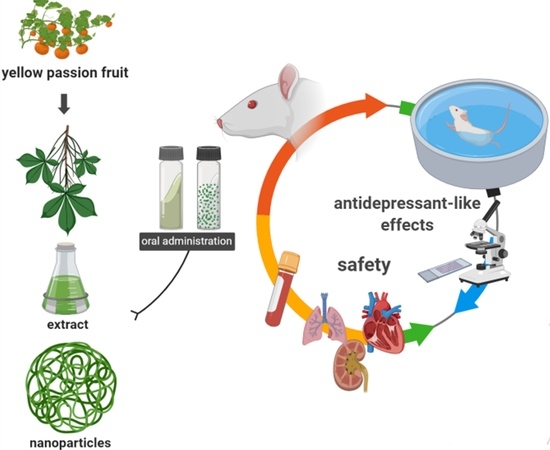
In Vivo Antidepressant Effect of Passiflora edulis f. flavicarpa into Cationic Nanoparticles: Improving Bioactivity and Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Leaf Extract
2.3. Standardization of Leaf Extract by LC-QqQ-MS/MS
2.4. Preparation of Extract-Loaded Nanoparticles
2.5. Characterization of Extract-Loaded Nanoparticles
2.5.1. Particle Size and Zeta Potential Measurements
2.5.2. Extract-Loading Efficiency
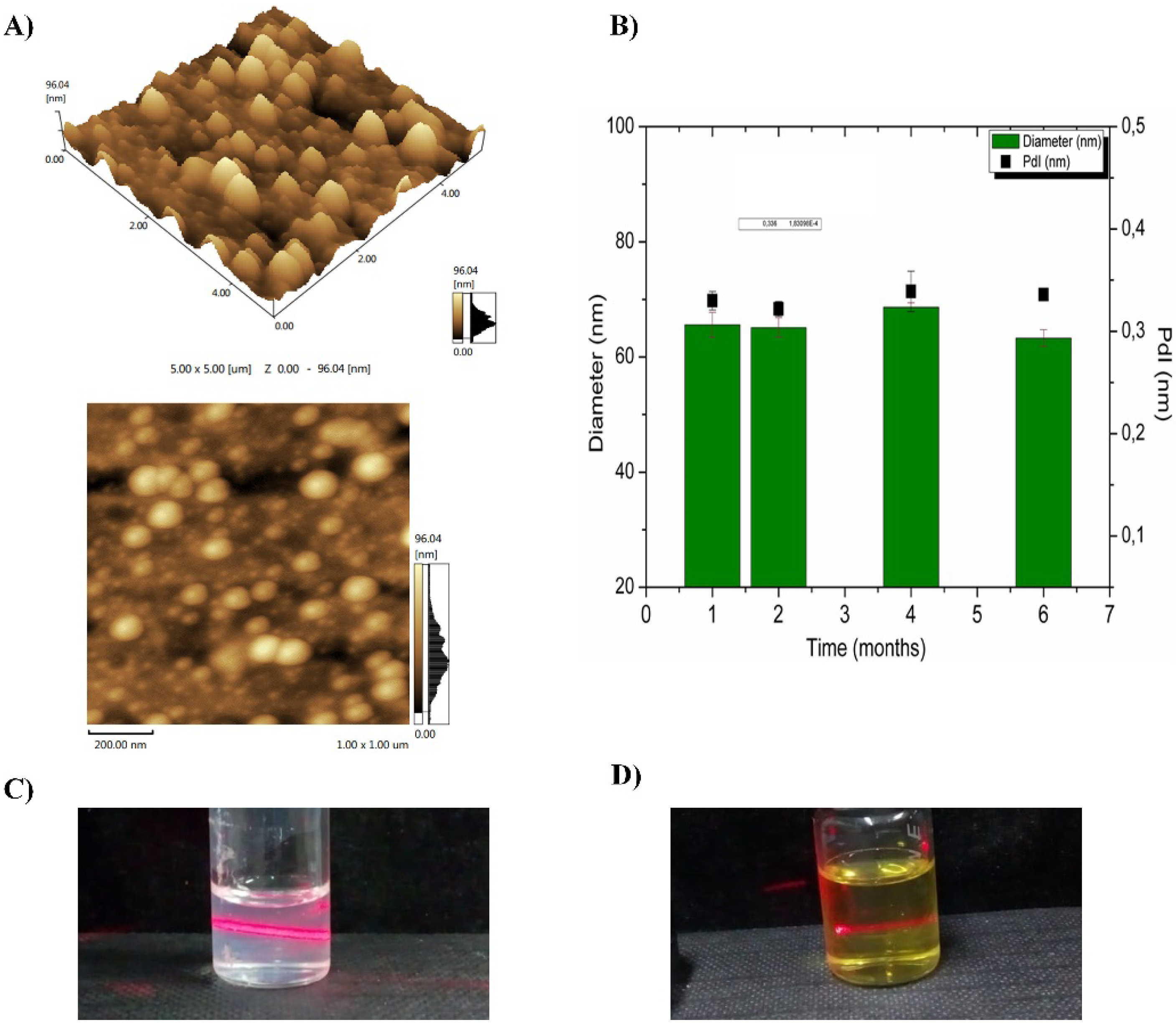
2.5.3. Atomic Force Microscopy (AFM)
2.5.4. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR)
2.5.5. Physicochemical Stability
2.6. In Vivo Experimental Procedures
2.6.1. Animals
2.6.2. In Vivo Biocompatibility of EP, NPB, and NPEP
2.6.3. Drug Treatment for Behavioral Assays
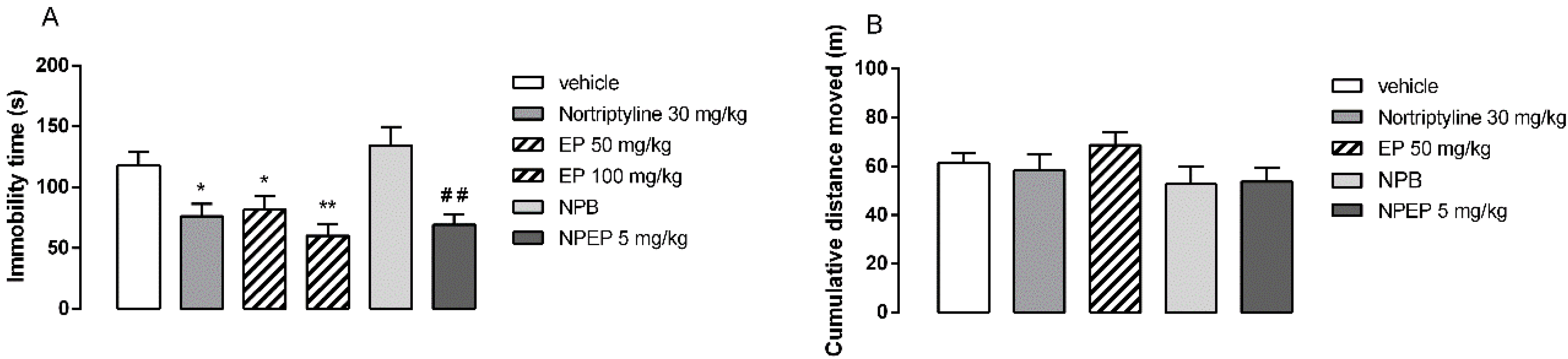
2.6.4. Forced Swim Test (FST)
2.6.5. Open Field Test
2.6.6. Statistical
3. Results and Discussion
3.1. Extract Standardization by LC-QqQ-MS/MS
3.2. Preparation and Characterization of Extract-Loaded Nanoparticles (NPEP)
3.2.1. Particle Size, Zeta Potential, and Encapsulation Efficiency
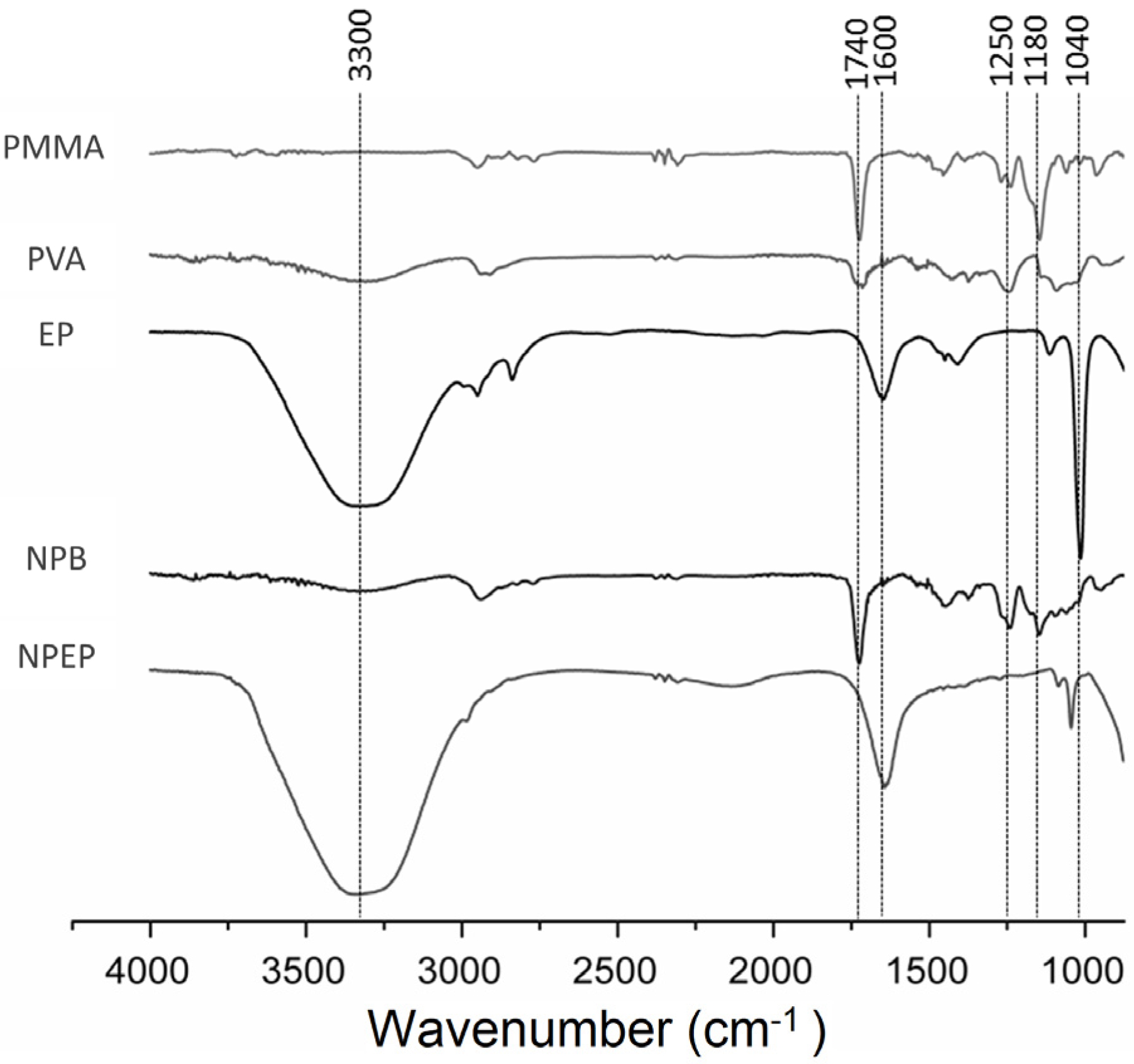
3.2.2. Attenuated Total Reflectance Fourier Transform Infrared (ATR-FTIR)
3.2.3. Atomic Force Microscopy (AFM)
3.2.4. Physicochemical Stability
3.3. In Vivo Biocompatibility Study
3.4. In Vivo Behavioral Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. The Global Burden of Disease: 2004 Update; WHO Document Production Services: Geneva, Switzerland, 2017; p. 24. [Google Scholar]
- Ayres, A.S.F.S.J.; Santos, W.B.; Junqueira-Ayres, D.D.; Costa, G.M.; Ramos, F.A.; Castellanos, L.; Alves, J.S.F.; Asth, L.; Medeiros, I.U.; Zucolotto, S.M.; et al. Monoaminergic neurotransmission is mediating the antidepressant-like effects of Passiflora edulis Sims fo. edulis. Neurosci. Lett. 2017, 660, 79–85. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, N.; Ren, J.; Wu, Y.; Wang, S.; Huang, X.; Yu, Y. Orientin improves depression-like behavior and BDNF in chronic stressed mice. Mol. Nutr. Food Res. 2015, 59, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- OECD Indicators. Health at a Glance; OECD Publishing: Paris, France, 2017; p. 191. [Google Scholar]
- Ortmann, C.F.; Réus, G.Z.; Ignácio, Z.M.; Abelaira, H.M.; Titus, S.E.; Carvalho, P.; Arent, C.O.; Santos, M.A.B.; Matias, B.I.; Martins, M.M.; et al. Enriched Flavonoid Fraction from Cecropia pachystachya Trécul Leaves Exerts Antidepressant-like Behavior and Protects Brain Against Oxidative Stress in Rats Subjected to Chronic Mild Stress. Neurotox. Res. 2016, 29, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, C.F.; Abelaira, H.M.; Réus, G.Z.; Ignácio, Z.M.; Chaves, V.C.; Santos, T.C.; Carvalho, P.; Carlessi, A.S.; Bruchchen, L.; Danielski, L.G.; et al. LC/QTOF profile and preliminary stability studies of an enriched flavonoid fraction of Cecropia pachystachya Trécul leaves with potential antidepressant-like activity. Biomed. Chromatogr. 2017, 31, 1–13. [Google Scholar] [CrossRef]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biotransformations and biological activities of hop flavonoids. Biotechnol. Adv. 2014, 33, 1063–1090. [Google Scholar] [CrossRef] [PubMed]
- Gugulothu, D.; Kulkarni, A.; Patravale, V.; Dandekar, P. PH-sensitive nanoparticles of curcumin-celecoxib combination: Evaluating drug synergy in ulcerative colitis model. J. Pharm. Sci. 2014, 103, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Nanoparticle mediated brain targeted delivery of gallic acid: In vivo behavioral and biochemical studies for improved antioxidant and antidepressant-like activity. Drug Deliv. 2012, 19, 378–391. [Google Scholar] [CrossRef]
- Farag, M.A.; Otify, A.; Porzel, A.; Michel, C.G.; Elsayed, A.; Wessjohann, L.A. Comparative metabolite profiling and fingerprinting of genus Passiflora leaves using a multiplex approach of UPLC-MS and NMR analyzed by chemometric tools. Anal. Bioanal. Chem. 2016, 408, 3125–3143. [Google Scholar] [CrossRef]
- Ayres, A.S.F.S.J.; De Araújo, L.L.S.; Soares, T.C.; Costa, G.M.; Reginatto, F.H.; Ramos, F.A.; Castellanos, L.; Schenkel, E.P.; Soares-Rachetti, V.P.; Zucolotto, S.M.; et al. Comparative central effects of the aqueous leaf extract of two populations of Passiflora edulis. Braz. J. Pharm. 2015, 25, 499–505. [Google Scholar] [CrossRef]
- Sena, L.M.; Zucolotto, S.M.; Reginatto, F.H.; Schenkel, E.P.; De Lima, T.C.M. Neuropharmacological activity of the pericarp of Passiflora edulis flavicarpa Degener: Putative involvement of C-glycosylflavonoids. Exp. Biol. Med. (Maywood) 2009, 234, 967–975. [Google Scholar] [CrossRef]
- Zucolotto, S.M.; Goulart, S.; Montanher, A.B.; Reginatto, F.H.; Schenkel, E.P.; Fröde, T.S. Bioassay-guided isolation of anti-inflammatory C-glucosylflavones from Passiflora edulis. Planta Med. 2009, 75, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Otify, A.; George, C.; Elsayed, A.; Farag, M.A. Function Mechanistic evidence of Passiflora edulis (Passifloraceae) anxiolytic activity in relation to its metabolite fingerprint as revealed via LC-MS and. Food Funct. 2015, 6, 3807–3817. [Google Scholar] [CrossRef]
- Mohamed, E.I.; Zaki, M.A.; Chaurasiya, N.D.; Owis, A.I.; AbouZid, S.; Wang, Y.; Avula, B.; Seida, A.A.; Tekwani, B.L. Monoamine oxidases inhibitors from Colvillea racemosa; Isolation, biological evaluation, and computational study. Fitoterapia 2019, 8, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Bello, O.M.; Ogbesejana, A.B.; Adetunji, C.O.; Oguntoye, S.O. Flavonoids isolated from Vitex grandfolia: An underutilized vegetable, exert monoamine A & B inhibitory and anti-inflammatory effects and structure activity relationship (SAR). Turk. J. Pharm. Sci. 2019, 16. [Google Scholar] [CrossRef]
- Can, Ö.D.; Özkay, Ü.D.; Üçel, U.I. Anti-depressant-like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur. J. Pharmacol. 2013, 699, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Solanki, P.; Mitra, S. Curcuminoid-loaded poly (methyl methacrylate) nanoparticles for cancer therapy. Int. J. Nanomed. 2017, 114, 101–105. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, N. Nanocarriers for the delivery of active ingredients and fractions extracted from natural products used in traditional Chinese medicine (TCM). Adv. Colloid Interface Sci. 2015, 221, 60–76. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, Y.-A.; Kannan, S.; Kannan, R.M. Targeting specific cells in the brain with nanomedicines for CNS therapies. J. Control. Release 2016, 240, 212–226. [Google Scholar] [CrossRef]
- Papa, S.; Ferrari, R.; De Paola, M.; Rossi, F.; Mariani, A.; Caron, I.; Sammali, E.; Peviani, M.; Dell’Oro, V.; Colombo, C.; et al. Polymeric nanoparticle system to target activated microglia/macrophages in spinal cord injury. J. Control. Release 2014, 174, 15–26. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar]
- Mendes, A.N.; Filgueiras, L.A.; Siqueira, M.R.P.; Barbosa, G.M.; Holandino, C.; Moreira, D.L.; Pinto, J.C.; Nele, M. Encapsulation of Piper cabralanum (Piperaceae) nonpolar extract in poly (methyl methacrylate) by miniemulsion and evaluation of increase in the effectiveness of antileukemic activity in K562 cells. Int. J. Nanomedicine 2017, 12, 8363–8373. [Google Scholar] [CrossRef] [PubMed]
- Dalcin, A.J.F.; Vizzotto, B.S.; Bochi, G.V.; Guarda, N.S.; Nascimento, K.; Sagrillo, M.R.; Moresco, R.N.; Schuch, A.P.; Ourique, A.F.; Gomes, P. Nanoencapsulation of the flavonoid dihydromyricetin protects against the genotoxicity and cytotoxicity induced by cationic nanocapsules. Colloids Surf. B Biointerfaces 2019, 173, 798–805. [Google Scholar] [CrossRef]
- Santos-Silva, A.M.; Caland, L.B.; Oliveira, A.L.C.S.L.; Araújo-Júnior, R.F.; Fernandes-Pedrosa, M.F.; Cornélio, A.M.; Silva-Júnior, A.A. Designing structural features of novel benznidazole-loaded cationic nanoparticles for inducing slow drug release and improvement of biological efficacy. Mater. Sci. Eng. C 2017, 78, 978–987. [Google Scholar]
- Santos-Silva, A.M.; Caland, L.B.; Nascimento, E.G.; Oliveira, A.L.C.S.L.; Araújo-Júnior, R.F.; Cornélio, A.M.; Fernandes-Pedrosa, M.F.; Silva-Júnior, A.A. Self-Assembled Benznidazole-Loaded Cationic Nanoparticles Containing Cholesterol/Sialic Acid: Physicochemical Properties, In Vitro Drug Release and In Vitro Anticancer Efficacy. Int. J. Mol. Sci. 2019, 20, 2350. [Google Scholar] [CrossRef]
- El-Nahas, A.E.; Allam, A.N.; El-Kamel, A.H. Mucoadhesive buccal tablets containing silymarin Eudragit-loaded nanoparticles: Formulation, characterisation and ex vivo permeation. J. Microencapsul. 2017, 34, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Gurushankar, K.; Gohulkumar, M.; Rajendra Prasad, N.; Krishnakumar, N. Synthesis, characterization and in vitro anti-cancer evaluation of hesperetin-loaded nanoparticles in human oral carcinoma (KB) cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 015006. [Google Scholar] [CrossRef]
- Puhl, A.C.; Fagundes, M.; Santos, K.C.; Polikarpov, I.; Silva, M.F.G.F.; Fernandes, J.B.; Vieira, P.C.; Forim, M.R. Preparation and characterization of polymeric nanoparticles loaded with the flavonoid luteolin, by using factorial design. Int. J. Drug Deliv. 2011, 3, 683–698. [Google Scholar]
- Gohulkumar, M.; Gurushankar, K.; Prasad, N.R.; Krishnakumar, N. Enhanced cytotoxicity and apoptosis-induced anticancer effect of silibinin-loaded nanoparticles in oral carcinoma (KB) cells. Mater. Sci. Eng. C 2014, 41, 274–282. [Google Scholar] [CrossRef]
- Wang, C.; Xu, F.; Shang, J.; Xiao, H.; Fan, W.; Dong, F.; Hu, J.; Zhou, J. Cycloartane triterpenoid saponins from water soluble of Passiflora edulis Sims and their antidepressant-like effects. J. Ethnopharmacol. 2013, 148, 812–817. [Google Scholar] [CrossRef]
- Křen, V.; Kubisch, J.; Sedmera, P.; Halada, P.; Přikrylová, V.; Jegorov, A.; Cvak, L.; Gebhardt, R.; Ulrichová, J.; Simánek, V. Glycosylation of silybin. J. Chem. Soc. Perkin Trans. 1997, 1, 2467–2474. [Google Scholar] [CrossRef]
- ICH. Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology, Q2 (R1), ICH, Geneva, Switzerland. 2005. Available online: https://pacificbiolabs.com/wp-content/uploads/2017/12/Q2_R1__Guideline-4.pdf (accessed on 10 June 2019).
- Moreira, E.A.; Pilon, A.C.; Andrade, L.E.; Lopes, N.P. New Perspectives on Chlorogenic Acid Accumulation in Harvested Leaf Tissue: Impact on Traditional Medicine Preparations. ACS Omega 2018, 3, 18380–18386. [Google Scholar] [CrossRef]
- ANVISA. Resolução da Diretoria Colegiada—RDC No. 166, de 25 de Julho de 2017 sobre a validação de métodos analíticos e outras providências. Diário da União 2017, 141, 87. [Google Scholar]
- Muniswamy, V.J.; Raval, N.; Gondaliya, P.; Tambe, V.; Kalia, K.; Tekade, R.K. ‘Dendrimer-Cationized-Albumin’ encrusted polymeric nanoparticle improves BBB penetration and anticancer activity of doxorubicin. Int. J. Pharm. 2019, 555, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Chetoni, P.; Burgalassi, S.; Monti, D.; Tampucci, S.; Tullio, V.; Cuffini, A.M.; Muntoni, E.; Spagnolo, R.; Zara, G.P.; Cavalli, R. Solid lipid nanoparticles as promising tool for intraocular tobramycin delivery: Pharmacokinetic studies on rabbits. Eur. J. Pharm. Biopharm. 2016, 109, 214–223. [Google Scholar] [CrossRef] [PubMed]
- OECD, Organization for Economic Co-operation and Development. Guidelines for Testing of Chemicals. Acute Oral Toxicity. Acute Toxic Class Method. Guide No. 423. 2008, pp. 1–14. Available online: http://www.oecd.org (accessed on 29 November 2018).
- McGrath, J.; Drummond, G.; McLachlan, E.; Kilkenny, C.; Wainwright, C. Editorial: Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef] [PubMed]
- Porsolt, R.; Pichon, M.L.; Jalfre, M. Depression: A new animal model sensitive to antidepressant treatments. Nature 1977, 266, 730–732. [Google Scholar] [CrossRef]
- Shi, Z.; Song, D.; Li, R.; Yang, H.; Qi, L.; Xin, G.; Wang, D.; Song, H.; Chen, J.; Hao, H.; et al. Identification of effective combinatorial markers for quality standardization of herbal medicines. J. Chromatogr. A 2014, 1345, 78–85. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, X.; Liu, E.; Sheng, L.; Qi, L.; Li, P. More accurate matrix-matched quantification using standard superposition method for herbal medicines. J. Chromatogr. A 2012, 1254, 43–50. [Google Scholar] [CrossRef]
- Schubert, S.; Delaney, J.T.; Schubert, U.S. Nanoprecipitation and nanoformulation of polymers: From history to powerful possibilities beyond poly(lactic acid). Soft Matter 2011, 7, 1581–1588. [Google Scholar] [CrossRef]
- Thioune, O.; Fessi, H.; Devissagueta, J.; Puisieux, F. Preparation of pseudolatex by nanoprecipitation: Influence of the solvent nature on intrinsic viscosity and interaction constant. Int. J. Pharm. 1997, 146, 233–238. [Google Scholar] [CrossRef]
- Varan, C.; Bilensoy, E. Cationic PEGylated polycaprolactone nanoparticles carrying post-operation docetaxel for glioma treatment. Beilstein J. Nanotechnol. 2017, 8, 1446–1456. [Google Scholar] [CrossRef] [PubMed]
- Margulis, K.; Magdassi, S.; Lee, H.S.; Macosko, C.W. Formation of curcumin nanoparticles by flash nanoprecipitation from emulsions. J. Colloid Interface Sci. 2014, 434, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Pasqualini, R.; Arap, W.; Ferrari, M. Intravascular delivery of particulate systems: Does geometry really matter? Pharm. Res. 2009, 26, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Fan, S.; Zheng, Y.; Liu, X.; Fang, W.; Chen, X.; Liao, W.; Jing, X.; Lei, M.; Tao, E.; Ma, Q.; et al. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018, 25, 1091–1102. [Google Scholar] [CrossRef]
- Chorny, M.; Fishbein, I.; Danenberg, H.D.; Golomb, G. Lipophilic drug loaded nanospheres prepared by nanoprecipitation: Effect of formulation variables on size, drug recovery and release kinetics. J. Control. Release 2002, 83, 389–400. [Google Scholar] [CrossRef]
- Venishetty, V.K.; Chede, R.; Komuravelli, R.; Adepu, L.; Sistla, R.; Diwan, P.V. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: A novel strategy to avoid intraduodenal administration. Colloids Surf. B Biointerfaces 2012, 95, 1–9. [Google Scholar] [CrossRef]
- Chow, S.F.; Wan, K.Y.; Cheng, K.K.; Wong, K.W.; Sun, C.C.; Baum, L.; Chow, A.H.L. Development of highly stabilized curcumin nanoparticles by flash nanoprecipitation and lyophilization. Eur. J. Pharm. Biopharm. 2015, 94, 436–449. [Google Scholar] [CrossRef]
- Wu, T.; Yen, F.; Lin, L.; Tsai, T.; Lin, C.; Cham, T. Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int. J. Pharm. 2008, 346, 160–168. [Google Scholar] [CrossRef]
- Dadashpour, M.; Firouzi-Amandi, A.; Pourhassan-Moghaddam, M.; Maleki, M.J.; Soozangar, N.; Jeddi, F.; Nouri, M.; Zarghami, N.; Pilehvar-Soltanahmadi, Y. Biomimetic synthesis of silver nanoparticles using Matricaria chamomilla extract and their potential anticancer activity against human lung cancer cells. Mater. Sci. Eng. C 2018, 92, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Devaki, K.; Beulah, U.; Akila, G.; Gopalakrishnan, V. Effect of aqueous extract of Passiflora edulis on biochemical and hematological parameters of Wistar albino rats. Toxicol. Int. 2012, 19, 63. [Google Scholar] [PubMed]
- Amaral, K.M.; Schenkel, E.; Langeloh, A. Avaliação da toxicidade reprodutiva dos extratos aquosos liofilizados de Passiflora alata dryander e Passiflora edulis sims em ratas Wistar. Acta Farm. Bonaer. 2001, 20, 215–220. [Google Scholar]
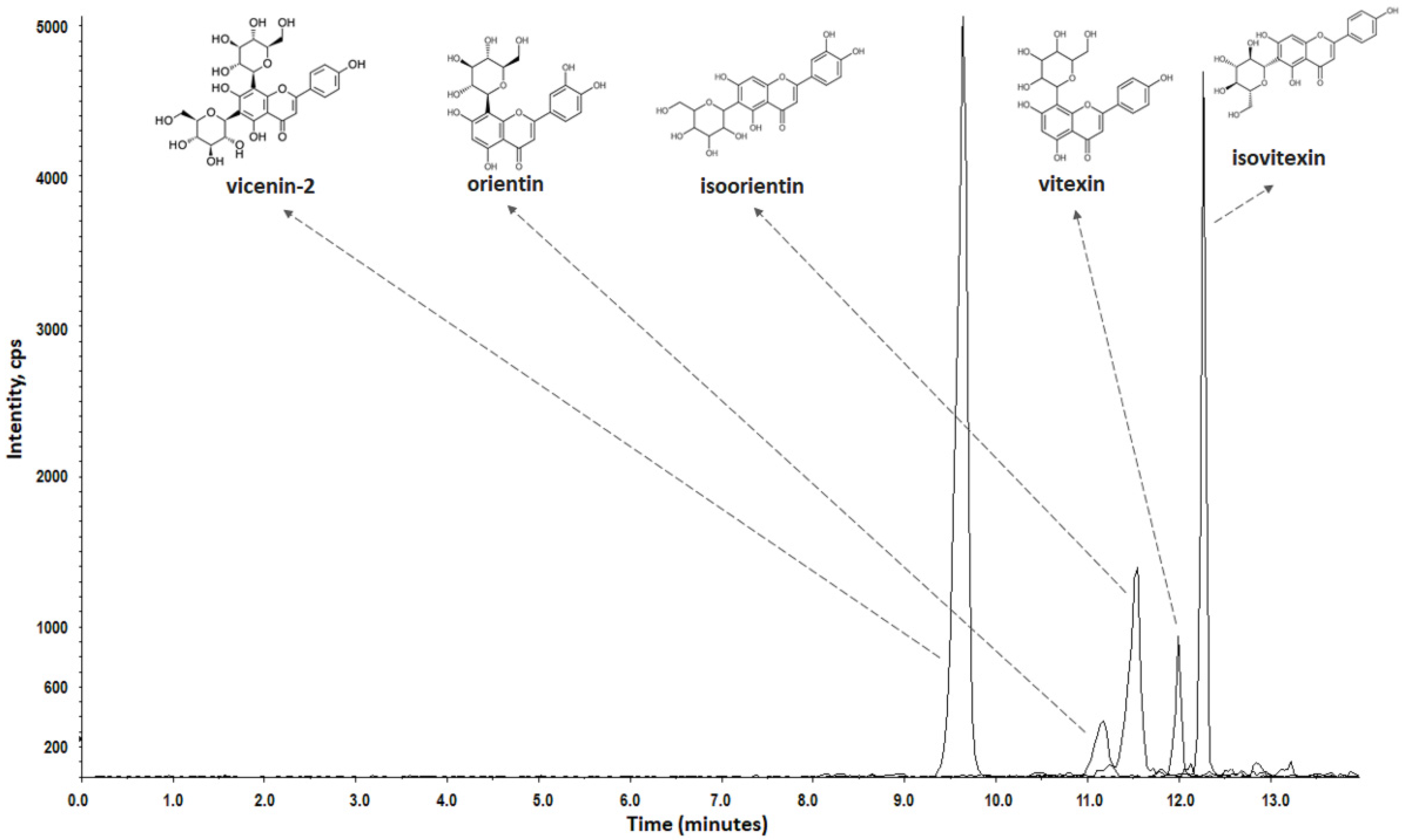
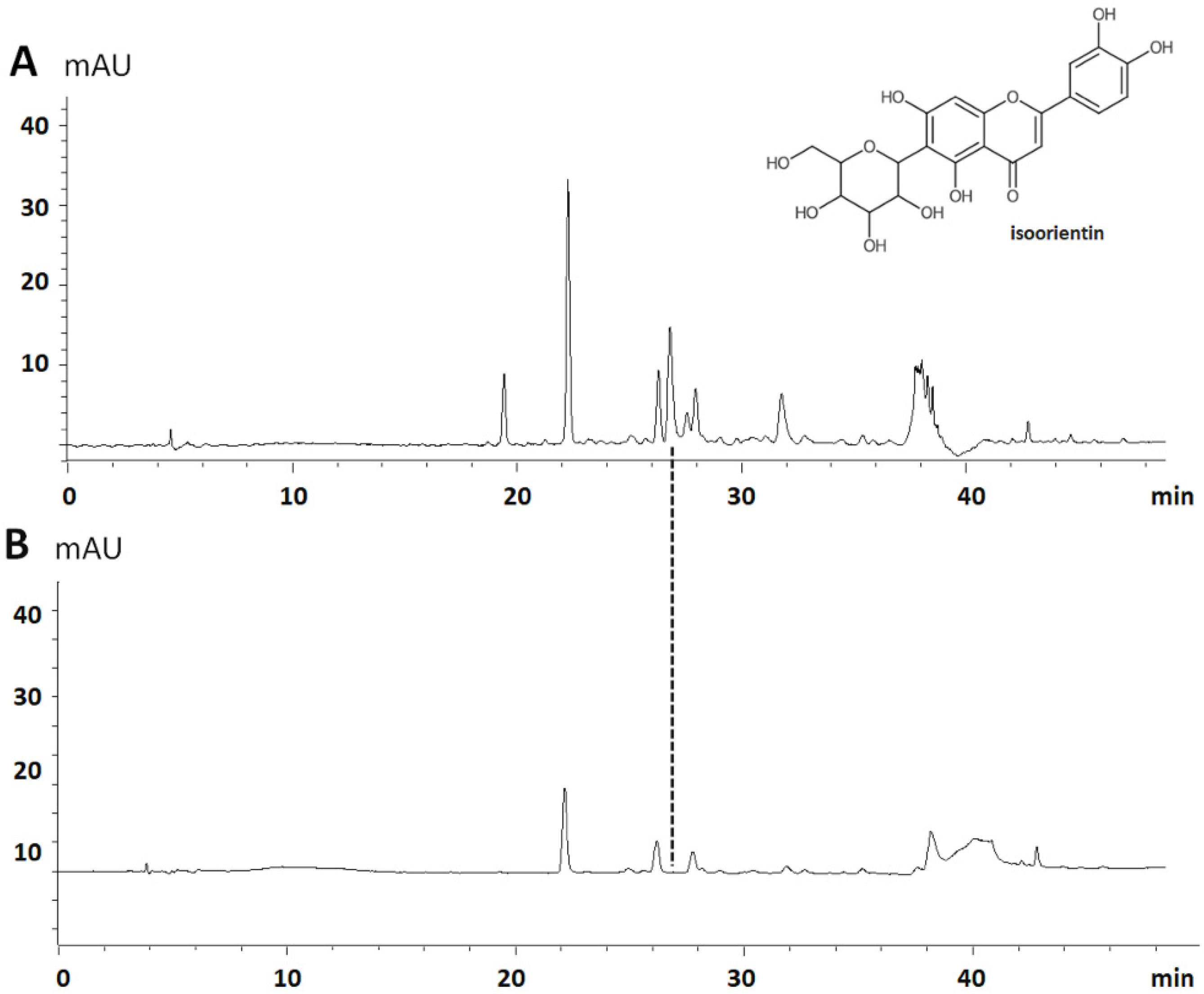

| Compounds | Linearity Range (ng·mL−1) | Calibration Equation | Correlation Factor (r) | LOD (ng·mL−1) | LOQ (ng·mL−1) |
|---|---|---|---|---|---|
| Vicenin-2 | 5–500 | y = 136.2x − 136.2 | 0.9960 | 1.66 | 5.0 |
| Orientin | 5–500 | y = 115.47x − 539.0 | 0.9973 | 1.66 | 5.0 |
| Isoorientin | 5–500 | y = 98.41x − 162.4 | 0.9993 | 1.66 | 5.0 |
| Vitexin | 5–500 | y = 340.0x − 501.0 | 0.9984 | 1.00 | 3.0 |
| Isovitexin | 5–500 | y = 264.94x + 667.0 | 0.9986 | 1.00 | 3.0 |
| Compounds | Concentration | Precision | Accuracy (Recovery) | |
|---|---|---|---|---|
| (ng·mL−1) | RSD (%) | (ng·mL−1) | SRE (%) | |
| Vicenin-2 | 10 | 3.7 | 10.1 | 1.0 |
| 50 | 1.8 | 51.9 | 3.9 | |
| 100 | 3.4 | 101.0 | 1.0 | |
| Orientin | 10 | 3.7 | 10.0 | 3.1 |
| 50 | 1.8 | 52.2 | −1.4 | |
| 100 | 3.4 | 102.1 | 0.5 | |
| Isoorientin | 10 | 3.7 | 10.3 | −1.9 |
| 50 | 1.8 | 49.3 | −0.8 | |
| 100 | 3.4 | 100.5 | 0.2 | |
| Vitexin | 10 | 1.9 | 10.2 | 1.6 |
| 50 | 2.6 | 51.1 | 2.2 | |
| 100 | 3.4 | 103.4 | 3.4 | |
| Isovitexin | 10 | 1.5 | 9.8 | −1.9 |
| 50 | 3.5 | 49.6 | −0.8 | |
| 100 | 2.4 | 100.2 | 0.2 | |
| Extract/Eudragit Ratio | Diameter (nm) ± SD | PdI (nm) ± SD | Zeta Potential (mV) ± SD |
|---|---|---|---|
| 0 | 106.2 ± 1.2 | 0.245 ± 0.03 | +35.7 ± 1.3 |
| 1:10 | 65.6 ± 2.1 | 0.330 ± 0.01 | +38.4 ± 1.2 |
| 1:5 | 90.7 ± 0.5 | 0.305 ± 0.01 | +40.4 ± 2.2 |
| 1:2.5 | 128.8 ± 0.2 | 0.129 ± 0.01 | +37.9 ± k0.4 |
| Analyte | Vehicle | NPB 1 | EP 1 (2 g/kg) | NPEP 1 (5 mg/kg) |
|---|---|---|---|---|
| Liver Function | ||||
| Total protein | 5.37 ± 0.29 | 5.00 ± 0.53 | 5.29 ± 0.43 | 4.79 ± 0.28 |
| Albumin | 2.28 ± 0.11 | 1.97 ± 0.09 | 2.30 ± 0.15 | 1.96 ± 0.05 |
| ALT 2 | 48.14 ± 10.07 | 37.40 ± 1.14 | 47.20 ± 8.50 | 37.4 ± 7.47 |
| AST 2 | 77.00 ± 11.65 | 95.20 ± 11.61 | 83.20 ± 11.20 | 70.20 ± 13.14 |
| Kidney Function | ||||
| Urea | 42.86 ± 10.79 | 38.8 ± 8.5 | 52.60 ± 6.5 | 43.4 ± 10.92 |
| Creatinine | 0.25 ± 0.07 | 0.40 ± 0.12 | 0.23 ± 0.06 | 0.33 ± 0.05 |
| Organs | Vehicle | NPB 1 | EP 1 (2 g/kg) | NPEP 1 (5 mg/kg) |
|---|---|---|---|---|
| Brain | 0.0126 ± 0.0003 | 0.0135 ± 0.0020 | 0.0128 ± 0.0005 | 0.0136 ± 0.0007 |
| Liver | 0.0518 ± 0.0030 | 0.0424 ± 0.0028 | 0.0484 ± 0.0116 | 0.0424 ± 0.0028 |
| Heart | 0.0055 ± 0.0008 | 0.0059 ± 0.0009 | 0.0064 ± 0.0014 | 0.0064 ± 0.0014 |
| Spleen | 0.0038 ± 0.0006 | 0.0044 ± 0.0016 | 0.0042 ± 0.0006 | 0.0041 ± 0.0010 |
| Kidneys | 0.0135 ± 0.0018 | 0.0104 ± 0.0008 | 0.0107 ± 0.0035 | 0.0111 ± 0.0011 |
| Weight variation | 1.02 ± 0.0206 | 1.04 ± 0.0715 | 1.01 ± 0.0154 | 1.01 ± 0.0247 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, J.S.F.; Silva, A.M.d.S.; da Silva, R.M.; Tiago, P.R.F.; de Carvalho, T.G.; de Araújo Júnior, R.F.; de Azevedo, E.P.; Lopes, N.P.; Ferreira, L.D.S.; Gavioli, E.C.; et al. In Vivo Antidepressant Effect of Passiflora edulis f. flavicarpa into Cationic Nanoparticles: Improving Bioactivity and Safety. Pharmaceutics 2020, 12, 383. https://doi.org/10.3390/pharmaceutics12040383
Alves JSF, Silva AMdS, da Silva RM, Tiago PRF, de Carvalho TG, de Araújo Júnior RF, de Azevedo EP, Lopes NP, Ferreira LDS, Gavioli EC, et al. In Vivo Antidepressant Effect of Passiflora edulis f. flavicarpa into Cationic Nanoparticles: Improving Bioactivity and Safety. Pharmaceutics. 2020; 12(4):383. https://doi.org/10.3390/pharmaceutics12040383
Chicago/Turabian StyleAlves, Jovelina Samara Ferreira, Alaine Maria dos Santos Silva, Rodrigo Moreira da Silva, Pamella Rebeca Fernandes Tiago, Thais Gomes de Carvalho, Raimundo Fernandes de Araújo Júnior, Eduardo Pereira de Azevedo, Norberto Peporine Lopes, Leandro De Santis Ferreira, Elaine Cristina Gavioli, and et al. 2020. "In Vivo Antidepressant Effect of Passiflora edulis f. flavicarpa into Cationic Nanoparticles: Improving Bioactivity and Safety" Pharmaceutics 12, no. 4: 383. https://doi.org/10.3390/pharmaceutics12040383
APA StyleAlves, J. S. F., Silva, A. M. d. S., da Silva, R. M., Tiago, P. R. F., de Carvalho, T. G., de Araújo Júnior, R. F., de Azevedo, E. P., Lopes, N. P., Ferreira, L. D. S., Gavioli, E. C., da Silva-Júnior, A. A., & Zucolotto, S. M. (2020). In Vivo Antidepressant Effect of Passiflora edulis f. flavicarpa into Cationic Nanoparticles: Improving Bioactivity and Safety. Pharmaceutics, 12(4), 383. https://doi.org/10.3390/pharmaceutics12040383