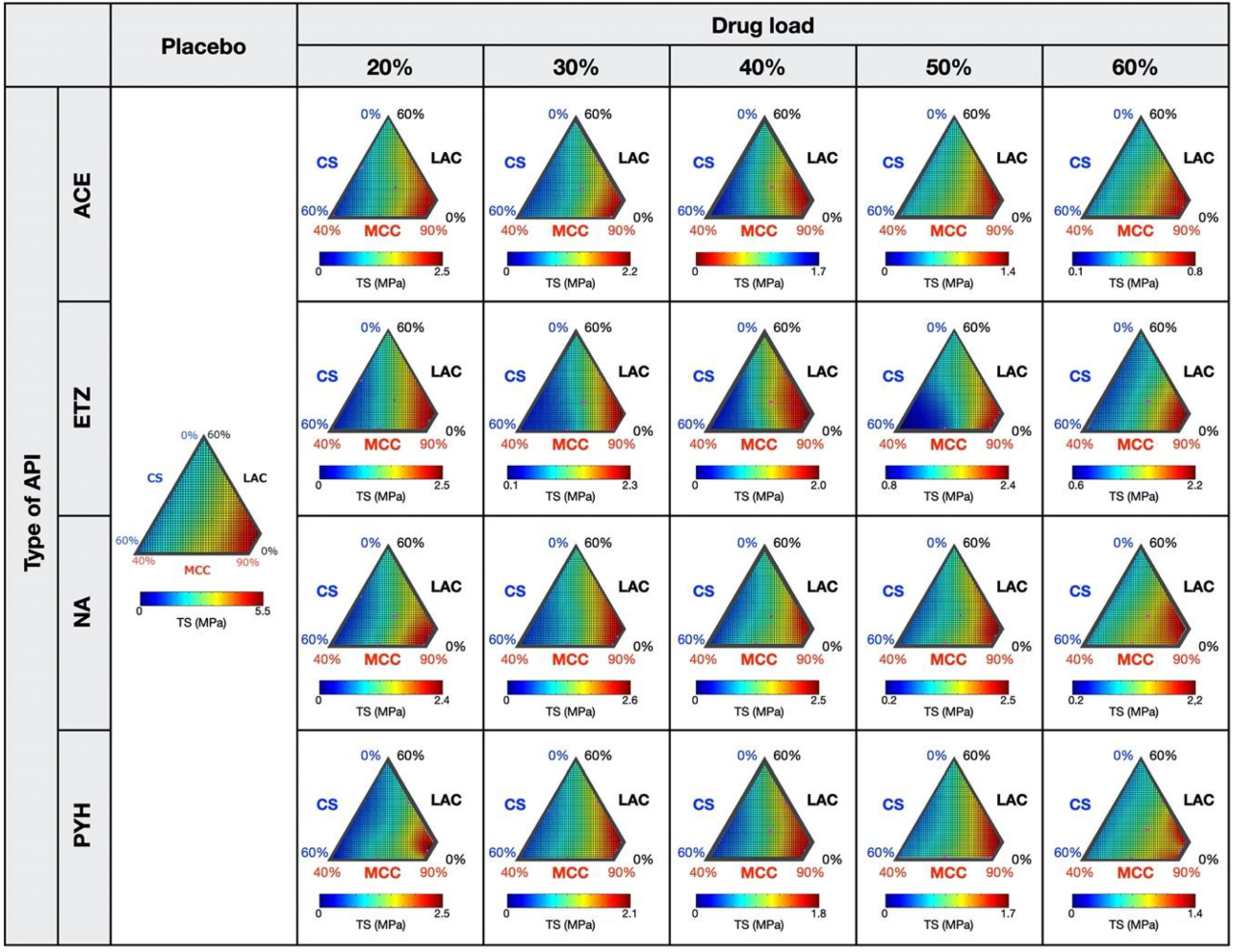
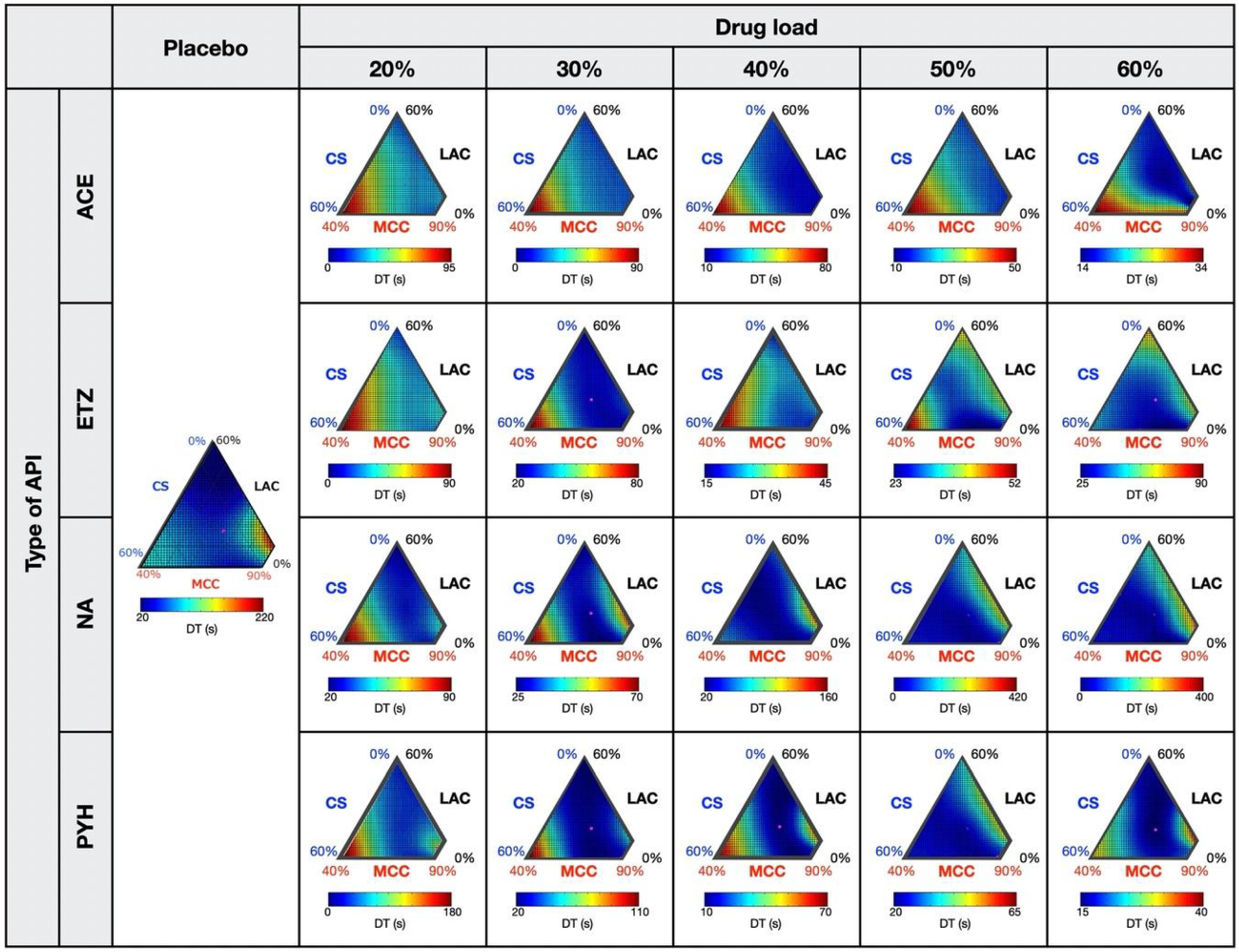
3.1. Response Surfaces for the TS and DT of Placebo- and API-Containing Tablets
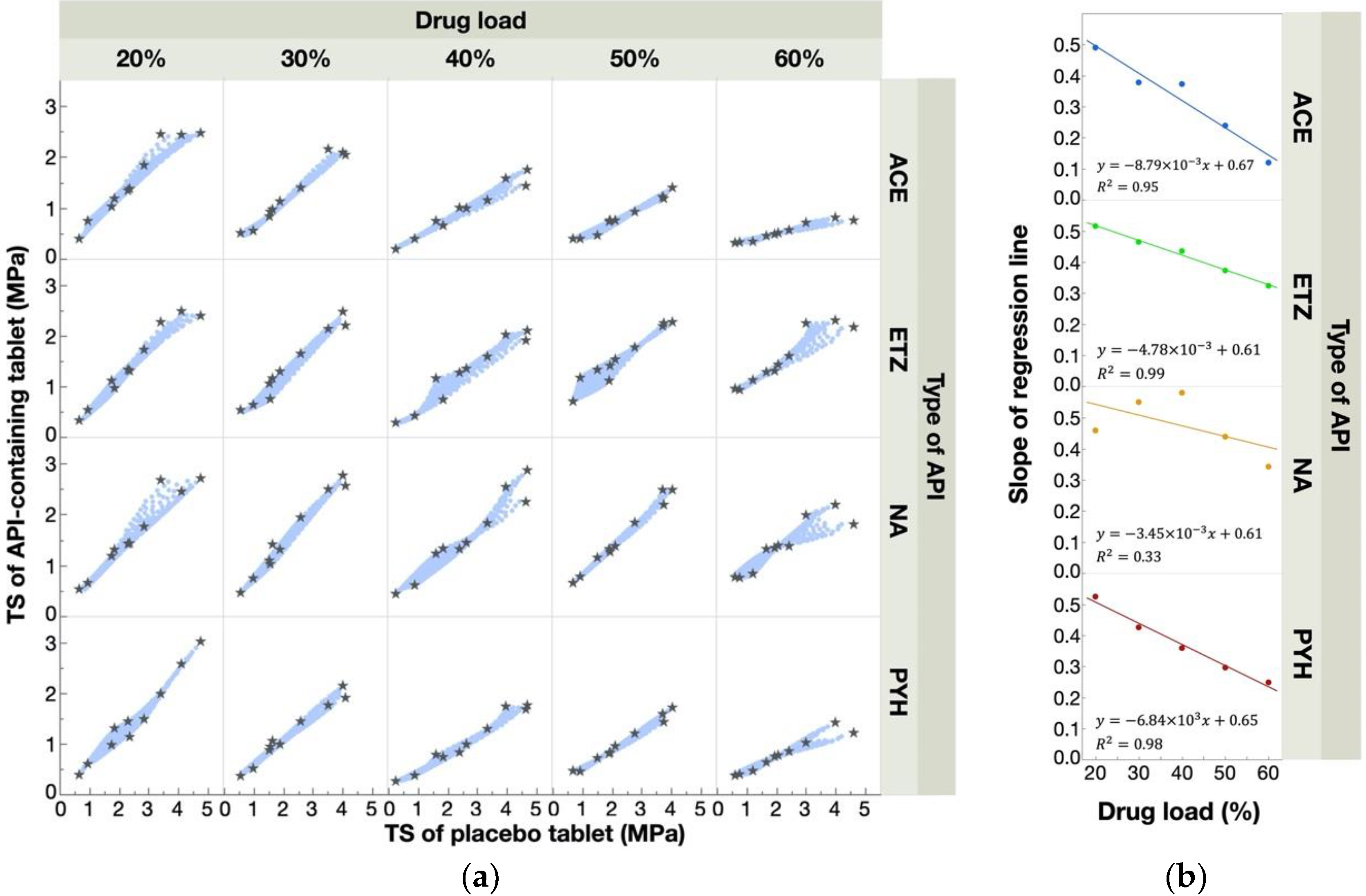
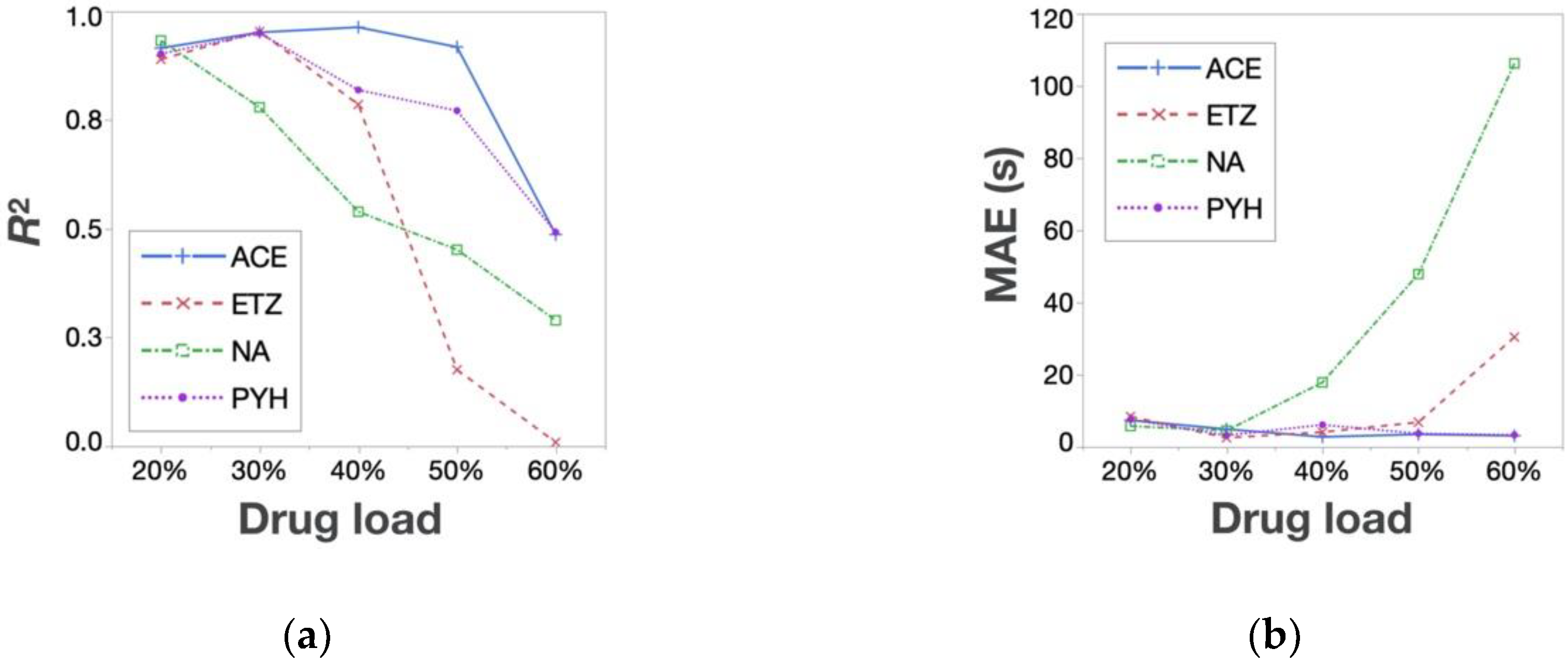
3.2. Relationships between Response Surfaces of Tablet Properties for Placebo- and API-Containing Tablets
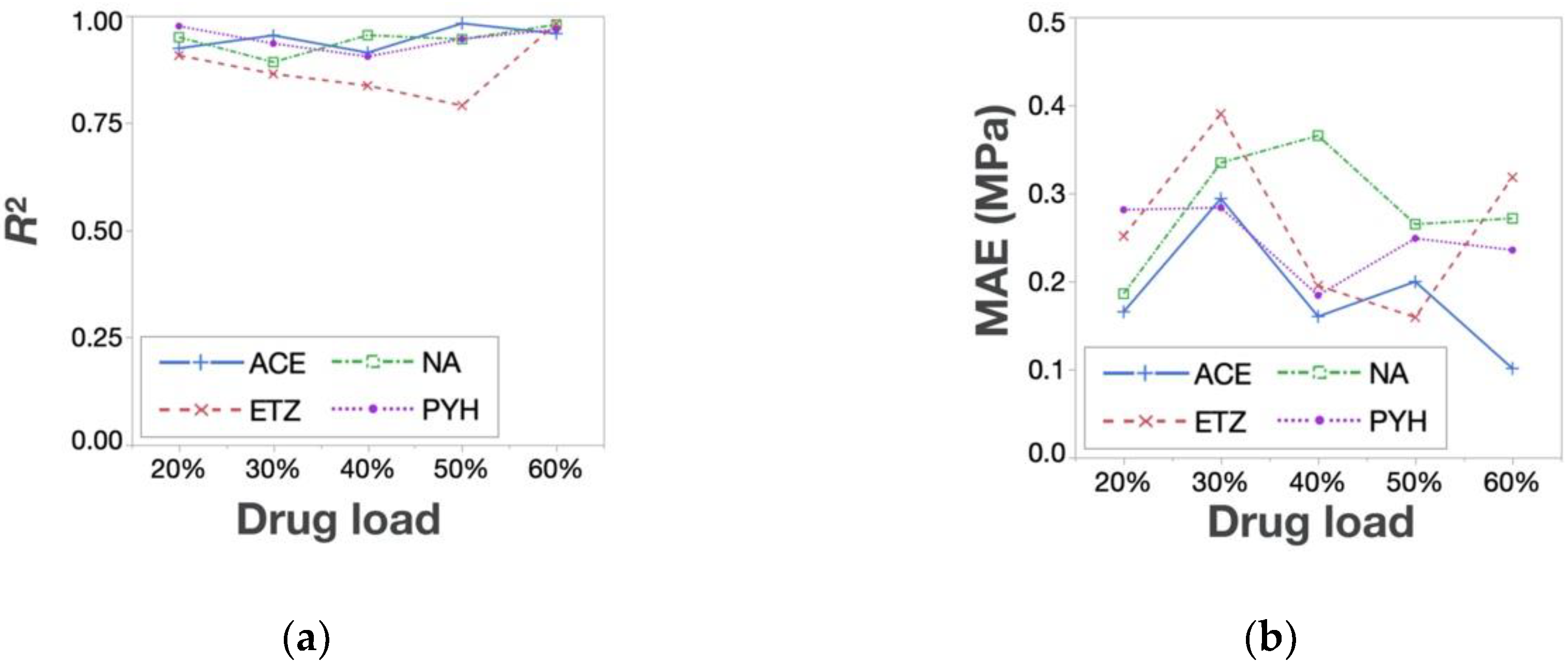
3.3. Prediction of Response Surfaces for Tablet Properties of API-Containing Tablets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef] [PubMed]
- The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH); ICH Harmonised Tripartite Guide, Pharmaceutical Development Q8(R2). 2009. Available online: https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf (accessed on 27 June 2020).
- Takayama, K.; Fujikawa, M.; Obata, Y.; Morishita, M. Neural network based optimization of drug formulations. Adv. Drug Deliv. Rev. 2003, 55, 1217–1231. [Google Scholar] [CrossRef]
- Djuris, J.; Medarevic, D.; Krstic, M.; Djuric, Z.; Ibric, S. Application of quality by design concepts in the development of fluidized bed granulation and tableting processes. J. Pharm. Sci. 2013, 102, 1869–1882. [Google Scholar] [CrossRef]
- Fahmy, R.; Kona, R.; Dandu, R.; Xie, W.; Claycamp, G.; Hoag, S.W. Quality by design I: Application of failure mode effect analysis (FMEA) and Plackett-Burman design of experiments in the identification of “main factors” in the formulation and process design space for roller-compacted ciprofloxacin hydrochloride immediat. AAPS PharmSciTech 2012, 13, 1243–1254. [Google Scholar] [CrossRef]
- Dumarey, M.; Goodwin, D.J.; Davison, C. Multivariate modelling to study the effect of the manufacturing process on the complete tablet dissolution profile. Int. J. Pharm. 2015, 486, 112–120. [Google Scholar] [CrossRef]
- Faulhammer, E.; Fink, M.; Llusa, M.; Lawrence, S.M.; Biserni, S.; Calzolari, V.; Khinast, J.G. Low-dose capsule filling of inhalation products: critical material attributes and process parameters. Int. J. Pharm. 2014, 473, 617–626. [Google Scholar] [CrossRef]
- Matji, A.; Donato, N.; Gagol, A.; Morales, E.; Carvajal, L.; Serrano, D.R.; Worku, Z.A.; Healy, A.M.; Torrado, J.J. Predicting the critical quality attributes of ibuprofen tablets via modelling of process parameters for roller compaction and tabletting. Int. J. Pharm. 2019, 565, 209–218. [Google Scholar] [CrossRef]
- Kikuchi, S.; Takayama, K. Multivariate statistical approach to optimizing sustained-release tablet formulations containing diltiazem hydrochloride as a model highly water-soluble drug. Int. J. Pharm. 2010, 386, 149–155. [Google Scholar] [CrossRef]
- Petrović, J.; Ibrić, S.; Betz, G.; Đurić, Z. Optimization of matrix tablets controlled drug release using Elman dynamic neural networks and decision trees. Int. J. Pharm. 2012, 428, 57–67. [Google Scholar] [CrossRef]
- Hayashi, Y.; Oshima, E.; Maeda, J.; Onuki, Y.; Obata, Y.; Takayama, K. Latent structure analysis of the process variables and pharmaceutical responses of an orally disintegrating tablet. Chem. Pharm. Bull. 2012, 60, 1419–1425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rambali, B.; Baert, L.; Massart, D.L. Using experimental design to optimize the process parameters in fluidized bed granulation on a semi-full scale. Int. J. Pharm. 2001, 220, 149–160. [Google Scholar] [CrossRef]
- Hayashi, Y.; Kikuchi, S.; Onuki, Y.; Takayama, K. Reliability evaluation of nonlinear design space in pharmaceutical product development. J. Pharm. Sci. 2012, 101, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Sovány, T.; Tislér, Z.; Kristó, K.; Kelemen, A.; Regdon, G. Estimation of design space for an extrusion–spheronization process using response surface methodology and artificial neural network modelling. Eur. J. Pharm. Biopharm. 2016, 106, 79–87. [Google Scholar] [CrossRef]
- Norioka, T.; Hayashi, Y.; Onuki, Y.; Andou, H.; Tsunashima, D.; Yamashita, K.; Takayama, K. A novel approach to establishing the design space for the oral formulation manufacturing process. Chem. Pharm. Bull. 2013, 61, 39–49. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazumder, S.; Pavurala, N.; Manda, P.; Xu, X.; Cruz, C.N.; Krishnaiah, Y.S.R. Quality by design approach for studying the impact of formulation and process variables on product quality of oral disintegrating films. Int. J. Pharm. 2017, 527, 151–160. [Google Scholar] [CrossRef]
- Liu, H.; Galbraith, S.C.; Ricart, B.; Stanton, C.; Smith-Goettler, B.; Verdi, L.; O’Connor, T.; Lee, S.; Yoon, S. Optimization of critical quality attributes in continuous twin-screw wet granulation via design space validated with pilot scale experimental data. Int. J. Pharm. 2017, 525, 249–263. [Google Scholar] [CrossRef]
- Leuenberger, H.; Puchkov, M.; Schneider, B. Right first time concept and workflow a paradigm shift for a smart & lean six-sigma development. Swiss J. Pharm. Ind. 2013, 35, 3–16. [Google Scholar]
- Millen, N.; Kovacevic, A.; Khera, L.; Djuris, J.; Ibric, S. Machine learning modelling of wet granulation scale-up using compressibility, compactibility and manufacturability parameters. Hem. Ind. Ind. 2019, 73, 155–168. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Z.; Su, Y.; Zhao, Q.; Li, X.; Ouyang, D. Deep learning for in vitro prediction of pharmaceutical formulations. Acta Pharm. Sin. B 2019, 9, 177–185. [Google Scholar] [CrossRef]
- Hayashi, Y.; Tsuji, T.; Shirotori, K.; Oishi, T.; Kosugi, A.; Kumada, S.; Hirai, D.; Takayama, K.; Onuki, Y. Relationships between response surfaces for tablet characteristics of placebo and API-containing tablets manufactured by direct compression method. Int. J. Pharm. 2017, 532, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Suzuki, T.; Kaseda, C.; Ohyama, K.; Takayama, K. Bootstrap re-sampling technique to evaluate the optimal formulation of theophylline tablets predicted by non-linear response surface method incorporating multivariate spline interpolation. Chem. Pharm. Bull. (Tokyo) 2007, 55, 586–593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tye, C.K.; Sun, C.C.; Amidon, G.E. Evaluation of the effects of tableting speed on the relationships between compaction pressure, tablet tensile strength, and tablet solid fraction. J. Pharm. Sci. 2005, 94, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Demiri, V.; Stranzinger, S.; Rinner, P.; Piller, M.; Sacher, S.; Lingitz, J.; Khinast, J.; Salar-Behzadi, S. Gluing Pills Technology: A novel route to multilayer tablet manufacturing. Int. J. Pharm. 2018, 548, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Law, Y.; Chakrabarti, S. Physical properties and compact analysis of commonly used direct compression binders. AAPS PharmSciTech 2004, 4, 489–499. [Google Scholar] [CrossRef]
- Fonteyne, M.; Wickström, H.; Peeters, E.; Vercruysse, J.; Ehlers, H.; Peters, B.-H.H.; Remon, J.P.; Vervaet, C.; Ketolainen, J.; Sandler, N.; et al. Influence of raw material properties upon critical quality attributes of continuously produced granules and tablets. Eur. J. Pharm. Biopharm. 2014, 87, 252–263. [Google Scholar] [CrossRef]
- Sangekar, S.A.; Sarli, M.; Sheth, P.R. Effect of moisture on physical characteristics of tablets prepared from direct compression excipients. J. Pharm. Sci. 1972, 61, 939–944. [Google Scholar] [CrossRef]
- Haware, R.V.; Tho, I.; Bauer-Brandl, A. Evaluation of a rapid approximation method for the elastic recovery of tablets. Powder Technol. 2010, 202, 71–77. [Google Scholar] [CrossRef]
- Hayashi, Y.; Oishi, T.; Shirotori, K.; Marumo, Y.; Kosugi, A.; Kumada, S.; Hirai, D.; Takayama, K.; Onuki, Y. Modeling of quantitative relationships between physicochemical properties of active pharmaceutical ingredients and tensile strength of tablets using a boosted tree. Drug Dev. Ind. Pharm. 2018, 44, 1090–1098. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Rozet, E.; Carlin, B.; Evrard, B. Understanding the impact of microcrystalline cellulose physicochemical properties on tabletability. Int. J. Pharm. 2015, 490, 47–54. [Google Scholar] [CrossRef]
- Khomane, K.S.; Bansal, A.K. Effect of particle size on in-die and out-of-die compaction behavior of ranitidine hydrochloride polymorphs. AAPS PharmSciTech 2013, 14, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Wünsch, I.; Friesen, I.; Puckhaber, D.; Schlegel, T.; Finke, J.H. Scaling tableting processes from compaction simulator to rotary presses—Mind the sub-processes. Pharmaceutics 2020, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Markl, D.; Zeitler, J.A. A review of disintegration mechanisms and measurement techniques. Pharm. Res. 2017, 34, 890–917. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.M.; Liew, C.V.; Heng, P.W.S. Review of disintegrants and the disintegration phenomena. J. Pharm. Sci. 2016, 105, 2545–2555. [Google Scholar] [CrossRef]
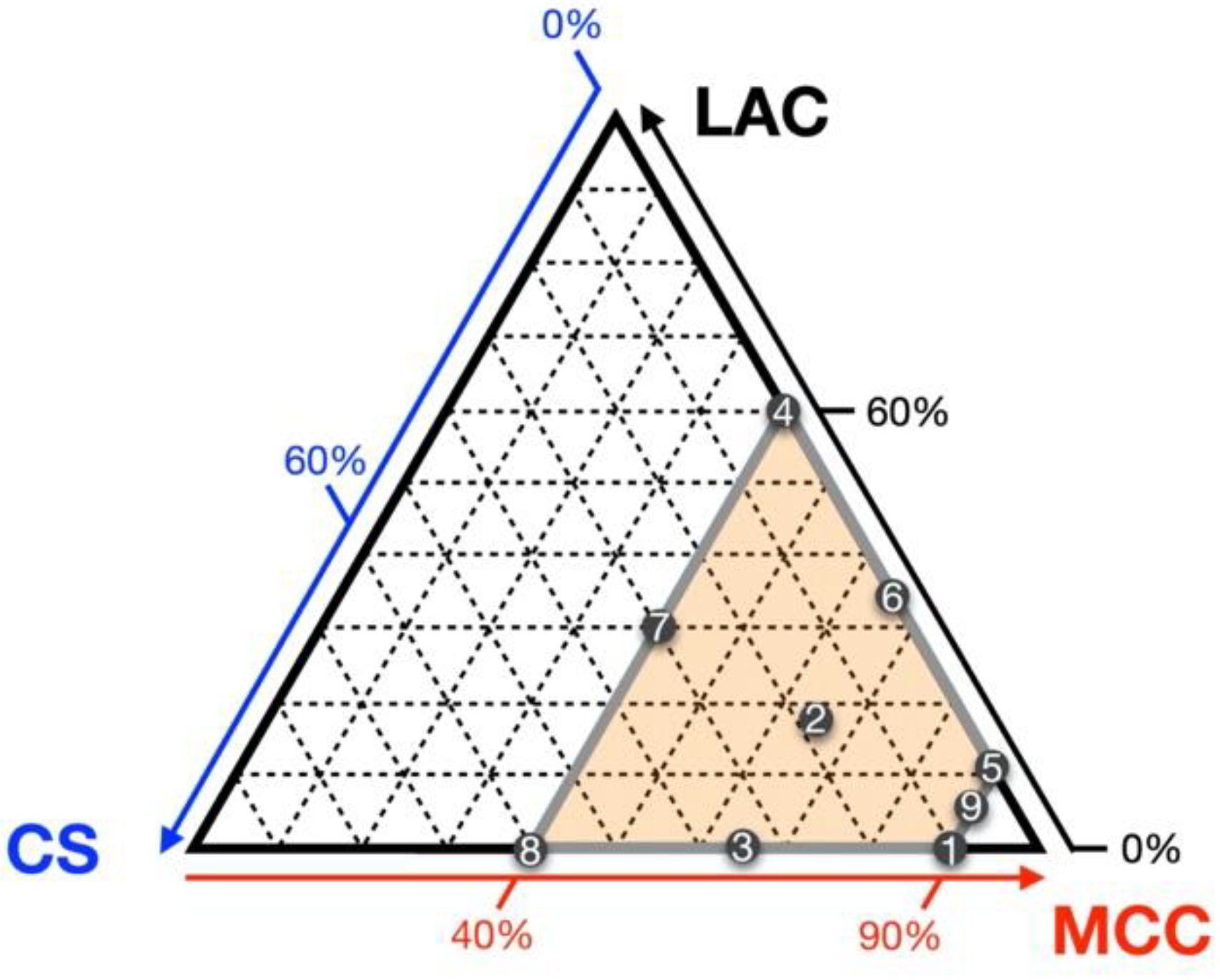

| Kinds | Component | Median Particle Size Diameter (μm) 1 | Particle Shape | Solubility 2 |
|---|---|---|---|---|
| API | ACE | 26.8 3 | Nonuniform 3 | Sparingly soluble in water |
| ETZ | 7.3 3 | Rod-shaped 3 | Practically insoluble in water | |
| NA | 33.7 3 | Nonuniform 3 | Sparingly soluble in water | |
| PYH | 47.5 3 | Square-shaped 3 | Freely soluble in water | |
| Excipients | LAC | 155 | Nonuniform | Freely soluble in water |
| CS | 107 | Spherical | Practically insoluble in water | |
| MCC | 66.9 | Fibrous and elongated | Practically insoluble in water | |
| Mg-St | 4.9 | Nonuniform | Practically insoluble in water |
| Rp. | LAC (%) | CS (%) | MCC (%) |
|---|---|---|---|
| 1 | 0 | 10 | 90 |
| 2 1 | 17.5 | 17.5 | 65 |
| 2 1 | 17.5 | 17.5 | 65 |
| 3 | 0 | 35 | 65 |
| 4 | 60 | 0 | 40 |
| 5 | 10 | 0 | 90 |
| 6 | 35 | 0 | 65 |
| 7 | 30 | 30 | 40 |
| 8 | 0 | 60 | 40 |
| 9 | 5 | 5 | 90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, Y.; Shirotori, K.; Kosugi, A.; Kumada, S.; Leong, K.H.; Okada, K.; Onuki, Y. A Precise Prediction Method for the Properties of API-Containing Tablets Based on Data from Placebo Tablets. Pharmaceutics 2020, 12, 601. https://doi.org/10.3390/pharmaceutics12070601
Hayashi Y, Shirotori K, Kosugi A, Kumada S, Leong KH, Okada K, Onuki Y. A Precise Prediction Method for the Properties of API-Containing Tablets Based on Data from Placebo Tablets. Pharmaceutics. 2020; 12(7):601. https://doi.org/10.3390/pharmaceutics12070601
Chicago/Turabian StyleHayashi, Yoshihiro, Kaede Shirotori, Atsushi Kosugi, Shungo Kumada, Kok Hoong Leong, Kotaro Okada, and Yoshinori Onuki. 2020. "A Precise Prediction Method for the Properties of API-Containing Tablets Based on Data from Placebo Tablets" Pharmaceutics 12, no. 7: 601. https://doi.org/10.3390/pharmaceutics12070601
APA StyleHayashi, Y., Shirotori, K., Kosugi, A., Kumada, S., Leong, K. H., Okada, K., & Onuki, Y. (2020). A Precise Prediction Method for the Properties of API-Containing Tablets Based on Data from Placebo Tablets. Pharmaceutics, 12(7), 601. https://doi.org/10.3390/pharmaceutics12070601






