Investigating the Suitability of High Content Image Analysis as a Tool to Assess the Reversibility of Foamy Alveolar Macrophage Phenotypes In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Assessment of Cell Vacuolation and Lipid Profiles
2.3. Quantitative High Content Analysis
2.4. Statistical Analysis
3. Results
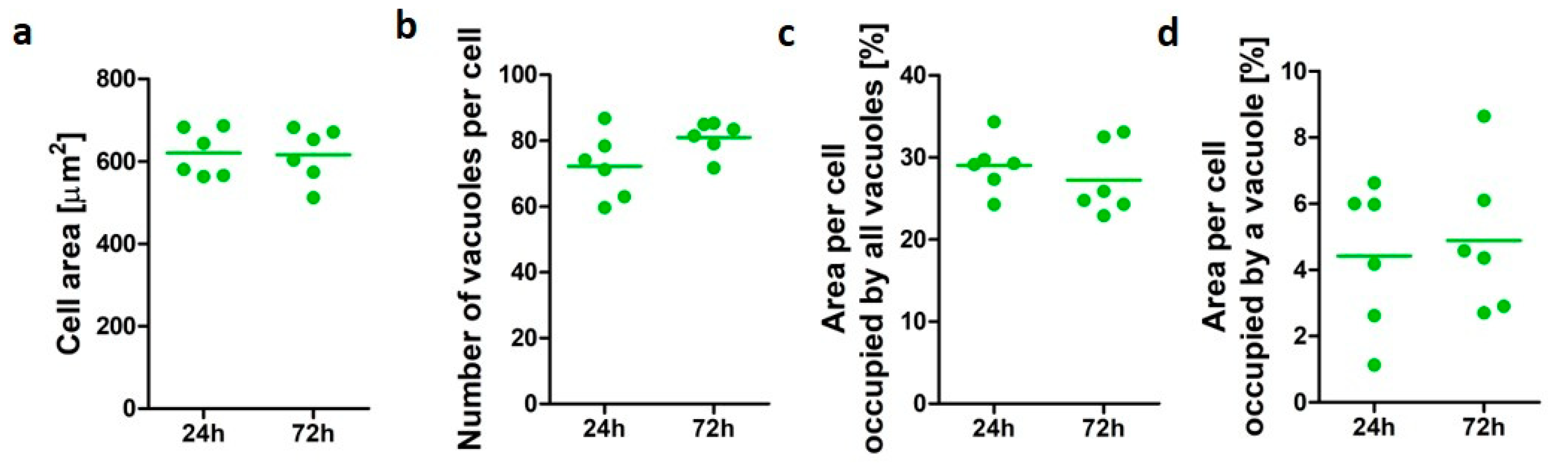
3.1. Characterization of PMA-Differentiated U937 Cells
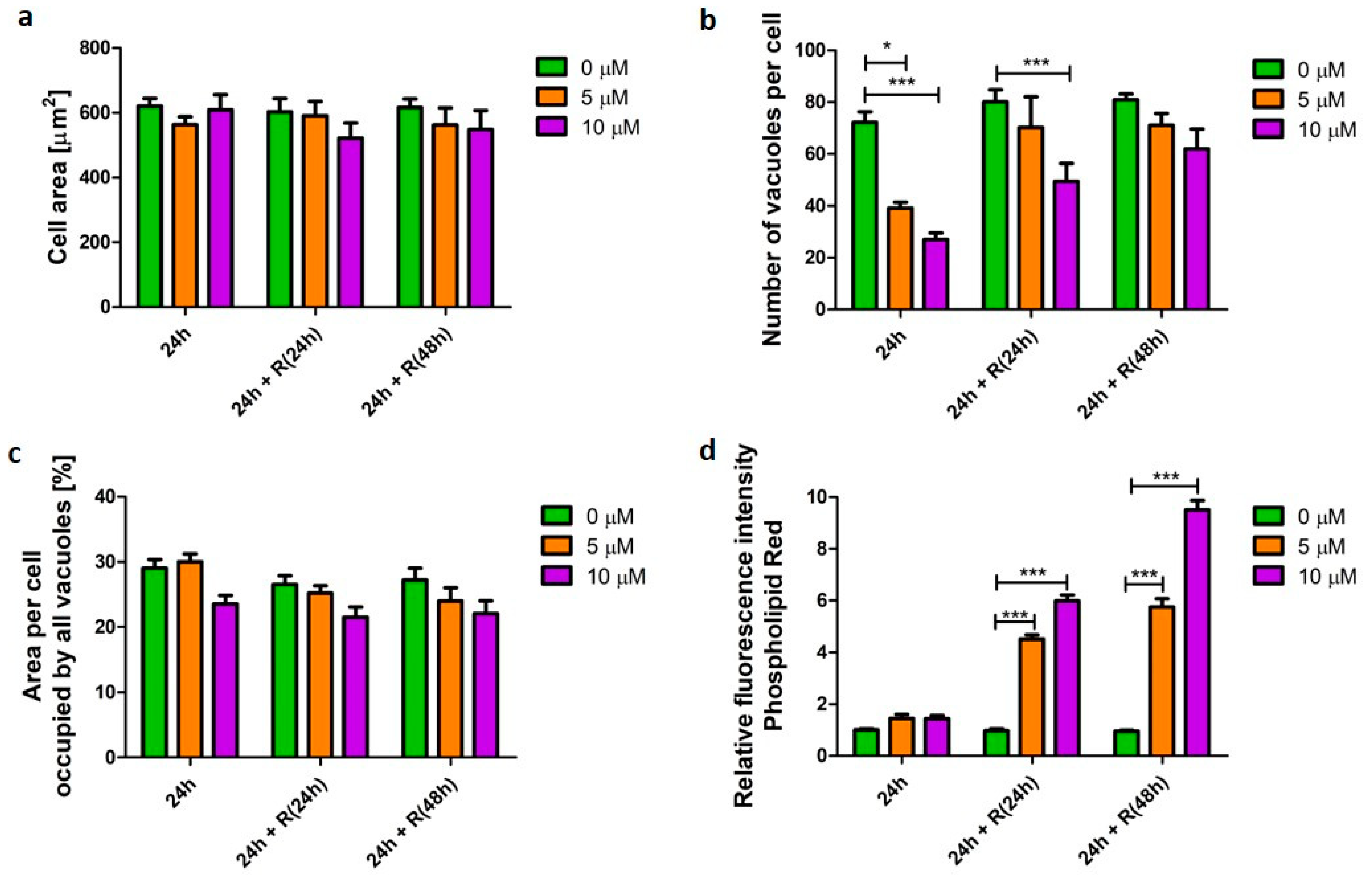
3.2. Phospholipidosis Phenotype
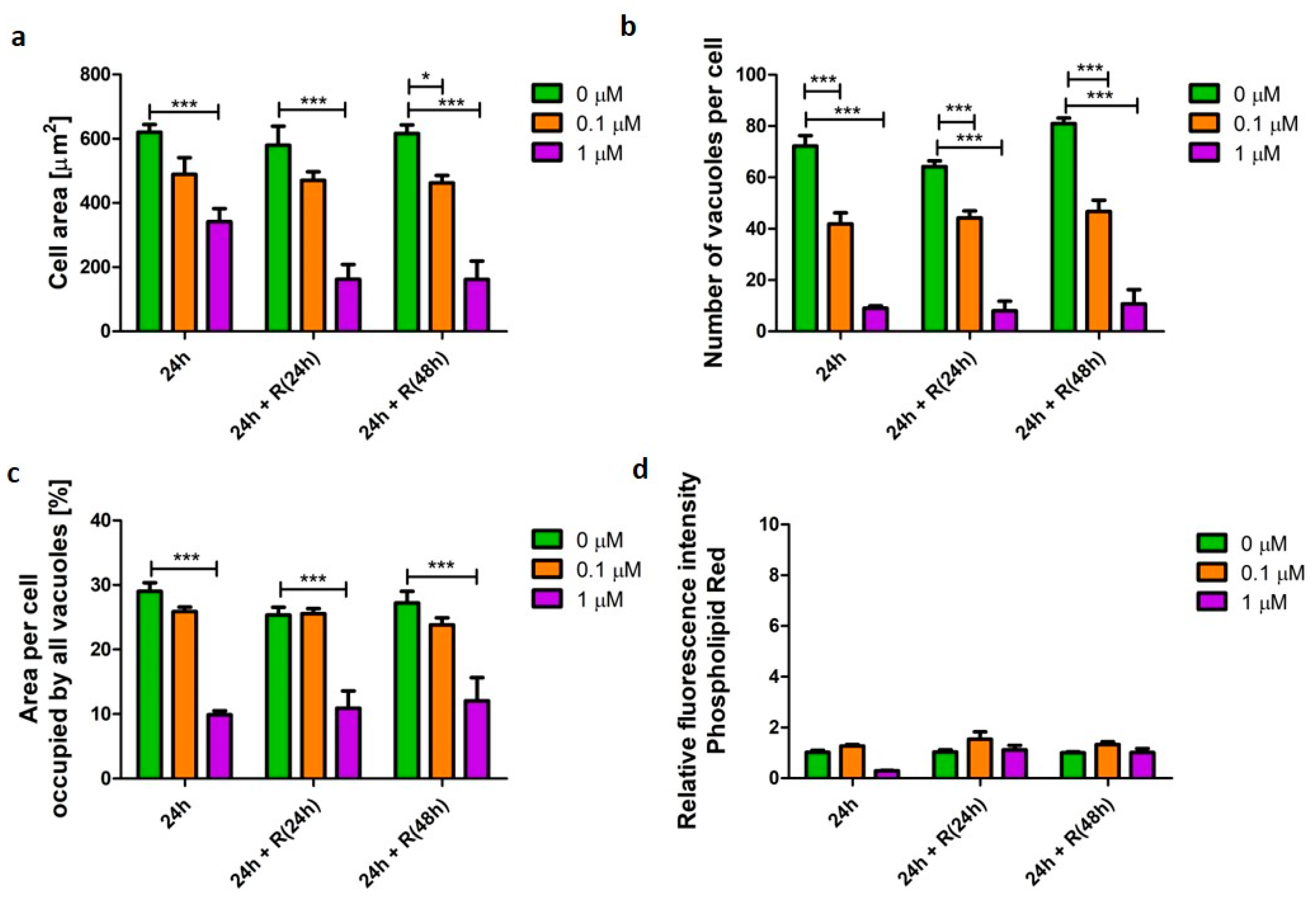
3.3. Pro-Apoptotic Phenotype
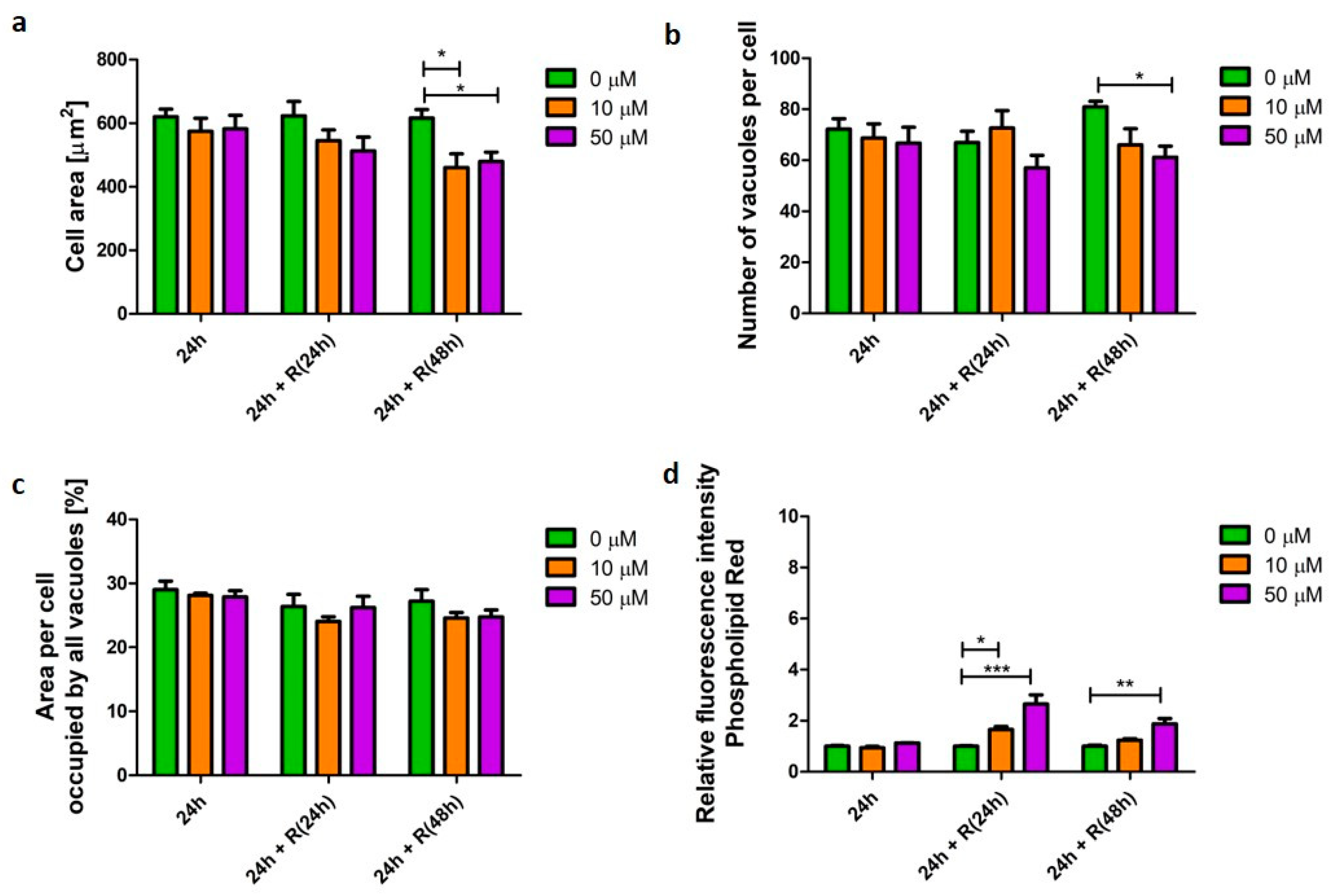
3.4. Corticosteroid Phenotype
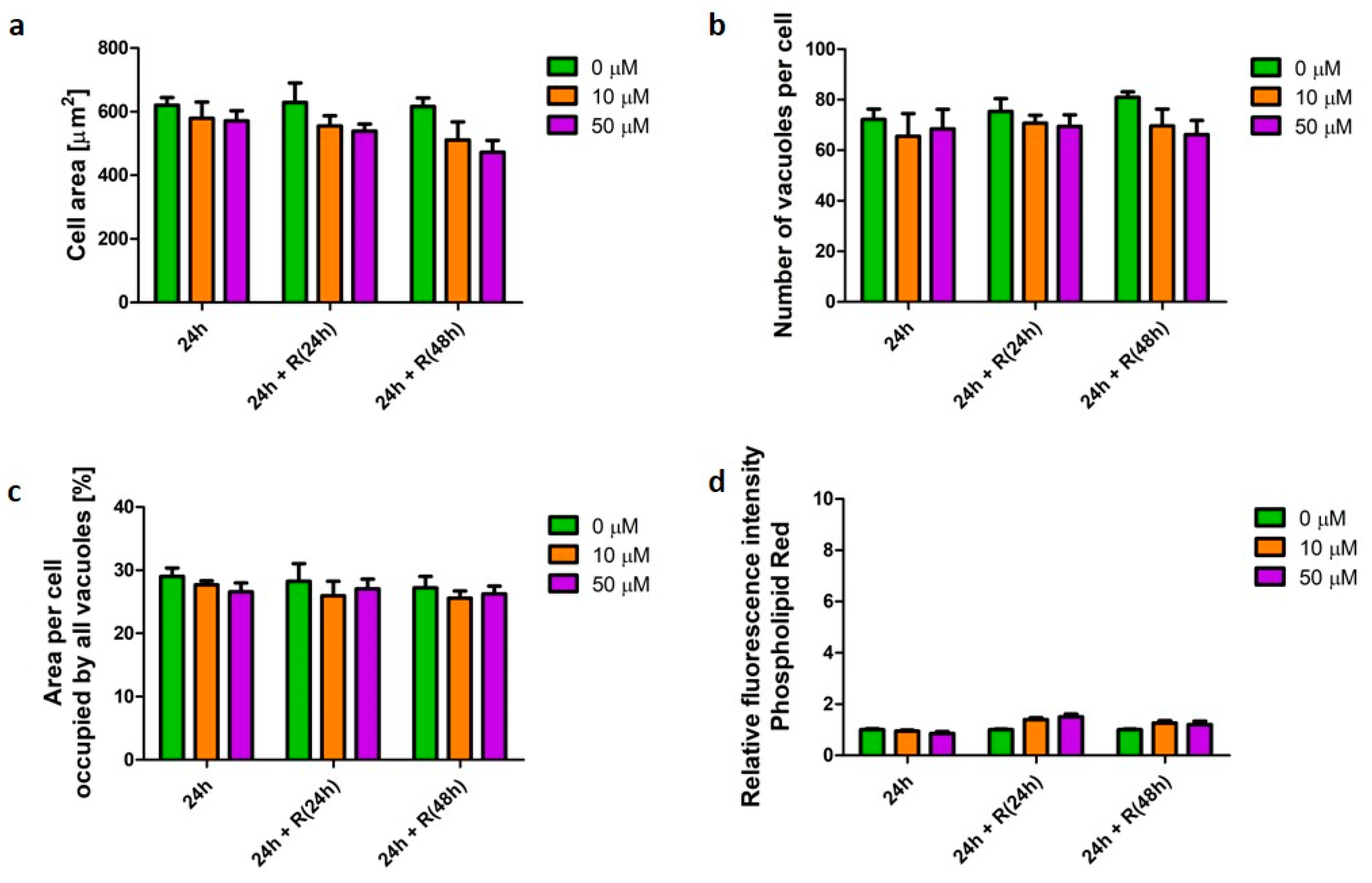
3.5. β2-Agonists Phenotype
4. Discussion
4.1. Phospholipidosis Phenotype Was Not Destructive for Macrophages
4.2. Apoptotic Phenotype Was Not Reversible
4.3. Corticosteroid and β2-Agonist Phenotypes Resolve over Time
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

References
- Joshi, N.; Walter, J.M.; Misharin, A.V. Alveolar Macrophages. Cell. Immunol. 2018, 330, 86–90. [Google Scholar] [CrossRef]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their role, activation and polarization in pulmonary diseases. Immunobiology 2018, 223, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Forbes, B.; O’Lone, R.; Allen, P.P.; Cahn, A.; Clarke, C.; Collinge, M.; Dailey, L.A.; Donnelly, L.E.; Dybowski, J.; Hassall, D.; et al. Challenges for inhaled drug discovery and development: Induced alveolar macrophage responses. Adv. Drug Deliv. Rev. 2014, 71, 15–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, D.J.; Williams, T.C.; Beck, S.L. Foamy macrophage responses in the rat lung following exposure to inhaled pharmaceuticals: A simple, pragmatic approach for inhaled drug development. J. Appl. Toxicol. 2014, 34, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Nikula, K.J.; McCartney, J.E.; McGovern, T.; Miller, G.K.; Odin, M.; Pino, M.V.; Reed, M.D. STP Position Paper:Interpreting the Significance of Increased Alveolar Macrophages in Rodents Following Inhalation of Pharmaceutical Materials. Toxicol. Pathol. 2014, 42, 472–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colerangle, J.B. Chapter 25-Preclinical Development of Nononcogenic Drugs (Small and Large Molecules). In A Comprehensive Guide to Toxicology in Nonclinical Drug Development (Second Edition); Faqi, A.S., Ed.; Academic Press: Boston, FL, USA, 2017; pp. 659–683. [Google Scholar]
- Da Silva, E.; Birkelund Sorli, J. Animal Testing for Acute Inhalation Toxicity: A Thing of the Past? Appl. Toxicol. 2018, 4, 89–90. [Google Scholar] [CrossRef]
- Jennings, P. The future of in vitro toxicology. Toxicology 2015, 29, 1217–1221. [Google Scholar] [CrossRef]
- Warheit, D.B.; Kreiling, R.; Levy, L.S. Relevance of the rat lung tumor response to particle overload for human risk assessment—Update and interpretation of new data since ILSI 2000. Toxicology 2016, 374, 42–59. [Google Scholar] [CrossRef]
- Henderson, R.F. Use of bronchoalveolar lavage to detect respiratory tract toxicity of inhaled material. Exp. Toxicol. Pathol. 2005, 57, 155–159. [Google Scholar] [CrossRef]
- Hoffman, E.; Patel, A.; Ball, D.; Klapwijk, J.; Millar, V.; Kumar, A.; Martin, A.; Mahendran, R.; Dailey, L.A.; Forbes, B.; et al. Morphometric Characterization of Rat and Human Alveolar Macrophage Cell Models and their Response to Amiodarone using High Content Image Analysis. Pharm. Res. 2017, 34, 2466–2476. [Google Scholar] [CrossRef] [Green Version]
- Wolkove, N.; Baltzan, M. Amiodarone pulmonary toxicity. Can. Respir. J. 2009, 16, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.L.; Sahai, E.A.; Yeo, M.; Bosch, M.; Dewar, A.; Olson, M.F. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat. Cell Biol. 2001, 3, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Howe, J.; Tan, K.S.W. Staurosporine-induced programmed cell death in Blastocystis occurs independently of caspases and cathepsins and is augmented by calpain inhibition. Microbiology 2010, 156, 1284–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colice, G.; Martin, R.J.; Israel, E.; Roche, N.; Barnes, N.; Burden, A.; Polos, P.; Dorinsky, P.; Hillyer, E.V.; Lee, A.J.; et al. Asthma outcomes and costs of therapy with extrafine beclomethasone and fluticasone. J. Allergy Clin. Immunol. 2013, 132, 45–54.e10. [Google Scholar] [CrossRef]
- Salbutamol. Meyler’s Side Effects of Drugs, 6th ed.; Aronson, J.K., Ed.; Elsevier: Oxford, UK, 2016; pp. 281–289. [Google Scholar]
- Hoffman, E.; Kumar, A.; Kanabar, V.; Arno, M.; Preux, L.; Millar, V.; Page, C.; Collins, H.; Mudway, I.; Dailey, L.A.; et al. In Vitro Multiparameter Assay Development Strategy toward Differentiating Macrophage Responses to Inhaled Medicines. Mol. Pharm. 2015, 12, 2675–2687. [Google Scholar] [CrossRef]
- Matheson, L.A.; Labow, R.S.; Santerre, J.P. Biodegradation of polycarbonate-based polyurethanes by the human monocyte-derived macrophage and U937 cell systems. J. Biomed. Mater. Res. 2002, 61, 505–513. [Google Scholar] [CrossRef]
- Kuroda, A.; Sugiyama, E.; Taki, H.; Mino, T.; Kobayashi, M. Interleukin-4 inhibits the gene expression and biosyntheis of cytosolic phospholipase A2 in lipopolysaccharide stimulated U937 macrophage cell line and freshly prepared adherent rheumatoid synovial cells. Biochem. Biophys Res. Commun. 1997, 230, 40–43. [Google Scholar] [CrossRef]
- Prieto, J.; Eklund, A.; Patarroyo, M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol. 1994, 156, 191–211. [Google Scholar] [CrossRef]
- Smirnova, L.; Harris, G.; Leist, M.; Hartung, T. Cellular resilience. Altex 2015, 32, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Bortner, C.D.; Cidlowski, J.A. Apoptotic volume decrease and the incredible shrinking cell. Cell Death Differ. 2002, 9, 1307. [Google Scholar] [CrossRef]
- Cummings, B.S.; Schnellmann, R.G. Measurement of cell death in mammalian cells. Curr. Protoc. Pharmacol. 2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, E.; Breznan, D.; Stals, S.; Jasinghe, V.J.; Gonçalves, D.; Girard, D.; Faucher, S.; Vincent, R.; Thierry, A.R.; Lavigne, C. Cytotoxicity assessment, inflammatory properties, and cellular uptake of Neutraplex lipid-based nanoparticles in THP-1 monocyte-derived macrophages. Nanobiomedicine 2017, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passmore, J.S.; Lukey, P.T.; Ress, S.R. The human macrophage cell line U937 as an in vitro model for selective evaluation of mycobacterial antigen-specific cytotoxic T-cell function. Immunology 2001, 102, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Hoffman, E.; Ball, D.; Klapwijk, J.; Steven, R.T.; Dexter, A.; Bunch, J.; Baker, D.; Murnane, D.; Hutter, V.; et al. Comparison of Oral, Intranasal and Aerosol Administration of Amiodarone in Rats as a Model of Pulmonary Phospholipidosis. Pharmaceutics 2019, 11, 345. [Google Scholar] [CrossRef] [Green Version]
- Reasor, M.J.; McCloud, C.M.; Beard, T.L.; Ebert, D.C.; Kacew, s.; Gardner, M.F.; Aldern, K.A.; Hostetler, K.Y. Comparative evaluation of amiodarone-induced phospholipidosis and drug accumulation in Fischer-344 and Sprague-Dawley rats. Toxicology 1996, 106, 139–147. [Google Scholar] [CrossRef]
- Morissette, G.; Ammoury, A.; Rusu, D.; Marguery, M.C.; Lodge, R.; Poubelle, P.E.; Marceau, F. Intracellular sequestration of amiodarone: Role of vacuolar ATPase and macroautophagic transition of the resulting vacuolar cytopathology. Br. J. Pharmacol. 2009, 157, 1531–1540. [Google Scholar] [CrossRef] [Green Version]
- Anderson, N.; Borlak, J. Drug-induced phospholipidosis. FEBS Lett. 2006, 580, 5533–5540. [Google Scholar] [CrossRef]
- Reasor, M.J.; McCloud, C.M.; DiMatteo, M.; Schafer, R.; Ima, A.; Lemaire, I. Effects of amiodarone-induced phospholipidosis on pulmonary host defense functions in rats. Soc. Exp. Biol. Med. (N.Y.) 1996, 211, 346–352. [Google Scholar] [CrossRef]
- McCloud, C.M.; Beard, T.L.; Kacew, S.; Reasor, M.J. In Vivo and In Vitro Reversibility of Chlorphentermine-Induced Phospholipidosis in Rat Alveolar Macrophages. Exp. Molecular Pathol. 1995, 62, 12–21. [Google Scholar] [CrossRef]
- Kramer, N.I.; Di Consiglio, E.; Blaauboer, B.J.; Testai, E. Biokinetics in repeated-dosing in vitro drug toxicity studies. Toxicology 2015, 30, 217–224. [Google Scholar] [CrossRef]
- Quaglino, D.; Ha, H.R.; Duner, E.; Bruttomesso, D.; Bigler, L.; Follath, F.; Realdi, G.; Pettenazzo, A.; Baritussio, A. Effects of metabolites and analogs of amiodarone on alveolar macrophages: Structure-activity relationship. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L438–L447. [Google Scholar] [CrossRef] [PubMed]
- Haller, T.; Cerrada, A.; Pfaller, K.; Braubach, P.; Felder, E. Polarized light microscopy reveals physiological and drug-induced changes in surfactant membrane assembly in alveolar type II pneumocytes. Biochim. Biophys. Acta (BBA) Biomembr. 2018, 1860, 1152–1161. [Google Scholar] [CrossRef]
- Belmokhtar, C.A.; Hillion, J.; Ségal-Bendirdjian, E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 2001, 20, 3354–3362. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, U.; Groscurth, P. Morphological Features of Cell Death. Physiol. 2004, 19, 124–128. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Geske, F.J.; Lieberman, R.; Strange, R.; Gerschenson, L.E. Early stages of p53-induced apoptosis are reversible. Cell Death Differ 2001, 8, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.L.; Yuen, K.L.; Tang, H.M.; Fung, M.C. Reversibility of apoptosis in cancer cells. Br. J. Cancer 2009, 100, 118–122. [Google Scholar] [CrossRef] [Green Version]
- Stepczynska, A.; Lauber, K.; Engels, I.H.; Janssen, O.; Kabelitz, D.; Wesselborg, S.; Schulze-Osthoff, K. Staurosporine and conventional anticancer drugs induce overlapping, yet distinct pathways of apoptosis and caspase activation. Oncogene 2001, 20, 1193–1202. [Google Scholar] [CrossRef] [Green Version]
- Yamaki, K.; Hong, J.; Hiraizumi, K.; Ahn, J.W.; Zee, O.; Ohuchi, K. Participation of various kinases in staurosporine induced apoptosis of RAW 264.7 cells. J. Pharm. Pharmacol. 2002, 54, 1535–1544. [Google Scholar] [CrossRef]
- Estrada-Reyes, E.; Del Rio-Navarro, B.E.; Rosas-Vargas, M.A.; Nava-Ocampo, A.A. Co-administration of salbutamol and fluticasone for emergency treatment of children with moderate acute asthma. Pediatric Allergy Immunol. 2005, 16, 609–614. [Google Scholar] [CrossRef]
- Johnson, M. The anti-inflammatory profile of fluticasone propionate. Allergy 1995, 50, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Wraight, J.M.; Hancox, R.J.; Herbison, G.P.; Cowan, J.O.; Flannery, E.M.; Taylor, D.R. Bronchodilator tolerance: The impact of increasing bronchoconstriction. Eur. Respir. J. 2003, 21, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Scientific rationale for inhaled combination therapy with long-acting β2-agonists and corticosteroids. Eur. Respir. J. 2002, 19, 182–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffman, E.; Murnane, D.; Hutter, V. Investigating the Suitability of High Content Image Analysis as a Tool to Assess the Reversibility of Foamy Alveolar Macrophage Phenotypes In Vitro. Pharmaceutics 2020, 12, 262. https://doi.org/10.3390/pharmaceutics12030262
Hoffman E, Murnane D, Hutter V. Investigating the Suitability of High Content Image Analysis as a Tool to Assess the Reversibility of Foamy Alveolar Macrophage Phenotypes In Vitro. Pharmaceutics. 2020; 12(3):262. https://doi.org/10.3390/pharmaceutics12030262
Chicago/Turabian StyleHoffman, Ewelina, Darragh Murnane, and Victoria Hutter. 2020. "Investigating the Suitability of High Content Image Analysis as a Tool to Assess the Reversibility of Foamy Alveolar Macrophage Phenotypes In Vitro" Pharmaceutics 12, no. 3: 262. https://doi.org/10.3390/pharmaceutics12030262
APA StyleHoffman, E., Murnane, D., & Hutter, V. (2020). Investigating the Suitability of High Content Image Analysis as a Tool to Assess the Reversibility of Foamy Alveolar Macrophage Phenotypes In Vitro. Pharmaceutics, 12(3), 262. https://doi.org/10.3390/pharmaceutics12030262




