Key-Protease Inhibition Regimens Promote Tumor Targeting of Neurotensin Radioligands
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide Analogs – Protease Inhibitors
Preparation and Quality Control of 99mTc-DT1, 99mTc-DT5 and 99mTc-DT6
2.2. In Vitro Assays
2.2.1. Cell Lines and Culture
2.2.2. Internalization Assay in WiDr Cells
2.3. Animal Studies
2.3.1. In Vivo Stability Tests
2.3.2. Induction of WiDr Xenografts in Mice
2.3.3. Biodistribution of 99mTc-Radiotracers in WiDr Xenograft-Bearing SCID Mice
2.3.4. Statistical Analysis
2.3.5. SPECT/CT Imaging of 99mTc-DT1 in WiDr Xenograft-Bearing SCID Mice
3. Results
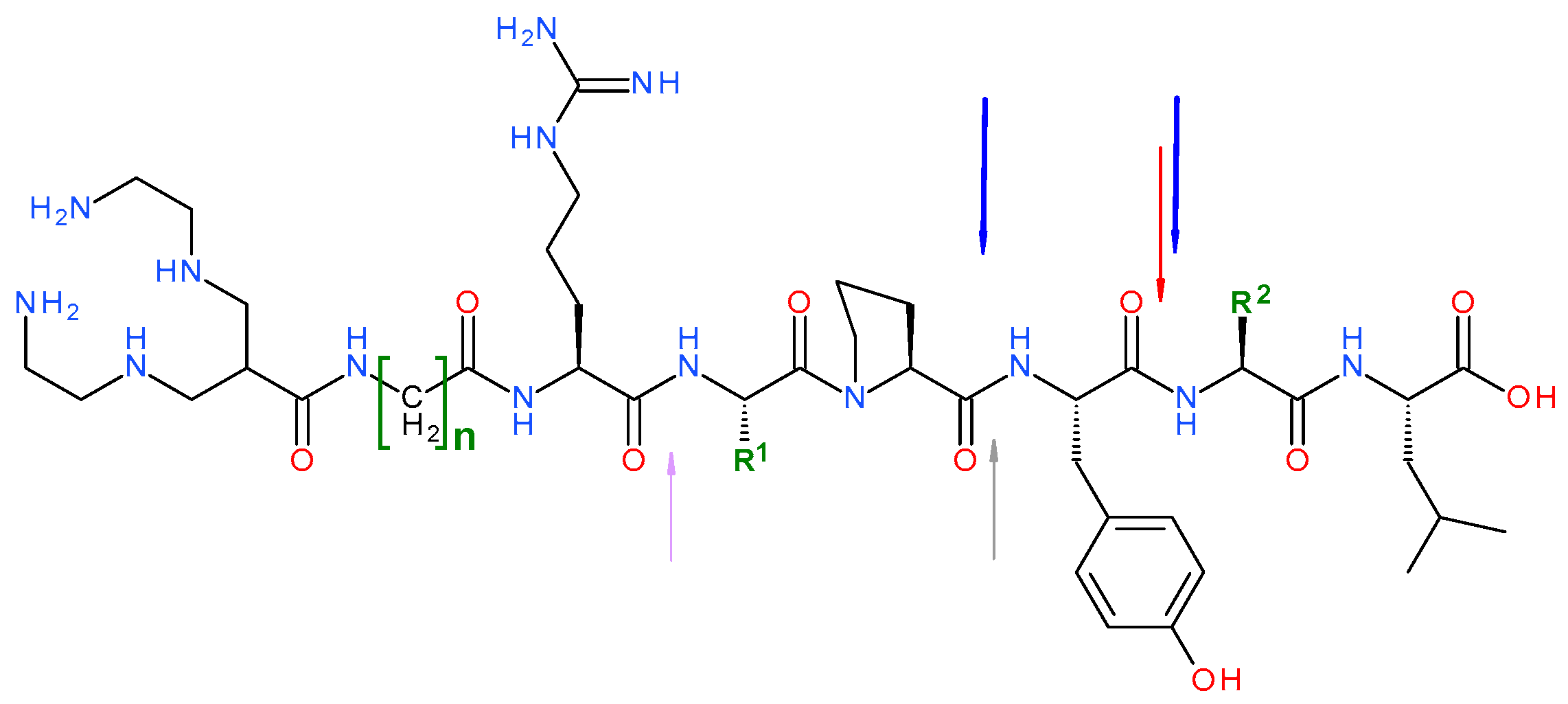
3.1. Peptides and Radioligands
3.2. In Vitro Assays
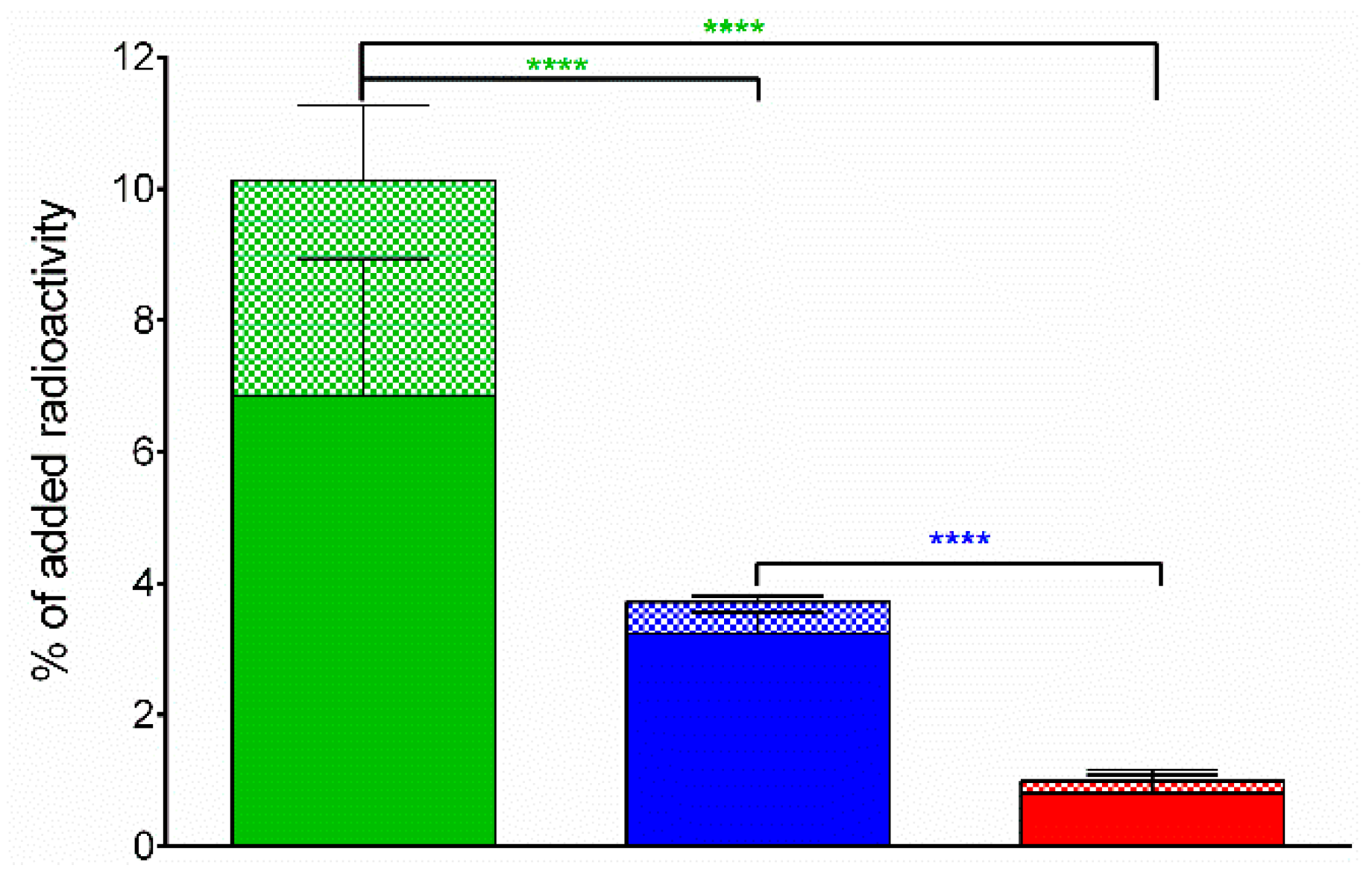
Comparative Internalization of 99mTc-DT1, 99mTc-DT5 or 99mTc-DT6 in WiDr Cells
3.3. In Vivo Comparison of 99mTc-DT1, 99mTc-DT5 or 99mTc-DT6
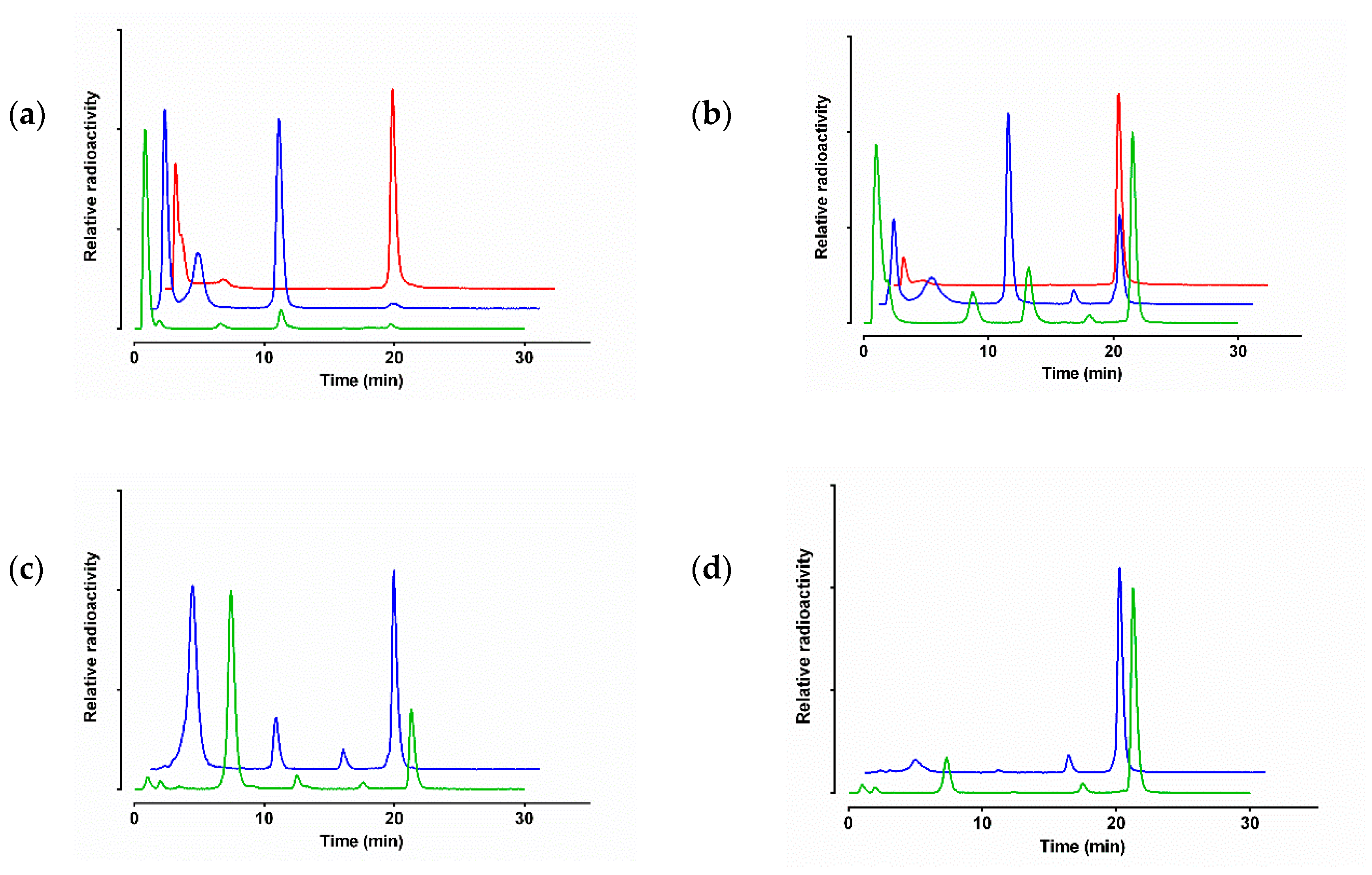
3.3.1. Stability of 99mTc-DT1, 99mTc-DT5 and 99mTc-DT6 in Mice
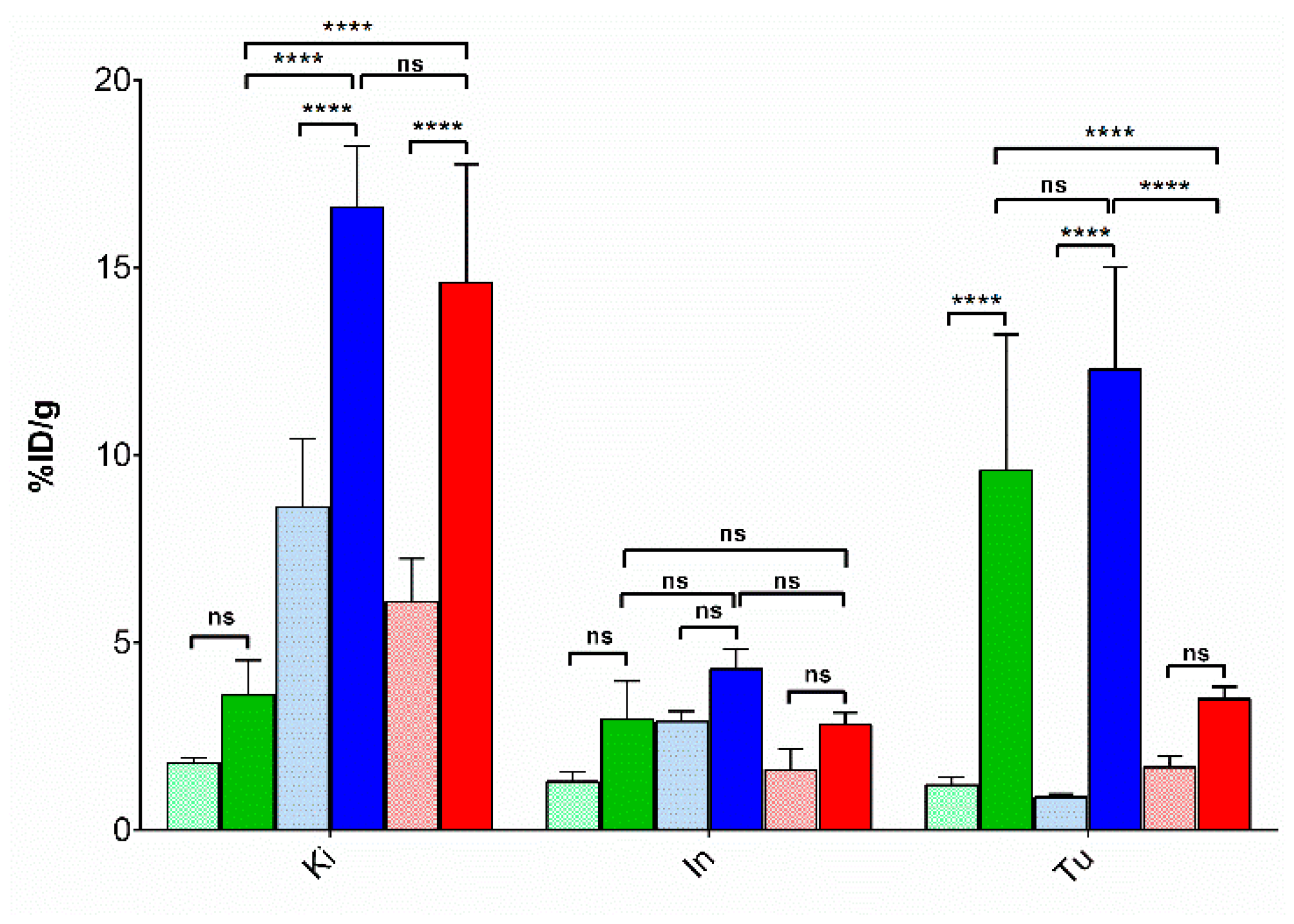
3.3.2. Biodistribution of 99mTc-DT1, 99mTc-DT5 and 99mTc-DT6 in WiDr Xenograft-Bearing Mice
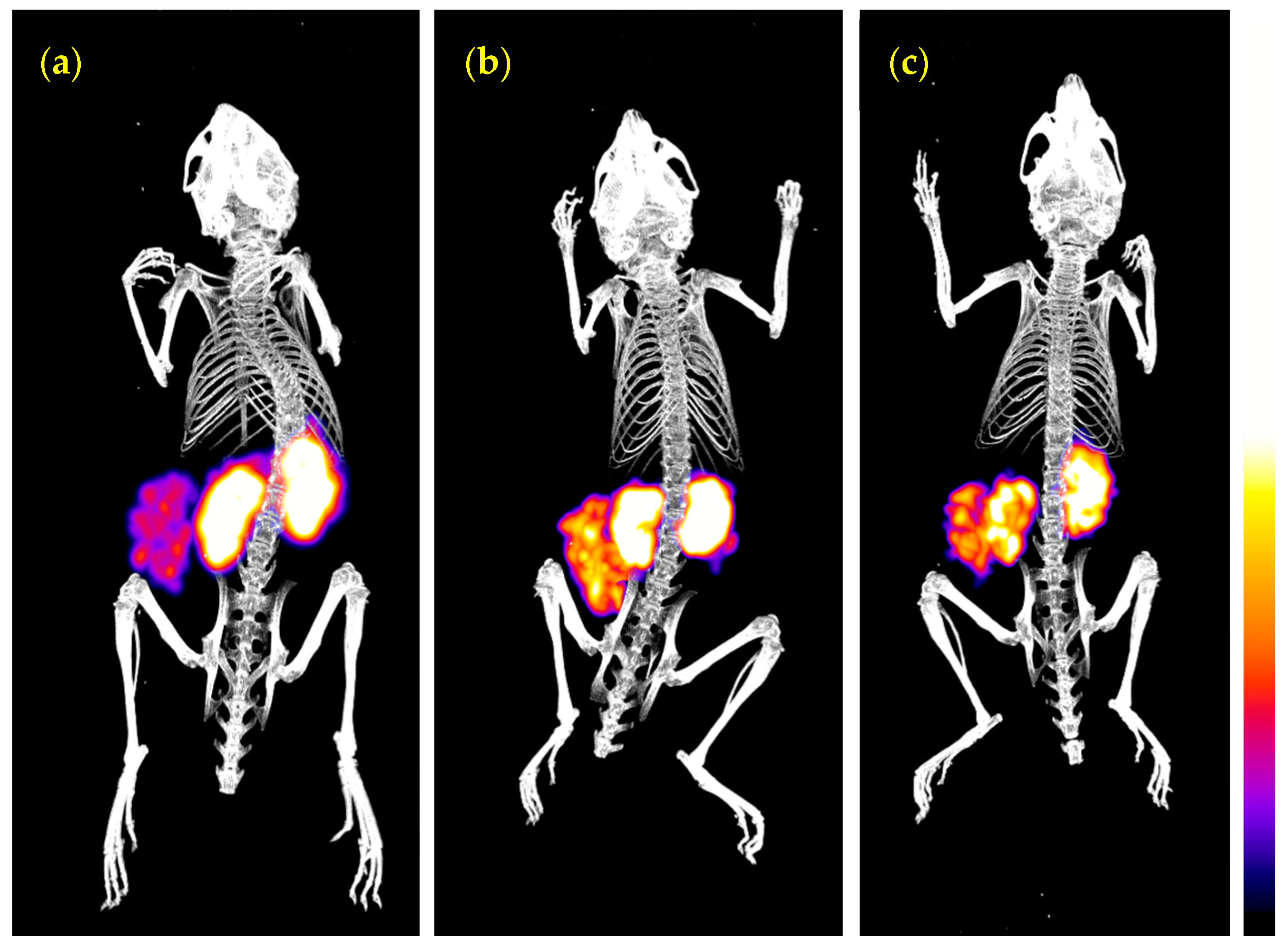
3.3.3. SPECT/CT of 99mTc-DT1 in WiDr Xenograft-Bearing Mice
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baum, R.P.; Kulkarni, H.R. Theranostics: From molecular imaging using Ga-68 labeled tracers and PET/CT to personalized radionuclide therapy—The Bad Berka experience. Theranostics 2012, 2, 437–447. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Breeman, W.A.; Kwekkeboom, D.J.; Valkema, R.; Krenning, E.P. Tumor imaging and therapy using radiolabeled somatostatin analogues. Acc. Chem. Res. 2009, 42, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Singh, A.; Kulkarni, H.R.; Schuchardt, C.; Müller, D.; Wester, H.J.; Maina, T.; Rosch, F.; van der Meulen, N.P.; Müller, C.; et al. From bench to bedside-the Bad Berka experience with first-in-human studies. Semin. Nucl. Med. 2019, 49, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for combined imaging and therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef]
- Kostelnik, T.I.; Orvig, C. Radioactive main group and rare earth metals for imaging and therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr. Rev. 2003, 24, 389–427. [Google Scholar] [CrossRef]
- Vincent, J.P.; Mazella, J.; Kitabgi, P. Neurotensin and neurotensin receptors. Trends Pharmacol. Sci. 1999, 20, 302–309. [Google Scholar] [CrossRef]
- Kitabgi, P. Functional domains of the subtype 1 neurotensin receptor (NTS1). Peptides 2006, 27, 2461–2468. [Google Scholar] [CrossRef]
- Kitabgi, P. Targeting neurotensin receptors with agonists and antagonists for therapeutic purposes. Curr. Opin. Drug Discov. Dev. 2002, 5, 764–776. [Google Scholar]
- Evers, B.M. Neurotensin and growth of normal and neoplastic tissues. Peptides 2006, 27, 2424–2433. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Friess, H.; Büchler, M.; Laissue, J. Neurotensin receptors: A new marker for human ductal pancreatic adenocarcinoma. Gut 1998, 42, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, J.; Townsend, C.M., Jr.; Thompson, J.C. Neurotensin regulates growth of human pancreatic cancer. Ann. Surg. 1993, 217, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, R.A.; Kim, S.; Zhang, Y.; Ethridge, R.T.; Murrilo, C.; Hellmich, M.R.; Evans, D.B.; Townsend, C.M., Jr.; Mark Evers, B. Gut peptide receptor expression in human pancreatic cancers. Ann. Surg. 2000, 231, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Waser, B.; Schaer, J.C.; Laissue, J.A. Neurotensin receptors in human neoplasms: High incidence in Ewing’s sarcomas. Int. J. Cancer 1999, 82, 213–218. [Google Scholar] [CrossRef]
- Gui, X.; Guzman, G.; Dobner, P.R.; Kadkol, S.S. Increased neurotensin receptor-1 expression during progression of colonic adenocarcinoma. Peptides 2008, 29, 1609–1615. [Google Scholar] [CrossRef]
- Morgat, C.; Chastel, A.; Molinie, V.; Schollhammer, R.; Macgrogan, G.; Velasco, V.; Malavaud, B.; Fernandez, P.; Hindie, E. Neurotensin receptor-1 expression in human prostate cancer: A pilot study on primary tumors and lymph node metastases. Int. J. Mol. Sci. 2019, 20, 1721. [Google Scholar] [CrossRef]
- Souaze, F.; Dupouy, S.; Viardot-Foucault, V.; Bruyneel, E.; Attoub, S.; Gespach, C.; Gompel, A.; Forgez, P. Expression of neurotensin and NT1 receptor in human breast cancer: A potential role in tumor progression. Cancer Res. 2006, 66, 6243–6249. [Google Scholar] [CrossRef]
- Nikolaou, S.; Qiu, S.; Fiorentino, F.; Simillis, C.; Rasheed, S.; Tekkis, P.; Kontovounisios, C. The role of neurotensin and its receptors in non-gastrointestinal cancers: A review. Cell Commun. Signal. 2020, 18, 68. [Google Scholar] [CrossRef]
- Granier, C.; van Rietschoten, J.; Kitabgi, P.; Poustis, C.; Freychet, P. Synthesis and characterization of neurotensin analogues for structure/activity relationship studies. Acetyl-neurotensin-(8--13) is the shortest analogue with full binding and pharmacological activities. Eur. J. Biochem. 1982, 124, 117–124. [Google Scholar] [CrossRef]
- García-Garayoa, E.; Allemann-Tannahill, L.; Blauenstein, P.; Willmann, M.; Carrel-Remy, N.; Tourwé, D.; Iterbeke, K.; Conrath, P.; Schubiger, P.A. In vitro and in vivo evaluation of new radiolabeled neurotensin(8-13) analogues with high affinity for nt1 receptors. Nucl. Med. Biol. 2001, 28, 75–84. [Google Scholar] [CrossRef]
- Maes, V.; García-Garayoa, E.; Blauenstein, P.; Tourwé, D. Novel 99mTc-labeled neurotensin analogues with optimized biodistribution properties. J. Med. Chem. 2006, 49, 1833–1836. [Google Scholar] [CrossRef] [PubMed]
- Alshoukr, F.; Rosant, C.; Maes, V.; Abdelhak, J.; Raguin, O.; Burg, S.; Sarda, L.; Barbet, J.; Tourwé, D.; Pelaprat, D.; et al. Novel neurotensin analogues for radioisotope targeting to neurotensin receptor-positive tumors. Bioconjug. Chem. 2009, 20, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Maina, T.; Nikolopoulou, A.; Stathopoulou, E.; Galanis, A.S.; Cordopatis, P.; Nock, B.A. [99mTc]demotensin 5 and 6 in the NTS1-R-targeted imaging of tumours: Synthesis and preclinical results. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1804–1814. [Google Scholar] [CrossRef]
- Nock, B.A.; Nikolopoulou, A.; Reubi, J.C.; Maes, V.; Conrath, P.; Tourwé, D.; Maina, T. Toward stable N4-modified neurotensins for NTS1-receptor-targeted tumor imaging with 99mTc. J. Med. Chem. 2006, 49, 4767–4776. [Google Scholar] [CrossRef] [PubMed]
- de Visser, M.; Janssen, P.J.; Srinivasan, A.; Reubi, J.C.; Waser, B.; Erion, J.L.; Schmidt, M.A.; Krenning, E.P.; de Jong, M. Stabilised 111In-labelled DTPA- and DOTA-conjugated neurotensin analogues for imaging and therapy of exocrine pancreatic cancer. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1134–1139. [Google Scholar] [CrossRef]
- Achilefu, S.; Srinivasan, A.; Schmidt, M.A.; Jimenez, H.N.; Bugaj, J.E.; Erion, J.L. Novel bioactive and stable neurotensin peptide analogues capable of delivering radiopharmaceuticals and molecular beacons to tumors. J. Med. Chem. 2003, 46, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Mascarin, A.; Valverde, I.E.; Mindt, T.L. Structure-activity relationship studies of amino acid substitutions in radiolabeled neurotensin conjugates. ChemMedChem 2016, 11, 102–107. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, W.; Zhou, Z.; Wagh, N.K.; Fan, W.; Brusnahan, S.K.; Garrison, J.C. Evaluation of DOTA-chelated neurotensin analogs with spacer-enhanced biological performance for neurotensin-receptor-1-positive tumor targeting. Nucl. Med. Biol. 2015, 42, 816–823. [Google Scholar] [CrossRef]
- Alshoukr, F.; Prignon, A.; Brans, L.; Jallane, A.; Mendes, S.; Talbot, J.N.; Tourwé, D.; Barbet, J.; Gruaz-Guyon, A. Novel DOTA-neurotensin analogues for 111In scintigraphy and 68Ga PET imaging of neurotensin receptor-positive tumors. Bioconjug. Chem. 2011, 22, 1374–1385. [Google Scholar] [CrossRef]
- Schubiger, P.A.; Allemann-Tannahill, L.; Egli, A.; Schibli, R.; Alberto, R.; Carrel-Remy, N.; Willmann, M.; Blauenstein, P.; Tourwé, D. Catabolism of neurotensins. Implications for the design of radiolabeling strategies of peptides. Q. J. Nucl. Med. 1999, 43, 155–158. [Google Scholar]
- Couder, J.; Tourwé, D.; Van Binst, G.; Schuurkens, J.; Leysen, J.E. Synthesis and biological activities of psi (CH2NH) pseudopeptide analogues of the C-terminal hexapeptide of neurotensin. Int. J. Pept. Protein Res. 1993, 41, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Mascarin, A.; Valverde, I.E.; Vomstein, S.; Mindt, T.L. 1,2,3-triazole stabilized neurotensin-based radiopeptidomimetics for improved tumor targeting. Bioconjug. Chem. 2015, 26, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Sparr, C.; Purkayastha, N.; Yoshinari, T.; Seebach, D.; Maschauer, S.; Prante, O.; Hubner, H.; Gmeiner, P.; Kolesinska, B.; Cescato, R.; et al. Syntheses, receptor bindings, in vitro and in vivo stabilities and biodistributions of DOTA-neurotensin(8-13) derivatives containing beta-amino acid residues—A lesson about the importance of animal experiments. Chem. Biodivers. 2013, 10, 2101–2121. [Google Scholar] [CrossRef] [PubMed]
- Maschauer, S.; Prante, O. Radiopharmaceuticals for imaging and endoradiotherapy of neurotensin receptor-positive tumors. J. Label. Compd. Radiopharm. 2018, 61, 309–325. [Google Scholar] [CrossRef]
- Fröberg, A.C.; van Eijck, C.H.; Verdijsseldonck, M.C.; Melis, M.; Bakker, H.; Krenning, E.P. Use of neurotensin analogue In-111-DTPA-neurotensin (IN-111-MP2530) in diagnosis of pancreatic adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, S392. [Google Scholar] [CrossRef]
- Gabriel, M.; Decristoforo, C.; Woll, E.; Eisterer, W.; Nock, B.; Maina, T.; Moncayo, R.; Virgolini, I. [99mTc]demotensin VI: Biodistribution and initial clinical results in tumor patients of a pilot/phase I study. Cancer Biother. Radiopharm. 2011, 26, 557–563. [Google Scholar] [CrossRef]
- Buchegger, F.; Bonvin, F.; Kosinski, M.; Schaffland, A.O.; Prior, J.; Reubi, J.C.; Blauenstein, P.; Tourwé, D.; Garcia Garayoa, E.; Bischof Delaloye, A. Radiolabeled neurotensin analog, 99mTc-NT-XI, evaluated in ductal pancreatic adenocarcinoma patients. J. Nucl. Med. 2003, 44, 1649–1654. [Google Scholar]
- Kitabgi, P.; De Nadai, F.; Rovere, C.; Bidard, J.N. Biosynthesis, maturation, release, and degradation of neurotensin and neuromedin N. Ann. N. Y. Acad. Sci. 1992, 668, 30–42. [Google Scholar] [CrossRef]
- Kitabgi, P.; Dubuc, I.; Nouel, D.; Costentin, J.; Cuber, J.C.; Fulcrand, H.; Doulut, S.; Rodriguez, M.; Martinez, J. Effects of thiorphan, bestatin and a novel metallopeptidase inhibitor JMV 390-1 on the recovery of neurotensin and neuromedin N released from mouse hypothalamus. Neurosci. Lett. 1992, 142, 200–204. [Google Scholar] [CrossRef]
- Checler, F.; Vincent, J.P.; Kitabgi, P. Degradation of neurotensin by rat brain synaptic membranes: Involvement of a thermolysin-like metalloendopeptidase (enkephalinase), angiotensin-converting enzyme, and other unidentified peptidases. J. Neurochem. 1983, 41, 375–384. [Google Scholar] [CrossRef]
- Schindler, L.; Bernhardt, G.; Keller, M. Modifications at Arg and Ile give neurotensin(8-13) derivatives with high stability and retained NTS1 receptor affinity. ACS Med. Chem. Lett. 2019, 10, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Skidgel, R.A.; Engelbrecht, S.; Johnson, A.R.; Erdös, E.G. Hydrolysis of substance P and neurotensin by converting enzyme and neutral endopeptidase. Peptides 1984, 5, 769–776. [Google Scholar] [CrossRef]
- Roques, B.P.; Noble, F.; Dauge, V.; Fournie-Zaluski, M.C.; Beaumont, A. Neutral endopeptidase 24.11: Structure, inhibition, and experimental and clinical pharmacology. Pharmacol. Rev. 1993, 45, 87–146. [Google Scholar]
- Roques, B.P. Zinc metallopeptidases: Active site structure and design of selective and mixed inhibitors: New approaches in the search for analgesics and anti-hypertensives. Biochem. Soc. Trans. 1993, 21, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Nock, B.A.; Maina, T.; Krenning, E.P.; de Jong, M. “To serve and protect”: Enzyme inhibitors as radiopeptide escorts promote tumor targeting. J. Nucl. Med. 2014, 55, 121–127. [Google Scholar] [CrossRef]
- Lymperis, E.; Kaloudi, A.; Sallegger, W.; Bakker, I.L.; Krenning, E.P.; de Jong, M.; Maina, T.; Nock, B.A. Radiometal-dependent biological profile of the radiolabeled gastrin-releasing peptide receptor antagonist SB3 in cancer theranostics: Metabolic and biodistribution patterns defined by neprilysin. Bioconjug. Chem. 2018, 29, 1774–1784. [Google Scholar] [CrossRef]
- Chatalic, K.L.; Konijnenberg, M.; Nonnekens, J.; de Blois, E.; Hoeben, S.; de Ridder, C.; Brunel, L.; Fehrentz, J.A.; Martinez, J.; van Gent, D.C.; et al. In vivo stabilization of a gastrin-releasing peptide receptor antagonist enhances PET imaging and radionuclide therapy of prostate cancer in preclinical studies. Theranostics 2016, 6, 104–117. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Krenning, E.P.; de Jong, M.; Maina, T. 99mTc-labeled gastrins of varying peptide chain length: Distinct impact of NEP/ACE-inhibition on stability and tumor uptake in mice. Nucl. Med. Biol. 2016, 43, 347–354. [Google Scholar] [CrossRef]
- Valkema, R.; Froberg, A.; Maina, T.; Nock, B.; de Blois, E.; Melis, M.; Konijnenberg, M.; Koolen, S.; Peeters, R.; de Herder, W.; et al. Clinical translation of the pepprotect concept: Improved detection of cancer and metastases, applied in medullary thyroid cancer patients with [111In]In-MG1 scanning during neprilysin inhibition. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, S701–S702. [Google Scholar]
- Suda, H.; Aoyagi, T.; Takeuchi, T.; Umezawa, H. Letter: A thermolysin inhibitor produced by actinomycetes: Phosphoramidon. J. Antibiot. (Tokyo) 1973, 26, 621–623. [Google Scholar] [CrossRef]
- Millar, J.A.; Derkx, F.H.; McLean, K.; Reid, J.L. Pharmacodynamics of converting enzyme inhibition: The cardiovascular, endocrine and autonomic effects of MK421 (enalapril) and MK521. Br. J. Clin. Pharmacol. 1982, 14, 347–355. [Google Scholar] [CrossRef]
- Maoret, J.J.; Pospai, D.; Rouyer-Fessard, C.; Couvineau, A.; Laboisse, C.; Voisin, T.; Laburthe, M. Neurotensin receptor and its MRNA are expressed in many human colon cancer cell lines but not in normal colonic epithelium: Binding studies and RT-PCR experiments. Biochem. Biophys. Res. Commun. 1994, 203, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Waser, B.; Schmassmann, A.; Laissue, J.A. Receptor autoradiographic evaluation of cholecystokinin, neurotensin, somatostatin and vasoactive intestinal peptide receptors in gastro-intestinal adenocarcinoma samples: Where are they really located? Int. J. Cancer 1999, 81, 376–386. [Google Scholar] [CrossRef]
- Paschoalin, T.; Carmona, A.K.; Rodrigues, E.G.; Oliveira, V.; Monteiro, H.P.; Juliano, M.A.; Juliano, L.; Travassos, L.R. Characterization of thimet oligopeptidase and neurolysin activities in B16F10-NEX2 tumor cells and their involvement in angiogenesis and tumor growth. Mol. Cancer 2007, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Berti, D.A.; Morano, C.; Russo, L.C.; Castro, L.M.; Cunha, F.M.; Zhang, X.; Sironi, J.; Klitzke, C.F.; Ferro, E.S.; Fricker, L.D. Analysis of intracellular substrates and products of thimet oligopeptidase in human embryonic kidney 293 cells. J. Biol. Chem. 2009, 284, 14105–14116. [Google Scholar] [CrossRef]
| 99mTc-DT1 | 99mTc-DT5 | 99mTc-DT6 | |
|---|---|---|---|
| Control | 1.8 ± 0.8 (n = 4) | 1.3 ± 0.2 (n = 2) | 55.1 ± 3.9 (n = 2) |
| PA | 26.4 ± 2.2 (n = 2) | 15.4 ± 5.1 (n = 2) | 89.3 ± 6.7 (n = 4) |
| Lis | 18.8 ± 2.5 (n = 3) | 28.8 ± 5.2 (n = 2) | - |
| PA+Lis | 72.3 ± 3.2 (n = 4) | 79.0 ± 1.7 (n = 2) | - |
| Tissue | 99mTc-DT1 – 4 h pi | ||||
|---|---|---|---|---|---|
| Block 1 | Controls | 4 h PA 2 | Lis 3 | PA+Lis 4 | |
| Blood | 0.07 ± 0.02 | 0.15 ± 0.01 | 0.12 ± 0.03 | 0.10 ± 0.01 | 0.13 ± 0.06 |
| Liver | 0.42 ± 0.02 | 0.42 ± 0.02 | 0.47 ± 0.05 | 0.39 ± 0.05 | 0.58 ± 0.12 |
| Heart | 0.05 ± 0.01 | 0.08 ± 0.00 | 0.10 ± 0.02 | 0.08 ± 0.02 | 0.10 ± 0.02 |
| Kidneys | 2.89 ± 0.56 | 1.80 ± 0.14 | 2.26 ± 0.30 | 2.24 ± 0.34 | 3.61 ± 0.93 |
| Stomach | 0.90 ± 0.25 | 1.89 ± 0.26 | 1.39 ± 0.12 | 1.18 ± 0.22 | 1.39 ± 0.73 |
| Intestines | 0.43 ± 0.14 | 1.30 ± 0.26 | 2.91 ± 0.30 | 1.72 ± 0.29 | 2.96 ± 1.02 |
| Spleen | 0.26±0.02 | 0.26 ± 0.06 | 0.56 ± 0.13 | 0.32 ± 0.07 | 1.01 ± 0.30 |
| Muscle | 0.02 ± 0.02 | 0.03 ± 0.01 | 0.05 ± 0.03 | 0.03 ± 0.01 | 0.07 ± 0.06 |
| Lungs | 0.23 ± 0.10 | 0.20 ± 0.03 | 0.42 ± 0.06 | 0.19 ± 0.02 | 0.67 ± 0.08 |
| Pancreas | 0.04 ± 0.01 | 0.08 ± 0.01 | 0.10 ± 0.02 | 0.06 ± 0.01 | 0.12 ± 0.02 |
| Tumor | 0.23 ± 0.09 | 1.20 ± 0.21 | 4.58 ± 0.47 | 1.60 ± 0.47 | 9.60 ± 3.62 |
| Tissue | 99mTc-DT5 – 4 h pi | ||
|---|---|---|---|
| Block 1,2 | Controls | PA+Lis 3 | |
| Blood | 0.45 ± 0.16 | 0.41 ± 0.12 | |
| Liver | 0.90 ± 0.20 | 1.12 ± 0.27 | |
| Heart | 0.16 ± 0.04 | 0.22 ± 0.05 | |
| Kidneys | 8.63 ± 1.80 | 16.62 ± 1.63 | |
| Stomach | 2.00 ± 0.35 | 2.02 ± 0.37 | |
| Intestines | 0.42 ± 0.01 | 2.90 ± 0.28 | 4.30 ± 0.52 |
| Spleen | 0.31 ± 0.09 | 0.86 ± 0.07 | |
| Muscle | 0.07 ± 0.02 | 0.11 ± 0.02 | |
| Lungs | 0.43 ± 0.10 | 1.10 ± 0.16 | |
| Pancreas | 0.16 ± 0.04 | 0.29 ± 0.03 | |
| Tumor | 0.13 ± 0.01 | 0.88 ± 0.08 | 12.29 ± 2.73 |
| Tissue | 99mTc-DT6 – 4 h pi | ||||
|---|---|---|---|---|---|
| Block 1 | Controls | 4 h PA 2 | Lis 3 | PA+Lis 4 | |
| Blood | 0.11 ± 0.09 | 0.10 ± 0.06 | 0.10 ± 0.03 | 0.08 ± 0.03 | 0.09 ± 0.00 |
| Liver | 0.36 ± 0.06 | 0.34 ± 0.07 | 0.42 ± 0.05 | 0.28 ± 0.01 | 0.45 ± 0.07 |
| Heart | 0.09 ± 0.04 | 0.08 ± 0.02 | 0.12 ± 0.03 | 0.07 ± 0.02 | 0.10 ± 0.02 |
| Kidneys | 10.83 ± 3.26 | 6.09 ± 1.15 | 11.14 ± 3.87 | 6.24 ± 1.56 | 14.61 ± 3.16 |
| Stomach | 0.93 ± 0.30 | 0.44 ± 0.11 | 0.87 ± 0.22 | 0.62 ± 0.22 | 0.89 ± 0.16 |
| Intestines | 0.46 ± 0.21 | 1.61 ± 0.55 | 2.52 ± 0.40 | 1.46 ± 0.32 | 2.81 ± 0.32 |
| Spleen | 0.48 ± 0.16 | 0.64 ± 0.14 | 1.71 ± 0.26 | 0.75 ± 0.09 | 1.84 ± 0.28 |
| Muscle | 0.03 ± 0.01 | 0.04 ± 0.03 | 0.05 ± 0.02 | 0.03 ± 0.01 | 0.09 ± 0.06 |
| Lungs | 0.54 ± 0.07 | 0.22 ± 0.06 | 1.26 ± 0.15 | 0.19 ± 0.03 | 1.27 ± 0.02 |
| Pancreas | 0.07 ± 0.04 | 0.10 ± 0.04 | 0.14 ± 0.02 | 0.07 ± 0.01 | 0.16 ± 0.02 |
| Tumor | 0.38 ± 0.15 | 1.68 ± 0.28 | 3.89 ± 0.71 | 2.15 ± 0.43 | 3.50 ± 0.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanellopoulos, P.; Kaloudi, A.; de Jong, M.; Krenning, E.P.; Nock, B.A.; Maina, T. Key-Protease Inhibition Regimens Promote Tumor Targeting of Neurotensin Radioligands. Pharmaceutics 2020, 12, 528. https://doi.org/10.3390/pharmaceutics12060528
Kanellopoulos P, Kaloudi A, de Jong M, Krenning EP, Nock BA, Maina T. Key-Protease Inhibition Regimens Promote Tumor Targeting of Neurotensin Radioligands. Pharmaceutics. 2020; 12(6):528. https://doi.org/10.3390/pharmaceutics12060528
Chicago/Turabian StyleKanellopoulos, Panagiotis, Aikaterini Kaloudi, Marion de Jong, Eric P. Krenning, Berthold A. Nock, and Theodosia Maina. 2020. "Key-Protease Inhibition Regimens Promote Tumor Targeting of Neurotensin Radioligands" Pharmaceutics 12, no. 6: 528. https://doi.org/10.3390/pharmaceutics12060528
APA StyleKanellopoulos, P., Kaloudi, A., de Jong, M., Krenning, E. P., Nock, B. A., & Maina, T. (2020). Key-Protease Inhibition Regimens Promote Tumor Targeting of Neurotensin Radioligands. Pharmaceutics, 12(6), 528. https://doi.org/10.3390/pharmaceutics12060528







