Optimal Connection for Tiotropium SMI Delivery through Mechanical Ventilation: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
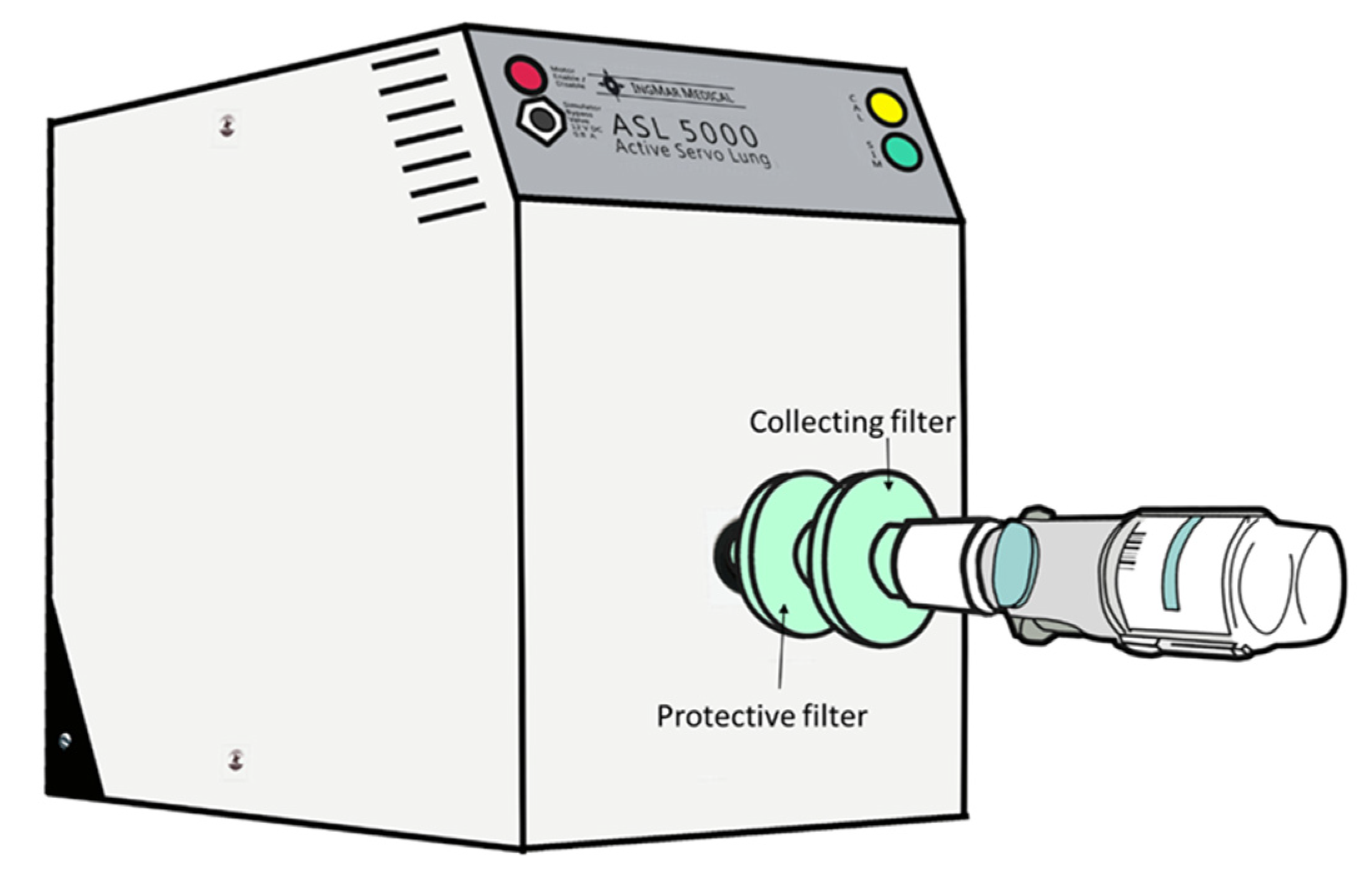
2.1. Lung Model
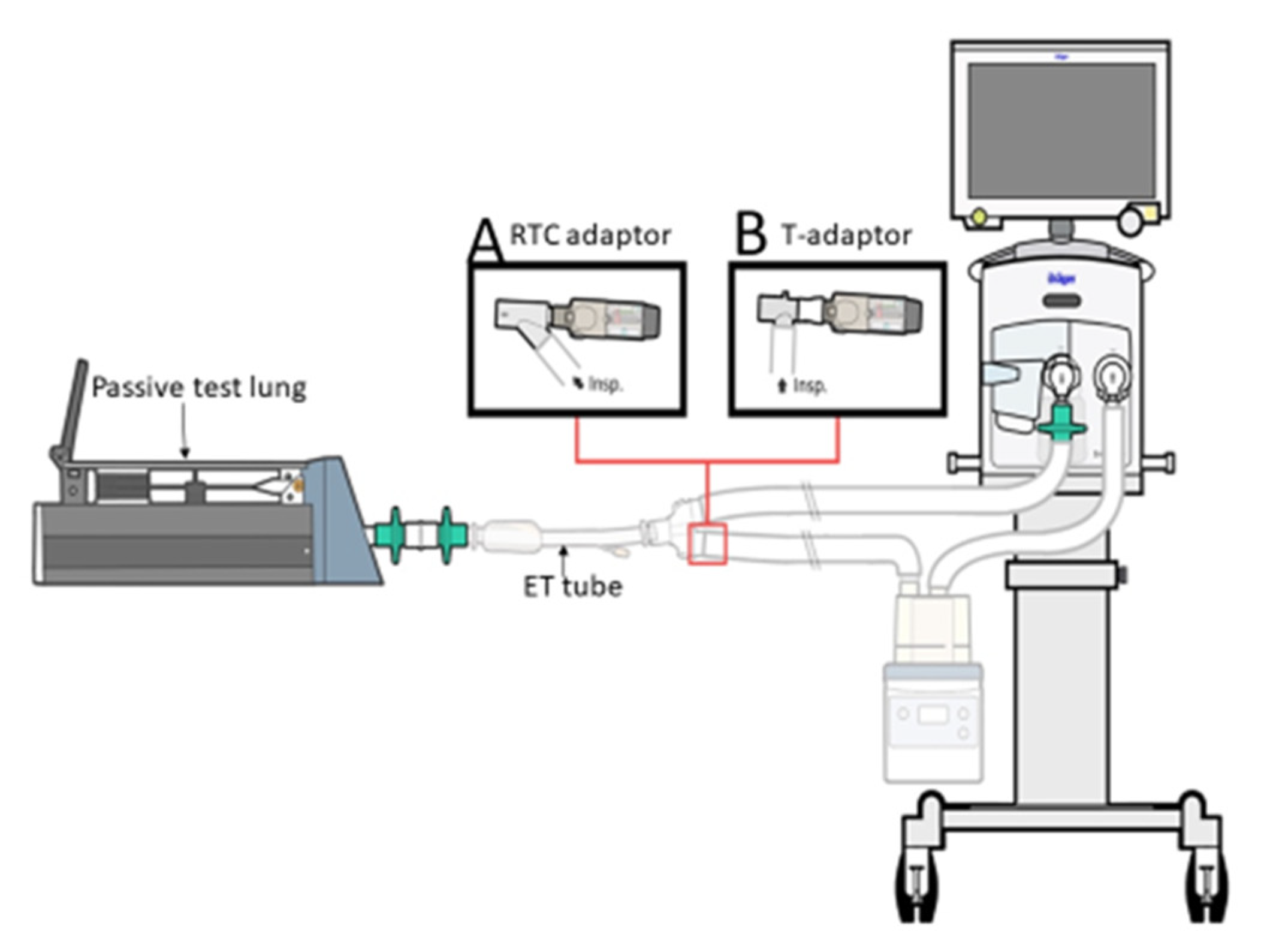
2.2. SMI Delivery in the Ventilator System
2.3. Drug Elution and Analysis of Tiotropium
2.4. Ventilator Parameter Changes
2.5. Statistical Analysis
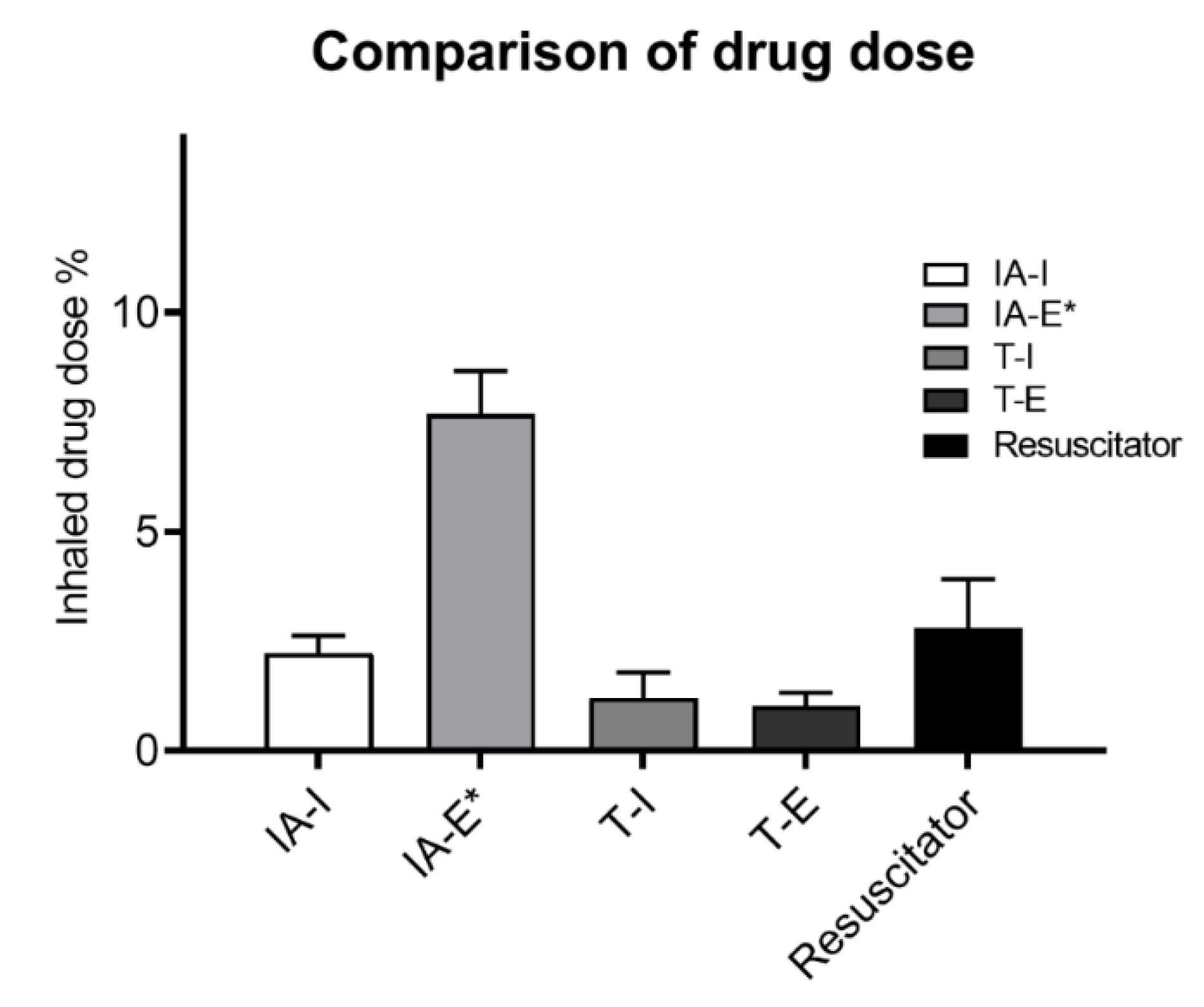
3. Results
4. Discussion
4.1. Influence of Adapter Selection
4.2. Influence of Actuation Timing
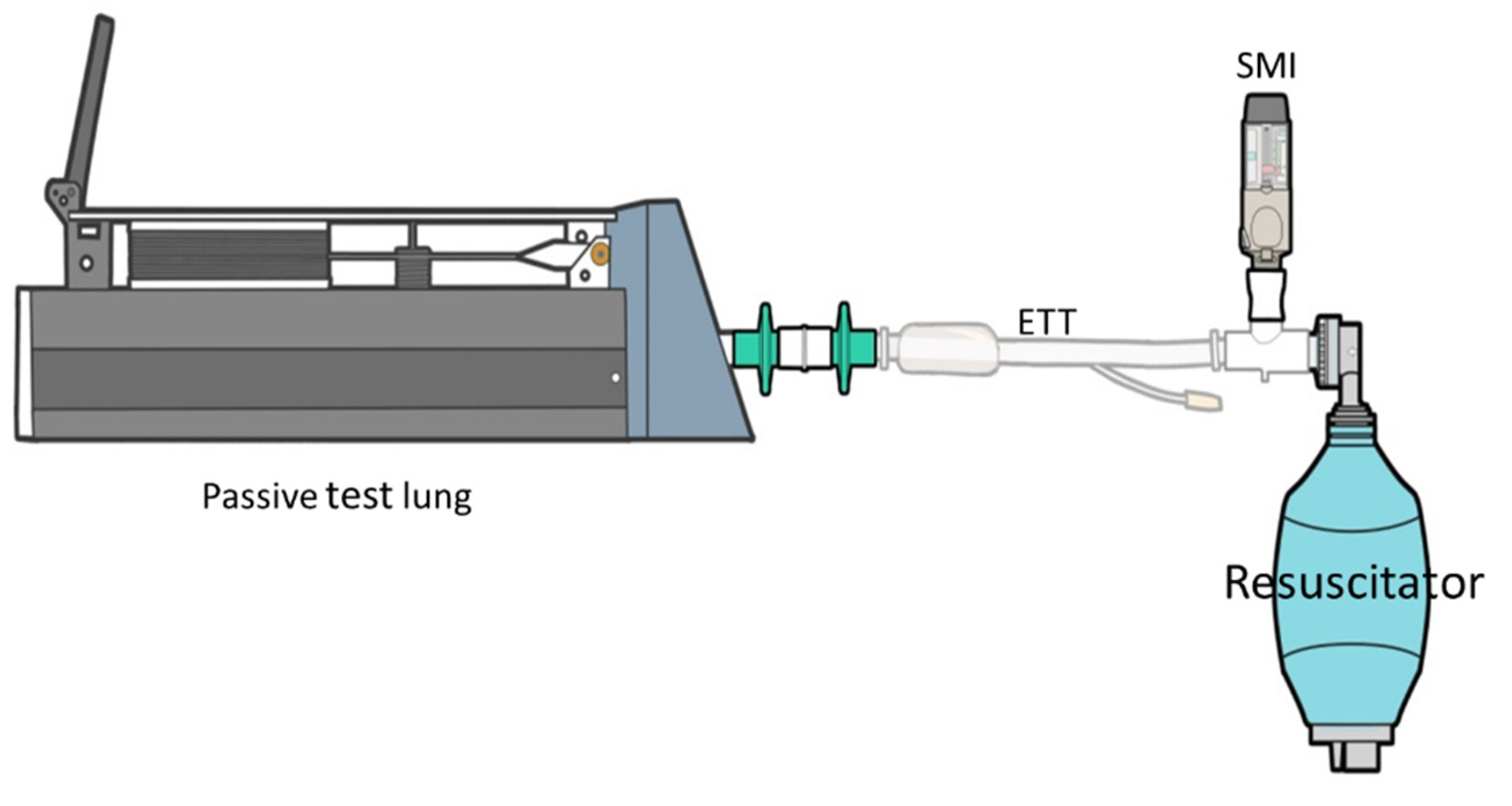
4.3. Manual Resuscitation Bag Delivery Technique
4.4. Clinical Implication
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Usmani, O.S. Treating the small airways. Respiration 2012, 84, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Lavorini, F.; Buttini, F.; Usmani, O.S. 100 Years of Drug Delivery to the Lungs. Handb. Exp. Pharmacol. 2019, 260, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Dalby, R.; Spallek, M.; Voshaar, T. A review of the development of Respimat® Soft Mist™ Inhaler. Int. J. Pharm. 2004, 283, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dhand, R.; Eicher, J.; Hänsel, M.; Jost, I.; Meisenheimer, M.; Wachtel, H. Improving usability and maintaining performance: Human-factor and aerosol-performance studies evaluating the new reusable Respimat inhaler. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Lyseng-Williamson, K.A.; Keating, G.M. Tiotropium Respimat® Soft Mist™ inhaler: A guide to its use in chronic obstructive pulmonary disease (COPD) in the EU. Drugs Ther. Perspect. 2015, 31, 39–44. [Google Scholar] [CrossRef]
- Ari, A. Aerosol Therapy in Pulmonary Critical Care. Respir. Care 2015, 60, 858. [Google Scholar] [CrossRef] [PubMed]
- Dellweg, D.; Wachtel, H.; Höhn, E.; Pieper, M.P.; Barchfeld, T.; Köhler, D.; Glaab, T. In vitro validation of a Respimat® adapter for delivery of inhaled bronchodilators during mechanical ventilation. J. Aerosol. Med. Pulm. Drug Deliv. 2011, 24, 285–292. [Google Scholar] [CrossRef] [PubMed]
- FDA. RTC 26-C In-line Aerosol Adapter. 2017. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf16/K162753.pdf (accessed on 1 March 2019).
- Elkady, E.F.; Tammam, M.H.; Elmaaty, A.A. Development and validation of RP-HPLC method for simultaneous estimation of tiotropium bromide, formoterol fumarate, and olodaterol HCL in bulk and metered dose aerosols: Application to olodaterol HCL forced degradation study and degradation kinetics. Chromatographia 2017, 80, 1749–1760. [Google Scholar] [CrossRef]
- Suggett, J.; Nagel, M. Assessment of a Ventilator Circuit Adapter to Be Used in Conjunction with the Respimat Soft Mist Inhaler (SMI). Available online: https://erj.ersjournals.com/content/50/suppl_61/PA2100 (accessed on 15 July 2019).
- Diot, P.; Morra, L.; Smaldone, G.C. Albuterol delivery in a model of mechanical ventilation. Comparison of metered-dose inhaler and nebulizer efficiency. Am. J. Respir. Crit. Care Med. 1995, 152, 1391–1394. [Google Scholar] [CrossRef] [PubMed]
- Ari, A.; Fink, J.B. Differential medical aerosol device and interface selection in patients during spontaneous, conventional mechanical and noninvasive ventilation. J. Aerosol. Med. Pulm. Drug Deliv. 2016, 29, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.R.; Wang, W.J.; Lin, T.H.; Wu, C.L.; Huang, S.H.; Wu, H.D.; Chen, C.C. In vitro evaluation of aerosol performance and delivery efficiency during mechanical ventilation between soft mist inhaler and pressurized metered-dose inhaler. Respir Care 2020. [Google Scholar] [CrossRef] [PubMed]
- Hochrainer, D.; Hölz, H.; Kreher, C.; Scaffidi, L.; Spallek, M.; Wachtel, H. Comparison of the aerosol velocity and spray duration of Respimat Soft Mist inhaler and pressurized metered dose inhalers. J. Aerosol. Med. 2005, 18, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Berlinski, A.; Ari, A.; Davies, P.; Fink, J.; Majaesic, C.; Reychler, G.; Tatla, T.; Amirav, I. Workshop Report: Aerosol Delivery to Spontaneously Breathing Tracheostomized Patients. J. Aerosol. Med. Pulm. Drug Deliv. 2017, 30, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.D.; Berlinski, A. Survey of aerosol delivery techniques to spontaneously breathing tracheostomized children. Respir. Care 2012, 57, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Dhand, R. How should aerosols be delivered during invasive mechanical ventilation? Respir. Care 2017, 62, 1343–1367. [Google Scholar] [CrossRef] [PubMed]
- Ari, A.; Harwood, R.J.; Sheard, M.M.; Fink, J.B. An in vitro evaluation of aerosol delivery through tracheostomy and endotracheal tubes using different interfaces. Respir. Care 2012, 57, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.P.; Brown, J.; Steed, K.P.; Reader, S.J.; Kladders, H. Lung deposition of fenoterol and flunisolide delivered using a novel device for inhaled medicines: Comparison of RESPIMAT with conventional metered-dose inhalers with and without spacer devices. Chest 1998, 113, 957–963. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, T.-P.; Chen, Y.-J.; Yang, T.-M.; Wang, S.-H.; Hung, M.-S.; Chiu, S.-H.; Li, H.-H.; Fink, J.B.; Lin, H.-L. Optimal Connection for Tiotropium SMI Delivery through Mechanical Ventilation: An In Vitro Study. Pharmaceutics 2020, 12, 291. https://doi.org/10.3390/pharmaceutics12030291
Fang T-P, Chen Y-J, Yang T-M, Wang S-H, Hung M-S, Chiu S-H, Li H-H, Fink JB, Lin H-L. Optimal Connection for Tiotropium SMI Delivery through Mechanical Ventilation: An In Vitro Study. Pharmaceutics. 2020; 12(3):291. https://doi.org/10.3390/pharmaceutics12030291
Chicago/Turabian StyleFang, Tien-Pei, Yu-Ju Chen, Tsung-Ming Yang, Szu-Hu Wang, Ming-Szu Hung, Shu-Hua Chiu, Hsin-Hsien Li, James B. Fink, and Hui-Ling Lin. 2020. "Optimal Connection for Tiotropium SMI Delivery through Mechanical Ventilation: An In Vitro Study" Pharmaceutics 12, no. 3: 291. https://doi.org/10.3390/pharmaceutics12030291
APA StyleFang, T.-P., Chen, Y.-J., Yang, T.-M., Wang, S.-H., Hung, M.-S., Chiu, S.-H., Li, H.-H., Fink, J. B., & Lin, H.-L. (2020). Optimal Connection for Tiotropium SMI Delivery through Mechanical Ventilation: An In Vitro Study. Pharmaceutics, 12(3), 291. https://doi.org/10.3390/pharmaceutics12030291






