Enhanced Transdermal Delivery of Concentrated Capsaicin from Chili Extract-Loaded Lipid Nanoparticles with Reduced Skin Irritation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Capsaicin Contents in Chili Extract
2.3. HPLC Analysis
2.4. Pre-Formulation for Capsaicin-Loaded Lipid-Based Nanoparticles
2.4.1. Lipid Selection for SLN and NLC
2.4.2. Preparation of SLN and NLC
2.4.3. Preparation of Chili Extract-Loaded Lipid Nanoparticle Incorporated in Hydrogel
2.5. Characterizing the Chili Extract-Loaded Nanodelivery System
2.5.1. Particle Size, Size Distribution and Zeta Potential Analysis
2.5.2. Determining Entrapment Efficiency (EE) and Loading Capacity (LC)
2.5.3. Transmission Electron Microscopy Analysis
2.5.4. Differential Scanning Calorimetry (DSC) Analysis
2.6. In Vitro Release Kinetics Study
2.7. In Vitro Skin Permeation Study
2.7.1. Drug Permeation
2.7.2. Skin Distribution
2.8. Hen’s Egg Test Chorioallantoic Membrane (HET-CAM) Assay
2.9. In Vitro Skin Irritation Test Using a Three-Dimensional EpiDerm Skin Model
2.9.1. MTT Assay on the 3D EpiDermTM Skin Model
2.9.2. ELISA Assay
2.10. Statistical Analysis
3. Results and Discussion
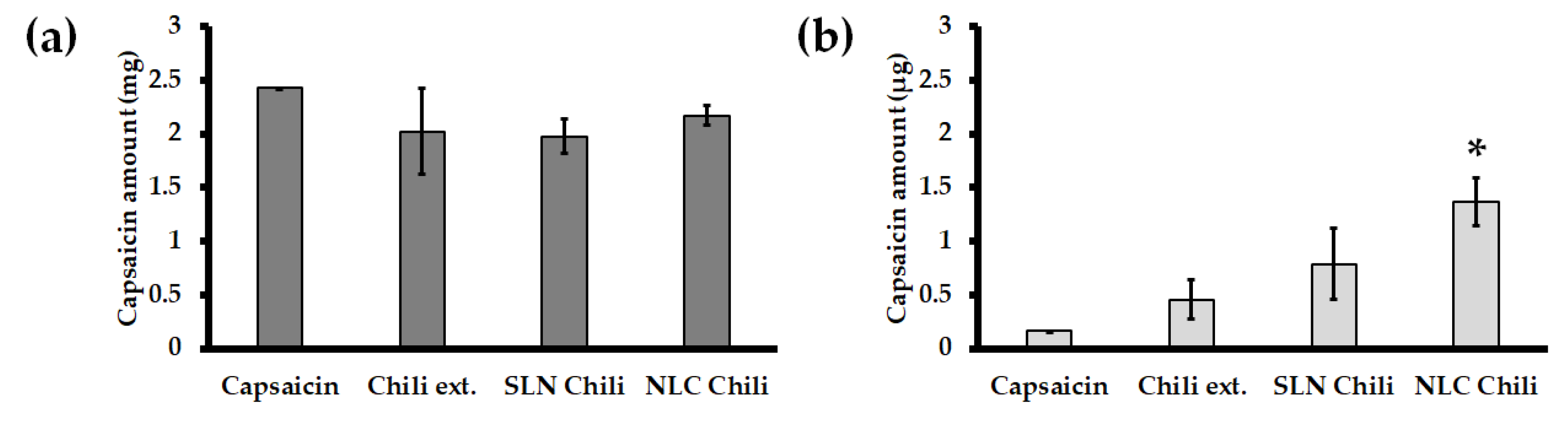
3.1. Capsaicin Contents in Chili Extract
3.2. Pre-Formulation for Capsaicin Loaded Lipid-Based Nanoparticles
3.2.1. Lipid Selection for SLN and NLC
3.2.2. Characterizing the Chili Extract-Loaded Nanodelivery System
3.2.3. Entrapment Efficiency (EE) and Loading Capacity (LC)
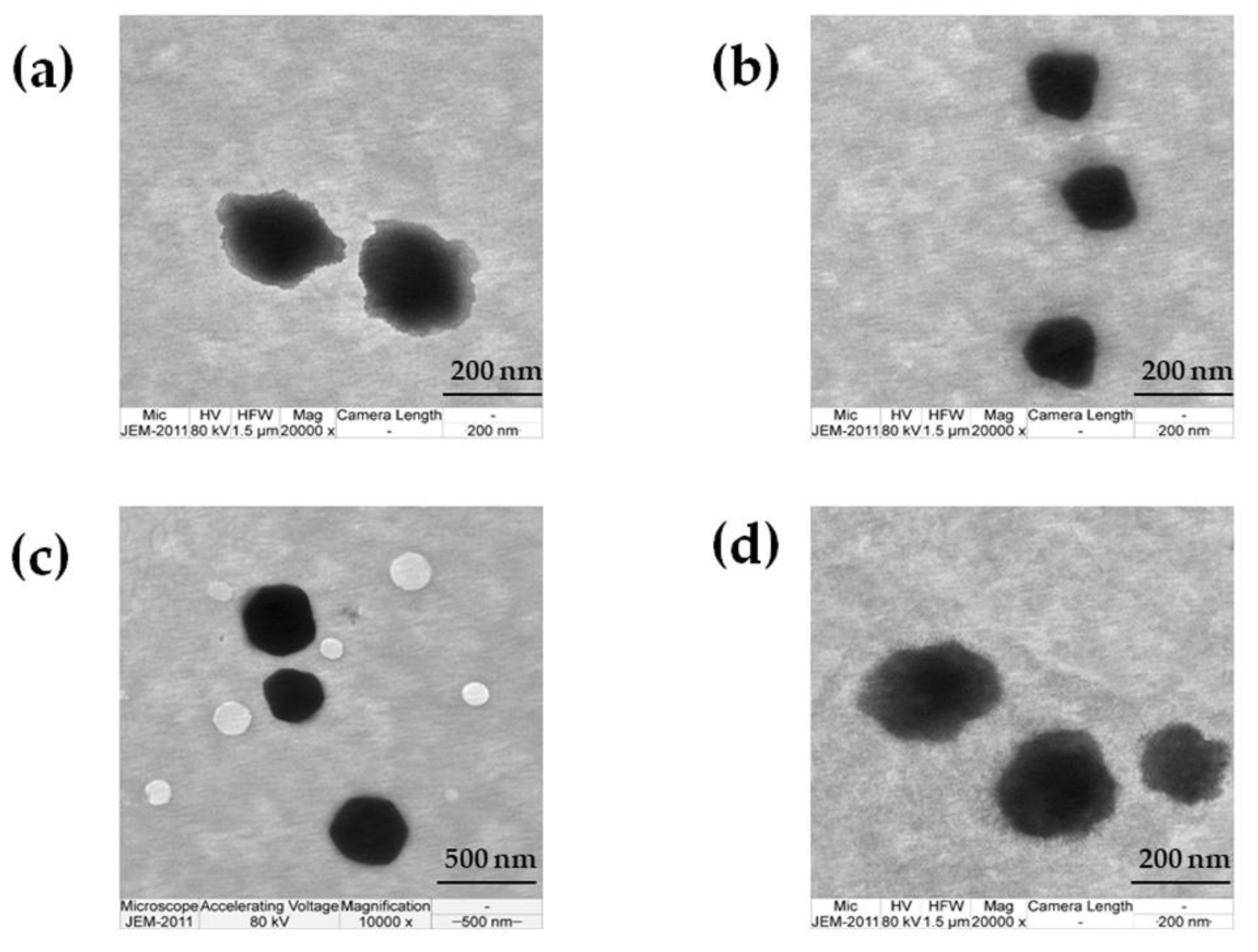
3.2.4. TEM Investigation
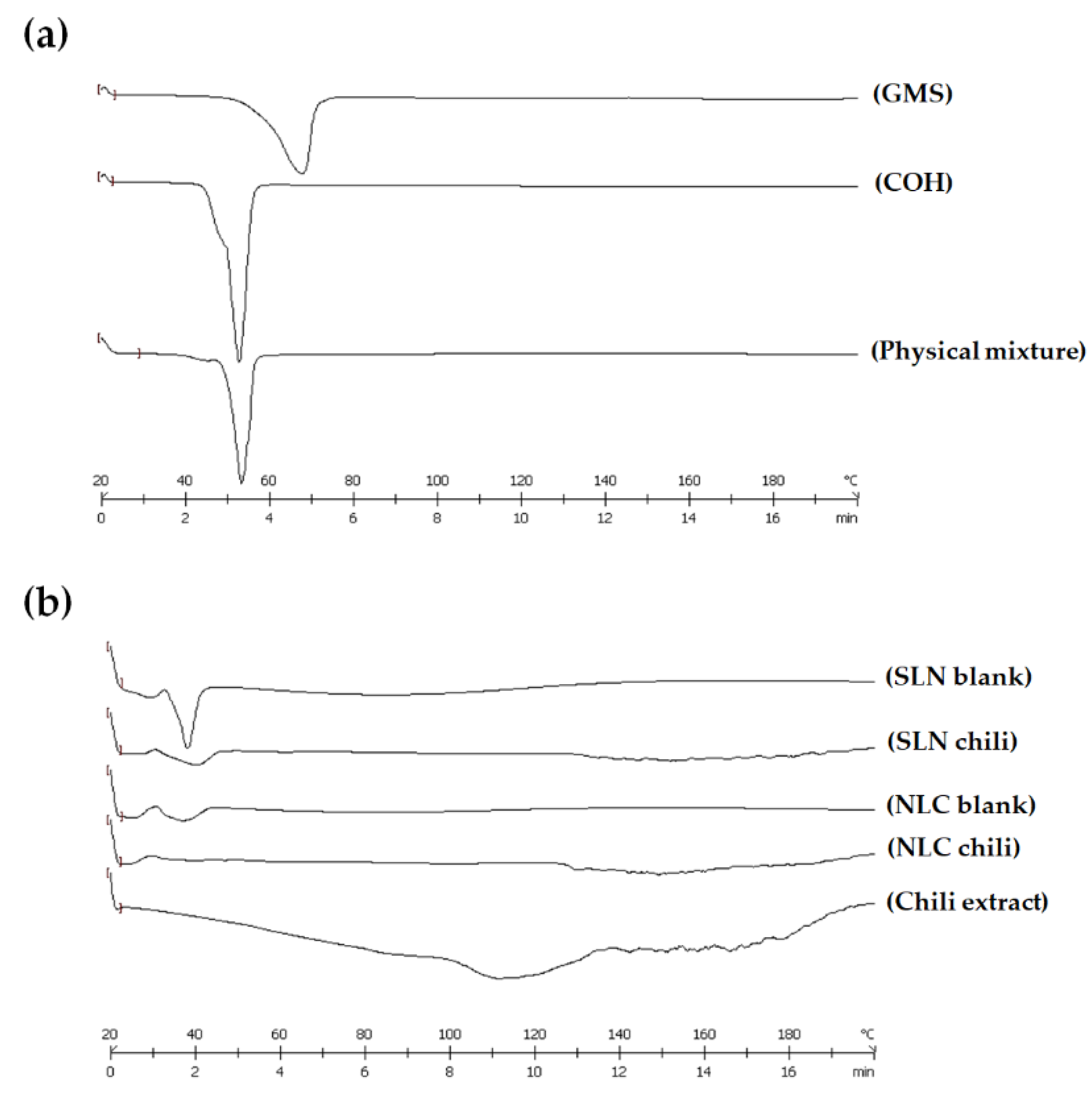
3.2.5. Differential Scanning Calorimetry (DSC) Analysis
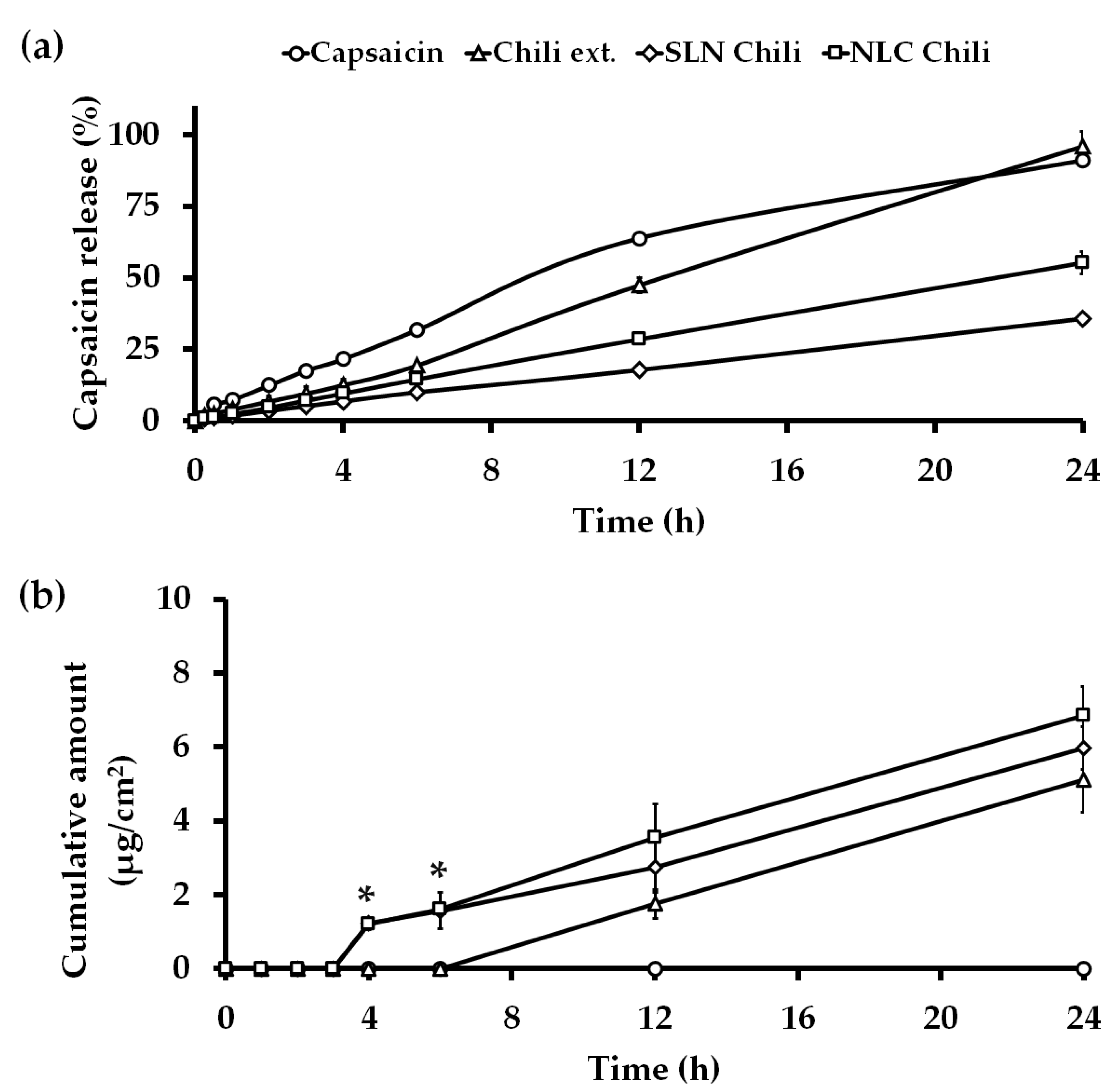
3.3. In Vitro Release Kinetics Study
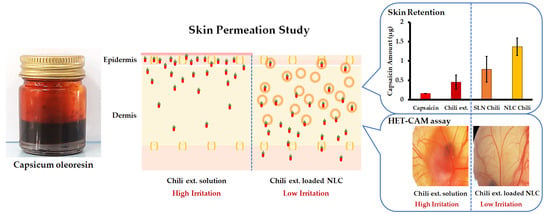
3.4. In Vitro Skin Permeation Study
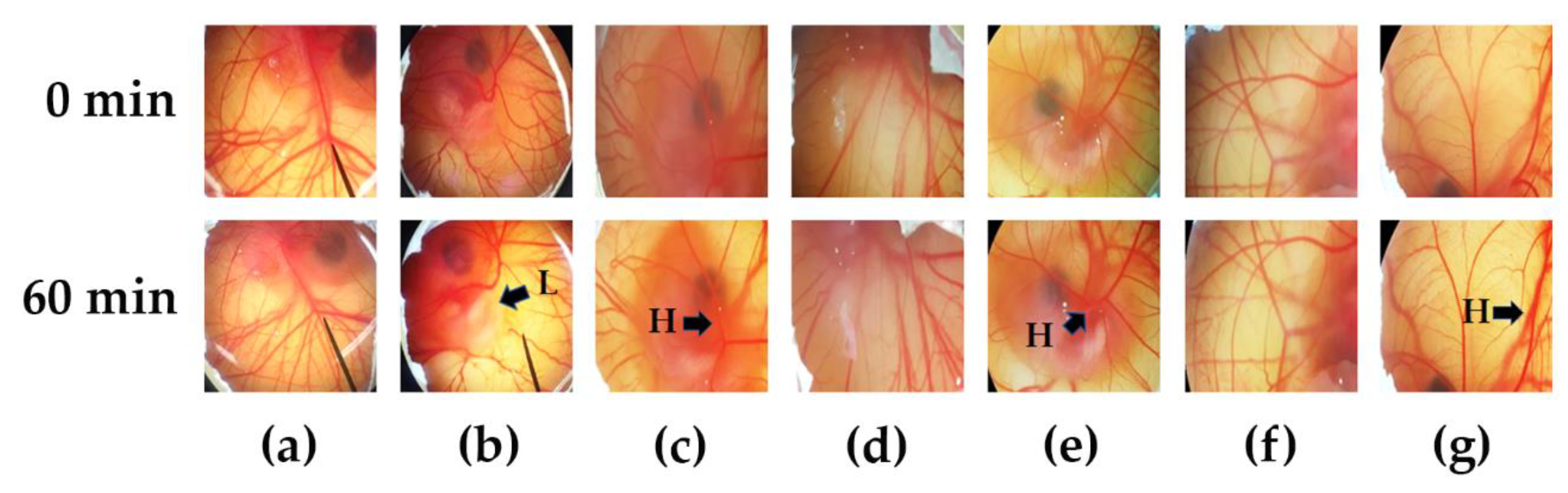
3.5. Irritation Properties of Chili Extract Determined by the HET-CAM Assay
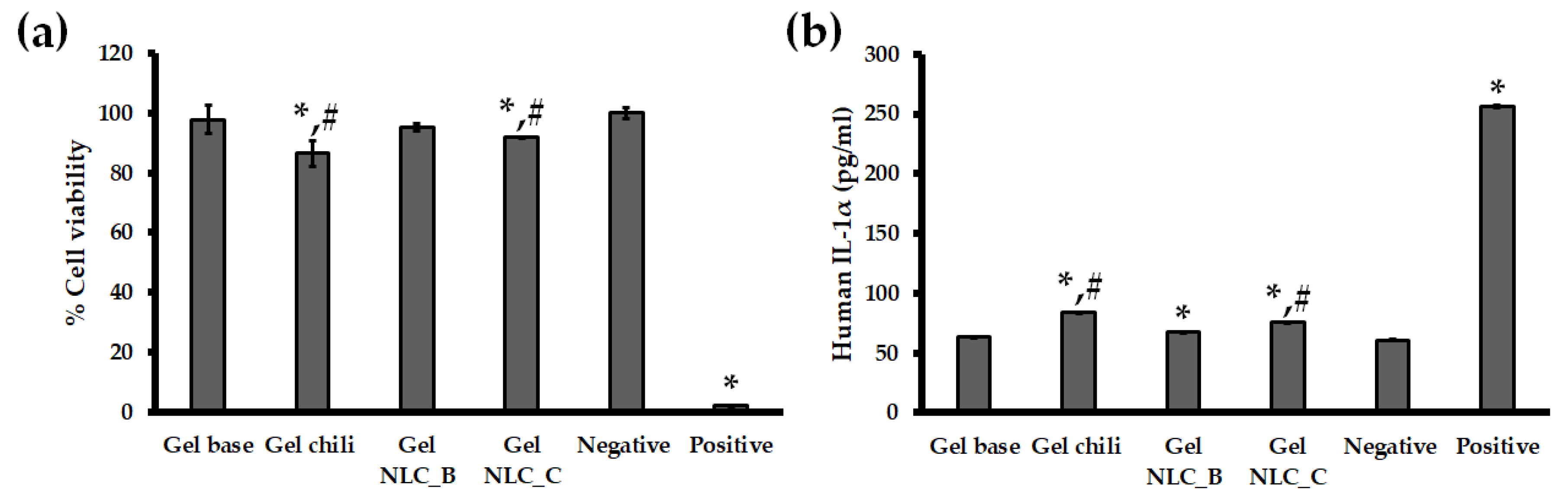
3.6. In Vitro Skin Irritation Test Using the EpiDermTM Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anogianaki, A.; Negrev, N.N.; Shaik, Y.B.; Castellani, M.L.; Frydas, S.; Vecchiet, J.; Tete, S.; Salini, V.; De Amicis, D.; De Lutiis, M.A.; et al. Capsaicin: An irritant anti-inflammatory compound. J. Biol. Reg. Homeos. Agents 2007, 21, 1–4. [Google Scholar]
- Contri, R.V.; Frank, L.A.; Kaiser, M.; Pohlmann, A.R.; Guterres, S.S. The use of nanoencapsulation to decrease human skin irritation caused by capsaicinoids. Int. J. Nanomed. 2014, 9, 951–962. [Google Scholar] [CrossRef][Green Version]
- Thapa, B.; Pepic, I.; Vanic, Z.; Basnet, P.; Skalko-Basnet, N. Topical delivery system for phytochemicals: Capsaicin and capsicum tincture. J. Pharm. Drug Dev. 2013, 1, 1–7. [Google Scholar] [CrossRef]
- Derry, S.; Moore, R.A. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Zempsky, W. Use of topical analgesics in treating neuropathic and musculoskeletal pain. Pain Med. News 2013, 9, 1–4. [Google Scholar]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Knotkova, H.; Pappagallo, M.; Szallasi, A. Capsaicin (TRPV1 Agonist) therapy for pain relief: Farewell or revival? Clin. J. Pain 2008, 24, 142–154. [Google Scholar] [CrossRef]
- Reyes-Escogido, M.D.L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef]
- Wang, X.R.; Gao, S.Q.; Niu, X.Q.; Li, L.J.; Ying, X.Y.; Hu, Z.J.; Gao, J.Q. Capsaicin-loaded nanolipoidal carriers for topical application: Design, characterization, and in vitro/in vivo evaluation. Int. J. Nanomed. 2017, 12, 3881–3898. [Google Scholar] [CrossRef]
- Sharma, A.; Jindal, M.; Aggarwal, G.; Jain, S. Development of a novel method for fabrication of solid lipid nanoparticles: Using high shear homogenization and ultrasonication. Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 265–274. [Google Scholar]
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br. J. Anaesth. 2011, 107, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Shareef, M.A.; Singal, P.; Sharma, G.; Negi, P.; Katare, O.P. Lipid-based capsaicin-loaded nano-colloidal biocompatible topical carriers with enhanced analgesic potential and decreased dermal irritation. J. Liposome Res. 2014, 24, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Gupta, M.; Vyas, S. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis. Artif. Cells Nanomed. Biotechnol. 2015, 43, 33–39. [Google Scholar] [CrossRef]
- Popescu, M.; Chiutu, L.; Mircioiu, C.; Dima, S. Capsaicin microemulsions: Preparation, characterization and in vitro release study. Farmacia 2014, 62, 58–68. [Google Scholar]
- Kim, J.H.; Ko, J.A.; Kim, J.T.; Cha, D.S.; Cho, J.H.; Park, H.J.; Shin, G.H. Preparation of a capsaicin-loaded nanoemulsion for improving skin penetration. J. Agric. Food Chem. 2014, 62, 725–732. [Google Scholar] [CrossRef]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Müller, R.H. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int. J. Pharm. 2004, 278, 71–77. [Google Scholar] [CrossRef]
- Hou, D.; Xie, C.; Huang, K.; Zhu, C. The production and characteristics of solid lipid nanoparticles (SLNs). Biomaterials 2003, 24, 1781–1785. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Chantaburanan, T.; Junyaprasert, V.B. Influence of state and crystallinity of lipid matrix on physicochemical properties and permeation of capsaicin-loaded lipid nanoparticles for topical delivery. J. Drug. Deliv. Sci. Technol. 2017, 39, 300–307. [Google Scholar] [CrossRef]
- Tatke, A.; Dudhipala, N.; Janga, K.Y.; Balguri, S.P.; Avula, B.; Jablonski, M.M.; Majumdar, S. In situ gel of triamcinolone acetonide-loaded solid lipid nanoparticles for improved topical ocular delivery: Tear kinetics and ocular disposition studies. Nanomaterials 2019, 9, 33. [Google Scholar] [CrossRef]
- NICEATM-ICCVAM. Evaluation of In Vitro Test Methods to Identify Ocular Corrosives and Severe Irritants. Available online: https://ntp.niehs.nih.gov/pubhealth/evalatm/test-method-evaluations/ocular/in-vitro/index.html (accessed on 26 March 2020).
- Vinardell, M.; Mitjans, M. Alternative methods for eye and skin irritation tests: An overview. J. Pharm. Sci. 2008, 97, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N.; Kemper, F. The HET-CAM test: An alternative to the Draize eye test. Food Chem. Toxicol. 1986, 24, 495–496. [Google Scholar] [CrossRef]
- Chaiyana, W.; Punyoyai, C.; Somwongin, S.; Leelapornpisid, P.; Ingkaninan, K.; Waranuch, N.; Srivilai, J.; Thitipramote, N.; Wisuitiprot, W.; Schuster, R.; et al. Inhibition of 5α-reductase, IL-6 secretion, and oxidation process of Equisetum debile Roxb. ex vaucher extract as functional food and nutraceuticals ingredients. Nutrients 2017, 9, 1105. [Google Scholar] [CrossRef] [PubMed]
- Ramezanli, T.; Michniak-Kohn, B.B. Development and Characterization of a Topical Gel Formulation of Adapalene-TyroSpheres and Assessment of Its Clinical Efficacy. Mol. Pharm. 2018, 15, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, J.; Lee, H.; Park, J.; Yoon, B.I.; Jin, S.M.; Park, K. Skin corrosion and irritation test of nanoparticles using reconstructed three-dimensional human skin model, EpiDerm™. Toxicol. Res. 2016, 32, 311–316. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Wu, S.; Ju, B.; Zhu, D.; Yan, Y.; Wang, M.; Hu, J. Preparation and evaluation of microemulsion-based transdermal delivery of Cistanche tubulosa phenylethanoid glycosides. Mol. Med. Rep. 2017, 15, 1109–1116. [Google Scholar] [CrossRef]
- Gupta, S.; Kesarla, R.; Chotai, N.; Misra, A.; Omri, A. Systematic approach for the formulation and optimization of solid lipid nanoparticles of efavirenz by high pressure homogenization using design of experiments for brain targeting and enhanced bioavailability. Biomed. Res. Int. 2017, 2017, 1–18. [Google Scholar] [CrossRef]
- Jatin, B.P.; Ramesh, D.P.; Soniwala, M.M.; Chacda, J.R. Solid Lipid Nanoparticles: Overview on Excipients. Asian J. Pharm. Technol. Innov. 2013, 1, 1–9. [Google Scholar]
- Schmidts, T.; Schlupp, P.; Gross, A.; Dobler, D.; Runkel, F. Required HLB determination of some pharmaceutical oils in submicron emulsions. J. Dispers. Sci. Technol. 2012, 33, 816–820. [Google Scholar] [CrossRef]
- Zhao, X.L.; Yang, C.R.; Yang, K.L.; Li, K.X.; Hu, H.Y.; Chen, D.W. Preparation and characterization of nanostructured lipid carriers loaded traditional Chinese medicine, zedoary turmeric oil. Drug Dev. Ind. Pharm. 2010, 36, 773–780. [Google Scholar] [CrossRef]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar] [CrossRef]
- Üner, M.; Wissing, S.A.; Yener, G.; Müller, R.H. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for application of ascorbyl palmitate. Pharmazie 2005, 60, 577–582. [Google Scholar] [PubMed]
- Muchow, M.; Maincent, P.; Müller, R.H. Lipid nanoparticles with a solid matrix (SLN®, NLC®, LDC®) for oral drug delivery. Drug Dev. Ind. Pharm. 2008, 34, 1394–1405. [Google Scholar] [CrossRef]
- Song, S.H.; Lee, K.M.; Kang, J.B.; Lee, S.G.; Kang, M.J.; Choi, Y.W. Improved skin delivery of voriconazole with a nanostructured lipid carrier-based hydrogel formulation. Chem. Pharm. Bull. 2014, 62, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Douglas, K.; Farber, J.; Flynn, S.; Gu, J.; Qin, V.; Shih, J.; Silberg, J.; Wamakima, A. Zero-Order Controlled Release Kenetics Through Polymer Matrices. Available online: https://pdfs.semanticscholar.org/41f6/0e17d70a31503e22d397095bf4d67758a18d.pdf (accessed on 26 May 2019).
- Chinsriwongkul, A.; Chareanputtakhun, P.; Ngawhirunpat, T.; Rojanarata, T.; Sila-On, W.; Ruktanonchai, U.; Opanasopit, P. Nanostructured lipid carriers (NLC) for parenteral delivery of an anticancer drug. AAPS PharmSciTech 2012, 13, 150–158. [Google Scholar] [CrossRef]
- Bhaskar, K.; Mohan, C.K.; Lingam, M.; Mohan, S.J.; Venkateswarlu, V.; Rao, Y.M.; Bhaskar, K.; Anbu, J.; Ravichandran, V. Development of SLN and NLC enriched hydrogels for transdermal delivery of nitrendipine: In vitro and in vivo characteristics. Drug Dev. Ind. Pharm. 2009, 35, 98–113. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Poloniae Pharm. 2010, 67, 217–223. [Google Scholar]
- Todo, H. Transdermal permeation of drugs in various animal species. Pharmaceutics 2017, 9, 33. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar]
- Gkeka, P.; Sarkisov, L.; Angelikopoulos, P. Homogeneous hydrophobic–hydrophilic surface patterns enhance permeation of nanoparticles through lipid membranes. J. Phys. Chem. Lett. 2013, 4, 1907–1912. [Google Scholar] [CrossRef]
- Contini, C.; Schneemilch, M.; Gaisford, S.; Quirke, N. Nanoparticle–membrane interactions. J. Exp. Nanosci. 2018, 13, 62–81. [Google Scholar] [CrossRef]
- Tan, Y.J.; Lee, C.S.; Er, H.M.; Lim, W.H.; Wong, S.F. In-vitro evaluation of griseofulvin loaded lipid nanoparticles for topical delivery. J. Drug Deliv. Sci. Technol. 2016, 31, 1–10. [Google Scholar] [CrossRef]
- Singh, S.; Singh, M.; Tripathi, C.B.; Arya, M.; Saraf, S.A. Development and evaluation of ultra-small nanostructured lipid carriers: Novel topical delivery system for athlete’s foot. Drug Deliv. Transl. Res. 2016, 6, 38–47. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method, OECD Guidelines for the Testing of Chemicals, Section 4. 2015. Available online: https://www.oecd-ilibrary.org/docserver/9789264242845-en.pdf?expires=1589480230&id=id&accname=guest&checksum=34A6EC101DE1E2670644038BA205081A (accessed on 20 March 2020).
- Kidd, D.; Johnson, M.; Clements, J. Development of an in vitro corrosion/irritation prediction assay using the EpiDermTM skin model. Toxicol. In Vitro 2007, 21, 1292–1297. [Google Scholar] [CrossRef]
- Costin, G.E.; Raabe, H.; Curren, R. In vitro safety testing strategy for skin irritation using the 3D reconstructed human epidermis. Rom. J. Biochem. 2009, 46, 165–186. [Google Scholar]
| Formulations | Lipid Phase | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| SLN 1:4 * | GBH + COH | 153.74 ± 6.72 | 0.39 ± 0.01 | −44.26 ± 1.22 |
| GBH + COH + chili | 176.80 ± 6.19 | 0.15 ± 0.07 | −33.64 ± 1.09 | |
| GMS + COH | 129.42 ± 5.80 | 0.33 ± 0.05 | −42.16 ± 0.77 | |
| GMS + COH + chili | 148.42 ± 4.80 | 0.22 ± 0.04 | −40.78 ± 0.68 | |
| GBH + SOH | 154.68 ± 11.87 | 0.46 ± 0.01 | −33.14 ± 1.31 | |
| GBH + SOH + chili | 154.66 ± 4.22 | 0.17 ± 0.02 | −26.60 ± 1.84 | |
| GMS + SOH | 113.74 ± 3.58 | 0.43 ± 0.06 | −34.94 ± 1.39 | |
| GMS + SOH + chili | 160.86 ± 5.47 | 0.19 ± 0.04 | −28.24 ± 0.69 | |
| NLC 1 4 * | GBH + COH + IPM | 150.98 ± 3.09 | 0.31 ± 0.02 | −35.40 ± 1.10 |
| GBH + COH + IPM + chili | 156.10 ± 2.82 | 0.11 ± 0.02 | −31.40 ± 1.45 | |
| GMS + COH + IPM | 139.34 ± 3.95 | 0.38 ± 0.03 | −34.64 ± 1.37 | |
| GMS + COH + IPM + chili | 148.50 ± 2.94 | 0.12 ± 0.03 | −29.58 ± 1.37 | |
| GBH + SOH + IPM | 150.42 ± 4.12 | 0.43 ±0.03 | −31.54 ± 1.96 | |
| GBH + SOH + IPM + chili | 154.86 ± 1.66 | 0.16 ± 0.05 | −30.90 ± 0.94 | |
| GMS + SOH + IPM | 126.84 ± 4.61 | 0.38 ± 0.06 | −30.92 ± 0.95 | |
| GMS + SOH + IPM + chili | 150.08 ± 2.16 | 0.16 ± 0.03 | −27.10 ± 0.91 |
| Methods | Formulations | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| Probe sonication | SLN | 1671.00 ± 643.07 | 0.97 ± 0.07 | −45.18 ± 1.34 |
| NLC | 502.24 ± 136.29 | 0.68 ± 0.08 | −45.80 ± 0.83 | |
| High pressure homogenizer | SLN | 129.42 ± 5.80 | 0.33 ± 0.05 | −42.16 ± 0.77 |
| NLC | 139.34 ± 3.95 | 0.38 ± 0.03 | −34.64 ± 1.37 |
| Capsaicin Conc. (%) | Formulation | Lipid Phase (Wax) | % EE | % LC |
|---|---|---|---|---|
| 0.075 | SLN | GBH + COH + chili | 78.33 ± 0.25 | 2.35 ± 0.01 |
| GMS + COH + chili | 82.23 ± 1.69 | 2.47 ± 0.05 | ||
| GBH + SOH + chili | 77.27 ± 5.98 | 2.32 ± 0.18 | ||
| GMS + SOH + chili | 76.49 ± 8.85 | 2.29 ± 0.27 | ||
| NLC | GBH + COH + IPM + chili | 85.99 ± 6.37 | 2.58 ± 0.19 | |
| GMS + COH + IPM + chili | 91.67 ± 4.02 | 2.75 ± 0.12 | ||
| GBH + SOH + IPM + chili | 84.65 ± 3.01 | 2.54 ± 0.09 | ||
| GMS + SOH + IPM + chili | 85.43 ± 1.29 | 2.56 ± 0.04 | ||
| 0.25 | SLN | GMS + COH + chili | 68.63 ± 0.54 | 6.86 ± 0.05 |
| NLC | GMS + COH + IPM + chili | 85.27 ± 0.12 | 8.53 ± 0.01 |
| Samples | Onset (°C) | Peak (°C) | Endset (°C) | Integral (mJ) | ΔH (J/g) | % Crystallinity |
|---|---|---|---|---|---|---|
| GMS | 57.81 | 66.77 | 70.83 | −1043.38 | −163.03 | - |
| COH | 48.06 | 51.35 | 55.69 | −1291.74 | −205.04 | - |
| Physical mixture | 49.61 | 52.15 | 56.11 | −719.92 | −110.76 | 100.00 |
| SLN blank | 34.75 | 37.88 | 40.97 | −123.57 | −14.54 | 65.64 |
| NLC blank | 34.88 | 38.72 | 42.81 | −52.26 | −6.15 | 39.66 |
| SLN chili | 30.91 | 39.84 | 45.24 | −61.72 | −7.09 | 15.33 |
| NLC chili | 30.49 | 35.21 | 44.19 | −10.81 | −1.26 | 3.13 |
| Formulation | Flux (µg/cm2 h) | Lag Time (h) | Permeability Coefficient (cm/h) × 10−5 | Q24h (µg) | Enhancement Ratio (Er) |
|---|---|---|---|---|---|
| Chili extract | 0.28 ± 0.05 | 5.95 ± 0.15 | 11.36 ± 1.90 | 9.05 ± 1.54 | 1.00 ± 0.00 |
| SLN chili | 0.37 ± 0.12 | 2.31 ± 0.07 * | 17.17 ± 3.31 * | 11.54 ± 1.78 | 1.39 ± 0.12 |
| NLC chili | 0.45 ± 0.16 | 2.30 ± 0.06 * | 21.02 ± 4.38 * | 13.68 ± 1.79 * | 1.70 ± 0.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anantaworasakul, P.; Chaiyana, W.; Michniak-Kohn, B.B.; Rungseevijitprapa, W.; Ampasavate, C. Enhanced Transdermal Delivery of Concentrated Capsaicin from Chili Extract-Loaded Lipid Nanoparticles with Reduced Skin Irritation. Pharmaceutics 2020, 12, 463. https://doi.org/10.3390/pharmaceutics12050463
Anantaworasakul P, Chaiyana W, Michniak-Kohn BB, Rungseevijitprapa W, Ampasavate C. Enhanced Transdermal Delivery of Concentrated Capsaicin from Chili Extract-Loaded Lipid Nanoparticles with Reduced Skin Irritation. Pharmaceutics. 2020; 12(5):463. https://doi.org/10.3390/pharmaceutics12050463
Chicago/Turabian StyleAnantaworasakul, Phunsuk, Wantida Chaiyana, Bozena B. Michniak-Kohn, Wandee Rungseevijitprapa, and Chadarat Ampasavate. 2020. "Enhanced Transdermal Delivery of Concentrated Capsaicin from Chili Extract-Loaded Lipid Nanoparticles with Reduced Skin Irritation" Pharmaceutics 12, no. 5: 463. https://doi.org/10.3390/pharmaceutics12050463
APA StyleAnantaworasakul, P., Chaiyana, W., Michniak-Kohn, B. B., Rungseevijitprapa, W., & Ampasavate, C. (2020). Enhanced Transdermal Delivery of Concentrated Capsaicin from Chili Extract-Loaded Lipid Nanoparticles with Reduced Skin Irritation. Pharmaceutics, 12(5), 463. https://doi.org/10.3390/pharmaceutics12050463