Adipocyte-Based Cell Therapy in Oncology: The Role of Cancer-Associated Adipocytes and Their Reinterpretation as Delivery Platforms
Abstract
1. Introduction
2. Current Cell-Based Approaches in Drug Delivery
Cell Types Used as Drug Delivery Vehicles
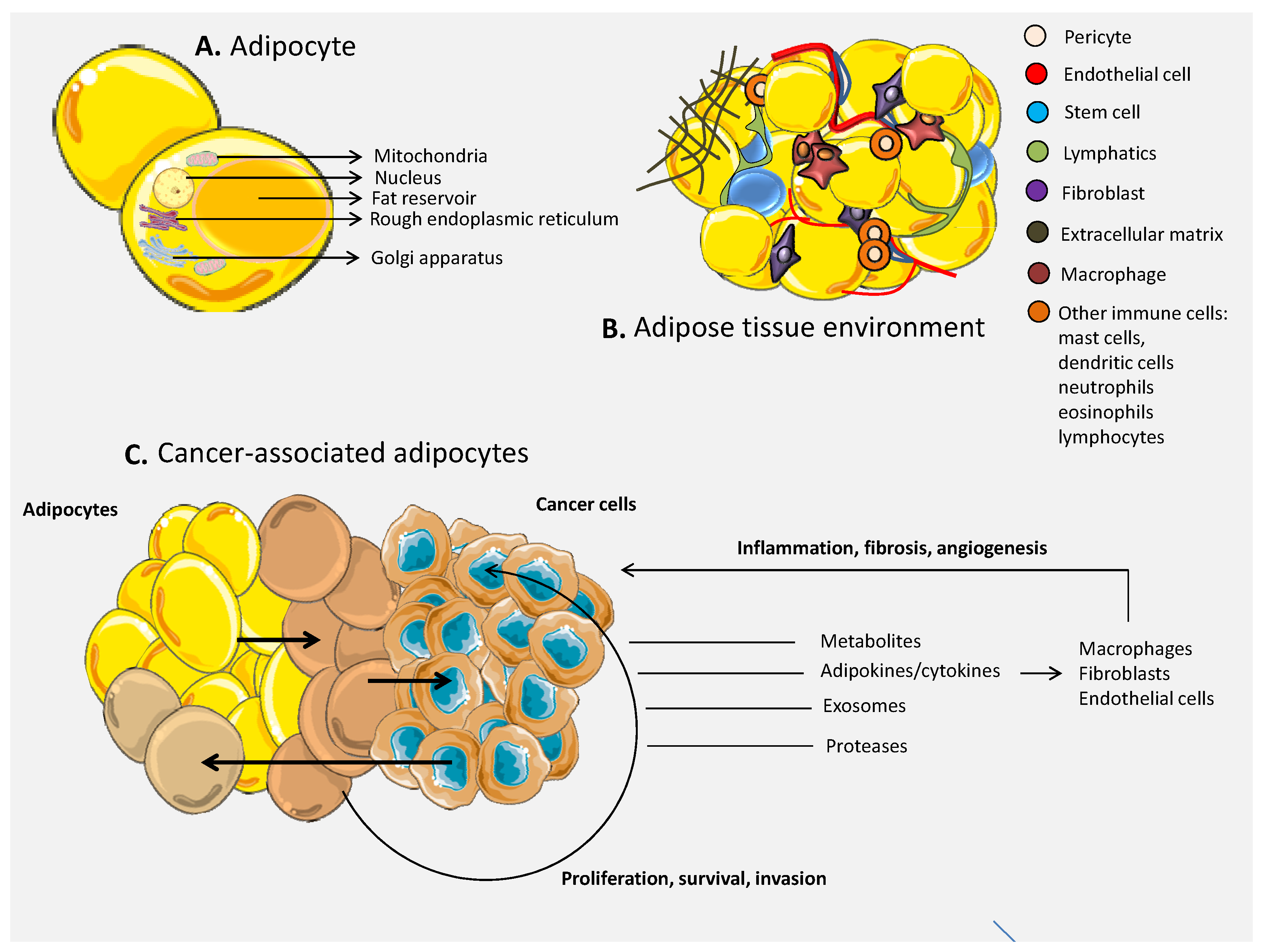
3. The Role of Adipocytes in Cancer
3.1. The Environment of the Adipose Tissue
3.2. The Link between Adipose Tissue and Cancer
3.3. Cells of the Immune System within the Adipose Tissue in Cancer
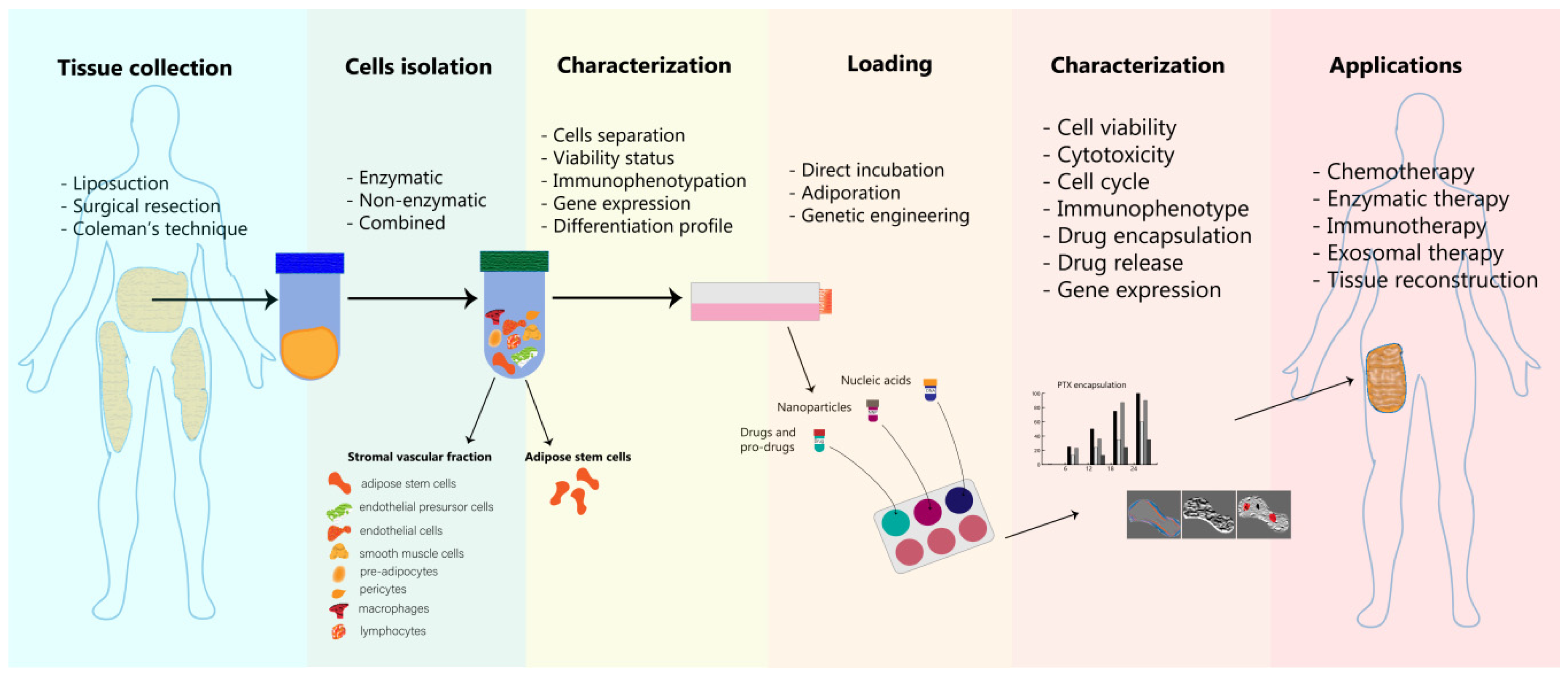
4. Adipose Cells as Delivery Platforms—Isolation, Loading and Characterization
4.1. Adipocytes Isolation and Processing
4.2. Adipose Cells’ Loading Strategies
4.3. Characterization of Engineered Adipose Tissue-Derived Cells
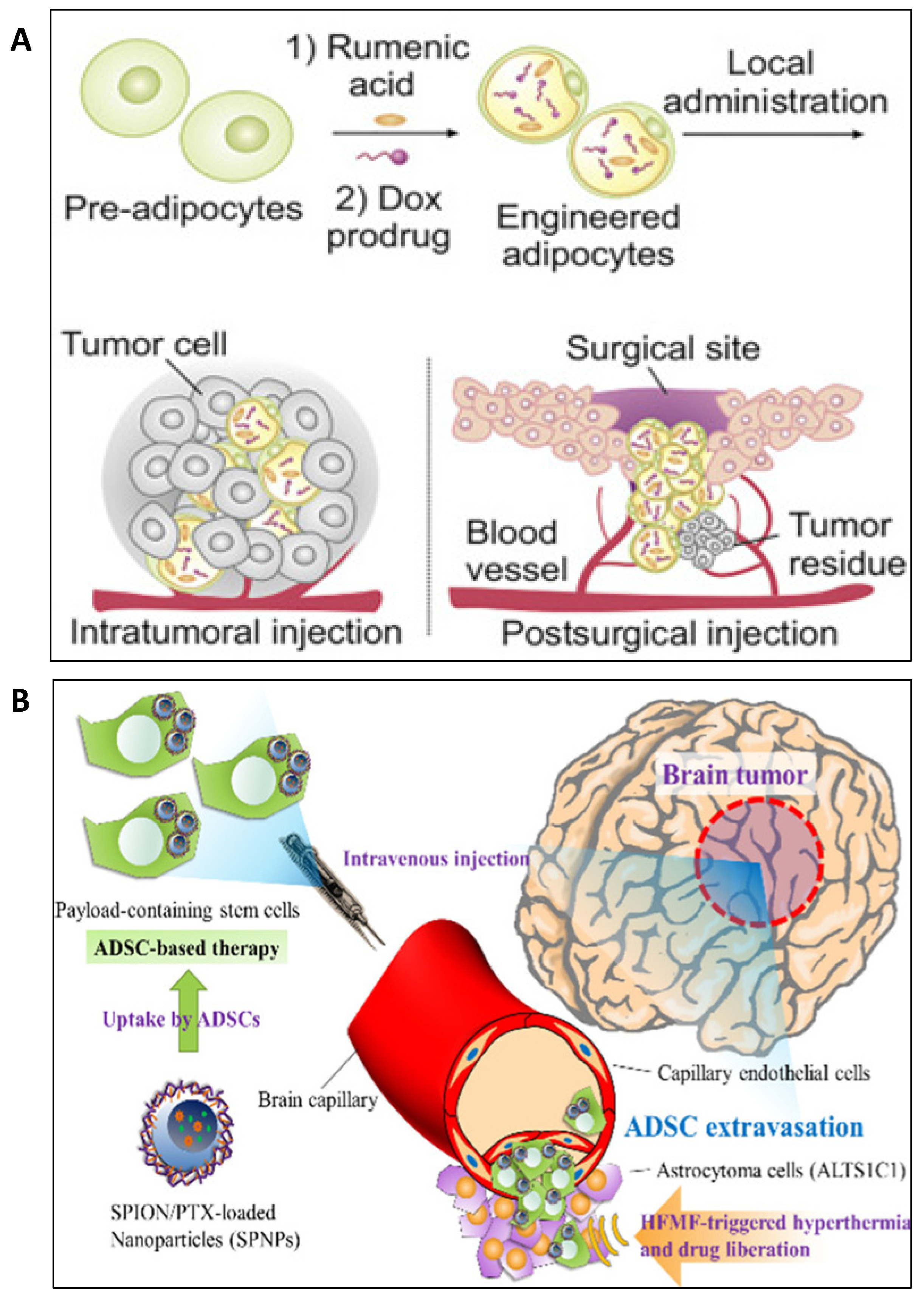
5. Applications of Engineered Adipose Tissue-Derived Cells in Oncology
6. Adipocyte-Based Drug Delivery in Oncology
7. Adipocyte Conjugated Nanoformulations for Drug Delivery in Oncology
8. Adipocytes-Derived Exosomes
9. Future Perspectives
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Boca, S.; Gulei, D.; Zimta, A.-A.; Onaciu, A.; Magdo, L.; Tigu, A.B.; Ionescu, C.; Irimie, A.; Buiga, R.P.; Berindan-Neagoe, I. Nanoscale delivery systems for microRNAs in cancer therapy. Cell. Mol. Life Sci. 2019, 77, 1059–1086. [Google Scholar] [CrossRef] [PubMed]
- Jurj, A.; Braicu, C.; Pop, L.-A.; Tomuleasa, C.; Gherman, C.D.; Berindan-Neagoe, I. The new era of nanotechnology, an alternative to change cancer treatment. Drug Des. Dev. Ther. 2017, 11, 2871–2890. [Google Scholar] [CrossRef] [PubMed]
- Irimie, A.I.; Sonea, L.; Jurj, A.; Mehterov, N.; Berindan-Neagoe, I.; Budisan, L.; Braicu, C.; Berindan-Neagoe, I. Future trends and emerging issues for nanodelivery systems in oral and oropharyngeal cancer. Int. J. Nanomed. 2017, 12, 4593–4606. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Gu, Z. Bioinspired and Biomimetic Nanomedicines. Accounts Chem. Res. 2019, 52, 1255–1264. [Google Scholar] [CrossRef]
- Wen, D.; Wang, J.; Driessche, G.V.D.; Chen, Q.; Zhang, Y.; Chen, G.; Li, H.; Soto, J.; Liu, M.; Ohashi, M.; et al. Adipocytes as Anticancer Drug Delivery Depot. Phys. B Condens. Matter 2019, 1, 1203–1214. [Google Scholar] [CrossRef]
- Fregni, G.; Quinodoz, M.; Möller, E.; Vuille, J.; Galland, S.; Fusco, C.; Martin, P.; Letovanec, I.; Provero, P.; Rivolta, C.; et al. Reciprocal modulation of mesenchymal stem cells and tumor cells promotes lung cancer metastasis. EBioMedicine 2018, 29, 128–145. [Google Scholar] [CrossRef]
- Muehlberg, F.; Song, Y.-H.; Krohn, A.; Pinilla, S.P.; Droll, L.H.; Leng, X.; Seidensticker, M.; Ricke, J.; Altman, A.M.; Devarajan, E.; et al. Tissue-resident stem cells promote breast cancer growth and metastasis. Carcinogenesis 2009, 30, 589–597. [Google Scholar] [CrossRef]
- Kandil, E.; Hauch, A.; Friedlander, P.; Sheng, M.; Tsumagari, K.; Saeed, A.; Gimble, J.M.; Rowan, B.G. A novel mouse model of metastatic thyroid carcinoma using human adipose tissue-derived stromal/stem cells. Anticancer. Res. 2013, 33. [Google Scholar]
- Kucerova, L.; Altanerova, V.; Matuskova, M.; Tyciakova, S.; Altaner, C. Adipose Tissue-Derived Human Mesenchymal Stem Cells Mediated Prodrug Cancer Gene Therapy. Cancer Res. 2007, 67, 6304–6313. [Google Scholar] [CrossRef]
- Takahara, K.; Ii, M.; Inamoto, T.; Komura, K.; Ibuki, N.; Minami, K.; Uehara, H.; Hirano, H.; Nomi, H.; Kiyama, S.; et al. Adipose-derived stromal cells inhibit prostate cancer cell proliferation inducing apoptosis. Biochem. Biophys. Res. Commun. 2014, 446, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Lu, I.-L.; Chiang, W.-H.; Lin, Y.-W.; Tsai, Y.-C.; Chen, H.-H.; Chang, C.-W.; Chiang, C.-S.; Chiu, H.-C. Tumortropic adipose-derived stem cells carrying smart nanotherapeutics for targeted delivery and dual-modality therapy of orthotopic glioblastoma. J. Control. Release 2017, 254, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Waters, R.; Alam, P.; Pacelli, S.; Chakravarti, A.R.; Ahmed, R.P.; Paul, A. Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater. 2017, 69, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Berindan-Neagoe, I.; Braicu, C.; Craciun, L.; Irimie, A.; Takahashi, Y.; Tomuleasa, C. Nanopharmacology in translational hematology and oncology. Int. J. Nanomed. 2014, 9, 3465–3479. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Onaciu, A.; Braicu, C.; Berindan-Neagoe, I.; Moldovan, A.; Știufiuc, R.I.; Buse, M.; Ciocan, C.; Buduru, S.; Berindan-Neagoe, I. Gold nanorods: From anisotropy to opportunity. An evolution update. Nanomedicine 2019, 14, 1203–1226. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Dong, H.; Zhang, C.; Mo, R. Cell-based drug delivery systems for biomedical applications. Nano Res. 2018, 11, 5240–5257. [Google Scholar] [CrossRef]
- Polyak, B.; Friedman, G. Magnetic targeting for site-specific drug delivery: Applications and clinical potential. Expert Opin. Drug Deliv. 2009, 6, 53–70. [Google Scholar] [CrossRef]
- Karimi, M.; Eslami, M.; Zangabad, P.S.; Mirab, F.; Farajisafiloo, N.; Shafaei, Z.; Ghosh, D.; Bozorgomid, M.; Dashkhaneh, F.; Hamblin, M.R. pH-Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 696–716. [Google Scholar] [CrossRef]
- Lutz, H.; Hu, S.; Dinh, P.-U.; Cheng, K. Cells and cell derivatives as drug carriers for targeted delivery. Med. Drug Discov. 2019, 3, 100014. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V.; Mitra, A.K. Next generation drug delivery: Circulatory cells-mediated nanotherapeutic approaches. Expert Opin. Drug Deliv. 2016, 14, 1–5. [Google Scholar] [CrossRef]
- Su, Y.; Xie, Z.; Kim, G.B.; Dong, C.; Yang, J. Design Strategies and Applications of Circulating Cell-Mediated Drug Delivery Systems. ACS Biomater. Sci. Eng. 2015, 1, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Chen, B. Combination of drugs and carriers in drug delivery technology and its development. Drug Des. Dev. Ther. 2019, 13, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Chessa, L.; Leuzzi, V.; Plebani, A.; Soresina, A.; Micheli, R.; D’Agnano, D.; Venturi, T.; Molinaro, A.; Fazzi, E.; Marini, M.; et al. Intra-Erythrocyte Infusion of Dexamethasone Reduces Neurological Symptoms in Ataxia Teleangiectasia Patients: Results of a Phase 2 Trial. Orphanet J. Rare Dis. 2014, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Hunault-Berger, M.; Leguay, T.; Huguet, F.; Lepretre, S.; Deconinck, E.; Ojeda-Uribe, M.; Bonmati, C.; Escoffre-Barbe, M.; Bories, P.; Himberlin, C.; et al. A Phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: The GRASPALL/GRAALL-SA2-2008 study. Am. J. Hematol. 2015, 90, 811–818. [Google Scholar] [CrossRef]
- Dale, G.L.; Kuhl, W.; Beutler, E. Incorporation of glucocerebrosidase into Gaucher’s disease monocytes in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 473–475. [Google Scholar] [CrossRef]
- Moran, N.F.; Bain, M.D.; Muqit, M.M.; Bax, B. Carrier Erythrocyte Entrapped Thymidine Phosphorylase Therapy For Mngie. Neurology 2008, 71, 686–688. [Google Scholar] [CrossRef]
- Skorokhod, O.A.; Garmaeva, T.T.; Vitvitsky, V.; Isaev, V.G.; Пapoвичникoвa, E.H.; Savchenko, V.G.; Ataullakhanov, F.I. Pharmacokinetics of erythrocyte-bound daunorubicin in patients with acute leukemia. Med. Sci. Monit. 2004, 10. [Google Scholar]
- Skorokhod, O.; Kulikova, E.V.; Galkina, N.M.; Medvedev, P.V.; Zybunova, E.E.; Vitvitsky, V.M.; Pivnik, A.V.; Ataullakhanov, F.I. Doxorubicin pharmacokinetics in lymphoma patients treated with doxorubicin-loaded eythrocytes. Haematol. 2007, 92, 570–571. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, Q.; Jiang, C.; Gu, Z. Platelet for drug delivery. Curr. Opin. Biotechnol. 2019, 58, 81–91. [Google Scholar] [CrossRef]
- Dai, L.; Gu, N.; Chen, B.-A.; Marriott, G. Human platelets repurposed as vehicles for in vivo imaging of myeloma xenotransplants. Oncotarget 2016, 7, 21076–21090. [Google Scholar] [CrossRef]
- Xu, P.; Zuo, H.; Zhou, R.; Wang, F.; Liu, X.; Ouyang, J.; Chen, B. Doxorubicin-loaded platelets conjugated with anti-CD22 mAbs: A novel targeted delivery system for lymphoma treatment with cardiopulmonary avoidance. Oncotarget 2017, 8, 58322–58337. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zuo, H.; Chen, B.; Wang, R.; Ahmed, A.; Hu, Y.; Ouyang, J. Doxorubicin-loaded platelets as a smart drug delivery system: An improved therapy for lymphoma. Sci. Rep. 2017, 7, 42632. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.K.; Mendizabal, A.; Parikh, S.H.; Szabolcs, P.; Driscoll, T.A.; Page, K.; Lakshminarayanan, S.; Allison, J.; Wood, S.; Semmel, D.; et al. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: Influence of cellular composition of the graft on transplantation outcomes. Blood 2008, 112, 2979–2989. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Montgomery, R.R. Platelets as delivery systems for disease treatments. Adv. Drug Deliv. Rev. 2010, 62, 1196–1203. [Google Scholar] [CrossRef]
- Abdelgawwad, M.S.; Cao, W.; Zheng, L.; Kocher, N.K.; Williams, L.A.; Zheng, X.L. Transfusion of Platelets Loaded With Recombinant ADAMTS13 (A Disintegrin and Metalloprotease With Thrombospondin Type 1 Repeats-13) Is Efficacious for Inhibiting Arterial Thrombosis Associated With Thrombotic Thrombocytopenic Purpura. Arter. Thromb. Vasc. Boil. 2018, 38, 2731–2743. [Google Scholar] [CrossRef]
- Shvidel, L.; Sigler, E.; Shtalrid, M.; Berrebi, A. Vincristine-loaded platelet infusion for treatment of refractory autoimmune hemolytic anemia and chronic immune thrombocytopenia: Rethinking old cures. Am. J. Hematol. 2006, 81, 423–425. [Google Scholar] [CrossRef]
- Xu, P.; Jiang, Y.; Zuo, H.; Liu, X.; Xia, T.; Zhou, R.; Chen, B.; Ouyang, J. Vincristine-loaded platelets coated with anti-CD41 mAbs: A new macrophage targeting proposal for the treatment of immune thrombocytopenia. Biomater. Sci. 2019, 7, 4568–4577. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Gilbert, J.B.; Kumar, S.; Gupta, V.; Cohen, R.E.; Rubner, M.F.; Mitragotri, S. Monocyte-mediated delivery of polymeric backpacks to inflamed tissues: A generalized strategy to deliver drugs to treat inflammation. J. Control. Release 2015, 199, 29–36. [Google Scholar] [CrossRef]
- Andón, F.T.; Digifico, E.; Maeda, A.; Erreni, M.; Mantovani, A.; Alonso, M.J.; Allavena, P. Targeting tumor associated macrophages: The new challenge for nanomedicine. Semin. Immunol. 2017, 34, 103–113. [Google Scholar] [CrossRef]
- Huang, M.-N.; Nicholson, L.T.; Batich, K.A.; Swartz, A.M.; Kopin, D.; Wellford, S.; Prabhakar, V.K.; Woroniecka, K.; Nair, S.K.; Fecci, P.E.; et al. Antigen-loaded monocyte administration induces potent therapeutic antitumor T cell responses. J. Clin. Investig. 2020, 130, 774–788. [Google Scholar] [CrossRef]
- Christensen, J.M.; Brat, G.A.; Johnson, K.E.; Chen, Y.; Buretta, K.J.; Cooney, D.S.; Brandacher, G.; Lee, W.P.A.; Li, X.; Sacks, J.M. Monocytes Loaded with Indocyanine Green as Active Homing Contrast Agents Permit Optical Differentiation of Infectious and Non-Infectious Inflammation. PLoS ONE 2013, 8, e81430. [Google Scholar] [CrossRef] [PubMed]
- Bressani, R.F.; Nowacek, A.S.; Singh, S.; Balkundi, S.; Rabinow, B.; McMillan, J.; Gendelman, H.E.; Kanmogne, G.D. Pharmacotoxicology of monocyte-macrophage nanoformulated antiretroviral drug uptake and carriage. Nanotoxicology 2010, 5, 592–605. [Google Scholar] [CrossRef]
- Brynskikh, A.M.; Zhao, Y.; Mosley, R.L.; Li, S.; Boska, M.D.; Klyachko, N.L.; Kabanov, A.V.; Gendelman, H.E.; Batrakova, E.V. Macrophage delivery of therapeutic nanozymes in a murine model of Parkinson’s disease. Nanomedicine 2010, 5, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.K.A.; Kolosnjaj-Tabi, J.; Bonneau, S.; Marangon, I.; Boggetto, N.; Aubertin, K.; Clement, O.; Bureau, M.F.; Luciani, N.; Gazeau, F.; et al. Magnetic and Photoresponsive Theranosomes: Translating Cell-Released Vesicles into Smart Nanovectors for Cancer Therapy. ACS Nano 2013, 7, 4954–4966. [Google Scholar] [CrossRef] [PubMed]
- Stephan, M.T.; Stephan, S.B.; Bak, P.; Chen, J.; Irvine, D.J. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials 2012, 33, 5776–5787. [Google Scholar] [CrossRef]
- Stephan, M.T.; Moon, J.J.; Um, S.H.; Bershteyn, A.; Irvine, D.J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat. Med. 2010, 16, 1035–1041. [Google Scholar] [CrossRef]
- Huber, A.; Dammeijer, F.; Aerts, J.G.J.V.; Vroman, H. Current State of Dendritic Cell-Based Immunotherapy: Opportunities for in vitro Antigen Loading of Different DC Subsets? Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; El-Zamarany, E.A.; Khedr, E.G.; El-Bahrawy, H.A.; El-Feky, O. Antigen-loaded dendritic cells triggers a specific cytotoxic T lymphocytes immune response against hepatocellular carcinoma: In vitro study. Clin. Transl. Oncol. 2018, 21, 636–645. [Google Scholar] [CrossRef]
- Gholamin, M.; Moaven, O.; Farshchian, M.; Mahmoudi, M.; Sankian, M.; Memar, B.; Forghani, M.N.; Malekzadeh, R.; Rajabi-Mashhadi, M.T.; Abbaszadegan, M.R. Induction of cytotoxic T lymphocytes primed with Tumor RNA-loaded Dendritic Cells in esophageal squamous cell carcinoma: Preliminary step for DC vaccine design. BMC Cancer 2010, 10, 261. [Google Scholar] [CrossRef]
- Wu, H.-H.; Zhou, Y.; Tabata, Y.; Gao, J.-Q. Mesenchymal stem cell-based drug delivery strategy: From cells to biomimetic. J. Control. Release 2019, 294, 102–113. [Google Scholar] [CrossRef]
- Huang, B.; Jiang, X.-C.; Zhang, T.-Y.; Hu, Y.-L.; Tabata, Y.; Chen, Z.; Pluchino, S.; Gao, J.-Q. Peptide modified mesenchymal stem cells as targeting delivery system transfected with miR-133b for the treatment of cerebral ischemia. Int. J. Pharm. 2017, 531, 90–100. [Google Scholar] [CrossRef]
- Zhang, T.-Y.; Huang, B.; Wu, H.-B.; Wu, J.-H.; Li, L.-M.; Li, Y.-X.; Hu, Y.-L.; Han, M.; Shen, Y.; Tabata, Y.; et al. Synergistic effects of co-administration of suicide gene expressing mesenchymal stem cells and prodrug-encapsulated liposome on aggressive lung melanoma metastases in mice. J. Control. Release 2015, 209, 260–271. [Google Scholar] [CrossRef]
- Pessina, A.; Coccè, V.; Pascucci, L.; Bonomi, A.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Ciusani, E.; Crovace, A.; Falchetti, M.L.; et al. Mesenchymal stromal cells primed with Paclitaxel attract and kill leukaemia cells, inhibit angiogenesis and improve survival of leukaemia-bearing mice. Br. J. Haematol. 2013, 160, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Morgante, L.; De Pascalis, I.; Coccè, V.; Bonomi, A.; Pascucci, L.; Alessandri, G.; Pessina, A.; et al. Mesenchymal stromal cells loaded with paclitaxel induce cytotoxic damage in glioblastoma brain xenografts. Stem Cell Res. Ther. 2015, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.R.; Saboeiro, A.P. Fat Grafting to the Breast Revisited: Safety and Efficacy. Plast. Reconstr. Surg. 2007, 119, 775–785. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-Assisted Lipotransfer for Cosmetic Breast Augmentation: Supportive Use of Adipose-Derived Stem/Stromal Cells. Aesthetic Plast. Surg. 2007, 32, 48–55. [Google Scholar] [CrossRef]
- Yoshimura, K.; Asano, Y.; Aoi, N.; Kurita, M.; Oshima, Y.; Sato, K.; Inoue, K.; Suga, H.; Eto, H.; Kato, H.; et al. Progenitor-Enriched Adipose Tissue Transplantation as Rescue for Breast Implant Complications. Breast J. 2010, 16, 169–175. [Google Scholar] [CrossRef]
- Spear, S.L.; Wilson, H.B.; Lockwood, M.D. Fat Injection to Correct Contour Deformities in the Reconstructed Breast. Plast. Reconstr. Surg. 2005, 116, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Furue, M.; Matsuda, T. Novel Strategy for Soft Tissue Augmentation Based on Transplantation of Fragmented Omentum and Preadipocytes. Tissue Eng. 2004, 10, 1672–1683. [Google Scholar] [CrossRef]
- Matsumoto, D.; Sato, K.; Gonda, K.; Takaki, Y.; Shigeura, T.; Sato, T.; Aiba-Kojima, E.; Iizuka, F.; Inoue, K.; Suga, H.; et al. Cell-assisted lipotransfer: Supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng. 2006, 12, 3375–3382. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Li, J.; Gao, J.; Ogawa, R.; Ou, C.; Yang, B.; Fu, B. Improvement of the Survival of Human Autologous Fat Transplantation by Using VEGF-Transfected Adipose-Derived Stem Cells. Plast. Reconstr. Surg. 2009, 124, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, H.E.; Block, J.E. Facial fat grafting with a prototype injection control device. Clin. Cosmet. Investig. Dermatol. 2013, 6, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Abbo, O.; Taurand, M.; Monsarrat, P.; Raymond, I.; Arnaud, E.; De Barros, S.; Auriol, F.; Galinier, P.; Casteilla, L.; Planat-Benard, V. Comparison between pediatric and adult adipose mesenchymal stromal cells. Cytotherapy 2017, 19, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Dige, A.; Hougaard, H.T.; Agnholt, J.; Pedersen, B.G.; Tencerova, M.; Kassem, M.; Krogh, K.; Lundby, L. Efficacy of Injection of Freshly Collected Autologous Adipose Tissue Into Perianal Fistulas in Patients With Crohn’s Disease. Gastroenterology 2019, 156, 2208–2216.e1. [Google Scholar] [CrossRef] [PubMed]
- Comella, K.; Silbert, R.; Parlo, M.; Comella, K.; Silbert, R.; Parlo, M. Effects of the intradiscal implantation of stromal vascular fraction plus platelet rich plasma in patients with degenerative disc disease. J. Transl. Med. 2017, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Planat-Benard, V.; Silvestre, J.-S.; Cousin, B.; André, M.; Nibbelink, M.; Tamarat, R.; Clergue, M.; Manneville, C.; Saillan-Barreau, C.; Duriez, M.; et al. Plasticity of Human Adipose Lineage Cells Toward Endothelial Cells. Circulation 2004, 109, 656–663. [Google Scholar] [CrossRef]
- Mattei, A.; Bertrand, B.; Jouve, E.; Blaise, T.; Philandrianos, C.; Grimaud, F.; Giraudo, L.; Aboudou, H.; Dumoulin, C.; Arnaud, L.; et al. Feasibility of First Injection of Autologous Adipose Tissue-Derived Stromal Vascular Fraction in Human Scarred Vocal Folds: A Nonrandomized Controlled Trial. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 355–363. [Google Scholar] [CrossRef]
- Shimizu, S.; Yamamoto, T.; Nakayama, S.; Hirakawa, A.; Kuwatsuka, Y.; Funahashi, Y.; Matsukawa, Y.; Takanari, K.; Toriyama, K.; Kamei, Y.; et al. Design of a single-arm clinical trial of regenerative therapy by periurethral injection of adipose-derived regenerative cells for male stress urinary incontinence in Japan: The ADRESU study protocol. BMC Urol. 2017, 17, 89. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Scherer, P.E. Adipose Tissue-Derived Factors: Impact on Health and Disease. Endocr. Rev. 2006, 27, 762–778. [Google Scholar] [CrossRef]
- Lee, M.-J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 2012, 34, 1–11. [Google Scholar] [CrossRef]
- Giralt, M.; Villarroya, F. White, Brown, Beige/Brite: Different Adipose Cells for Different Functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Cozzo, A.; Fuller, A.M.; Makowski, L. Contribution of Adipose Tissue to Development of Cancer. Compr. Physiol. 2017, 8, 237–282. [Google Scholar] [CrossRef]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef]
- Frontini, A.; Giordano, A.; Cinti, S. Endothelial cells of adipose tissues. Cell Cycle 2012, 11, 2765–2766. [Google Scholar] [CrossRef] [PubMed]
- Gogg, S.; Nerstedt, A.; Boren, J.; Smith, U. Human adipose tissue microvascular endothelial cells secrete PPARgamma ligands and regulate adipose tissue lipid uptake. JCI Insight. 2019, 4. [Google Scholar] [CrossRef]
- Chu, D.-T.; Phuong, T.N.T.; Tien, N.L.B.; Tran, K.; Bui, L.M.; Van Thanh, V.; Anh, P.G.; Pham, V.-H.; Nga, V.T. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J. Clin. Med. 2019, 8, 917. [Google Scholar] [CrossRef]
- Wang, S.; Su, X.; Xu, M.; Xiao, X.; Li, X.; Li, H.; Keating, A.; Zhao, R.C. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res. Ther. 2019, 10, 117. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. International Agency for Research on Cancer Handbook Working Group Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Yang, H.P.; Pike, M.C.; McCann, S.E.; Yu, H.; Xiang, Y.-B.; Wolk, A.; Wentzensen, N.; Weiss, N.S.; Webb, P.; et al. Type I and II Endometrial Cancers: Have They Different Risk Factors? J. Clin. Oncol. 2013, 31, 2607–2618. [Google Scholar] [CrossRef]
- Dougan, M.M.; Hankinson, S.E.; Vivo, I.D.; Tworoger, S.S.; Glynn, R.J.; Michels, K.B. Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. Int. J. Cancer 2015, 137, 625–637. [Google Scholar] [CrossRef]
- Hoyo, C.; Cook, M.B.; Kamangar, F.; Freedman, N.D.; Whiteman, D.; Bernstein, L.; Brown, L.M.; Risch, H.A.; Ye, W.; Sharp, L.; et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: A pooled analysis from the International BEACON Consortium. Int. J. Epidemiol. 2012, 41, 1706–1718. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Wang, X.; Wang, J.; Yan, Z.-P.; Cheng, J.; Gong, G.; Li, G. Body Mass Index and Risk of Gastric Cancer: A Meta-analysis of a Population with More Than Ten Million from 24 Prospective Studies. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Wang, J.; Yan, Z.-P.; Luo, J. Excess body weight and the risk of primary liver cancer: An updated meta-analysis of prospective studies. Eur. J. Cancer 2012, 48, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.T.; Newton, C.C.; Freedman, N.D.; Koshiol, J.; Alavanja, M.C.; Freeman, L.E.B.; Buring, J.E.; Chan, A.T.; Chong, D.Q.; Datta, I.; et al. Body Mass Index, Waist Circumference, Diabetes, and Risk of Liver Cancer for U.S. Adults. Cancer Res. 2016, 76, 6076–6083. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Y. Body mass index and risk of renal cell cancer: A dose-response meta-analysis of published cohort studies. Int. J. Cancer 2014, 135, 1673–1686. [Google Scholar] [CrossRef] [PubMed]
- McTigue, K.; McTigue, K.M.; Fidler, C.J.; Neaton, J.D.; Chang, Y.; Fried, L.F.; Liu, S.-M.; Kuller, L.H. Hypertension and obesity and the risk of kidney cancer in 2 large cohorts of US men and women. Hypertension 2014, 63, 934–941. [Google Scholar] [CrossRef]
- Wallin, A.; Larsson, S.C. Body mass index and risk of multiple myeloma: A meta-analysis of prospective studies. Eur. J. Cancer 2011, 47, 1606–1615. [Google Scholar] [CrossRef] [PubMed]
- Niedermaier, T.; Behrens, G.; Schmid, D.; Schlecht, I.; Fischer, B.; Leitzmann, M. Body mass index, physical activity, and risk of adult meningioma and glioma. Neurology 2015, 85, 1342–1350. [Google Scholar] [CrossRef]
- Genkinger, J.; Spiegelman, N.; Anderson, K.E.; Bernstein, L.; Brandt, P.A.V.D.; Calle, E.E.; English, D.R.; Folsom, A.R.; Freudenheim, J.L.; Fuchs, C.S.; et al. A pooled analysis of 14 cohort studies of anthropometric factors and pancreatic cancer risk. Int. J. Cancer 2011, 129, 1708–1717. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Tang, Y.; Wang, S.; Wang, C.; Li, Y.; Su, X.; Tian, J.; Tian, Y.; Pan, J.; et al. Synergistic Chemo–Photothermal Therapy of Breast Cancer by Mesenchymal Stem Cell-Encapsulated Yolk–Shell GNR@HPMO-PTX Nanospheres. ACS Appl. Mater. Interfaces 2016, 8, 17927–17935. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.; Tyson, M.; Egger, M.; Heller, R.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Munsell, M.F.; Sprague, B.L.; Berry, N.A.; Chisholm, G.; Trentham-Dietz, A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol. Rev. 2014, 36, 114–136. [Google Scholar] [CrossRef]
- Brinton, L.A.; Cook, M.B.; McCormack, V.; Johnson, K.C.; Olsson, H.; Casagrande, J.T.; Cooke, R.; Falk, R.T.; Gapstur, S.M.; Gaudet, M.M.; et al. Anthropometric and Hormonal Risk Factors for Male Breast Cancer: Male Breast Cancer Pooling Project Results. J. Natl. Cancer Inst. 2014, 106, djt465. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Epidemiological Studies of Ovarian C. Ovarian cancer and body size: Individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLoS Med. 2012, 9, e1001200. [Google Scholar]
- Kitahara, C.M.; McCullough, M.L.; Franceschi, S.; Rinaldi, S.; Wolk, A.; Neta, G.; Adami, H.O.; Anderson, K.; Andreotti, G.; Freeman, L.E.B.; et al. Anthropometric Factors and Thyroid Cancer Risk by Histological Subtype: Pooled Analysis of 22 Prospective Studies. Thyroid 2016, 26, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Institute NC. Obesity and Cancer. 2017. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/obesity-fact-sheet (accessed on 28 February 2020).
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Neuhouser, M.L.; Agurs-Collins, T.; Zanetti, K.A.; Cadmus-Bertram, L.; Dean, L.T.; Drake, B. Impact of Obesity on Cancer Survivorship and the Potential Relevance of Race and Ethnicity. J. Natl. Cancer Inst. 2013, 105, 1344–1354. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Huang, L.; Li, C. Leptin: A multifunctional hormone. Cell Res. 2000, 10, 81–92. [Google Scholar] [CrossRef]
- Bjorbaek, C. Leptin Signaling in the Central Nervous System and the Periphery. Recent Prog. Horm. Res. 2004, 59, 305–331. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.L.D.G.; Haynes, W. Obesity-related hypertension: Is there a role for selective leptin resistance? Curr. Hypertens. Rep. 2004, 6, 230–235. [Google Scholar] [CrossRef]
- Hukshorn, C.J.; Saris, W.H. Leptin and energy expenditure. Curr. Opin. Clin. Nutr. Metab. Care. 2004, 7, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Chagnon, Y.C.; Rankinen, T.; Snyder, E.E.; Weisnagel, S.J.; Pérusse, L.; Bouchard, C. The Human Obesity Gene Map: The 2002 Update. Obes. Res. 2003, 11, 313–367. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Surmacz, E. Leptin and cancer. J. Cell. Physiol. 2006, 207, 12–22. [Google Scholar] [CrossRef]
- Ambrosini, G.; Nath, A.K.; Sierra-Honigmann, M.R.; Flores-Riveros, J. Transcriptional Activation of the Human Leptin Gene in Response to Hypoxia. J. Boil. Chem. 2002, 277, 34601–34609. [Google Scholar] [CrossRef] [PubMed]
- Grosfeld, A.; André, J.; Mouzon, S.H.-D.; Berra, E.; Pouysségur, J.; Guerre-Millo, M. Hypoxia-inducible Factor 1 Transactivates the Human Leptin Gene Promoter. J. Boil. Chem. 2002, 277, 42953–42957. [Google Scholar] [CrossRef]
- Cao, R.; Brakenhielm, E.; Wahlestedt, C.; Thyberg, J.; Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl. Acad. Sci. USA 2001, 98, 6390–6395. [Google Scholar] [CrossRef]
- Artwohl, M.; Roden, M.; Hölzenbein, T.; Freudenthaler, A.; Waldhäusl, W.; Baumgartner-Parzer, S.M. Modulation by leptin of proliferation and apoptosis in vascular endothelial cells. Int. J. Obes. 2002, 26, 577–580. [Google Scholar] [CrossRef][Green Version]
- Dieudonné, M.-N.; Machinal-Quelin, F.; Serazin-Leroy, V.; Leneveu, M.-C.; Pecquery, R.; Giudicelli, Y. Leptin mediates a proliferative response in human MCF7 breast cancer cells. Biochem. Biophys. Res. Commun. 2002, 293, 622–628. [Google Scholar] [CrossRef]
- Hardwick, J.C.H.; Brink, G.R.V.D.; Offerhaus, G.; Van Deventer, S.J.; Peppelenbosch, M. Leptin is a growth factor for colonic epithelial cells. Gastroenterology 2001, 121, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Candelaria, P.V.; Rampoldi, A.; Harbuzariu, A.; Gonzalez-Perez, R.R. Leptin signaling and cancer chemoresistance: Perspectives. World J. Clin. Oncol. 2017, 8, 106–119. [Google Scholar] [CrossRef]
- Han, G.; Wang, L.; Zhao, W.; Yue, Z.; Zhao, R.; Li, Y.; Zhou, X.; Hu, X.; Liu, J. High expression of leptin receptor leads to temozolomide resistance with exhibiting stem/progenitor cell features in gliobalastoma. Cell Cycle 2013, 12, 3833–3840. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, R.R.; Xu, Y.; Guo, S.; Watters, A.; Zhou, W.; Leibovich, J. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFκB/HIF-1α activation. Cell. Signal. 2010, 22, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Katira, A.; Tan, P.H. Evolving role of adiponectin in cancer-controversies and update. Cancer Boil. Med. 2016, 13, 101–119. [Google Scholar] [CrossRef]
- Moschovi, M.; Trimis, G.; Vounatsou, M.; Katsibardi, K.; Margeli, A.; Damianos, A.; Chrousos, G.; Papassotiriou, I. Serial Plasma Concentrations of Adiponectin, Leptin, and Resistin During Therapy in Children With Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. 2010, 32, e8–e13. [Google Scholar] [CrossRef] [PubMed]
- Taliaferro-Smith, L.; Nagalingam, A.; Zhong, D.; Zhou, W.; Saxena, N.K.; Sharma, D. LKB1 is required for adiponectin-mediated modulation of AMPK–S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene 2009, 28, 2621–2633. [Google Scholar] [CrossRef]
- Saxena, N.K.; Sharma, D. Metastasis suppression by adiponectin: LKB1 rises up to the challenge. Cell Adhes. Migr. 2010, 4. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Baek, A.; Hwang, J.-E.; Choi, Y.A.; Jeong, J.; Lee, M.-S.; Cho, D.H.; Lim, J.-S.; Kim, K.I.; Yang, Y. Adiponectin-Activated AMPK Stimulates Dephosphorylation of AKT through Protein Phosphatase 2A Activation. Cancer Res. 2009, 69, 4018–4026. [Google Scholar] [CrossRef]
- Nieman, K.M.; Kenny, H.A.; Penicka, C.V.; Ladányi, A.; Buell-Gutbrod, R.; Zillhardt, M.R.; Romero, I.L.; Carey, M.S.; Mills, G.B.; Hotamisligil, G.S.; et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011, 17, 1498–1503. [Google Scholar] [CrossRef]
- Martinez-Outschoorn, U.; Sotgia, F.; Lisanti, M.P. Power surge: Supporting cells “fuel” cancer cell mitochondria. Cell Metab. 2012, 15, 4–5. [Google Scholar] [CrossRef]
- Meyer, K.A.; Neeley, C.K.; Baker, N.A.; Washabaugh, A.R.; Flesher, C.G.; Nelson, B.S.; Frankel, T.L.; Lumeng, C.N.; Lyssiotis, C.A.; Wynn, M.L.; et al. Adipocytes promote pancreatic cancer cell proliferation via glutamine transfer. Biochem. Biophys. Rep. 2016, 7, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Miéville, P.; Warren, C.M.; Saghafinia, S.; Li, L.; Peng, M.-W.; Hanahan, D. Metabolic Symbiosis Enables Adaptive Resistance to Anti-angiogenic Therapy that Is Dependent on mTOR Signaling. Cell Rep. 2016, 15, 1144–1160. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, E.C.; Van Houten, B. Metabolic symbiosis in cancer: Refocusing the Warburg lens. Mol. Carcinog. 2012, 52, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; Le Gonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S.; et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res. 2016, 76, 4051–4057. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, B.; Li, Z.; Li, J.; Sun, S.; Sun, S. Cancer-associated adipocytes: Key players in breast cancer progression. J. Hematol. Oncol. 2019, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zhai, M.; Wu, S.; Hu, X.-G.; Hua, Z.; Sun, H.; Guo, J.; Zhang, W.; Wang, Z. Adipocyte-derived stem cell-based gene therapy upon adipogenic differentiation on microcarriers attenuates type 1 diabetes in mice. Stem Cell Res. Ther. 2019, 10, 36. [Google Scholar] [CrossRef]
- Wu, B.; Sun, X.; Gupta, H.B.; Yuan, B.; Li, J.; Ge, F.; Chiang, H.C.; Zhang, X.; Zhang, C.; Zhang, D.; et al. Adipose PD-L1 Modulates PD-1/PD-L1 Checkpoint Blockade Immunotherapy Efficacy in Breast Cancer. Oncoimmunology 2018, 7, e1500107. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Meulle, A.; Salles, B.; Le Gonidec, S.; Garrido, I.; et al. Cancer-Associated Adipocytes Exhibit an Activated Phenotype and Contribute to Breast Cancer Invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef]
- Ye, M.; Gong, S. Drug Loaded Adipocytes: Sugar-Coated Bullets for Cancer. Phys. B Condens. Matter 2019, 1, 1104–1105. [Google Scholar] [CrossRef]
- Rocha, P.M.; Santo, V.E.; Gomes, M.E.; Reis, R.L.; Mano, J.F. Encapsulation of adipose-derived stem cells and transforming growth factor-β1 in carrageenan-based hydrogels for cartilage tissue engineering. J. Bioact. Compat. Polym. 2011, 26, 493–507. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Jeong, J.-H. Clinical Application of Adipose Stem Cells in Plastic Surgery. J. Korean Med Sci. 2014, 29, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Glass, G.E.; Ferretti, P. Adipose-Derived Stem Cells in Aesthetic Surgery. Aesthetic Surg. J. 2018, 39, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H.; Yang, J.-M.; Park, S.-B.; Kwak, S.-J.; Park, J.-S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
- Gimble, J.M. Adipose tissue-derived therapeutics. Expert Opin Biol Ther. 2003, 3, 705–713. [Google Scholar] [CrossRef]
- Fraser, J.K.; Wulur, I.; Alfonso, Z.; Hedrick, M.H. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006, 24, 150–154. [Google Scholar] [CrossRef]
- Dahl, J.A.; Duggal, S.; Coulston, N.; Millar, D.; Melki, J.; Shahdadfar, A.; Brinchmann, J.E.; Collas, P. Genetic and epigenetic instability of human bone marrow mesenchymal stem cells expanded in autologous serum or fetal bovine serum. Int. J. Dev. Boil. 2008, 52, 1033–1042. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Schreml, S.; Babilas, P.; Fruth, S.; Orsó, E.; Schmitz, G.; Mueller, M.B.; Nerlich, M.; Prantl, L. Harvesting human adipose tissue-derived adult stem cells: Resection versus liposuction. Cytotherapy 2009, 11, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.L.Q.; Coleman, S.R.; Cui, X.; Ferguson, R.E.H.; Vasconez, H.C. Autologous Fat Grafts Harvested and Refined by the Coleman Technique: A Comparative Study. Plast. Reconstr. Surg. 2008, 122, 932–937. [Google Scholar] [CrossRef]
- Ferraro, G.A.; De Francesco, F.; Tirino, V.; Cataldo, C.; Rossano, F.; Nicoletti, G.; D’Andrea, F. Effects of a New Centrifugation Method on Adipose Cell Viability for Autologous Fat Grafting. Aesthet. Plast. Surg. 2010, 35, 341–348. [Google Scholar] [CrossRef]
- Iyyanki, T.; Hubenak, J.; Liu, J.; Chang, E.I.; Beahm, E.K.; Zhang, Q. Harvesting Technique Affects Adipose-Derived Stem Cell Yield. Aesthet. Surg. J. 2015, 35, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Bertozzi, N. Isolation of Ready-to-Use Adipose-Derived Stem Cell (ASC) Pellet for Clinical Applications and a Comparative Overview of Alternate Methods for ASC Isolation. Curr. Protoc. Stem Cell Boil. 2017, 41, 1F.17.1–1F.17.12. [Google Scholar] [CrossRef] [PubMed]
- Bora, P.; Majumdar, A.S. Adipose tissue-derived stromal vascular fraction in regenerative medicine: A brief review on biology and translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef]
- Senesi, L.; De Francesco, F.; Farinelli, L.; Manzotti, S.; Gagliardi, G.; Papalia, G.F.; Riccio, M.; Gigante, A. Mechanical and Enzymatic Procedures to Isolate the Stromal Vascular Fraction From Adipose Tissue: Preliminary Results. Front. Cell Dev. Boil. 2019, 7, 88. [Google Scholar] [CrossRef]
- Zhu, M.; Cohen, S.R.; Hicok, K.C.; Shanahan, R.K.; Strem, B.M.; Yu, J.C.; Arm, D.M.; Fraser, J.K. Comparison of Three Different Fat Graft Preparation Methods. Plast. Reconstr. Surg. 2013, 131, 873–880. [Google Scholar] [CrossRef]
- Tremolada, C.; Colombo, V.; Ventura, C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr. Stem Cell Rep. 2016, 2, 304–312. [Google Scholar] [CrossRef]
- Ueberreiter, K.; Tanzella, U.; Cromme, F.; Doll, D.; Krapohl, B.D. One stage rescue procedure after capsular contracture of breast implants with autologous fat grafts collected by water assisted liposuction (“BEAULI Method”). GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2013, 2. [Google Scholar]
- Trovato, L.; Monti, M.; Del Fante, C.; Cervio, M.; Lampinen, M.; Ambrosio, L.; Redi, C.A.; Perotti, C.; Kankuri, E.; Ambrosio, G.; et al. A New Medical Device Rigeneracons Allows to Obtain Viable Micro-Grafts From Mechanical Disaggregation of Human Tissues. J. Cell. Physiol. 2015, 230, 2299–2303. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Mannucci, S.; Conti, G.; Prè, E.D.; Sbarbati, A.; Riccio, M. A Non-Enzymatic Method to Obtain a Fat Tissue Derivative Highly Enriched in Adipose Stem Cells (ASCs) from Human Lipoaspirates: Preliminary Results. Int. J. Mol. Sci. 2018, 19, 2061. [Google Scholar] [CrossRef]
- Bellei, B.; Migliano, E.; Tedesco, M.; Caputo, S.; Picardo, M. Maximizing non-enzymatic methods for harvesting adipose-derived stem from lipoaspirate: Technical considerations and clinical implications for regenerative surgery. Sci. Rep. 2017, 7, 10015. [Google Scholar] [CrossRef] [PubMed]
- Alstrup, T.; Eijken, M.; Bohn, A.B.; Møller, B.; Damsgaard, T.E. Isolation of Adipose Tissue-Derived Stem Cells: Enzymatic Digestion in Combination with Mechanical Distortion to Increase Adipose Tissue-Derived Stem Cell Yield from Human Aspirated Fat. Curr. Protoc. Stem Cell Boil. 2018, 48, e68. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Widgerow, A.D.; Banyard, D.; Toranto, J.; Wirth, G.A.; Paydar, K.; Tussardi, I.T.; Evans, G.R. Strategic Sequences in Fat Graft Survival. Ann. Plast. Surg. 2015, 74, 376–382. [Google Scholar] [CrossRef]
- Olenczak, J.; Seaman, S.A.; Lin, K.Y.; Pineros-Fernandez, A.; Davis, C.E.; Salopek, L.S.; Peirce, S.M.; Cottler, P.S. Effects of Collagenase Digestion and Stromal Vascular Fraction Supplementation on Volume Retention of Fat Grafts. Ann. Plast. Surg. 2017, 78, S335–S342. [Google Scholar] [CrossRef] [PubMed]
- Raposio, E.; Caruana, G.; Bonomini, S.; Libondi, G. A novel and effective strategy for the isolation of adipose-derived stem cells: Minimally manipulated adipose-derived stem cells for more rapid and safe stem cell therapy. Plast. Reconstr. Surg. 2014, 133, 1406–1409. [Google Scholar]
- Tropel, P.; Noël, D.; Platet, N.; Legrand, P.; Benabid, A.-L.; Berger, F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp. Cell Res. 2004, 295, 395–406. [Google Scholar] [CrossRef]
- Coccè, V.; Brini, A.T.; Gianni’, A.; Sordi, V.; Berenzi, A.; Alessandri, G.; Tremolada, C.; Versari, S.; Bosetto, A.; Pessina, A. A Nonenzymatic and Automated Closed-Cycle Process for the Isolation of Mesenchymal Stromal Cells in Drug Delivery Applications. Stem Cells Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Doornaert, M.; De Maere, E.; Colle, J.; Declercq, H.; Taminau, J.; Lemeire, K.; Berx, G.; Blondeel, P. Xenogen-free isolation and culture of human adipose mesenchymal stem cells. Stem Cell Res. 2019, 40, 101532. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Artuso, S.; D’Angelo, C.; Porru, M.; D’Amico, F.; Bielli, A.; Gentile, P.; Cervelli, V.; Leonetti, C.; Orlandi, A. Adipose-derived stem cell-mediated paclitaxel delivery inhibits breast cancer growth. PLoS ONE 2018, 13, e0203426. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, A.; Coccè, V.; Cavicchini, L.; Sisto, F.; Dossena, M.; Balzarini, P.; Portolani, N.; Ciusani, E.; Parati, E.; Alessandri, G.; et al. Adipose Tissue-Derived Stromal Cells Primed in Vitro with Paclitaxel Acquire Anti-Tumor Activity. Int. J. Immunopathol. Pharmacol. 2013, 26, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Cocce, L.B.V. Fluorescent Immortalized Human Adipose Derived Stromal Cells (hASCs-TS/GFP+) for Studying Cell Drug Delivery Mediated by Microvesicles. Anti-Cancer Agents Med. Chem. 2017, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Jin, L.; Caglayan, H.; Chen, J.; Xing, G.; Zheng, C.; Doan-Nguyen, V.; Kang, Y.; Engheta, N.; Kagan, C.R.; et al. Improved Size-Tunable Synthesis of Monodisperse Gold Nanorods through the Use of Aromatic Additives. ACS Nano 2012, 6, 2804–2817. [Google Scholar] [CrossRef]
- Granneman, J.G. Delivery of DNA into Adipocytes within Adipose Tissue. Adv. Struct. Saf. Stud. 2008, 423, 191–195. [Google Scholar] [CrossRef]
- Fisher, P.D.; Brambila, C.J.; McCoy, J.R.; Kiosses, W.B.; Mendoza, J.M.; Oh, J.; Yung, B.S.; Schultheis, K.; Smith, T.R.F.; Broderick, K.E. Adipose tissue: A new target for electroporation-enhanced DNA vaccines. Gene Ther. 2017, 24, 757–767. [Google Scholar] [CrossRef]
- Rostami, M.; Haidari, K.; Shahbazi, M. Genetically Engineered Adipose Mesenchymal Stem Cells Using HIV-Based Lentiviral Vectors as Gene Therapy for Autoimmune Diseases. Cell. Reprogramm. 2018, 20, 337–346. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Prot. Immunol. 2001, 21, A.3B.1–A.3B.2. [Google Scholar] [CrossRef]
- Durandt, C.; Van Vollenstee, F.A.; Dessels, C.; Kallmeyer, K.; De Villiers, D.; Murdoch, C.; Potgieter, M.; Pepper, M.S. Novel flow cytometric approach for the detection of adipocyte subpopulations during adipogenesis. J. Lipid Res. 2016, 57, 729–742. [Google Scholar] [CrossRef]
- Ussar, S.; Lee, K.; Dankel, S.N.; Boucher, J.; Haering, M.-F.; Kleinridders, A.; Thomou, T.; Xue, R.; Macotela, Y.; Cypess, A.M.; et al. ASC-1, PAT2, and P2RX5 are cell surface markers for white, beige, and brown adipocytes. Sci. Transl. Med. 2014, 6, 247ra103. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.K.; Tan, C.S.; Chan, K.L.; Goesantoso, G.G.; Chan, X.H.D.; Chan, E.; Yin, J.; Yeo, C.R.; Khoo, C.M.; So, J.B.-Y.; et al. Identification of specific cell-surface markers of adipose-derived stem cells from subcutaneous and visceral fat depots. Stem Cell Rep. 2014, 2, 171–179. [Google Scholar] [CrossRef]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Bouraoui, L.; Gutiérrez, J.; Navarro, I.; Alvarez, I.N. Regulation of proliferation and differentiation of adipocyte precursor cells in rainbow trout (Oncorhynchus mykiss). J. Endocrinol. 2008, 198, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef] [PubMed]
- De Melo, N.; McGinlay, S.; Markus, R.; Macri-Pellizzeri, L.; Symonds, M.E.; Ahmed, I.; Sottile, V. Live Simultaneous Monitoring of Mineral Deposition and Lipid Accumulation in Differentiating Stem Cells. Biomimetics 2019, 4, 48. [Google Scholar] [CrossRef]
- Yuan, C.; Chakraborty, S.; Chitta, K.K.; Subramanian, S.; Lim, T.E.; Han, W.; Prakash, K.N.B.; Sugii, S. Fast Adipogenesis Tracking System (FATS)—A robust, high-throughput, automation-ready adipogenesis quantification technique. Stem Cell Res. Ther. 2019, 10, 38. [Google Scholar] [CrossRef]
- Riss, T.; Hook, B.; Duellman, S. Evaluation of real time cell viability assays multiplexed with other methods. Toxicol. Lett. 2015, 238, S179–S180. [Google Scholar] [CrossRef]
- Pozarowski, P.; Darzynkiewicz, Z. Analysis of Cell Cycle by Flow Cytometry. Checkpoint Controls Cancer 2004, 281, 301–312. [Google Scholar] [CrossRef]
- Philippé, J.; De Sitter, S.; De Ridder, L.; Cornelissen, M. Annexin V expression in apoptotic peripheral blood lymphocytes: An electron microscopic evaluation. Apoptosis 2002, 7, 41–47. [Google Scholar] [CrossRef]
- Wankhade, U.; Rane, S.G. Flow Cytometry Assisted Isolation of Adipose Tissue Derived Stem Cells. Adv. Struct. Saf. Stud. 2017, 1566, 17–24. [Google Scholar] [CrossRef]
- Johnson, S.; Nguyen, V.; Coder, D. Assessment of Cell Viability. Curr. Protoc. Cytom. 2013, 64, 9.2.1–9.2.26. [Google Scholar] [CrossRef] [PubMed]
- Tekkeli, S.E.K.; Kiziltas, M.V. Current HPLC Methods for Assay of Nano Drug Delivery Systems. Curr. Top. Med. Chem. 2017, 17, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Werling, N.; Satkunanathan, S.; Thorpe, R.; Zhao, Y. Systematic Comparison and Validation of Quantitative Real-Time PCR Methods for the Quantitation of Adeno-Associated Viral Products. Hum. Gene Ther. Methods 2015, 26, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Charrier, S.; Ferrand, M.; Zerbato, M.; Précigout, G.; Viornery, A.; Bucher-Laurent, S.; Benkhelifa-Ziyyat, S.; Merten, O.W.; Perea, J.; Galy, A. Quantification of lentiviral vector copy numbers in individual hematopoietic colony-forming cells shows vector dose-dependent effects on the frequency and level of transduction. Gene Ther. 2010, 18, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.A.; Yun, J.-W.; Joo, K.M.; Lee, J.Y.; Kwak, P.A.; Lee, Y.E.; You, J.-R.; Kwon, E.; Kim, W.H.; Wang, K.-C.; et al. Preclinical Biosafety Evaluation of Genetically Modified Human Adipose Tissue-Derived Mesenchymal Stem Cells for Clinical Applications to Brainstem Glioma. Stem Cells Dev. 2016, 25, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Salehi, H.; Oskuee, R.K.; Mohammadpour, A.; Mirzaei, H.R.; Sharifi, M.; Salarinia, R.; Darani, H.Y.; Mokhtari, M.; Masoudifar, A.; et al. The therapeutic potential of human adipose-derived mesenchymal stem cells producing CXCL10 in a mouse melanoma lung metastasis model. Cancer Lett. 2018, 419, 30–39. [Google Scholar] [CrossRef]
- Altaner, C.; Altanerova, V.; Cihova, M.; Ondicova, K.; Rychlý, B.; Bačiak, L.; Mravec, B. Complete regression of glioblastoma by mesenchymal stem cells mediated prodrug gene therapy simulating clinical therapeutic scenario. Int. J. Cancer 2013, 134, 1458–1465. [Google Scholar] [CrossRef]
- Altanerova, V.; Cihova, M.; Babič, M.; Rychlý, B.; Ondicova, K.; Mravec, B.; Altaner, C. Human adipose tissue-derived mesenchymal stem cells expressing yeast cytosinedeaminase::uracil phosphoribosyltransferase inhibit intracerebral rat glioblastoma. Int. J. Cancer 2011, 130, 2455–2463. [Google Scholar] [CrossRef]
- Cavarretta, I.; Altanerova, V.; Matuskova, M.; Kucerova, L.; Culig, Z.; Altaner, C. Adipose Tissue–derived Mesenchymal Stem Cells Expressing Prodrug-converting Enzyme Inhibit Human Prostate Tumor Growth. Mol. Ther. 2009, 18, 223–231. [Google Scholar] [CrossRef]
- Abrate, A.; Buono, R.; Canu, T.; Esposito, A.; Del Maschio, A.; Lucianò, R.; Bettiga, A.; Colciago, G.; Guazzoni, G.; Benigni, F.; et al. Mesenchymal stem cells expressing therapeutic genes induce autochthonous prostate tumour regression. Eur. J. Cancer 2014, 50, 2478–2488. [Google Scholar] [CrossRef] [PubMed]
- You, M.-H.; Kim, W.-J.; Shim, W.; Lee, S.-R.; Lee, G.; Choi, S.; Kim, D.-Y.; Kim, Y.M.; Kim, H.; Han, S.-U. Cytosine deaminase-producing human mesenchymal stem cells mediate an antitumor effect in a mouse xenograft model. J. Gastroenterol. Hepatol. 2009, 24, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, L.; Skolekova, S.; Demkova, L.; Bohovic, R.; Matuskova, M. Long-term efficiency of mesenchymal stromal cell-mediated CD-MSC/5FC therapy in human melanoma xenograft model. Gene Ther. 2014, 21, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-H.; Peng, B.-Y.; Chang, C.-C.; Dubey, N.K.; Lo, W.-C.; Cheng, H.-C.; Wang, J.R.; Wei, H.-J.; Deng, W.-P. Tumor-Targeted Immunotherapy by Using Primary Adipose-Derived Stem Cells and an Antigen-Specific Protein Vaccine. Cancers 2018, 10, 446. [Google Scholar] [CrossRef]
- Ito, M.; Bujo, H.; Takahashi, K.; Arai, T.; Tanaka, I.; Saito, Y. Implantation of primary cultured adipocytes that secrete insulin modifies blood glucose levels in diabetic mice. Diabetology 2005, 48, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Kakimoto, K.; Goto, M.; Higuchi, K. Novel Therapeutic Approach Using Drug-loaded Adipose-derived Stem Cells for Pancreatic Cancer. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mangraviti, A.; Tzeng, S.Y.; Gullotti, D.; Kozielski, K.L.; Kim, J.E.; Seng, M.; Abbadi, S.; Schiapparelli, P.; Sarabia-Estrada, R.; Vescovi, A.; et al. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials 2016, 100, 53–66. [Google Scholar] [CrossRef]
- Balcells, L.; Fornaguera, C.; Brugada-Vilà, P.; Guerra-Rebollo, M.; Meca-Cortés, Ó.; Martínez, G.; Rubio, N.; Blanco, J.; Santamaría, J.; Cascante, A.; et al. SPIONs’ Enhancer Effect on Cell Transfection: An Unexpected Advantage for an Improved Gene Delivery System. ACS Omega 2019, 4, 2728–2740. [Google Scholar] [CrossRef]
- Jiang, X.; Fitch, S.; Wang, C.; Wilson, C.; Li, J.; Grant, G.A.; Yang, F. Nanoparticle engineered TRAIL-overexpressing adipose-derived stem cells target and eradicate glioblastoma via intracranial delivery. Proc. Natl. Acad. Sci. USA 2016, 113, 13857–13862. [Google Scholar] [CrossRef]
- Jurj, A.; Zanoaga, O.; Braicu, C.; Lazar, V.; Tomuleasa, C.; Irimie, A.; Berindan-Neagoe, I. A Comprehensive Picture of Extracellular Vesicles and Their Contents. Molecular Transfer to Cancer Cells. Cancers 2020, 12, 298. [Google Scholar] [CrossRef]
- Gulei, D.; Irimie, A.I.; Cojocneanu, R.; Schultze, J.; Berindan-Neagoe, I. Exosomes—Small Players, Big Sound. Bioconj. Chem. 2018, 29, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Gulei, D.; Petrut, B.; Ţigu, A.B.; Onaciu, A.; Fischer-Fodor, E.; Atanasov, A.G.; Ionescu, C.; Berindan-Neagoe, I. Exosomes at a glance—Common nominators for cancer hallmarks and novel diagnosis tools. Crit. Rev. Biochem. Mol. Boil. 2018, 53, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Gulei, D.; Raduly, L.; Broseghini, E.; Ferracin, M.; Berindan-Neagoe, I. The extensive role of miR-155 in malignant and non-malignant diseases. Mol. Asp. Med. 2019, 70, 33–56. [Google Scholar] [CrossRef]
- Bica-Pop, C.; Cojocneanu, R.; Magdo, L.; Raduly, L.; Gulei, D.; Berindan-Neagoe, I. Overview upon miR-21 in lung cancer: Focus on NSCLC. Cell. Mol. Life Sci. 2018, 75, 3539–3551. [Google Scholar] [CrossRef]
- Braicu, C.; Cătană, C.; Calin, G.A.; Berindan-Neagoe, I. NCRNA Combined Therapy as Future Treatment Option for Cancer. Curr. Pharm. Des. 2014, 20, 6565–6574. [Google Scholar] [CrossRef]
- Pop-Bica, C.; Gulei, D.; Cojocneanu, R.; Braicu, C.; Petrut, B.; Berindan-Neagoe, I. Understanding the Role of Non-Coding RNAs in Bladder Cancer: From Dark Matter to Valuable Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 1514. [Google Scholar] [CrossRef]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-K.; Finniss, S.; Cazacu, S.; Bucris, E.; Ziv-Av, A.; Xiang, C.; Bobbitt, K.; Rempel, S.A.; Hasselbach, L.; Mikkelsen, T.; et al. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget 2013, 4, 346–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Choi, Y.; Kim, K.; Koo, H.J.; Choi, J. Quantification of Unknown Nanoscale Biomolecules Using the Average-Weight-Difference Method. Appl. Sci. 2019, 9, 130. [Google Scholar] [CrossRef]
- Klingelhutz, A.J.; Gourronc, F.A.; Chaly, A.; Wadkins, D.A.; Burand, A.; Markan, K.; Idiga, S.O.; Wu, M.; Potthoff, M.J.; Ankrum, J.A. Scaffold-free generation of uniform adipose spheroids for metabolism research and drug discovery. Sci. Rep. 2018, 8, 523. [Google Scholar] [CrossRef]
- Chusyd, D.E.; Wang, N.; Huffman, D.M.; Nagy, T. Relationships between Rodent White Adipose Fat Pads and Human White Adipose Fat Depots. Front. Nutr. 2016, 3, 1164. [Google Scholar] [CrossRef] [PubMed]
- Gabriely, I.; Ma, X.H.; Yang, X.M.; Atzmon, G.; Rajala, M.W.; Berg, A.H.; Scherer, P.; Rossetti, L.; Barzilai, N. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: An adipokine-mediated process? Diabetes 2002, 51, 2951–2958. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, R.; Allison, D.B.; Huffman, D.M.; Ma, X.; Atzmon, G.; Einstein, F.H.; Fishman, S.; Poduval, A.D.; McVei, T.; Keith, S.W.; et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell 2008, 7, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.S.; Liaw, L.; Reagan, M.R. In vitro tissue-engineered adipose constructs for modeling disease. BMC Biomed. Eng. 2019, 1, 1–19. [Google Scholar] [CrossRef]
- Ren, B.; Betz, V.M.; Thirion, C.; Salomon, M.; Klar, R.M.; Jansson, V.; Müller, P.E.; Betz, O.B. Gene activated adipose tissue fragments as advanced autologous biomaterials for bone regeneration: Osteogenic differentiation within the tissue and implications for clinical translation. Sci. Rep. 2019, 9, 224. [Google Scholar] [CrossRef]
- Zhang, Z.; Ortiz, O.; Goyal, R.; Kohn, J. 13—Biodegradable Polymers. In Handbook of Polymer Applications in Medicine and Medical Devices; Modjarrad, K., Ebnesajjad, S., Eds.; William Andrew Publishing: Oxford, UK, 2014; pp. 303–335. [Google Scholar]
| Cell Type | Advantages | Drug/Compound Loaded/Functionalized | Pathology | Clinical Trial Phase | Ref. |
|---|---|---|---|---|---|
| Erythrocytes | Dexamethasone Sodium Phosphate (DSP) | Ataxia Telangiectasia | Phase 2 | [23] | |
| L-asparaginase | (a) Philadelphia Chromosome-Negative Acute Lymphoblastic Leukemia | Phase 2 | [24] | ||
| (b) Acute Myeloblastic Leukemia | Phase 2 | NCT01810705 | |||
| (c) Pancreatic Cancer and Progressive Metastatic Pancreatic Carcinoma | Phase 1 and Phase 2 | NCT01523808 and NCT02195180 | |||
| Glucocerebrosidase | Gaucher’s Disease | [25] | |||
| Thymidine phosphorylase | Mitochondrial Neuro-gastrointestinal Encephalopathy (MNGIE) | [26] | |||
| Daunorubicin | Acute Leukemia | [27] | |||
| Doxorubicin | Lymphoma | [28] | |||
| Platelets | Epidoxorubicin | Myeloma | [30] | ||
| Doxorubicin | Lymphoma | [31,32] | |||
| Factor VIII/Factor IX | Hemophilia | [33,34] | |||
| ADAMTS13 (A Disintegrin and Metalloprotease with Thrombospondin Type 1 Repeats—13) | Arterial Thrombosis Associated with Thrombotic Thrombocytopenic Purpura | [35] | |||
| Vincristine | (a) Refractory Autoimmune Hemolytic Anemia and Chronic Immune Thrombocytopenia | [36] | |||
| (b) Immune Thrombocytopenia | [37] | ||||
| Monocytes and Macrophages | Protein/Peptide Antigens | Cancer | [40] | ||
| Indocyanine Green (Contrast agent) | Inflammation | [38,41] | |||
| Efavirenz, Ritonavir, Indinavir | Retroviral Infection | [42] | |||
| Nano-formulated Catalase | Parkinson’s Disease | [43] | |||
| Photosensitizer (mTHPC) and Magnetic Nanoparticles (NPs) | Cancer | [44] | |||
| T-cells | Maleimide-functionalized NPs | Prostate Cancer | [45] | ||
| Drug (small molecules)-loaded Liposomes/Multilamellar Lipid NPs/Lipid-coated Polymer NPs | Melanoma | [46] | |||
| Chimeric Antigen Receptor (CAR)—Anti-CD19 CAR-T | (a) Acute Lymphoblastic Leukemia (NCT03366324) | Phase 1 | NCT03016377 | ||
| (b) Immune System Diseases, Immunoproliferative Disorders | Phase 2 | NCT03016377 | |||
| Dendritic cells |
| Antigen (tumor cell lysate) | Hepatocellular Carcinoma [48] | [48] | |
| Tumor RNA | Esophageal Squamous Cell Carcinoma [49] | [49] | |||
| Allogeneic Apoptotic-Necrotic Melanoma Cells | Melanoma | Phase 1 | NCT00515983 | ||
| Stem cells | Pancreatic Precursor Cells | Type 1 Diabetes | Phase 2 | NCT02239354 | |
| MiR-133b | Cerebral Ischemia | [51] | |||
| Suicide Genes | Aggressive Lung Melanoma Metastases | [52] | |||
| Paclitaxel | Leukemia and Glioblastoma | [53,54] | |||
| IL-12 | Advanced Head and Neck Cancer | Phase 1 | NCT02079324 | ||
| CCL5 Promoter | Advanced Gastrointestinal Cancer | Phase 1/2 | NCT02008539 |
| Important roles of adipose tissue | Expenditure of the body energy Food intake behavior Immune functions Reproduction Hematopoiesis Lymphopoiesis |
| Types of adipose tissue | Visceral Subcutaneous Intramuscular |
| Subtype of adipose tissue | White Brown Beige |
| Types of cells found within the adipose tissue | Adipocytes Endothelial cells Fibroblasts Connective tissue cells Pericytes Progenitor and Stem cells Macrophages Mast cells Dendritic cells Neutrophils Eosinophils Lymphocytes |
| Cancers linked with obesity | Endometrial cancer [80,81] Esophageal adenocarcinoma [82] Gastric cardia cancer [83] Liver cancer [84,85] Kidney cancer [86,87] Multiple myeloma [88] Meningioma [89] Pancreatic cancer [90] Gallbladder cancer [91] Breast cancer [92,93,94] Ovarian cancer [95] Thyroid cancer [96] |
| Molecular mechanisms behind cancer and obesity [97] | Chronic local inflammation Increased amount of estrogen High level of insulin and insulin-like growth factor-1 (IGF-1) in the blood Increased secretion of adipokines with roles in cell proliferation Indirect effect of adipocytes in modulating pathways involved in cell growth |
| Principal adipokines with a role in cancer progression [100] | Adiponectin Leptin |
| Principal signaling molecules secreted by both adipocytes and immune cells with a role in cancer progression [100] | Tumor necrosis factor alpha (TNF-α) Interleukin-6 (IL-6) Resistin Visfatin Chemokine monocyte chemoattractant protein (MCP-1) Plasminogen activator inhibitor-1 (PAI-1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, R.; Onaciu, A.; Moldovan, C.; Zimta, A.-A.; Gulei, D.; Paradiso, A.V.; Lazar, V.; Berindan-Neagoe, I. Adipocyte-Based Cell Therapy in Oncology: The Role of Cancer-Associated Adipocytes and Their Reinterpretation as Delivery Platforms. Pharmaceutics 2020, 12, 402. https://doi.org/10.3390/pharmaceutics12050402
Munteanu R, Onaciu A, Moldovan C, Zimta A-A, Gulei D, Paradiso AV, Lazar V, Berindan-Neagoe I. Adipocyte-Based Cell Therapy in Oncology: The Role of Cancer-Associated Adipocytes and Their Reinterpretation as Delivery Platforms. Pharmaceutics. 2020; 12(5):402. https://doi.org/10.3390/pharmaceutics12050402
Chicago/Turabian StyleMunteanu, Raluca, Anca Onaciu, Cristian Moldovan, Alina-Andreea Zimta, Diana Gulei, Angelo V. Paradiso, Vladimir Lazar, and Ioana Berindan-Neagoe. 2020. "Adipocyte-Based Cell Therapy in Oncology: The Role of Cancer-Associated Adipocytes and Their Reinterpretation as Delivery Platforms" Pharmaceutics 12, no. 5: 402. https://doi.org/10.3390/pharmaceutics12050402
APA StyleMunteanu, R., Onaciu, A., Moldovan, C., Zimta, A.-A., Gulei, D., Paradiso, A. V., Lazar, V., & Berindan-Neagoe, I. (2020). Adipocyte-Based Cell Therapy in Oncology: The Role of Cancer-Associated Adipocytes and Their Reinterpretation as Delivery Platforms. Pharmaceutics, 12(5), 402. https://doi.org/10.3390/pharmaceutics12050402







