Preparation and Optimization of In Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity
Abstract
1. Introduction
2. Materials
3. Methods
3.1. Preparation of RO-EA Based NTS
3.1.1. Experimental Design
3.1.2. Characterization of NTS Formulations
Vesicles Shape, and Size Distribution
Determination of Zeta Potential
Entrapment Efficiency
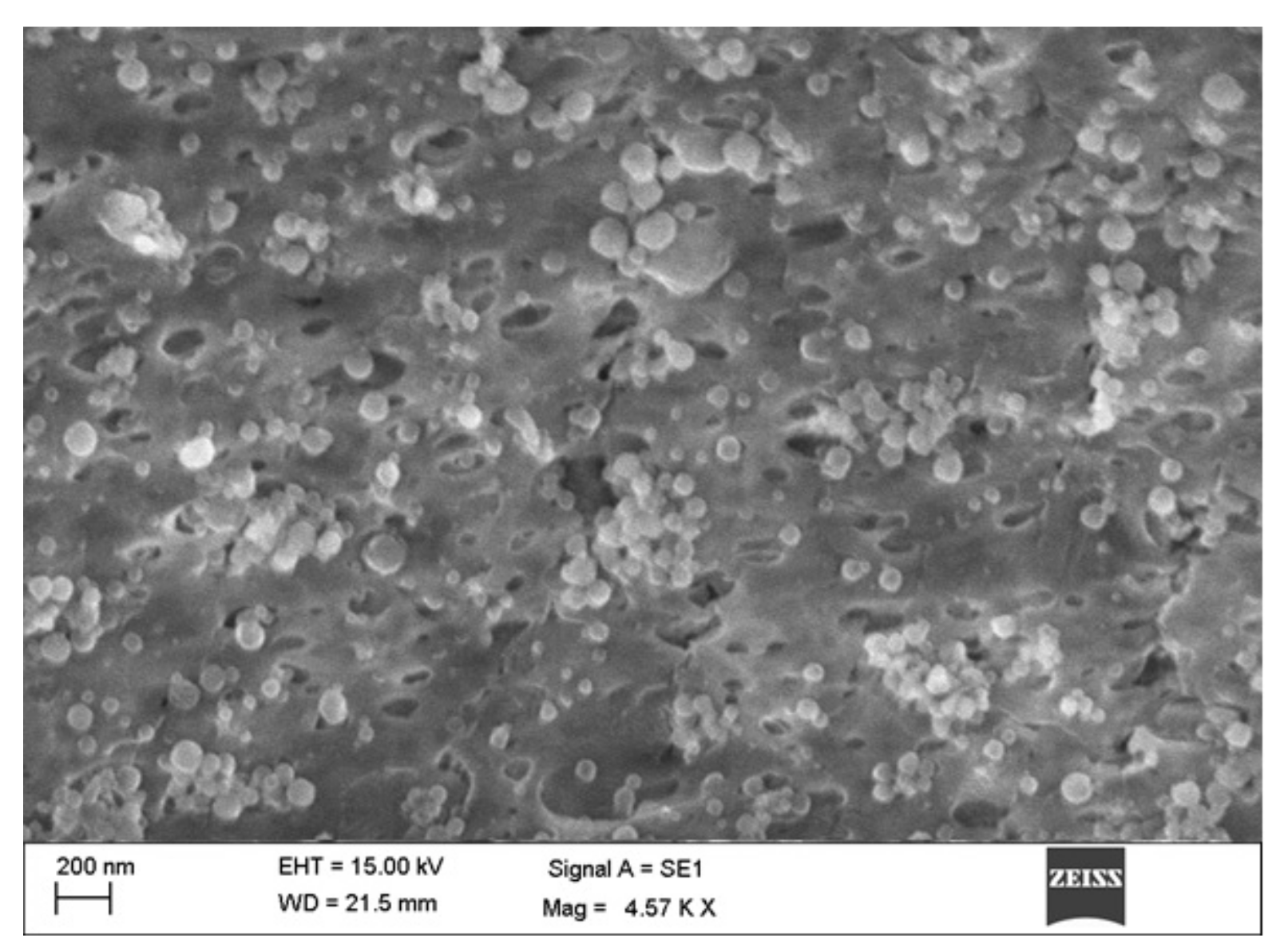
Surface Morphology
Stability Studies/Stability Index
3.2. Preparation of RO-NTS–Loaded In Situ Gel
3.2.1. Determination of Gelation Temperature
3.2.2. Determination of Viscosity
3.2.3. In-Vitro Release Studies
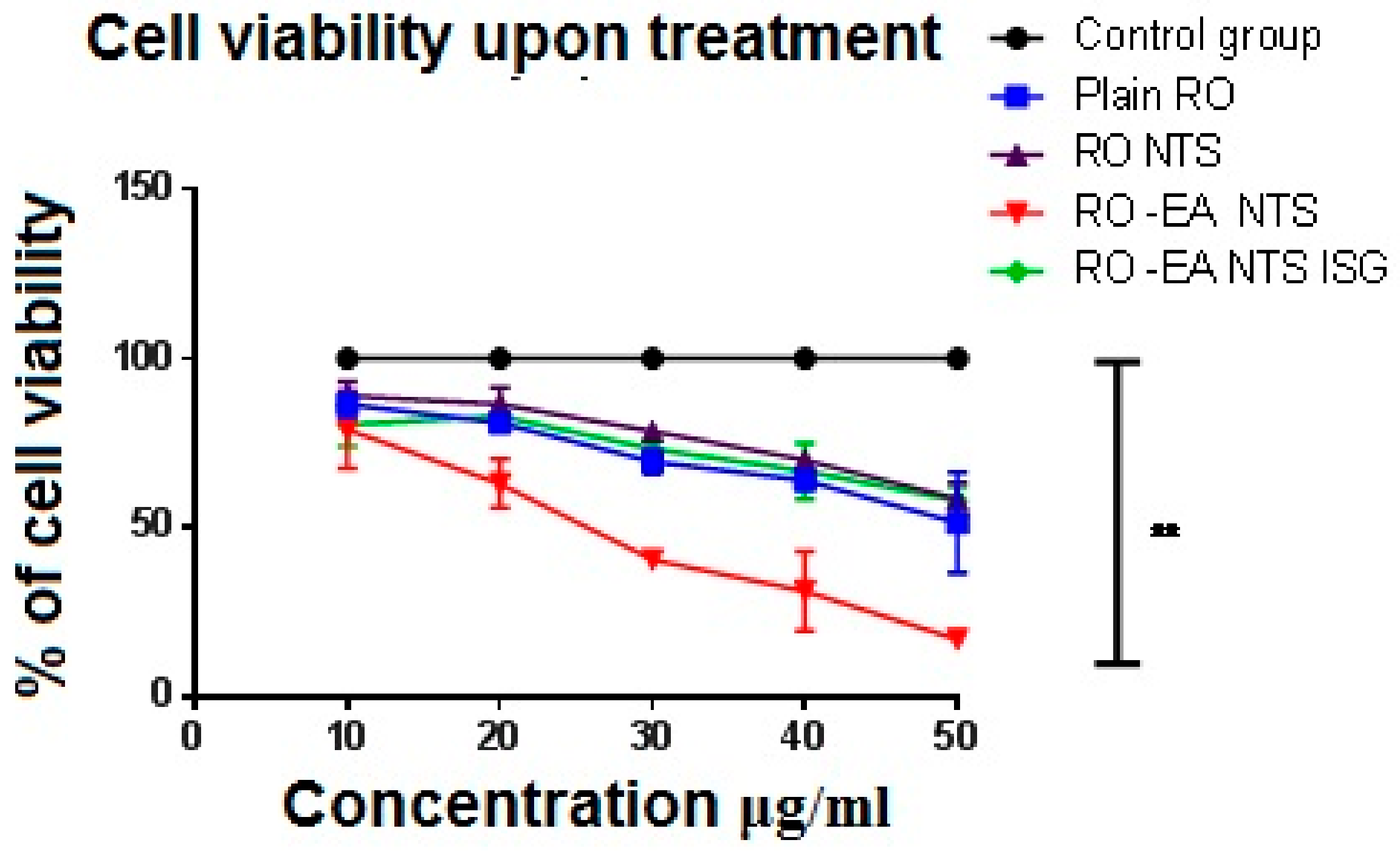
3.2.4. In Vitro Cell Viability Assay
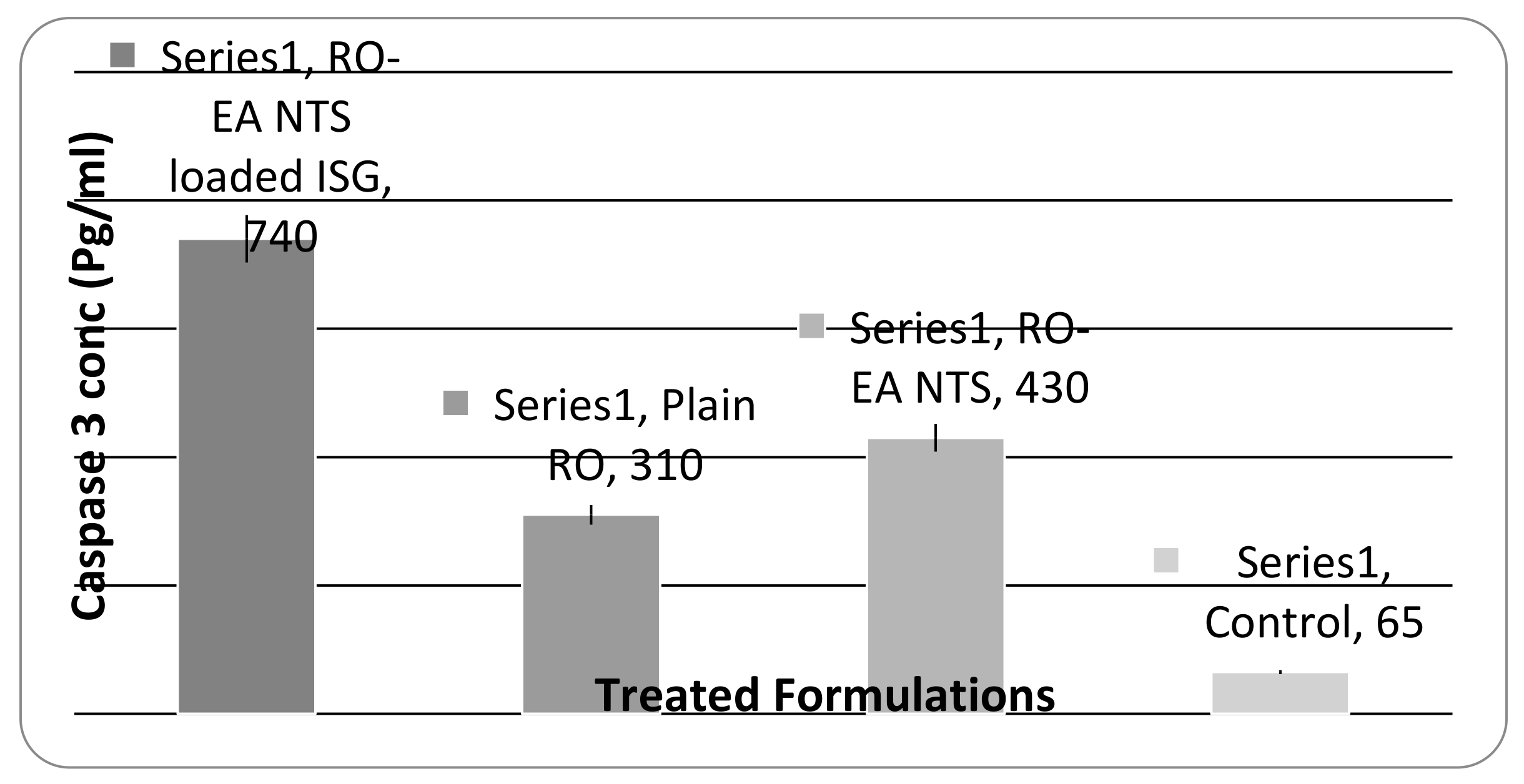
3.2.5. Caspase-3 Enzyme Assay
3.2.6. Statistical Analysis
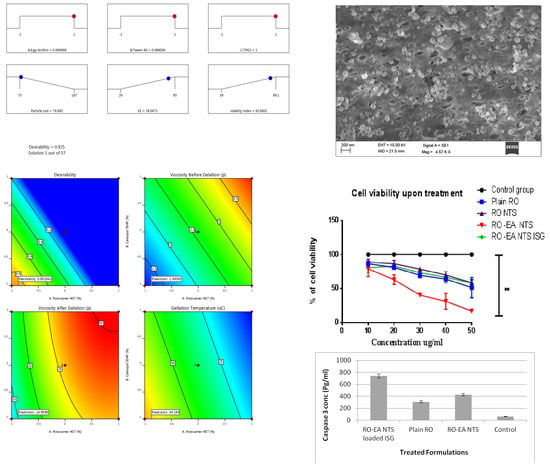
4. Results
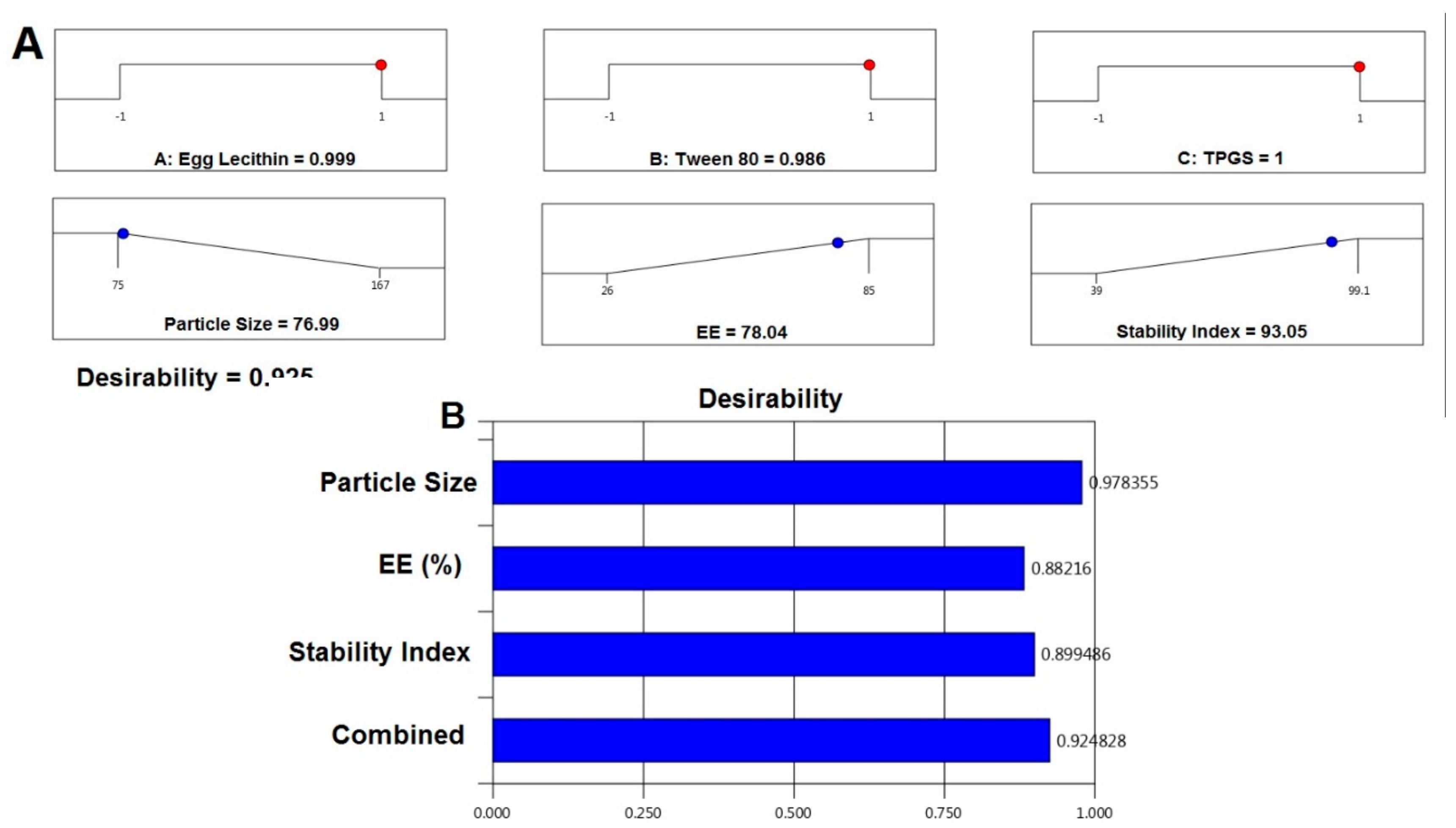
4.1. Preparation of NTS
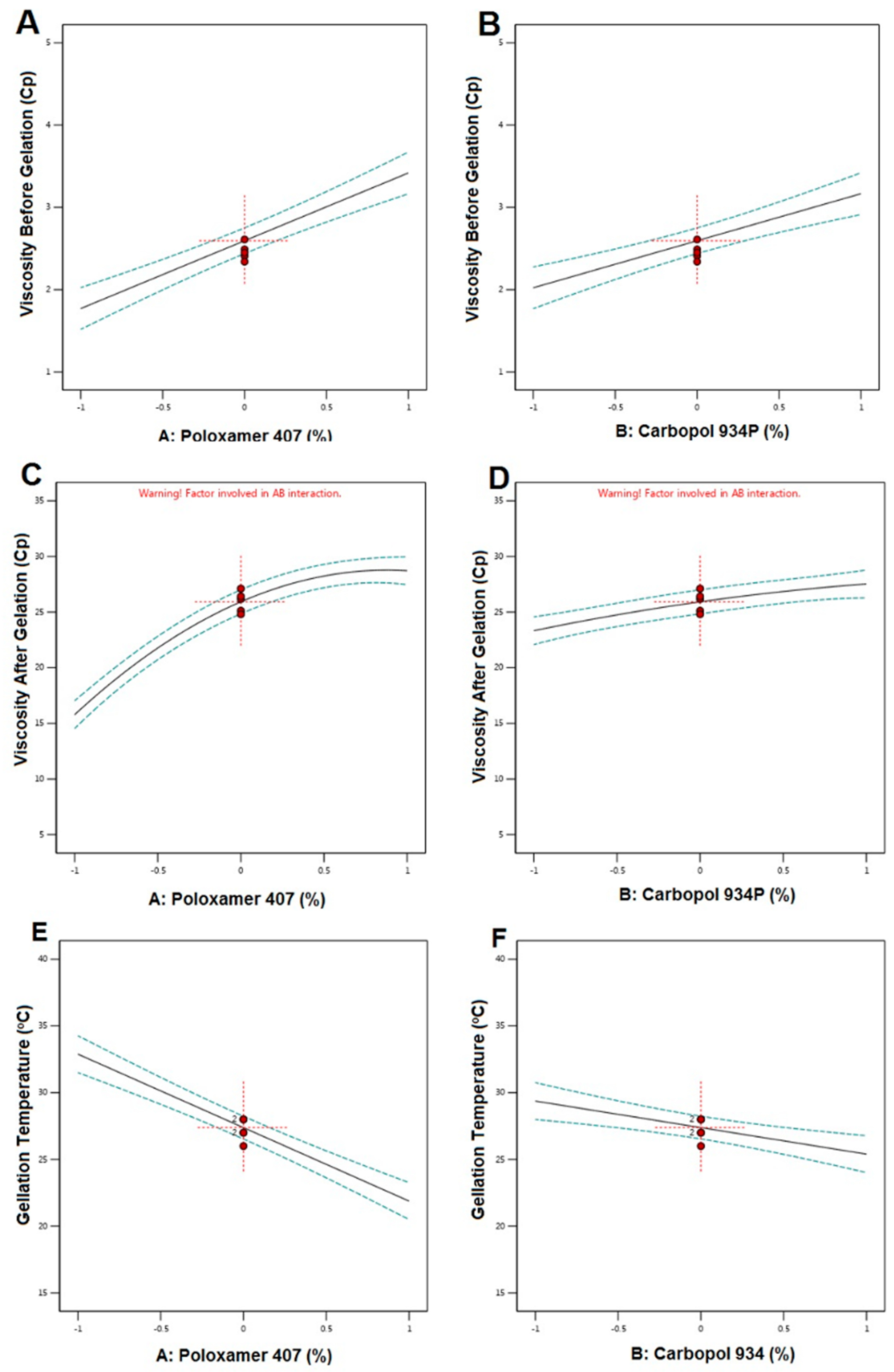
4.2. Preparation of RO-EA NTS Loaded In Situ Gel
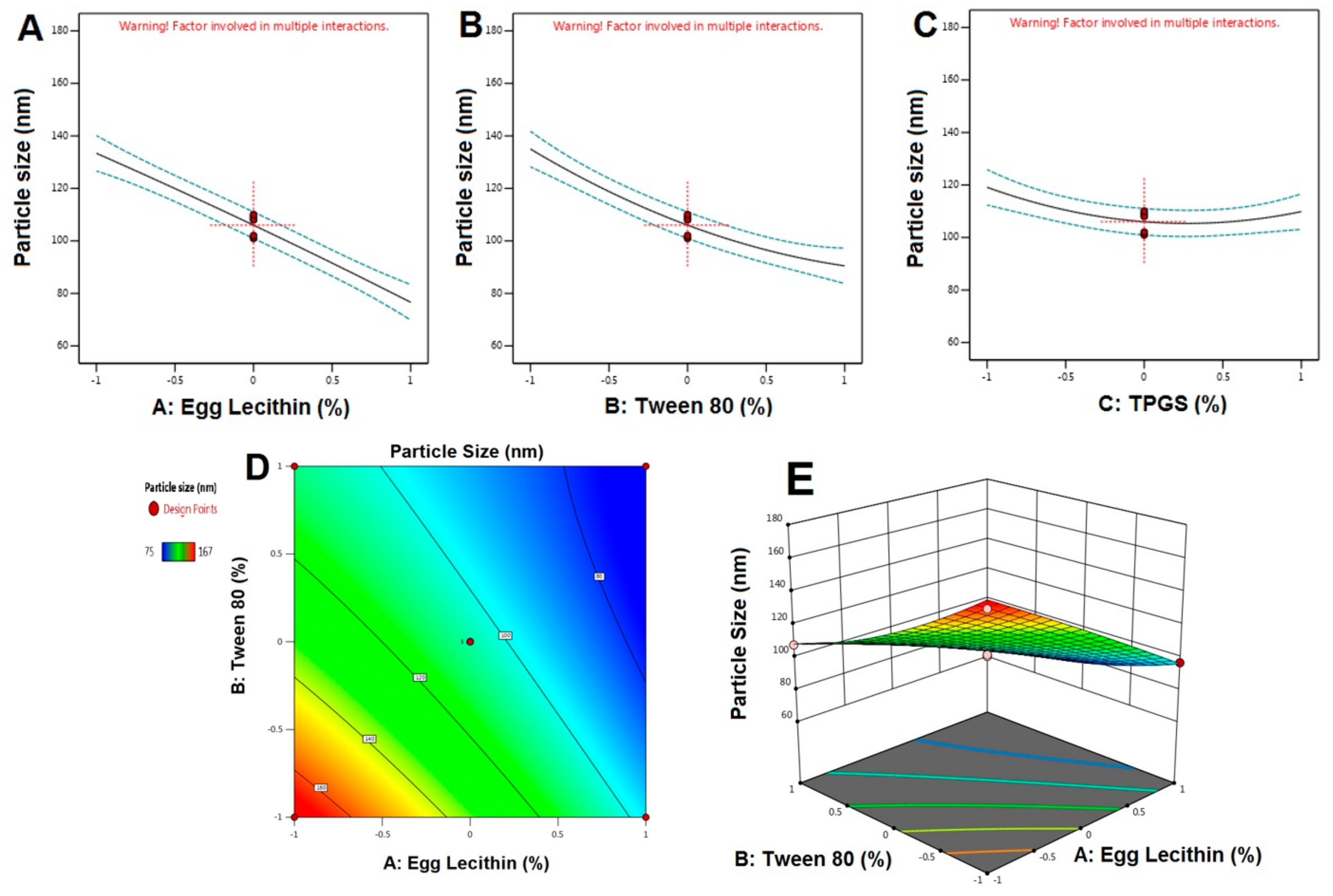
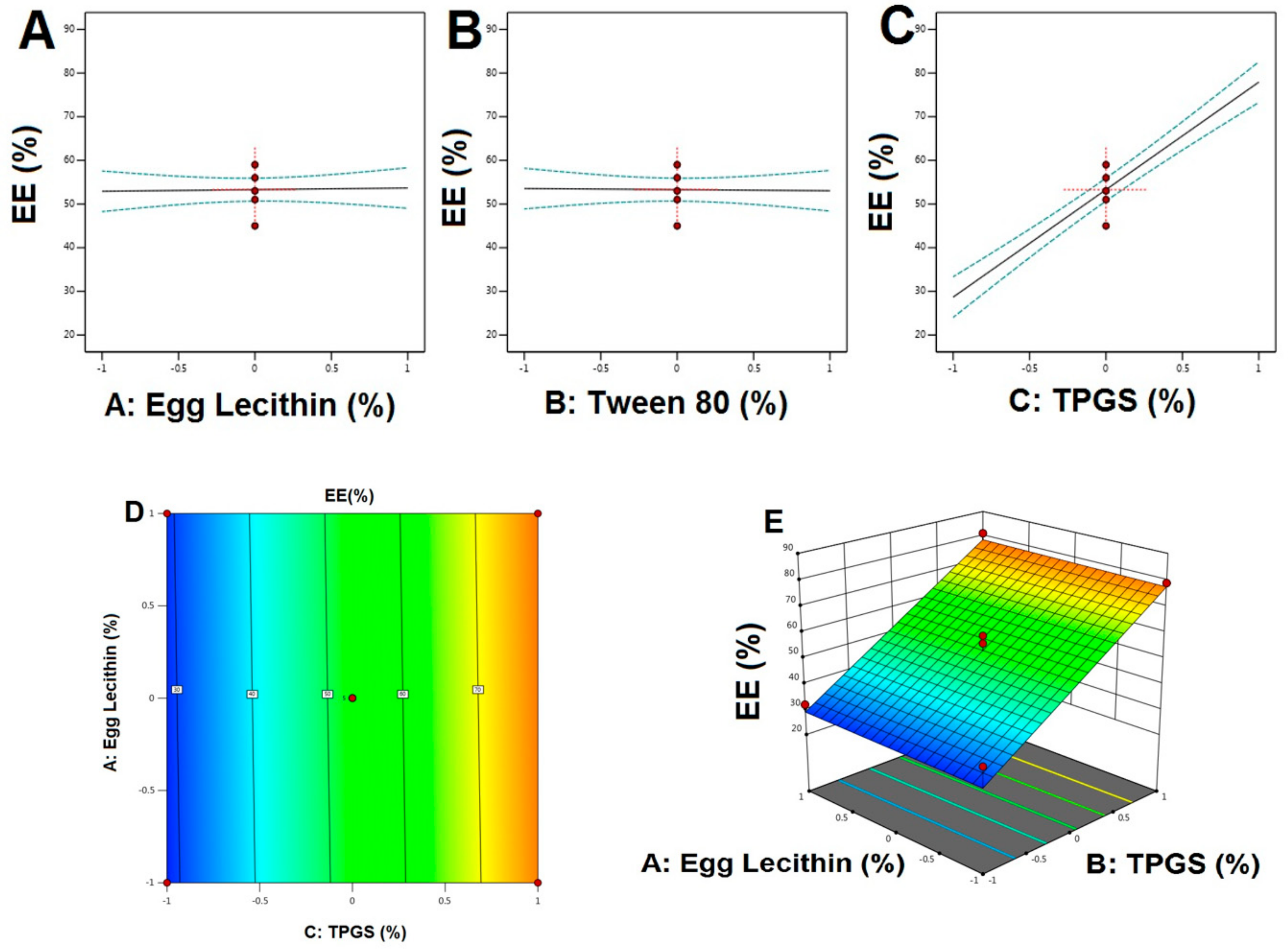
4.3. Characterization of NTS Formulations
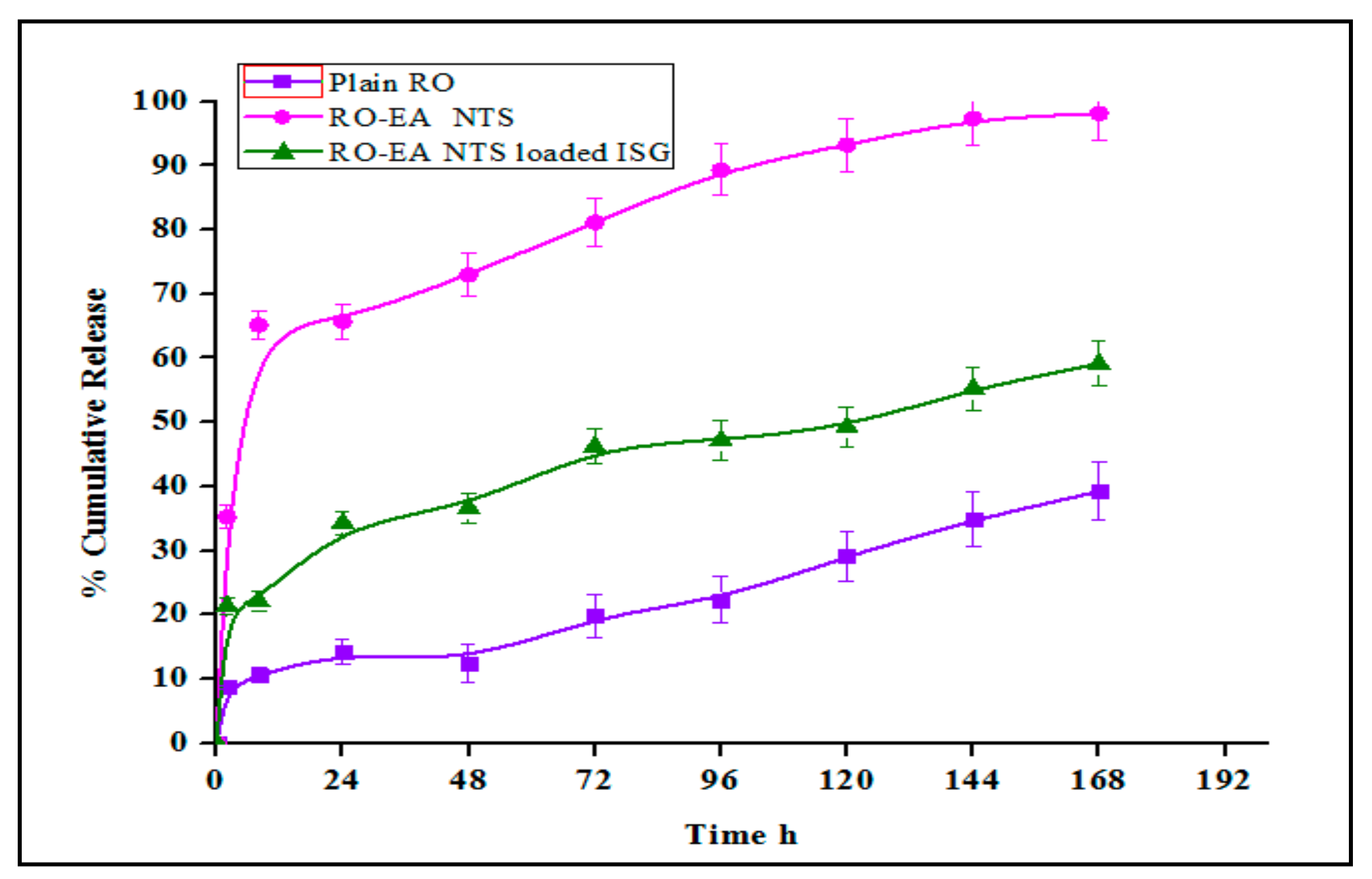
4.4. In-Vitro Release Studies
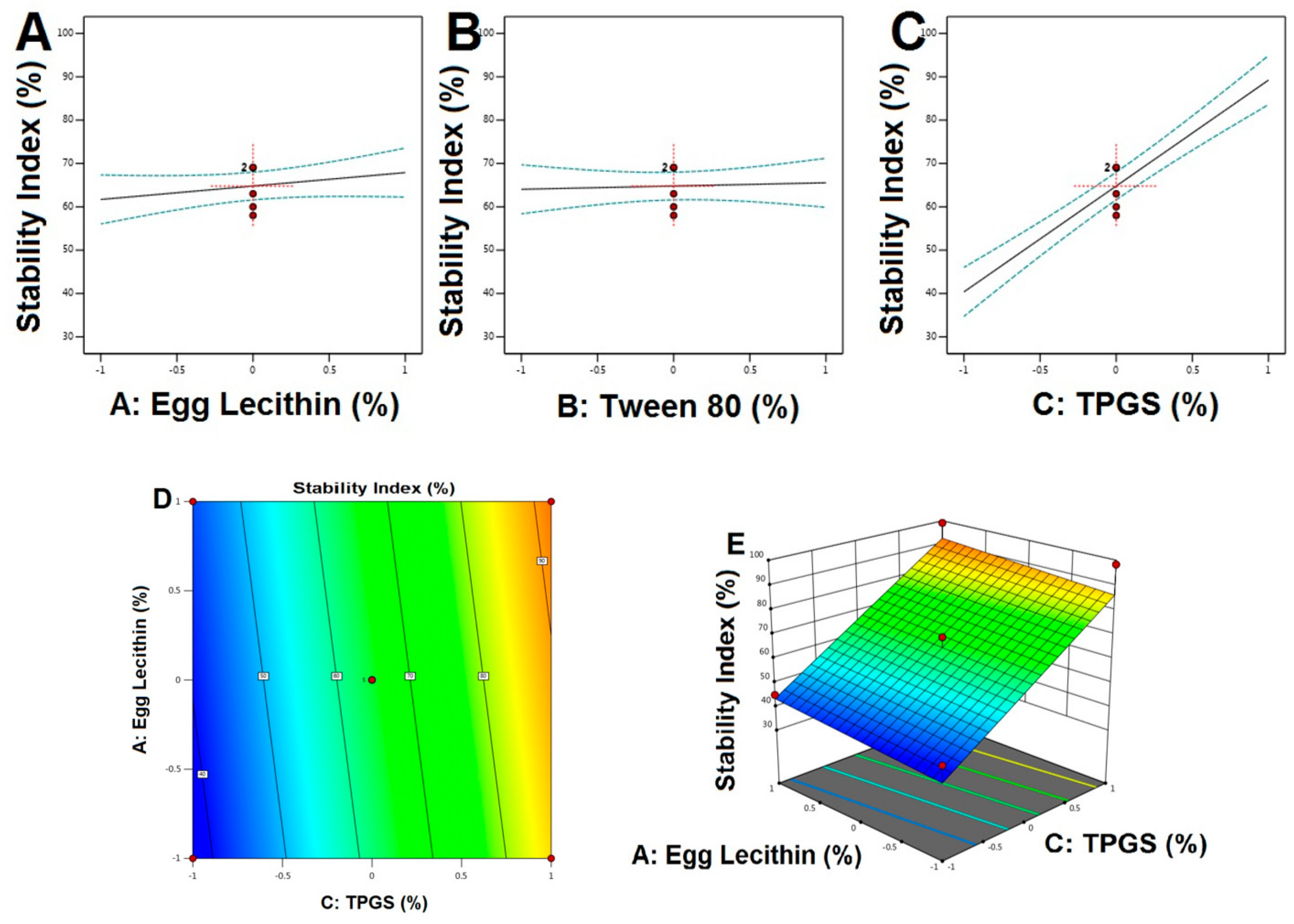
4.5. Stability Studies
4.6. In Vitro Cell Viability Assay
4.7. Caspase-3 Enzyme Assay
5. Discussion
5.1. Formulation Optimization of RO EA Based NTS
5.2. Preparation of RO-NTS Loaded In Situ Gel
5.3. SEM, Average Particle Size, and Zeta Potential
5.4. Stability Studies
5.5. In Vitro Drug Release, Cell Viability Studies, and Caspase-3 Enzyme Assay
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Matsuoka, H.; Kuroiwa, Y.; Sato, K.; Tanaka, S.; Yoshida, T. Dysphagia following various degrees of surgical resection for oral cancer. Ann. Otol. Rhinol. Laryngol. 1992, 101, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Souto, E.B. Nanostructured lipid carrier-based hydrogel formulations for drug delivery: A comprehensive review. Expert Opin. Drug Deliv. 2009, 6, 165–176. [Google Scholar] [CrossRef]
- Feleszko, W.; Mlynarczuk, I.; Balkowiec-Iskra, E.Z.; Czajka, A.; Switaj, T.; Stoklosa, T.; Giermasz, A.; Jako’bisiak, M. Lovastatin potentiates antitumor activity and attenuates cardiotoxicity of doxorubicin in three tumor models in mice. Clin. Cancer Res. 2000, 6, 2044–2052. [Google Scholar]
- Rozados, V.R.; Hinrichsen, L.I.; Binda, M.M.; Gervasoni, S.I.; Matar, P.; Bonfil, R.D.; Scharovsky, O.G. Lovastatin enhances the antitumoral and apoptotic activity of doxorubicin in murine tumor models. Oncol. Rep. 2008, 19, 1205–1211. [Google Scholar] [CrossRef]
- Roudier, E.; Mistafa, O.; Stenius, U. Statins induce mammalian target of rapamycin (mTOR)-mediated inhibition of Akt signaling and sensitize p53 deficient cells to cytostatic drugs. Mol. Cancer Ther. 2006, 5, 2706–2715. [Google Scholar] [CrossRef]
- Stirewalt, D.L.; Appelbaum, F.R.; Willman, C.L.; Zager, R.A.; Banker, D.E. Mevastatin can increase toxicity in primary AMLs exposed to standard therapeutic agents, but statin efficacy is not simply associated with ras hotspot mutations or overexpression. Leuk. Res. 2003, 27, 133–145. [Google Scholar] [CrossRef]
- Fujiwara, K.; Tsubaki, M.; Yamazoe, Y.; Nishiura, S.; Kawaguchi, T.; Ogaki, M.; Nishinobo, M.; Shimamoto, K.; Moriyama, K.; Nishida, S. Fluvastatin induces apoptosis on human tongue carcinoma cell line HSC-3, Yakugaku Zasshi. J. Pharm. Soc. Jpn. 2008, 128, 153–158. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G. Biological activity and characteristics of triamcinolone-acetonide formulated with the self-regulating drug carriers, Transfersomes. Biochim. Biophys. Acta 2003, 1614, 156–164. [Google Scholar] [CrossRef]
- Aburahma, M.H. Bile salts-containing vesicles: Promising pharmaceutical carriers for oral delivery of poorly water-soluble drugs and peptide/protein-based therapeutics or vaccines. Drug Deliv. 2014, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Aggarwal, G.; Singla, S.; Arora, R. Transfersomes: A novel vesicular carrier for enhanced transdermal delivery of sertraline: Development, characterization and performance evaluation. Sci. Pharm. 2012, 80, 1061–1080. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pathak, K.; Bali, V. Ultra-adaptable nanovesicular systems: A carrier for systemic delivery of therapeutic agents. Drug Discov. Today 2012, 17, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, B.; Chellan, V.R.; Natarajan, T.S.; Ranganathan, H.; Siyad, A. Self nano emulsifying drug delivery system (SNEDDS) of Rosuvastatin calcium: Design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf. B 2013, 112, 337–343. [Google Scholar]
- Zeybek, N.D.; Gulcelik, N.E.; Kaymaz, F.F.; Sarisozen, C.; Vural, I.; Bodur, E.; Canpinar, H.; Usman, A.; Asan, E. Rosuvastatin induces apoptosis in cultured human papillary thyroid cancer cells. J. Endocrinol. 2011, 210, 105–115. [Google Scholar] [CrossRef] [PubMed]
- El Sharkawi, F.Z.; El Shemy, H.A.; Khaled, H.M. Possible anticancer activity of rosuvastatine, doxazosin, repaglinide and oxcarbazepin. Asian Pac. J. Cancer Prev. 2014, 15, 199–203. [Google Scholar] [CrossRef]
- Brown, M.; Hart, C.; Tawadros, T.; Ramani, V.; Sangar, V.; Lau, M.; Clarke, N. The differential effects of statins on the metastatic behaviour of prostate cancer. Br. J. Cancer 2012, 106, 1689–1696. [Google Scholar] [CrossRef]
- Dorai, T.; Aggarwal, B.B. Role of chemopreventive agents in cancer theraphy. Cancer Lett. 2004, 215, 129–140. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef]
- Nirmal, H.B.; Bakliwal, S.R.; Pawar, S.P. In-Situ gel: New trends in Controlled and Sustained Drug Delivery System. Int. J. Pharm Tech Res. 2010, 2, 1398–1408. [Google Scholar]
- Ito, T.; Yeo, Y.; Highley, C.B.; Bellas, E.; Benitez, C.A.; Kohane, D.S. The prevention of peritoneal adhesions by in situ cross-linking hydrogels of hyaluronic acid and cellulose derivatives. Biomaterials 2007, 28, 975–983. [Google Scholar] [CrossRef]
- Taheri, A.; Atyabi, F.; Dinarvnd, R. Temperature-responsive and biodegradable PVA: PVP k30: Poloxamer 407 hydrogel for controlled delivery of human growth hormone (hGH). J. Pediatr. Endocrinol. Metab. 2011, 24, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Qushawy, M.; Nasr, A.; Abd-Alhaseeb, M.; Swidan, S. Design, optimization and characterization of a transfersomal gel using miconazole nitrate for the treatment of candida skin infections. Pharmaceutics 2018, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Khajeh, M. Application of Box-Behnken design in the optimization of a magnetic nanoparticle procedure for zinc determination in analytical samples by inductively coupled plasma optical emission spectrometry. J. Hazard Mater. 2009, 172, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ping, Q.; Sun, G.; Jiao, C. Lecithin vesicular carriers for transdermal delivery of cyclosporine. Int. J. Pharm. 2000, 194, 201–207. [Google Scholar] [CrossRef]
- El Maghraby, G.M.M.; Williams, A.C.; Barry, B.W. Skin delivery from ultradeformable liposomes: Refinement of surfactant concentration. J. Pharm. Pharmacol. 1999, 51, 1123–1134. [Google Scholar] [CrossRef]
- Rimple, S.; Munish, A.; Harmanmeet, K. Thiolated pectin nanoparticles: Preparation, characterization and ex vivo corneal permeation study. Carbohydr. Polym. 2012, 87, 1606–1610. [Google Scholar]
- Bunjes, H.; KWestesen, K.; Koch, M.H. Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. Int. J. Pharm. 1996, 129, 159–173. [Google Scholar] [CrossRef]
- Bhatia, M.; Ahuja, M. Psyllium arabinoxylan: Carboxymethylation, characterization and evaluation for nanoparticulate drug delivery. Int. J. Biol. Macromol. 2015, 72, 495–501. [Google Scholar] [CrossRef]
- Miller, S.C.; Donovan, M.D. Effect of poloxamer 407 gel on the miotic activity of pilocarpine nitrate in rabbits. Int. J. Pharm. 1982, 12, 147–152. [Google Scholar] [CrossRef]
- Badgujar, S.D.; Sontakke, M.A.; Narute, D.R.; Karmarkar, R.R.; Tupkar, S.V.; Barhate, S.D. Formulation and evaluation of sumatriptan succinate nasal in situ gel using fulvic acid as novel permeation enhancer. Int. J. Pharm. Res. Dev. 2010, 2, 1–8. [Google Scholar]
- Dabhi, M.R.; Nagori, S.A.; Gohel, M.C.; Parikh, R.K.; Sheth, N.R. Formulation development of smart gel periodontal drug delivery system for local delivery of chemotherapeutic agents with application of experimental design. Drug Deliv. 2010, 17, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Wamorkar, V.; Varma, M.M.; Manjunath, S.Y. Formulation and evaluation of stomach specific in situ gel of metoclopramide using natural, bioodegradable Polymers. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 193–201. [Google Scholar]
- Zhang, X.; Liu, J.; Qiao, H.; Liu, H.; Ni, J.; Zhang, W.; Shi, Y. Formulation optimization of dihydroartemisinin nanostructured lipid carrier using response surface methodology. Powder Technol. 2010, 197, 120–128. [Google Scholar] [CrossRef]
- Chien, M.H.; Ying, T.H.; Hsieh, Y.S.; Chang, Y.C.; Yeh, C.M.; Ko, J.L.; Yang, S.F. Dioscorea nipponica Makino inhibits migration and invasion of human oral cancer HSC-3 cells by transcriptional inhibition of matrix metalloproteinase-2 through modulation of CREB and AP-1 activity. Food Chem. Toxicol. 2012, 50, 558–566. [Google Scholar] [CrossRef]
- Lin, C.C. Berberine induces apoptosis in human HSC-3 oral cancer cells via simultaneous activation of the death receptor-mediated and mitochondrial pathway. Anticancer Res. 2007, 27, 3371–3378. [Google Scholar]
- Joshi, R.; Kulkarni, R. Chaudhari, In-vitro and Ex-vivo evaluation of Raloxifene hydrochloride delivery using nano-transfersome based formulations. J. Drug Deliv. Sci. Technol. 2018, 45, 151–158. [Google Scholar] [CrossRef]
- Patil, P.; Khairnar, G.; Naik, J. Preparation and statisticaloptimization of Losartan Potassium loaded nanoparticles using Box behnken factorial design: Microreactor precipitation. Chem. Eng. Res. Des. 2015, 104, 98–109. [Google Scholar] [CrossRef]
- Khairnar, G.; Mokale, V.; Naik, J. Formulation and development of nateglinide loaded sustained release ethyl cellulose microspheres by O/W solvent emulsification technique. J. Pharm. Innov. 2014, 44, 411–422. [Google Scholar] [CrossRef]
- Siepmann, F.; Hoffmann, A.; Leclercq, B.; Carlin, B.; Siepmann, J. How to adjust desired drug release patterns from ethylcellulose-coated dosage forms. J. Control. Release 2007, 119, 182–189. [Google Scholar] [CrossRef]
- Alkhalidi, H.M.; Naguib, G.H.; Kurakula, M.; Hamed, M.T.; Attar, M.H.; Almatrook, Z.H.; Aldryhim, A.Y.; Bahmdan, R.H.; Khallaf, R.A.; El Sisi, A.M.; et al. In vitro and preclinical assessment of factorial design based nanoethosomal transdermal film formulation of mefenamic acid to overcome barriers to its use in relieving pain and inflammation. J. Drug Deliv. Sci. Technol. 2018, 48, 450–456. [Google Scholar] [CrossRef]
- Naveen, N.R.; Gopinath, C.; Rao, D.S. Design expert supported mathematical optimization of repaglinide gastro retentive floating tablets: In vitro and in vivo evaluation, Future. J. Pharm. Sci. 2017, 3, 140–147. [Google Scholar]
- Chaudharya, H.; Kohlib, K.; Kumar, V. Nano-transfersomes as a novel carrier for transdermal delivery. Int. J. Pharm. 2013, 454, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A. Formulation and optimization of nanotransfersomes using experimental design technique for accentuated transdermal delivery of valsartan. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Madane, R.G.; Mahajan, H.S. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: Design, characterization, and in vivo study. Drug Deliv. 2016, 23, 1326–1334. [Google Scholar]
- Liu, Q.; Deng, R.; Li, S.; Li, X.; Li, K.; Kebaituli, G.; Li, X.; Liu, R. Ellagic acid protects against neuron damage in ischemic stroke through regulating the ratio of Bcl-2/Bax expression. Appl. Physiol. Nutr. Metab. 2017, 42, 855–860. [Google Scholar] [CrossRef]
- Fahmy, U.A. Augmentation of fluvastatin cytotoxicity against prostate carcinoma PC3 cell line utilizing alpha lipoic–ellagic acid nanostructured lipid carrier formula. AAPS PharmSciTech 2018, 19, 3454–3461. [Google Scholar] [CrossRef]

| Independent Variables | Levels | Dependent Variables | Constraints | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Concentration of Egg lecithin (%)-X1 | 0 | 10 | 25 | Particle Size (nm) | Minimum |
| Concentration of Tween 80 (%)-X2 | 0 | 10 | 20 | EE (%) | Maximum |
| Concentration of TPGS (%)-X3 | 0 | 10 | 15 | Stability Index (%) | Maximum |
| Independent Variables | Levels | Dependent Variables | Constraints | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| X1: P 407 (% w/v) | 05 | 15 | 20 | Y1: Viscosity before gelation (Cp *) | Minimum |
| X2: Carbopol 934P (% w/v) | 0.2 | 0.5 | 1.0 | Y2: Viscosity after gelation (Cp) | Maximum |
| Y3: Gelation temperature (oC) | Maximum | ||||
| Run | Factor 1 X1: Egg Lecithin % | Factor 2 X2: Tween 80 % | Factor 3 TPGS % | Response Y1 Particle Size (nm) | Response Y2 EE % | Response Y3 Stability index % |
|---|---|---|---|---|---|---|
| 1 | 0 | −1 | 1 | 141 | 85 | 85 |
| 2 | −1 | 0 | 1 | 137 | 79 | 98.3 |
| 3 | 0 | 0 | 0 | 110 | 51 | 63 |
| 4 | 1 | 0 | −1 | 85 | 32 | 45 |
| 5 | 0 | 1 | −1 | 101 | 28 | 41 |
| 6 | −1 | 1 | 0 | 108 | 49 | 58 |
| 7 | −1 | 0 | −1 | 152 | 36 | 44 |
| 8 | 0 | 0 | 0 | 102 | 59 | 69 |
| 9 | 0 | 0 | 0 | 109 | 56 | 69.1 |
| 10 | 0 | 1 | 1 | 93 | 74 | 82 |
| 11 | −1 | −1 | 0 | 167 | 49 | 55 |
| 12 | 1 | 0 | 1 | 80 | 81 | 99.1 |
| 13 | 0 | 0 | 0 | 101 | 45 | 58 |
| 14 | 0 | −1 | −1 | 150 | 26 | 39 |
| 15 | 1 | −1 | 0 | 97 | 48 | 66 |
| 16 | 0 | 0 | 0 | 108 | 53 | 60 |
| 17 | 1 | 1 | 0 | 75 | 55 | 70 |
| Factors | PD | EE | SI | |||
|---|---|---|---|---|---|---|
| F-Value | p-Value | F-Value | p-Value | |||
| Model | 56.34 | <0.0001 * | 43.39 | <0.0001 | 43.39 | <0.0001 |
| A-Egg lecithin | 284.92 | <0.0001 * | 2.06 | 0.1747 | 2.06 | 0.1747 |
| B-Tween 80 | 175.19 | <0.0001 * | 0.1207 | 0.7339 | 0.1207 | 0.7339 |
| C-TPGS | 7.57 | 0.0285 * | 127.99 | <0.0001* | 127.99 | <0.0001 * |
| AB | 15.14 | 0.0060 * | ||||
| AC | 1.11 | 0.3279 | ||||
| BC | 0.0111 | 0.9192 | ||||
| A2 | 0.1862 | 0.6790 | ||||
| B2 | 8.49 | 0.0226 | ||||
| C2 | 13.46 | 0.0080 * | ||||
| Name | Goal | Lower Limit | Upper Limit |
|---|---|---|---|
| A: Egg lecithin | is in range | −1 | 1 |
| B: Tween 80 | is in range | −1 | 1 |
| C: TPGS | is in range | −1 | 1 |
| Particle size | minimize | 75 | 167 |
| EE | maximize | 26 | 85 |
| Stability index | maximize | 39 | 99.1 |
| Run | Factor 1 | Factor 2 | Response 1 | Response 2 | Response 3 |
|---|---|---|---|---|---|
| A: Poloxamer 407 | B: Carbopol 934P | Viscosity Before Gelation | Viscosity After Gelation | Gelation Temperature | |
| % | % | Cp | Cp | oC | |
| 1 | 20 | 0.5 | 3.82 ± 0.12 | 28.14 ± 0.12 | 21 |
| 2 | 20 | 0.2 | 3.01 ± 0.19 | 26.12 ± 0.11 | 24 |
| 3 | 15 | 0.5 | 2.49 ± 0.38 | 26.17 ± 0.15 | 28 |
| 4 | 15 | 0.5 | 2.61 ± 0.11 | 27.1 ± 0.77 | 26 |
| 5 | 15 | 0.5 | 2.41 ± 0.36 | 25.12 ± 0.11 | 27 |
| 6 | 5 | 0.2 | 1.32 ± 0.87 | 14.51 ± 0.47 | 33 |
| 7 | 15 | 0 | 1.89 ± 0.22 | 21.12 ± 0.17 | 30 |
| 8 | 20 | 1 | 4.06 ± 0.14 | 29.86 ± 0.51 | 20 |
| 9 | 15 | 0.5 | 2.45 ± 0.55 | 26.41 ± 0.78 | 27 |
| 10 | 15 | 1.5 | 3.25 ± 0.47 | 28.26 ± 0.77 | 23 |
| 11 | 5 | 1 | 2.93 ± 0.71 | 17.47 ± 0.89 | 31 |
| 12 | 15 | 0.5 | 2.34 ± 0.78 | 24.81 ± 0.63 | 28 |
| 13 | 0 | 0.5 | 1.15 ± 0.45 | 8.51 ± 0.13 | 38 |
| Factors | Y1 | Y2 | Y3 | |||
|---|---|---|---|---|---|---|
| F-value | p-Value | F-value | p-Value | F-value | p-Value | |
| Model | 62.80 | <0.0001 | 89.48 | <0.0001 | 72.05 | <0.0001 |
| A-Poloxamer 407 | 84.79 | <0.0001 | 322.58 | <0.0001 | 127.48 | <0.0001 |
| B-Carbopol 934P | 40.81 | <0.0001 | 33.97 | 0.0006 | 16.61 | 0.0022 |
| AB | 0.1465 | 0.7133 | ||||
| A2 | 90.69 | <0.0001 | ||||
| B2 | 1.65 | 0.2397 | ||||
| Formulation | Particle Size | Residual Drug Content | |||||
|---|---|---|---|---|---|---|---|
| Storage Conditions | 0 Day | 15 Days | 30 Days | 0 Day | 15 Days | 30 Days | |
| RO-EA NTS | 4.0 ± 1 °C | 105 ± 2.1 | 108 ± 4.5 | 107 ± 1.3 | 99.4 ± 0.11 | 97.4 ± 0.61 | 95.5 ± 0.31 |
| 25 ± 2 °C | 104 ± 1.1 | 107 ± 3.5 | 106 ± 3.2 | 98.4 ± 0.81 | 97.4 ± 0.41 | 96.2 ± 0.25 | |
| RO-EA NTS IN SITU GEL | 4.0 ± 1 °C | 109 ± 6.1 | 108 ± 1.1 | 108 ± 5.1 | 99.1 ± 0.31 | 98.4 ± 0.21 | 99.3 ± 0.71 |
| 25 ± 2 °C | 109 ± 2.6 | 109 ± 4.1 | 106 ± 4.1 | 99.4 ± 0.31 | 99.5 ± 0.11 | 99.1 ± 0.71 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosny, K.M.; Rizg, W.Y.; Khallaf, R.A. Preparation and Optimization of In Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity. Pharmaceutics 2020, 12, 263. https://doi.org/10.3390/pharmaceutics12030263
Hosny KM, Rizg WY, Khallaf RA. Preparation and Optimization of In Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity. Pharmaceutics. 2020; 12(3):263. https://doi.org/10.3390/pharmaceutics12030263
Chicago/Turabian StyleHosny, Khaled M., Waleed Y. Rizg, and Rasha A. Khallaf. 2020. "Preparation and Optimization of In Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity" Pharmaceutics 12, no. 3: 263. https://doi.org/10.3390/pharmaceutics12030263
APA StyleHosny, K. M., Rizg, W. Y., & Khallaf, R. A. (2020). Preparation and Optimization of In Situ Gel Loaded with Rosuvastatin-Ellagic Acid Nanotransfersomes to Enhance the Anti-Proliferative Activity. Pharmaceutics, 12(3), 263. https://doi.org/10.3390/pharmaceutics12030263