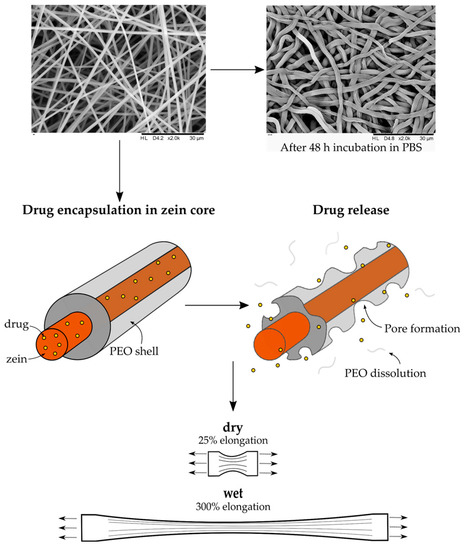
Highly Elastic and Water Stable Zein Microfibers as a Potential Drug Delivery System for Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Microfiber Mats
2.3. Surface Morphology
2.4. Wettability
2.5. Water Vapor Sorption
2.6. Water Stability
2.7. Solid State Characterization
2.8. Mechanical Characterization
2.9. Drug Loading and Release
2.10. Agar Diffusion Assay
2.11. Cell Safety
2.12. Statistical Analysis
3. Results
3.1. Fiber Diameter, Surface Morphology and Electrospinnability
3.2. Wettability
3.3. Water Vapor Sorption
3.4. Water Stability
3.5. Solid State Characterization
3.6. Mechanical Characterization
3.7. Drug Loading and Release
3.8. Agar Diffusion Assay
3.9. Cell Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A




References
- Olsson, M.; Jarbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Darwin, E.; Tomic-Canic, M. Healing Chronic Wounds: Current Challenges and Potential Solutions. Curr. Dermatol. Rep. 2018, 7, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.; Green, J. Review of patients' experiences with fungating wounds and associated quality of life. J. Wound Care 2013, 22, 265–266. [Google Scholar] [CrossRef]
- Gaspar-Pintiliescu, A.; Stanciuc, A.M.; Craciunescu, O. Natural composite dressings based on collagen, gelatin and plant bioactive compounds for wound healing: A review. Int. J. Biol. Macromol. 2019, 138, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.X.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J.W. Recent advances in electrospun nanofibers for wound healing. Nanomedicine-Uk 2017, 12, 1335–1352. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Designing electrospun nanofiber mats to promote wound healing-a review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Han, D.; Steckl, A.J. Coaxial Electrospinning Formation of Complex Polymer Fibers and their Applications. Chempluschem 2019, 84, 1453–1497. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, X.; He, H.W.; Yu, M.; Ning, X.; Long, Y.Z. Solvent-free electrospinning: Opportunities and challenges. Polym. Chem-Uk 2017, 8, 333–352. [Google Scholar] [CrossRef]
- Wang, Y.; Padua, G.W. Nanoscale Characterization of Zein Self-Assembly. Langmuir 2012, 28, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Labib, G. Overview on zein protein: A promising pharmaceutical excipient in drug delivery systems and tissue engineering. Expert Opin. Drug. Deliv. 2018, 15, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Li, H.-M.; Hu, X.; Guo, P.; Fu, P.; Xu, L.; Zhang, X.-Z. Antioxidant properties and possible mode of action of corn protein peptides and zein peptides. J. Food Biochem. 2010, 34, 44–60. [Google Scholar] [CrossRef]
- Miyoshi, T.; Toyohara, K.; Minematsu, H. Preparation of ultrafine fibrous zein membranes via electrospinning. Polym. Int. 2005, 54, 1187–1190. [Google Scholar] [CrossRef]
- Paliwal, R.; Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef]
- Kanjanapongkul, K.; Wongsasulak, S.; Yoovidhya, T. Investigation and Prevention of Clogging During Electrospinning of Zein Solution. J. Appl. Polym. Sci. 2010, 118, 1821–1829. [Google Scholar] [CrossRef]
- Selling, G.W.; Biswas, A.; Patel, A.; Walls, D.J.; Dunlap, C.; Wei, Y. Impact of solvent on electrospinning of zein and analysis of resulting fibers. Macromol. Chem. Phys. 2007, 208, 1002–1010. [Google Scholar] [CrossRef]
- Zhang, J.F.; Wang, Y.; Liao, S.; Lallier, T.; Wen, Z.T.; Xu, X. Photo-cross-linked Antibacterial Zein Nanofibers Fabricated by Reactive Electrospinning and its Effects against Streptococcus mutans. Oral Health Dent. Stud. 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Jiang, Q.R.; Yang, Y.Q. Water-Stable Electrospun Zein Fibers for Potential Drug Delivery. J. Biomat. Sci-Polym. E 2011, 22, 1393–1408. [Google Scholar] [CrossRef]
- Yao, C.; Li, X.S.; Song, T.Y. Electrospinning and crosslinking of zein nanofiber mats. Abstr. Pap. Am. Chem. Sci. 2006, 231, 380–385. [Google Scholar] [CrossRef]
- Song, T.Y.; Yao, C.; Li, X.S. Electrospinning of zein/chitosan composite fibrous membranes. Chin. J. Polym. Sci. 2010, 28, 171–179. [Google Scholar] [CrossRef]
- Wen, H.F.; Yang, C.; Yu, D.G.; Li, X.Y.; Zhang, D.F. Electrospun zein nanoribbons for treatment of lead-contained wastewater. Chem. Eng. J. 2016, 290, 263–272. [Google Scholar] [CrossRef]
- Vogt, L.; Liverani, L.; Roether, J.A.; Boccaccini, A.R. Electrospun Zein Fibers Incorporating Poly(glycerol sebacate) for Soft Tissue Engineering. Nanomaterials 2018, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Feng, H.T.; He, J.M.; Li, C.; Mao, X.; Xie, D.M.; Ao, N.J.; Chu, B. Coaxial electrospun zein nanofibrous membrane for sustained release. J. Biomat. Sci-Polym. E 2013, 24, 1923–1934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.J.; Jia, Y.Q.; Han, H.S.; Sun, D.H. Preparation and Evaluation of Electrospun Zein/HA Fibers Based on Two Methods of Adding HA Nanoparticles. J. Bionic Eng. 2014, 11, 115–124. [Google Scholar] [CrossRef]
- Koombhongse, S.; Liu, W.X.; Reneker, D.H. Flat polymer ribbons and other shapes by electrospinning. J. Polym. Sci. Pol. Phys. 2001, 39, 2598–2606. [Google Scholar] [CrossRef]
- Arslan, E.; Koc, M.H.; Uysal, O.; Dikecoglu, B.; Topal, A.E.; Garifullin, R.; Ozkan, A.D.; Dana, A.; Hermida-Merino, D.; Castelletto, V.; et al. Supramolecular Peptide Nanofiber Morphology Affects Mechanotransduction of Stem Cells. Biomacromolecules 2017, 18, 3114–3130. [Google Scholar] [CrossRef]
- Miri, M.A.; Movaffagh, J.; Najafi, M.B.H.; Najafi, M.N.; Ghorani, B.; Koocheki, A. Optimization of Elecrospinning Process of Zein Using Central Composite Design. Fiber Polym. 2016, 17, 769–777. [Google Scholar] [CrossRef]
- Shi, C.; Xi, S.X.; Han, Y.C.; Zhang, H.; Liu, J.S.; Li, Y.Q. Structure, rheology and electrospinning of zein and poly(ethylene oxide) in aqueous ethanol solutions. Chin. Chem. Lett. 2019, 30, 305–310. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Y.; Zheng, M.; Dunne, F.; Liu, T.; Fu, X.W.; Kong, L.; Pan, S.Y.; Zhong, W.H. Morphology engineering of protein fabrics for advanced and sustainable filtration. J. Mater. Chem. A 2018, 6, 21585–21595. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhao, M.; Barker, S.A.; Belton, P.S.; Craig, D.Q.M. A spectroscopic and thermal investigation into the relationship between composition, secondary structure and physical characteristics of electrospun zein nanofibers. Mat. Sci. Eng. C-Mater. 2019, 98, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Chen, L.Y. Fabrication and characterization of novel assembled prolamin protein nanofabrics with improved stability, mechanical property and release profiles. J. Mater. Chem. 2012, 22, 21592–21601. [Google Scholar] [CrossRef]
- Selling, G.W.; Woods, K.K.; Biswas, A. Electrospinning formaldehyde-crosslinked zein solutions. Polym. Int. 2011, 60, 537–542. [Google Scholar] [CrossRef]
- Selling, G.W.; Woods, K.K.; Sessa, D.; Biswas, A. Electrospun Zein Fibers Using Glutaraldehyde as the Crosslinking Reagent: Effect of Time and Temperature. Macromol. Chem. Phys. 2008, 209, 1003–1011. [Google Scholar] [CrossRef]
- Selling, G.W.; Woods, K.K.; Biswas, A. Electrospun zein fibers using glyoxal as the crosslinking reagent. J. Appl. Polym. Sci. 2012, 123, 2651–2661. [Google Scholar] [CrossRef]
- Xu, W.J.; Karst, D.; Yang, W.; Yang, Y.Q. Novel zein-based electrospun fibers with the water stability and strength necessary for various applications. Polym. Int. 2008, 57, 1110–1117. [Google Scholar] [CrossRef]
- Ochoa Machiste, E.; Segale, L.; Conti, S.; Fasani, E.; Albini, A.; Conte, U.; Maggi, L. Effect of UV light exposure on hydrophilic polymers used as drug release modulators in solid dosage forms. J. Drug Deliv. Sci. Technol. 2005, 15, 151–157. [Google Scholar] [CrossRef]
- Janga, K.Y.; King, T.; Ji, N.; Sarabu, S.; Shadambikar, G.; Sawant, S.; Xu, P.; Repka, M.A.; Murthy, S.N. Photostability Issues in Pharmaceutical Dosage Forms and Photostabilization. AAPS PharmSciTech 2018, 19, 48–59. [Google Scholar] [CrossRef]
- Diridollou, S.; Patat, F.; Gens, F.; Vaillant, L.; Black, D.; Lagarde, J.M.; Gall, Y.; Berson, M. In vivo model of the mechanical properties of the human skin under suction. Skin Res. Technol. 2000, 6, 214–221. [Google Scholar] [CrossRef]
- Lawton, J.W. Plasticizers for zein: Their effect on tensile properties and water absorption of zein films. Cereal Chem. 2004, 81, 1–5. [Google Scholar] [CrossRef]
- Lai, H.M.; Padua, G.W.; Wei, L.S. Properties and microstructure of zein sheets plasticized with palmitic and stearic acids. Cereal Chem. 1997, 74, 83–90. [Google Scholar] [CrossRef]
- Vega-Lugo, A.C.; Lim, L.T. Effects of poly(ethylene oxide) and pH on the electrospinning of whey protein isolate. J. Polym. Sci. Pol. Phys. 2012, 50, 1188–1197. [Google Scholar] [CrossRef]
- Kurusu, R.S.; Demarquette, N.R. Surface modification to control the water wettability of electrospun mats. Int. Mater. Rev. 2019, 64, 249–287. [Google Scholar] [CrossRef]
- Lin, T.K.; Zhong, L.L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int J. Mol. Sci. 2018, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Haryanto; Kim, S.; Kim, J.H.; Kim, J.O.; Ku, S.; Cho, H.; Han, D.H.; Huh, P. Fabrication of poly(ethylene oxide) hydrogels for wound dressing application using E-beam. Macromol. Res. 2014, 22, 131–138. [Google Scholar] [CrossRef]
- Acevedo, F.; Hermosilla, J.; Sanhueza, C.; Mora-Lagos, B.; Fuentes, I.; Rubilar, M.; Concheiro, A.; Alvarez-Lorenzo, C. Gallic acid loaded PEO-core/zein-shell nanofibers for chemopreventive action on gallbladder cancer cells. Eur. J. Pharm. Sci. 2018, 119, 49–61. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Tort, S.D.; Tugcu-Demiröz, F.N.; Yıldız, S.; Acartürk, F. Effects of UV Exposure Time on Nanofiber Wound Dressing Properties During Sterilization. J. Pharm. Innov. 2019. [Google Scholar] [CrossRef]
- Zupancic, S.; Preem, L.; Kristl, J.; Putrins, M.; Tenson, T.; Kocbek, P.; Kogermann, K. Impact of PCL nanofiber mat structural properties on hydrophilic drug release and antibacterial activity on periodontal pathogens. Eur. J. Pharm. Sci. 2018, 122, 347–358. [Google Scholar] [CrossRef]
- Kayaci, F.; Uyar, T. Electrospun zein nanofibers incorporating cyclodextrins. Carbohydr. Polym. 2012, 90, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S.; Gimenez, E.; Lagarona, J.M. Characterization of the morphology and thermal properties of zein prolamine nanostructures obtained by electrospinning. Food Hydrocoll. 2008, 22, 601–614. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Ocio, M.J.; Lagaron, J.M. Novel antimicrobial ultrathin structures of zein/chitosan blends obtained by electrospinning. Carbohyd. Polym. 2009, 77, 261–266. [Google Scholar] [CrossRef]
- Wongsasulak, S.; Tongsin, P.; Intasanta, N.; Yoovidhya, T. Effect of glycerol on solution properties governing morphology, glass transition temperature, and tensile properties of electrospun zein film. J. Appl. Polym. Sci. 2010, 118, 910–919. [Google Scholar] [CrossRef]
- Hussien, E.M. HPLC method validation for modernization of the tetracycline hydrochloride capsule USP monograph. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 239–244. [Google Scholar] [CrossRef]
- Young, J.E.; Matyska, M.T.; Azad, A.K.; Yoc, S.E.; Pesek, J.J. Separation Differences Among Phenyl Hydride, UDC Cholesterol and Bidentate C8 Stationary Phases for Stability Indicating Methods of Tetracyclines: Journal of Liquid Chromatography & Related Technologies. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 926–942. [Google Scholar] [CrossRef]
- Smith, R.; Russo, J.; Fiegel, J.; Brogden, N. Antibiotic Delivery Strategies to Treat Skin Infections When Innate Antimicrobial Defense Fails. Antibiotics 2020, 9, 56. [Google Scholar] [CrossRef]
- Ahmad, A.; Zachariasen, C.; Christiansen, L.E.; Graesboll, K.; Toft, N.; Matthews, L.; Damborg, P.; Agerso, Y.; Olsen, J.E.; Nielsen, S.S. Pharmacodynamic modelling of in vitro activity of tetracycline against a representative, naturally occurring population of porcine Escherichia coli. Acta Vet. Scand. 2015, 57, 79. [Google Scholar] [CrossRef]
- Heman-Ackah, S.M. Comparison of tetracycline action on Staphylococcus aureus and Escherichia coli by microbial kinetics. Antimicrob. Agents Chemother. 1976, 10, 223–228. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, C.; Lin, W.F.; Hu, R.D.; Wang, Q.M.; Chen, H.; Li, L.Y.; Chen, S.F.; Zheng, J. Binding characteristics between polyethylene glycol (PEG) and proteins in aqueous solution. J. Mater. Chem. B 2014, 2, 2983–2992. [Google Scholar] [CrossRef]
- Zhang, H.; Xi, S.; Han, Y.; Liu, L.; Dong, B.; Zhang, Z.; Chen, Q.; Min, W.; Huang, Q.; Li, Y.; et al. Determining electrospun morphology from the properties of protein–polymer solutions. Soft Matter 2018, 14, 3455–3462. [Google Scholar] [CrossRef] [PubMed]
- Romero-Bastida, C.A.; Martin-Polo, M.O.; Velazquez, G.; Torres, J.A. Effect of plasticizer, ph and hydration on the mechanical and barrier properties of zein and ethylcellulose films. Cienc. y Tecnol. Aliment. 2004, 4, 251–256. [Google Scholar] [CrossRef]
- Liao, I.; Chew, S.; Leong, K. Aligned core–shell nanofibers delivering bioactive proteins. Nanomedicine-Uk 2006, 1, 465–471. [Google Scholar] [CrossRef] [PubMed]
- T.E.C.o.A.S. Breakpoint Tables for Interpretation of MICs and Zone Diameters. 2020, Volume 10. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 18 April 2020).
- Juniarti, D.E.; Samadi, K.; Sudirman, A. Differences in cytotoxicity between 5% tetracycline hydrochloride and 15% EDTA as root canal irrigant. Dent. J. 2008, 41, 67–69. [Google Scholar] [CrossRef][Green Version]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 356–366. [Google Scholar] [CrossRef]
- Fael, H.; Demirel, A.L. Nisin/polyanion layer-by-layer films exhibiting different mechanisms in antimicrobial efficacy. RSC Adv. 2020, 10, 10329–10337. [Google Scholar] [CrossRef]
- Shukla, A.; Fleming, K.E.; Chuang, H.F.; Chau, T.M.; Loose, C.R.; Stephanopoulos, G.N.; Hammond, P.T. Controlling the release of peptide antimicrobial agents from surfaces. Biomaterials 2010, 31, 2348–2357. [Google Scholar] [CrossRef]
- Jennings, J.A.; Beenken, K.E.; Parker, A.C.; Smith, J.K.; Courtney, H.S.; Smeltzer, M.S.; Haggard, W.O. Polymicrobial Biofilm Inhibition Effects of Acetate-Buffered Chitosan Sponge Delivery Device. Macromol. Biosci. 2016, 16, 591–598. [Google Scholar] [CrossRef]







| Sample | Core | Shell | Flowrate (mL/h) | Voltage (kV) | Distance (cm) | ||
|---|---|---|---|---|---|---|---|
| Core | Shell | Injector | Collector | ||||
| (Z)PEO | Zein | PEO | 700 | 500 | 4.5 | −0.6 | 18 |
| (Z)E | Zein | EtOH | 385 | 185 | 18.8 | −2.7 | 23 |
| (Z+T)PEO | Zein+T | PEO | 700 | 500 | 4.5 | −0.6 | 18 |
| (Z+PEO)PEO | Zein+PEO | PEO | 700 | 500 | 4.5 | −0.6 | 18 |
| (Z+PEO)E | Zein+PEO | EtOH | 700 | 300 | 6 | −1 | 18 |
| (Z+PEO+T)PEO | Zein+PEO+T | PEO | 700 | 500 | 4.5 | −0.6 | 18 |
| (Z+SA)PEO | Zein+Stearic acid | PEO | 700 | 500 | 4.8 | −1.8 | 23 |
| (Z+SA)E | Zein+Stearic acid | EtOH | 400 | 110 | 15.5 | −3.5 | 13 |
| (Z+SA+T)PEO | Zein+Stearic acid+T | PEO | 700 | 500 | 4.8 | −1.8 | 23 |
| Sample | Diameter (µm ± SD) | Morphology | Contact Angle (° ± SD) | Vapor Sorption (%) |
|---|---|---|---|---|
| (Z)PEO | 1.5 ± 0.3 | Tubular fibers | 64.0 ± 15.4 | 26.5 |
| (Z)E | N/A | Beads | N/A | N/A |
| (Z+T)PEO | 1.6 ± 0.5 | Tubular fibers | 68.9 ± 16.7 | 23.1 |
| (Z+PEO)PEO | 1.2 ± 0.1 | Tubular fibers | 47.1 ± 4.7 | 24.3 |
| (Z+PEO)E | 1.6 ± 0.5 | Ribbon fibers | 110.6 ± 9.1 *** | 8.2 |
| (Z+PEO+T)PEO | 1.6 ± 0.2 | Tubular fibers | 59.7 ± 17.6 | 21.5 |
| (Z+SA)PEO | 1.4 ± 0.2 | Mixed fibers | 72.1 ± 7.4 | 22.4 |
| (Z+SA)E | N/A | Beads | N/A | N/A |
| (Z+SA+T)PEO | 1.4 ± 0.3 | Mixed fibers | 100.6 ± 8.4 *** | 24.3 |
| Sample | Young’s Modulus (MPa ± SD) | Tensile Strength at Break (kPa ± SD) | Elongation at Break (% ± SD) | |||
|---|---|---|---|---|---|---|
| Dry | Wet | Dry | Wet | Dry | Wet | |
| (Z)PEO | 1.5 ± 1.0 | 0.9 ± 0.4 | 54.6 ± 13.2 | 34.4 ± 17.1 | 22.7 ± 3.4 | 304.9 ± 105.8 |
| (Z+T)PEO | 4.3 ± 1.8 | 1.0 ± 0.9 | 85.1 ± 23.4 | 50.0 ± 47.6 | 16.7 ± 1.5 | 284.2 ± 74.7 |
| (Z+PEO)PEO | 4.2 ± 2.6 | 1.1 ± 0.4 | 75.2 ± 19.0 | 24.6 ± 7.1 | 17.4 ± 1.3 | 58.5± 22.4 *** |
| (Z+PEO+T)PEO | 1.8 ± 0.9 | 0.5 ± 0.2 | 69.1 ± 20.5 | 44.2 ± 34.9 | 18.4 ± 4.1 | 265.1 ± 54.6 |
| (Z+SA)PEO | 1.6 ± 0.3 | 0.2 ± 0.1 | 58.0 ± 17.8 | 22.7 ± 5.3 | 19.3 ± 0.8 | 411.2 ± 54.3 |
| (Z+SA+T)PEO | 1.6 ± 1.0 | 1.2 ± 0.3 | 51.6 ± 12.9 | 18.7 ± 6.6 | 14.6 ± 1.7 | 24.0 ± 11.5 *** |
| Sample | Drug Load (%) | TDL (%) | EE (% ± SD) | |
|---|---|---|---|---|
| Fresh | 4 Months | |||
| (Z+T)PEO | 5 | 4.5 | 99.1 ± 6.2 | 89.6 ± 1.6 |
| (Z+PEO+T)PEO | 5 | 4.5 | 95.0 ± 1.4 | 93.0 ± 0.4 |
| (Z+SA+T)PEO | 5 | 4.5 | 89.0 ± 0.3 | 90.6 ± 0.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetova, A.; Lanno, G.-M.; Kogermann, K.; Malmsten, M.; Rades, T.; Heinz, A. Highly Elastic and Water Stable Zein Microfibers as a Potential Drug Delivery System for Wound Healing. Pharmaceutics 2020, 12, 458. https://doi.org/10.3390/pharmaceutics12050458
Akhmetova A, Lanno G-M, Kogermann K, Malmsten M, Rades T, Heinz A. Highly Elastic and Water Stable Zein Microfibers as a Potential Drug Delivery System for Wound Healing. Pharmaceutics. 2020; 12(5):458. https://doi.org/10.3390/pharmaceutics12050458
Chicago/Turabian StyleAkhmetova, Alma, Georg-Marten Lanno, Karin Kogermann, Martin Malmsten, Thomas Rades, and Andrea Heinz. 2020. "Highly Elastic and Water Stable Zein Microfibers as a Potential Drug Delivery System for Wound Healing" Pharmaceutics 12, no. 5: 458. https://doi.org/10.3390/pharmaceutics12050458
APA StyleAkhmetova, A., Lanno, G.-M., Kogermann, K., Malmsten, M., Rades, T., & Heinz, A. (2020). Highly Elastic and Water Stable Zein Microfibers as a Potential Drug Delivery System for Wound Healing. Pharmaceutics, 12(5), 458. https://doi.org/10.3390/pharmaceutics12050458