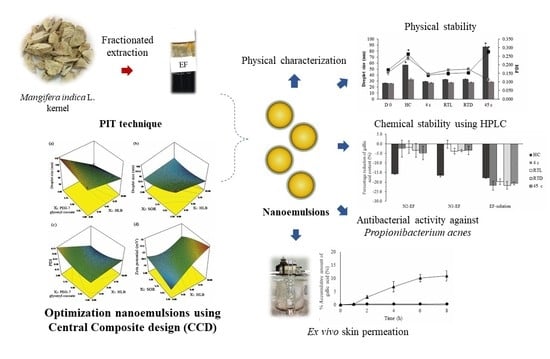
Optimization of Mangifera indica L. Kernel Extract-Loaded Nanoemulsions via Response Surface Methodology, Characterization, Stability, and Skin Permeation for Anti-Acne Cosmeceutical Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of M. indica Kernel Extract
2.3. Preparation of Nanoemulsions
2.4. Experimental Design
2.5. Optimization and Verification Procedures
2.6. Formulation of the Extract-Loaded Nanoemulsions
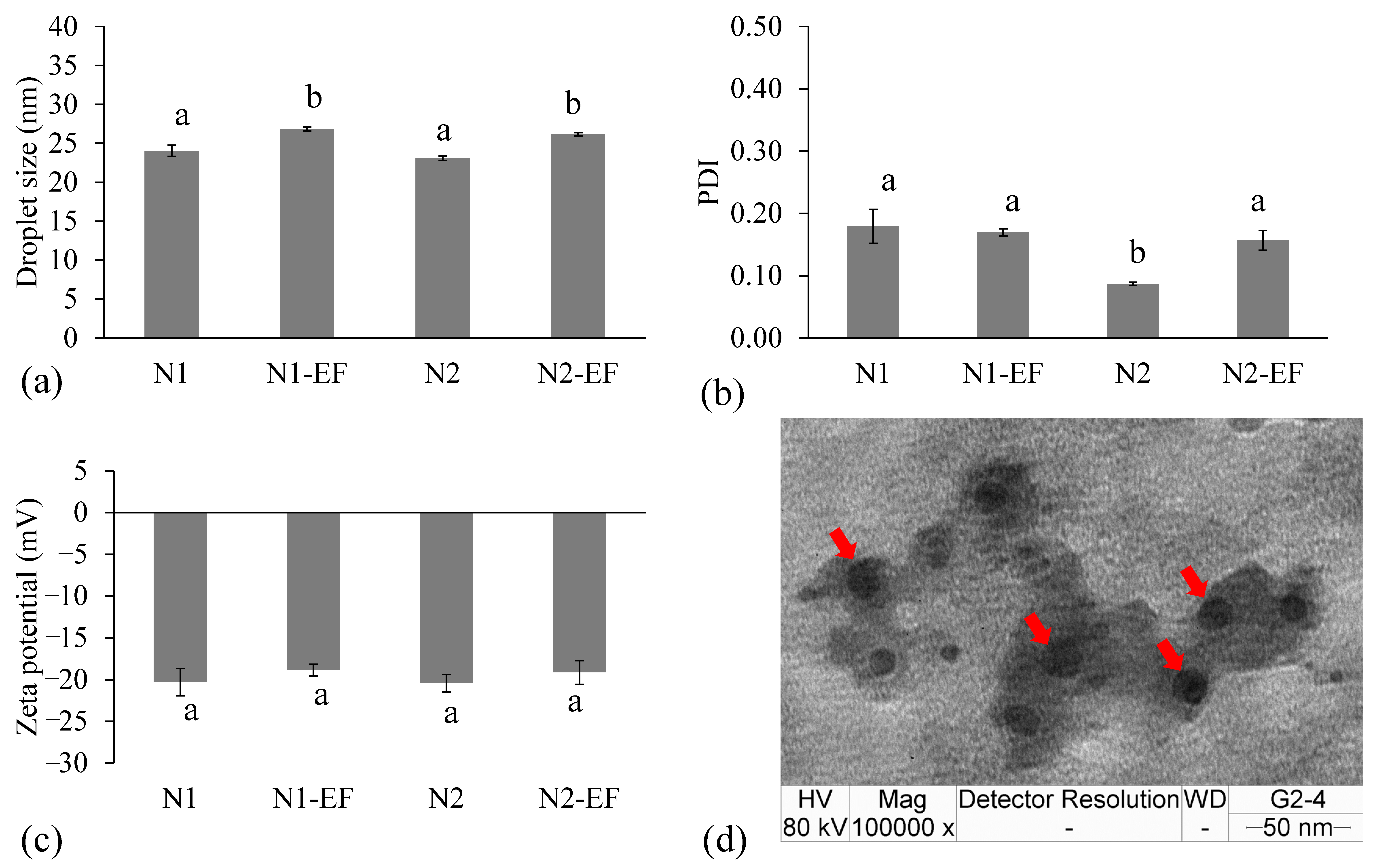
2.7. Determination of Droplet Size, Polydispersity Index (PDI), and Zeta Potential
2.8. Transmission Electron Microscopic Analysis
2.9. Stability Study
2.9.1. Storage Conditions
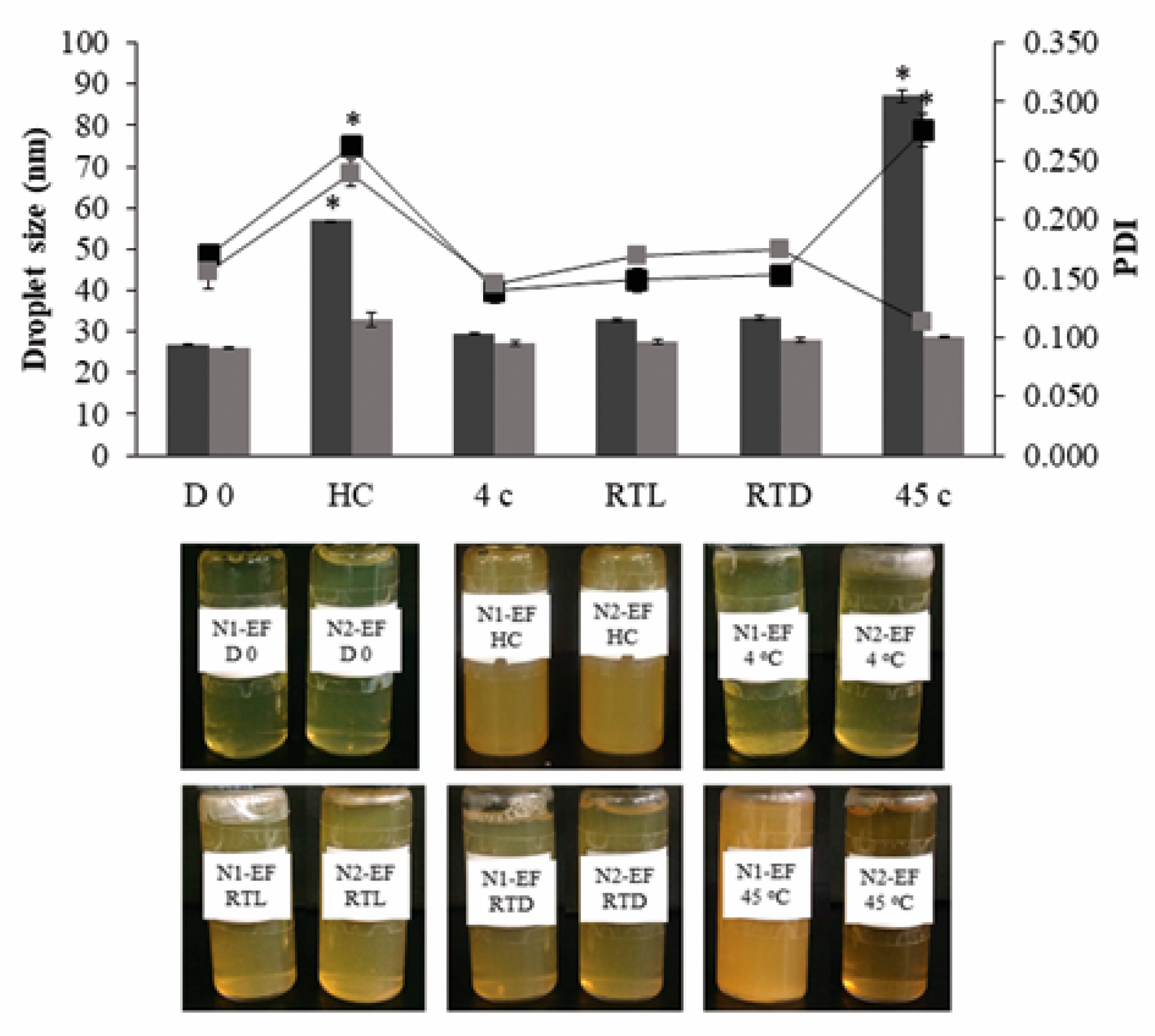
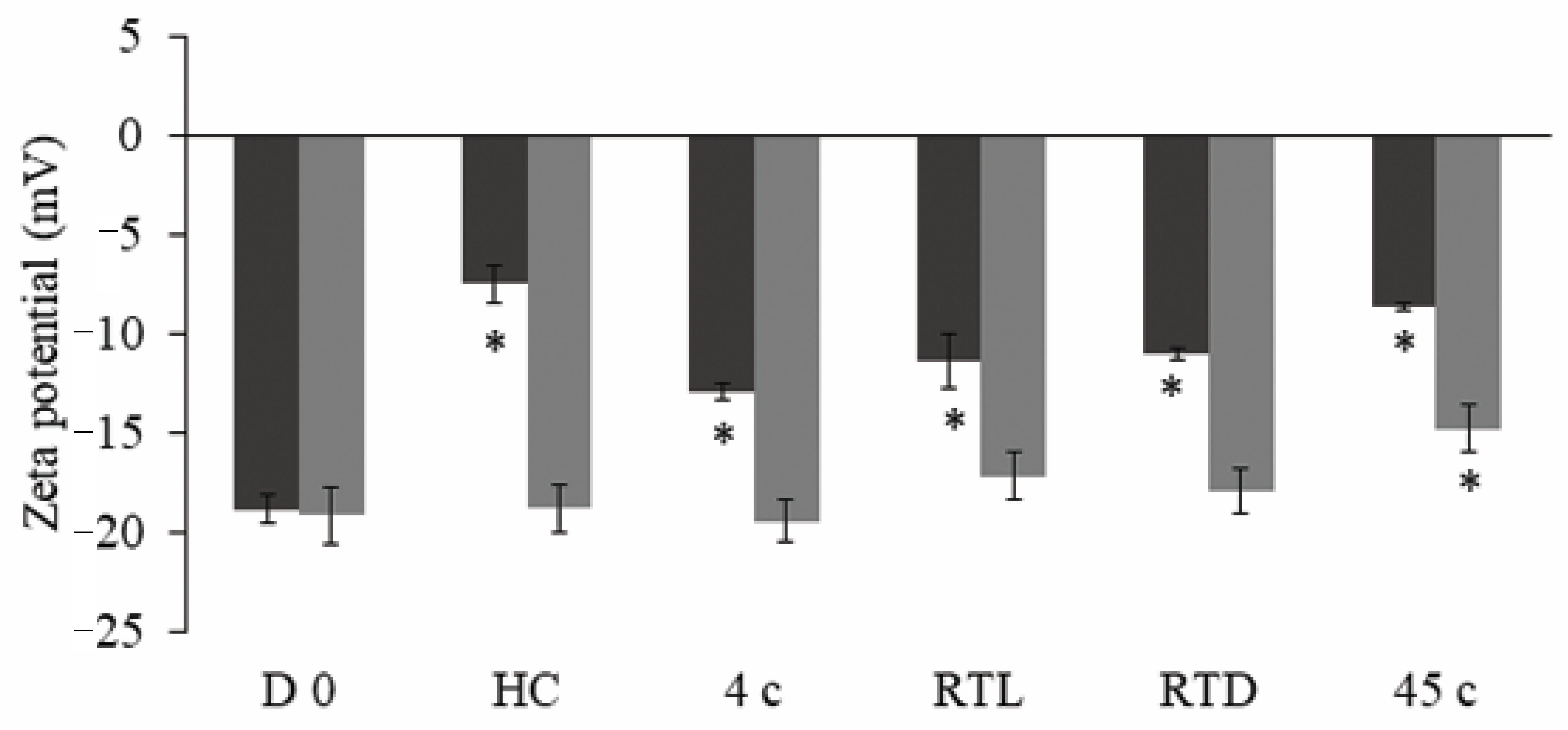
2.9.2. Physical Stability
2.9.3. Chemical Stability
2.9.4. Stability of Antibacterial Activity against P. acnes
2.10. Ex Vivo Skin Permeation Experiment
2.11. Statistical Analysis
3. Results
3.1. Optimization of Nanoemulsions and Data Analysis
3.1.1. Droplet Size
3.1.2. Polydispersity Index (PDI)
3.1.3. Zeta Potential
3.2. Optimization of Responses for Formulating Safflower Oil-Based Nanoemulsions
3.3. Formulation and Characterization of Nanoemulsions and EF-Loaded Nanoemulsions
3.4. Stability Study
3.5. Ex Vivo Skin Permeation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tan, J.K.L.; Bhate, K.A. Global perspective on the epidemiology of acne. Br. J. Dermatol. 2015, 172, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Dessionioti, C.; Katsambas, A.D. The role of Propionibacterium acnes in acne pathogenesis: Facts and controversies. Clin. Dermatol. 2010, 28, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Poomanee, W.; Chaiyana, W.; Mueller, M.; Viernstein, H.; Khunkitti, W.; Leelapornpisid, P. In-vitro investigation of anti-acne properties of Mangifera indica L. kernel extract and its mechanism of action against Propionibacterium acnes. Anaerobe 2018, 52, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Krautheim, A.; Gollinick, H.P.M. Acne: Topical treatment. Clin. Dermatol. 2004, 22, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Eady, E.A.; Gloor, M.; Leyden, J.J. Propionibacterium acnes resistance: A worldwide problem. Dermatology 2003, 206, 54–56. [Google Scholar] [CrossRef]
- Kosmadaki, M.; Katsambas, A. Topical treatment of acne. Clin. Dermatol. 2017, 35, 173–178. [Google Scholar] [CrossRef]
- Chomnawang, M.T.; Surassmo, S.; Nukoolkarn, V.S.; Gritsanapan, W. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J. Ethnopharmacol. 2005, 10, 330–333. [Google Scholar] [CrossRef]
- Tsai, T.H.; Tsai, T.H.; Wu, W.H.; Tseng, J.T.; Tsai, P. In vitro antimicrobial and anti-inflammatory effects of herbs against Propionibacterium acnes. Food Chem. 2010, 119, 964–968. [Google Scholar] [CrossRef]
- Zainol, S.; Basri, M.; Basri, H.B.; Shamsuddin, A.F.; Abdul-Gani, S.S.; Karjiban, R.A.; Abdul-Malek, E. Formulation optimization of a palm-based nanoemulsion system containing levodopa. Int. J. Mol. Sci. 2012, 13, 13049–13064. [Google Scholar] [CrossRef]
- Lovelyn, C.; Attama, A.A. Current state of nanoemulsions in drug delivery. J. Biomater. Nanobiotechnol. 2011, 2, 626–639. [Google Scholar] [CrossRef]
- Zorzi, G.K.; Caregnato, F.; Moreira, J.C.; Teixeira, H.F.; Carvalho, E.L. Antioxidant effect of nanoemulsions containing extract of achyrocline satureioides (Lam) D.C.-Asteraceae. AAPS PharmSciTech 2016, 17, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Choi, D. Current application of phytocompound-based nanocosmeticals for beauty and skin therapy. Int. J. Nanomed. 2016, 1, 1987–2007. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, P.; Andre, V.; Rieger, J.; Kuhnle, A. Nano-emulsion formulation by emulsion phase inversion. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251, 53–58. [Google Scholar] [CrossRef]
- Ostertag, F.; Weiss, J.; McClements, D.J. Low-energy formulation of edible nanoemulsions: Factors influencing droplet size produced by emulsion phase inversion. J. Colloid Interface Sci. 2012, 388, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yukuyama, M.N.; Ghisleni, D.D.M.; Pinto, T.J.A.; Bou-Chacra, N.A. Nanoemulsion: Process selection and application in cosmetics—Review. Int. J. Cosmet. Sci. 2016, 38, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Rizza, L.; Drechsler, M.; Bonina, F. Nanoemulsions as vehicles for topical administration of glycyrrhetic acid: Characterization and in vitro and in vivo evaluation. Drug Deliv. 2010, 17, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Chuesiang, P.; Siripatrawan, U.; Sanguandeekul, R.; McLandsborough, L.; McClement, D.J. Optimization of cinnamon oil nanoemulsions using phase inversion temperature method: Impact of oil phase composition and surfactant concentration. J. Colloid Interface Sci. 2018, 514, 208–216. [Google Scholar] [CrossRef]
- Woitiski, C.B.; Veiga, F.; Ribeiro, A.; Neufeld, R. Design of optimization of nanoparticles integrating biomaterials for orally dosed insulin. Eur. J. Pharm. Biopharm. 2009, 73, 25–33. [Google Scholar] [CrossRef]
- Masoumi, H.R.F.; Basri, M.; Samiun, W.S.; Izadiyan, Z.; Lim, C.J. Enhancement of encapsulation efficiency of nanoemulsion-containing aripiprazole for the treatment of schizophrenia using mixture experimental design. Int. J. Nanomed. 2015, 10, 6469–6476. [Google Scholar] [CrossRef]
- Khammuang, S.; Sarnthima, R. Antioxidant and antibacterial activities of selected varieties of Thai mango seed extract. Pak. J. Pharm. Sci. 2011, 24, 37–42. [Google Scholar]
- Jiamboonsri, P.; Pithayanukul, P.; Bavovada, R.; Chomnawang, M.T. The Inhibitory Potential of Thai mango seed kernel extract against Methicillin-resistant Staphylococcus aureus. Molecules 2011, 16, 6255–6270. [Google Scholar] [CrossRef] [PubMed]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; Anneo, A.D. Multifaceted health benefits of Mangifera indica L. (mango): The inestimable value of orchards recently planted in Sicilian rural areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Leanpolchareanchai, J.; Padois, K.; Falson, F.; Bavovada, R.; Pithayanukul, P. Microemulsion system for topical delivery of Thai mango seed kernel extract: Development, physicochemical characterization, and ex vivo skin permeation study. Molecules 2014, 19, 17107–17129. [Google Scholar] [CrossRef] [PubMed]
- Nakpanich, N.; Chaiyana, W.; Leelapornpisid, P. Antioxidant activities and stability of seed kernel extracts from mango (Mangifera indica Linn.) cultivated in Northern Thailand. Chiang Mai J. Sci. 2017, 44, 573–578. [Google Scholar]
- Jahurul, M.H.; Zaidul, I.S.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.L.; Norulaini, N.A.N.; Sahena, F.; Omar, A.K.M. Mango (Mangifera indica L.) by-products and their valuable components: A review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; Brito, E.S.D.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and quantitation of polyphenolic compounds in bark, kernel, leaves, and peel of mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Maisuthisakul, P.; Gordon, M.H. Characterization and storage stability of the extract of Thai mango (Mangifera indica Linn. Cultivar Chok-Anan) seed kernels. J. Food Sci. Technol. 2014, 51, 1453–1462. [Google Scholar] [CrossRef]
- Chaiyana, W.; Rades, T.; Okonogi, S. Characterization and in vitro permeation study of microemulsions and liquid crystalline systems containing the anticholinesterase alkaloidal extract from Tabernaemontana divaricate. Int. J. Pharm. 2013, 452, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Jahanzad, F.; Crombie, G.; Innes, R.; Sajjadi, S. Catastrophic phase inversion via formation of multiple emulsions; a prerequisite for formulation of fine emulsions. Chem. Eng. Sci. 2009, 87, 492–498. [Google Scholar]
- Kotta, S.; Khan, A.W.; Ansari, S.H.; Sharma, R.K.; Ali, J. Formulation of nanoemulsion: A comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015, 22, 455–466. [Google Scholar] [CrossRef]
- Ribeiro, R.C.A.; Barreto, S.M.A.; Ostrosky, E.A.; Rocha-Filho, P.A.; Verissimo, L.M.; Ferrari, M. Production and characterization of cosmetic nanoemulsions containing Opuntia ficus-indica (L.) mill extract as moisturizing agent. Molecule 2015, 20, 2492–2509. [Google Scholar] [CrossRef]
- Ibrahim, N.; Raman, I.A.; Yusop, M.R. Effects of functional group of non-ionic surfactants on the stability of emulsion. Malays. J. Anal. Sci. 2015, 19, 261–267. [Google Scholar]
- Nantarat, T.; Chansakaow, S.; Leelapornpisid, P. Optimization, characterization and stability of essential oils blend loaded nanoemulsions by PIC technique for anti-tyrosinase activity. Int. J. Pharm. Pharm. Sci. 2015, 7, 308–312. [Google Scholar]
- Sato, T.; Ruch, R. Stabilization of Colloidal Dispersion by Polymer Adsorption; Mercel Dekker Inc.: New York, NY, USA, 1980. [Google Scholar]
- Rao, J.; McClements, D.J. Stabilization of phase inversion temperature nanoemulsions by surfactant displacement. J. Agric. Food Chem. 2010, 58, 7059–7066. [Google Scholar] [CrossRef] [PubMed]
- Prow, T.W.; Grice, J.E.; Lin, L.L.; Faye, R.; Butler, M.; Becker, W.; Wurm, E.M.T.; Yoong, C.; Robertson, T.A.; Soyer, H.P.; et al. Nanoparticles and microparticles for skin drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 470–491. [Google Scholar] [CrossRef] [PubMed]
- Layden, J.J. New understandings of the pathogenesis of acne. J. Am. Acad. Dermatol. 1995, 32, S15–S25. [Google Scholar] [CrossRef]
- Graham, G.M.; Farrar, M.D.; Cruse-Sawyer, J.E.; Holland, K.T.; Ingham, E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br. J. Dermatol. 2004, 150, 421–428. [Google Scholar] [CrossRef]
- Farrar, M.D.; Ingram, E. Acne: Inflammation. Clin. Dermatol. 2004, 22, 380–384. [Google Scholar] [CrossRef]
- Sinha, P.; Srivastava, S.; Mishra, N.; Singh, D.K.; Luqman, S.; Chanda, D.; Yadav, N.P. Development, optimization, and characterization of a novel tea tree oil nanogel using response surface methodology. Drug Dev. Ind. Pharm. 2016, 42, 1434–1445. [Google Scholar] [CrossRef]

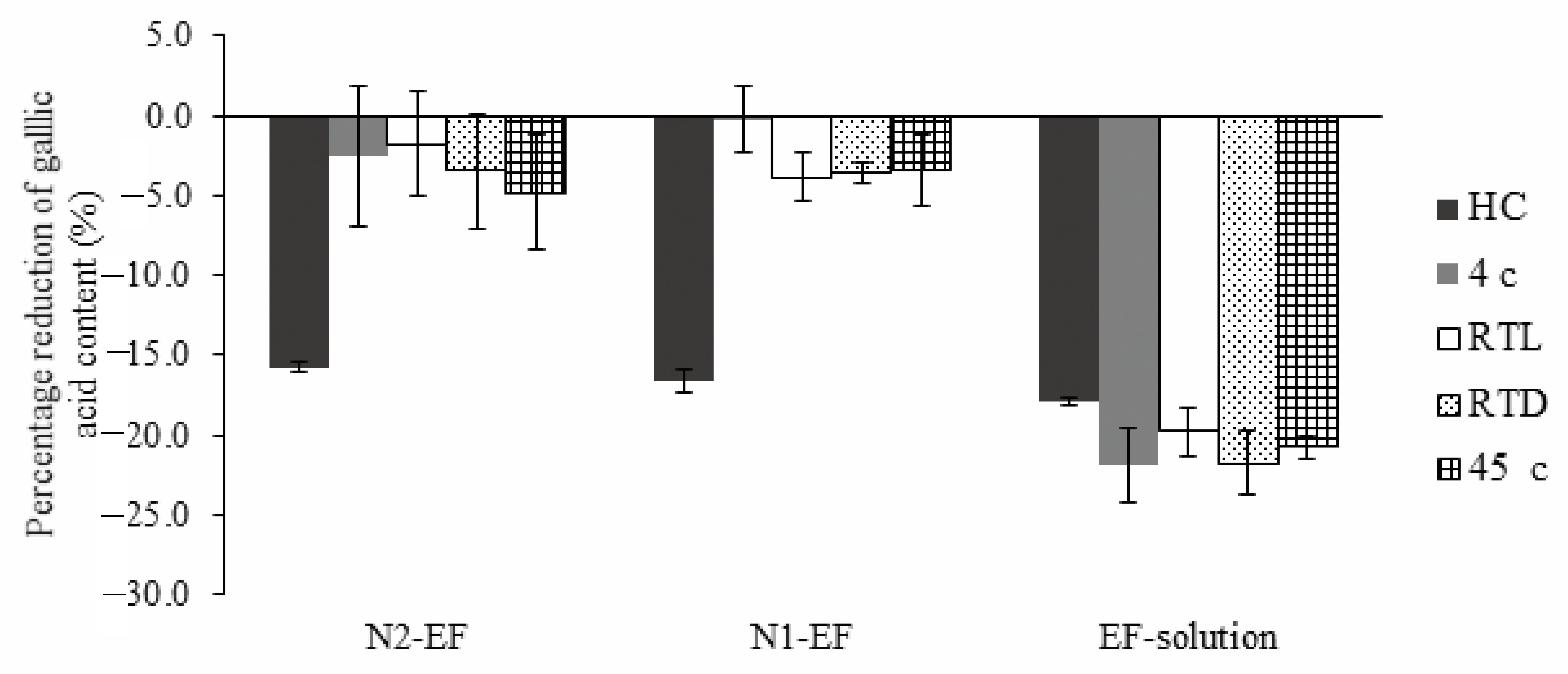
 ) N2-EF and (
) N2-EF and ( ) EF-solution along with cumulative amount of gallic acid through skin per unit surface area after 8 h (Q8) in different layers (b); RC—receiver compartment, SC—stratum corneum, and VED—viable epidermis dermis; (■) N2-EF and (■) EF-solution (mean ± SD; n = 3).
) EF-solution along with cumulative amount of gallic acid through skin per unit surface area after 8 h (Q8) in different layers (b); RC—receiver compartment, SC—stratum corneum, and VED—viable epidermis dermis; (■) N2-EF and (■) EF-solution (mean ± SD; n = 3).
 ) N2-EF and (
) N2-EF and ( ) EF-solution along with cumulative amount of gallic acid through skin per unit surface area after 8 h (Q8) in different layers (b); RC—receiver compartment, SC—stratum corneum, and VED—viable epidermis dermis; (■) N2-EF and (■) EF-solution (mean ± SD; n = 3).
) EF-solution along with cumulative amount of gallic acid through skin per unit surface area after 8 h (Q8) in different layers (b); RC—receiver compartment, SC—stratum corneum, and VED—viable epidermis dermis; (■) N2-EF and (■) EF-solution (mean ± SD; n = 3).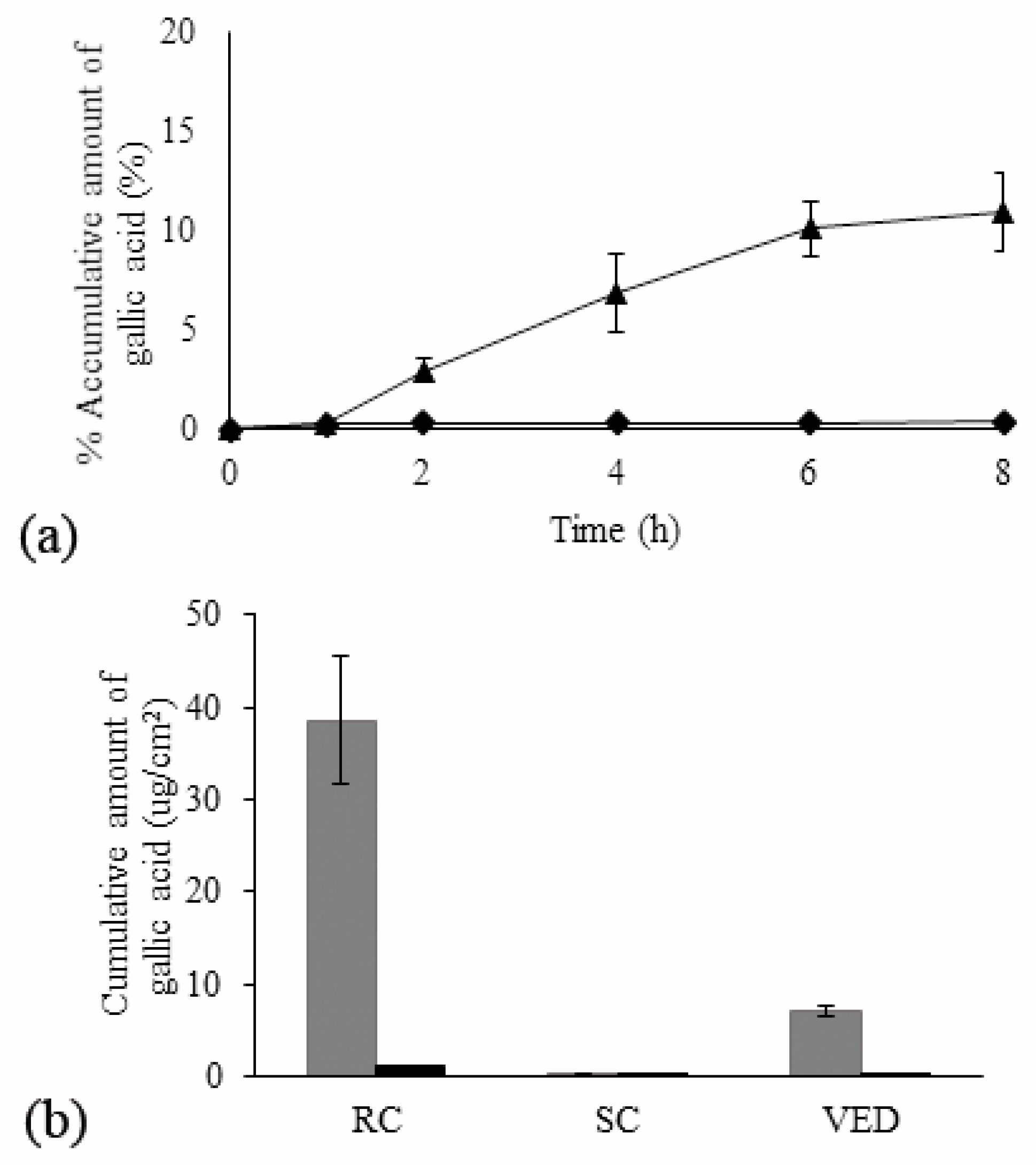
| Independent Variables | Code Level | ||||
|---|---|---|---|---|---|
| Axial (−α) | Low | Center | High | Axial (+α) | |
| HLB value (X1) | 8.40 | 8.8 | 9.4 | 10.0 | 10.4 |
| PEG-7 glyceryl cocoate (X2: %) | 0.66 | 1 | 1.5 | 2 | 2.34 |
| Surfactant-to-oil ratio: SOR (X3) | 1.33 | 1.5 | 1.75 | 2 | 2.17 |
| Run | Block | Independent Variables | Response Variables | ||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Droplet Size (Y1: nm) | Polydispersity Index (Y2) | Zeta Potential (Y3: mV) | ||
| 1 | 1 | 9.4 | 0.66 | 1.75 | 57.3 ± 0.4 | 0.49 ± 0.01 | −28.6 ± 0.8 |
| 2 | 1 | 10.0 | 2.0 | 2.00 | 17.8 ± 0.8 | 0.13 ± 0.01 | −26.4 ± 2.9 |
| 3 | 1 | 9.4 | 1.5 | 1.75 | 52.3 ± 0.6 | 0.33 ± 0.20 | −26.8 ± 0.5 |
| 4 | 1 | 9.4 | 1.5 | 2.17 | 69.2 ± 0.9 | 0.26 ± 0.04 | −24.2 ± 1.3 |
| 5 | 1 | 10.4 | 1.5 | 1.75 | 21.2 ± 1.0 | 0.14 ± 0.01 | −12.3 ± 0.4 |
| 6 | 1 | 10.0 | 2.0 | 1.50 | 22.9 ± 0.6 | 0.10 ± 0.02 | −13.1 ± 2.9 |
| 7 | 1 | 9.4 | 1.5 | 1.75 | 52.3 ± 0.6 | 0.33 ± 0.20 | −26.8 ± 0.5 |
| 8 | 1 | 10.0 | 1.0 | 2.00 | 94.8 ± 0.5 | 0.33 ± 0.16 | −24.1 ± 0.7 |
| 9 | 1 | 8.8 | 1.0 | 2.00 | 55.6 ± 0.7 | 0.39 ± 0.09 | −28.9 ± 0.1 |
| 10 | 1 | 9.4 | 1.5 | 1.75 | 52.3 ± 0.6 | 0.33 ± 0.20 | −26.8 ± 0.5 |
| 11 | 1 | 8.4 | 1.5 | 1.75 | 97.9 ± 0.4 | 0.50 ± 0.01 | −25.4 ± 0.3 |
| 12 | 1 | 8.8 | 2.0 | 2.00 | 86.2 ± 0.2 | 0.61 ± 0.01 | −33.3 ± 0.2 |
| 13 | 1 | 9.4 | 1.5 | 1.75 | 52.3 ± 0.6 | 0.33 ± 0.20 | −26.8 ± 0.5 |
| 14 | 1 | 9.4 | 1.5 | 1.75 | 52.3 ± 0.6 | 0.33 ± 0.20 | −26.8 ± 0.5 |
| 15 | 1 | 9.4 | 1.5 | 1.33 | 121.3 ± 1.1 | 0.79 ± 0.09 | −19.9 ± 0.5 |
| 16 | 1 | 9.4 | 1.5 | 1.75 | 52.3 ± 0.6 | 0.33 ± 0.20 | −26.8 ± 0.5 |
| 17 | 1 | 9.4 | 2.34 | 1.75 | 71.4 ± 0.5 | 0.90 ± 0.10 | −17.5 ± 0.7 |
| 18 | 1 | 8.8 | 2.0 | 1.50 | 167.3 ± 7.1 | 0.85 ± 0.27 | −30.7 ± 0.5 |
| 19 | 1 | 10.0 | 1.0 | 1.50 | 217.0 ± 12.1 | 1.00 ± 0.01 | −22.2 ± 0.5 |
| 20 | 1 | 8.8 | 1.0 | 1.50 | 137.6 ± 5.5 | 0.69 ± 0.04 | −35.1 ± 0.2 |
| Surfactant Systems | Ratio of Cetereth-20:PEG-40:Sorbitan Oleate | HLB Value |
|---|---|---|
| 1 | 1:2:4 | 8.4 |
| 2 | 1:3.75:5 | 8.8 |
| 3 | 1:4:4 | 9.4 |
| 4 | 1:2:2 | 10.0 |
| 5 | 7:5:4 | 10.4 |
| Regression Coefficient | Droplet Size (Y1, nm) | PDI (Y2) | Zeta Potential (Y3, mV) |
|---|---|---|---|
| 0 | 59.27 | 0.34 | −0.040 |
| X1 | −27.40 | −0.16 | −0.013 |
| X2 | −2.66 | 0.014 | - |
| X3 | −16.63 | −0.075 | −0.006 |
| X12 | - | - | −0.006 |
| X22 | - | 0.094 | - |
| X32 | 15.72 | - | - |
| X12 | −22.57 | −0.12 | - |
| X13 | 23.34 | - | 0.009 |
| X23 | - | - | - |
| R2 | 0.938 | 0.883 | 0.836 |
| Adjusted R2 | 0.907 | 0.839 | 0.789 |
| Regression (p-value) | <0.0001 a | <0.0001 a | <0.0001 a |
| Types of Effects | Variables | Droplet Size (Y1) | PDI (Y2) | Zeta Potential (Y3) | |||
|---|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | F-Value | p-Value | ||
| Main effects | X1 | 61.48 | <0.0001 | 45.46 | <0.0001 | 46.90 | <0.0001 |
| X2 | 0.58 | 0.462 b | 0.35 | 0.5656 b | - | - | |
| X3 | 22.64 | 0.0005 | 10.49 | 0.0065 | 10.60 | 0.0058 | |
| Quadratic effects | X12 | - | - | - | - | 13.55 | 0.0025 |
| X22 | - | - | 19.55 | 0.0007 | - | - | |
| X32 | 21.77 | 0.0007 | - | - | - | - | |
| Interaction effects | X12 | 22.33 | 0.0005 | 13.57 | 0.0028 | - | - |
| X13 | 23.89 | 0.0004 | - | - | 13.87 | 0.0023 | |
| X23 | - | - | - | - | - | - | |
| Response | Predicted Value | Actual Value | %Prediction Error |
|---|---|---|---|
| Y1: Droplet size (nm) | 20 | 20.78 | 3.75 |
| Y2: Polydispersity index (PDI) | 0.12 | 0.12 | 0 |
| Y3: Zeta potential (mV) | −20.47 | −21.14 | 3.17 |
| Storage Condition | N1-EF | N2-EF | EF-Solution | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| D 0 | 3.13 | 12.50 | 3.13 | 12.50 | 1.56 | 12.50 |
| HC | 3.13 | 12.50 | 3.13 | 12.50 | 3.13 | 12.50 |
| 4 °C | 3.13 | 12.50 | 3.13 | 12.50 | 3.13 | 12.50 |
| RTL | 3.13 | 12.50 | 3.13 | 12.50 | 3.13 | 12.50 |
| RTD | 3.13 | 12.50 | 3.13 | 12.50 | 3.13 | 12.50 |
| 45 °C | 3.13 | 12.50 | 3.13 | 12.50 | 3.13 | 12.50 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poomanee, W.; Khunkitti, W.; Chaiyana, W.; Leelapornpisid, P. Optimization of Mangifera indica L. Kernel Extract-Loaded Nanoemulsions via Response Surface Methodology, Characterization, Stability, and Skin Permeation for Anti-Acne Cosmeceutical Application. Pharmaceutics 2020, 12, 454. https://doi.org/10.3390/pharmaceutics12050454
Poomanee W, Khunkitti W, Chaiyana W, Leelapornpisid P. Optimization of Mangifera indica L. Kernel Extract-Loaded Nanoemulsions via Response Surface Methodology, Characterization, Stability, and Skin Permeation for Anti-Acne Cosmeceutical Application. Pharmaceutics. 2020; 12(5):454. https://doi.org/10.3390/pharmaceutics12050454
Chicago/Turabian StylePoomanee, Worrapan, Watcharee Khunkitti, Wantida Chaiyana, and Pimporn Leelapornpisid. 2020. "Optimization of Mangifera indica L. Kernel Extract-Loaded Nanoemulsions via Response Surface Methodology, Characterization, Stability, and Skin Permeation for Anti-Acne Cosmeceutical Application" Pharmaceutics 12, no. 5: 454. https://doi.org/10.3390/pharmaceutics12050454
APA StylePoomanee, W., Khunkitti, W., Chaiyana, W., & Leelapornpisid, P. (2020). Optimization of Mangifera indica L. Kernel Extract-Loaded Nanoemulsions via Response Surface Methodology, Characterization, Stability, and Skin Permeation for Anti-Acne Cosmeceutical Application. Pharmaceutics, 12(5), 454. https://doi.org/10.3390/pharmaceutics12050454