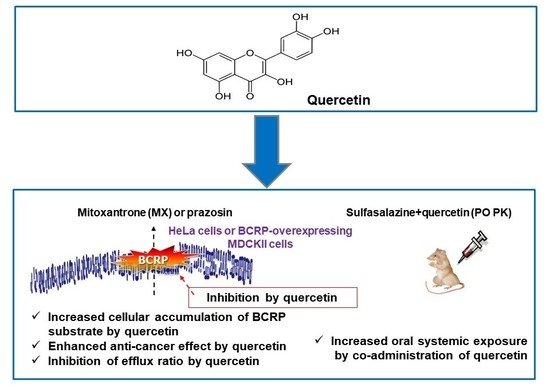
Quercetin Is a Flavonoid Breast Cancer Resistance Protein Inhibitor with an Impact on the Oral Pharmacokinetics of Sulfasalazine in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. RT-PCR Analysis
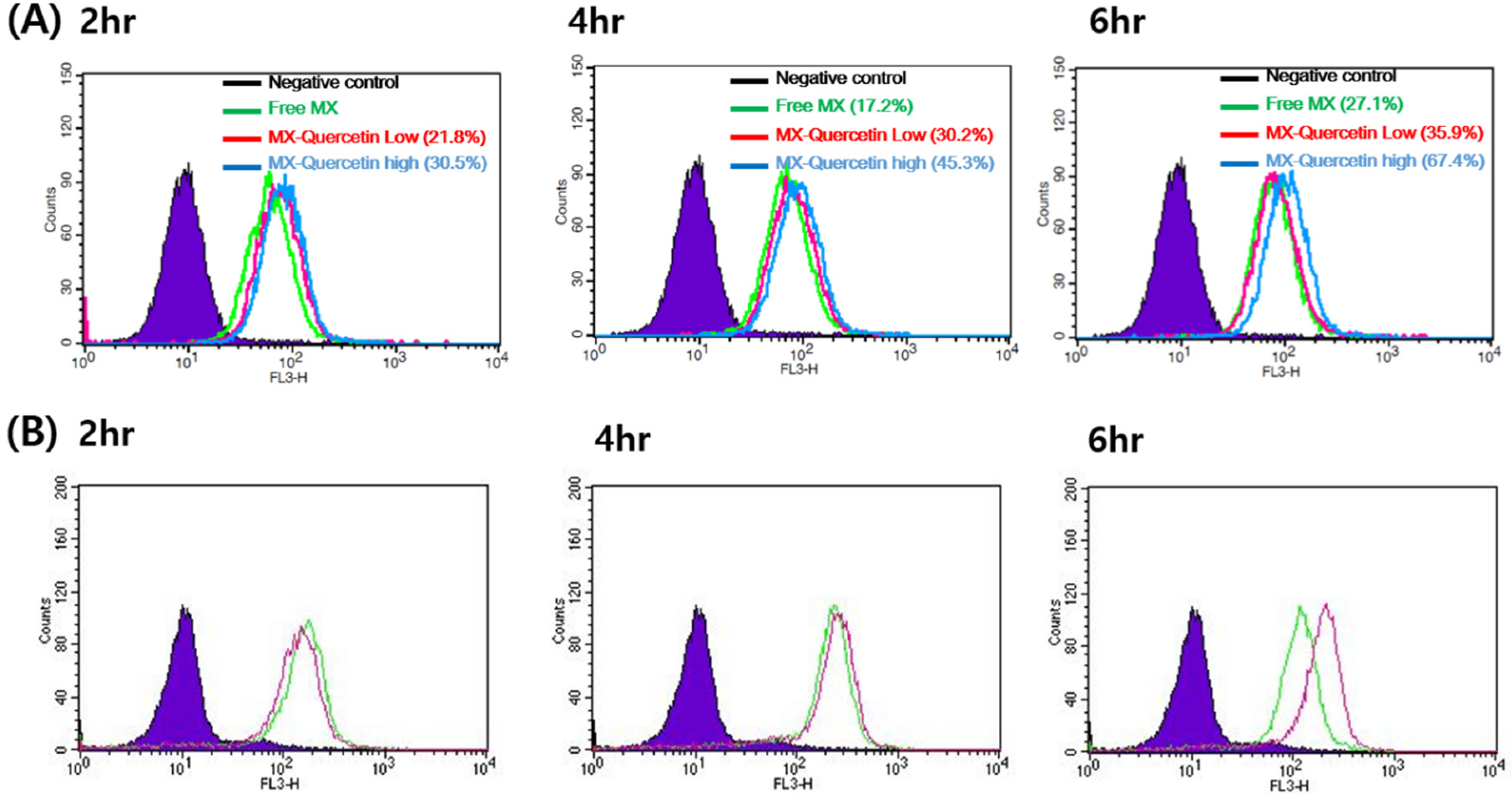
2.4. FACS-Cellular Accumulation Study
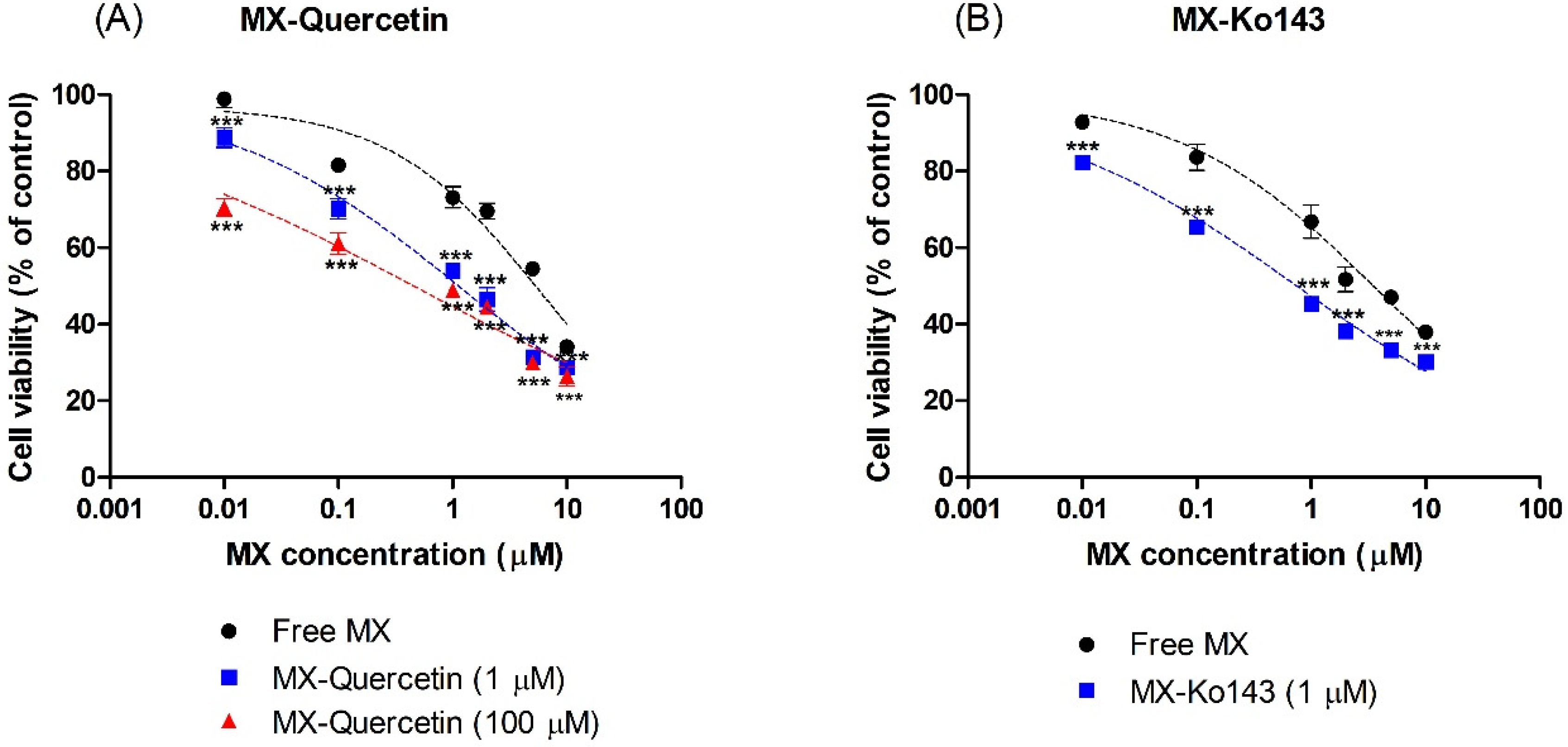
2.5. Cytotoxicity Assay
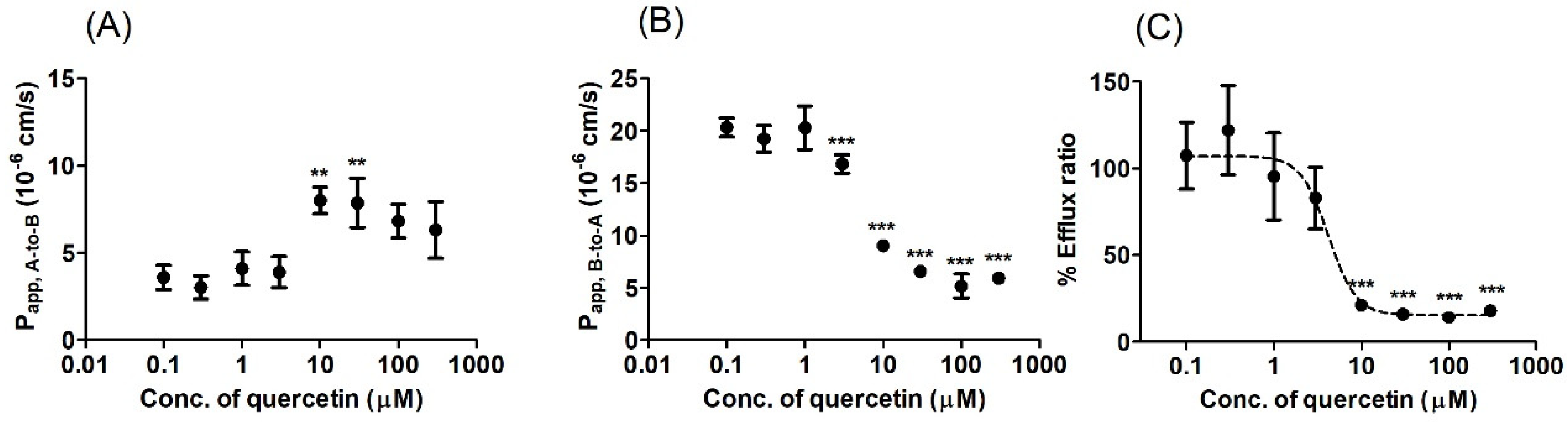
2.6. Bi-Directional Transport Study
2.7. Experimental Animals
2.8. Oral Pharmacokinetic Study in Rats
2.9. Quantification Using LC-MS/MS
2.10. Data Analysis
2.10.1. In Vitro Kinetic Analysis
2.10.2. Non-Compartmental Pharmacokinetic Analysis
2.11. Statistical Analysis
3. Results
3.1. FACS-Cellular Accumulation Study
3.2. Cytotoxicity of Mitoxantrone in the Presence of Quercetin
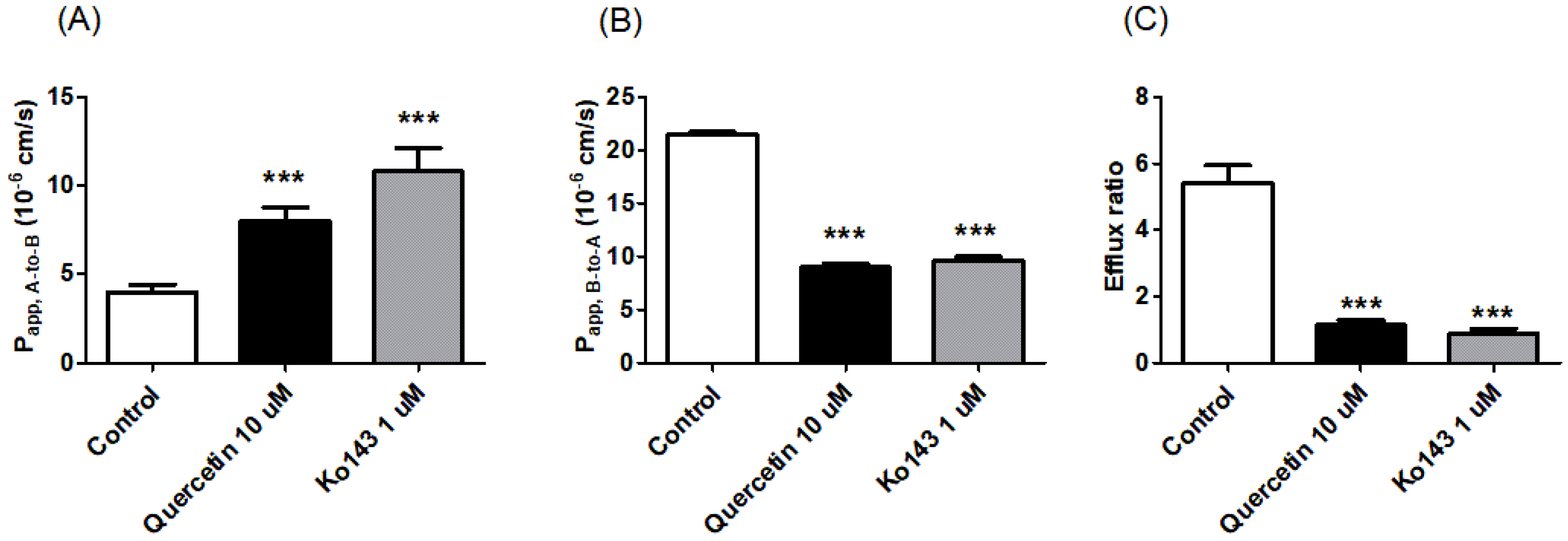
3.3. Bi-Directional Transport Study in MDCKII/BCRP Cells
3.4. Oral Pharmacokinetic Study in Rats with or without Quercetin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hertog, M.; Hollman, P.; Katan, M. Flavonol and flavone content of vegetables and fruits. In Flavonols and Flavones in Foods and Their Relation with Cancer and Coronary Heart Disease Risk; CIP-Data Koninklijke Bibliotheek: The Hague, The Netherlands, 1994. [Google Scholar]
- Hertog, M.G.L.; Hollman, P.C.H.; Van De Putte, B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J. Agric. Food Chem. 1993, 41, 1242–1246. [Google Scholar] [CrossRef]
- Kelly, G.S. Quercetin. Altern. Med. Rev. 2011, 16, 172–195. [Google Scholar] [PubMed]
- Sampson, L.; Rimm, E.; Hollman, P.C.; De Vries, J.H.; Katan, M.B. Flavonol and flavone intakes in US health professionals. J. Am. Diet. Assoc. 2002, 102, 1414–1420. [Google Scholar] [CrossRef]
- De Vrie, J.H.; Janssen, P.; Hollman, P.C.; Van Staveren, W.A.; Katan, M.B. Consumption of quercetin and kaempferol in free-living subjects eating a variety of diets. Cancer Lett. 1997, 114, 141–144. [Google Scholar] [CrossRef]
- Formica, J.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Takahama, U. Inhibition of lipoxygenase-dependent lipid peroxidation by quercetin: Mechanism of antioxidative function. Phytochemistry 1985, 24, 1443–1446. [Google Scholar] [CrossRef]
- Lamson, D.W.; Brignall, M.S. Antioxidants and cancer, part 3: Quercetin. Altern. Med. Rev. J. Clin. Ther. 2000, 5, 196–208. [Google Scholar]
- Ohnishi, E.; Bannai, H. Quercetin potentiates TNF-induced antiviral activity. Antivir. Res. 1993, 22, 327–331. [Google Scholar] [CrossRef]
- De La Lastra, A.; Martin, M.; Motilva, V. Antiulcer and Gastroprotective Effects of Quercetin: A Gross and Histologic Study. Pharmacology 1994, 48, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Vida, R.G.; Fittler, A.; Somogyi-Végh, A.; Poór, M. Dietary quercetin supplements: Assessment of online product informations and quantitation of quercetin in the products by high-performance liquid chromatography. Phytother. Res. 2019, 33, 1912–1920. [Google Scholar] [CrossRef]
- Geller, A.I.; Shehab, N.; Weidle, N.J.; Lovegrove, M.C.; Wolpert, B.J.; Timbo, B.B.; Mozersky, R.P.; Budnitz, D.S. Emergency Department Visits for Adverse Events Related to Dietary Supplements. N. Engl. J. Med. 2015, 373, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.; Real, R.; Pérez, M.; Mendoza, G.; Prieto, J.G.; Merino, G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J. Pharm. Sci. 2010, 99, 598–617. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.R.; Khurana, A.; Bale, S.; Ravirala, R.; Reddy, V.S.S.; Mohankumar, M.; Godugu, C. Natural flavonoids silymarin and quercetin improve the brain distribution of co-administered P-gp substrate drugs. SpringerPlus 2016, 5, 1618. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-S.; Piao, Y.-J.; Kang, K.-W. Effects of quercetin on the bioavailability of doxorubicin in rats: Role of CYP3A4 and P-gp inhibition by quercetin. Arch. Pharmacal Res. 2011, 34, 607–613. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.; Zeng, S. Involvement of P-glycoprotein in regulating cellular levels of Ginkgo flavonols: Quercetin, kaempferol, and isorhamnetin. J. Pharm. Pharmacol. 2005, 57, 751–758. [Google Scholar] [CrossRef]
- Van Zanden, J.J.; Wortelboer, H.M.; Bijlsma, S.; Punt, A.; Usta, M.; Van Bladeren, P.J.; Rietjens, I.M.C.M.; Cnubben, N.H. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem. Pharmacol. 2005, 69, 699–708. [Google Scholar] [CrossRef]
- Chae, Y.-J.; Cho, K.H.; Yoon, I.-S.; Noh, C.-K.; Lee, H.-J.; Park, Y.; Ji, E.; Seo, M.-D.; Maeng, H.-J. Vitamin D Receptor-Mediated Upregulation of CYP3A4 and MDR1 by Quercetin in Caco-2 cells. Planta Medica 2015, 82, 121–130. [Google Scholar] [CrossRef]
- Ni, Z.; Bikadi, Z.; Rosenberg, M.F.; Mao, Q. Structure and function of the human breast cancer resistance protein (BCRP/ABCG2). Curr. Drug Metab. 2010, 11, 603–617. [Google Scholar] [CrossRef]
- Sesink, A.L.; Arts, I.C.; De Boer, V.C.; Breedveld, P.; Schellens, J.H.; Hollman, P.C.; Russel, F.G. Breast cancer resistance protein (Bcrp1/Abcg2) limits net intestinal uptake of quercetin in rats by facilitating apical efflux of glucuronides. Mol. Pharmacol. 2005, 67, 1999–2006. [Google Scholar] [CrossRef]
- Pick, A.; Müller, H.; Mayer, R.; Haenisch, B.; Pajeva, I.; Weigt, M.; Bönisch, H.; Müller, C.E.; Wiese, M. Structure–activity relationships of flavonoids as inhibitors of breast cancer resistance protein (BCRP). Bioorg. Med. Chem. 2011, 19, 2090–2102. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Morris, M.E. Flavonoids Are Inhibitors of Breast Cancer Resistance Protein (ABCG2)-Mediated Transport. Mol. Pharmacol. 2004, 65, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.; Sagawa, K.; Morris, M.E. Flavonoids chrysin and benzoflavone, potent breast cancer resistance protein inhibitors, have no significant effect on topotecan pharmacokinetics in rats or mdr1a/1b (–/–) mice. Drug Metab. Dispos. 2004, 33, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zamek-Gliszczynski, M.J.; Bedwell, D.W.; Bao, J.Q.; Higgins, J.W. Characterization of SAGE Mdr1a (P-gp), Bcrp, and Mrp2 Knockout Rats Using Loperamide, Paclitaxel, Sulfasalazine, and Carboxydichlorofluorescein Pharmacokinetics. Drug Metab. Dispos. 2012, 40, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Kusuhara, H.; Furuie, H.; Inano, A.; Sunagawa, A.; Yamada, S.; Wu, C.; Fukizawa, S.; Morimoto, N.; Ieiri, I.; Morishita, M.; et al. Pharmacokinetic interaction study of sulphasalazine in healthy subjects and the impact of curcumin as an in vivo inhibitor of BCRP. Br. J. Pharmacol. 2012, 166, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Zaher, H.; Khan, A.A.; Palandra, J.; Brayman, T.G.; Yu, L.; Ware, J.A. Breast Cancer Resistance Protein (Bcrp/abcg2) Is a Major Determinant of Sulfasalazine Absorption and Elimination in the Mouse. Mol. Pharm. 2006, 3, 55–61. [Google Scholar] [CrossRef]
- Song, Y.-K.; Park, J.E.; Oh, Y.; Hyung, S.; Jeong, Y.-S.; Kim, M.-S.; Lee, W.; Chung, S.-J. Suppression of Canine ATP Binding Cassette ABCB1 in Madin-Darby Canine Kidney Type II Cells Unmasks Human ABCG2-Mediated Efflux of Olaparib. J. Pharmacol. Exp. Ther. 2018, 368, 79–87. [Google Scholar] [CrossRef]
- Kang, J.-W.; Cho, H.-J.; Lee, H.J.; Jin, H.-E.; Maeng, H.-J. Polyethylene glycol-decorated doxorubicin/carboxymethyl chitosan/gold nanocomplex for reducing drug efflux in cancer cells and extending circulation in blood stream. Int. J. Boil. Macromol. 2019, 125, 61–71. [Google Scholar] [CrossRef]
- Irvine, J.D.; Takahashi, L.; Lockhart, K.; Cheong, J.; Tolan, J.W.; Selick, H.E.; Grove, J.R. MDCK (Madin-Darby Canine Kidney) Cells: A Tool for Membrane Permeability Screening. J. Pharm. Sci. 1999, 88, 28–33. [Google Scholar] [CrossRef]
- Merino, G.; Van Herwaarden, A.E.; Wagenaar, E.; Jonker, J.W.; Schinkel, A.H. Sex-Dependent Expression and Activity of the ATP-Binding Cassette Transporter Breast Cancer Resistance Protein (BCRP/ABCG2) in Liver. Mol. Pharmacol. 2005, 67, 1765–1771. [Google Scholar] [CrossRef]
- Tanaka, Y.; Slitt, A.L.; Leazer, T.M.; Maher, J.M.; Klaassen, C.D. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem. Biophys. Res. Commun. 2004, 326, 181–187. [Google Scholar] [CrossRef]
- Yung-Chi, C.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef]
- To, K.K.; Robey, R.; Zhan, Z.; Bangiolo, L.; Bates, S.E. Upregulation of ABCG2 by romidepsin via the aryl hydrocarbon receptor pathway. Mol. Cancer Res. 2011, 9, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Ikai, A.; Watanabe, M.; Sowa, Y.; Kishimoto, M.; Yanagisawa, A.; Fujiwara, H.; Otsuji, E.; Sakai, A.T. Phosphorylated retinoblastoma protein is a potential predictive marker of irinotecan efficacy for colorectal cancer. Int. J. Oncol. 2016, 48, 1297–1304. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lepist, E.-I.; Phan, T.K.; Roy, A.; Tong, L.; MacLennan, K.; Murray, B.; Ray, A.S. Cobicistat Boosts the Intestinal Absorption of Transport Substrates, Including HIV Protease Inhibitors and GS-7340, In Vitro. Antimicrob. Agents Chemother. 2012, 56, 5409–5413. [Google Scholar] [CrossRef]
- FDA. In Vitro Metabolism and Transporter Mediated Drug-Drug Interaction Studies: Guidance for Industry; US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). 2017. Available online: https://www.fda.gov/media/108130/download (accessed on 24 October 2017).
- Zhou, L.; Schmidt, K.; Nelson, F.R.; Zelesky, V.; Troutman, M.D.; Feng, B. The effect of breast cancer resistance protein (Bcrp) and P-glycoprotein (Mdr1a/1b) on the brain penetration of flavopiridol, Gleevec, prazosin and PF-407288 in mice. Drug Metab. Dispos. 2009, 37, 946–955. [Google Scholar] [CrossRef]
- Cooray, H.C.; Janvilisri, T.; Van Veen, H.W.; Hladky, S.B.; A Barrand, M. Interaction of the breast cancer resistance protein with plant polyphenols. Biochem. Biophys. Res. Commun. 2004, 317, 269–275. [Google Scholar] [CrossRef]
- Tachibana, T.; Kato, M.; Watanabe, T.; Mitsui, T.; Sugiyama, Y. Method for predicting the risk of drug–drug interactions involving inhibition of intestinal CYP3A4 and P-glycoprotein. Xenobiotica 2009, 39, 430–443. [Google Scholar] [CrossRef]
- Khaled, K.A.; El-Sayed, Y.M.; Al-Hadiya, B.M. Disposition of the Flavonoid Quercetin in Rats After Single Intravenous and Oral Doses. Drug Dev. Ind. Pharm. 2003, 29, 397–403. [Google Scholar] [CrossRef]
- Murota, K.; Terao, J. Antioxidative flavonoid quercetin: Implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003, 417, 12–17. [Google Scholar] [CrossRef]
- Radominska-Pandya, A.; Little, J.M.; Pandya, J.T.; Tephly, T.R.; King, C.D.; Barone, G.W.; Raufman, J.-P. UDP-glucuronosyltransferases in human intestinal mucosa. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1998, 1394, 199–208. [Google Scholar] [CrossRef]
- Crespy, V.; Morand, C.; Manach, C.; Besson, C.; Demigne, C.; Remesy, C. Part of quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal lumen. Am. J. Physiol. Content 1999, 277, G120–G126. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, O.Q.P.; Zuo, Z.; Chow, M.S. Pharmacokinetics and Modeling of Quercetin and Metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Tomaru, A.; Morimoto, N.; Morishita, M.; Takayama, K.; Fujita, T.; Maeda, K.; Kusuhara, H.; Sugiyama, Y. Studies on the intestinal absorption characteristics of sulfasalazine, a breast cancer resistance protein (BCRP) substrate. Drug Metab. Pharmacokinet. 2012, 28, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Mandery, K.; Bujok, K.; Schmidt, I.; Keiser, M.; Siegmund, W.; Balk, B.; Koenig, J.; Fromm, M.F.; Glaeser, H. Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem. Pharmacol. 2010, 80, 1746–1753. [Google Scholar] [CrossRef]

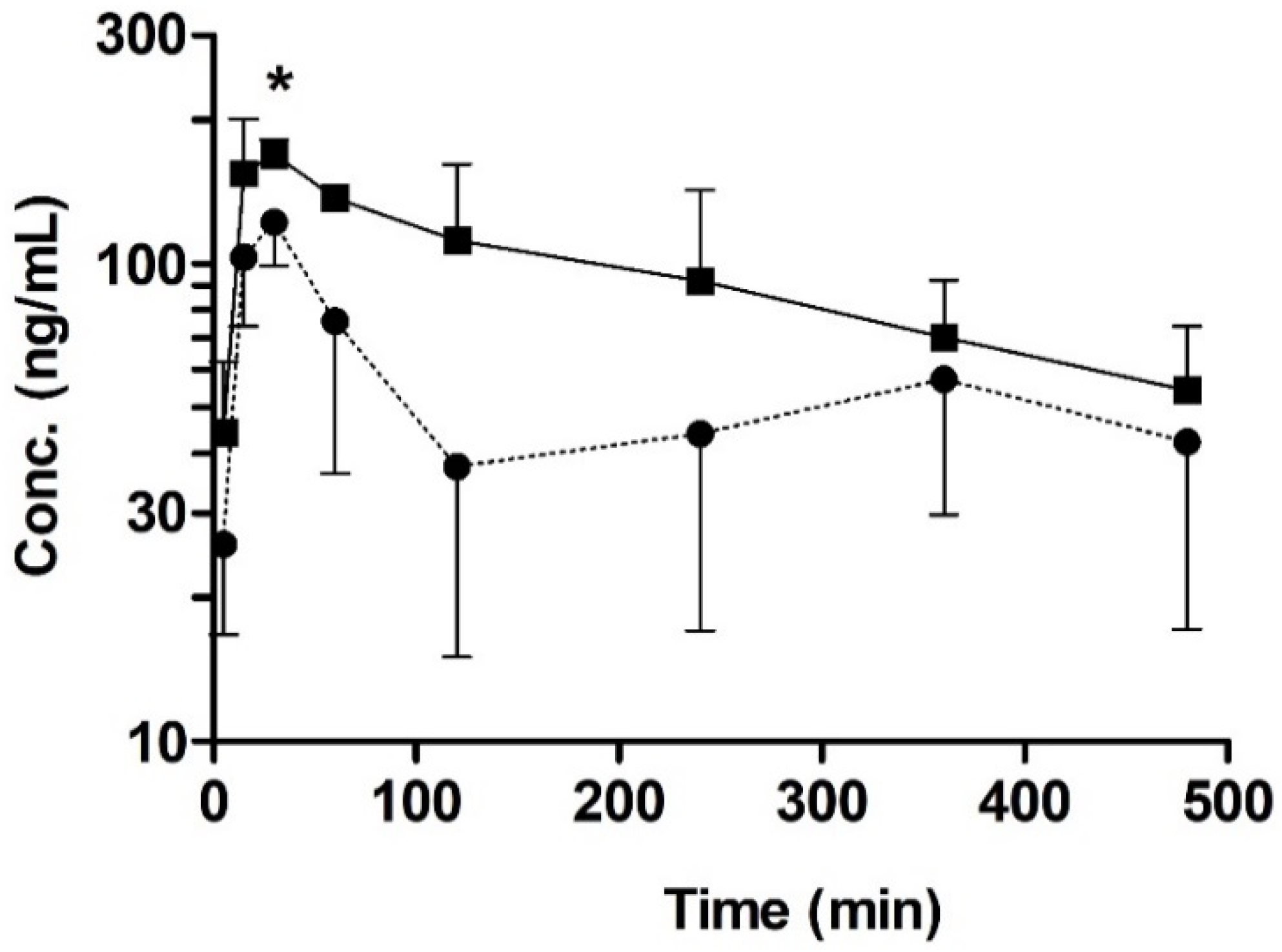
| Parameter | Control | Pre-Administration Group (10 mg/kg Quercetin) |
|---|---|---|
| t1/2 (min) | 383 ± 111 | 242 ± 80.7 |
| tmax (min) | 30 ± 0 | 22.5 ± 8.70 |
| Cmax (ng/mL) | 122 ± 23.2 | 179 ± 23.0 * |
| AUC8h(min∙ng/mL) | 25700 ± 9980 | 44500 ± 11800 |
| CL/F (mL/min/kg) | 52.5 ± 33.5 | 33.2 ± 10.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-K.; Yoon, J.-H.; Woo, J.K.; Kang, J.-H.; Lee, K.-R.; Oh, S.H.; Chung, S.-J.; Maeng, H.-J. Quercetin Is a Flavonoid Breast Cancer Resistance Protein Inhibitor with an Impact on the Oral Pharmacokinetics of Sulfasalazine in Rats. Pharmaceutics 2020, 12, 397. https://doi.org/10.3390/pharmaceutics12050397
Song Y-K, Yoon J-H, Woo JK, Kang J-H, Lee K-R, Oh SH, Chung S-J, Maeng H-J. Quercetin Is a Flavonoid Breast Cancer Resistance Protein Inhibitor with an Impact on the Oral Pharmacokinetics of Sulfasalazine in Rats. Pharmaceutics. 2020; 12(5):397. https://doi.org/10.3390/pharmaceutics12050397
Chicago/Turabian StyleSong, Yoo-Kyung, Jin-Ha Yoon, Jong Kyu Woo, Ju-Hee Kang, Kyeong-Ryoon Lee, Seung Hyun Oh, Suk-Jae Chung, and Han-Joo Maeng. 2020. "Quercetin Is a Flavonoid Breast Cancer Resistance Protein Inhibitor with an Impact on the Oral Pharmacokinetics of Sulfasalazine in Rats" Pharmaceutics 12, no. 5: 397. https://doi.org/10.3390/pharmaceutics12050397
APA StyleSong, Y.-K., Yoon, J.-H., Woo, J. K., Kang, J.-H., Lee, K.-R., Oh, S. H., Chung, S.-J., & Maeng, H.-J. (2020). Quercetin Is a Flavonoid Breast Cancer Resistance Protein Inhibitor with an Impact on the Oral Pharmacokinetics of Sulfasalazine in Rats. Pharmaceutics, 12(5), 397. https://doi.org/10.3390/pharmaceutics12050397